
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Trop. Dis., 20 September 2024
Sec. Emerging Tropical Diseases
Volume 5 - 2024 | https://doi.org/10.3389/fitd.2024.1375087
This article is part of the Research TopicScrub Typhus & Its Changing DynamicsView all 6 articles
 Barath Prashanth Sivasubramanian1*
Barath Prashanth Sivasubramanian1* Abul Hasan Shadali Abdul Khader2
Abul Hasan Shadali Abdul Khader2 Diviya Bharathi Ravikumar3
Diviya Bharathi Ravikumar3 Francis Vino Dominic Savio4*
Francis Vino Dominic Savio4* Umabalan Thirupathy5
Umabalan Thirupathy5 Varshini Thiruvadi6
Varshini Thiruvadi6 Rhea Prasad3
Rhea Prasad3 Hema Thokala7
Hema Thokala7 Husna Qadeer3
Husna Qadeer3 Dhiraj Poragal Venkataperumal3
Dhiraj Poragal Venkataperumal3 Ashima Gupta8
Ashima Gupta8 Nagaraj Sanchitha Honganur9
Nagaraj Sanchitha Honganur9 Raghavendra Tirupathi10
Raghavendra Tirupathi10Scrub typhus, a zoonotic disease caused by Orientia tsutsugamushi and transmitted by chiggers, predominantly affects the Asia-Pacific region. Complications of Scrub Typhus involve multiple systems, including cardiovascular (pericarditis, arrhythmia, myocarditis), respiratory (acute respiratory distress syndrome), hepatic (hepatitis), and renal (azotemia). In this review, we comprehensively focused on the cardiac manifestations caused due to scrub typhus. Scrub typhus-induced pericarditis should be suspected in patients residing in endemic regions presenting with fever, thrombocytopenia, and pericardial effusion. If undetected, it frequently leads to cardiomegaly, pericardial effusion, and congestive heart failure. Heart failure with scrub typhus commonly occurs following myocardial inflammation, particularly in patients with pre-existing cardiac disorders. Scrub typhus myocarditis is a relatively rare, but serious cardiac complication with a high mortality rate of up to 24.0%. Arrhythmias arise due to the involvement of the interventricular septum, coronary artery, or cardiac valves causing variable ECG findings including sinus arrhythmia, T wave changes, and QTc interval prolongation. Atrial fibrillation due to scrub typhus is associated with a 1.3 fold increase in 3-month mortality. These cardiac complications are mainly assessed using electrocardiography (ECG) and echocardiography. Serology is the primary diagnostic tool for O. tsutsugamushi. While the Scrub Typhus Detect IFA test offers 100% sensitivity, the Weil Felix test is specific and cost-effective. Nested PCR and ELISA are effective for early detection but are limited to resource-rich settings. Diagnostic difficulties arise from nonspecific symptoms and current testing limitations. Vaccine development using extracellular vesicles, nanoparticles, and subunit vaccines shows promise. Combined therapy with doxycycline and azithromycin is recommended for cardiac complications, alongside guideline-directed therapy. The review underscores the need for heightened clinical awareness and prompt management of scrub typhus, especially in endemic regions. It also highlights the necessity for further research into the pathogenesis of cardiac involvement and the development of more effective diagnostic tools and treatments.
Scrub typhus is a zoonotic illness caused by Orientia tsutsugamushi, transmitted by the bite of trombiculid mite larvae (chiggers). The disease is most frequently reported within the “tsutsugamushi triangle” of the Asia-Pacific region, which encompasses more than 8 million km², including Thailand, India, China, Korea, Philippines, Taiwan, Sri Lanka, and extending from the Russian Far East in the north, to Pakistan in the west, Australia in the south, and Japan in the east (1). The pathophysiology of O tsutsugamushi involves vasculitis due to the infection of endothelial cells, leading to perivascular infiltration of T cells and monocytes or macrophages. Subsequently, a wide range of inflammatory responses occurs, with endothelial and non-endothelial cells producing various cytokines. These cytokines can have both beneficial effects, such as antimicrobial activity, and detrimental effects, causing tissue destruction in the infected host. This immune response’s dual nature can lead to severe complications such as hepatitis, renal failure, meningoencephalitis, and respiratory failure, including acute respiratory distress syndrome (ARDS) and myocarditis (2). The bacterial dissemination and replication during early infection need to be better understood (3). Vascular involvement is hypothesized to occur by downregulating the expression of glycoprotein-96 in the endothelial cells and phagocytes and by neutralizing the host’s immune response (4). Orientia infection is also proposed to affect CCR2, which drives blood monocytes into the lung, accelerating bacterial replication and the development of inflammation of the pulmonary interstitium (5). C-type lectin receptors, CCR7/dendritic cell-mediated mechanisms are also involved in immune dysregulation in scrub typhus (3, 6, 7). TNF receptors played an intrinsic role in CD8+ T cell activation, revealing the protective immunity of TNF against O. tsutsugamushi infection (8). Scrub typhus can increase several interleukins (IL-1alpha/beta, IL-4, IL-6, IL-7, IL10, IL-11, IL-18, and IL-24), chemokines (CXCL8, CCL2/MCP1, CCL5/RANTES, and CCL17), growth factors (NODAL, CNTF, and CSF2/GM-CSF), and TNFSF13B (9, 10). This can disrupt the B/T cell microenvironment and dysregulation of B cell responses during active infection (11). The cytokine signatures can be used as depletion targets in future experiments (12).
Scrub typhus is known to produce several cardiotoxic, hepatotoxic, and nephrotoxic metabolites (13). The incidence of complicated scrub typhus increases with delayed diagnosis and treatment of scrub typhus (14). Untreated infections can cause multi-organ involvement and raise the cost of treatment (15). The most common complications are hepatitis (40.5%), thrombocytopenia (28.4%), acute respiratory distress syndrome (20.5%), acute kidney injury (19.2%), meningitis (16.4%), shock (16.2%), and myocarditis (15.5%) (16, 17). Scrub typhus can persist for longer durations in the affected organs (18). Cardiac involvement due to scrub typhus is attributed to macrophage activation, immune-mediated or direct invasion of cardiomyocytes, and electrolyte alterations (4, 19–22). Table 1 depicts the Cardiac manifestation of scrub typhus.
Several studies are being published in the native languages of endemic areas and studies that rely on national surveillance systems often face missing data due to unreported cases. Most studies are conducted on a small sample size with a single-center design. Due to a paucity of literature on cardiovascular compilations, there is a delay in timely diagnosis (23, 24). Scrub typhus is a rapidly emerging public health threat but no national protocols for prevention exist in Southeast Asia (25).
This article aims to provide a comprehensive review of the literature on the cardiac manifestation of scrub typhus to aid clinicians in timely diagnosis by ordering the ideal tests and optimal treatment modalities.
Scrub typhus is endemic to a vast area known as the “tsutsugamushi triangle,” encompassing over 8 million km² of the Asia-Pacific region. This includes countries like Thailand, India, China, Korea, the Philippines, Taiwan, and Sri Lanka, stretching from the Russian Far East in the north to Pakistan in the west, Australia in the south, and Japan in the east. Within this region, specific locations like the northeastern states of India, Fujian province in China, the northern region of Thailand, and the southernmost part of Taiwan exhibit higher disease prevalence (1, 16). In South Korea, scrub typhus is most common in the southern and western regions, with a peak incidence in the later months of the year (26). The disease is also concentrated in the western province of Sri Lanka and limited to northern Queensland, Australia (27, 28). Epidemiology across various countries with respective prefectures and seasonal pattern have been described in the below.
The disease poses a significant public health threat, with an estimated one million cases annually. While primarily concentrated within the tsutsugamushi triangle, sporadic cases have been identified outside this region, underscoring the importance of understanding its global distribution (1) (Table 2).
Pericarditis is an additional noteworthy cardiac complication linked with scrub typhus. It frequently leads to cardiomegaly and, if untreated, can progress to pericardial effusion and congestive heart failure (26). Recent studies by Karthik et al. and Chin et al. have observed pericardial effusion in 51% and 20% of their respective patient populations. However, there is no mention of the development of cardiac tamponade in the studies (27, 28). In patients residing in endemic regions presenting with fever, thrombocytopenia, and pericardial effusion, clinical suspicion of scrub-typhus pericarditis should be taken into consideration (29). In certain cases, perimyocarditis can also occur and poses a diagnostic challenge due to its nonspecific presentation (30).
The diagnosis entails confirming the presence of pericardial effusion and serological tests such as the immunofluorescence assay (IFA) or the IgM enzyme-linked immunosorbent assay (ELISA) (26). The recently developed tool of PCR-based detection of tissue or tissue fluid can also aid in the diagnosis of scrub typhus (26, 29). Other supportive findings include an increase in inflammatory markers (erythrocyte sedimentation rate/C-reactive protein), ST-elevation in the anterior leads of the ECG, and a hypervascular epicardial region observed in CT angiography (30). Treatment options consist of doxycycline and azithromycin as an alternative if any side effects develop with doxycycline (29). Timely suspicion and appropriate management are crucial for achieving clinical improvement and reducing mortality (26).
Cardiac failure in patients with scrub typhus commonly occurs following myocardial inflammation. Younger patients often had a moderate reduction in ejection fraction and patients with predisposed cardiac disorders had a higher risk of developing heart failure. The presentation of heart failure characterized by dyspnea and features of fluid overload, may or may not be present (31–36). Even rare presentations such as Takotsubo cardiomyopathy and acute cor pulmonale with predominant lung involvement are seen with scrub typhus (31, 37). Due to atypical presentation and absence of physical signs, a high index of suspicion is necessary to diagnose heart failure before the development of cardiogenic shock (14, 38, 39). This warrants the identification of scrub typhus, chest X-ray, BNP (or NT-proBNP), and echocardiogram workup in suspected patients (28, 36, 40). 18.5% of patients with scrub typhus had detectable features of congestive heart failure on Chest X-ray (40). An echocardiogram is recommended to assess cardiac chamber size, wall thickness, and ventricular function in suspected myocarditis (41). Patients with CK-MB >25 U/L had a significantly longer length of hospital stay (36). If the diagnosis is uncertain, an endomyocardial biopsy can be obtained, but we exercise caution in patients with heart failure to further prevent the development of cardiogenic shock (42).
Management of acute heart failure from scrub typhus includes hemodynamic monitoring, stabilization with fluid resuscitation, vasopressor support, GDMT-guided therapy, and appropriate antibiotics such as doxycycline (31, 34, 42–44). As glucocorticoid is often used in severe sepsis, low doses of the same can be used in life threatening infections (45). One study reported a dramatic improvement in scrub typhus when steroids and chloramphenicol were used together (46). In our hospital, we have observed prompt improvements with the use of steroids for refractory shock (severe myocarditis), impending adult respiratory distress syndrome, or repeated seizures (severe encephalitis). However, as steroids inhibit the development of immunity against scrub typhus, the duration of use should be minimized (47). ECMO has also been effective in treating patients with complications (14). A repeat echo, 2 weeks following recovery is recommended to assess the resolution of Ejection Fraction and heart enlargement (31, 35, 44).
During acute infection, arrhythmia occurs due to the involvement of the interventricular septum, coronary artery, or cardiac valves (48). Table 3 depicts different ECG findings encountered in Scrub Typhus.
We focused on atrial fibrillation due to scrub typhus because it is a poor prognostic factor, with a 1.3-fold increase in 3-month mortality associated with new-onset AF (49). About 1% of patients with scrub typhus are found to have new-onset AF and the majority (87.2%) were >60 years of age. Regardless of occurrence, these patients were also more likely to have a previous diagnosis of cardiovascular disease, with a 1.67 times higher risk in patients with prior hypertension, a 1.9 times higher risk in ischemic heart disease, and a 1.5 times higher risk of heart failure in developing AF (49–51). Atrial fibrillation was seen in patients with an older age, male sex, fever, leukocytosis, and abnormal renal and liver functions (50, 51). However, the management of atrial fibrillation remains the same. In patients with the acute phase of infection, it is important to perform serial ECG monitoring (52) and in patients with suspected atrial fibrillation baseline cardiac enzymes (mainly CK MB) and Echocardiogram are needed. These will aid in identifying myocarditis as Paroxysmal AF is a strong predictor of myocarditis (70% sensitivity and 84% specificity) (28).
For treatment of atrial fibrillation, we recommend taking appropriate measures to maintain hemodynamic stability including ICU admission (52). Achieving rate control is key in the treatment through the use of agents like calcium channel blockers and/or beta-blockers. If rate control is hard to achieve or the patient is symptomatic, attain rhythm control through cardioversion or antiarrhythmic agents (amiodarone, flecainide, propafenone, dofetilide, and ibutilide) (53). Prompt treatment of scrub typhus with oral or intravenous antibiotics (doxycycline, azithromycin, or rifampicin) prevents serious cardiac complications (48, 52, 54). In patients with hemodynamic instability, intravenous antibiotics are preferred to achieve therapeutic levels (55). In addition to this, monitoring the metabolic panel is essential to prevent further complications (52).
Myocarditis is defined as the inflammation of the cardiac muscle that causes chest pain, palpitations, and heart failure and is often accompanied by changes in the electrocardiogram, and elevated levels of inflammatory and cardiac biomarkers (56). Myocarditis is relatively rare and has been reported in only a few cases of scrub typhus and varies in severity from mild to fulminant, with some cases requiring mechanical circulatory support (14, 30, 44–46). The incidence of myocarditis in scrub typhus varies and has been reported to range from 8% to 14% (27, 28, 57–63) and a recent systematic review indicated an incidence of 4.3% (23, 27, 28, 62). Scrub typhus myocarditis is a serious cardiac complication with a mortality rate as high as 24.0% (23). Certain risk factors have been identified that can increase the risk of acute myocarditis, including paroxysmal atrial fibrillation (OR= 2.85; p= 0.02), elevated levels of total bilirubin (OR= 1.2; p= 0.04), and a shorter duration of symptoms before presentation (mean duration of illness= 6.8d vs 8.3d OR= 0.69; p= 0.04) (28, 30). The other risk factors that contribute to a poor prognosis with scrub typhus include liver disease (OR= 62.70; p=0.011), elevated C-reactive protein (OR= 122.69; p= 0.002), elevated bilirubin levels (RR= 9.28; p= 0.02), and elevated creatinine levels (RR= 43.9; p= 0.003) (28, 64). Identifying and acknowledging these risk factors promptly facilitates risk stratification and enables timely management (28, 64). The exact mechanism of myocarditis in scrub typhus is not well understood, and further research is needed for better understanding (14).
Patients with myocarditis can present with nonspecific symptoms like fever, myalgia, palpitations, and exertional dyspnea or even with cardiogenic shock and sudden cardiac death (65, 66). Thus, in cases of systemic infection with scrub typhus and concomitant new cardiovascular dysfunction or elevated cardiac enzymes, myocarditis should be suspected (45). Various tests like ECG, echocardiography, endomyocardial biopsy, and cardiac MRI aid in the diagnosis of acute myocarditis in scrub typhus (27, 44, 45). ST abnormalities on ECG may serve as an initial manifestation in the majority of patients (44). In a prospective study, ECG changes such as T wave inversion (p= 0.02) and QRS changes (p< 0.001) correlated with the occurrence of myocarditis (27). Cardiac biomarker- Troponin T is utilized as the most sensitive biomarker to indicate myocyte injury in patients with suspected myocarditis. The diagnosis of myocarditis is considered if myocardial injury coincides with global myocardial dysfunction (27). Echocardiography to detect impaired left ventricular function is needed in suspected cases of myocarditis (27). Endomyocardial biopsy has proven helpful in establishing the diagnosis in certain cases, as highlighted in a recent case report by Park et al. (14, 42, 67). A special indication of endomyocardial biopsy includes patients who develop acute decompensated heart failure of unknown etiology (less than 2 weeks in duration) (68). This technique has its limitations, such as invasiveness in hemodynamically unstable patients, difficulties in acquiring adequate specimens, and low sensitivity (45). Recently, cardiac MRI has been increasingly utilized for the diagnosis and prognostic assessment of myocarditis. This imaging modality offers three key advantages- it indirectly assesses cardiac function, aids in guiding myocardial biopsy at suitable sites, such as focal regions identified on the cardiac MRI, and facilitates the distinction between myocarditis and myocardial infarction based on the delayed gadolinium enhancement pattern in the myocardium (45, 69). Treatment for scrub typhus-induced myocarditis is doxycycline 100 mg IV or orally twice daily for 7-14 days and it has demonstrated efficacy in multiple cases (14, 44, 45). Interestingly, in certain severe cases, the addition of intravenous chloramphenicol to doxycycline is necessary (66). Low-dose glucocorticoids have been used in conjunction with antibiotics for severe myocarditis in a few cases with improvement (17, 47). However, this remains controversial as steroids inhibit the development of immunity against scrub typhus, and increase the risk of scrub relapse, and post-typhus asthenia. Also, in-vitro studies have shown steroids do not increase bacteria counts yet further in vivo research is warranted to support this line of management (70). In cases of fulminant progression that does not respond to medical management, the successful and prompt implementation of VA ECMO has proven to be beneficial (14, 46).
Scrub typhus can cause ischemic events through endothelial dysfunction and increase proinflammatory mediators which further worsens this event. It can also cause systemic vasculitis or can also cause plaque stability (71). Patients who have high-risk factors for acute coronary syndrome can also develop myocardial ischemia during the illness. Men and patients of 35-49 years of age had a 2-fold increased risk of developing acute coronary syndrome. The risk of ACS was also associated with diabetes (adjusted HR =2.77, 95% CI 2.04 to 3.76), hypertension (adjusted HR 1.88, 95% CI 1.38 to 2.76), and previous coronary artery disease (adjusted HR=1.53, 95% CI 1.03 to 2.27). However, they also remain independent risk factors of ACS after adjusting for covariates (72). During 6 month follow-up, patients who had scrub typhus had a 3-fold significant increase in the risk of developing ACS (95% CI 1.47 to 7.70) (72). As the pathogenesis involves systemic vasculitis, young patients have presented with no atherosclerotic blockage on coronary angiography but exhibit symptoms of acute coronary syndrome. Additionally, they show elevated cardiac enzymes and EKG changes (73). There are also cases of scrub typhus where thrombotic occlusion was identified and percutaneous intervention where necessary to stabilize the patient (74, 75). We recommend treating patients with appropriate antibiotics and depending on coronary angiography findings further management should be planned.
BCNIE (blood culture-negative infective endocarditis) can manifest in up to 31% of all patients with infective endocarditis with significant diagnostic and therapeutic difficulties. Scrub typhus-induced endocarditis is extremely rare and presents as blood-culture negative IE (76). However, based on clinical suspicion and endemicity in the region, clinicians should consider workup for scrub typhus as a possible etiology (77). In a case report by Yu et al. in 2016, chloramphenicol (1g q12h iv drip for 10 days) may be appropriate for scrub typhus-associated infective endocarditis. However, further large-scale prospective randomized controlled trials are necessary to validate this finding (77).
Serology is the primary diagnostic tool for O. tsutsugamushi infection. IgM antibody titer increases by the end of the 1st week of the infection, while IgG antibody peaks by the end of the 2nd week. The Weil-Felix test is the most affordable serological test to detect antibodies for various Proteus species. A titer of 1:320 or above, or a fourfold increase from 1:50, is considered positive (76). However, the test has a low sensitivity of 33% at a breakpoint titer of 1:80 but with 100% specificity and positive predictive value (78, 79). Indirect immunofluorescence antibody (IFA) is an expensive, specialized test to confirm infection before seroconversion (80, 81). The lateral flow rapid test and Scrub Typhus Detect use recombinant 56-kDa antigen and have shown a strong potential for diagnosing scrub typhus patients with 100% sensitivity and 92% specificity for IgM. This prototype product can help clinicians diagnose patients rapidly, accurately, and easily, allowing them to provide timely care (82). Some commercial laboratories in India offer the immunochromatographic test (ICT) that serves as a rapid diagnostic test, with the advantage of being inexpensive (83). Replacing fluorescein with peroxidase, indirect immunoperoxidase eliminates the need for a fluorescent microscope, making it beneficial in a resource-poor setting (84). Western immunoblot assay is an effective serodiagnostic tool for large-scale screening and confirming serologic diagnosis. It uses sodium dodecyl sulfate-gel electrophoresed and electro-blotted antigens and helps analyze cross-reactive strains (80). The most abundant and immunodominant protein is employed in a recombinant protein-based enzyme-linked immunosorbent assay, which has been developed to detect Orientia-specific antibodies in serum. Four prototype strains of Karp were used to generate three recombinant protein antigens, namely TA763 (r56C1), Kato (r56Kt), and Gilliam (r56Gm) Orientia. Chimeric proteins were compared to titers of serum samples against Karp, Kato, Gilliam, and TA763 strains. These new proteins had similar reactivity to parent proteins and were recognized by 14 Orientia strains, making them useful for diagnosis and vaccination. Chimeric C1 was identified as a potential substitute for parent proteins to diagnose Orientia infection and as a vaccine candidate with broad protective efficacy (81). It can be regarded as an enhanced, practical, and affordable substitute for the gold standard IFA in rapid diagnosis and seroprevalence (85). A cell-based ELISA technique was used to test serum samples from ST-positive rats and monoclonal antibodies. The accuracy, sensitivity, and specificity of this technique were 96.3%, 98.6%, and 84.6%, respectively. The results were consistent with those of immunofluorescence assays. This technique is safe, easy to operate, and does not require specialized equipment (86). Rickettsial culture can be obtained from buffy coats of heparinized blood, defibrinated whole blood, plasma, and skin biopsy. Different methods, such as MRC 5 cells, Vero cell culture, BHK21, etc., are used for this purpose, with Vero or L929 cells enabling quicker and more effective isolation (87). The average time required for culture isolation of Rickettsia species is four weeks (88). However, bacterial culture for O. tsutsugamushi is a tedious process requiring biosafety level 3 containment and significant technical expertise. Due to its complexity, it is primarily used for research purposes in reference laboratories.
Polymerase Chain Reaction (PCR) molecular detection using skin rash, lymph node, or blood samples is an efficient and sensitive method for diagnosing scrub typhus. Real-time PCR assays based on GroEL provide a more quantitative result, while nested PCR is one hundred times more sensitive than single PCR (76, 89, 90). Combined with IFA, nested PCR has a sensitivity of 82.2% and specificity of 100% (91) and other studies also show similar findings (87, 92, 93).
A tool was created to distinguish severe fever with thrombocytopenia syndrome (SFTS) from scrub typhus. It uses low CRP, thrombocytopenia, and leukopenia as variables. A score of >=2 has a specificity of 96.1% and a sensitivity of 93.1% for SFTS (89). Recently, the highly conserved O. tsutsugamushi 60 kDa GroEL chaperonin produced by E. coli was the focus of an immunochromatographic antigen detection test kit (ICT AgTK). Polyclonal antibodies, including a rGroEL-specific monoclonal antibody, were used as antigen detection reagents. In in-house validation studies, the test showed potential with a sensitivity, specificity, and accuracy of 85%, 100%, and 95%, respectively, compared to the combined clinical characteristics and standard IFA. This test holds promise for on-the-spot and early diagnosis of scrub typhus (90). In a recent report by Zuan Zhan et al, metagenomic next-generation has been used to diagnose scrub typhus in a patient with fever, multiorgan dysfunction, and negative serology (94). It has the advantage of having the ability to diagnose scrub typhus early in the course of illness (95). The investigations are summarized in Table 4.
The increase in outbreaks both within and outside the Tsutsugamushi Triangle, the emergence of antibiotic-resistant strains of Orientia, and the emergence of new Orientia species (101). During the 1990s, genotypes in Thailand that were resistant to chloramphenicol and doxycycline were identified (101, 102). The strains AFSC-3 and AFSC-4 of the pathogen are identified as resistant to doxycycline (102). Proposed mechanisms to explain resistance include dormant organisms within patients subjected to repeated or long-term exposure to antibiotics. Additionally, the supplementation of poultry feed in Thailand with antibiotics may result in the development of drug-resistant strains of chiggers. The ingestion of antibiotics by mites during their feeding on their rodent host, which frequently consumes grain designated for poultry, is a plausible consequence (101). The occurrence of treatment failures has also prompted reports of potential doxycycline resistance in India and South Korea (103). It is important to note that the prophylactic administration of doxycycline has failed to prevent breakthrough infections and can lead to the emergence of resistant strains (101). Despite this, there is no evidence of extensive presence or prevalent distribution of scrub typhus strains that are resistant to antibiotics (101).
When it comes to treating cardiac complications, our recommendation aligns with Varghese et al. The study by Varghese et al. was a prospective, multicenter, randomized trial with a large sample size, and concluded that combination therapy with azithromycin and doxycycline is better than monotherapy when it comes to severe sepsis and organopathies (104). We recommend combination therapy, especially in patients with multiple organ involvement and in those with multiple comorbidities that further increase their risk for cardiac complications (104). In patients who have not yet developed cardiac complications but are from endemic regions of scrub typhus and have an eschar on presentation, we recommend empirical therapy (103) as either a monotherapy or as a combination therapy (47, 104). In regions where doxycycline resistance is not a concern, drugs such as doxycycline, rifampicin, or azithromycin can be used (47). Rifampicin and doxycycline combination therapy showed more effectiveness and longer fever clearance than monotherapy (105). Since rifampicin is an integral part of antitubercular therapy, it should be kept as a last resort. In pregnant women, azithromycin is preferred over doxycycline. For the treatment of nonpregnant adults, oral doxycycline is comparable to iv azithromycin in treating scrub typhus (106). When treating an undifferentiated fever in clinic practice, cefotaxime is not a preferred therapy for scrub typhus. This is because when used in combination therapy, it has demonstrated an antagonistic effect with azithromycin and doxycycline. The reason behind the antagonism is not well understood (47). Table 5 depicts commonly used antimicrobial agents in scrub typhus infection.
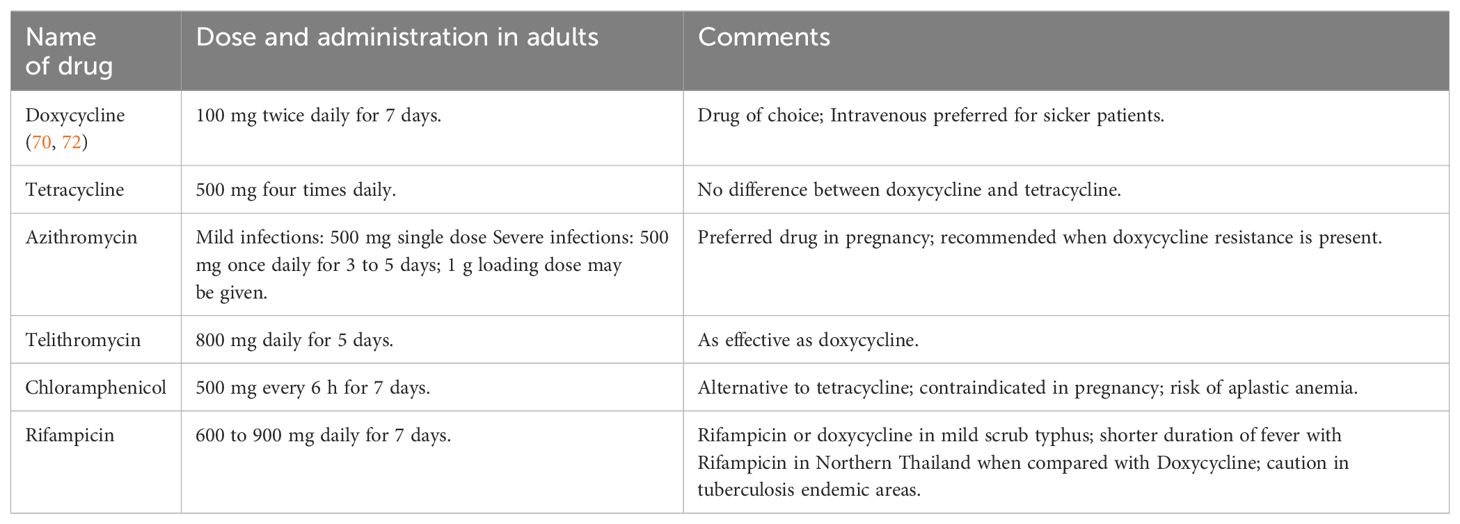
Table 5. Commonly used antimicrobial agents in scrub typhus infection (123).
Traditional ayurvedic and Chinese phytochemicals containing compounds (ZINC8214635, ZINC32793028, ZINC08101133, ZINC85625167, ZINC06018678, and ZINC13377938) have demonstrated successful inhibition of Orientia tsutsugamushi (107). Newer compounds and antibiotic discovery are underway. The CDC recommends avoiding contact with infected chiggers (25). General measures against vectors include minimizing exposure through clothes avoiding walking through dense vegetation, sitting in bare ground or grass, and use of insecticides and insect repellants. Appropriate clothing to avoid getting bitten in sun-exposed areas. Personal hygiene is advised and removal of clothing and thorough cleaning of skin and clothes with detergent can reduce the risk of infection. Postexposure prophylaxis with doxycycline is currently not recommended (1, 108). However, prophylactic medications of doxycycline, chloramphenicol, or tetracycline are provided in the endemic areas (single dose every 5 days for a total of 35 days) (1, 109). Measures to control rodents that attract these vectors are also recommended (108).
The existence of numerous strains of Orientia tsutsugamushi hinders the development of a broad and lasting immune response. Efforts to prepare a vaccine have been ongoing since World War II. A recent study has highlighted that the CD-1 outbred mice model proves valuable for understanding host susceptibility and facilitating future vaccine studies (110). Table 6 depicts the evolution of vaccines for scrub typhus. Field trials and large-scale attempts, such as the Tyburn operation and studies by the Japanese, were not fruitful, each demonstrating setbacks, particularly the waning of immunity or the acquisition of a fatal infection post-vaccination (112–115). Vaccine preparation has been attempted through formalin-inactivated pathogens, live attenuated strains, irradiated strains, and using antibiotics and live vaccines together. However, all these strategies have failed to provide either long-term immunity for homologous strains or any immunity against heterologous strains (115–122). This encouraged a shift towards the development of subunit vaccines (112).
Over the last two decades, the focus on scrub typhus subunit vaccine involved the production of recombinant proteins (kDa antigens) and their incorporation into DNA vaccine candidates through cloning (124, 125). Research on the 56-kDa protein of Rickettsia tsutsugamushi explored TSA56 (56-kDa type-specific antigen), a key outer membrane protein, as a potential vaccine candidate for generating immunity against scrub typhus. Kp r56 showed strong immunogenicity, triggering immune responses and IFN-γ production (126). The plasmid DNA vaccine pKarp56, pKarp110, 47 kDa gene fragment, and the fused antigen Sta56-47 demonstrated the ability to elicit robust immune responses (124, 127–129). Additionally, a novel recombinant antigen derived from the conserved regions of the 56 kDa type-specific antigen (cTSA56) provided superior protection against both homologous and heterologous genotypes (130). Recent studies also explored the protective effects of ScaA immunization and Zinc oxide nanoparticles (ZNPs) as a novel vaccine carrier system for O. tsutsugamushi (131, 132). Subsequently, dual-antigen subunit vaccine nanoparticles (47 kD and 56 kD) also highlight their potential as a promising vaccine strategy (133).
The intranasal rec56 vaccines elicited a higher IgG response than the intramuscular route (134). Supporting this, Park et al. demonstrate the development of an intranasal vaccine targeting Orientia tsutsugamushi’s outer-membrane protein (OMPOT), intranasal vaccination boosts cell-mediated and protective immunity in pulmonary compartments (135). A model vaccine, utilizing extracellular vesicles (EVs) derived from Salmonella expressing DNA sequences of full-length Ot proteins (TSA56, ScaA, ScaC, ScaD, and ScaE), showed promising results. Inoculation with EVs from TSA56-expressing cells protected mice from Salmonella-induced illness, suggesting the potential for scrub typhus immunizations based on T-cell immune response (136).
We conducted a review of the literature to raise awareness on cardiac complications of scrub typhus. Our review is limited to strain-specific epidemiological patterns and treatment options based on disease severity. The early identification of at-risk cases is key to managing these complications, particularly patients from endemic regions presenting with eschars. IFA is the gold standard for investigation and rapid ICT kits are invaluable in resource-limited areas. The approach to the individual cardiac complications is decided based on the presentation and investigations such as ECG and echocardiography findings of the patients. We recommend combined therapy with doxycycline and azithromycin for treating cardiac complications in addition to the guideline-directed therapy for the cardiac event. Vaccine development using extracellular vesicles, nanoparticles, and subunit vaccines containing recombinant proteins has demonstrated potential. However further research is needed to design a vaccine that can provide a long-lasting immunity against scrub typhus.
BS: Writing – original draft, Writing – review & editing. AA: Writing – review & editing, Writing – original draft. DR: Writing – review & editing, Writing – original draft. FD: Writing – original draft, Writing – review & editing. UT: Writing – review & editing, Writing – original draft. VT: Writing – review & editing, Writing – original draft. RP: Writing – original draft. HT: Writing – original draft. HQ: Writing – original draft. DP: Writing – original draft. AG: Writing – original draft. NH: Writing – original draft. RT: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Xu G, Walker DH, Jupiter D, Melby PC, Arcari CM. A review of the global epidemiology of scrub typhus. PloS Negl Trop Dis. (2017) 11:e0006062. doi: 10.1371/journal.pntd.0006062
2. Singh OB, Panda PK. Scrub typhus. Treasure Island, Florida: StatPearls Publishing (2024). Available at: https://www.ncbi.nlm.nih.gov/books/NBK558901/.
3. Liang Y, Wang H, Gonzales C, Thiriot J, Sunyakumthorn P, Melby PC, et al. CCR7/dendritic cell axis mediates early bacterial dissemination in Orientia tsutsugamushi-infected mice. Front Immunol. (2022) 13:1061031. doi: 10.3389/fimmu.2022.1061031
4. Rajapakse S, Rodrigo C, Fernando D. Scrub typhus: pathophysiology, clinical manifestations and prognosis. Asian Pac J Trop Med. (2012) 5:261–4. doi: 10.1016/S1995-7645(12)60036-4
5. Petermann M, Orfanos Z, Sellau J, Gharaibeh M, Lotter H, Fleischer B, et al. CCR2 deficiency impairs ly6Clo and ly6Chi monocyte responses in orientia tsutsugamushi infection. Front Immunol. (2021) 12:670219. doi: 10.3389/fimmu.2021.670219
6. Fisher J, Card G, Liang Y, Trent B, Rosenzweig H, Soong L. Orientia tsutsugamushi selectively stimulates the C-type lectin receptor Mincle and type 1-skewed proinflammatory immune responses. PloS Pathog. (2021) 17:e1009782. doi: 10.1371/journal.ppat.1009782
7. Fisher J, Gonzales C, Chroust Z, Liang Y, Soong L. Orientia tsutsugamushi infection stimulates syk-dependent responses and innate cytosolic defenses in macrophages. Pathogens. (2022) 12:1. doi: 10.3390/pathogens12010053
8. Liang Y, Fisher J, Gonzales C, Trent B, Card G, Sun J, et al. Distinct role of TNFR1 and TNFR2 in protective immunity against orientia tsutsugamushi infection in mice. Front Immunol. (2022) 13:867924. doi: 10.3389/fimmu.2022.867924
9. Ge H, Farris CM, Tong M, Maina A, Richards AL. Transcriptional profiles of cytokines and chemokines reveal important pro-inflammatory response from endothelial cells during Orientia tsutsugamushi infection. Microbes Infect. (2019) 21:313–20. doi: 10.1016/j.micinf.2019.01.002
10. Soong L, Wang H, Shelite TR, Liang Y, Mendell NL, Sun J, et al. Strong type 1, but impaired type 2, immune responses contribute to Orientia tsutsugamushi-induced pathology in mice. PloS Negl Trop Dis. (2014) 8:e3191. doi: 10.1371/journal.pntd.0003191
11. Gonzales C, Liang Y, Fisher J, Card G, Sun J, Soong L. Alterations in germinal center formation and B cell activation during severe Orientia tsutsugamushi infection in mice. PloS Negl Trop Dis. (2023) 17:e0011090. doi: 10.1371/journal.pntd.0011090
12. Luce-Fedrow A, Chattopadhyay S, Chan TC, Pearson G, Patton JB, Richards AL. Comparison of lethal and nonlethal mouse models of orientia tsutsugamushi infection reveals T-cell population-associated cytokine signatures correlated with lethality and protection. Trop Med Infect Dis. (2021) 6:1. doi: 10.3390/tropicalmed6030121
13. Choi S, Ahn DH, Yoo MG, Lee HJ, Cho SB, Park HB, et al. Urine metabolite of mice with orientia tsutsugamushi infection. Am J Trop Med Hyg. (2023) 108:296–304. doi: 10.4269/ajtmh.20-1608
14. Park H, Lim Y, Kim MC, Kim SE, Jeong IS, Choi YD, et al. Case report: fulminant myocarditis successfully treated with extracorporeal membrane oxygenation in ikeda strain orientia tsutsugamushi infection. Front Cardiovasc Med. (2021) 8:795249. doi: 10.3389/fcvm.2021.795249
15. John KJ, George TK, Joy M, John B, Abraham OC, Prasad J. Costs & outcomes of hospitalized scrub typhus infection in a tertiary hospital in south India. Indian J Med Res. (2023) 157:559–67. doi: 10.4103/ijmr.IJMR_3917_20
16. Devasagayam E, Dayanand D, Kundu D, Kamath MS, Kirubakaran R, Varghese GM. The burden of scrub typhus in India: A systematic review. PloS Negl Trop Dis. (2021) 15:e0009619. doi: 10.1371/journal.pntd.0009619
17. Ravikumar DB, Sivasubramanian BP, Shanmugam SN, Krishnaswamy V, Rabaan A, Al-Tawfig JA, et al. Multifaceted realities of scrub typhus: a case series from southern India. Infez Med. (2023) 31:384–93. doi: 10.53854/liim-3103-12
18. Soong L, Mendell NL, Olano JP, Rockx-Brouwer D, Xu G, Goez-Rivillas Y, et al. An intradermal inoculation mouse model for immunological investigations of acute scrub typhus and persistent infection. PloS Negl Trop Dis. (2016) 10:e0004884. doi: 10.1371/journal.pntd.0004884
19. Garg D, Manesh A. Neurological facets of scrub typhus: A comprehensive narrative review. Ann Indian Acad Neurol. (2021) 24:849–64. doi: 10.4103/aian.aian_739_21
20. Cho NH, Choi CY, Seong SY. Down-regulation of gp96 by Orientia tsutsugamushi. Microbiol Immunol. (2004) 48:297–305. doi: 10.1111/j.1348-0421.2004.tb03510.x
21. Trent B, Fisher J, Soong L. Scrub typhus pathogenesis: innate immune response and lung injury during orientia tsutsugamushi infection. Front Microbiol. (2019) 10:2065. doi: 10.3389/fmicb.2019.02065
22. Parchani A, Krishnan Vs G, Kumar VKS. Electrocardiographic changes in dengue fever: A review of literature. Int J Gen Med. (2021) 14:5607–14. doi: 10.2147/IJGM.S328755
23. Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PloS Negl Trop Dis. (2015) 9:e0003971. doi: 10.1371/journal.pntd.0003971
24. Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: A systematic review. PloS Negl Trop Dis. (2017) 11:e0005838. doi: 10.1371/journal.pntd.0005838
25. CDC. Centers for disease control and prevention (2021). Available online at: https://www.cdc.gov/typhus/scrub/index.html.
26. Chang JH, Ju MS, Chang JE, Park YS, Han WS, Kim IS, et al. Pericarditis due to Tsutsugamushi disease. Scand J Infect Dis. (2000) 32:101–2. doi: 10.1080/00365540050164344
27. Karthik G, Sudarsan TI, Peter JV, Sudarsanam T, Varghese GM, Kundavaram P, et al. Spectrum of cardiac manifestations and its relationship to outcomes in patients admitted with scrub typhus infection. Pediatr Crit Care Med. (2018) 7:16–23. doi: 10.5492/wjccm.v7.i1.16
28. Chin JY, Kang KW, Moon KM, Kim J, Choi YJ. Predictors of acute myocarditis in complicated scrub typhus: an endemic province in the Republic of Korea. Korean J Intern Med. (2018) 33:323–30. doi: 10.3904/kjim.2016.303
29. Chanprasertpinyo W, Diewsurin J, Thongsri T, Road S. Scrub typhus pericarditis: A case report. Available online at: https://www.idthai.org/2015/journal/_file_ar_fulltext/file_ar2291d2ec3b3048d1a6f86c2c4591b7e0.pdf.
30. Lee NJ, Shih HI, Lin CH, Hsu HC. Scrub typhus complicated with fulminant perimyocarditis. J Acute Med. (2023) 13:84–8. doi: 10.6705/j.jacme.202306_13(2).0006
31. Ray A, Nangia V, Chatterji RS, Dalal N. Scrub typhus infection presenting as acute heart failure: A case report and systematic review of literature of cardiopulmonary involvement in scrub typhus infection. Lung India. (2016) 33:439–43. doi: 10.4103/0970-2113.184923
32. Rajapakse S, Weeratunga P, Sivayoganathan S, Fernando SD. Clinical manifestations of scrub typhus. Trans R Soc Trop Med Hyg. (2017) 111:43–54. doi: 10.1093/trstmh/trx017
33. Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al. Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care. Health Technol Assess. (2009) 13:1–207. doi: 10.3310/hta13320
34. Ammirati E, Frigerio M, Adler ED, Basso C, Birnie DH, Brambatti M, et al. Management of acute myocarditis and chronic inflammatory cardiomyopathy. Circ Heart Fail. (2020) 13:e007405. doi: 10.1161/CIRCHEARTFAILURE.120.007405
35. Sittiwangkul R, Pongprot Y, Silviliarat S, Oberdorfer P, Jittamala P, Sirisanthana V. Acute fulminant myocarditis in scrub typhus. Ann Trop Paediatr. (2008) 28:149–54. doi: 10.1179/146532808X302189
36. Pannu AK, Debnath MK, Sharma N, Biswal M, Vijayvergia R, Bhalla A, et al. Circulating cardiac biomarkers and echocardiographic abnormalities in patients with scrub typhus: A prospective cohort study from a tertiary care center in North India. J Vector Borne Dis. (2021) 58:193–8. doi: 10.4103/0972-9062.321754
37. Mohanty S, Harsha KN, Kalale N. Takotsubo cardiomyopathy in pediatric scrub typhus. Indian Pediatr. (2021) 58:78–9. doi: 10.1007/s13312-021-2101-1
38. Dave M, Nagaraja T, Sareen M, Kumar R, Saini R, Veerwal R. Scrub typhus and myocarditis: A rare complication. IJCP. (2022) 32:35–7.
39. Kumar M, Krishnamurthy S, Delhikumar CG, Narayanan P, Biswal N, Srinivasan S. Scrub typhus in children at a tertiary hospital in southern India: clinical profile and complications. J Infect Public Health. (2012) 5:82–8. doi: 10.1016/j.jiph.2011.11.001
40. Charoensak A, Chawalparit O, Suttinont C, Niwattayakul K, Losuwanaluk K, Silpasakorn S, et al. Scrub typhus: chest radiographic and clinical findings in 130 Thai patients. J Med Assoc Thai. (2006) 89:600–7.
41. Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. (2013) 34:2636–48. doi: 10.1093/eurheartj/eht210
42. Yotsukura M, Aoki N, Fukuzumi N, Ishikawa K. Review of a case of tsutsugamushi disease showing myocarditis and confirmation of Rickettsia by endomyocardial biopsy. Jpn Circ J. (1991) 55:149–53. doi: 10.1253/jcj.55.149
43. Bharathi S, Jayachandran S, Senthil N, Sujatha S. Scrub typhus causing myocarditis and ARDS: A case report. Heart India. (2013) 1:85. doi: 10.4103/2321-449x.122785
44. Avasthi GL, Patel P, Kuka R, Mahajan R. Acute fulminant myocarditis as a rare manifestation in complicated scrub typhus: A case report. IHJ Cardiovasc Case Rep (CVCR). (2018) 2:S18–20. doi: 10.1016/j.ihjccr.2018.07.009
45. Ki YJ, Kim DM, Yoon NR, Kim SS, Kim CM. A case report of scrub typhus complicated with myocarditis and rhabdomyolysis. BMC Infect Dis. (2018) 18:551. doi: 10.1186/s12879-018-3458-1
46. Jung YH, Minjoo LEE, Kyoung-Hwa LEE, Ji-Hoon LEE, Seok-Hyung KIM, Byoung-Kwon LEE. Acute fulminant myocarditis recovered from electro-mechanical dissociation in scrub typhus. Ewha Med J. (2016) 39:1–5. doi: 10.12771/emj.2016.39.1.1
47. Chung MH, Lee JS, Im JH. Antibiotic combination therapy for severe scrub typhus: is it necessary? Infect Chemother. (2023) 55:179–84. doi: 10.3947/ic.2023.0055
48. Fang CY, Dennis DT, Lee JB. Electrocardiographic changes in scrub typhus patients. Southeast Asian J Trop Med Public Health. (1977) 8:503–9.
49. Jang SY, Kang KW, Kim JH, Kim B, Chin JY, Park SH, et al. New-onset atrial fibrillation predicting for complicating cardiac adverse outcome in scrub typhus infection. Clin Cardiol. (2019) 42:1210–21. doi: 10.1002/clc.23276
50. Choi SW, Yun NR, Choi DH, Ki YJ, Kim SW, Kim CM, et al. Scrub typhus and abnormal electrocardiography. Am J Trop Med Hyg. (2019) 100:399–404. doi: 10.4269/ajtmh.17-0565
51. Son MK, Lim NK, Cho MC, Park HY. Incidence and risk factors for atrial fibrillation in korea: the national health insurance service database (2002-2010). Korean Circ J. (2016) 46:515–21. doi: 10.4070/kcj.2016.46.4.515
52. Gupta H, Parchani A, Choudhury A, Pathania M, Bairwa M. Atrial fibrillation in scrub typhus: A series of four cases. Cureus. (2022) 14:e25338. doi: 10.7759/cureus.25338
53. Gutierrez C, Blanchard DG. Diagnosis and treatment of atrial fibrillation. Am Fam Physician. (2016) 94:442–52.
54. Gupta S, Jesrani G, Gaba S, Gupta M. Scrub typhus manifesting as electrocardiographic disturbance: A case report and review of literature. Turk J Emerg Med. (2022) 22:47–50. doi: 10.4103/2452-2473.336103
55. Thipmontree W, Tantibhedhyangkul W, Silpasakorn S, Wongsawat E, Waywa D, Suputtamongkol Y. Scrub typhus in northeastern Thailand: eschar distribution, abnormal electrocardiographic findings, and predictors of fatal outcome. Am J Trop Med Hyg. (2016) 95:769–73. doi: 10.4269/ajtmh.16-0088
56. Feldman AM, McNamara D. Medical progress. N Engl J Med. (2000) 343:19. doi: 10.1056/NEJM200011093431908
57. Gaba S, Gupta M, Singla N, Singh R. Clinical outcome and predictors of severity in scrub typhus patients at a tertiary care hospital in Chandigarh, India. J Vector Borne Dis. (2019) 56:367–72. doi: 10.4103/0972-9062.302041
58. Narvencar KPS, Rodrigues S, Nevrekar RP, Dias L, Dias A, Vaz M, et al. Scrub typhus in patients reporting with acute febrile illness at a tertiary health care institution in Goa. Indian J Med Res. (2012) 136:1020–4.
59. Kumar R, Thakur S, Bhawani R, Kanga A, Ranjan A. Clinical profile and complications of scrub typhus: hospital-based study in sub-himalayan region. J Assoc Physicians India. (2016) 64:30–4.
60. Kim DM, Kim SW, Choi SH, Yun NR. Clinical and laboratory findings associated with severe scrub typhus. BMC Infect Dis. (2010) 10:108. doi: 10.1186/1471-2334-10-108
61. Lamichhane P, Pokhrel KM, Alghalyini B, Zaidi ARZ, Alshehery MZ, Khanal K, et al. Epidemiology, clinical characteristics, diagnosis, and complications of scrub typhus infection in Nepal: a systematic review. Ann Med Surg (Lond). (2023) 85:5022–30. doi: 10.1097/MS9.0000000000001259
62. Bhargava A, Kaushik R, Kaushik RM, Sharma A, Ahmad S, Dhar M, et al. Scrub typhus in Uttarakhand & adjoining Uttar Pradesh: Seasonality, clinical presentations & predictors of mortality. Indian J Med Res. (2016) 144:901–9. doi: 10.4103/ijmr.IJMR_1764_15
63. Emergence of scrub typhus in northern India: experience from tertiary care hospital. Available online at: https://www.klimikdergisi.org/en/2021/01/05/emergence-of-scrub-typhus-in-northern-India-experience-from-tertiary-care-hospital/.
64. Lim HK, Wang JM. Scrub typhus: seven-year experience and literature review. J Acute Med. (2018) 8:99–108. doi: 10.6705/j.jacme.201809_8(3).0003
65. Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. (2006) 113:876–90. doi: 10.1161/CIRCULATIONAHA.105.584532
66. Premaratna R, Loftis AD, Chandrasena TGAN, Dasch GA, de Silva HJ. Rickettsial infections and their clinical presentations in the Western Province of Sri Lanka: a hospital-based study. Int J Infect Dis. (2008) 12:198–202. doi: 10.1016/j.ijid.2007.06.009
67. Jeong MH, Ahn YK, Gill GC, Park JH, Cho JG, Park JC, et al. Tsutsugamushi myocarditis with congestive heart failure and persistent atrial standstill. Jpn Circ J. (1996) 60:382–8. doi: 10.1253/jcj.60.382
68. Ahmed T, Goyal A. Endomyocardial biopsy. Treasure Island, Florida: StatPearls Publishing (2023). Available at: https://www.ncbi.nlm.nih.gov/books/NBK557597/.
69. Olimulder MAGM, van Es J, Galjee MA. The importance of cardiac MRI as a diagnostic tool in viral myocarditis-induced cardiomyopathy. Neth Heart J. (2009) 17:481–6. doi: 10.1007/BF03086308
70. Kim CO, Huh AJ, Yeom JS, Lee KS, Chin BS, Han SH, et al. Lack of effect of dexamethasone on growth of Orientia tsutsugamushi Gilliam in mouse L929 cells. Yonsei Med J. (2011) 52:624–9. doi: 10.3349/ymj.2011.52.4.624
71. Kim DG, Kim JW, Choi YS, Kim SH, Kim SM, Park CG, et al. Acute myocardial infarction following scrub typhus infection. Int J Cardiol. (2007) 114:e18–20. doi: 10.1016/j.ijcard.2006.07.131
72. Chung WS, Lin CL, Hsu WH, Kao CH. Scrub typhus increases the risk of developing acute coronary syndrome: a nationwide cohort study. Heart. (2014) 100:1844–50. doi: 10.1136/heartjnl-2014-306181
73. Pradeesh A, Vasudevan B, Sharma N, Verma R. A rare case of scrub typhus vasculitis presenting as acute coronary syndrome diagnosed by skin manifestations. Indian J Dermatol Venereol Leprol. (2022) 88:184–7. doi: 10.25259/IJDVL_158_20
74. Chen Y, Guo Z, Wang L, Cheng N, Wang C. The first case report of acute myocardial infarction in young adult caused by scrub typhus. Medicine. (2023) 102:e35271. doi: 10.1097/MD.0000000000035271
75. Levine HD. Pathologic study of thirty-one cases of scrub typhus fever with especial reference to the cardiovascular system. Am Heart J. (1946) 31:314–28. doi: 10.1016/0002-8703(46)90313-4
76. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. (2015) 36:3075–128. doi: 10.1093/eurheartj/ehv319
77. Yu S, Yu X, Zhou B, Liu D, Wang M, Zhang H, et al. Tsutsugamushi disease presenting with aortic valve endocarditis: a case report and literature review. Am J Cardiovasc Dis. (2016) 6:185–90.
78. Isaac R, Varghese GM, Mathai E, Joseph I. Scrub typhus: prevalence and diagnostic issues in rural Southern India. Clin Infect Dis. (2004) 39:1395–6. doi: 10.1086/424748
79. Kularatne SAM, Gawarammana IB. Validity of the Weil-Felix test in the diagnosis of acute rickettsial infections in Sri Lanka. Trans R Soc Trop Med Hyg. (2009) 103:423–4. doi: 10.1016/j.trstmh.2008.11.020
80. Pradutkanchana J, Silpapojakul K, Paxton H, Pradutkanchana S, Kelly DJ, Strickman D. Comparative evaluation of four serodiagnostic tests for scrub typhus in Thailand. Trans R Soc Trop Med Hyg. (1997) 91:425–8. doi: 10.1016/S0035-9203(97)90266-2
81. Chao CC, Huber ES, Porter TB, Zhang Z, Ching WM. Analysis of the cross-reactivity of various 56 kDa recombinant protein antigens with serum samples collected after Orientia tsutsugamushi infection by ELISA. Am J Trop Med Hyg. (2011) 84:967–72. doi: 10.4269/ajtmh.2011.10-0545
82. Chao CC, Zhangm Z, Weissenberger G, Chen HW, Ching WM. Lateral flow rapid test for accurate and early diagnosis of scrub typhus: A febrile illness of historically military importance in the pacific rim. Mil Med. (2017) 182:369–75. doi: 10.7205/MILMED-D-16-00091
83. Lee KD, Moon C, Oh WS, Sohn KM, Kim BN. Diagnosis of scrub typhus: introduction of the immunochromatographic test in Korea. Korean J Intern Med. (2014) 29:253–5. doi: 10.3904/kjim.2014.29.2.253
84. Kelly DJ, Wong PW, Gan E, Lewis GE Jr. Comparative evaluation of the indirect immunoperoxidase test for the serodiagnosis of rickettsial disease. Am J Trop Med Hyg. (1988) 38:400–6. doi: 10.4269/ajtmh.1988.38.400
85. Chao CC, Zhang Z, Belinskaya T, Thipmontree W, Tantibhedyangkul W, Silpasakorn S, et al. An ELISA assay using a combination of recombinant proteins from multiple strains of Orientia tsutsugamushi offers an accurate diagnosis for scrub typhus. BMC Infect Dis. (2017) 17:413. doi: 10.1186/s12879-017-2512-8
86. Liao CC, Tsai CH, Lo HR, Lin PR, Lin CC, Chao YC. Development of a scrub typhus diagnostic platform incorporating cell-surface display technology. Front Immunol. (2021) 12:761136. doi: 10.3389/fimmu.2021.761136
87. Paris DH, Shelite TR, Day NP, Walker DH. Unresolved problems related to scrub typhus: a seriously neglected life-threatening disease. Am J Trop Med Hyg. (2013) 89:301–7. doi: 10.4269/ajtmh.13-0064
88. Chakraborty S, Sarma N. Scrub typhus: an emerging threat. Indian J Dermatol. (2017) 62:478–85. doi: 10.4103/ijd.IJD_388_17
89. Park SW, Lee CS, Kim JH, Bae IG, Moon C, Kwak YG, et al. Severe fever with thrombocytopenia syndrome: comparison with scrub typhus and clinical diagnostic prediction. BMC Infect Dis. (2019) 19:174. doi: 10.1186/s12879-019-3773-1
90. Indrawattana N, Aiumurai P, Sae-Lim N, Seesuay W, Reamtong O, Chongsa-Nguan M, et al. GroEL chaperonin-based assay for early diagnosis of scrub typhus. Diagnostics (Basel). (2022) 12. doi: 10.3390/diagnostics12010136
91. Kim DM, Park G, Kim HS, Lee JY, Neupane GP, Graves S, et al. Comparison of conventional, nested, and real-time quantitative PCR for diagnosis of scrub typhus. J Clin Microbiol. (2011) 49:607–12. doi: 10.1128/JCM.01216-09
92. Prakash JAJ. Scrub typhus: risks, diagnostic issues, and management challenges. Res Rep Trop Med. (2017) 8:73–83. doi: 10.2147/RRTM.S105602
93. Lim C, Blacksell SD, Laongnualpanich A, Kantipong P, Day NPJ, Paris DH, et al. Optimal cutoff titers for indirect immunofluorescence assay for diagnosis of scrub typhus. J Clin Microbiol. (2015) 53:3663–6. doi: 10.1128/JCM.01680-15
94. Zhan Z, Li CF, Liu J, Cao CS, Huang L. Metagenomic next-generation sequencing to diagnose atypical severe scrub typhus. J Microbiol Immunol Infect. (2022) 55:556–7. doi: 10.1016/j.jmii.2021.12.002
95. Liu X, Zhang Y, Zhang J, Lou Z, Xia H, Lu Z. The early diagnosis of scrub typhus by metagenomic next-generation sequencing. Front Public Health. (2021) 9:755228. doi: 10.3389/fpubh.2021.755228
96. Aronoff DM, Watt G. Prevalence of relative bradycardia in Orientia tsutsugamushi infection. Am J Trop Med Hyg. (2003) 68:477–9. doi: 10.4269/ajtmh.2003.68.477
97. Manappallil RG, Nambiar J, Anil R. Afebrile scrub typhus infection with cardiac manifestation. BMJ Case Rep. (2021) 14. doi: 10.1136/bcr-2020-240223
98. Views PDF. Available online at: https://www.idthai.org/2015/journal/index.php/journal/aritcles/664/abstract.
99. Watt G, Kantipong P, Jirajarus K. Acute scrub typhus in Northern Thailand: EKG changes. Southeast Asian J Trop Med Public Health. (2002) 33:312–3.
100. Dalal P, Singla N. An atypical case of complete heart block in scrub typhus. Trop Doct. (2022) 52:351–3. doi: 10.1177/00494755221074545
101. Luce-Fedrow A, Lehman ML, Kelly DJ, Mullins K, Maina AN, Stewart RL, et al. A review of scrub typhus (Orientia tsutsugamushi and related organisms): then, now, and tomorrow. Trop Med Infect Dis. (2018) 3:2. doi: 10.3390/tropicalmed3010008
102. Watt G, Chouriyagune C, Ruangweerayud R, Watcharapichat P, Phulsuksombati D, Jongsakul K, et al. Scrub typhus infections poorly responsive to antibiotics in northern Thailand. Lancet. (1996) 348:86–9. doi: 10.1016/S0140-6736(96)02501-9
103. Gaba S, Gupta M, Gaba R, Lehl SS. Scrub typhus: An update. Curr Trop Med Rep. (2021) 8:133–40. doi: 10.1007/s40475-021-00234-5
104. Varghese GM, Dayanand D, Gunasekaran K, Kundu D, Wyawahare M, Sharma N, et al. Intravenous doxycycline, azithromycin, or both for severe scrub typhus. N Engl J Med. (2023) 388:792–803. doi: 10.1056/NEJMoa2208449
105. Watt G, Kantipong P, Jongsakul K, Watcharapichat P, Phulsuksombati D, Strickman D. Doxycycline and rifampicin for mild scrub-typhus infections in northern Thailand: a randomised trial. Lancet. (2000) 356:1057–61. doi: 10.1016/S0140-6736(00)02728-8
106. Hwang JH, Kim MJ, Im YJ, Moon SJ, Kim JH, Lee MG, et al. Treatment outcomes of oral doxycycline versus intravenous azithromycin in adults hospitalized with scrub typhus: A retrospective study using inverse probability treatment weighting (IPTW) propensity analysis. Travel Med Infect Dis. (2023) 52:102525. doi: 10.1016/j.tmaid.2022.102525
107. Basharat Z, Akhtar U, Khan K, Alotaibi G, Jalal K, Abbas MN, et al. Differential analysis of Orientia tsutsugamushi genomes for therapeutic target identification and possible intervention through natural product inhibitor screening. Comput Biol Med. (2022) 141:105165. doi: 10.1016/j.compbiomed.2021.105165
108. Pautu L, Lalmalsawma P, Vanramliana, Balasubramani K, Balabaskaran Nina P, Rosangkima G, et al. Seroprevalence of scrub typhus and other rickettsial diseases among the household rodents of Mizoram, North-East India. Zoonoses Public Health. (2023) 70:269–75. doi: 10.1111/zph.13025
109. Olson JG, Bourgeois AL, Fang RC, Coolbaugh JC, Dennis DT. Prevention of scrub typhus. Prophylactic administration of doxycycline in a randomized double blind trial. Am J Trop Med Hyg. (1980) 29:989–97. doi: 10.4269/ajtmh.1980.29.989
110. Thiriot J, Liang Y, Fisher J, Walker DH, Soong L. Host transcriptomic profiling of CD-1 outbred mice with severe clinical outcomes following infection with Orientia tsutsugamushi. PloS Negl Trop Dis. (2022) 16:e0010459. doi: 10.1371/journal.pntd.0010459
111. Koh GCKW, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. (2010) 82:368–70. doi: 10.4269/ajtmh.2010.09-0233
112. Valbuena G, Walker DH. Approaches to vaccines against Orientia tsutsugamushi. Front Cell Infect Microbiol. (2012) 2:170. doi: 10.3389/fcimb.2012.00170
113. Buckland FE, Dudgeon A. Scrubtyphus vaccine; large-scale production. Lancet. (1945) 2:734–7. doi: 10.1016/S0140-6736(45)91070-1
114. Walker WT. Scrub typhus vaccine; its effect on 16 cases incubating the disease. Br Med J. (1947) 1:484–7. doi: 10.1136/bmj.1.4501.484
115. Berge TO, Gauld RL, Kitaoka M. A field trial of a vaccine prepared from the Volner strain of Rickettsia tsutsugamushi. Am J Hyg. (1949) 50:337–42. doi: 10.1093/oxfordjournals.aje.a119366
116. Choi Y, Kim KS, Kim TY, Cheong HS, Ahn BY. Long-term egg-yolk adaptation of the Orientia tsutsugamushi for preparation of a formalinized immunogen. Vaccine. (2006) 24:1438–45. doi: 10.1016/j.vaccine.2005.07.113
117. Kekcheyeva N. A living chemo-vaccine prepared from rickettsia tsutsugamushi. Acta Med Biol. (1967) 15:113–6.
118. Smadel JE, Ley HL Jr, Diercks FH, Paterson PY, Wisseman CL Jr, Traub R. Immunization against scrub typhus: duration of immunity in volunteers following combined living vaccine and chemoprophylaxis. Am J Trop Med Hyg. (1952) 1:87–99. doi: 10.4269/ajtmh.1952.1.87
119. Eisenberg GH Jr, Osterman JV. Gamma-irradiated scrub typhus immunogens: broad-spectrum immunity with combinations of rickettsial strains. Infect Immun. (1979) 26:131–6. doi: 10.1128/iai.26.1.131-136.1979
120. Eisenberg GH Jr, Osterman JV. Experimental scrub typhus immunogens: gamma-irradiated and formalinized rickettsiae. Infect Immun. (1977) 15:124–31. doi: 10.1128/iai.15.1.124-131.1977
121. Eisenberg GH Jr, Osterman JV. Gamma-irradiated scrub typhus immunogens: development and duration of immunity. Infect Immun. (1978) 22:80–6. doi: 10.1128/iai.22.1.80-86.1978
122. Jerrells TR, Palmer BA, Osterman JV. Gamma-irradiated scrub typhus immunogens: development of cell-mediated immunity after vaccination of inbred mice. Infect Immun. (1983) 39:262–9. doi: 10.1128/iai.39.1.262-269.1983
123. Peter JV, Sudarsan TI, Prakash JAJ, Varghese GM. Severe scrub typhus infection: Clinical features, diagnostic challenges and management. Pediatr Crit Care Med. (2015) 4:244–50. doi: 10.5492/wjccm.v4.i3.244
124. Chattopadhyay S, Richards AL. Scrub typhus vaccines: past history and recent developments. Hum Vaccin. (2007) 3:73–80. doi: 10.4161/hv.3.3.4009
125. Walker DH, Mendell NL. A scrub typhus vaccine presents a challenging unmet need. NPJ Vaccines. (2023) 8:11. doi: 10.1038/s41541-023-00605-1
126. Chattopadhyay S, Jiang J, Chan TC, Manetz TS, Chao CC, Ching WM, et al. Scrub typhus vaccine candidate Kp r56 induces humoral and cellular immune responses in cynomolgus monkeys. Infect Immun. (2005) 73:5039–47. doi: 10.1128/IAI.73.8.5039-5047.2005
127. Ni YS, Chan TC, Chao CC, Richards AL, Dasch GA, Ching WM. Protection against scrub typhus by a plasmid vaccine encoding the 56-KD outer membrane protein antigen gene. Am J Trop Med Hyg. (2005) 73:936–41. doi: 10.4269/ajtmh.2005.73.936
128. Niu D, Chen W, Zhang X, Chen M, Cui H, Wei W, et al. Immunogenicity of a 40kDa fragment of the 47kDa recombinant protein and DNA vaccine from Karp strain of Orientia tsutsugamushi. Ann N Y Acad Sci. (2003) 990:527–34. doi: 10.1111/j.1749-6632.2003.tb07423.x
129. Yu Y, Wen B, Wen B, Niu D, Chen M, Qiu L. Induction of protective immunity against scrub typhus with a 56-kilodalton recombinant antigen fused with a 47-kilodalton antigen of Orientia tsutsugamushi Karp. Am J Trop Med Hyg. (2005) 72:458–64. doi: 10.4269/ajtmh.2005.72.458
130. Kim HI, Ha NY, Kim G, Min CK, Kim Y, Yen NTH, et al. Immunization with a recombinant antigen composed of conserved blocks from TSA56 provides broad genotype protection against scrub typhus. Emerg Microbes Infect. (2019) 8:946–58. doi: 10.1080/22221751.2019.1632676
131. Ha NY, Sharma P, Kim G, Kim Y, Min CK, Choi MS, et al. Immunization with an autotransporter protein of Orientia tsutsugamushi provides protective immunity against scrub typhus. PloS Negl Trop Dis. (2015) 9:e0003585. doi: 10.1371/journal.pntd.0003585
132. Ha NY, Shin HM, Sharma P, Cho HA, Min CK, Kim HI, et al. Generation of protective immunity against Orientia tsutsugamushi infection by immunization with a zinc oxide nanoparticle combined with ScaA antigen. J Nanobiotechnology. (2016) 14:76. doi: 10.1186/s12951-016-0229-2
133. Park J, Zhang Z, Belinskaya T, Tsoras AN, Chao CC, Jiang L, et al. Dual-antigen subunit vaccine nanoparticles for scrub typhus. Pathogens. (2023) 12:35. doi: 10.3390/pathogens12121390
134. Choi S, Jeong HJ, Ju YR, Gill B, Hwang KJ, Lee J. Protective immunity of 56-kDa type-specific antigen of Orientia tsutsugamushi causing scrub typhus. J Microbiol Biotechnol. (2014) 24:1728–35. doi: 10.4014/jmb.1407.07048
135. Park SM, Gu MJ, Ju YJ, Cheon IS, Hwang KJ, Gill B, et al. Intranasal Vaccination with Outer-Membrane Protein of Orientia tsutsugamushi induces Protective Immunity Against Scrub Typhus. Immune Netw. (2021) 21:e14. doi: 10.4110/in.2021.21.e14
136. Cho H, Lee WH, Kim YK, Kim KS. Extracellular vesicle-associated antigens as a new vaccine platform against scrub typhus. Biochem Biophys Res Commun. (2020) 523:602–7. doi: 10.1016/j.bbrc.2020.01.014
137. Card WI, Walker JM. Scrub-typhus vaccine; field trial in South-east Asia. Lancet. (1947) 1:481–3. doi: 10.1016/S0140-6736(47)91989-2
139. Smadel JE, Ley HL Jr, Diercks FH, Traub R, Tipton VJ, Frick LP. Immunization against scrub typhus. I. Combined living vaccine and chemoprophylaxis in volunteers. Am J Hyg. (1951) 53:317–25.
140. Kekcheyeva N. Preventive immunization against tsutsugamushi fever. J Hyg Epidemiol Microbiol Immunol. (1968) 12:14–7.
141. Seong SY, Huh MS, Jang WJ, Park SG, Kim JG, Woo SG, et al. Induction of homologous immune response to Rickettsia tsutsugamushi Boryong with a partial 56-kilodalton recombinant antigen fused with the maltose-binding protein MBP-Bor56. Infect Immun. (1997) 65:1541–5. doi: 10.1128/iai.65.4.1541-1545.1997
142. Seong SY, Kim HR, Huh MS, Park SG, Kang JS, Han TH, et al. Induction of neutralizing antibody in mice by immunization with recombinant 56 kDa protein of Orientia tsutsugamushi. Vaccine. (1997) 15:1741–7. doi: 10.1016/S0264-410X(97)00112-6
Keywords: scrub typhus (tsutsugamushi disease), Orientia species, cardiac complications, pericarditis, myocarditis, arrhythmia, heart failure, mite-borne disease
Citation: Sivasubramanian BP, Abdul Khader AHS, Ravikumar DB, Dominic Savio FV, Thirupathy U, Thiruvadi V, Prasad R, Thokala H, Qadeer H, Venkataperumal DP, Gupta A, Honganur NS and Tirupathi R (2024) Comprehensive review on cardiac manifestation of scrub typhus. Front. Trop. Dis 5:1375087. doi: 10.3389/fitd.2024.1375087
Received: 01 February 2024; Accepted: 22 July 2024;
Published: 20 September 2024.
Edited by:
Suzanne Donovan, University of California, Los Angeles, United StatesReviewed by:
Yohei Sato, Jikei University School of Medicine, JapanCopyright © 2024 Sivasubramanian, Abdul Khader, Ravikumar, Dominic Savio, Thirupathy, Thiruvadi, Prasad, Thokala, Qadeer, Venkataperumal, Gupta, Honganur and Tirupathi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barath Prashanth Sivasubramanian, YmFyYXRocHJhc2hhbnRoMTgxOTZAZ21haWwuY29t; Francis Vino Dominic Savio, ZnJhbmNpcy52aW5vQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.