- 1Parasite Host Biology Group, ICMR-National Institute of Malaria Research, Delhi, India
- 2Health Services, District Malaria Office Kokrajhar, Assam, India
Introduction: The diagnosis of infectious reservoirs in malaria (gametocytes) is necessary, especially in low-density infections and asymptomatic malaria patients. The gametocyte stage is a surrogate marker for infection of P. falciparum malaria in healthy individuals. The early detection of infectious gametocytes and treatment will strengthen our efforts in curbing transmission. The nested PCR and real-time quantitative PCR (RT-qPCR) methods have been demonstrated for the diagnosis of infectious gametocyte reservoirs. In this study, RDT, blood smear microscopy, and nested-PCR were used for the detection of P. falciparum and P. vivax, and compared with RT-qPCR detection of Pfg27 gametocyte biomarker gene.
Methods: In the present cross-sectional study, 356 human blood samples were collected from endemic areas of Kokrajhar Assam (asymptomatic and symptomatic malaria patients) for malaria diagnosis.
Results: A total of 8.42%(30/356) incidence of malaria was observed. Malaria patients were observed to be both symptomatic, 80%(24/30; 13Pf+11Pv), and asymptomatic, 20%(6 (4Pf +2Pv)). More than 64%(11/17) of Pf and 92.3%(12/13) of Pv infections were observed in children and the adolescent population (age <20 years) by RDT, microscopy, nested PCR, and RT-qPCR methods. The prevalence of Pf infection was 4.77%(17/356) by RT-qPCR method. Of 16 the Pf positive samples 81.25%(13/16) were symptomatic and 18.75%(3/16) were asymptomatic. One asymptomatic individual was found positive for Pf infection by the RT-qPCR method.
Conclusion: The findings from this research study revealed that the routine microscopy and RDT methods are insufficient for detecting all asymptomatic malaria and gametocyte infectious reservoirs. The early detection of infectious P. falciparum gametocytes and the treatment of patients will be helpful in preventing the transmission of malaria.
Introduction
The five Plasmodium species- Plasmodium falciparum (Pf), P. vivax (Pv), P. malariae (Pm), P. ovale (Po), and P. knowlesi (Pk) are known to cause malaria in humans (1). Approximately 90% of the world’s malaria mortality has been attributed to infection with P. falciparum and thus this strain poses a great threat to public health (2). Globally, ~ 241 million malaria cases and 6,27,000 malaria deaths were estimated, which is ~ 14 million more cases and 69,000 more deaths in 2020 compared to 2019 (3). Malaria is a major health burden in India, especially affecting the population living in rural, tribal, and forest areas. In its fight against malaria, India has already launched a national framework for malaria elimination and is aiming to eliminate malaria by 2030 (4). There are certain hurdles that threaten this ambitious malaria elimination program, among them asymptomatic carriers of the malaria parasite in the rural, tribal, and forest populations; drug resistance; and insecticide resistance hindering vector control. Detection of asymptomatic carriers of the malaria parasite requires a robust and efficient diagnostic tool capable of detecting low-density infections, something that is generally lacking in remote areas. In such areas, the only choice for P. falciparum malaria detection is by Pfhrp2/Pfhrp3 antigen-based rapid diagnostic tests (RDTs) kits in addition to microscopy, which requires highly skilled personnel. Recently, the Pfhrp2/Pfhrp3-based RDT kits are facing challenges due to the deletion of the Pfhrp2/Pfhrp3 genes in P. falciparum parasites (5, 6). Moreover, these RDTs are based on the target proteins that are expressed only during the asexual stages of P. falciparum. Detection of sexual stages, particularly early detection of gametocytes is highly desirable, but due to lack of trained microscopists, identification and differentiation of gametocyte stages (I-V) remains a challenge. There is also a dearth of highly sensitive RDT-like tools to detect the sexually infectious stages of P. falciparum- gametocytes. Gametocyte maturation is classically divided into five distinct morphological stages (I-V) that lasts about 8-12 days in P. falciparum, and only the mature and infectious stage V is released into the bloodstream. These infectious gametocytes are taken up by the mosquito vector with the feeding blood and further mature into infectious sporozoites after completing their developmental cycle in the mosquito (7). Therefore, gametocytes require timely detection in human hosts to appropriately modulate the gametocidal drug administration in an efficient manner. Due to relatively lower gametocyte densities than asexual parasites, submicroscopic infections remain undetected in malaria-endemic areas leading to continued transmission (8). The application of molecular biology tools has led to the detection of submicroscopic gametocyte carriage and examination of human infectious reservoirs (9). Even low-density gametocyte asymptomatic reservoirs are potential carriers for transmission of malaria (10–12). There are variations in the time of appearance of these gametocytes in asymptomatic and symptomatic infections (13). Gametocytes are widely known as surrogate markers for malaria transmission in a healthy population (10). The amplification of RNA transcripts of Pf gametocyte-specific Pfg27 gene by real-time-quantitative PCR (RT-qPCR) assay is a potential tool for the detection of gametocytes (10, 14, 15). The PF3D7_1302100 coding Pfg27 gene is expressed in the early stage (I-III) and plays an important role in gametocytogenesis (16). In the present study, gametocyte-specific marker Pfg27 gene was explored in an RT-qPCR assay and compared with the traditionally used tools such as RDTs, microscopy, and nested-PCR, in malaria-endemic northeastern Indian states.
Methods
Sample collection
Due to the COVID-19 pandemic situation during 2020-22 sample collection of malaria patients from the field sites were difficult. However, a mass survey was conducted in the highly malaria-endemic region of Kokrajhar, Assam, following COVID-19 appropriate protocols. The study was approved by the ICMR-National Institute of Malaria Research Institutional Ethics Committee (IEC No. ECR/NIMR/EC/2019/306). Before collecting blood samples, informed consent was taken from all adults and, in the case of minors, their respective legal parent/guardian’s approval was included in the study. Blood samples were collected in RNAlater® (Thermo Fisher, USA) and transported in a cold chain to the laboratory. The thick and thin blood smears were prepared and one drop of blood was used for detection of P. falciparum and P. vivax malaria by RDT kit method. The RDT-positive patients were treated as per the national drug policy of India. Individuals having body temperature ≥ 37.5 °C were considered symptomatic/febrile, and individuals with no fever history for the previous 48 hrs. were considered asymptomatic/afebrile (17). The thick and thin blood smears were stained with Giemsa stain and examined at 100X magnification for detection of Plasmodium parasites. The slides were read for the presence of P. falciparum parasites against a background of 200 WBCs in a thick film (18–20).
Nucleic acids isolation
Three punched discs from dried blood spots were used for DNA isolation by QIAamp DNA kit, Cat. No. 51306 (Qiagen, Germany), as per manufacturer protocol. Briefly, the discs were incubated overnight in lysis buffer (ATL buffer). Buffer AL was added followed by the addition of 100% ethanol to precipitate the DNA. The mixture was loaded on a QIAmp mini spin column and centrifuged at 8000 rpm in a collection tube. The spin column was washed with buffers AW1 and AW2. The isolated DNA was eluted in 50 µl of elution buffer and stored at -20 °C, until further use. The RNA isolation was accomplished by Nucleospin RNA Blood kit, Macherey-Nagel, Germany as per manufacturer’s protocol. Briefly, 200 µl of whole blood was incubated for 15 min. at room temperature (RT) in buffer DL for lysis with proteinase K. RNA was bound in a spin column and desalted, followed by DNA digestion using rDNase provided in the kit. Total RNA was washed thoroughly using wash buffers and RNA was eluted in RNase-free H2O, and stored at -20 °C until further use.
First-strand cDNA synthesis
The extracted RNA was converted to cDNA using the prime script 1st strand cDNA synthesis kit (Takara Bio, Japan), according to the manufacturer’s instructions. Briefly, template RNA (~ 1µg) was added to a reaction mix containing Oligo dT primer (50 µM) and dNTP mix (10 mM each) and incubated for 5 min. at 65 °C, followed by instant cooling on ice. Then, 10 µl of the template RNA primer mixture is added in a new reaction mix (20 µl) containing 4 µl of 5X PrimeScript buffer and 200 U/µl of PrimeScript RTase. The reaction mixture was incubated at 30 °C for 10 min followed by incubation at 42 °C for 40 min. The first strand cDNA was directly used as a template for RT-qPCR of the Pfg27 gene.
Quantitative amplification of Pfg27 gene and nested PCR for Pf and Pv
RT-qPCR was performed in a final reaction volume of 25 µl constituting 5 µl of cDNA from patient samples, 500 nM of each primers- forward 5’- CTTAGCAAGGATCCTGAGAAGTTT-3’ and reverse 5’-GTTGACAATGTTATCTTGGACACGT-3’, 10 µl of 2X SYBR Green master mix (Thermo Fisher Scientific, USA). The RT-qPCR conditions were as follows: 95 °C for 5 min, 40 cycles 95 °C for 15 sec, 55 °C for 1 min. The amplifications were performed in CFX-96 Connect Real-Time PCR System (BioRad, USA) (21). Further a nested PCR was performed for detection of P. falciparum and P. vivax malaria parasites using previously reported PCR primers and conditions by Snounou et al. 1993 (22).
Results
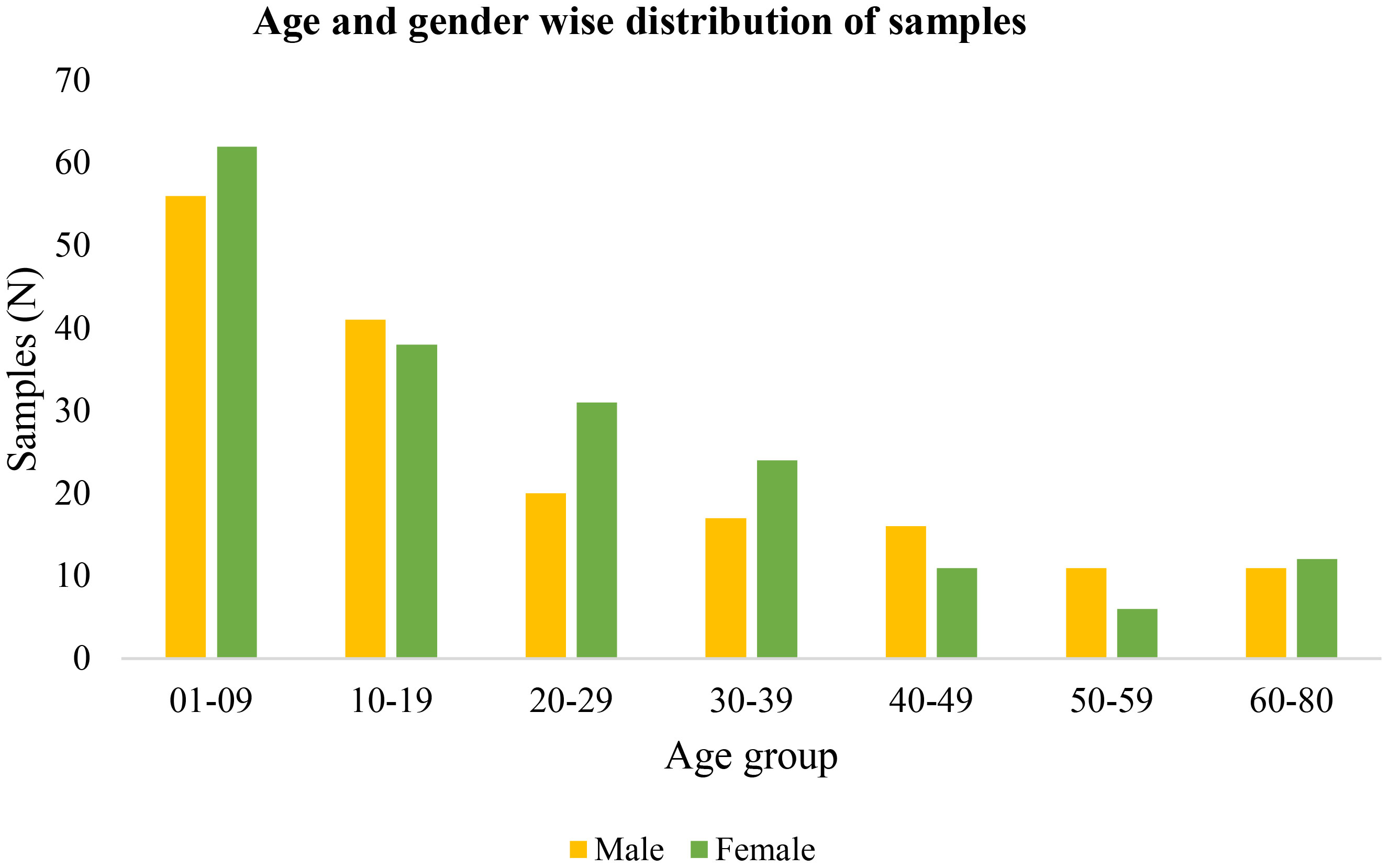
During the mass survey, blood samples of 356 malaria-suspected individuals from highly malaria-endemic districts of Kokrajhar, Assam from 2020 to 2022 were collected. There was no major difference in gender proportions among the study subjects, which comprised 172(48.31%) men/boys and 184(51.68%) women/girls (Figure 1). The major age group in the mass survey was children and adolescents (>50%), while one-third of the total samples were from children below 10 years of age. A total of 30(8.14%) (17Pf+13Pv) malaria cases were detected by RT-qPCR (Table 1). Of the 356 individuals, 33(9.27%) were symptomatic and 323(97.73%) were asymptomatic (Table 1).
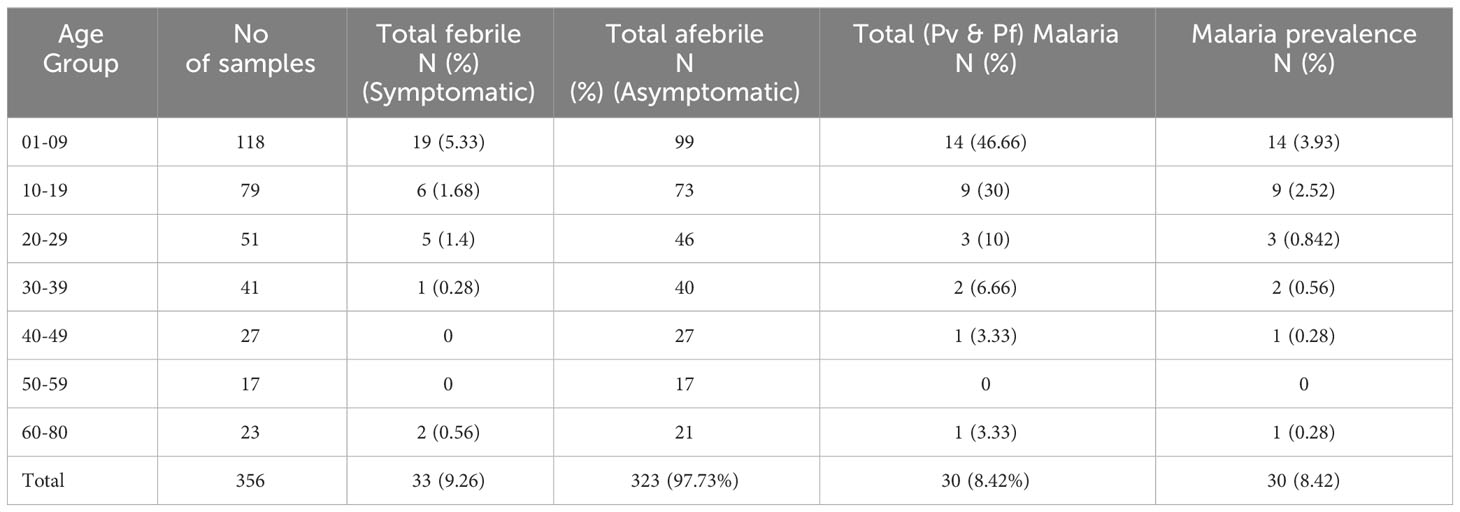
Table 1 Age-group sorted Pf and Pv infection by nested-PCR and RT-qPCR and their prevalence (n=356).
Among the 17 Pf infections, 11(67.7%) belonged to the child and adolescent population (age <20 years); whereas in the case of Pv infection, 92.3%(12/13) were observed in children (0-9 years of age) (Table 2). This was notable that among the 30 malaria positive cases 76.66%(11Pf+12Pv) infections were observed only in children and adolescent population (age <20 years) (Table 2). Of the 356 samples, RDT, microscopy and nested PCR detected (Pf- 16(4.5%) infections, whereas 17(4.77%) Pf positive cases were detected by the RT-qPCR method. The RT-qPCR method targeted for detection of infectious gametocytes could only detect one additional asymptomatic malaria case (aged 30-39), which was not detected by previously established methods (RDT, microscopy, and nested PCR). It is important to note that of the 16 Pf samples, 81.25%(13/16) were from symptomatic individuals, while 18.75%(3/16) were from asymptomatic patients, as observed by RDT and microscopy, nested PCR method. Whereas the RT-qPCR method detected 23.52% (4/17) Pf cases from asymptomatic individuals. The detection limit of the Pf gametocyte stage by RT-qPCR method is comparatively higher than RDT, microscopy, and nested PCR methods (Table 2), but not statistically significant, which may be due to the the limited sample size.
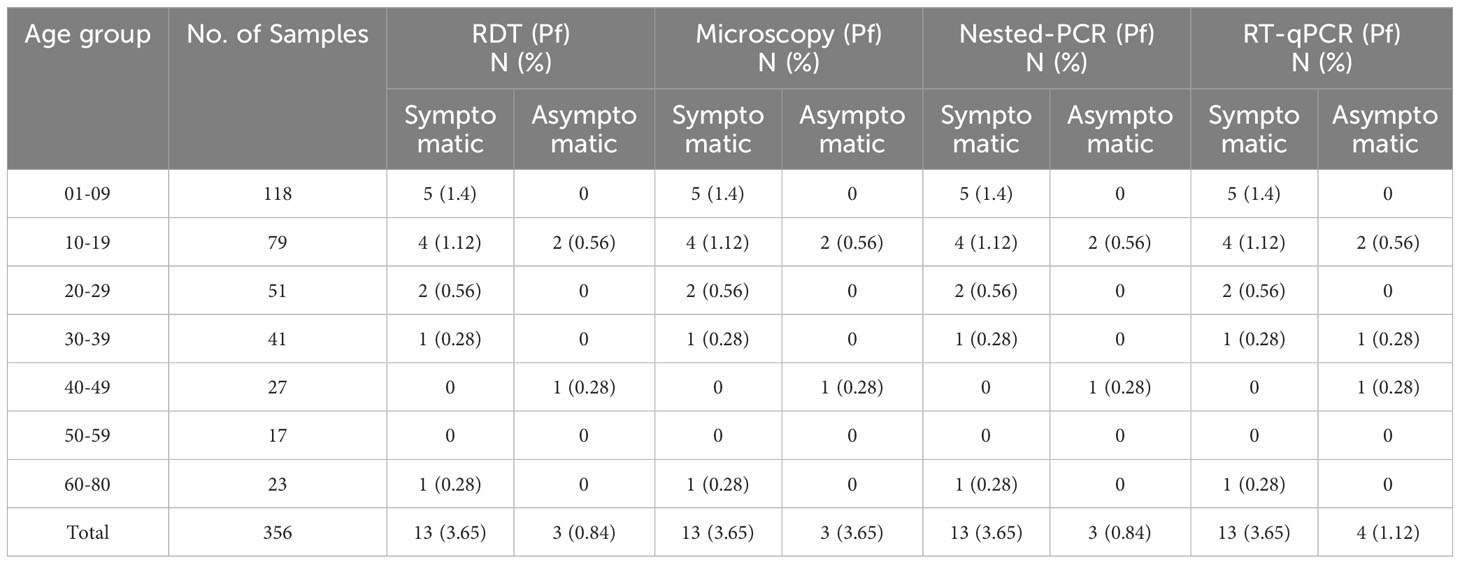
Table 2 Age-group sorted detection of P. falciparum infection in symptomatic and asymptomatic populations by RDT, microscopy, nested PCR, and RT-qPCR methods (n=356).
In the context of P. vivax, RDT, microscopy, and nested PCR methods detected 3.65%(13/356) (Pv) positive cases. As with the case of Pf infections, majority of the Pv samples 69.23%(9/13) detected, belonged to children <10 years of age. All the children of age <10 years who were Pv positive had fever as a symptom. The asymptomatic Pv cases were observed in 15.38%(2/13) individuals (Table 3). Further, no sample was observed positive for Pv in individuals with age >30 years (Figure 2). The prevalence of Pv was observed to be 3.56%(13/356) by RDT, microscopy and nested-PCR methods in the studied subjects. There was no sensitivity difference between RDT, microscopy, and nested-PCR methods for Pv.
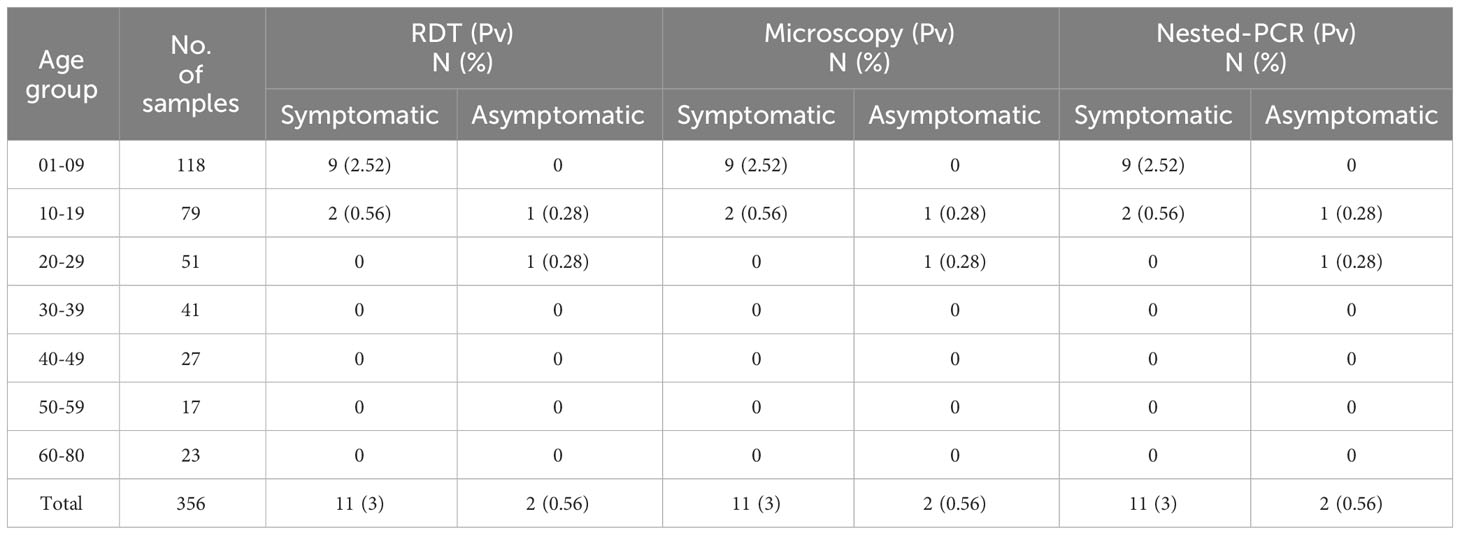
Table 3 Age-group sorted detection of P. vivax infection in symptomatic and asymptomatic patients by RDT, microscopy, and nested PCR methods (n=356).
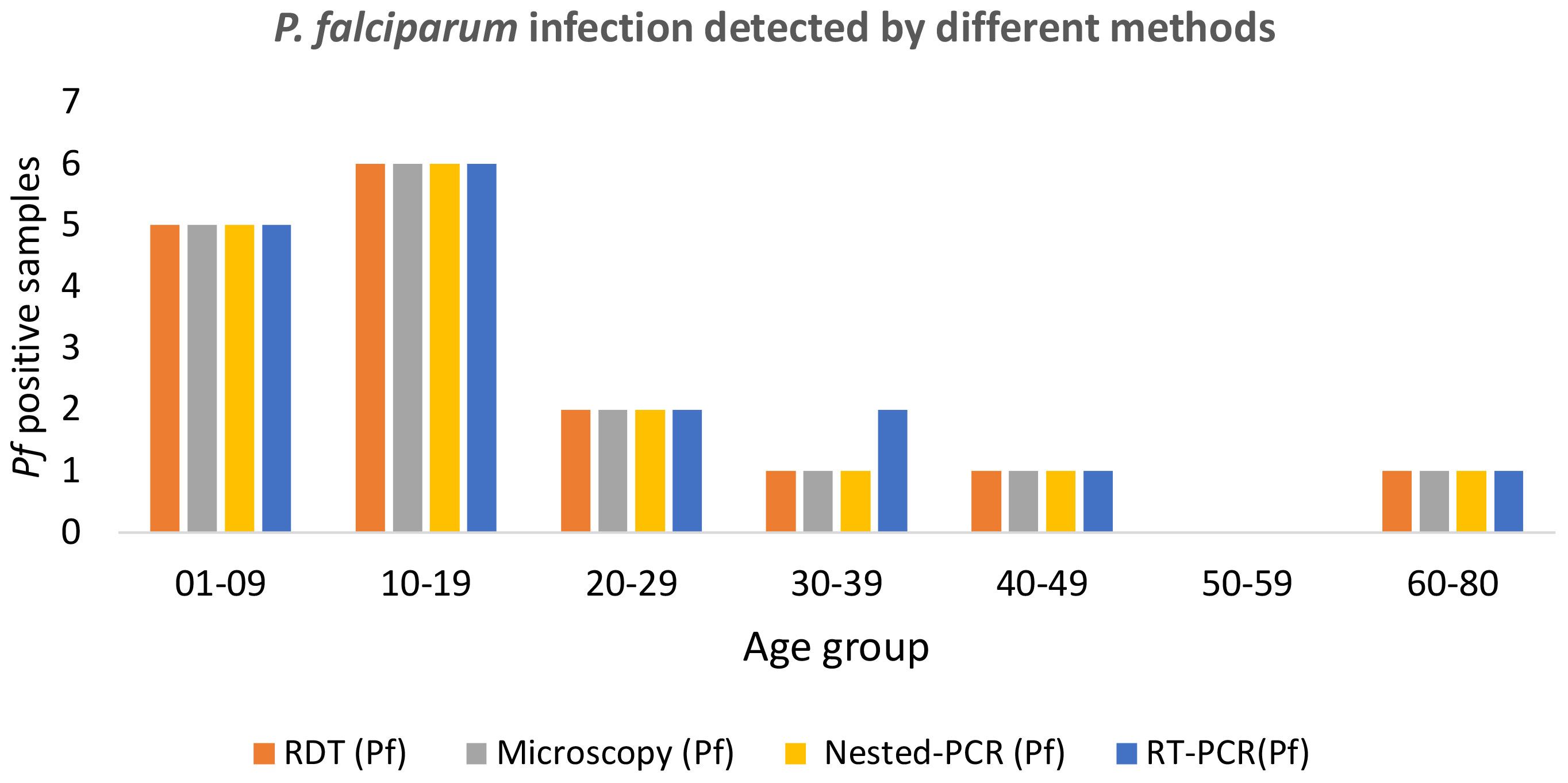
Figure 2 Age-group sorted P. falciparum infection was detected by different methods such as RDT, microscopy, nested PCR, and RT-qPCR.
Discussion
Malaria in India is still a public health problem and P. falciparum is a predominant malaria parasite (> 50%) along with P. vivax (23). While malaria cases have progressively decreased in India, the endemic areas with asymptomatic and low-density transmission result in continued transmission in healthy individuals (11, 24, 25). Identification and appropriate control of infectious gametocytes is critical in curbing transmission because even at one gametocyte/μl of blood, mosquitoes can transmit the malaria parasite to another healthy individual (26). The asymptomatic and low-gametocytaemia individuals with P. falciparum malaria are important reservoirs and are active carriers for the transmission of malaria infection in the community. The government of India is focusing on malaria elimination by clinical case management and vector control (27). The RDTs are potential tool for surveillance of symptomatic malaria patients in the field but asymptomatic or low-density P. falciparum infections are often missed by RDTs (3, 28). The asymptomatic, low-density malaria parasites and low gametocytemic reservoir patients pose a significant challenge to the elimination of malaria from India by 2030 (11, 25). The PF3D7_1302100 coding Pfg27 gene is expressed in early stages (I-III) and play important role in male and female gametocytogenesis. It is important to note that the Pf gametocyte maturation from stage I to V takes up to 8-12 days; and only stage V is released into the bloodstream. Compared to other asexual stages of the parasite in humans, the infectious gametocytes are potential biomarkers for the detection of the Pf malaria parasite, as they are more stable compared to other asexual stages of Plasmodium in humans. Therefore, diagnosis of infectious gametocytes of P. falciparum and treatment is necessary for blocking of transmission in healthy individuals.
This study presents the latest malaria endemicity data from malaria-endemic areas of northeastern India. In a recent study, from different malaria-endemic states of India (Chennai, Nadiad, Rourkela) 3-8 % malaria prevalence was reported (17). The present study also observed a prevelance of Pf infection 4.77%(17/356) in Assam, India, which is similar to previously reported data from different states of India [Assam (6.50%), Andhra Pradesh (9.17%), Bihar (8.23%), Chhattisgarh (2.24%), Gujarat (1.92%), Karnataka (13.14%), Madhya Pradesh (23.76%), Maharashtra (30.15%), Odisha (6.52%), Punjab (0.17%), Tamil Nadu (1.66%), and Uttar Pradesh (9.40%)] (29). A similar prevalence of Pv was observed at 3.65%(13/356) and concurred with previous findings of Pv prevalence (29). Our results suggested that the prevalence of malaria by RDT and RT-qPCR molecular approach is similar. Among the 30 malaria positive cases, symptomatic 80% (13Pf+11Pv) and asymptomatic 20%(4Pf+2Pv) infections were observed by RDT, microscopy, nested-PCR, and RT-qPCR methods; however, 30-80% of asymptomatic malaria cases have been reported in previous studies from malaria endemic areas of India (17, 30). Our study presents a lower proportion of asymptomatic malaria cases from malaria-endemic areas. It is important to note that our study found the majority of malaria-positive cases from the age groups of children and adolescents (>50%), which indicated the vulnerability of these age groups to malarial infections. Moreover, children below 10 years of age also do not have protective immunity as in the case of adults in malaria-endemic areas.
The RDT, microscopy, and conventional PCR techniques are less sensitive compared to the RT-qPCR assay to detect gametocytes (10). Therefore, RT-qPCR approaches should be routinely performed to screen Pf malaria diagnosis in addition to RDT and gold standard microscopy method (31). The Pfg27 gene-targeted RT-qPCR technique is a potential tool for early (gametocyte stage I-III) detection and treatment of malaria for curbing transmission (9, 32–35). Further studies are required with a significant number of malaria samples for statistically sound conclusions. The isolation of RNA, cDNA preparation, and RT-qPCR are labor intensive and require well-established laboratories for the detection of gametocyte reservoirs (36). This technique requires a skilled molecular biologist along with a well-curated molecular biology lab, which suggests that RT-qPCR is not applicable in the field. Therefore, we need to develop an advance gametocyte-specific serodiagnosis tool. Gametocyte-specific serodiagnosis in the field will be helpful in early diagnosis of gametocyte infectious reservoirs and will aid in curbing transmission for malaria elimination (7, 16).
Conclusions
The findings of the research study revealed that the routine gold standard microscopy and RDT method is insufficient for detecting all low-density Pf parasites and asymptomatic gametocyte infectious reservoirs. Therefore, in relation to malaria control and elimination, RT-qPCR would be a good approach for early detection of asymptomatic infectious Pf gametocyte reservoirs. The detection of infectious gametocytes will be helpful in curbing the transmission of malaria, its control, and elimination.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved by the ICMR-National Institute of Malaria Research Institutional Ethics Committee (IEC No. ECR/NIMR/EC/2019/306). Before collecting blood samples, informed consent was taken from all adults and, in the case of minors, their legal respective parent/guardian approval was included in the study.
Author contributions
RD: Conceptualization, Funding acquisition, Writing – original draft. KV: Data curation, Writing – review & editing. LK: Data curation, Writing – review & editing. KS: Writing – review & editing. GK: Software, Writing – review & editing. IH: Methodology, Writing – review & editing. JG: Resources, Visualization, Writing – review & editing. KP: Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Project funding support obtained from the Indian Council of Medical Research (ICMR) (Project Id: 2020-9512).
Acknowledgments
The authors are thankful to the P Gupta, P Gautam, Ankita, YS Tomar, DP Singh, P. Das, B. Ishlam, A Gupta, and Kripanand for technical support from ICMR-NIMR, Delhi and District Malaria Office, Kokrajhar, Assam, India.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sato S. Plasmodium-a brief introduction to the parasites causing human malaria and their basic biology. J Physiol Anthropol. (2021) 40, 1–13. doi: 10.1186/s40101-020-00251-9
2. Harris LM, Monsell KR, Noulin F, Famodimu MT, Smargiasso N, Damblon C, et al. G-quadruplex DNA motifs in the malaria parasite Plasmodium falciparum and their potential as novel antimalarial drug targets. Antimicrobial Agents Chemother. (2018) 62, 1–14. doi: 10.1128/aac.01828-17
3. Organization WH. World malaria report 2022. Geneva, Switzerland: World Health Organization (2022).
4. Ghosh SK, Rahi M. Malaria elimination in India-The way forward. J Vector Borne Dis. 56(1):32–40. doi: 10.4103/0972-9062.257771
5. Bharti PK, Chandel HS, Ahmad A, Krishna S, Udhayakumar V, Singh N. Prevalence of pfhrp2 and/or pfhrp3 Gene Deletion in Plasmodium falciparum Population in Eight Highly Endemic States in India. PloS One. (2016) 11:e0157949. doi: 10.1371/journal.pone.0157949
6. Kumar N, Pande V, Bhatt R, Shah NK, Mishra N, Srivastava B, et al. Genetic deletion of HRP2 and HRP3 in Indian Plasmodium falciparum population and false negative malaria rapid diagnostic test. Acta Tropica. (2013) 125:119–21. doi: 10.1016/j.actatropica.2012.09.015
7. Gebru T, Ajua A, Theisen M, Esen M, Ngoa UA, Issifou S, et al. Recognition of Plasmodium falciparum mature gametocyte-infected erythrocytes by antibodies of semi-immune adults and malaria-exposed children from Gabon. Malaria J. (2017) 16:176. doi: 10.1186/s12936-017-1827-7
8. Tigu F, Gebremaryam T, Desalegn A. Seasonal profile and five-year trend analysis of malaria prevalence in Maygaba health center, Welkait district, Northwest Ethiopia. J Parasitol Res. (2021) 2021:6727843. doi: 10.1155/2021/6727843
9. Bousema T, Drakeley C. Epidemiology and Infectivity of Plasmodium falciparum and Plasmodium vivax Gametocytes in Relation to Malaria Control and Elimination. Clin Microbiol Rev. (2011) 24:377–410. doi: 10.1128/CMR.00051-10
10. Koepfli C, Yan G. Plasmodium gametocytes in field studies: do we measure commitment to transmission or detectability? Trends Parasitol. (2018) 34:378–87. doi: 10.1016/j.pt.2018.02.009
11. Kumar G, Shankar H, Pasi S, Kaur J. Asymptomatic and low-density Plasmodium infections in India: an unexplored link. Pathog Global Health. (2022) 116:465–6. doi: 10.1080/20477724.2022.2113304
12. Nguitragool W, Mueller I, Kumpitak C, Saeseu T, Bantuchai S, Yorsaeng R, et al. Very high carriage of gametocytes in asymptomatic low-density Plasmodium falciparum and P. vivax infections in western Thailand. Parasites Vectors. (2017) 10:1–9. doi: 10.1186/s13071-017-2407-y
13. Nilsson SK, Childs LM, Buckee C, Marti M. Targeting human transmission biology for malaria elimination. PloS Pathog. (2015) 11:e1004871. doi: 10.1371/journal.ppat.1004871
14. Lima GF, Levi JE, Geraldi MP, Sanchez MCA, Segurado AA, Hristov AD, et al. Malaria diagnosis from pooled blood samples: comparative analysis of real-time PCR, nested PCR and immunoassay as a platform for the molecular and serological diagnosis of malaria on a large-scale. Memorias do Instituto Oswaldo Cruz. (2011) 106:691–700. doi: 10.1590/S0074-02762011000600008
15. Daniel K, Anthony F, Ebony L, Gabrielle K, Beka RA, Ashley NB, et al. From genes to biomarkers: understanding the biology of malaria gametocytes and their detection. In: Mahmut Ç, editor. Genetic Polymorphisms. IntechOpen, Rijeka (2021).
16. Camarda G, Bertuccini L, Singh SK, Salzano AM, Lanfrancotti A, Olivieri A, et al. Regulated oligomerisation and molecular interactions of the early gametocyte protein Pfg27 in Plasmodium falciparum sexual differentiation. Int J Parasitol. (2010) 40:663–73. doi: 10.1016/j.ijpara.2009.11.006
17. van Eijk AM, Sutton PL, Ramanathapuram L, Sullivan SA, Kanagaraj D, Priya GSL, et al. The burden of submicroscopic and asymptomatic malaria in India revealed from epidemiology studies at three varied transmission sites in India. Sci Rep. (2019) 9:17095. doi: 10.1038/s41598-019-53386-w
18. Alam MS, Mohon AN, Mustafa S, Khan WA, Islam N, Karim MJ, et al. Real-time PCR assay and rapid diagnostic tests for the diagnosis of clinically suspected malaria patients in Bangladesh. Malaria J. (2011) 10:175. doi: 10.1186/1475-2875-10-175
19. Palmer CJ, Lindo JF, Klaskala WI, Quesada JA, Kaminsky R, Baum MK, et al. Evaluation of the OptiMAL test for rapid diagnosis of Plasmodium vivax and Plasmodium falciparum malaria. J Clin Microbiol. (1998) 36:203–6. doi: 10.1128/JCM.36.1.203-206.1998
20. Warhurst DC, Williams J. ACP Broadsheet no 148. July 1996. Laboratory diagnosis of malaria. J Clin Pathol. (1996) 49:533. doi: 10.1136/jcp.49.7.533
21. Brancucci NM, Bertschi NL, Zhu L, Niederwieser I, Chin WH, Wampfler R, et al. Heterochromatin protein 1 secures survival and transmission of malaria parasites. Cell Host Microbe. (2014) 16:165–76. doi: 10.1016/j.chom.2014.07.004
22. Snounou G, Viriyakosol S, Zhu XP, Lucilia WJ, Rosario V, Thaithong S, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. (1993) 61:315–20. doi: 10.1016/0166-6851(93)90077-B
23. Anvikar AR, Shah N, Dhariwal AC, Sonal GS, Pradhan MM, Ghosh SK, et al. Epidemiology of plasmodium vivax malaria in India. Am J Trop Med Hyg. (2016) 95:108–20. doi: 10.4269/ajtmh.16-0163
24. Kumari P, Sinha S, Gahtori R, Yadav CP, Pradhan MM, Rahi M, et al. Prevalence of asymptomatic malaria parasitemia in Odisha, India: a challenge to malaria elimination. Am J Trop Med Hygiene. (2020) 103:1510. doi: 10.4269/ajtmh.20-0018
25. Singh A, Bhandari S, Das A, Bharti PK. Asymptomatic low-density Plasmodium falciparum infections: a challenge in malaria elimination in India. J Infect Public Health. (2021), 14(11):1600–2. doi: 10.1016/j.jiph.2021.08.032
26. Churcher TS, Bousema T, Walker M, Drakeley C, Schneider P, Ouédraogo AL, et al. Predicting mosquito infection from Plasmodium falciparum gametocyte density and estimating the reservoir of infection. Elife. (2013) 2:e00626. doi: 10.7554/eLife.00626.011
27. Pradhan MM, Pradhan S, Dutta A, Shah NK, Valecha N, Joshi PL, et al. Impact of the malaria comprehensive case management programme in Odisha, India. PloS One. (2022) 17:e0265352. doi: 10.1371/journal.pone.0265352
28. Saha S, Narang R, Deshmukh P, Pote K, Anvikar A, Narang P. Diagnostic efficacy of microscopy, rapid diagnostic test and polymerase chain reaction for malaria using bayesian latent class analysis. Indian J Med Microbiol. (2017) 35:376–80. doi: 10.4103/ijmm.IJMM_17_199
29. Deora N, Yadav CP, Pande V, Sinha A. A systematic review and meta-analysis on sub-microscopic Plasmodium infections in India: Different perspectives and global challenges. Lancet Regional Health - Southeast Asia. (2022) 2, 1–19. doi: 10.1016/j.lansea.2022.05.001
30. Chourasia MK, Raghavendra K, Bhatt RM, Swain DK, Meshram HM, Meshram JK, et al. Additional burden of asymptomatic and sub-patent malaria infections during low transmission season in forested tribal villages in Chhattisgarh, India. Malaria J. (2017) 16:320. doi: 10.1186/s12936-017-1968-8
31. Shamseddin J, Ghanbarnejad A, Zakeri A, Abedi F, Khojasteh S, Turki H. Molecular method is essential to identify asymptomatic malaria reservoirs: A successful experience in the malaria elimination program in Iran. Diagnostics. (2022) 12:3025. doi: 10.3390/diagnostics12123025
32. Eksi S, Morahan BJ, Haile Y, Furuya T, Jiang H, Ali O, et al. Plasmodium falciparum gametocyte development 1 (Pfgdv1) and gametocytogenesis early gene identification and commitment to sexual development. PloS Pathog. (2012) 8:e1002964. doi: 10.1371/journal.ppat.1002964
33. Gadalla AAH, Siciliano G, Farid R, Alano P, Ranford-Cartwright L, McCarthy JS, et al. Real-time PCR assays for detection and quantification of early P. falciparum gametocyte stages. Sci Rep. (2021) 11:19118. doi: 10.1038/s41598-021-97456-4
34. Tadesse FG, Slater HC, Chali W, Teelen K, Lanke K, Belachew M, et al. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis. (2018) 66:1883–91. doi: 10.1093/cid/cix1123
35. Alemayehu A. Biology and epidemiology of Plasmodium falciparum and Plasmodium vivax gametocyte carriage: Implication for malaria control and elimination. Parasite Epidemiol Control. (2023) 21:e00295. doi: 10.1016/j.parepi.2023.e00295
Keywords: Plasmodium falciparum, malaria, RT-qPCR, gametocyte detection, Pfg27- gametocyte biomarker gene
Citation: Das R, Vashisht K, Kori L, Singh K, Kumar G, Hasan I, Gam J and Pandey KC (2024) Detection of the infective Plasmodium falciparum gametocytes by RT-qPCR assay from a malaria-endemic region of Northeastern India. Front. Trop. Dis 5:1366462. doi: 10.3389/fitd.2024.1366462
Received: 06 January 2024; Accepted: 12 February 2024;
Published: 08 March 2024.
Edited by:
Murilo Vieira Silva, Federal University of Uberlandia, BrazilReviewed by:
Dhaneswar Prusty, Central University of Rajasthan, IndiaRaja Babu Singh Kushwah, Texas A and M University, United States
Fnu Vikky, Clemson University, United States
Copyright © 2024 Das, Vashisht, Kori, Singh, Kumar, Hasan, Gam and Pandey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ram Das, cmFtZGFzOUBnbWFpbC5jb20=
 Ram Das
Ram Das Kapil Vashisht
Kapil Vashisht Lokesh Kori
Lokesh Kori Kuldeep Singh
Kuldeep Singh Gaurav Kumar
Gaurav Kumar Izazul Hasan
Izazul Hasan