
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 06 March 2024
Sec. Neglected Tropical Diseases
Volume 5 - 2024 | https://doi.org/10.3389/fitd.2024.1358514
 Herald Midzi1,2*
Herald Midzi1,2* Thajasvarie Naicker2
Thajasvarie Naicker2 Arthur Vengesai3
Arthur Vengesai3 Emilia T. Choto4
Emilia T. Choto4 Petros Muchesa5
Petros Muchesa5 Maritha Kasambala6
Maritha Kasambala6 Tariro L. Mduluza-Jokonya2,7
Tariro L. Mduluza-Jokonya2,7 Victor Muleya3
Victor Muleya3 Elliot Nyagumbo8
Elliot Nyagumbo8 Donald Tafirenyika Kapanga8
Donald Tafirenyika Kapanga8 Lucy Mabaya8
Lucy Mabaya8 Francisca Mutapi9
Francisca Mutapi9 Takafira Mduluza1,2
Takafira Mduluza1,2Background: Metabolomics approaches are indispensable tools in infection biomarker discovery efforts as they shed light on the underlying pathophysiological mechanisms of disease. In this study, we analysed plasma metabolites that can be used as biomarkers of urogenital schistosomiasis in pre-school aged children below the age of five.
Methods: A case-control study was conducted involving 82 pre-school aged children that were age- and sex-matched. Urine samples were collected for three consecutive days to detect S. haematobium infection using urine filtration. Blood samples were also collected and processed to obtain plasma. Beckman Coulter AU480 chemistry analyser and commercial metabolite kits were used for profiling biomarkers in plasma samples. Descriptive statistics and MetaboAnalyst tool, were used for metabolite analysis. For the determination of diagnostic efficiency of plasma biomarkers, the area under the curve (AUC) was calculated from receiver operating characteristic curves at 95% CI.
Results: Succinic acid, glucose-6-phosphate, phosphatidylcholine, alanine and creatinine levels in plasma were significantly associated with urogenital schistosomiasis (p<0.005) at the population level. Significant increase in concentration at 1.5-fold change (FC) threshold was highest for glucose-6-phosphate with FC value of 2.02 followed by creatinine, albumin and phosphatidylcholine. Creatinine was significantly downregulated with a FC value of 1.98. Of the six dysregulated metabolic pathways, glucose and sucrose metabolism were predominantly affected. Glucose-6-phosphate had the highest AUC (0.81), sensitivity (88.85%) and specificity (90.37%). Phosphatidylcholine and succinic acid also had AUC values greater than 0.7.
Conclusion: Urogenital schistosomiasis affects the energy-related metabolic pathways in pre-school aged children. Glucose-6-phosphate was identified as a potential indicator of infection at the population level. Furthermore, we recommend intensive validation of schistosome metabolite biomarkers.
Urogenital schistosomiasis caused by Schistosoma haematobium, is one of the major neglected tropical diseases (1–3). The World Health Organization (WHO) revealed that 251.4 million cases of schistosomiasis are recorded annually at a global scale, and more than 50% of the cases are predominantly children living in Africa (4, 5). The need for a well-understood pathophysiological mechanism of schistosomiasis, together with accurate disease diagnosis, are crucial elements for the successful control and elimination of the disease. In this regard, there has been an increase in concerted efforts aimed at identifying infection biomarkers to be utilised in the development of diagnostic tools and identification of therapeutic targets (6, 7).
Despite the availability of evidence on the rising morbidity and mortality rates of urogenital schistosomiasis in children (8), there has been a dearth of studies on schistosomiasis in pre-school aged children (PSAC) under the age of five. This knowledge gap has largely been attributed to a number of factors including, difficulties in obtaining parasitological samples, poor diagnostic tools and limited knowledge on risk factors for the age group (9, 10). One of the questions that remains unanswered within this age group is the effect of schistosome infection on the clinically relevant metabolic processes (11). Furthermore, these challenges have been exacerbated by the non-specificity of morbidity biomarkers associated with schistosomiasis in paediatrics (6). The non-specificity of these biomarkers is due to physiological, biochemical and immunological changes and their determination is even more complex in co-infections (12).
Currently, the gold standard method for diagnosing urogenital schistosomiasis is microscopic detection of eggs in urine (13). A major caveat of this technique is the low sensitivity in the detection of light infections that are prevalent in the pre-school aged group and in low schistosome transmission areas (14, 15). Severe metabolic alterations may have occurred to the paediatric patient by the time the eggs are detected (16). On the other hand the standard technique is not able to identify single worm or same sex schistosome infections, as the eggs are only produced when both male and female pair up (17). To mitigate the shortcomings that are associated with the microscopic technique, several methods such as, the polymerase chain reaction and detection of circulating anodic and cathodic antigens have since been developed (18, 19). However, these methods are expensive and often require expertise and complex instrumentation to execute making them unsuitable for use in resource-limited settings. Therefore, finding diagnostic biomarkers with high sensitivity that can detect schistosome infection earlier would be key in reducing the burden of the disease (6).
Spectrometry based metabolomics approaches have expanded rapidly in the field of science for the systematic identification, visualisation and characterisation of infection biomarkers (20, 21). The feasibility of metabolomics in the discovery of biomarkers falls on the premise that metabolites are the key elements in biological pathways and in the presence of a disease, notable changes will occur in these metabolic pathways (22). Consequently, parallel assessment of multiple metabolites during infection enables identification of potent biomarkers that can be used in the development of cost-effective and highly sensitive diagnostic tools (7, 23).
The analysis of host metabolite composition is a holistic approach in identifying novel or potential infection biomarkers. These biomarkers may be indicative of the host’s response to infection and hence, shed light on the underlying pathophysiological mechanisms in urogenital schistosomiasis in pre-school aged children (PSAC) (24). The scarcity of information pertaining to the pathological alterations at the metabolic level in S. haematobium infection in PSAC has provided an impetus for the current investigation. This study aims to profile plasma metabolites that can be used as infection biomarkers in PSAC below the age five.
Ethical approval for the study was granted by the Medical Research Council of Zimbabwe (MRCZ/A/2711). Permission to conduct the study in Mupfure was obtained from the District Medical Officer and local leaders. The study objectives and conduct were explained to the participant’s guardian or parent in Shona and English. It was established that the partaking in the study was voluntary. Written and signed informed consent were attained from the guardian or parent for inclusion of their child in the study.
The study was conducted in Mupfure village located in the Shamva District, Mashonaland Central province of Zimbabwe. This district is located at an elevation of 952.92 meters above sea level and has a subtropical, dry winter climate with annual average temperature and rainfall of 24.6°C and 126.17 millimetres respectively (25). Shamva is an agriculturally based region and people mainly survive on cash crop, food crop and livestock sales (26). The study site is named after the Mupfure river that joins the Sanyati river which flows northwards and drains into the Zambezi river (27). Furthermore, the study site is an high endemic region for S. haematobium infection with a percentage prevalence of the parasitic infection being greater than 50%, as reported previously in a national survey (28).
The current matched case-control study emanated from an ongoing metabolomics project to understand schistosomiasis in children under five years of age, and the cohort was selected at baseline. Human-model based studies using metabolomics for biomarker discovery of infectious diseases, have previously reported that a minimum of 50 samples that are age- and sex-matched should be used to determine metabolite biomarkers of infection, whilst in animal-based studies a minimum of 10 animals may be used (29, 30). As a result, we therefore applied the Buderer’s statistical method for sample size determination (31). This study included 82 children (41 uninfected and 41 S. haematobium infected), aged 2-5 years and age-sex matched. Participants that were positive for schistosomiasis during the study were treated with a single dose (40mg/kg) of praziquantel in-line with WHO treatment guidelines.
Participants were given 100 ml urine collecting bottles or sterile urine bag (Romsons® Paediatric Urine collecting Bag, India). Urine samples were collected from participants for three consecutive days between 1000hrs and 1400 hrs (32). A faecal sample was also collected on one occasion. S. haematobium infection was detected using the urine filtration technique in which 10 ml urine sample was filtered through a nitrile filter with a pore size of 12 µm. 5% iodine solution was added to the filter. In the presence of infection, the oval eggs (110-170 µm length by 40-70 µm width) with a terminal spine are visualised under a light microscope. S. haematobium egg intensity was expressed as the number of eggs per 10 ml urine. For diagnosis of S. mansoni infections that were excluded from the study, a Kato Katz thick smear was prepared from a faecal sample by straining the sample through a 250 µm mesh wire and 41.7 mg of the residue was placed on a glass slide using a Kato Katz template. Cellophane coverslip drenched in 50% glycerine–malachite green was used to produce thick smears. The slide was examined within 60 minutes of preparation to detect soil transmitted helminths and thereafter re-examined after 24 hours for S. mansoni ova (114 to 180 µm length by 45-70 µm width) with lateral spine under a light microscope. Blood samples were collected into 5 ml lavender top EDTA/K2 tubes (Ideal® Blood Collection Tubes, USA) and cold centrifuged for 10 minutes at 1000X for 15 minutes to obtain plasma.
Targeted metabolites were selected based on our earlier study and availability of methods or commercial kits (29). Beckman Coulter AU480 chemistry analyser was used to determine the concentrations of metabolites according to the manufacturer’s instructions. Protein analytical profiles were used to measure total protein, albumin and alanine. Urea and creatinine were measured by measuring urea nitrogen and pricrate-based tests respectively. The lipid profile was used to measure the total cholesterol, triglyceride, high density lipoprotein and low-density lipoprotein. All these tests were conducted simultaneously from 300µl per sample. Glucose-6-phosphate colourimetric assay kit (Abcam® ab83426, India) was used to determine the concentration of glucose-6-phosphate by measuring optical density (OD) values at 450 nanometres (nm). Citrate and succinate concentration were determined using MAK057 Citrate assay kit and MAK335B Succinate assay kit (Merck, USA) by measuring OD values at 570nm.
Data were entered into an Excel spreadsheet, checked for entry errors and transferred into Statistical Package for Social Scientist (SPSS) version 29.0.1 for descriptive statistics and MetaboAnalyst (version 5.0, USA) for comprehensive metabolite analysis including functional interpretation and biomarker identification using R-statistics. The association between plasma metabolites and urogenital schistosomiasis was determined using the Wilcoxon Mann Whitney rank test. Box plots were generated to show the distribution and the mean concentrations of metabolites in both S. haematobium infected and control samples. The FC of plasma metabolites were determined at FC threshold of 1.5 and Log2(FC). The Pearson’s correlation coefficient was used to calculate the association amongst metabolites during schistosomiasis. A p-value of less than 0.05 (p < 0.05) was considered significant during the analysis. For generation of heatmap for correlations of plasma metabolites, variables were compared by chi-squared test and the p-values were visualised on a log scale [-log10(p value)]. Metabolic pathway enrichment analysis was used to predict the effect of the altered plasma metabolite on metabolic pathways. The diagnostic performance of plasma metabolites was assessed by calculating sensitivity, specificity, negative likelihood ratio (NLR) and positive likelihood ratio (PLR) at 50% disease prevalence. Area under the curve (AUC) was calculated from receiver operating characteristic (ROC) curves at 95% CI to determine the diagnostic efficiency plasma biomarkers.
An overview of characteristics of PSAC who partook in the current study is presented in Table 1. For the 82 study participants, the median age was 4 years (IQR: 3) and the mean for height and weight were 1.01 (SD: 0.11) and 15.99 (SD: 3.74) respectively. A high proportion of the participants (70.7%) had light S. haematobium infection compared to those who had heavy infections and the average egg count per 10 ml urine was 30.47.
Table 2 shows association between thirteen plasma metabolites with urogenital schistosomiasis and their mean concentrations in PSAC. Pearson bivariate correlation analysis showed a significant association of the infection with five plasma metabolites namely glucose-6-phosphate (p = 0.008), succinate (p = 0.034), phosphatidylcholine (p = 0.041), creatinine (p = 0.042) and alanine (p = 0.049). However, high-density lipoprotein, low-density lipoproteins, cholesterol, triglycerides, albumin, total proteins and citrate were not significantly associated (p > 0.05) with S. haematobium infection. Furthermore, the distribution of metabolites in both infected and healthy participants are shown in box plots categorically as protein, lipid and energy related metabolites in Figures 1-3.
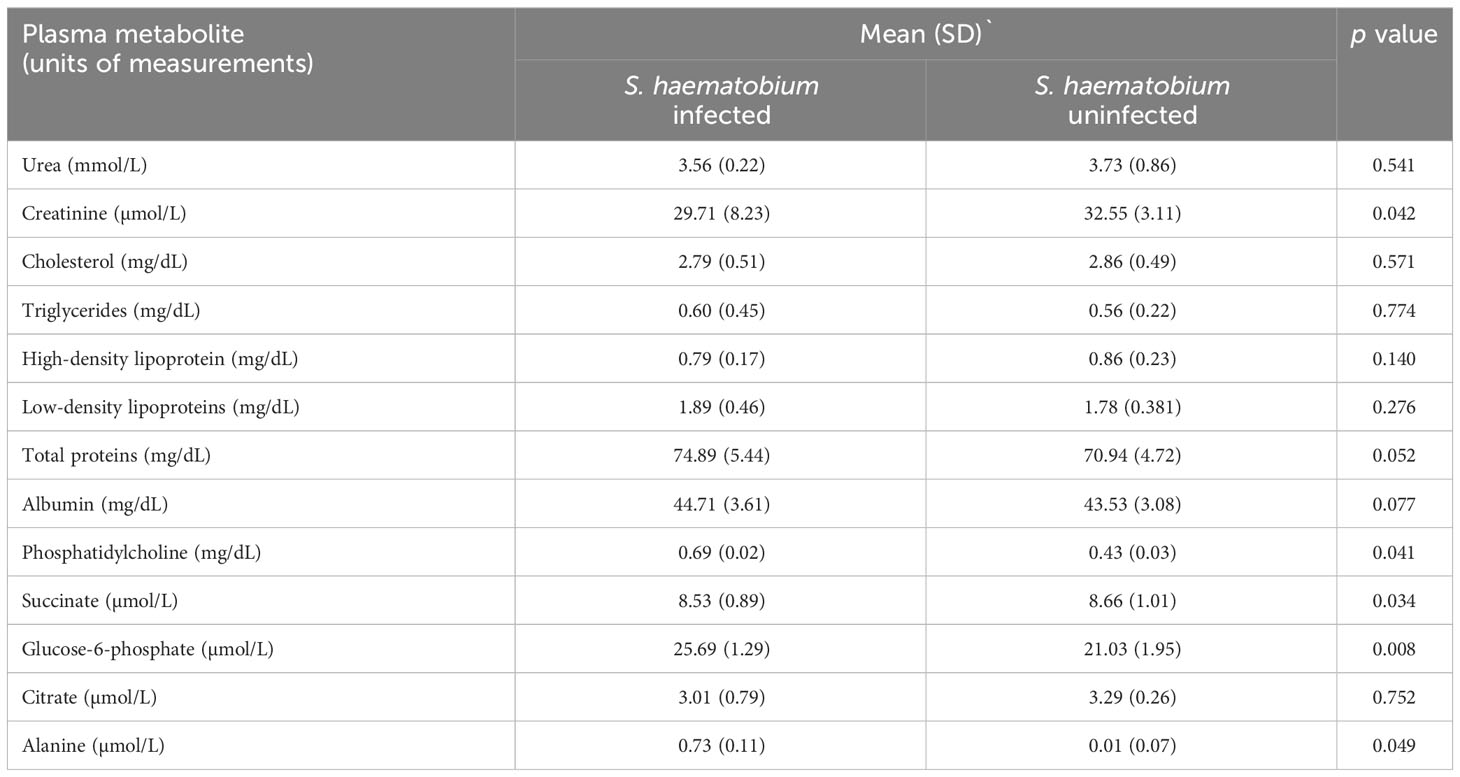
Table 2 Mean concentrations of plasma metabolites and association with urogenital schistosomiasis in PSAC.
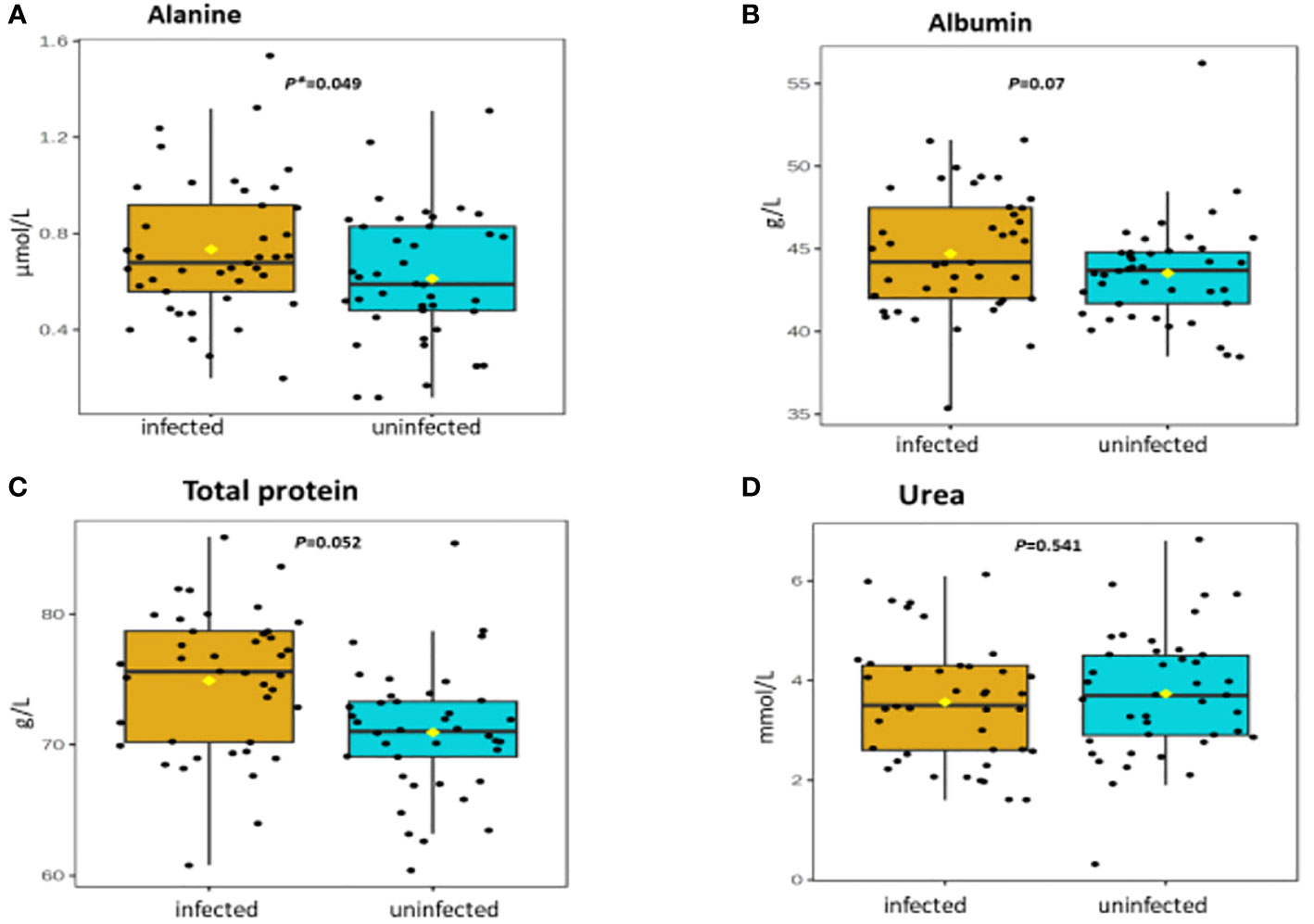
Figure 1 (A-D) Distribution and concentration of protein-related plasma metabolites in S. haematobium infected and uninfected pre-school aged children. (*) indicates p value smaller than 0.05 (p<0.05) and (***) indicate p value smaller than 0.001 (p<0.001).
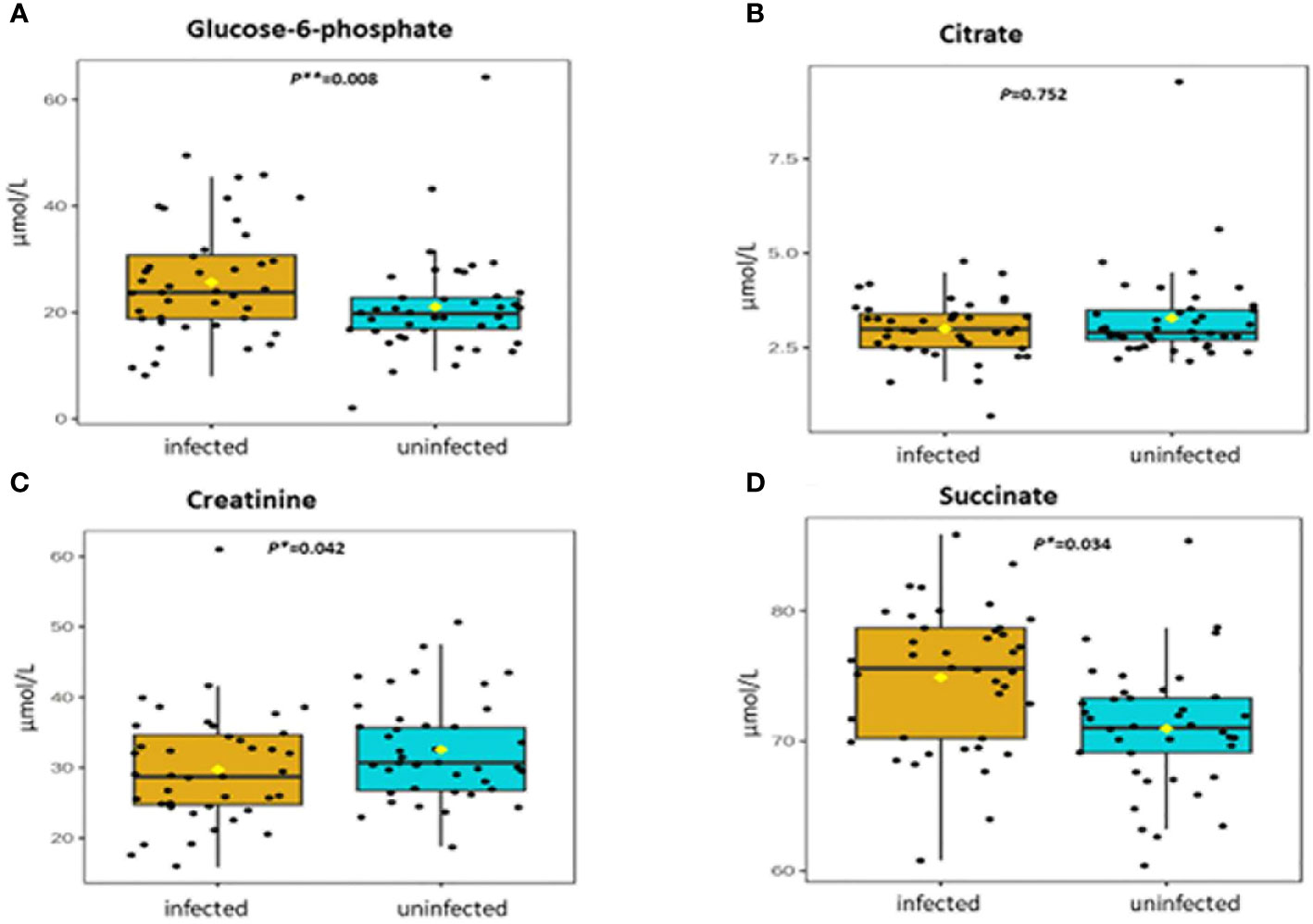
Figure 2 (A-D) Distribution and concentration of energy-related plasma metabolites in S. haematobium infected and uninfected pre-school aged children. (*) indicates p value smaller than 0.05 (p<0.05) and (***) indicate p value smaller than 0.001 (p<0.001).
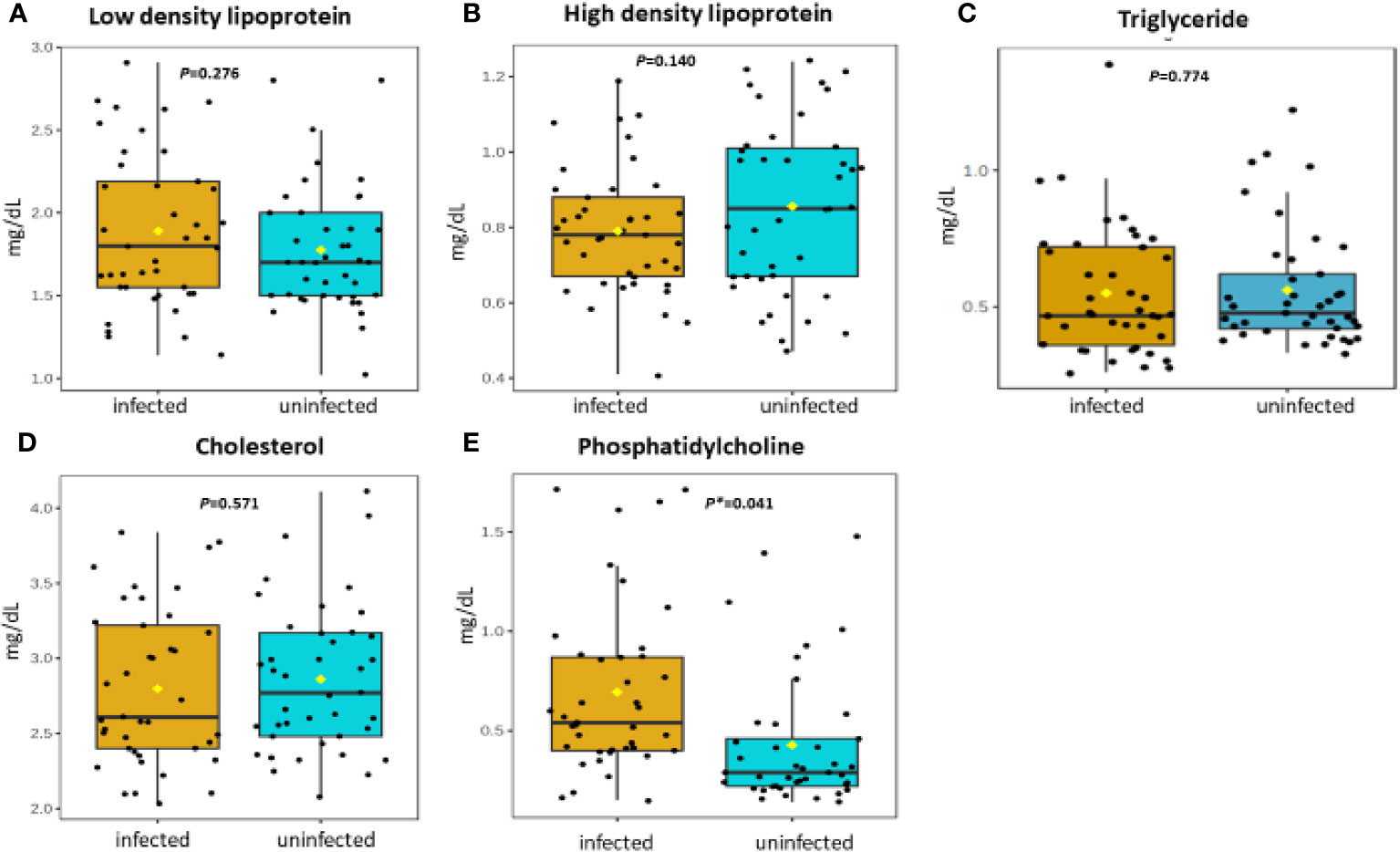
Figure 3 (A-E) Distribution and concentration of lipid-related plasma metabolites in S. haematobium infected and uninfected pre-school aged children. (*) indicates p value smaller than 0.05 (p<0.05) and (***) indicate p value smaller than 0.001 (p<0.001).
The correlation of plasma metabolites with each other during urogenital schistosomiasis is shown in correlation matrix in Figure 4. There was a significant positive correlation between high density lipoprotein and urea; succinate and glucose-6-phosphate; succinate and total protein; cholesterol and high-density lipoprotein with p values of 0.004; 0.005; 0.015 and 0.021 respectively. Inversely, significant negative correlation (p < 0.005) was observed between triglycerides and high-density lipoprotein; urea and triglycerides. The remaining plasma metabolites (phosphatidylcholine, alanine, citrate, low density lipoproteins, total proteins and albumin) were not correlated with each other during schistosomiasis in children below the age of five.
Shown in Table 3 are concentration FC values for the metabolites during S. haematobium infection. The threshold value of 1.5 was used to determine the FC values in metabolite concentration and the values greater than or equal were considered significant (p < 0.05). Six out of thirteen plasma metabolites were significantly altered during urogenital schistosomiasis. Glucose-6-phosphate, albumin and phosphatidylcholine were significantly upregulated with FC values of 2.06, 1.64 and 1.62 respectively. Creatinine, succinate and high-density lipoprotein had their concentrations downregulated significantly with FC values of 1.98, 1.85 and 1.78 respectively. There was no statistical significance in concentration change for citrate, low-density lipoprotein, alanine, total proteins, cholesterol, triglycerides and urea. Figure 5 is a metabolic impact pathway analysis due to changes in metabolite concentration during urogenital schistosomiasis. Six metabolic pathways were altered with glucose and sucrose metabolism predominantly.
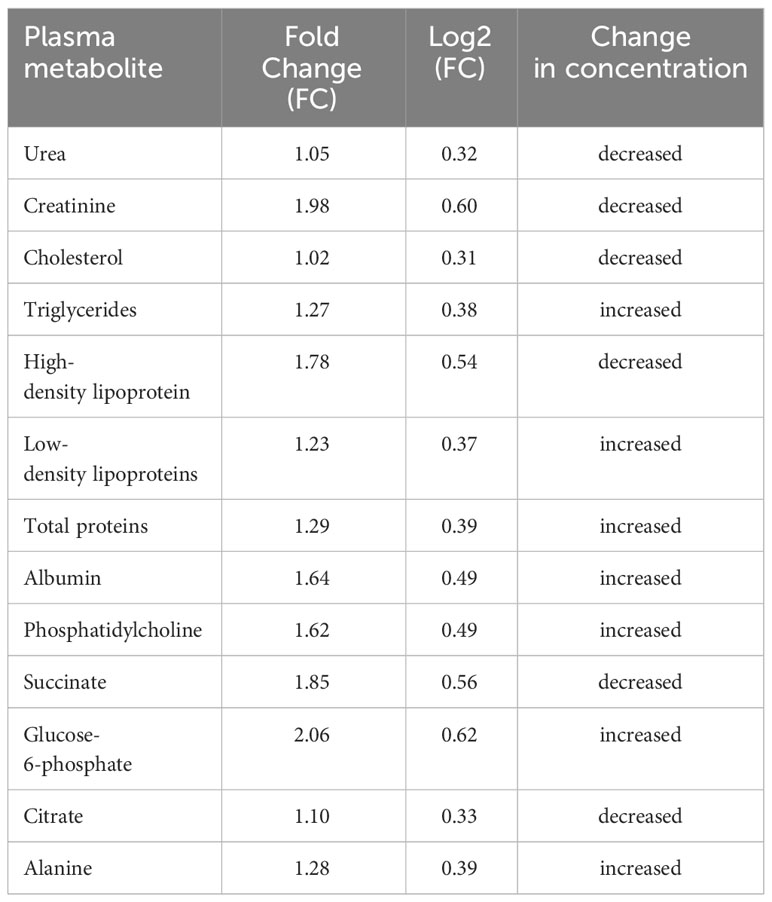
Table 3 Fold change in concentration at 1.5 threshold for plasma metabolites during S. haematobium infection in pre-school aged children.
Shown in Table 4 are diagnostic accuracy and performance values for the plasma metabolites during schistosome infection. The sensitivity and specificity values were highest for glucose-6-phosphate (88.85% and 90.37%) followed by phosphatidylcholine, succinate and creatinine respectively whilst triglycerides had lowest sensitivity and specificity. Glucose-6-phosphate was the strongest indicator of infection in PSAC with AUC value of 0.81 whilst phosphatidylcholine and succinate were fair indicators with AUC of 0.79 and 0.73 respectively. Creatinine was a poor marker of infection with AUC of 0.68 and the remaining metabolites had AUC less than 0.60.
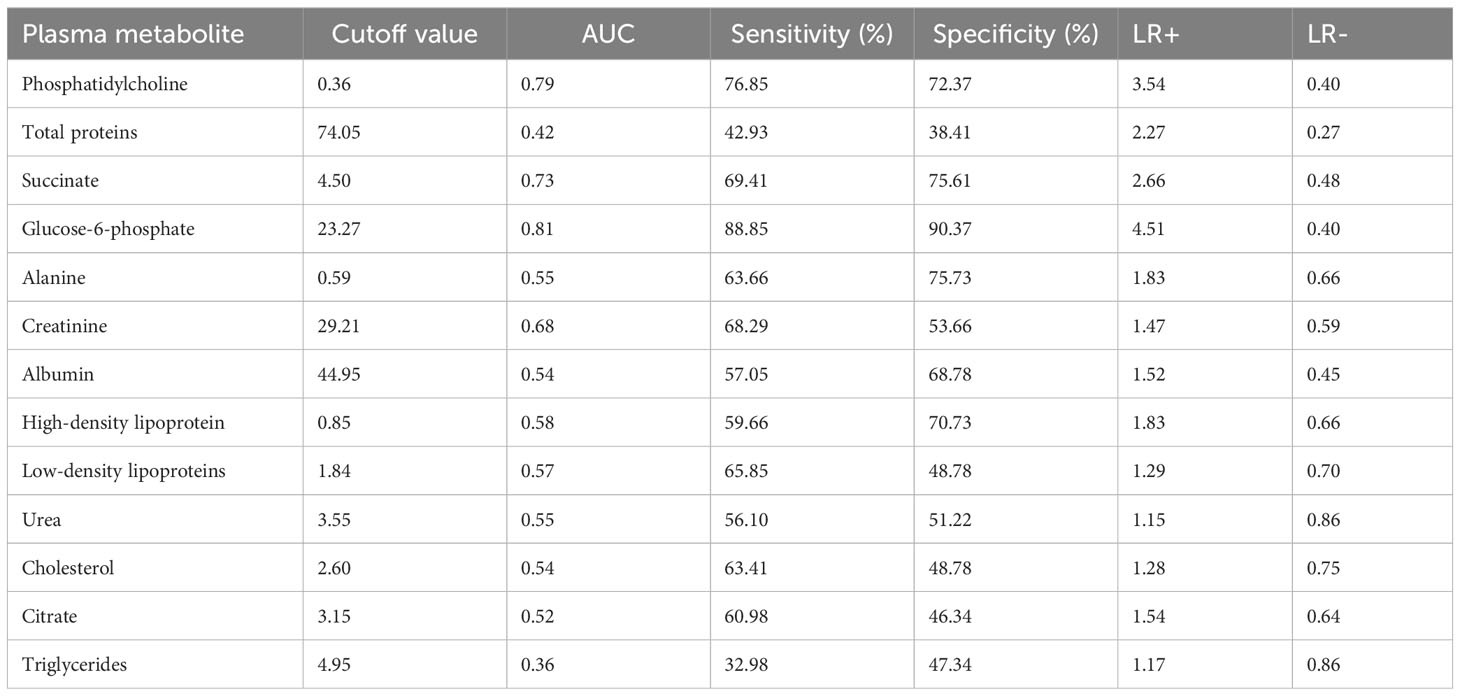
Table 4 Assessment of plasma metabolite biomarkers in the detection of S. haematobium infection in pre-school aged children.
Metabolomics is a more holistic approach in the identification of biomarkers. In this study, selected plasma metabolites were analysed in samples from PSAC with urogenital schistosomiasis and those without the infection. Additionally, their impact on various biochemical pathways was determined.
Five plasma metabolites (glucose-6-phosphate, succinate, phosphatidylcholine, alanine and creatinine) were associated with S. haematobium infection in PSAC at the population level. However, the ranges in plasma concentration of these metabolites showed considerable overlap between infected and uninfected individual children which precludes their use as robust biomarkers of infection. The concentration of glucose-6-phospate was significantly increased during schistosome infection. Glucose-6-phosphate is a crucial metabolite in the mainstream of carbohydrate metabolism; particularly in the glycolytic pathway, the pentose phosphate pathway, glycogen synthesis, hexosamine pathway, and glucose production depending on dietary or hormonal conditions. Consequently, its alteration has a significant impact on all its connected metabolic pathways (33). The findings obtained in this study are strikingly similar to those obtained in a study conducted by Osakunor and colleagues, who reported a 2-fold increase in glycolytic pathway metabolites glucose-6-phosphate and 3-phoshoglycerate. Osakunor and colleagues concluded that these metabolite changes are consistent with parasite-related clinical manifestations of malnutrition and poor growth observed in schistosome-infected children (34).
Phosphatidylcholine was the second most upregulated metabolite during urogenital schistosomiasis. Phosphatidylcholine is a phospholipid that is found ubiquitously in mammalian cell membranes and plays a pivotal role in both proliferative growth and programmed cell death (35, 36). Several mechanistic investigations on the choline phospholipids revealed that the metabolite promotes carcinogenesis through induction of genomic and autophagy processes that allow the survival of tumour cells using minimum energetic sources (37, 38). During chronic urogenital schistosomiasis, bladder cancer manifestations may occur as a result of inflammation and healing of granulomas thus damaging ureteric musculature (39).
Amongst the most downregulated metabolites, and associated with urogenital schistosomiasis, was creatinine which is a catabolic product formed by the degradation of creatine (N-aminoiminomethyl-N-methyl glycine), a nitrogen-containing compound synthesised from amino acids containing guanidine and phosphate groups that are mainly found in the muscles. Creatine plays a critical role in providing energy through the phosphoryl transferase activity of creatine kinase. Poor development of cognitive skills is one of the effects of urogenital schistosomiasis in paediatrics. These clinical manifestations can be attributed to the downregulation of creatine. This is underscored by reports from several studies indicating that low brain creatine content is associated with voluntary or involuntary movement disorders that can lead to autism, epilepsy, cognitive and motor development delays (40–43). This is espoused by a number of different studies which have further demonstrated an improvement in brain bioenergetics and neuroprotective benefits, including cognition upon dietary supplementation with creatine (44–47). Therefore, the addition of creatine in chemotherapeutic treatment of urogenital schistosomiasis may attenuate mental fatigue and improve cognition.
High-density lipoproteins are a family of complex metabolites that can exhibit fundamentally diverse metabolic functions that include, transportation of cholesterol from peripheral tissues to the liver for disposal and steroidogenic tissues for hormone production (48, 49). The current data in our study revealed a down regulation of high-density lipoproteins. Our findings are similar to a separate lipodomics study conducted by Zinsou and colleagues (50), who reported that circulating levels of lipid species associated with cholesterol-rich lipoprotein particles were significantly reduced in schistosome-infected individuals in an intensity-dependent manner. Identical findings were also reported in several investigations in humans with S. mansoni species which showed the occurrence of low lipid profile or dyslipidemia in patients as compared with healthy groups (51, 52).
Succinate is an intermediate metabolite of the Krebs cycle which is one the critical process in cellular respiration (53). In the current study, succinate was down regulated and statistically different between schistosome infected and uninfected PSAC. Severe inflammation of the bladder is one of the clinical symptoms of urogenital schistosomiasis and succinate has been reported to accumulate in certain pathophysiological situations associated with inflammation and metabolic stress (54, 55). Furthermore, the metabolite has been shown to be a mediator between microbial metabolism and intestinal mucosal immune cell development (56). Moderate concentrations of succinate are required to stimulate inflammation to clear pathogens, however down regulation of the metabolite results in poor activation of the innate immunity that drives inflammatory responses during infection (57).
It is clear from the metabolic impact data that urogenital schistosomiasis in PSAC, dysregulates more than six metabolic pathways. This metabolic dysregulation may consequently lead to severe pathological symptoms. Our observations show that sucrose, glucose and tricarboxylic acid cycle (Kreb’s cycle) were predominantly affected. Similarly, in a separate study investigating metabolic changes during schistosomiasis, using mice models, Wu et al. (58) reported significant elevation of urinary 3-ureidopropionate and most importantly the disturbance of glycolysis where glucose and sucrose are the main initiating metabolites and depression of tricarboxylic acid cycle. In another separate metabolomics study, Wang et al. (59) revealed that tricarboxylic acid cycle and its intermediates such as citrate and succinate and pyruvate were dysregulated upon infection. Taken together, these findings from elsewhere, are in agreement with the metabolomic impact assessment observations of our current investigation.
It is noteworthy that purine synthesis and steroid synthesis were amongst the dysregulated metabolic pathways observed in this study during urogenital schistosomiasis. Previous studies have highlighted the production of orthologous enzymes involved in the conversion of progesterone and pregnenolone to estriol and estrone androsterone in schistosome infection (60–63). Oliveira et al. (64) reported disturbance in oestradiol, testosterone and progesterone in hamster models during schistosome infection and these hormones may largely contribute to sterility and infertility in female urogenital schistosomiasis.
The current study assessed the diagnostic performance of selected plasma metabolites during S. haematobium infection in PSAC and five indicators of infection were identified namely; glucose-6-phosphate, phosphatidylcholine, succinate, alanine and creatinine. Glucose-6-phosphate had highest specificity, sensitivity and AUC value thus a valuable infection indicator in the discrimination between S. haematobium infected and healthy individuals at a population level. Thus, there is a need to explore this metabolite and its dynamics during schistosomiasis for it to be inclusively used as a biomarker in PSAC. Considering the poor sensitivity that is associated with the diagnostic tools that are currently applied in parasitological diagnosis for low intensity schistosome infections and the challenges bedevilling the cost prohibitive, more sensitive tools such as PCR, metabolomics provides a channel for the development of cost effective and highly sensitive metabolite based rapid diagnostic tools (65, 66).
In this current study, we acknowledge confounding factors such as co-infections, diet and environmental conditions have been reported to impact plasma metabolite composition and concentration (67–69). Additionally, variations in fold concentration change for metabolites have been shown to be influenced by type of biofluid sample and metabolomic technique used for the analysis (70, 71). Nevertheless, this study reveals potential biomarkers of infection during urogenital schistosomiasis in PSAC. Furthermore, our investigation provides insight on alteration of metabolic pathways as a consequence of S. haematobium infection which helps to explain some of the pathophysiological changes and clinical symptom manifestations in the disease state for this particular young age group.
In this study, five plasma metabolites were significantly associated with urogenital schistosomiasis with glucose-6-phosphate and creatinine being most upregulated and downregulated respectively. As this field of metabolomics is gaining momentum in helminths research and biomarker discovery, we recommend further research to determine metabolite profiles in urine in PSAC and to further validate plasma metabolites especially glucose-6-phosphate as it showed potential as an indicator for use at the population level.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Medical Research Council of Zimbabwe (MRCZ). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
HM: Conceptualisation, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing, Resources. TN: Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing. AV: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. EC: Formal analysis, Investigation, Writing – review & editing. PM: Data curation, Formal analysis, Investigation, Visualisation, Writing – original draft, Writing – review & editing. MK: Formal analysis, Investigation, Methodology, Writing – review & editing. TM-J: Conceptualisation, Data curation, Formal analysis, Investigation, Writing – review & editing. VM: Formal analysis, Software, Visualisation, Writing – review & editing, Methodology. EN: Data curation, Funding acquisition, Methodology, Project administration, Resources, Software, Writing – review & editing. DK: Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Writing – review & editing. LM: Data curation, Funding acquisition, Methodology, Project administration, Resources, Software, Writing – review & editing. FM: Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualisation, Writing – review & editing. TM: Conceptualisation, Funding acquisition, Investigation, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, Methodology.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The proposed study ideation emanated from a study supported by the Royal Society Grants ICA_R1_201.
The authors give special thanks to both parents/guardians and PSAC who participated in the study. We extend our gratification to the nurses and community health workers at Mupfure clinic for their professional assistance during the study. We also wholeheartedly thank FM for the funding of the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou X-N. Schistosomiasis. Nat Rev Dis Primers. (2018) 4:1–19. doi: 10.1038/s41572-018-0013-8
2. Paul JF, Verma S, Berry K. Urinary schistosomiasis. Emergency Med J. (2002) 19:483–4. doi: 10.1136/emj.19.5.483
3. Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. (2000) 77:41–51. doi: 10.1016/S0001-706X(00)00122-4.
4. Schistosomiasis . Available online at: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis (Accessed November 23, 2023).
5. Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. (2015) 19:196–205. doi: 10.1016/j.bjid.2014.11.004
6. Silva-Moraes V, Ferreira JMS, Coelho PMZ, Grenfell RFQ. Biomarkers for schistosomiasis: Towards an integrative view of the search for an effective diagnosis. Acta Tropica. (2014) 132:75–9. doi: 10.1016/j.actatropica.2013.12.024
7. Araújo R, Bento LFN, Fonseca TAH, Von Rekowski CP, da Cunha BR, Calado CRC. Infection biomarkers based on metabolomics. Metabolites. (2022) 12:92. doi: 10.3390/metabo12020092
8. Osakunor DNM. Paediatric schistosomiasis: dynamics and consequences. PLoS Negl Trop Dis. (2020) 12(12):e0006144. doi: 10.7488/era/841
9. Osakunor DNM, Woolhouse MEJ, Mutapi F. Paediatric schistosomiasis: What we know and what we need to know. PloS Negl Trop Dis. (2018) 12:0.0006144-55. doi: 10.1371/journal.pntd.0006144
10. Faust CL, Osakunor DNM, Downs JA, Kayuni S, Stothard JR, Lamberton PHL, et al. Schistosomiasis control: leave no age group behind. Trends Parasitol. (2020) 36:582–91. doi: 10.1016/j.pt.2020.04.012
11. Isaiah PM, Sólveig Palmeirim M, Steinmann P. Epidemiology of pediatric schistosomiasis in hard-to-reach areas and populations: a scoping review. Infect Dis Poverty. (2023) 12:37. doi: 10.1186/s40249-023-01088-x
12. Molehin AJ. Current understanding of immunity against schistosomiasis: impact on vaccine and drug development. Res Rep Trop Med. (2020) 11:119–28. doi: 10.2147/RRTM.S274518
13. Ephraim RKD, Duah E, Andrews JR, Bogoch II. Ultra–low-cost urine filtration for schistosoma haematobium diagnosis: A proof-of-concept study. Am J Trop Med Hygiene. (2014) 91:544. doi: 10.4269/ajtmh.14-0221
14. Chala B. Advances in diagnosis of schistosomiasis: focus on challenges and future approaches. IJGM. (2023) 16:983–95. doi: 10.2147/IJGM.S391017
15. Fangyu LI, Hua HE. Assessing the accuracy of diagnostic tests. Shanghai Arch Psychiatry. (2018) 30:207–12. doi: 10.11919/j.issn.1002-0829.218052
16. Bustinduy AL, Wright S, Joekes EC, Kabatereine NB, Reinhard-Rupp J, King CH, et al. One hundred years of neglect in paediatric schistosomiasis. Parasitology. (2017) 144:1613–23. doi: 10.1017/S0031182017000014
17. Lu D-B, Deng Y, Ding H, Liang Y-S, Webster JP. Single-sex schistosome infections of definitive hosts: Implications for epidemiology and disease control in a changing world. PloS Pathog. (2018) 14:e1006817. doi: 10.1371/journal.ppat.1006817
18. Cavalcanti MG, Cunha AFA, Peralta JM. The advances in molecular and new point-of-care (POC) diagnosis of schistosomiasis pre- and post-praziquantel use: in the pursuit of more reliable approaches for low endemic and non-endemic areas. Front Immunol. (2019) 10:858. doi: 10.3389/fimmu.2019.00858
19. Ludolf F, Patrocínio PR, Corrêa-Oliveira R, Gazzinelli A, Falcone FH, Teixeira-Ferreira A, et al. Serological screening of the Schistosoma mansoni adult worm proteome. PloS Negl Trop Dis. (2014) 8:e2745. doi: 10.1371/journal.pntd.0002745
20. Segers K, Declerck S, Mangelings D, Heyden YV, Eeckhaut AV. Analytical techniques for metabolomic studies: a review. Bioanalysis. (2019) 11:2297–318. doi: 10.4155/bio-2019-0014
21. Preidis GA, Hotez PJ. The newest “Omics”—Metagenomics and metabolomics—Enter the battle against the neglected tropical diseases. PloS Negl Trop Dis. (2015) 9:e0003382. doi: 10.1371/journal.pntd.0003382
22. Alarcon-Barrera JC, Kostidis S, Ondo-Mendez A, Giera M. Recent advances in metabolomics analysis for early drug development. Drug Discovery Today. (2022) 27:1763–73. doi: 10.1016/j.drudis.2022.02.018
23. Legido-Quigley C. Metabolite-biomarker investigations in the life cycle of and infection with. Schistosoma Parasitol. (2010) 137:1425–35. doi: 10.1017/S0031182010000545
24. Moco S, Buescher JM. Metabolomics: going deeper, going broader, going further. Methods Mol Biol. (2023) 2554:155–78. doi: 10.1007/978-1-0716-2624-5_11
25. Weather and Climate. Shamva, Mashonaland Central, ZW Climate Zone, Monthly Averages, Historical Weather Data . Available online at: https://tcktcktck.org/Zimbabwe/mashonaland-central/shamva (Accessed July 14, 2023).
26. Rubhara TT, Oduniyi OS, Mudhara M, Akwasi AM. Analysis of household food expenditure patterns. A case of Shamva district Zimbabwe. Future Food: J Food Agric Soc. (2020) 8:8–21. doi: 10.17170/kobra-202003241099
27. Ravengai S, Love D, Mabvira-Meck M, Musiwa K, Moyce W. Water quality in an abandoned gold mining belt, Beatrice, Sanyati Valley, Zimbabwe. Phys Chem Earth Parts A/B/C. (2005) 30:826–31. doi: 10.1016/j.pce.2005.08.026
28. Midzi N, Mduluza T, Chimbari MJ, Tshuma C, Charimari L, Mhlanga G, et al. Distribution of schistosomiasis and soil transmitted helminthiasis in Zimbabwe: towards a national plan of action for control and elimination. PloS Negl Trop Dis. (2014) 8:0003014-32>. doi: 10.1371/journal.pntd.0003014
29. Midzi H, Vengesai A, Muleya V, Kasambala M, Mduluza-Jokonya TL, Chipako I, et al. Metabolomics for biomarker discovery in schistosomiasis: A systematic scoping review. Front Trop Dis. (2023) 4:1108317. doi: 10.3389/fitd.2023.1108317
30. Adebayo AS, Mundhe SD, Awobode HO, Onile OS, Agunloye AM, Isokpehi RD, et al. Metabolite profiling for biomarkers in Schistosoma haematobium infection and associated bladder pathologies. PloS Negl Trop Dis. (2018) 12:e0006452. doi: 10.1371/journal.pntd.0006452
31. Buderer NM. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med. (1996) 3:895–900. doi: 10.1111/j.1553-2712.1996.tb03538.x
32. Doehring E, Feldmeier H, Daffalla AA. Day-to-day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan. Ann Trop Med Parasitol. (1983) 77:587–94. doi: 10.1080/00034983.1983.11811757
33. Rajas F, Gautier-Stein A, Mithieux G. Glucose-6 phosphate, a central hub for liver carbohydrate metabolism. Metabolites. (2019) 9:282. doi: 10.3390/metabo9120282
34. Osakunor DNM, Mduluza T, Osei-Hyiaman D, Burgess K, Woolhouse MEJ, Mutapi F. Schistosoma haematobium infection is associated with alterations in energy and purine-related metabolism in preschool-aged children. PloS Negl Trop Dis. (2020) 14:e0008866. doi: 10.1371/journal.pntd.0008866
35. Saito R de F, Andrade LN de S, Bustos SO, Chammas R. Phosphatidylcholine-derived lipid mediators: the crosstalk between cancer cells and immune cells. Front Immunol. (2022) 13:768606. doi: 10.3389/fimmu.2022.768606
36. Ridgway ND. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit Rev Biochem Mol Biol. (2013) 48:20–38. doi: 10.3109/10409238.2012.735643
37. Zheng Y, Rodrik V, Toschi A, Shi M, Hui L, Shen Y, et al. Phospholipase D couples survival and migration signals in stress response of human cancer cells. J Biol Chem. (2006) 281:15862–8. doi: 10.1074/jbc.M600660200
38. Jackowski S. Cell cycle regulation of membrane phospholipid metabolism. J Biol Chem. (1996) 271:20219–22. doi: 10.1074/jbc.271.34.20219
39. Zaghloul MS, Zaghloul TM, Bishr MK, Baumann BC. Urinary schistosomiasis and the associated bladder cancer: update. J Egyptian Natl Cancer Institute. (2020) 32:44. doi: 10.1186/s43046-020-00055-z
40. Stockler-Ipsiroglu S, van Karnebeek CDM. Cerebral creatine deficiencies: a group of treatable intellectual developmental disorders. Semin Neurol. (2014) 34:350–6. doi: 10.1055/s-0034-1386772
41. Braissant O, Henry H, Béard E, Uldry J. Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids. (2011) 40:1315–24. doi: 10.1007/s00726-011-0852-z
42. Saraiva ALL, Ferreira APO, Silva LFA, Hoffmann MS, Dutra FD, Furian AF, et al. Creatine reduces oxidative stress markers but does not protect against seizure susceptibility after severe traumatic brain injury. Brain Res Bull. (2012) 87:180–6. doi: 10.1016/j.brainresbull.2011.10.010
43. Joncquel-Chevalier Curt M, Voicu P-M, Fontaine M, Dessein A-F, Porchet N, Mention-Mulliez K, et al. Creatine biosynthesis and transport in health and disease. Biochimie. (2015) 119:146–65. doi: 10.1016/j.biochi.2015.10.022
44. Avgerinos KI, Spyrou N, Bougioukas KI, Kapogiannis D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp Gerontol. (2018) 108:166–73. doi: 10.1016/j.exger.2018.04.013
45. Benton D, Donohoe R. The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br J Nutr. (2011) 105:1100–5. doi: 10.1017/S0007114510004733
46. McMorris T, Mielcarz G, Harris RC, Swain JP, Howard A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol Dev Cognit B Aging Neuropsychol Cognit. (2007) 14:517–28. doi: 10.1080/13825580600788100
47. Rae C, Digney AL, McEwan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trial. Proc Biol Sci. (2003) 270:2147–50. doi: 10.1098/rspb.2003.2492
48. Jomard A, Osto E. High density lipoproteins: metabolism, function, and therapeutic potential. Front Cardiovasc Med. (2020) 7:39. doi: 10.3389/fcvm.2020.00039
49. Graham A. Modulation of the cellular microRNA landscape: contribution to the protective effects of high-density lipoproteins (HDL). Biology. (2023) 12:1232. doi: 10.3390/biology12091232
50. Zinsou JF, Janse JJ, Honpkehedji YY, Dejon-Agobé JC, García-Tardón N, Hoekstra PT, et al. Schistosoma haematobium infection is associated with lower serum cholesterol levels and improved lipid profile in overweight/obese individuals. PloS Negl Trop Dis. (2020) 14:e0008464. doi: 10.1371/journal.pntd.0008464
51. Wolde M, Berhe N, Medhin G, Chala F, van Die I, Tsegaye A. Inverse associations of schistosoma mansoni infection and metabolic syndromes in humans: A cross-sectional study in Northeast Ethiopia. Microbiol Insights. (2019) 12:1178636119849934. doi: 10.1177/1178636119849934
52. da Fonseca CSM, Filho AAP, dos Santos BS, da Silva CA, Domingues ALC, Owen JS, et al. Human plasma lipid modulation in schistosoma mansoni depends on apolipoprotein E polymorphism. PloS One. (2014) 9:e101964. doi: 10.1371/journal.pone.0101964
53. Connors J, Dawe N, Van Limbergen J. The role of succinate in the regulation of intestinal inflammation. Nutrients. (2018) 11:25. doi: 10.3390/nu11010025
54. Akram M. Citric acid cycle and role of its intermediates in metabolism. Cell Biochem Biophys. (2014) 68:475–8. doi: 10.1007/s12013-013-9750-1
55. Tretter L, Patocs A, Chinopoulos C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim Biophys Acta. (2016) 1857:1086–101. doi: 10.1016/j.bbabio.2016.03.012
56. Wei Y, Ma X, Zhao J, Wang X, Gao C. Succinate metabolism and its regulation of host-microbe interactions. Gut Microbes. (2023) 15:2190300. doi: 10.1080/19490976.2023.2190300
57. Harber KJ, de Goede KE, Verberk SGS, Meinster E, de Vries HE, van Weeghel M, et al. Succinate is an inflammation-induced immunoregulatory metabolite in macrophages. Metabolites. (2020) 10:372. doi: 10.3390/metabo10090372
58. Wu J, Xu W, Ming Z, Dong H, Tang H, Wang Y. Metabolic changes reveal the development of schistosomiasis in mice. PloS Negl Trop Dis. (2010) 4:e807. doi: 10.1371/journal.pntd.0000807
59. Wang Y, Utzinger J, Xiao S-H, Xue J, Nicholson JK, Tanner M, et al. System level metabolic effects of a Schistosoma japonicum infection in the Syrian hamster. Mol Biochem Parasitol. (2006) 146:1–9. doi: 10.1016/j.molbiopara.2005.10.010
60. Vale N, Gouveia MJ, Rinaldi G, Santos J, Santos LL, Brindley PJ, et al. The role of estradiol metabolism in urogenital schistosomiasis-induced bladder cancer. Tumour Biol. (2017) 39:1010428317692247. doi: 10.1177/1010428317692247
61. Botelho MC, Alves H, Richter J. Estrogen catechols detection as biomarkers in schistosomiasis induced cancer and infertility. Lett Drug Des Discovery. (2017) 14:135–8. doi: 10.2174/1570180813666160720165057
62. Santos J, Gouveia MJ, Vale N, Delgado M de L, Gonçalves A, da Silva JMT, et al. Urinary estrogen metabolites and self-reported infertility in women infected with Schistosoma haematobium. PloS One. (2014) 9:e96774. doi: 10.1371/journal.pone.0096774
63. Correia da Costa JM, Vale N, Gouveia MJ, Botelho MC, Sripa B, Santos LL, et al. Schistosome and liver fluke derived catechol-estrogens and helminth associated cancers. Front Genet. (2014) 5:444. doi: 10.3389/fgene.2014.00444
64. Oliveira KC, Cardoso R, Dos Santos AC, Fernandes R, Botelho MC. Imbalance of steroid hormones in hamsters infected with schistosoma mansoni. Endocr Metab Immune Disord Drug Targets. (2019) 19:1122–6. doi: 10.2174/1871530319666190529121204
65. Ajibola O, Gulumbe BH, Eze AA, Obishakin E. Tools for detection of schistosomiasis in resource limited settings. Med Sci. (2018) 6. doi: 10.3390/medsci6020039
66. Bracewell-Milnes T, Saso S, Abdalla H, Nikolau D, Norman-Taylor J, Johnson M, et al. Metabolomics as a tool to identify biomarkers to predict and improve outcomes in reproductive medicine: a systematic review. Hum Reprod Update. (2017) 23:723–36. doi: 10.1093/humupd/dmx023
67. Siener R, Hesse A. The effect of different diets on urine composition and the risk of calcium oxalate crystallisation in healthy subjects. Eur Urol. (2002) 42:289–96. doi: 10.1016/s0302-2838(02)00316-0
68. Alwis US, Haddad R, Monaghan TF, Abrams P, Dmochowski R, Bower W, et al. Impact of food and drinks on urine production: A systematic review. Int J Clin Pract. (2020) 74:e13539. doi: 10.1111/ijcp.13539
69. Javan Balegh Marand A, Van Koeveringe GA, Janssen D, Vahed N, Vögeli T-A, Heesakkers J, et al. Urinary microbiome and its correlation with disorders of the genitourinary system. Urol J. (2021) 18:259–70. doi: 10.22037/uj.v16i7.5976
70. Smith L, Villaret-Cazadamont J, Claus SP, Canlet C, Guillou H, Cabaton NJ, et al. Important considerations for sample collection in metabolomics studies with a special focus on applications to liver functions. Metabolites. (2020) 10:104. doi: 10.3390/metabo10030104
Keywords: metabolites, plasma, biomarkers, S. haematobium, Pre-school aged children
Citation: Midzi H, Naicker T, Vengesai A, Choto ET, Muchesa P, Kasambala M, Mduluza-Jokonya TL, Muleya V, Nyagumbo E, Kapanga DT, Mabaya L, Mutapi F and Mduluza T (2024) Plasma metabolite profiling for S. haematobium biomarkers of infection in pre-school aged children in Shamva District, Zimbabwe. Front. Trop. Dis 5:1358514. doi: 10.3389/fitd.2024.1358514
Received: 19 December 2023; Accepted: 08 February 2024;
Published: 06 March 2024.
Edited by:
Jeremy Foster, New England Biolabs, United StatesReviewed by:
Pedro Manuel Ferreira, New University of Lisbon, PortugalCopyright © 2024 Midzi, Naicker, Vengesai, Choto, Muchesa, Kasambala, Mduluza-Jokonya, Muleya, Nyagumbo, Kapanga, Mabaya, Mutapi and Mduluza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Herald Midzi, bWlkemloZXJhbGRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.