
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Trop. Dis. , 20 March 2024
Sec. Neglected Tropical Diseases
Volume 5 - 2024 | https://doi.org/10.3389/fitd.2024.1354031
This article is part of the Research Topic The Role of Diagnostics in Eliminating Schistosomiasis as a Public Health Problem: Trend and Need View all 3 articles
 Silvio Wallemacq1*
Silvio Wallemacq1* Ahalieyah Anantharajah2
Ahalieyah Anantharajah2 Pamela Baldin3
Pamela Baldin3 Jean-Cyr Yombi1,4
Jean-Cyr Yombi1,4 Julien De Greef1,4†
Julien De Greef1,4† Leïla Belkhir1,4†
Leïla Belkhir1,4†Schistosomiasis is common in many tropical regions and poses a risk for the local population and travelers. In travelers, most of schistosomiasis are described as acute. We report the epidemiological, clinical, and laboratory characteristics associated with an outbreak of asymptomatic schistosomiasis among nonimmune Belgian school travelers in Rwanda. Schistosomiasis was diagnosed by serology in 12 out of the 15 (80%) travelers who swam in the lake nearly 2 years after a single exposure to freshwater at Kivu Lake, Rwanda. None showed signs of acute or chronic schistosomiasis. Eosinophilia was present in only 1 of them. Schistosoma mansoni eggs were not found in any infected patient. This report of an outbreak of asymptomatic schistosomiasis imported from Lake Kivu highlights the risk for travelers of acquiring the infection with only a short and single exposure, and provides strong arguments for routine serological screening for schistosomiasis in all individuals who have had any freshwater contact in endemic areas, irrespective of symptoms or laboratory findings.
Schistosomiasis is a parasitic disease, prevalent in tropical and subtropical areas, especially in the setting of inadequate sanitation. It is caused by blood flukes of the Schistosoma genus. The parasite’s life cycle requires certain types of freshwater snails as intermediate host (Biomphalaria glabrata being the main host of Schistosoma mansoni). The release of cercariae (the infectious form of the parasite) from the snail leads to contamination of water. Individuals can become infected when larval forms of the parasite penetrate the skin during contact with infested water, mainly close to where snails are found or where infected people defecate into water (1). In European travelers, expatriates and migrants, acquisition of Schistosoma species occurred mostly in Africa (95%), followed by South America (1.4%) and Southeast Asia (1.4%) (2). On the African continent, most infections occurred in West Africa (36%) followed by Central Africa (28%) and East Africa (27%). In Africa, the five most frequently named exact location of contamination are Lake Victoria, Lake Malawi, lakes near Lubumbashi (Democratic Republic of the Congo), river Nile, and Lake Kivu (2). In Rwanda and around Lake Kivu, S. mansoni is the only endemic species (3, 4).
Data about schistosomiasis among travelers returning from endemic areas are increasing, but most outbreak reports are based on acute symptomatic diseases among travelers (5–7). This report describes an uncommon outbreak of completely asymptomatic schistosomiasis among travelers.
We report a case series of asymptomatic schistosomiasis among a group of Belgian pupils after travelling to Rwanda. A timeline with relevant events from exposure to diagnostic and treatment can be found on Figure 1.
The 20 year old index case was admitted to hospital for acute appendicitis 18 months after the school trip to Rwanda. He had no relevant medical history. During surgery, several small white lesions were fortuitously discovered on the surface of the liver. Histologic examination of perioperative liver biopsies revealed presence of non-caseous granulomas, eosinophilic infiltration and evidence of hepatobiliary disease in the surrounding hepatic parenchyma. No fungal organisms, mycobacteria nor Schistosoma eggs were seen on initial microscopy examination. He was referred to the department of infectious diseases for assessment. Blood tests showed no inflammation nor eosinophilia and liver enzymes were within normal range. Interferon-Gamma Release Assay for detection of Mycobacterium tuberculosis infection was negative. Liver structure was normal on ultrasound examination with no evidence of focal lesions and subsequent FDG PET-CT showed no hypermetabolic foci. Finally, thorough patient history revealed that the patient had bathed in Lake Kivu (in Kibuye, Rwanda) during a school trip to Rwanda 18 months previously, suggesting the diagnosis of schistosomiasis. High titers of antibodies against Schistosoma spp (ELISA ratio at 3.88 (<1) and 1/640 (<1/160) by IHA) confirmed the diagnosis.
Examination of stools and urine (without concentration techniques) did not find Schistosoma eggs. Further histological examination of the liver biopsy samples revealed calcified Schistosoma eggs in the center of granulomas (Figure 2).
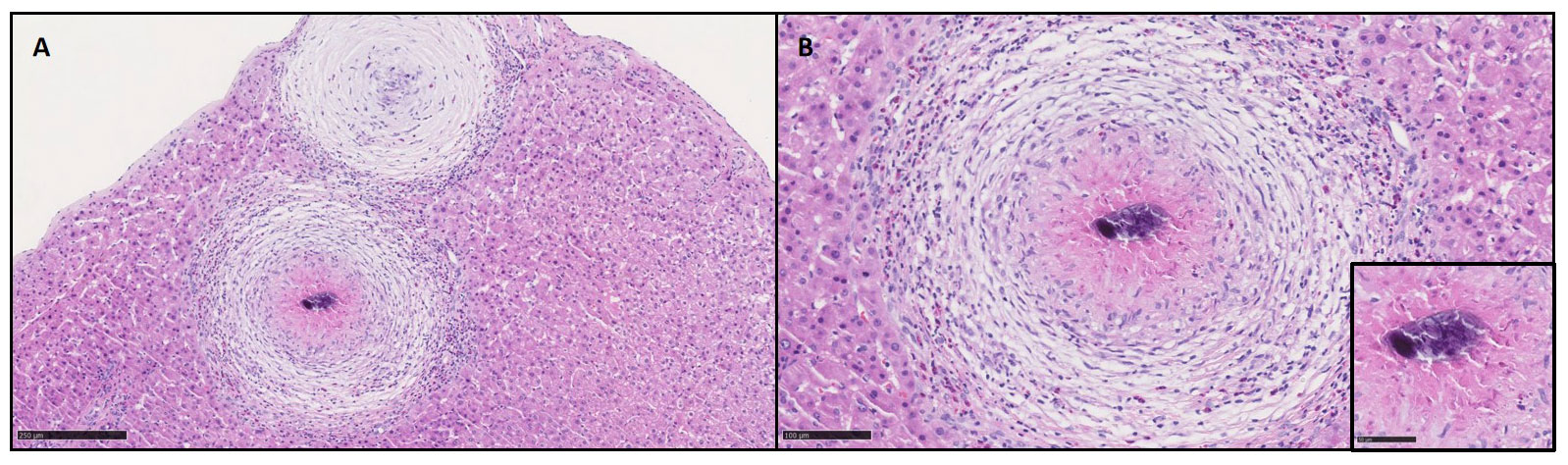
Figure 2 Hepatic parenchyma presented some granulomas composed by an aggregation of macrophages and inflammatory cells (A, H&E 10x). The granuloma was surrounded by a rich infiltration of inflamatory cells rich in eosinophils (B, H&E 20x) and centered by calcified schistosoma eggs (insert, H&E 40x).
As other members of the travel group had also bathed in the Kivu Lake, local public health authorities screened all of them for Schistosoma infection.
All the subjects who travelled to Rwanda with the index patient were summoned by the Local public health authorities to answer a questionnaire about exposure to infested water, the number of freshwater contacts, the exact geographical swimming locations, the presence of acute symptoms (specifically fever, cough, skin rash, weakness, headache, and abdominal pain) or chronic symptoms, screening tests by serology and stool examination and the treatments received. Subjects were also questioned on travels to schistosomiasis endemic regions other than Rwanda. A subject was considered to have a Schistosoma infection if Schistosoma eggs were observed in stools or a Schistosoma serology was positive. To detect Schistosoma eggs in feces, a 3 stool sample was processed for microscopic detection on a direct smear, without concentration technique.
Schistosoma serology was performed in two different laboratories (the Hospital University Laboratory of Brussels (LHUB) and the Institute of Tropical Medicine (ITM) in Antwerp). Detection of IgG by ELISA using S. mansoni soluble egg antigen and IgM by ELISA using S. mansoni adult worm antigen (Euroimmun Kit, Lübeck, Germany) were performed in LHUB, and indirect haemagglutination assay using S. mansoni adult worm antigen–covered sheep red blood cells (Biosynex Bilharziosis Kit by Fumouze Laboratories, Levallois Perret, France) and ELISA using a mix of S. mansoni soluble egg and soluble adult worm antigens (Bordier Kit, Crissier, Switzerland) were performed in ITM.
Table 1 summarizes the exposure to infested water, the presence of symptoms, the serology results and the treatment received for each traveler. 16 travelers who were exposed to infested water were between 20 and 21 years old, and 1 traveler had 38 years old.
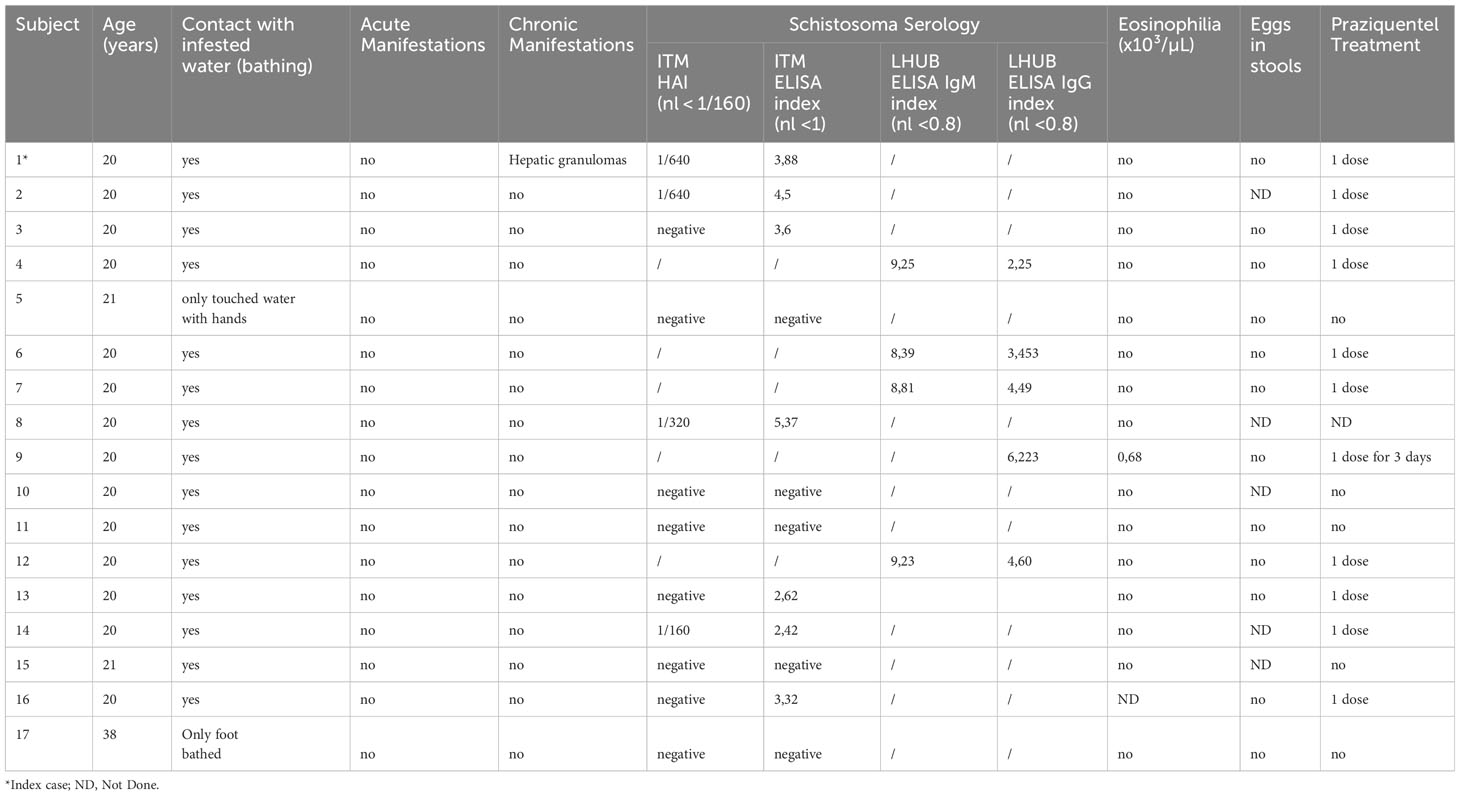
Table 1 Clinical and biological data of the travelers who had exposure with infested water during the school trip to Rwanda.
Among the 27 travelers, 6 of them could not be reached. Of those who answered the questionnaire, 15 reported having bathed in Lake Kivu, 2 reported having had hand or foot contact with the lake water, and 4 reported having had no contact with lake water. For each individual, only a single exposure to the water was reported, and bathing time lasted 20 minutes to 1 hour. Among the 15 bathers, 12 (80%) had positive Schistosoma serology at the time of the report (18-20 months after exposure). None of them had developed acute or chronic symptoms. Only one of the infected travelers had mild eosinophilia (0.68 x10³/µL). Schistosoma mansoni eggs were not found in any infected patient’s stools, but 5 of them declined the exam. No schistosomiasis was detected in subjects who had not bathed in Lake Kivu (n=4).
All but one of the infected travelers were treated by praziquantel (40 mg/kg per day in two divided doses for one day), as the worm burden was assumed to be low. Only one of the travelers, who presented moderate eosinophilia, was treated with praziquantel for 3 consecutive days. There were no specific follow-up performed given the lack of symptomatology and the irrelevance of post treatment serologic monitoring.
We report an outbreak of schistosomiasis in travelers after bathing off the Rwandan shore of Lake Kivu, with a very high infection rate of 12/15 (80%). This high rate may indicate a high density of cercariae in the lake water of that area.
In travelers, the absolute risk of schistosomiasis has not been prospectively determined in large series. Among 3528 mostly asymptomatic travelers and immigrants attending the Tropical Diseases unit in Toronto, 48 (1.5%) were found to be infected with schistosomiasis (8). Incidence of acute infection reported in cluster series is much higher, ranging from 32% in a report on travelers having bathed in Lake Malawi, to 97% in a report on travelers who had showered under a waterfall in Dogon country, Mali (7, 9). These rates are comparable to the rate in our report.
In contrast to other publications reporting schistosomiasis in association with tourism (10–12), in our study, none of the 12 infected patients presented with acute schistosomiasis. This illustrates the risk of acquiring asymptomatic disease among travelers, and emphasizes the need for screening after exposure to freshwater in endemic areas.
In fact, most infected individuals do not develop symptomatic illness. The natural course of the infection depends on the age at the first exposure, the intensity of subsequent exposures, the development of immunity against repeated infection, and genetic susceptibility (1, 13).
Infected travelers present mainly acute Katayama syndrome, which is a systemic hypersensitivity reaction to Schistosoma antigens and circulating immune complexes that occur three to eight weeks after infection. This happens almost exclusively among nonimmune hosts such as travelers and is usually observed in approximately half of infected individuals (14). On the contrary, chronic infection related to schistosomiasis is most common among individuals in endemic areas with repeated exposure, and may lead to intestinal involvement, portal hypertension, genitourinary or neurological complications. However, chronic infection has been reported in individuals with brief exposure such as travelers (15, 16). In an Israeli report of 137 cases of infected travelers (17), 26% of those who were initially asymptomatic eventually developed chronic symptomatic schistosomiasis.
Accordingly, it is highly recommended to treat asymptomatic travelers in order to prevent the development of potential chronic disease. Current CDC guidelines (18) recommend serologic screening 8-10 weeks after having been exposed to freshwater in high-risk areas.
A peculiar aspect of this present study is the clinical presentation of the index patient in whom diagnosis was made by the incidental finding of hepatic granuloma, which is the early stage of the hepatic schistosomiasis. This is the result of an early immune reaction to ova that are embolized and trapped in the presinusoidal periportal spaces of the liver, producing a local granulomatous inflammation. The progression into hepatofibrosis and secondary portal hypertension requires several years and is rarely seen in travelers (15).
Schistosomiasis infection diagnosis can sometimes be challenging, even in patients presenting with acute symptoms. Stool and urine examinations for ova are frequently negative, particularly in travelers, who generally have a low parasite burden. Sensitivity can be increased with the use of concentration techniques (19), but which are often not available in non-endemic settings. In the 2 laboratory institutions that examined travelers’ stools in this report, concentration techniques are available for urine examination but are not routinely used for stools examination. Showing an increased sensitivity compared to microscopy, RT-PCR for Schistosoma detection is a promising diagnostic tool and may be helpful to discriminate between past and acute infection (20, 21). However, RT-PCR for Schistosoma is still not available in clinical routine in Belgium. Diagnosis often relies on serologic testing, which although is known to be very sensitive, becomes positive only 6 to 12 weeks or more after exposure (22). Most routine techniques are designed to detect IgG or IgM against Soluble Worm Antigen or Soluble Egg Antigen by ELISA, IHA or immunofluorescence. Most assays stay positive for at least two years after effective treatment, and often much longer (22), whereas eosinophilia, if present, usually normalizes after 6 weeks (23, 24). So, unlike serological tests, eosinophil count monitoring can be useful for follow-up.
There is no vaccine or prophylactic treatment available for travelers to endemic areas of Schistosoma. Preventive measures include avoiding wading, swimming, or other contact with freshwater in disease-endemic countries. Although repellents have been suggested to prevent penetration of the skin by the cercariae (25, 26) this only reduces the risk but does not provide full protection. Some experts advise to take one dose of praziquantel after potential exposure, as a post-exposure prophylaxis. However, it can confer a false sense of security, as praziquantel is not effective against young worms. As an example, acute Katayama complicated with early neuroschistosomiasis was reported in 2006 14 days after bathing in Malawi Lake, even after intake of one dose of praziquantel (27).
Kivu Lake is known to be a risk area for schistosomiasis, but there are still tourism companies in this area that offer water activities including swimming in the lake.
This study emphasizes the need for professional pre-travel advice, particularly for long-term travelers in schistosomiasis-endemic areas.
In conclusion, schistosomiasis is a relevant clinical challenge for travelers to tropical and subtropical areas, and may frequently be underdiagnosed in asymptomatic travelers. Information before travelling to prevent exposure as well as screening of asymptomatic travelers returning from schistosomiasis-endemic areas are the cornerstones of schistosomiasis prevention in travel medicine.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent has been obtained from the index case for the publication of the data included in this article. The institutional ethical board of Cliniques Universitaires Saint-Luc and UCLouvain University approved the study (CEHF 2022/24OCT/402). Due to the retrospective design of the study, and according to Belgian and local ethics law, informed consent was not requested from the other cases because they were not described individually.
SW: Conceptualization, Investigation, Writing – original draft. AA: Data curation, Methodology, Supervision, Writing – review & editing. PB: Data curation, Methodology, Writing – review & editing. JCY: Supervision, Writing – review & editing. JDG: Conceptualization, Methodology, Supervision, Writing – review & editing. LB: Conceptualization, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank the COCOM for their assistance in calling all the travelers and asking for a screening test. We thank Dr. Mariana Andrade, M.D., who provided editorial assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. (2006) 368:1106–18. doi: 10.1016/S0140-6736(06)69440-3
2. Lingscheid T, Kurth F, Clerinx J, Marocco S, Trevino B, Schunk M, et al. Schistosomiasis in European travelers and migrants: analysis of 14 years TropNet surveillance data. Am J Trop Med Hyg. (2017) 97:567–74. doi: 10.4269/ajtmh.17-0034
3. Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. (2014) 383:2253–64. doi: 10.1016/S0140-6736(13)61949-2
4. Rujeni N, Morona D, Ruberanziza E, Mazigo HD. Schistosomiasis and soil-transmitted helminthiasis in Rwanda: an update on their epidemiology and control. Infect Dis Poverty. (2017) 6:8. doi: 10.1186/s40249-016-0212-z
5. Leshem E, Maor Y, Meltzer E, Assous M, Schwartz E. Acute schistosomiasis outbreak: clinical features and economic impact. Clin Infect Dis. (2008) 47:1499–506. doi: 10.1086/593191
6. Steiner F, Ignatius R, Friedrich-Jaenicke B, Dieckmann S, Harms G, Poppert S, et al. Acute schistosomiasis in European students returning from fieldwork at Lake Tanganyika, Tanzania. J Travel Med. (2013) 20:380–3. doi: 10.1111/jtm.12069
7. Visser LG, Polderman AM, Stuiver PC. Outbreak of schistosomiasis among travelers returning from Mali, West Africa. Clin Infect Dis. (1995) 20:280e5. doi: 10.1093/clinids/20.2.280
8. Boggild AK, Yohanna S, Keystone JS, Kain KC. Prospective analysis of parasitic infections in Canadian travelers and immigrants. J Travel Med. (2006) 13:138e44. doi: 10.1111/j.1708-8305.2006.00032.x
9. Cetron MS, Chitsulo L, Sullivan JJ, Pilcher J, Wilson M, Noh J, et al. Schistosomiasis in Lake Malawi. Lancet. (1996) 348:1274e8. doi: 10.1016/S0140-6736(96)01511-5
10. Cnops L, Huyse T, Maniewski U, Soentjens P, Bottieau E, Van Esbroeck M, et al. Acute Schistosomiasis With a Schistosoma mattheei Schistosoma haematobium Hybrid Species in a Cluster of 34 Travelers Infected in South Africa. Clin Infect Dis. (2021) 72:1693. doi: 10.1093/cid/ciaa312
11. Bou A, Gascón J, Eugenia Valls M, Corachán M. Katayama fever in Spanish tourists: analysis of 25 cases. Med Clin (Barc). (2001) 116:220–2. doi: 10.1016/S0025-7753(01)71776-9
12. Kager PA, Schipper HG. Acute schistosomiasis: fever and eosinophilia, with or without urticaria, after a trip to Africa. Ned Tijdschr Geneeskd. (2001) 145:220–5.
13. Abel L, Demenais F, Prata A, Souza AE, Dessein A. Evidence for the segregation of a major gene in human susceptibility/resistance to infection by Schistosoma mansoni. Am J Hum Genet. (1991) 48:959.
14. Bottieau E, Clerinx J, de Vega MR, Van den Enden E, Colebunders R, Van Esbroeck M, et al. Imported Katayama fever: clinical and biological features at presentation and during treatment. J Infect. (2006) 52:339. doi: 10.1016/j.jinf.2005.07.022
15. Clerinx J, Van Gompel A. Schistosomiasis in travellers and migrants. Travel Med Infect Dis. (2011) 9:6. doi: 10.1016/j.tmaid.2010.11.002
16. Blanchard TJ. Schistosomiasis. Travel Med Infect Dis. (2004) 2:5. doi: 10.1016/j.tmaid.2004.02.011
17. Meltzer E, Artom G, Marva E, Assous MV, Rahav G, Schwartzt E. Schistosomiasis among travelers: new aspects of an old disease. Emerg Infect Dis. (2006) 12:1696–700. doi: 10.3201/eid1211.060340
18. Centers for Disease Control and Prevention (CDC). Perspectives: screening asymptomatic returned travelers. In: Nemhauser J, editor. CDC Yellow Book 2024: Health Information for International Travel. Oxford University Press, New York (2023). p. 790–7.
19. Glinz D, Silué KD, Knopp S, Lohourignon LK, Yao KP, Steinmann P, et al. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PloS Negl Trop Dis. (2010) 4:e754. doi: 10.1371/journal.pntd.0000754
20. Guegan H, Fillaux J, Charpentier E, Robert-Gangneux F, Chauvin P, Guemas E, et al. Real-time PCR for diagnosis of imported schistosomiasis. PloS Negl Trop Dis. (2019) 13:e0007711. doi: 10.1371/journal.pntd.0007711
21. Wichmann D, Panning M, Quack T, Kramme S, Burchard GD, Grevelding C, et al. Diagnosing schistosomiasis by detection of cell-free parasite DNA in human plasma. PloS Negl Trop Dis. (2009) 3:e422. doi: 10.1371/journal.pntd.0000422
22. Jones ME, Mitchell RG, Leen CL. Long seronegative window in schistosoma infection. Lancet. (1992) 340:1549. doi: 10.1016/0140-6736(92)92805-P
23. Bierman WF, Wetsteyn JC, van Gool T. Presentation and diagnosis of imported schistosomiasis: relevance of eosinophilia, microscopy for ova, and serology. J Travel Med. (2005) 12:9–13. doi: 10.2310/7060.2005.00003
24. Cnops L, Huyse T, Maniewski U, Soentjens P, Bottieau E, Van Esbroeck M, et al. Acute Schistosomiasis With a Schistosoma mattheei × Schistosoma haematobium Hybrid Species in a Cluster of 34 Travelers Infected in South Africa. Clin Infect Dis. (2021) 72:1693–8. doi: 10.1093/cid/ciaa312
25. Salafsky B, Ramaswamy K, He XY, Li J, Shibuya T. Development and evaluation of LIPODEET, a new long-acting formulation of N, N-diethyl-m-toluamide (deet) for the prevention of schistosomiasis. Am J Trop Med Hyg. (1999) 61:743–50. doi: 10.4269/ajtmh.1999.61.743
26. Negm AY, Ibrahim IR, El-Temsahy MM, El-Azzouni MZ. Effect of topical agents on cercariae of Schistosoma mansoni. J Egypt Soc Parasitol. (2004) 34:903–13.
Keywords: Schistosoma, travelers, Kivu Lake, Rwanda, case report
Citation: Wallemacq S, Anantharajah A, Baldin P, Yombi J-C, De Greef J and Belkhir L (2024) Case report: Imported asymptomatic schistosomiasis among Belgian school travelers to Rwanda. Front. Trop. Dis 5:1354031. doi: 10.3389/fitd.2024.1354031
Received: 11 December 2023; Accepted: 22 February 2024;
Published: 20 March 2024.
Edited by:
Roch Christian Johnson, CIFRED UAC, BeninReviewed by:
Sammy Ohene-Aboagye, Rush University, United StatesCopyright © 2024 Wallemacq, Anantharajah, Baldin, Yombi, De Greef and Belkhir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvio Wallemacq, c2lsdmlvLndhbGxlbWFjcUBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.