- 1Department of Biosecurity, Ecosystems and Veterinary Public Health, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, Kampala, Uganda
- 2Department of Veterinary Pharmacy, Clinical and Comparative Medicine, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, Kampala, Uganda
- 3Department of Disease Control, School of Veterinary Medicine, The University of Zambia, Lusaka, Zambia
- 4School of Women and Gender Studies, College of Humanities, Makerere University, Kampala, Uganda
- 5Department of Biomolecular Resources and Bio-lab Sciences, College of Veterinary Medicine, Animal Resources and Biosecurity, Makerere University, Kampala, Uganda
- 6Department of Forestry and Wildlife Management, Inland Norway University of Applied Sciences (INN), Koppang, Norway
- 7Department of Arctic and Marine Biology, UiT, The Arctic University of Norway, Tromsø, Norway
Introduction: This study examines rabies incidence and associated risk factors at the interface between wildlife and human communities near Pian-Upe game reserve in Eastern Uganda. We hypothesized that human settlements in closer proximity to the reserve would exhibit higher rabies risk compared to those located further away.
Methods: Using a standard questionnaire, households within <4, 4-14, and >14 km from the Pian Upe game reserve in Bukedea District were interviewed. Data on socio-demographic characteristics, recent rabid animal bites, and suspected human and livestock rabies cases for the past year (2023) were collected after seeking informed consent. Descriptive statistics were used to analyze socio-demographic information and incidence data, while separate binomial generalized linear models with log-link function were used to identify predictors of rabies incidence and mortality.
Results: Between January and March 2023, 302 participants were interviewed. Respondents had an average age of 44 years with 34% (n=103) being female. All households owned at least one dog, though only 47% (n=142) had vaccinated their dogs against rabies in the past year. Additionally, 39% (n=118) of respondents used dogs for hunting. Rabies annual incidence increased with decreasing distance to the game reserve (7.5 to 15.7% for humans, for the dogs, and 5.0 to 9.8% for livestock, including cows, goats and sheep). Significant factors associated with rabies in humans included primary education level (aRR=3.8, 95%CI 1.0-23.7) and grazing livestock in the reserve (aRR=5.30, 95%CI 1.0-40.3). Mortality was associated with fetching firewood from the game reserve (aRR=4.7, 95%CI 1.3-17.5).
Conclusions: This study reveals that there is an increased risk of rabies for domesticated animals and people located within proximity to the game reserve. Further efforts to prevent the spread of rabies could include increasing education and awareness for communities along with targeted dog vaccination in settlements surrounding wildlife protected areas.
Background
Rabies, a disease caused by a negative sense single-stranded RNA virus in the family Rhabdoviridae, is an acute viral infection of the brain that results in high mortality for humans and other warm-blooded animals (1, 2). The disease occurs when saliva or brain tissue from an infected animal comes into direct contact with mucus membranes or broken skin (generally via a bite). Despite vaccination campaigns and post-exposure prophylaxis, rabies continues to kill approximately 50,000-61,000 people globally each year (1, 3). Over 95% of these fatal rabies cases occur in Asia and Africa (4). For example, rabies claimed approximately 21,000-25,000 lives in Africa during 2015 (5). In Uganda, a total of 14,865 annual dog bites were reported in 2021 (6) and rabies claimed approximately 36 lives per year (7).
The global estimates for rabies incidence and prevalence in Africa are not well documented. A meta-analysis by Wobessi et al. reports misrepresented incidence of rabies in Africa for exposed humans, dogs and other species as 83.4%, 44.1% and 33.8%, respectively, and the prevalence as 33.8%, 19.8% and 3.6% (8). In Uganda, the prevalence has been over-estimated to be 78.9%, 66.7% and 84.6% by Omodo et al (9) in dogs, jackals and livestock (goats, sheep, and cows), respectively (9). The exact statistics on rabies prevalence among humans in Uganda is lacking.
Rabies prevention and control programs, such as mass dog vaccination, sterilization, and post-exposure prophylaxis (PEP), have been performed seasonally in Uganda. However, due to limited funding, various endemic parts of Uganda do not receive these programs. Consequently, this leads to sub-optimal coverage according to the GARC, OIE, FAO recommendations and ensures that transmission of the virus continues in rural parts of Uganda. Limited PEP availability, awareness, and access makes it even more difficult to provide timely treatment for those that are infected. Only two out of ten people bitten by a suspected rabid animal have access to PEP in Uganda. This is due to many factors, including cost and long distances to hospitals. For example, PEP is approximately 40 USD in Uganda, yet citizens live on less than 1 USD per day. Also, it takes an average of three hours to reach the nearest hospital (Atutur) from Pian sub-counties (i.e. Kocheka).
People living in Pian Upe are largely nomadic pastoralists with poor access to health facilities. Practices and beliefs of these pastoralists, such as grazing cows with dogs, fetching firewood in the game reserve (GR), and frequent hunting (generally twice a week) predispose them and their animals (dogs and livestock) to close encounters with wildlife.
Approximately 99% of human rabies cases are transmitted by rabid domestic dogs (2), while a relatively small proportion of human cases are caused by wildlife. For example, a study in South Africa revealed that black-backed jackals (Canis mesomelas) contributed to 0.14% of rabies cases in the country during 2016 (10). Anecdotal findings in Bukedea region show frequent jackal and dog bites in humans, especially for communities near Pian Upe GR. This may be a potential risk for Rabies spillover, leading to epidemics in communities surrounding GRs and national parks. The aim of this study was to determine community reported incidence and mortality estimates of rabies at the wildlife-dog-livestock-human interface of Pian Upe GR in Bukedea, Eastern Uganda. We hypothesized that households near the GR experience more rabies cases than their counterparts situated more than 14km away.
Materials and methods
Study design
A cross-sectional cohort study was conducted among households from six selected sub-counties of the Bukedea district, Eastern Uganda. People that reported having been bitten by a dog were followed retrospectively for one year to identify the incidence of clinical rabies cases. Demographic and practice related factors were recorded as potential exposure variables.
Study setting
Six subcounties in this pastoral setting were studied. Three sub-counties were located >14 km from the Pian Upe GR, including Bukedea Trading center (2,360 households), Kocheka (3,510 households), and Kidongole (3,268 households). Three sub-counties were located ≤ 14 kms from the GR, including Aminit (2,115 households), Kamutur (2,265 households), and Kangole sub-county (2,300 households) (Figure 1). The common husbandry practice in Bukedea district is communal grazing, where animals are left to freely graze on shared land (called “Aaro”). Cows and other livestock can be left in Aaro for many weeks without the herdsman’s attendance. Instead, they are left to be taken care of by livestock guardian dogs, which protect against attacks from wildlife (especially jackals). Sub-counties near to the GR practice hunting more frequently than those further away from the GR, including hunting small animals like squirrels and edible rats.
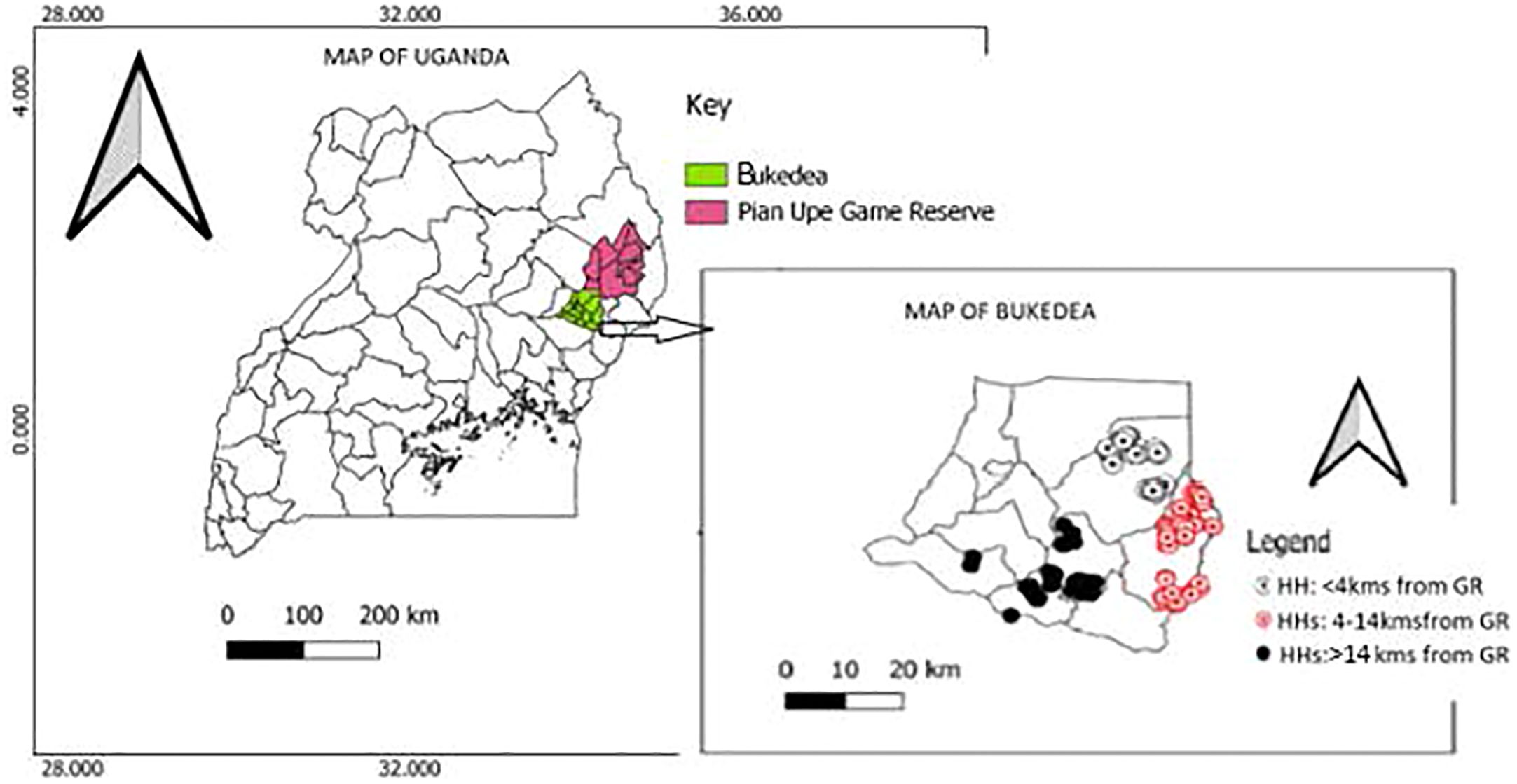
Figure 1 Map showing households studied in Bukedea district (Eastern Uganda) based on their proximity to the Pian Upe Game Reserve.
Study population and selection criteria
This study was conducted among households in Bukedea district between January 2023-March 2023. A household was defined as either a unit made up of a father, mother and children or a caretaker and children living together in a home. Interactions between domestic animals (dogs or livestock) and wildlife were quantified if habitat was shared at least once a week (either for food or water). We stratified households by distance to the GR since the latter variable is a potential effect modifier of rabies risk. Households that owned either dogs (following January 2022) or dogs and livestock were included in the study. Those participants who were interrupted by neighbors during the interview or lost interest in responding to certain questions were excluded from the study to minimize information bias.
Sampling strategy
Six sub-counties were purposively selected from Bukedea district based on geographical distance to the Pian Upe GR. Parishes were purposively selected from each sub-county. Villages from each parish were selected randomly using systematic random sampling based on the Local Council Record Book. From each Village, a household was picked purposively based on whether it had livestock interacting with wildlife and dogs.
Sample size estimation
Modified Daniel’s formula was used (Wayne Daniel, 2013) with alpha = 5% and a minimum statistical power of 80% considering finite population correction and a precision of 5%. We were able to interview 302 households from six parishes of Bukedea district, Eastern Uganda.
Data collection method
A semi-structured questionnaire (Supplementary material, S1) designed by a web based mobile App kobo collect toolbox version 2022.3.6 (11) was used to collect face to face data by four trained research assistants and the principal investigator. The variables collected fell under three main categories, including 1) socio-demographic characteristics of study participants, 2) risk factors of rabies at the wildlife-livestock-human interface, and 3) incidence and mortality of rabies.
Variables
The dependent variables were 1) community reported incidence and 2) mortality attributed to rabies in households of Bukedea district. A rabies case was defined as a household member, previously bitten by either a suspected rabid dog or jackal, who later developed signs of rabies including weakness, discomfort, abnormal behaviour (barking) and hydrophobia.
Incidence was measured as the proportion of community members that had been bitten by a suspected rabid dog or jackal in the last year and had developed clinical signs of rabies divided by the total sample size, computed to the base of 1000 persons or animals as illustrated in Equation 1.
Mortality was defined as the proportion of individuals that were bitten by a suspected rabid dog or jackal in the last year and had died of rabies (following classical signs of rabies) in this time period divided by the total sample size, computed to the base of 1000 as illustrated in Equation 2.
The predictors included 1) socio-demographic factors [age, sex, occupation, education level and religion], 2) geographic factors [distance to the GR], and 3) practice factors [hunting, grazing in a GR, history of dog vaccination, fetching firewood and water from the GR].
Statistical analysis
All statistical tests were carried out using STATA version 14.2. Socio-demographic and practice characteristics were analyzed descriptively as percentages, frequencies for categorical data, and medians, lower quartile and upper quartile for numerical data. Stratified analysis for descriptive statistics was done based on distance to the GR. Statistical comparisons were done using Chi-square and Fisher’s exact test, F-test and Kruskal Wallis ANOVA. Community-reported incidence and mortality of rabies among livestock, humans, wildlife and dogs were analyzed descriptively using Venn diagrams.
Incidence of Rabies was modelled using bivariable and multivariable binomial generalized linear model using the log link function for risk ratios. The response variable was incidence in humans, while the predictors were religion, education level, firewood source, grazing area, and sources of water stratified over location to the GR.
A similar regression model was used for rabies mortality. In this model, the outcome variable was mortality, while predictors were 1) rabies post-exposure prophylaxis status, firewood source, grazing area, water source, and education level.
Quality control
Data entry was checked using validation checks and “must enter” check points in Kobo collect tool (11). Statistical analysis was performed in parallel by two statisticians for quality outputs.
Ethical clearance
Ethical clearance was obtained from the Makerere University School of Public Health Research Ethics Committee (SPHREC-2023-509). Permission to collect data was obtained from Bukedea district Chief Administrative officer through Bukedea District Veterinary Officer. The study was fully explained in mother tongue (Ateso) and English to the participant before written informed consent for voluntary participation was obtained. Interpretation in the local language (Ateso) was provided when the respondent in a household was not well versed in English.
Results
Study flow diagram
There were 302 households in this study, including those located furthest away from the GR (>14km; n=161), near the GR (4-14km; n=90) and bordering the GR (<4kms; n=51) (Figure 2).
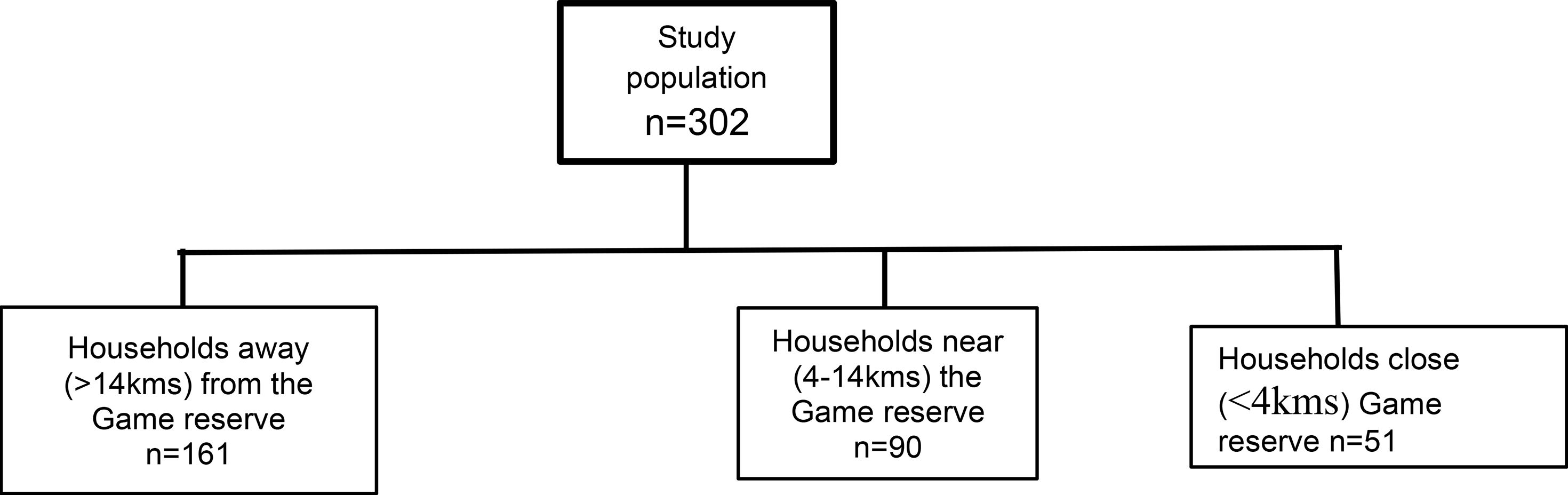
Figure 2 Study flow diagram showing the distribution of households studied and their distance to the Pian Upe Game Reserve.
Socio-demographic and practice-related characteristics of study participants
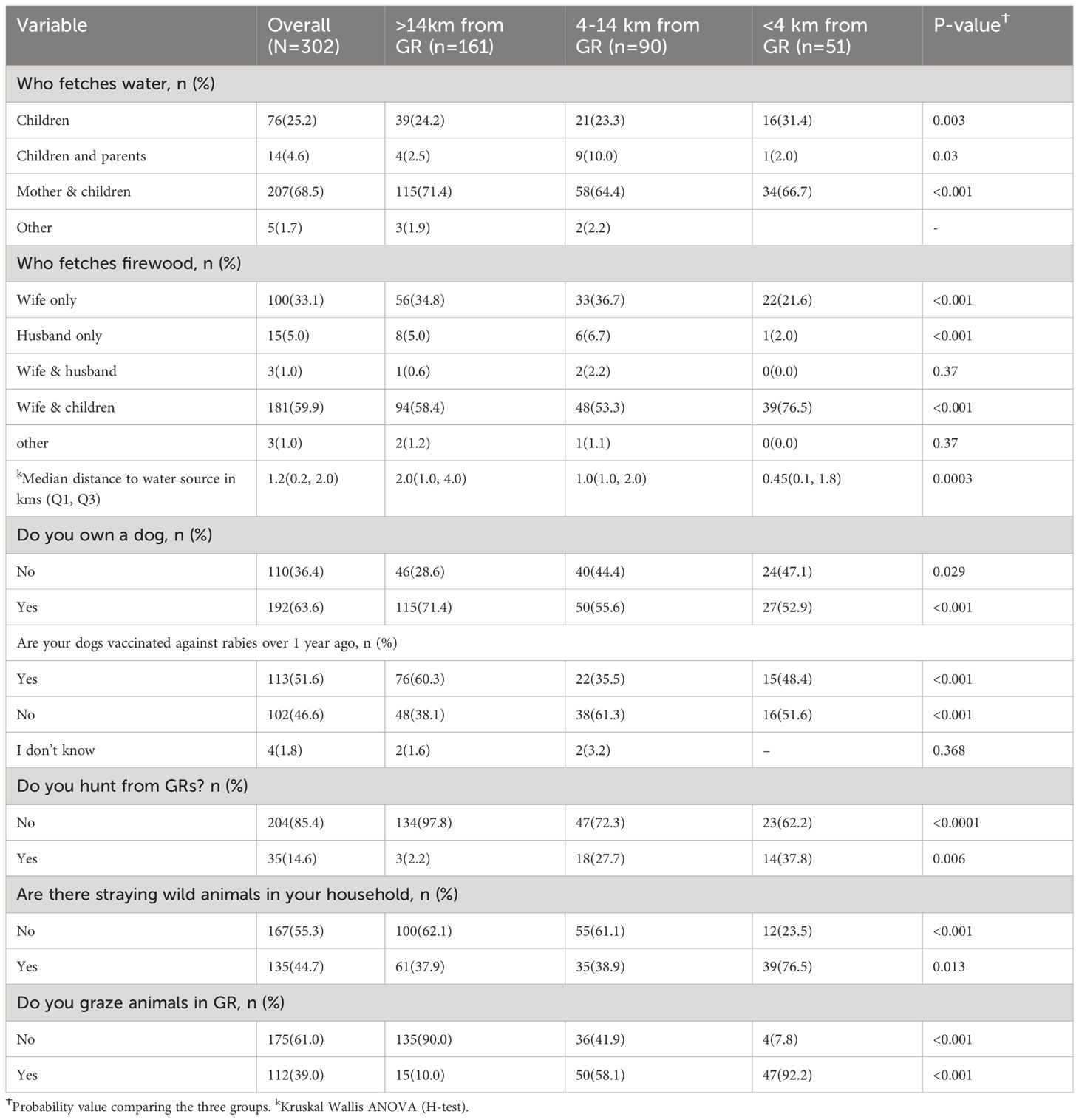
The mean age of study participants was 44 years, with 34% (n=104) of the households being headed by females. About 8% (n=25) of the study participants had not attended any formal education. The majority of participants were Anglicans (52%, n=80) and Catholics (30%, n=51) (Table 1). In the case of families that were situated<4 km to the GR, they were predominantly women and children who fetch water and firewood (Table 2).

Table 1 Socio-demographic characteristics of study participants in Bukedea district, Eastern Uganda.
In this study population, 64% (n=192) of the households owned dogs, with 47% (n=102) of them not having ever vaccinated them against rabies during the previous year. The percentage of unvaccinated dogs was even higher (61%, n=38) in families living 4-14 km to the GR. Similarly, 92% (n=47) of the households living <4 km to the GR reported practices such as hunting and grazing livestock in the Pian Upe GR (Table 2).
Incidence of rabies among households in Bukedea Eastern Uganda
The incidence of rabies was reported to be the same among humans and dogs (89 cases per 1000 persons or dogs per year; 95%CI 62-127). Approximately 66 rabies cases per 1000 animals per year (95%CI 43-101) were reported in livestock (Figure 3).
Relationship of proximity to Pian Upe GR and incidence of rabies among households in Bukedea (Eastern Uganda)
Households closer to the GR experienced significantly more rabies cases than those that were located further away (incidence increased with decreasing distance to the GR). Incidence increased from 75 to 157 per 1000 humans and dogs per year, respectively (p<0.001), and from 50 to 98 per 1000 animals per year among livestock (p<0.001) (Figure 4).
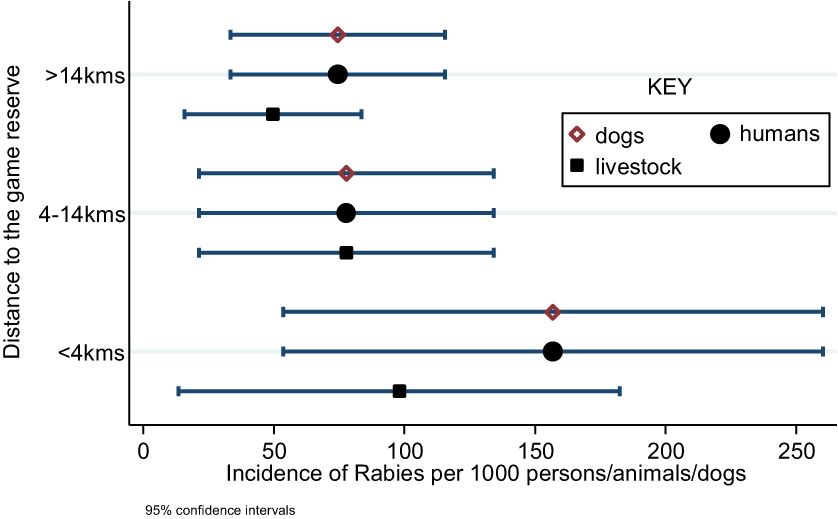
Figure 4 Incidence of rabies by distance to the Pian Upe Game Reserve in Bukedea district, Eastern Uganda.
Mortality due to rabies among households in Bukedea district, Eastern Uganda
A similar trend was observed for rabies mortality (Figure 4). Mortality due to rabies in households increased with proximity to the Pian Upe GR. Among livestock, the mortality increased from 49.7 to 98.0 per 1000 animals per year (p<0.001), while human mortality increased from 43.5 to 55.6 per 1000 persons per year (p=0.21) (Figure 5).
Incidence and mortality of rabies among livestock, dogs and humans in Bukedea district, Eastern Uganda
About 3% (n=9) of households reported co-occurrence [H=9] of rabies cases in humans (H), livestock (L), and dogs (D) (Figure 6). Only 6% (n=18) of households reported co-occurrence of rabies in humans and dogs [H=18], while no cases were reported to occur from the same household between humans and livestock [H=0] or livestock and dogs [=0].
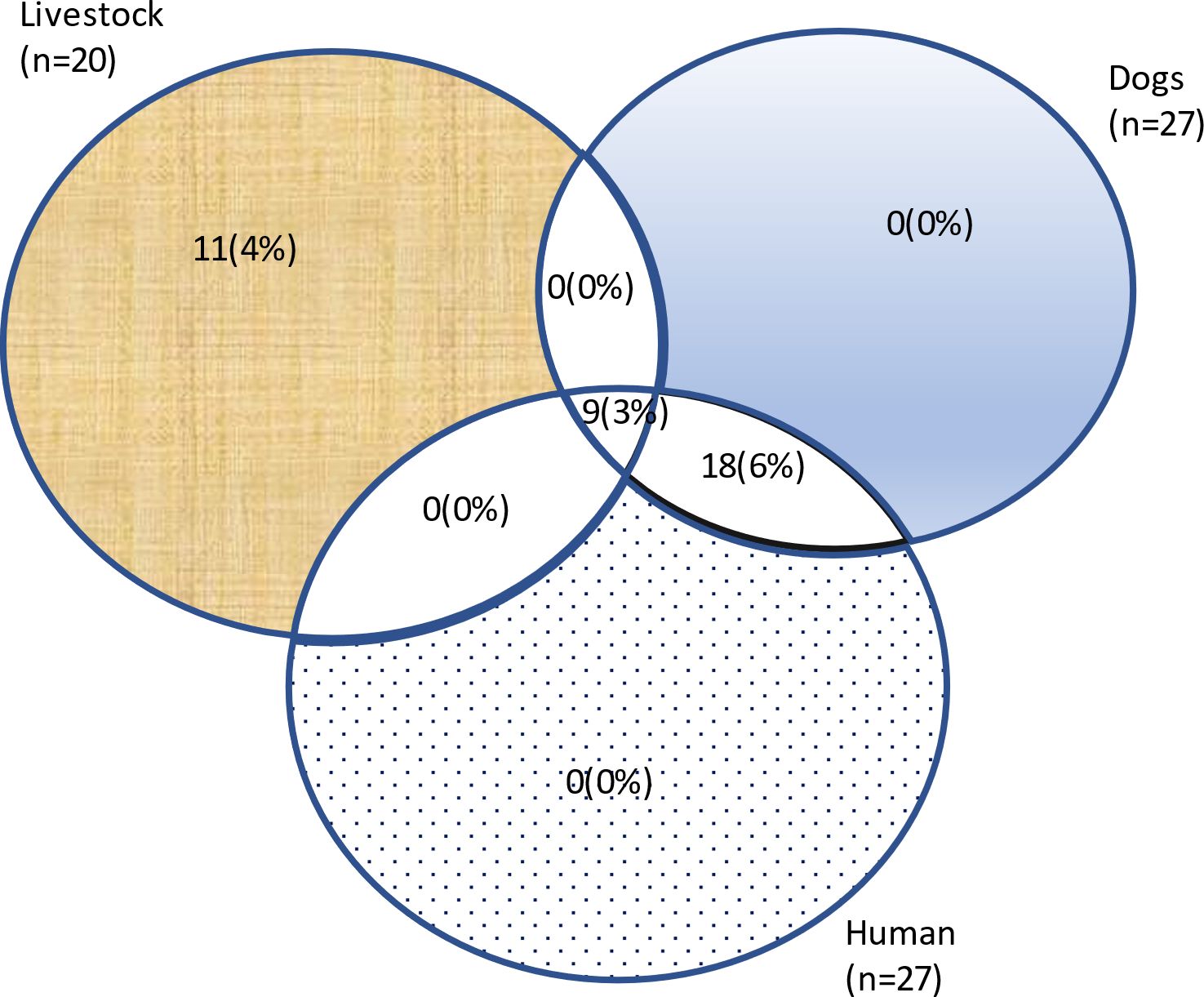
Figure 6 Co-occurrence of rabies in livestock, humans, and dogs among households in Bukedea district, Eastern Uganda.
Mortality was 4% (n=12) for humans and 6% (n=18) for livestock. Only 1% (n=2) of the households showed co-occurrence of rabies mortality in humans and livestock, [H=2].
Factors associated with rabies incidence among households of Bukedea, Eastern Uganda
Households located near the GR had a higher risk of rabies than those further away (>14 km). Predisposing factors at <4 km and 4-14 km distances included 1) primary level of education (aRR[95%CI] =3.8[1.0-23.7]) and grazing in GR (5.3[1.0-40.3]). Conversely, primary and secondary levels of education at >14 km and 4-14 km distance from the GR decreased the risk of rabies at: (aRR0.1[95%CI 0.04-0.26]), (aRR=0.07[95%CI 0.12-0.30]) and (aRR=0.07[95%CI 0.02-0.31]), (aRR=0.09[95%CI 0.01-0.56]), respectively (Figure 7).
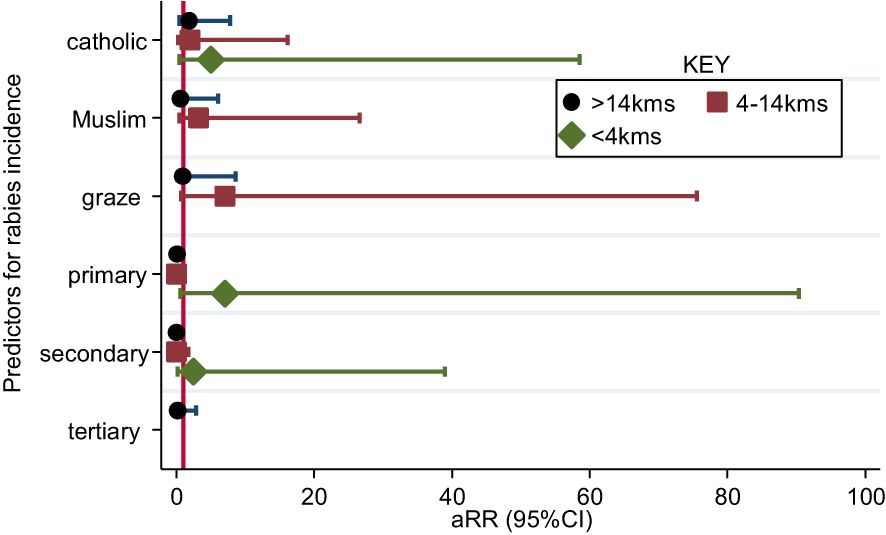
Figure 7 Factors associated with incidence of rabies in the Bukedea district, Uganda. y Variables on the y-axis: graze=Grazing in GR; primary=Primary Education; secondary=Secondary education; tertiary=Tertiary education.
Factors associated with rabies mortality in households of Bukedea, Eastern Uganda
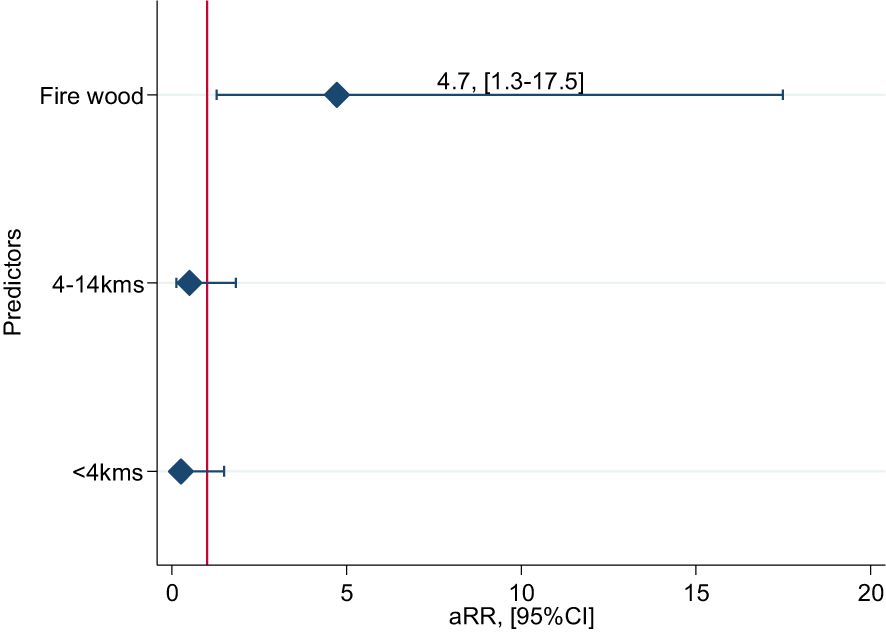
People fetching firewood from the Pian Upe GR were 4.7 times more likely to be exposed to rabies than households that did not fetch firewood from the GR (Figure 8; aRR=4.7; 95%CI 1.3-17.5). In this multivariable model, PEP use was not associated with Rabies mortality.
Discussion
In this study, we hypothesized that rabies risk would be higher in communities near wildlife protected areas (GR). Our findings support this hypothesis, as households closer to Pian Upe GR had higher rabies incidence, possibly due to more frequent interactions between livestock, dogs and wildlife. Rabies in wildlife can be a threat to humans and domestic animals due to human demography, animal translocations, and environmental alterations; however, efforts to control wildlife associated rabies is largely lacking in Uganda (12). The communally owned grazing lands in Bukedea (Aaro) act as contact points between livestock, dogs, humans, and wildlife. Most of these Aaro´s are close to the GR, giving wildlife (especially jackals) a greater chance of preying on livestock, facilitating close contact between dogs, jackals, and livestock. Anecdotal reports from community members and our own observations during data collection confirmed that jackals were frequently seen near livestock in the Aaro and near livestock tethered at home (at least 3-4 times a week), especially along the border between the GR and human settlements. Other studies show similar observations. For example, in the Mymensingh district of Bangladesh, jackals and dogs have been implicated in the transmission of rabies to domestic ruminants (13). However, Bangladesh has a far higher burden of rabies than Uganda. This is likely due to the larger population, with approximately 165.7 million compared to Uganada’s 49.2 million people (14), and the location, as it is situated between China and India, and is ranked first and second globally in rabies burden (15). The similarity concerning jackal and dog distribution in the two countries lies in somewhat similar climatic conditions and forest cover that acts as habitat for these canids (16).
In our study, we found a mean incidence of 8.9% for rabies cases among humans and dogs and 6.6% in livestock. This is much higher than the reported national rabies incidence in Uganda (0.04% (6). This discrepancy could be due to the risk-based sampling that we carried out as opposed to population wide sampling done from national epidemiological data (6). The large discrepancy between the two numbers implies a neglected rabies risk at the interface between wildlife, dogs, livestock, and humans. The incidence is less than 10%, which may be regarded as a low risk. However, as untreated rabies cases are fatal, every single case is of high public and economic importance for the household.
A study by Millán et al. 2013 (17) reported a similar sero-prevalence of rabies in dogs (12.7-28.6 %) in Queen Elizabeth national park (17). The two protected areas (Pian Upe GR and Queen Elizabeth national park) host the largest numbers of canines in Uganda, increasing the possibility for rabies spillover through bites between the wild canines and dogs, along with canine-human interactions (18). Furthermore, a study in Zimbabwe reported rabies transmission between jackals (Canis adustus and Canis mesomelas) and dogs at the wildlife-human interface (19). Jackals were the most implicated wildlife species, accounting for 91% of wildlife rabies cases (20). The unmet need for rabies control in wildlife is likely to make the rabies elimination agenda unattainable by 2030 in some parts of Africa, especially those that surround wildlife protected areas.
In the current study, mortality ranged between 44-56 cases per 1000 persons and 50-98 cases per 1000 livestock. These findings, especially those for humans, are higher than estimates across Uganda (16 deaths per 1000 persons per year (21). However, Nyasulu et al. (21) used Uganda rabies weekly surveillance data, which is not representative for some communities that are in rural and hard-to-reach areas. We also think that the elevated figures of mortality are due to risk-based sampling carried out in our study, since we expect more rabies cases at the wildlife-dog-livestock-human interface. Furthermore, our findings for livestock did not agree with the study by Omodo et al. (9), which reported lower incidence of rabies in cattle and goats using laboratory specimens collected between 2015-2022 in Uganda. The discrepancy could have been brought about by differences in geographical areas, given that they picked scattered regions in the country where the risk of rabies varied greatly (9).
In this study, 3% (n=9) of households reported co-occurrence of rabies in humans, livestock, and dogs. This clearly indicates that transmission is linked between all three species in these communities. Culturally, in this pastoralist setting, dogs are often used for herding in Aaro, where interactions with wildlife (especially jackals) occur frequently. This fact is supported by similar observations in selected districts in Tanzania, such as Ulanga and Kilombero, where jackals were rampantly biting livestock and human beings (22). We also report co-occurrence of rabies between dogs and humans, but not between dogs and livestock. This suggests that livestock can become infected from sources other than dogs, which points the suspicion to the black backed jackal, which is rampant in Pian Upe GR (23). This assertion is further supported by the co-occurrence of mortality between wildlife and livestock, and between wildlife and humans. Reporting of rabies cases in livestock is not often emphasized in Uganda, but our study suggests that there is a significant need for this, especially among pastoralist communities. Rabies has been previously reported in cattle in Ethiopia (24). Our study emphasizes that spillover events are most likely to hinder the success of the global dog-mediated rabies elimination target for 2030 (25).
In our study, primary level of education, grazing, and fetching firewood in the GR were associated with rabies cases in households near to the GR. Primary level of education may only be associated with less awareness and knowledge about rabies (26). Grazing and fetching firewood in GR might be associated with rabies cases since these practices increase the possibility for wildlife-dog-human interaction (27). Additionally, those who fetch firewood and allow their animals to graze from the GR are by default staying near the GR. Hence, they find themselves further away from the hospital and have less access to timely post exposure prophylaxis. The low socio-economic status of these households also reduces their ability to afford PEP (28). There are no similar studies in Uganda, with the exception of a study in Wakiso and Kampala districts by Kisaka and coworkers (2020), where they found that secondary level of education was associated with compliance to rabies pre-clinical control guidelines (29). There are volunteer groups, like the Uganda veterinary student outreach program, that go out to communities to implement rabies education efforts in primary and secondary schools (30), but these programs seem inadequate due to poor funding and political priority deficiencies.
One limitation for our study is that it lacks generalizability for households without dogs and livestock, given that these were criteria for household selection. However, having conducted a risk-based sampling, our findings may be applicable to households and communities neighboring wildlife protected areas in Uganda, and Africa in general. We also had to rely on reports given by household heads for rabies prevalence data, rather than using laboratory-confirmed cases. This approach may suffer from recall bias along with differences in knowledge of rabies symptoms between households. Nevertheless, rabies is a very recognizable and devastating disease, which may be well recorded in people’s memory. Thus, our estimates of rabies burden depict a proxy measure that warrants further investigation.
Conclusion
The risk of rabies at the wildlife-dog-livestock-human interface in Uganda is a neglected health threat. Both rabies incidence and rabies mortality among humans, dogs and livestock increased as proximity to the Pian Upe Game Reserve increased.
Those with primary level of education, fetching firewood in the GR and grazing their livestock in the GR had higher risk for rabies. This implies that communities of Bukedea need to exercise more caution on rabies prevention strategies than the rest of neighboring communities. More studies are necessary to carry out rabies surveillance in wildlife cannids of Pian Upe game reserve. Furthermore, increased sensitization for communities neighboring wildlife reserve and national parks is highly urgent to address rabies prevention and control.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical clearance was obtained from the Makerere University School of Public Health Research Ethics Committee (SPHREC-2023-509). Permission to collect data was obtained from Bukedea district Chief Administrative officer (CAO) through Bukedea District Veterinary Officer (DVO). A written informed consent for voluntary participation was fully explained in mother language and English to the participant before data collection. Interpretation to a local language (Ateso) was carried out when the respondent in a household was not well conversant with English.
Author contributions
CA: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. JOA: Conceptualization, Supervision, Visualization, Writing – review & editing, Writing – original draft. MTa: Data curation, Project administration, Validation, Writing – review & editing. PS: Conceptualization, Visualization, Writing – review & editing. MM: Conceptualization, Data curation, Methodology, Visualization, Writing – review & editing. CK: Conceptualization, Methodology, Supervision, Writing – review & editing. OT: Conceptualization, Supervision, Writing – review & editing. SS: Conceptualization, Supervision, Visualization, Writing – review & editing. FM: Conceptualization, Visualization, Writing – review & editing. KB: Visualization-Presentation of published work, Writing – review and editing. MTr: Conceptualization, Writing – original draft, Methodology, thorough Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, and/or publication of this article. We acknowledge funding obtained from Norwegian Agency for Development Cooperation (NORAD) through the NORHED-II Program and the project Climate Change and Infectious Diseases - A One Health Approach (CIDIMOH), grant number 68802. Also, thanks go to NORPART funded project titled: Promoting Sustainability of Higher Education through Global Collaboration, Student Program Development, Mobility and Training (EDUPROMO) which enabled the travel of the corresponding author to Norway to harness skills and obtain an ambient environment for guiding the writing of this article. Further, great thanks to Makerere University Research and Innovation Fund (PhD RIF) for part of data collection.
Acknowledgments
We highly acknowledge households of Bukedea that participated in providing data. Great thanks go to Dr. Angeki Simon who provided community entry through Chief Administrative officer, District Veterinary Officer Bukedea. We are indebted to Research Assistants, Agora Paul, Kelvin Bwambale, Arthur Mirimu and Alexander Ndyamuhaki who actively participated in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2024.1272141/full#supplementary-material
References
1. Nel L, Scott T, Wright N, Mollentze N, Markotter W, Sabeta C, et al. (2012). Rabies and rabies control in African regions, in: Compendium of the OIE Global Conference on Rabies Control 7–9 September 2011 Incheon-Seoul (Republic of Korea). Paris, France: OIE (World Organisation for Animal Health) (2012).
2. WHO. WHO expert consultation on rabies: third report. Geneva, Switzerland: World Health Organization (2018).
3. Deribe K, Meribo K, Gebre T, Hailu A, Ali A, Aseffa A, et al. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasites Vectors. (2012) 5:1–15. doi: 10.1186/1756-3305-5-240
4. Muhammad-Bashir B, Sani AM, Ademola OP, Peterside K, Maryam M, Jenna F. Prevalence and demographic distribution of canine rabies in Plateau state, Nigeria, 2004–2009. Anim Health Product. (2016) 64(1):127–36.
5. Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, et al. Estimating the global burden of endemic canine rabies. PloS Negl Trop Dis. (2015) 9(4):e0003709. doi: 10.1371/journal.pntd.0003709
6. Akusekera I, Namayanja J, Okello PE. Dying rabid: adopting compulsory mass dog vaccination to reduce human deaths from dog rabies in Uganda: policy brief. Uganda National Institute of Public Health: Quarterly Epidemiological Bulletin: October-December, 2021. (2021) 6:1–5.
7. Masiira B, Makumbi I, Matovu JK, Ario AR, Nabukenya I, Kihembo C, et al. Long term trends and spatial distribution of animal bite injuries and deaths due to human rabies infection in Uganda, 2001-2015. PloS One. (2018) 13(8):e0198568. doi: 10.1371/journal.pone.0198568
8. Wobessi JNS, Kenmoe S, Mahamat G, Belobo JTE, Emoh CPD, Efietngab AN, et al. Incidence and seroprevalence of rabies virus in humans, dogs and other animal species in Africa, a systematic review and meta-analysis. One Health. (2021) 13:100285. doi: 10.1016/j.onehlt.2021.100285
9. Omodo M, Gardela J, Namatovu A, Nakanjako MF, Okurut AR, Sekamatte M, et al. Geographic distribution of laboratory-confirmed cases of rabies in domestic and wild animals based on passive surveillance records in Uganda: 2015-2022. (2023). doi: 10.21203/rs.3.rs-2832463/v1
10. Ngoepe E, Chirima JG, Mohale D, Mogano K, Suzuki T, Makita K, et al. Rabies outbreak in black-backed jackals (Canis mesomelas), South Africa, 2016. Epidemiol Infect. (2022) 150:e137. doi: 10.1017/S0950268821002685
11. Lakshminarasimhappa M. Web-based and smart mobile app for data collection: Kobo Toolbox/Kobo collect. J Indian Library Assoc. (2022) 57:72–9.
12. Slate D, Rupprecht CE, Rooney JA, Donovan D, Lein DH, Chipman RB. Status of oral rabies vaccination in wild carnivores in the United States. Virus Res. (2005) 111(1):68–76. doi: 10.1016/j.virusres.2005.03.012
13. Noman Z, Anika T, Haque Z, Rahman A, Ward M, Martínez-López B. Risk factors for rabid animal bites: a study in domestic ruminants in Mymensingh district, Bangladesh. Epidemiol Infect. (2021) 149:e76. doi: 10.1017/S095026882100056X
14. Parkhurst JO, Rahman SA, Ssengooba F. Overcoming access barriers for facility-based delivery in low-income settings: insights from Bangladesh and Uganda. J health popul Nutr. (2006) 24:438.
15. Tao X-Y, Li M-L, Wang Q, Baima C, Hong M, Li W, et al. The reemergence of human rabies and emergence of an Indian subcontinent lineage in Tibet, China. PloS Negl Trop Dis. (2019) 13(1):e0007036. doi: 10.1371/journal.pntd.0007036
16. Chaudhury M, Kristjanson PM, Kyagazze F, Naab JB, Neelormi S. Participatory gender-sensitive approaches for addressing key climate change-related research issues: Evidence from Bangladesh, Ghana, and Uganda. Department of Agriculture and Ecology, Faculty of Life Sciences, University of Copenhagen, Frederiksberg, Denmark:: CCAFS Working Paper (2012).
17. Millán J, Chirife AD, Kalema-Zikusoka G, Cabezón O, Muro J, Marco I, et al. Serosurvey of dogs for human, livestock, and wildlife pathogens, Uganda. Emerg Infect Dis. (2013) 19(4):680. doi: 10.3201/eid1904.121143
18. Haruna KK, Rusoke T. Evaluation of Man-Lion Conflicts on Conservation Status of Lions (Panthera leo) in Queen Elizabeth National Park (QENP), Kasese district western Uganda. (2019).
19. Bingham J, Foggin CM, Wandeler AI, Hill F. The epidemiology of rabies in Zimbabwe. 2. Rabies in jackals (Canis adustus and Canis mesomelas). Onderstepoort J. Vet. Res. (1999) 66:11–23.
20. Pfukenyi D, Pawandiwa D, Makaya P, Ushewokunze-Obatolu U. A retrospective study of wildlife rabies in Zimbabwe, between 1992 and 2003. Trop Anim Health Product. (2009) 41:565–72. doi: 10.1007/s11250-008-9224-4
21. Nyasulu PS, Weyer J, Tschopp R, Mihret A, Aseffa A, Nuvor SV, et al. Rabies mortality and morbidity associated with animal bites in Africa: a case for integrated rabies disease surveillance, prevention and control: a scoping review. BMJ Open. (2021) 11(12):e048551. doi: 10.1136/bmjopen-2020-048551
22. Bardosh K, Sambo M, Sikana L, Hampson K, Welburn SC. Eliminating rabies in Tanzania? Local understandings and responses to mass dog vaccination in Kilombero and Ulanga districts. PloS Negl Trop Dis. (2014) 8(6):e2935. doi: 10.1371/journal.pntd.0002935
23. Terblanche R. Ongediertes: A critical qualitative study of farmer–black-backed jackal conflict and its management around the Square Kilometre Array core site in the Northern Cape, South Africa. Stellenbosch: Stellenbosch University (2020).
24. Gumi B, Girma S, Mohamed H, Deresa A. Rabies outbreak among livestock in a pastoralist community, Southern Ethiopia. Ethiop J Health Sci. (2018) 28(6):805–8. doi: 10.4314/ejhs.v28i6.16
25. Wallace RM, Undurraga EA, Blanton JD, Cleaton J, Franka R. Elimination of dog-mediated human rabies deaths by 2030: needs assessment and alternatives for progress based on dog vaccination. Front vet Sci. (2017) 4:9. doi: 10.3389/fvets.2017.00009
26. Kankya C, Dürr S, Hartnack S, Warembourg C, Okello J, Muleme J, et al. Awareness, knowledge, and perceptions regarding rabies prevention among rural communities in Masaka district, central Uganda: A qualitative study. Front vet Sci. (2022) 9. doi: 10.3389/fvets.2022.863526
27. Hikufe EH, Freuling CM, Athingo R, Shilongo A, Ndevaetela E-E, Helao M, et al. Ecology and epidemiology of rabies in humans, domestic animals and wildlife in Namibia, 2011-2017. PloS Negl Trop Dis. (2019) 13(4):e0007355. doi: 10.1371/journal.pntd.0007355
28. Shantavasinkul P, Wilde H. Postexposure prophylaxis for rabies in resource-limited/poor countries. Adv Virus Res. (2011) 79:291–307. doi: 10.1016/B978-0-12-387040-7.00013-5
29. Kisaka S, Makumbi FE, Majalija S, Bangirana A, Thumbi S. Epidemiology and preclinical management of dog bites among humans in Wakiso and Kampala districts, Uganda: Implications for prevention of dog bites and rabies. PloS One. (2020) 15(9):e0239090. doi: 10.1371/journal.pone.0239090
Keywords: rabies, wildlife-human interface, Uganda, Pian-Upe, Bukedea, cross-sectional cohort study
Citation: Atuheire CGK, Okwee-Acai J, Taremwa M, Ssajjakambwe P, Munyeme M, Kankya C, Terence O, Ssali SN, Mwiine FN, Buhler KJ and Tryland M (2024) Households neighboring wildlife protected areas may be at a higher risk of rabies than those located further away: a community-based cross-sectional cohort study at Pian Upe game reserve, Bukedea district, Eastern Uganda. Front. Trop. Dis 5:1272141. doi: 10.3389/fitd.2024.1272141
Received: 24 August 2023; Accepted: 22 January 2024;
Published: 22 April 2024.
Edited by:
Catherine A. Gordon, The University of Queensland, AustraliaReviewed by:
Carlos Landaeta-Aqueveque, University of Concepcion, ChileKatharina Sophia Kreppel, Institute of Tropical Medicine Antwerp, Belgium
Copyright © 2024 Atuheire, Okwee-Acai, Taremwa, Ssajjakambwe, Munyeme, Kankya, Terence, Ssali, Mwiine, Buhler and Tryland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Collins G. K. Atuheire, Y29sbGluc2F0dWhlaXJlQGdtYWlsLmNvbQ==
 Collins G. K. Atuheire
Collins G. K. Atuheire James Okwee-Acai2
James Okwee-Acai2 Martha Taremwa
Martha Taremwa Paul Ssajjakambwe
Paul Ssajjakambwe Musso Munyeme
Musso Munyeme Clovice Kankya
Clovice Kankya Frank N. Mwiine
Frank N. Mwiine Morten Tryland
Morten Tryland