
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 28 November 2023
Sec. Neglected Tropical Diseases
Volume 4 - 2023 | https://doi.org/10.3389/fitd.2023.1301485
This article is part of the Research Topic Female Genital Schistosomiasis: Research Needed to Raise Awareness and Deliver Action View all 14 articles
Background: Schistosomiasis affects many parts of the human body including those not usually accessible during routine clinical follow-up. We investigated the presence of schistosomiasis in routine tissue specimens sent to the only public histopathology laboratory in KwaZulu-Natal, South Africa.
Methods: The catchment area for the Department of Anatomical Pathology constitutes 11 million people in 10 districts. We retrospectively reviewed all the histopathology reports for occurrence of schistosomiasis between 1 January 2015 and 30 June 2020.
Results: Schistosomiasis was identified in the appendix, uterine cervix, urinary bladder, lung, liver, fallopian tubes and prostate. During the study period, 725 cases had a diagnosis of schistosomiasis confirmed on histopathology, which equals 0.3% of the total number of specimens sent to the laboratory. Female genital schistosomiasis represented 49.1% (356/725) of the schistosomiasis cases of which 25.1% (182) were from the uterine cervix and 24% (174) from the fallopian tubes. The appendix had 39.7% (289) of all the cases of schistosomiasis. Other organs were urinary bladder (4.4%, 32), lung (3.2%, 23) and liver (2.6%, 19). There were two cases of schistosomiasis in the prostate and four cases in the anorectal region. The main three indications for taking biopsies were acute appendicitis, cervical intraepithelial neoplasia, and sterilization. Majority of the schistosomiasis cases (312) were from eThekwini/Durban metropolitan district, however this represented only 1.2% (312/25 111) of the specimens received from eThekwini/Durban. The districts with the highest percentage positive cases were uMkhanyakude (43/965, 4.5%), followed by Ugu (129/5 251, 2.6%), and King Cetshwayo districts (132/5 360, 2.5%).
Conclusion: Clinicians in the KwaZulu-Natal public health sector hospitals did not suspect schistosomiasis when they submitted patient samples for histopathological investigations. The study indicates the prevalence and the diversity of the body organs affected by schistosomiasis.
Schistosomiasis (Bilharzia), is an acute and chronic tropical disease caused by trematodes of the genus Schistosoma (1). The schistosome parasite is transmitted through a fresh water snail intermediate host with the human being the definitive host (1). Worldwide, schistosomiasis continues to cause a public health problem with 251.4 million individuals requiring preventative treatment for schistosomiasis in 2021 (2). The disease is present in 78 countries and preventive chemotherapy is required in 51 endemic countries with moderate to high transmission (2). Approximately 90% of those who need preventative chemotherapy live in Africa (3, 4).
Although schistosomiasis has been successfully controlled in many countries, its burden remains high in sub-Saharan countries (1, 5). Transmission of the disease is dependent on the particular snail host (1). The distribution of schistosomiasis in South Africa is based on temperature suitability and lack of full access to protected water for all uses, including recreational swimming and contact with fresh water in connection with household chores or incidental seasonal contact, such as crossing a flooded stream (6–8). This has resulted in the disease being prevalent in the Mpumalanga, Gauteng, KwaZulu-Natal, North West, Limpopo, and Eastern Cape Provinces (6–8). The Limpopo, Mpumalanga, and KwaZulu-Natal provinces have an estimated 70% of the national burden in South Africa (6).
The World Health Organisation (WHO) had advocated for the elimination of schistosomiasis and soil-transmitted helminthiases by 2030 (8). Several community-based studies on schistosomiasis have been undertaken in KwaZulu-Natal over the past ten years and show that the disease is still prevalent (8–10).
There are several species of Schistosoma, however, in Sub-Saharan Africa, Schistosoma haematobium accounts for two-thirds of the schistosomiasis cases (11). The diagnosis of schistosomiasis is largely based on the identification of parasite ova on urine or stool microscopy, however, polymerase chain reaction (PCR), serology and immunological tests for antigens may be done (2). Systematic histopathology studies on schistosomiasis have found that schistosoma ova may be found in various organs including the female genital tract, gastrointestinal tract, lungs and brain (12). Tissue diagnosis of schistosomiasis may be undertaken; however, the skill set required and invasive nature of this diagnostic approach limits its utility (11, 13).
The tissue reaction patterns to Schistosoma ova have been described and include granulomatous inflammation, activated T-cells, eosinophils and/or fibrosis (14–16). Most of reactions were described prior to the HIV pandemic. However, schistosome infections have been found to be associated with increased HIV transmission (17–19). Studies on HIV progression in schistosomiasis have been contradictory (20, 21).
A better understanding of the geographic and anatomical distribution of schistosomiasis is important in controlling the disease in the general population and to better manage the individual patient. In this study, we aim to determine the prevalence and anatomical distribution of schistosomiasis in the histopathological surgical biopsies, and the geographic distribution of schistosomiasis in KwaZulu-Natal hospitals, South Africa.
This is a retrospective laboratory-based study conducted in accordance with the regulations of the National Health Laboratory Service (NHLS), and the approval of the Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal (Ref: BREC/00001997/2020). Patient names and other identifiers were removed, and unique link numbers were assigned to each case.
The National Health Laboratory Service (NHLS), Department of Anatomical Pathology Laboratory is the only public anatomical pathology laboratory in KwaZulu-Natal province, South Africa and is located at Inkosi Albert Luthuli Central Hospital (IALCH), Durban, South Africa. Private laboratories serve the more affluent populations of the province.
We used the Systemized Nomenclature of Medicine (SNOMED) codes in searches at the NHLS Corporate Data Warehouse (CDW) of all the cases referred to IALCH in for the period 01 January 2015 to 30 June 2020 (66 months). The search terms were “Schisto* or Bilharzia” and organ of origin was noted (appendix, uterine cervix, urinary bladder, lung, liver, fallopian tubes and prostate). A second search was performed for all specimens in these organs. Information, such as hospital name (where specimen was obtained), patient age, gender, and organ sampled were recorded on a data sheet.
The specimens were routinely fixed in 10% buffered formaldehyde solution. Once in the laboratory, the tissues were grossed and processed using a vacuum infiltration processor (VIP). Paraffin-embedded tissue blocks were sectioned at 4µm thickness. The sections were stained with hematoxylin and eosin following standard protocol. Individual stained slides were analyzed by pathologists who issued the final report. An incidental finding is defined as something found, not related to the reason the doctor ordered the test.
KwaZulu-Natal is located in the east of South Africa. It is bounded to the east by the Indian Occean, to the west by Lesotho and the Free State province, to the north by eSwatini and Mozambique, to the south by the Eastern Cape province, and to the north-west by the Mpumalanga province. KwaZulu-Natal Province covers an area of 94 361km² and is divided into 11 health districts. The province has a population of 11.1 million (2016 census) (22). The catchment area for the Department of Anatomical Pathology is the 10 of the 11 health districts in the KwaZulu-Natal province. Amajuba district (population 0.5 million) did not send specimens to our laboratory during the study period (22).
Data were processed using the following software programs: Excel 2000 (Microsoft, Redmond, WA) and SPSS (version 28.0; SPSS, Chicago, IL). Means and frequencies (%) were used to describe patients’ characteristics. For categorical variables, comparisons were performed by either Fisher’s exact test or χ2 test. For strength of association, a p value of ≤ 0.05 was considered significant.
During the study period (66 months), 209 579 specimens were examined at the Department of Anatomical Pathology, Inkosi Albert Luthuli Central Hospital, eThekwini/Durban. Schistosomiasis was only identified in the appendix, uterine cervix, urinary bladder, lung, liver, fallopian tubes, anorectal region and prostate. The histopathological specimens came from 58 hospitals in 10 (of the 11) health districts in KwaZulu-Natal province (Figure 1). The mean age of the biopsied patients was 36.5 years (SD 16.0). Overall, 80.2% (168 082/209 579) of the patients in the study were females and 19.4% (40 658/209 579) were males. Specimens were referred for investigation on suspicion of malignancy, postmortems, sterilization (tubal ligation), clinical suspicion of ectopic pregnancy (salpingectomies), appendicitis (appendectomies), and cervical intraepithelial neoplasia (from punch biopsies to hysterectomies). Only one clinician queried schistosomiasis, the specimen was from the bladder, the rest (724/725, 99.9%) were incidental findings.
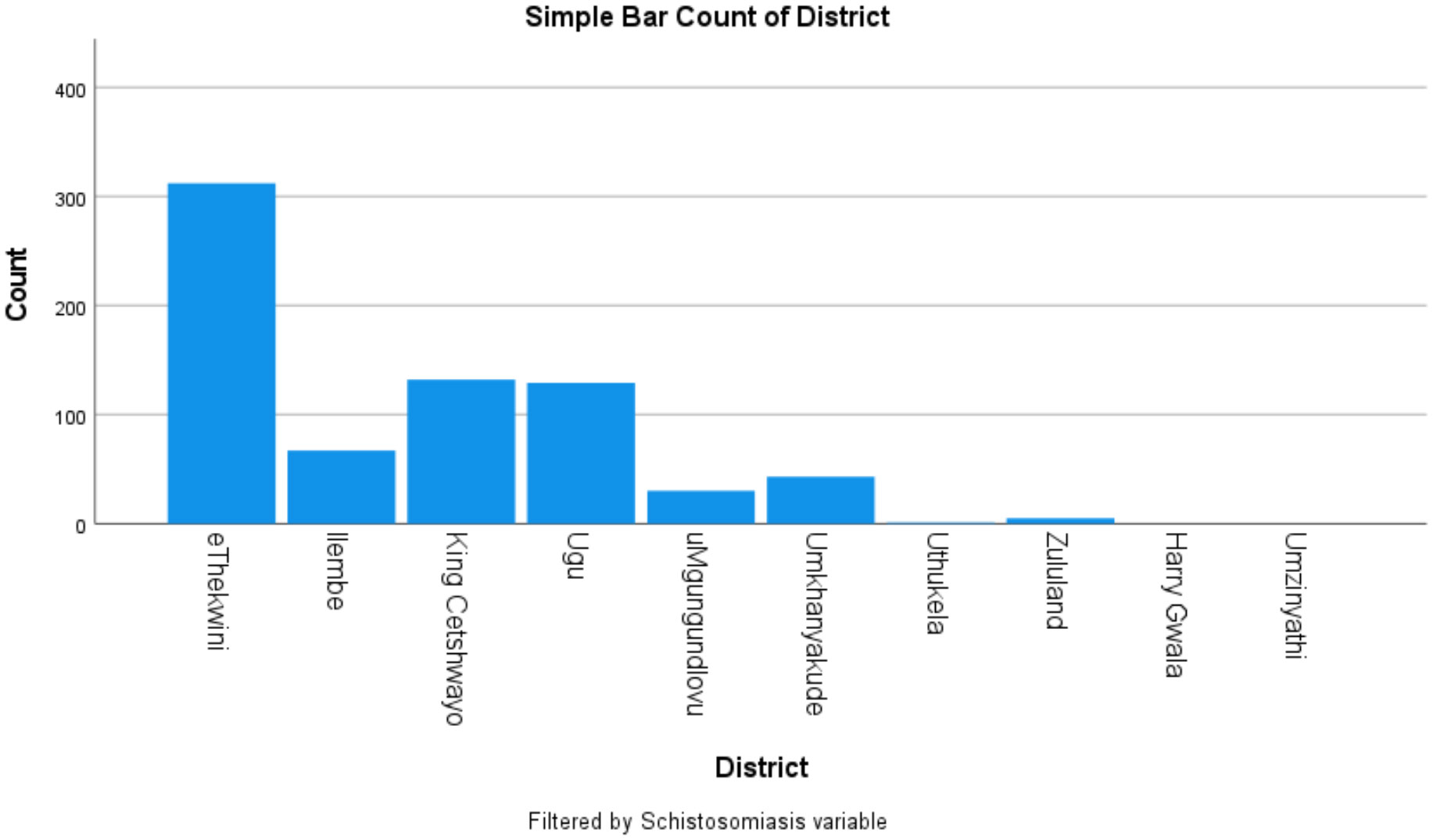
Figure 1 Distribution of Schistosomiasis cases by district Amajuba District did not send specimens during the study period.
Of the 725 schistosomiasis cases, 264 (36.4%) were males and 461 (63.6%) were females. Table 1 shows that 49.5% (356/725) schistosomiasis cases were found most often in the female genital tract, and secondly in the appendix. Amongst the total number of specimens received in the department only 0.3% (725/209 579) had schistosomiasis confirmed on histopathology. Figure 2 shows the histopathological features of schistosomiasis in fallopian tubes and appendix specimens.
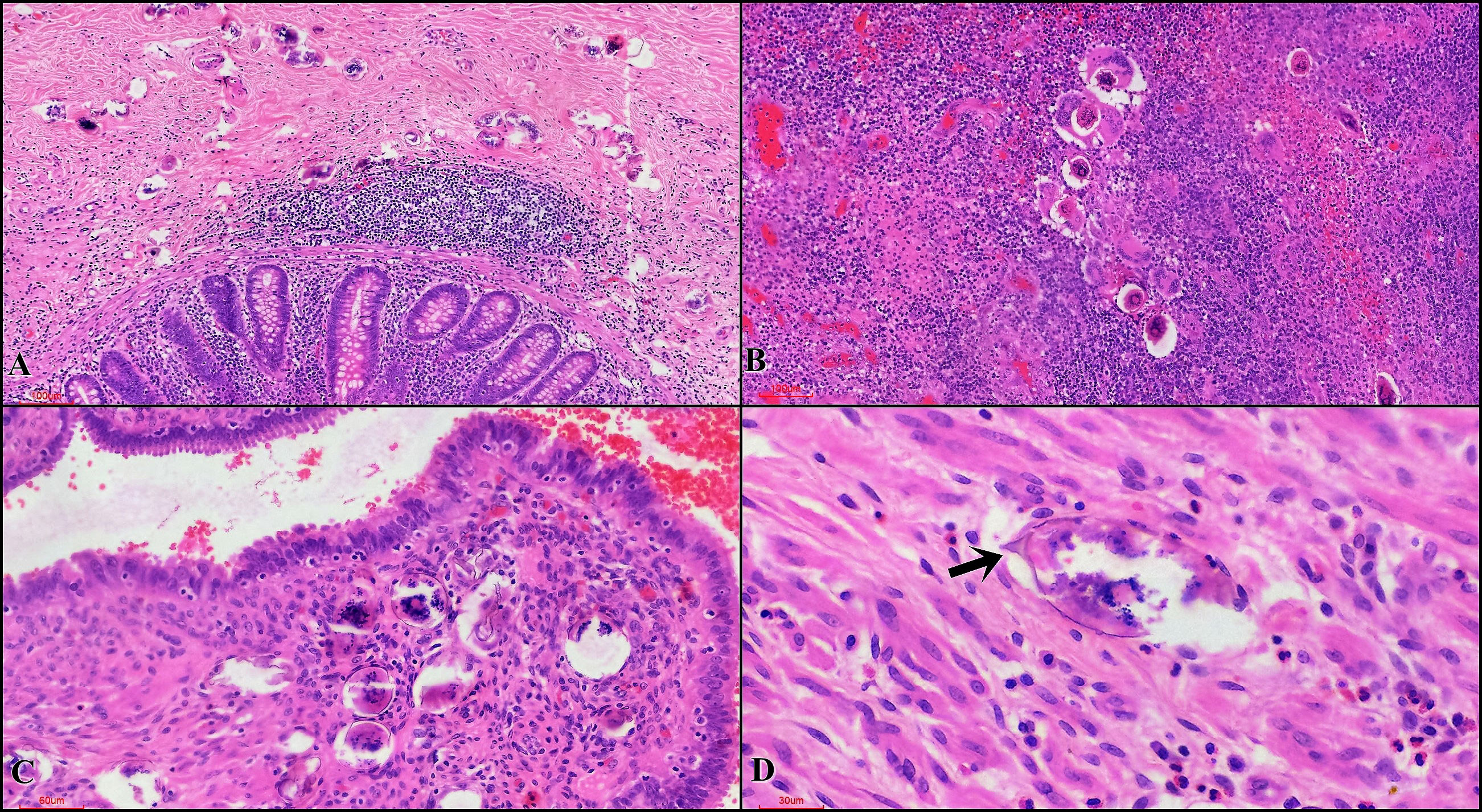
Figure 2 Histopathological features of schistosomiasis in acute appendicitis and ectopic pregnancy cases (A) Appendiceal tissue showing transmural Schistosoma eggs. Absence of inflammatory reaction around the eggs is noted (H&E, 10x (magnification)) (B) Appendix showing mucosal Schistosoma eggs with granulomatous response (H&E, 20x). (C) Fallopian tube showing mucosal Schistosoma eggs with no associated inflammatory response (H&E, x20) (D) Muscularis showing an egg with small terminal spine (arrow) (H&E, x40).
Female genital schistosomiasis cases were found in the uterine cervix 25.1% (182/725) and in the fallopian tubes 24% (174/725). The fallopian tubes were submitted either alone as a salpingectomy (2.3%; 457/19 556) or tubal ligation specimen (70.3%; 13 756/19 556), or as part of a more complex specimen from a hysterectomy and/or oophorectomy procedure (27.3%; 5 343/19 556). Tubal ectopic pregnancies accounted for 14.4% (25) of all the tubal schistosomiasis cases. Amongst those who had a fallopian tube specimen, ectopic pregnancy was found more often in those who had schistosomiasis than those who did not (Age-adjusted odds ratio 7.3, 95% CI 4.6-11.7, p<0.001).
Table 1 shows that there is significantly higher prevalence of schistosomiasis in males compared to females (p <0.001), appendicectomy and liver specimens contributed significantly to that. The appendix had 39.7% (289/725) of all the cases of schistosomiasis. Other organs were urinary bladder 4.4% (32/725), lung 3.2% (23/725) and liver 2.6% (19/725). There were 2 cases of schistosomiasis in the prostate and 4 cases in the anorectal region). Figure 2 indicates that schistosomiasis was sometimes seen with an associated inflammatory response and in some cases elicited a meagre response.
Sixty four percent (457/725) of the patients with schistosomiasis were female and 36% (259/725) were males (in 9 people gender was not reported). The patients with schistosomiasis (mean age 31.1,SD 13.4) were younger than those without, (mean age 36.6, SD16.0, p<0.001). Majority of patients (84%) with schistosomiasis were between the ages of 10 and 50 years (Figure 3). In the age group 10-19 years 91% (126/138) of the schistosomiasis cases were found during appendectomies.
Of the hospitals, King Edward Hospital (eThekwini/Durban) contributed most schistosomiasis cases 22.4% (70/312) (Figure 4) and most of the schistosomiasis cases (312), were from eThekwini metropolitan district as shown in Figure 4, however representing only 1.2% (312/25 111) of the specimens received from eThekwini, from these organs. Figure 5 shows that the districts with the highest percentage positive cases were uMkhanyakude 4.5% (43/965), followed by Ugu 2.6% (129/5 251), and King Cetshwayo districts 2.5% (132/5 360).
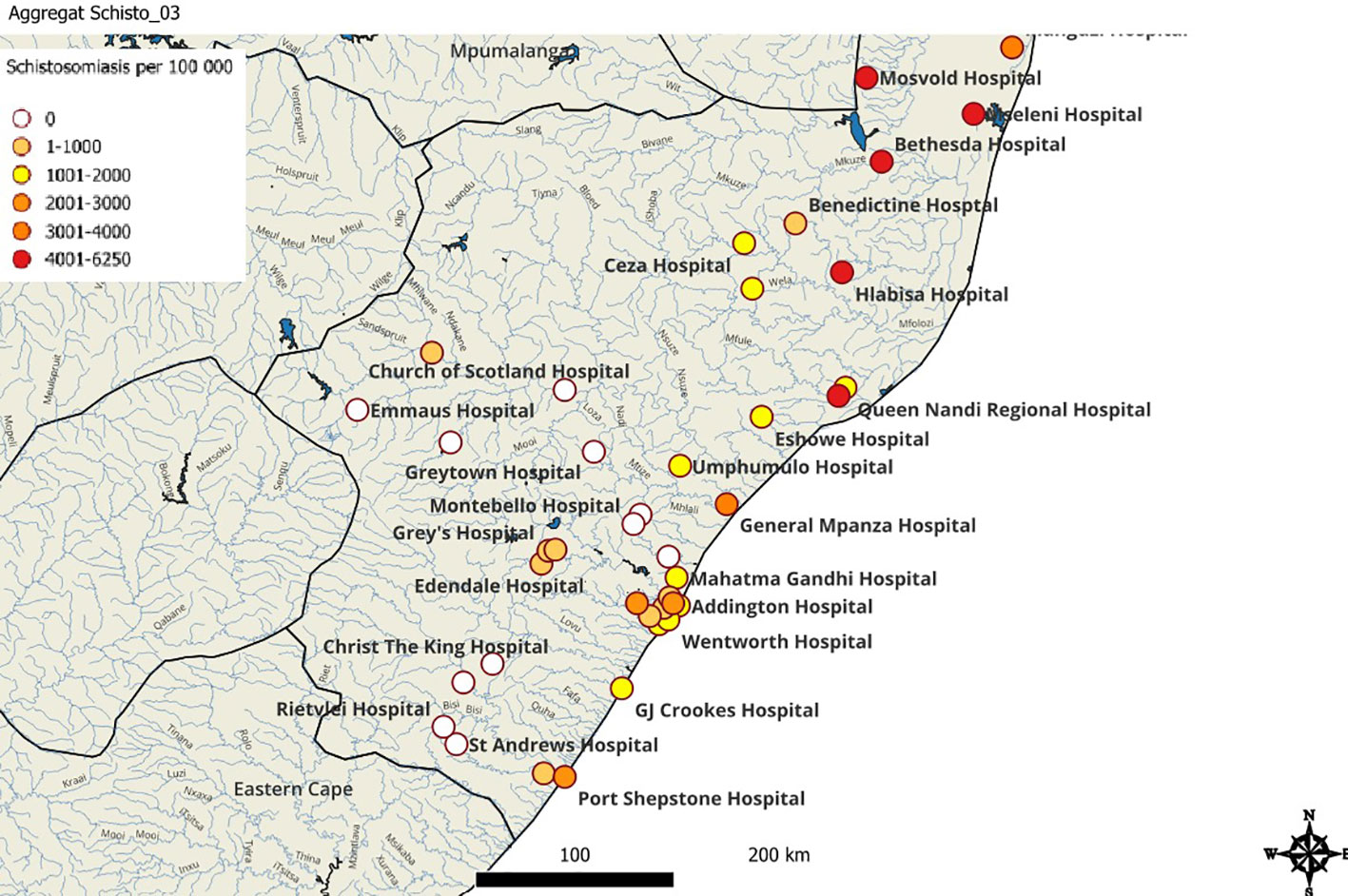
Figure 5 The geographical distribution of tissue schistosomiasis in KwaZulu-Natal. Schistosomiasis cases per 100 000 specimens (coloured dots) are extrapolated from the study data.
Clinicians in the KwaZulu-Natal public health sector hospitals did not suspect schistosomiasis when they submitted patient samples for histopathological investigations. Amongst these incidental findings, genital schistosomiasis was the most common and ectopic pregnancy was found more often in those who had schistosomiasis than those who did not. Secondly, schistosomiasis was found in the appendix, mostly in boys between the ages of 10 and 19 years. This study shows that schistosomiasis may be found in urban patients, either due to patient migration or referral from rural places, although urban transmission cannot be precluded (23).
The results from this retrospective study confirm the existence of schistosomiasis in patients attending the KwaZulu-Natal public healthcare sector. Confirming previous research, schistosomiasis was predominantly diagnosed as an incidental finding when a biopsy was done for other indications (12). The main indications for the biopsies in this study include cervical intraepithelial neoplasia (CIN), sterilization, acute appendicitis, and ectopic pregnancy. Patients presenting for cervical intraepithelial neoplasia (CIN) and tubal ligation are likely to have schistosomiasis discovered as an incidental finding as they may be asymptomatic. Clinical presentation of acute appendicitis or ectopic pregnancy secondary to schistosomiasis are indistinguishable from other causes and routine hematological and biochemical analyses (24–27).
Many studies have investigated schistosomiasis in school-aged children (8, 28–30). In this study, we have collected data from a wider age range; and have higher prevalence in boys than in girls. Schistosomiasis was also found more often in the young than in the old. However, from the age of 30 years, there is a higher prevalence of schistosomiasis among females. In a systematic review and meta-analysis study by Ayabina et al., gender-related differences in the prevalence were attributed to differences in gender roles in recreational and domestic water contact (31). Our study further reaffirms the gender-related differences in prevalence of schistosomiasis, however determining the causes for the differences is beyond the scope of this study.
There have been several studies exploring schistosomiasis in KwaZulu-Natal among school girls and these studies have shown a high prevalence of schistosomiasis in the coastal districts of KwaZulu-Natal (6, 28, 32). Previous research has indicated that uMkhanyakude, Ugu, and King Cetshwayo districts have areas highly endemic for schistosomiasis. This study further supports the current evidence. In addition, this study has demonstrated schistosomiasis in histopathology samples in the eThekwini/Durban metropolitan hospitals. Urban transmission of schistosomiasis has been documented in a number of countries (33–37). Urban district hospitals such as Prince Mshiyeni Memorial Hospital and RK Khan Hospital demonstrated a high number of schistosomiasis cases which could indicate local transmission. Tertiary and quaternary referral hospitals such as King Edward and Inkosi Albert Luthuli Central Hospitals equally demonstrated a high number of schistosomiasis cases. Although some of the cases seen at tertiary and quaternary hospitals would have been referrals from the rural areas, specimens such as appendicectomies and uterine cervical biopsies are done in local hospitals with few exceptions. As a result of this, urban transmission in the eThekwini/Durban metropolitan area cannot be precluded.
The majority of the schistosomiasis positive specimens were from the female genital tract, appendix and the urinary bladder. The indications for fallopian tube removal were for sterilization, ectopic pregnancy, and as part of a hysterectomy specimen. Tubal ectopic pregnancy and infertility are known sequelae of untreated schistosomal tubal disease (38). The manifestations of tubal schistosomiasis range from mild reaction to severe fibrotic granulomatous reaction which may impair tubal motility and patency, thus predisposing to ectopic pregnancy and infertility (38). Our findings further support that schistosomiasis may be a risk factor for ectopic pregnancy.
The appendix demonstrated the highest prevalence of schistosomiasis amongst the key organs. Notably, the indication for appendicectomy specimens was documented as acute appendicitis and some as part of hemicolectomy specimens, and in no instance was schistosomiasis a diagnostic consideration clinically. The role of schistosomiasis of the appendix in the pathogenesis of acute appendicitis remains unclear. The current thinking however is that schistosomiasis may cause acute appendicitis due to immunologic granulomatous response, excessive obstructive fibrosis, and ischemic alterations induced by egg emboli. The obstruction and ischemia then subsequently compromise the mucosal immunity, leading to bacterial infection (39, 40). The presence of the Schistosoma parasite does not always give rise to acute appendicitis as not all cases of schistosomiasis in the appendix are associated with appendicitis (39, 41). Numerous case reports and series have been documented on schistosomal appendicitis (24, 39–44). The possibility of parasitic infection having occurred years ago and a recent bacterial agent being able to provoke the present appendicitis have also been raised (45). Distinguishing schistosomal appendicitis from appendicitis associated with presence of Schistosoma eggs may thus not be entirely possible. Suffice to say however that the diagnosis must be entertained in patients from schistosomiasis-endemic areas with features of acute appendicitis or recurrent, dexterous abdominal pain (46).
Specimens from the cervix were mostly from patients with squamous cell carcinoma, high-grade squamous intraepithelial neoplasia, or persistent low-grade squamous intraepithelial neoplasia detected on Pap smears before the biopsy. Some of the specimens were from hysterectomy specimens for either fibroid uterus or endometrial carcinoma. The lower female genital tract (uterine cervix) was more frequently found to be positive for schistosomiasis relative to the upper female genital tract (fallopian tubes). This finding in our study is consistent with findings in other retrospective histopathology studies carried out in Egypt, Malawi, Mozambique, South Africa, Tanzania, and Zimbabwe which have also showed that genital involvement most often occurs in the cervix and less frequently in the fallopian tube (12, 47–50). Colposcopy features of schistosomiasis are well described and documented, however, none of our cases had schistosomiasis as a comorbid clinical diagnosis at the time of biopsy (51). The lack of consideration of schistosomiasis by colposcopists/gynecologists in KwaZulu-Natal, which is a schistosomiasis endemic area, is of some concern as this may be an indicator that patients are deprived of early diagnosis and treatment with subsequent complications.
The other organs demonstrating deposition of schistosoma ova in our cohort were the urinary bladder, lung, liver, prostate, and anorectal region. Whilst these organs had a smaller contribution, it is notable that the urinary bladder was amongst the organs with a higher percentage of schistosomiasis. Furthermore, pulmonary schistosomiasis is not an uncommon disease and it can occur in both acute and chronic phases of schistosomiasis (52). Majority of the patients in the acute phase are asymptomatic with a few presenting clinically as Katayama fever. Chronic pulmonary schistosomiasis presents with non-specific clinical and imaging findings related to pulmonary fibrosis, pulmonary hypertension and subsequent cor pulmonale (53).
We have demonstrated the wide distribution of schistosomiasis. Although we did not identify cases of neuroschistosomiasis in the period under review, it is worth noting that central nervous system schistosomiasis is a well-described entity that presents with neurological symptoms (54).
Though this study has shown that schistosomiasis is still prevalent in KwaZulu-Natal, it is important to note that this is based purely on histopathology samples and thus is not a true reflection of schistosomiasis burden in the community. For preclusion of schistosomiasis several sections should be done as the schistosoma eggs lie in clusters and may be missed (55). The histopathological technician was not alerted of this differential diagnosis by the clinician; hence this study certainly is an under-estimate of the true presence of schistosomiasis.
The figures obtained in this study also may not be a true reflection of the burden of disease in KwaZulu-Natal as most but not all specimens were sent to the Department of Anatomical Pathology laboratory.
Prior studies have shown S. haematobium as the most prevalent species in KwaZulu-Natal (9, 56). The other schistosome species, namely S. mansoni and S. japonicum are not endemic in KwaZulu-Natal (9, 56). In our study, this was not analyzed as the species were not differentiated in routine histopathology dyes.
The exact indication for the samples was often not given and could not be readily deduced from the pathology reports.
Schistosomiasis is still present in the community and remains a significant public health challenge.
South Africa needs to urgently implement programs towards the elimination of Neglected Tropical Diseases if it is to meet the WHO target of 2030 to end suffering from schistosomiasis. Clinicians should consider schistosomiasis as a differential diagnosis and when the technician is making sections for histopathological analyses, serial sections should be presented to the histopathologist. Furthermore, investigations are required to validate or disapprove findings suggestive of schistosomiasis transmission in the eThekwini/Durban metropolitan area.
The data analyzed in this study is subject to the following licenses/restrictions: The datasets analyzed during the current analysis are not publicly available due to them containing information that could compromise patient privacy including patient’s first and last name, gender, location and test conducted. Requests to access these datasets should be directed to https://aarms.nhls.ac.za/NHLS_AARMS/Public/Default.aspx.
The studies involving humans were approved by Biomedical Research Ethics Committee (BREC). University of KwaZulu-Natal. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
AM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. GN: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. EK: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. EK is funded by European Research Council under the European Union’s Horizon 2020/ERC Grant agreement no. 101057853 (DUALSAVE-FGS).
We thank Dinesh Sookhdeo and Roy Manyaira for data support. We would also like to thank Carmelo Pistilli, for his mapping of the geographical distribution of tissue schistosomiasis in KwaZulu-Natal.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. World Health Organization. Investing to overcome the global impact of neglected tropical diseases: Third WHO report on neglected tropical diseases 2015. Genève, Switzerland: World Health Organization (2015) p. 154–61. p.
2. World Health Organization. Schistosomiasis: Fact sheet (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis.
3. Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis (2015) 19(2). doi: 10.1016/j.bjid.2014.11.004
4. Gabrielli AF, Garba Djirmay A. Schistosomiasis in Europe. Curr Trop Med Rep (2023) 10(3):79–87. doi: 10.1007/s40475-023-00286-9
5. Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop (2000) 77(1). doi: 10.1016/S0001-706X(00)00122-4
6. Moodley I, Kleinschmidt I, Sharp B, Craig M, Appleton C. Temperature-suitability maps for schistosomiasis in South Africa. Ann Trop Med Parasitol (2003) 97(6). doi: 10.1179/000349803225001445
7. National Institute for Communicable Diseases (NICD). Schistosomiasis (Bilharzia) Frequently Asked Questions (2018). Available at: https://www.nicd.ac.za/wp-content/uploads/2017/03/Schistosomiasis-Bilharzia_20181104_Final.pdf.
8. Sacolo-Gwebu H, Chimbari M, Kalinda C. Prevalence and risk factors of schistosomiasis and soil-transmitted helminthiases among preschool aged children (1–5 years) in rural KwaZulu-Natal, South Africa: a cross-sectional study. Infect Dis Poverty (2019) 8(1). doi: 10.1186/s40249-019-0561-5
9. Christensen EE, Taylor M, Zulu SG, Lillebo K, Gundersen SG, Holmen S, et al. Seasonal variations in schistosoma haematobium egg excretion in school-age girls in rural Kwazulu-Natal province, South Africa. South Afr Med J (2018) 108(4):352–5. doi: 10.7196/SAMJ.2018.v108i4.12775
10. Magaisa K, Taylor M, Kjetland EF, Naidoo PJ. A review of the control of schistosomiasis in South Africa. S Afr J Sci (2015) 111(Number 11/12). doi: 10.17159/sajs.2015/20140427
11. Olveda DU, Li Y, Olveda RM, Lam AK, Chau TNP, Harn DA, et al. Bilharzia: pathology, diagnosis, management and control. Trop Med Surg (2013) 1(4). doi: 10.4172/2329-9088.1000135
12. Swai B, Poggensee G, Mtweve S, Krantz I. Female genital schistosomiasis as an evidence of a neglected cause for reproductive ill-health: a retrospective histopathological study from Tanzania. BMC Infect Dis (2006) 6(1). doi: 10.1186/1471-2334-6-134
13. Kjetland EF, Leutscher PDC, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol (2012) 28(2):58–65. doi: 10.1016/j.pt.2011.10.008
14. von Lichtenberg F. Schistosomiasis. In: Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE, editors. Pathology of Infectious Diseases. Stamford, Connecticut: Appleton & Lange (1997). p. 1537–51.
15. Kleppa E, Ramsuran V, Zulu S, Karlsen GH, Bere A, Passmore J-AS, et al. Effect of female genital schistosomiasis and anti-schistosomal treatment on monocytes, CD4+ T-cells and CCR5 expression in the female genital tract. PloS One (2014) 9(6):e98593. doi: 10.1371/journal.pone.0098593. LUTY AJF.
16. Poggensee G, Reimert CM, Nilsson L-A, Jamaly S, Sjastad A, Roald B, et al. Diagnosis of female genital schistosomiasis by indirect disease markers: determination of eosinophil cationic protein, neopterin and IgA in vaginal fluid and swab eluates. Acta Trop (1996) 62(4):269–80. doi: 10.1016/S0001-706X(96)00028-9
17. Changalucha JM, Andreasen A, Johnson WD, Kalluvya SE, Fitzgerald DW, Downs JA, et al. Association of schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg (2012) 87(5):868–73. doi: 10.4269/ajtmh.2012.12-0395
18. Downs JA, Claudia J, Dee HE, McGeehan M, Khan H, Marenga A, et al. Schistosomiasis and human immunodeficiency virus in men in Tanzania. Am J Trop Med Hyg (2017) 96(4):856–62. doi: 10.4269/ajtmh.16-0897
19. Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS (2006) 20(4):593–600. doi: 10.1097/01.aids.0000210614.45212.0a
20. Colombe S, Corstjens PLAM, de Dood CJ, Miyaye D, Magawa RG, Mngara J, et al. HIV-1 viral loads are not elevated in individuals co-infected with Schistosoma spp. After adjustment for duration of HIV-1 infection. Front Immunol (2018) 9(SEP):2005. doi: 10.3389/fimmu.2018.02005
21. Wall KM, Kilembe W, Vwalika B, Dinh C, Livingston P, Lee YM, et al. Schistosomiasis is associated with incident HIV transmission and death in Zambia. PLoS Negl Trop Dis (2018) 12(12):e0006902. doi: 10.1371/journal.pntd.0006902
22. Statistics South Africa. Provincial profile: KwaZulu-Natal Community Survey 2016. Pretoria (2018). Available at: http://cs2016.statssa.gov.za/wp-content/uploads/2018/07/KZN.pdf.
23. Johnson CL, Appleton CC. Urban schistosomiasis transmission in Pietermaritzburg, South Africa. South Afr J Epidemiol Infect (2005) 20(3):103–10. doi: 10.1080/10158782.2005.11441244
24. Zacarias M, Pizzol D, de Miranda H, Colangelo AC, Veronese N, Smith L. Schistosomal appendicitis: Case series and systematic literature review. PLoS Negl Trop Dis (2021) 15(6):e0009478. doi: 10.1371/journal.pntd.0009478
25. Nemungadi TG, Kleppa E, van Dam GJ, Corstjens PLAM, Galappaththi-Arachchige HN, Pillay P, et al. Female genital schistosomiasis lesions explored using circulating anodic antigen as an indicator for live schistosoma worms. Front Trop Dis (2022) 3:821463/full. doi: 10.3389/fitd.2022.821463/full
26. Sommerfelt I, Ndhlovu P, Taylor M, Naidoo S, Pillay P, Haaland H, et al. Health professionals’ knowledge about female genital schistosomiasis. A qualitative investigation in a schistosomiasis endemic area in South Africa. SSM - Qual Res Heal (2023) 3:100292. doi: 10.1016/j.ssmqr.2023.100292
27. UNAIDS. No more neglect: Female genital schistosomiasis and HIV. Geneva, Switzerland (2019). Available at: https://www.unaids.org/sites/default/files/media_asset/female_genital_schistosomiasis_and_hiv_en.pdf.
28. De Boni L, Msimang V, De Voux A, Frean J. Trends in the prevalence of microscopically-confirmed schistosomiasis in the South African public health sector, 2011–2018. PLoS Negl Trop Dis (2021) 15(9):e0009669. doi: 10.1371/journal.pntd.0009669. Zhou X-N, editor.
29. Kabuyaya M, Chimbari MJ, Manyangadze T, Mukaratirwa S. Schistosomiasis risk factors based on the infection status among school-going children in the Ndumo area, uMkhanyakude district, South Africa. South Afr J Infect Dis (2017) 32(2):67–72. doi: 10.4102/sajid.v32i2.56
30. Lothe A, Zulu N, Øyhus AO, Kjetland EF, Taylor M. Treating schistosomiasis among South African high school pupils in an endemic area, a qualitative study. BMC Infect Dis (2018) 18(1):239. doi: 10.1186/s12879-018-3102-0
31. Ayabina DV, Clark J, Bayley H, Lamberton PHL, Toor J, Hollingsworth TD. Gender-related differences in prevalence, intensity and associated risk factors of Schistosoma infections in Africa: A systematic review and meta-analysis. PLoS Negl Trop Dis (2021) 15(11):e0009083. doi: 10.1371/journal.pntd.0009083
32. Livingston M, Pillay P, Zulu SG, Sandvik L, Kvalsvig JD, Gagai S, et al. Mapping Schistosoma haematobium for Novel Interventions against Female Genital Schistosomiasis and Associated HIV Risk in KwaZulu-Natal, South Africa. Am J Trop Med Hyg (2021) 104(6):2055–64. doi: 10.4269/ajtmh.20-0679
33. Kapito-tembo AP, Mwapasa V, Meshnick SR, Samanyika Y, Banda D, Bowie C, et al. Prevalence Distribution and Risk Factors for Schistosoma hematobium Infection among School Children in Blantyre, Malawi. PLoS Negl Trop Dis (2009) 3(1):e361. doi: 10.1371/journal.pntd.0000361
34. Jinabhai CC, Taylor M, Coutsoudis A, Coovadia HM, Tomkins AM, Sullivan KR. Epidemiology of helminth infections: implications for parasite control programmes, a South African perspective. Public Heal Nutr (2001) 4(6):1211–9. doi: 10.1079/PHN2001180
35. Okoli EI, Odaibo AB. Urinary schistosomiasis among schoolchildren in Ibadan, an urban community in south-western Nigeria. Trop Med Int Heal (1999) 4(4):308–15. doi: 10.1046/j.1365-3156.1999.00388.x
36. Firmo JO, Lima Costa MF, Guerra HL, Rocha RS. Urban schistosomiasis: morbidity, sociodemographic characteristics and water contact patterns predictive of infection. Int J Epidemiol (1996) 25(6):1292–300. doi: 10.1093/ije/25.6.1292
37. Brooker S, Alexander N, Geiger S, Moyeed RA, Stander J, Fleming F, et al. Contrasting patterns in the small-scale heterogeneity of human helminth infections in urban and rural environments in Brazil. Int J Parasitol (2006) 36(10–11):1143–51. doi: 10.1016/j.ijpara.2006.05.009
38. Bland KG, Gelfand M. The effects of schistosomiasis on the the fallopian tubes in the African female. BJOG Int J Obstet Gynaecol (1970) 77(11):1024–7. doi: 10.1111/j.1471-0528.1970.tb03451.x
39. Satti MB, Tamimi DM, Al Sohaibani MO, Al Quorain A. Appendicular schistosomiasis: a cause of clinical acute appendicitis? J Clin Pathol (1987) 40(4):424–8. doi: 10.1136/jcp.40.4.424
40. Terada T. Schistosomal appendicitis: incidence in Japan and a case report. World J Gastroenterol (2009) 15(13):1648–9. doi: 10.3748/wjg.15.1648
41. Badmos K, Komolafe A, Rotimi O. Schistosomiasis presenting as acute appendicitus. East Afr Med J (2007) 83(10). doi: 10.4314/eamj.v83i10.9464
42. Ojo OS, Udeh SC, Odesanmi WO. Review of the histopathological findings in appendices removed for acute appendicitis in Nigerians. J R Coll Surg Edinb (1991) 36(4):245–8.
43. Hasan A, Elhussiny MEA, Nagaty ME, Eid M, Elias A-A-K, Abdulmohaymen A, et al. Clinico-pathological profile of schistosomal appendicitis detected in surgically resected appendices: A retrospective study. Int J Surg Open (2023) 54:100606. doi: 10.1016/j.ijso.2023.100606
44. Malallah H, Al-Onaizi T, Shuaib A, Alsharaf K, Behbehani A. Schistosomiasis as a cause of acute appendicitis in non-endemic areas. Int J Surg (2017) 47:S25. doi: 10.1016/j.ijsu.2017.08.137
45. Rivasi F, Pampiglione S. Appendicitis associated with presence of Schistosoma haematobium eggs: an unusual pathology for Europe. Rep three cases. APMIS (2006) 114(1):72–6. doi: 10.1111/j.1600-0463.2006.apm_314.x
46. Adebamowo CA, Ladipo JK, Ajao OG, Akang EEU. Schistosomiasis of the appendix. Br J Surg (2005) 78(10):1219–21. doi: 10.1002/bjs.1800781023
47. Ross MD, Blair DM, Weber MC, Gelfand M. Distribution and extent of schistosomiasis in female pelvic organs, with special reference to the genital tract, as determined at autopsy. Am J Trop Med Hyg (1971) 20(6):846–9. doi: 10.4269/ajtmh.1971.20.846
48. Edington GM, Nwabuebo I, Junaid TA. The pathology of schistosomiasis in Ibadan, Nigeria with special reference to the appendix, brain, pancreas and genital organs. Trans R Soc Trop Med Hyg (1975) 69(1):153–62. doi: 10.1016/0035-9203(75)90027-9
49. van Raalte JA, Venkataramaiah NR, Shaba JK. Bilharziasis of the female genital tract in Tanzania. East Afr Med J (1981) 58(7):543–7.
50. Wright ED, Chiphangwi J, Hutt MSR. Schistosomiasis of the female genital tract. A histopathological study of 176 cases from Malawi. Trans R Soc Trop Med Hyg (1982) 76(6):822–9. doi: 10.1016/0035-9203(82)90118-3
51. Norseth HM, Ndhlovu PD, Kleppa E, Randrianasolo BS, Jourdan PM, Roald B, et al. The colposcopic atlas of schistosomiasis in the lower female genital tract based on studies in Malawi, Zimbabwe, Madagascar and South Africa. PLoS Negl Trop Dis (2014) 8(11):e3229. doi: 10.1371/journal.pntd.0003229
52. Houlder EL, Costain AH, Cook PC, MacDonald AS. Schistosomes in the lung: immunobiology and opportunity. Front Immunol (2021) 12:635513/full. doi: 10.3389/fimmu.2021.635513/full
53. Niemann T, Marti HP, Duhnsen SH, Bongartz G. Pulmonary schistosomiasis – imaging features. J Radiol Case Rep (2010) 4(9). doi: 10.3941/jrcr.v4i9.482
54. Coyle CM. Schistosomiasis of the nervous system. Handb Clin Neurol (2013) 114:271–81. doi: 10.1016/B978-0-444-53490-3.00022-4
55. Kjetland EF, Poggensee G, Helling-Giese G, Richter J, Sjaastad A, Chitsulo L, et al. Female genital schistosomiasis due to Schistosoma haematobium Clinical and parasitological findings in women in rural Malawi. Acta Trop (1996) 62(4):239–55. doi: 10.1016/S0001-706X(96)00026-5
56. Saathoff E, Olsen A, Magnussen P, Kvalsvig JD, Becker W, Appleton CC. Patterns of Schistosoma haematobium infection, impact of praziquantel treatment and re-infection after treatment in a cohort of schoolchildren from rural KwaZulu-Natal/South Africa. BMC Infect Dis (2004) 4(1):40. doi: 10.1186/1471-2334-4-40
Keywords: schistosomiasis, bilharzia, neglected tropical diseases, tissue diagnosis, histopathology
Citation: Mwazha A, Nhlonzi GB and Kjetland EF (2023) Presence of tissue schistosomiasis in KwaZulu-Natal, South Africa: a retrospective histopathologic review. Front. Trop. Dis 4:1301485. doi: 10.3389/fitd.2023.1301485
Received: 25 September 2023; Accepted: 07 November 2023;
Published: 28 November 2023.
Edited by:
Joseph Daniel Turner, Liverpool School of Tropical Medicine, United KingdomReviewed by:
Chiaka Anumudu, University of Ibadan, NigeriaCopyright © 2023 Mwazha, Nhlonzi and Kjetland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Absalom Mwazha, YW13YXpoYUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.