- 1Graduate School of Life Sciences, Faculty of Science, Utrecht University, Utrecht, Netherlands
- 2Veterinary Medicine, Department Population Health Sciences, Utrecht University, Utrecht, Netherlands
- 3Centre for Zoonoses and Environmental Microbiology, National Institute for Public Health and the Environment, Bilthoven, Netherlands
Various arthropod vectors are responsible for the transmission of pathogens that cause serious diseases in humans. Some important pathogens are transmitted by mosquitoes during blood-feeding, for example the well-known parasite causing malaria, and viruses-causing diseases such as dengue, chikungunya, and Zika virus fever. In contrast, very little is known about the potential of mosquitoes to transmit pathogenic bacteria. Hitherto, only a few bacteria have occasionally been suggested to be spread by mosquitoes, but this is not widely known nor accepted, and literature on this topic is limited. The aim of this study was to review the literature about the possible role of mosquitoes in the transmission of the bacterium F. tularensis, the causal agent of tularaemia, which has been proposed by several experts. Available primary articles investigating this possible vector role of mosquitoes were analysed and evaluated based on four vector incrimination criteria. This demonstrated that several studies had indeed found indications of a correlation between mosquito bites and tularaemia, and that the results of some other studies suggested that such a vector role for mosquitoes might exist. However, conclusive evidence of a causal relationship was not found, nor irrefutable proof that mosquitoes can actually transmit this bacterium during blood-feeding. This literature review has provided an overview of the current relevant literature, shows that future studies should focus on gaining more insight into other explanations for the correlation between mosquito bites and tularaemia, and that the certainty with which some authors write about the vector role of mosquitoes is not entirely justified.
1 Introduction
Globally, about 17% of human infectious diseases result from infection with pathogenic bacteria, viruses, or parasites that are transmitted by arthropods, so called “vectors” (1). Such vector-borne diseases occur in all parts of the world and are spread by a wide variety of mainly blood-feeding insects and acari (2). Mosquitoes play an important role in the transmission of parasites and viruses. Worldwide, the mosquito-borne disease with the highest mortality in humans is malaria, with approximately 220 million cases and 400,000 deaths a year (3). The mosquito-borne disease with the highest morbidity is dengue, with an estimated 100 million–400 million infections annually (4, 5). Mosquitoes can also transmit pathogens to companion animals, livestock, and wildlife (6). For example, equine encephalitis viruses are generally spread to horses by Culex spp. (7), whereas avian malaria, caused by several Plasmodium spp., is transmitted to birds by mosquitoes from many genera (8). Likewise, dirofilarial parasites, which cause canine heartworm disease or subcutaneous dirofilariasis in both cats and dogs, are also transmitted by various species of mosquitoes (9). Rift Valley fever virus usually circulates between cattle and mosquitoes, causing diseases in cattle, but occasionally spillover to humans occurs when mosquitoes switch host species.
Interestingly, none of the commonly known mosquito-borne pathogens are of bacterial origin: they are all viruses or parasites, including helminths and protozoa. In contrast, transmission of bacterial pathogens has been demonstrated, or is even common, for some other vectors such as ticks (10).
Francisella tularensis, the causal agent of tularaemia, is a bacterium that has been associated with transmission by mosquitoes already since the early 20th century (11). The aim of this study was to review the available evidence for the claim that mosquitoes play a role in the transmission of F. tularensis.
2 Tularaemia and the possible transmission of Francisella tularensis by mosquitoes
2.1 Causative agent of tularaemia
The bacterium F. tularensis, a Gram-negative coccobacillus, is the causative agent of the disease tularaemia (12). Tularaemia is also known as “rabbit fever”, as it is not a disease unique to humans. The pathogen is found in over 250 other animal species and is especially pathogenic to rabbits, hares, and rodents. Tularaemia is on the list of notifiable diseases in many countries because of the severity of the disease and the low infectious dose of the bacterium. Because of the low infectious dose, it is even considered a potential bioweapon because it is also relatively easily transmitted via aerosols (12).
Nowadays, four subspecies of F. tularensis are distinguished, of which only two are clinically relevant for humans (13). These are the ssp. tularensis (also known as subtype A), and ssp. holarctica (also known as subtype B). The former is almost exclusively present in North America, highly virulent, and, therefore, responsible for the most severe cases of human disease. The latter is present in the whole northern hemisphere, as well as in Australia, and is the main cause of human cases of tularaemia in northern Europe (14, 15). Very little is known about a third subspecies, ssp. mediasiatica, which has been found in Asia (16). The fourth subspecies, ssp. novicida, is found worldwide, but is rarely seen as a causal agent of human disease. However, this subspecies does cause severe disease in some other mammals, including mice, and is therefore repeatedly used in experimental research using animal models (17).
2.2 Manifestations of tularaemia and routes of infection
The clinical presentation and severity of tularaemia in humans is not only determined by the subspecies causing the disease, but also largely depends on the route of infection (12, 13, 15). There are six types of disease manifestation related to different ways of exposure to the bacteria, which—in their turn—are closely connected to specific reservoirs of F. tularensis that are all—direct or indirect—of animal origin. Five manifestations of tularaemia are relatively uncommon. By far the most common manifestations of tularaemia are ulceroglandular and glandular infections, which both result from infections via the skin (12, 13). Infection via the skin takes place through direct inoculation with contaminated sources (e.g., skin wound while handling an infected animal) or via an arthropod bite (see below). In fact, aquatic ecosystems are considered an important source of F. tularensis, as the bacteria have often been found in spring water and lakes and also in private water wells and community water supplies (15). From this aquatic source, a “passive” uptake of the bacterium can occur via small lesions of the skin. The water itself is believed to become contaminated by infected animals, especially lagomorphs and small rodents, for example via their faeces or carcasses. So, animals—living or dead—are considered the main reservoir, and, therefore, also the main source of the bacteria. The oropharyngeal infection occurs via ingestion of contaminated food (undercooked meat of infected animals, residues on wild berries, etc.) or drinking of contaminated water from different sources. This route of infection can also lead to an intestinal infection. A pneumonic or respiratory form of tularaemia usually occurs after inhalation of the bacteria, via aerosols from contaminated water, dust, or hay. A common cause of contamination via hay is the presence of a carcass of an infected lagomorph or rodent. Oculoglandular infection can occur after eye contact with infected droplets of water or blood, or by rubbing the eye with contaminated fingers; this may occur after handling an infected animal. The most severe form of tularaemia is the typhoidal form, which is a systemic infection; the precise mode of infection is not clear yet.
As mentioned above, the bacterium can also be acquired via the bite of an haematophagous arthropod vector, such as a tick or a deerfly, as has been shown in many human infections with F. tularensis ssp. tularensis in northern America (15). Interestingly, in outbreaks in Sweden and Finland, mosquitoes rather than ticks or flies are suspected of being involved in the transmission of F. tularensis ssp. holarctica to humans (15). Concerning mosquitoes, two distinct transmission routes, zoonotic and water borne, are suggested in the literature. The former is the transmission of F. tularensis by a mosquito from an animal reservoir to a person. The latter refers to the situation in which water is the source of F. tularensis that the mosquito transmits to a person. This is part of the water-borne transmission described by Hennebique et al. (2019) (15).
2.3 Criteria for vector incrimination
Of all the currently known pathogens that cause disease in vertebrates, only a small proportion has been shown to be transmitted by mosquitoes. Furthermore, only a few of the many mosquito species known today are generally considered to be true vectors of specific pathogens. The latter raises the question of what is required to incriminate mosquitoes as vectors of certain pathogens.
In the process of incrimination, various aspects of the interactions between mosquito, pathogen, host, and environment are evaluated. These aspects can roughly be divided into four criteria.
2.3.1 Vector competence
When a mosquito is able to pick up a pathogen, to support its replication and/or development, and to transfer it to an uninfected host animal, it can be regarded as a competent biological vector (18–20). It is called a competent mechanical vector when it transmits pathogens without supporting replication or development. This former vector competence is modulated by intrinsic factors of both the mosquito and the pathogen. These include physiological as well as behavioural characteristics, such as the mosquito’s immune response and the duration and frequency of blood-feeding. And, obviously, the mosquito must live long enough for the pathogen to replicate and to reach the infective stage (18, 20). In addition, the genetic characteristics of both the mosquito and the pathogen have influence on the vector competence; for example, one strain of a certain mosquito species can have a lower or higher susceptibility to a certain pathogen than another and, similarly, one strain of a pathogen species is more successful in evading the mosquito immune system than another (21).
Demonstration of vector competence is complicated. In the laboratory it can be partly demonstrated with experiments showing that a specific mosquito species is susceptible to infection with the pathogen and can efficiently transmit it via blood-feeding (18–20). An experiment would need to include the following three steps: (1) allowing uninfected mosquitoes to feed on an infected vertebrate animal; (2) allowing these mosquitoes to subsequently feed on an uninfected animal and then monitoring whether or not that animal develops symptoms; and (3) detecting or isolating the pathogen from the newly infected animal. Due to the differences between species and populations, vector competence studies should preferably use the mosquito species or population suspected of being a vector, and the specific pathogen responsible for the disease in those animals to which transmission is being investigated. And even then, mosquitoes that are competent vectors for a pathogen in the laboratory may be inadequate vectors in natural conditions, as some intrinsic factors, such as mosquito behaviour and longevity, also depend to some extent on extrinsic factors, such as temperature and humidity.
2.3.2 Isolation from wild mosquitoes
To incriminate a certain mosquito species as vector of a certain pathogen there must also be repeated demonstration of wild mosquitoes of that species, collected in natural conditions, harbouring the infective stage of the same pathogen species as is isolated from infected vertebrate hosts in the same geographic region (18, 19). Usually, it is considered enough proof if about 1% of the wild mosquito population is shown to be infected (20). Detection of the pathogen specifically in the mosquito salivary glands is considered an even stronger indication of a possible vector role (22).
2.3.3 Direct contact
In addition to being competent as a vector, for a true vector role, the mosquito must actually feed on the host species to transmit the pathogen. Moreover, it should be demonstrated that direct contact between the two is not uncommon and, preferably, also that the mosquito species has a preference for feeding on the specific vertebrate host species (18–20, 22). Such proof can be established by capturing mosquitoes on the host species during blood-feeding, or by detecting a blood meal from the vertebrate host species inside the abdomen of a captured mosquito.
2.3.4 Sympatric association
Finally, there must also be a spatial and temporal relationship between the mosquito population and clinical or subclinical infection of vertebrate hosts by the pathogen (18–20, 22). This means that the occurrence of the disease in the host species should overlap with both the geographic distribution and the seasonal occurrence of the specific mosquito population.
2.4 Evaluation by criteria
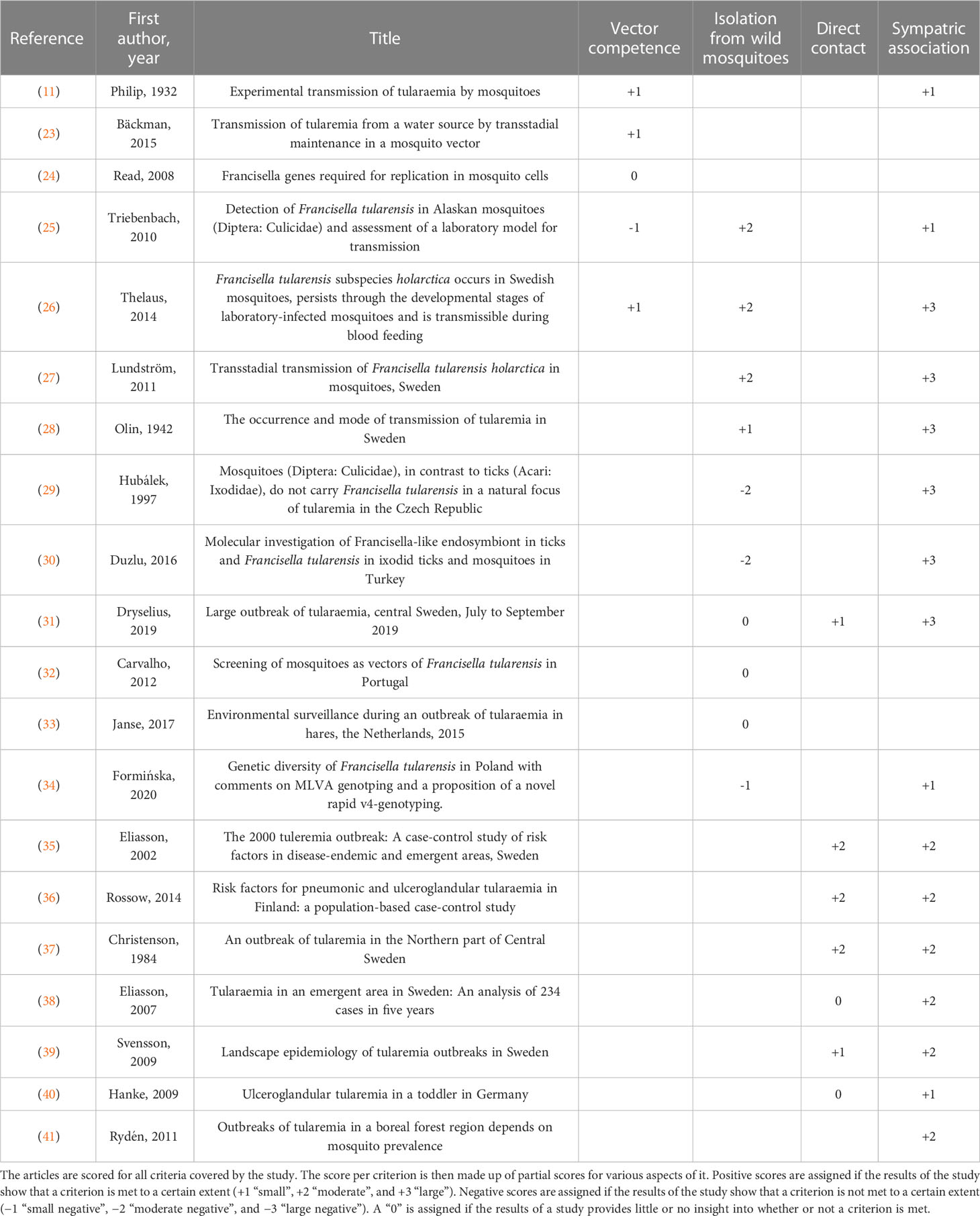
Using the four vector incrimination criteria presented above, 20 primary studies on the possible role of mosquitoes in the transmission of F. tularensis were evaluated to assess what evidence has been found to confirm or rather to invalidate the incrimination of mosquitoes as vectors of this bacterium. A summary of the evaluation of all publications is shown in Table 1.
2.4.1 Criterion 1: vector competence
As early as at the beginning of the 20th century, it was proposed that there is a link between mosquitoes and the spread of human tularaemia. Already in 1932, Philip et al. (11) had performed a series of experiments to investigate the vector competence of mosquitoes to transmit the bacterium that was then still known as Bacterium tularense. Since then, various aspects of vector competence of mosquitoes for these bacteria were investigated by multiple research groups, using various subspecies of the bacterium, several species of mosquitoes, and with various laboratory techniques. The experiments and results of these studies are listed below, starting with experiments with artificial infection of vector cells and hosts, followed by those in which adult mosquitoes fed on F. tularensis-infected blood and ending with experiments in which mosquito larvae were bred in F. tularensis-contaminated aquatic environments.
2.4.1.1 Artificial infection of vector cells and hosts
In the 1932 study by Philip et al. (11), healthy guinea pigs were injected intraperitoneally with mosquito homogenate and monitored for symptoms of disease, although this was not an experiment to test whether or not the animals would become infected. At that time, it was already generally presumed that the animals could be infected in this way, and lacking the more specific molecular tests used nowadays, this was simply the principal method to confirm that mosquitoes used in experiments were in fact carrying B. tularense. Indeed, when Aedes aegypti, which had previously been allowed to feed on B. tularense-infected guinea pigs, was injected into healthy guinea pigs these animals became ill and showed tularaemia-specific symptoms. In the later experiments of Bäckman et al. (2015) (23), mice were injected with F. tularensis-positive mosquito homogenates, that is, from adult Ae. aegypti exposed to F. tularensis ssp. holarctica during larval stage. Of the eight mice used in the experiment, three showed symptoms of tularaemia within 5 days; real-time PCR for the lpnA gene on the spleen homogenate of these three animals was found to be positive.
In one of the experiments by Philip et al. (1932) (11), adult Ae. aegypti were allowed to feed on B. tularense-infected guinea pigs. Mosquito eggs, which were laid 5–12 days after the blood-feeding, were collected and injected into five healthy “test” guinea pigs. None of those “test” guinea pigs became infected, although it was shown that these mosquitoes did carry viable bacteria (by injection of homogenates into healthy guinea pigs, as mentioned above).
In 2008, Read et al. (24) published about experiments where Anopheles gambiae-derived haemocyte-like cells were incubated with F. tularensis ssp. novicida. It was shown that the bacteria could enter and infect these cells. Moreover, the longer the incubation of cells with F. tularensis ssp. novicida lasted, the more colony-forming units were found after incubation of lysed cells on a plate.
2.4.1.2 Adult mosquitoes fed on Francisella tularensis-infected blood (zoonotic transmission)
Triebenbach et al. (2010) (25) allowed 167 naive individual adults of either An. gambiae or Ae. aegypti to take an artificial blood meal from a vial that contained F. tularensis ssp. novicida. At 0 hours, 2 hours, 48 hours, and 72 hours after blood-feeding, subsets of mosquitoes were harvested for PCR analysis. At every time point, most individuals (i.e., ≥ 85%) tested positive for the F. tularensis-specific fopA gene. However, the amount of bacterial DNA found in the mosquitoes decreased with time after the contaminated blood meal. Thelaus et al. (2014) (26) performed a similar experiment with naive adult Ae. aegypti, but used vials of blood containing F. tularensis ssp. holarctica. In this study, five out of nine batches (with 1–8 mosquitoes each) tested positive for the F. tularensis lpnA gene with real-time PCR within 48 hours of the blood meal. Data on the amount of DNA measured were not provided.
Philip et al. (1932) (11) allowed naive mosquitoes of a laboratory strain of Ae. aegypti, as well as adults of other mosquito species that had been captured as larvae and pupae in the field (i.e., in Montana, USA), to feed on guinea pigs that carried B. tularense. To check if the mosquitoes had taken up the bacteria, in total 98 mosquito homogenates were made at different time intervals from 1 day to 35 days after the infected blood meal and injected into healthy guinea pigs. These animals were then monitored for symptoms of the disease. Tularaemia was observed in 28 of 81 guinea pigs. This demonstrated that some individuals of the Ae. aegypti laboratory strain and of various locally captured mosquito species (mainly Aedes species and Theobaldia incidens—now known as Culiseta incidens) had taken up B. tularense and carried viable bacteria for at least several days. T. incidens even appeared to have carried viable bacteria up to 35 days. About 80 years later, Thelaus et al. (2014) (26) allowed 33 naive adult Ae. aegypti to take a blood meal from mice that had been infected with F. tularensis ssp. holarctica 3 days earlier. PCR analysis of these mosquitoes 2 weeks after this blood-feeding showed that 7 out of 12 (58%) observed blood meals resulted in transfer of bacteria to these mosquitoes.
In another experiment presented by Philip et al. (1932) (11), adult mosquitoes of both field-collected Aedes species and laboratory-reared Ae. aegypti were allowed to feed on guinea pigs that had been infected with B. tularense in the laboratory. At various time points between 4 days and 15 days after the infective meal, the mosquitoes were allowed to take a second blood meal, this time on a healthy test guinea pig. The mosquitoes were then homogenised, after which the suspension was injected into other healthy control guinea pigs. All test and control guinea pigs were monitored for symptoms of disease. None of the five test guinea pigs that served as host for the second blood meal developed symptoms of disease, whereas 7 of 10 control animals (70%) that had been injected with the mosquito homogenate died with tularaemia. Triebenbach et al. (2010) (25) allowed a total of 33 adult An. gambiae and 14 adult Ae. aegypti to feed on healthy anaesthetised mice, 3 days after a F. tularensis ssp. novicida-contaminated artificial blood meal. The six mice were monitored for 7 days but none of them developed symptoms of disease. After euthanasia their blood and spleens appeared to be all negative for the F. tularensis fopA gene on PCR analysis. In a subsequent part of the experiment of Thelaus et al. (2014) (26), described above, the 33 adult Ae. aegypti that had been allowed to feed on F. tularensis ssp. holarctica-infected mice were all allowed to feed on five naive mice, 4 days after the first (infective) blood meal. Those new mice were monitored for signs of disease but none of them became ill or tested positive for F. tularensis after euthanasia, although four of the seven mosquitoes that had fed on them were later found to be positive for the lpnA gene by PCR analysis.
Philip et al. (1932) (11) also conducted experiments to test for mechanical transmission of the B. tularense by Ae. aegypti. First, naive mosquitoes of various Aedes species were interrupted during their blood meal on an infected guinea pig and then transferred to cages with healthy but immobilised test guinea pigs and allowed to resume feeding on those test animals. All second blood meals started within 15 minutes after interruption of the first blood meal. After completion of the second blood meal, the mosquitoes were homogenised and injected in healthy control guinea pigs. All guinea pigs were monitored. In the reported experiments, 29 of 31 control animals (94%) that were injected with mosquito homogenate died of tularaemia, indicating that almost all mosquitoes had taken up F. tularensis with the first blood meal. In contrast, only one of the eight test guinea pigs (12.5%) on which mosquitoes were allowed to resume feeding died with tularaemia. On this particular animal, eight Ae. aegypti individuals had completed their interrupted blood meal.
In another experiment described by Philip et al. (1932) (11), adult Ae. aegypti that had fed on infected guinea pigs were sedated and crushed by slapping them on a carefully shaved area of the skin of healthy test guinea pigs. In some cases, the mosquitoes were also rubbed on the clipped but intact skin. Of the 16 test guinea pigs that had been exposed via crushing of mosquitoes with or without subsequent rubbing on the skin, two animals (12.5%) became infected and died of tularaemia: on one animal the mosquito had only been crushed by slapping, and on the other animal the mosquito had also been rubbed on the skin. Philip et al. (1932) (11) also collected fresh excrement droplets from the walls of the containers in which mosquitoes were held for 24 hours up to 9 days after blood-feeding on infected guinea pigs. After the excrement was rubbed on the scraped skin of healthy test-guinea pigs, 3 out of 16 test animals (19%) became infected.
2.4.1.3 Mosquito larvae bred in Francisella tularensis-contaminated water (water-borne transmission)
Interestingly, F. tularensis could also be detected in various life stages of mosquitoes when the larvae were bred in water contaminated with the bacterium.
The 2010 publication of Triebenbach et al. (25) described the exposure of laboratory-reared larvae of An. gambiae and Ae. aegypti to a fluorescent laboratory strain of F. tularensis ssp. novicida, with the subsequent detection of fluorescence in the larvae of both mosquito species. Moreover, qPCR of the exposed larvae showed that about half of the samples were positive for the F. tularensis-specific fopA gene. However, pupae and adults, which had been exposed during their larval stage, did not contain significant amounts of F. tularensis DNA.
Thelaus et al. (2014) (26) exposed Ae. aegypti larvae to F. tularensis ssp. holarctica in three independent experiments using 59–68 individuals. At three stages in development, individuals were harvested for analysis. With real-time PCR analysis, the F. tularensis-specific lpnA gene could be detected in all stages. The percentage of positive individuals was highest for larvae (69% ± 27%, fourth-instar larvae harvested 5 days after exposure) and lowest for adult mosquitoes (25% ± 5%, harvested 14–16 days after exposure). Haemolymph, collected from pupae and adults, was positive for the lpnA gene in 29% ± 4% and 19% ± 2% of the samples, respectively. A similar result of the same research group was published by Bäckman et al. (2015) (23). They reported that 33 of 140 (± 24%) homogenates of adult Ae. aegypti that were exposed to F. tularensis ssp. holarctica during the larval stage were positive for the lpnA gene using PCR analysis. However, after incubation of homogenates on agar plates, no bacterial growth was detected.
Thelaus et al. (26) also exposed Ae. aegypti larvae to fluorescent F. tularensis ssp. holarctica and allowed them after reaching adulthood to feed on vials with artificial blood meals. Real-time PCR analysis of the blood in the vials was positive for the lpnA gene in 20% ± 4% of the vials, and contamination with the bacteria was confirmed with fluorescence microscopy. However, incubation of blood on plates did not result in any colonies. Finally, Thelaus et al. (2014) (26) also allowed 184 mosquitoes that had been exposed to F. tularensis during their larval stage to feed upon healthy anesthetised mice. In total, 58 blood meals were observed. With PCR analysis, both mouse DNA and the F. tularensis-specific lpnA gene were detected in 11 of the mosquitoes, but none of the 14 mice developed symptoms of tularaemia within the following 25 days, nor were any traces of F. tularensis DNA found within these mice.
2.4.2 Criterion 2: isolation from wild mosquitoes
In several studies, adult mosquitoes or earlier life stages were collected in tularaemia-endemic regions and tested for the presence of F. tularensis (DNA). In addition, water collected from the same area was also tested in some studies.
2.4.2.1 Detection of Francisella tularensis in mosquitoes reared from field collected larvae
Lundström et al. (2011) (27) collected larvae of Aedes, Culiseta, and Culex species from water reservoirs in a tularaemia-endemic region in Sweden. After rearing in the laboratory, over 300 adults were obtained. These were divided over 48 pools based on place of collection, species, and sex. Analysis with real-time PCR showed that the F. tularensis-specific lpnA gene could be detected in 14 of these 48 pools (29%). From 11 positive pools, enough DNA could be isolated for sequencing, yielding a high sequence similarity with the F. tularensis genome. DNA from four pools contained a 30-bp deletion specific to F. tularensis ssp. holarctica, that is pools of Aedes punctor, Aedes vexans, and Aedes sticticus, and a mixed pool with both Culex pipiens and Culex torrentium. Water from reservoirs in five of the eight containers in which the larvae were collected also tested positive for the F. tularensis lpnA gene, and from four of those, positive mosquitoes had been collected. The water from the other three containers was negative for the gene, although F. tularensis DNA was detected in mosquito pools from these containers.
2.4.2.2 Detection of Francisella tularensis in field-collected adult mosquitoes
Olin (1942) (28) collected, in a tularaemia-endemic region in Sweden, approximately 50 mosquitoes that were all similar in appearance and characterised as Aedes cinereus. Mosquito homogenate was injected subcutaneously into two healthy guinea pigs, as this was the principal method to test whether or not mosquitoes were carrying the bacterium. Both guinea pigs became ill: one died after 7 days and the other was euthanised. At autopsy, both animals showed pathological changes typical of tularaemia, and bacteria with identical phenotype as those isolated from human tularaemia patients could be isolated from their blood. Hubálek and Halouzka (1997) (29) collected 9,167 mosquitoes in a tularaemia-endemic region in the Czechia. Over 85% of the individuals in a random subsample appeared to be Aedes spp., i.e., Aedes cantans (45%), Ae. vexans (30%), Ae. cinereus (5%), and Ae. sticticus (4%). Homogenised pools of 25–100 mosquitoes were injected subcutaneously in healthy mice. None of the mice developed the disease and attempts to find F. tularensis on plates with homogenised spleens of dead animals failed. In contrast, F. tularensis was recovered from some species of ticks collected in the same area. Triebenbach et al. (2010) (25) captured approximately 2,600 adult mosquitoes at five locations in a region of Alaska with low incidence of human tularaemia but where tularaemia is endemic in wildlife populations. Random pools of 10 mosquitoes (mixed species) were tested by real-time PCR for the presence of the F. tularensis-specific fopA gene. Of all pooled samples, 30% were found to be positive. The predominant mosquito species in 40 pools with sufficient mosquito DNA to sequence were Cs. incidens, Culiseta alaskaensis, Culiseta impatiens, Ochlerotatus communis, Ochlerotatus fitchii, Ochlerotatus pionips, Ochlerotatus excrucians, or Ae. vexans. In 2004, Thelaus et al. (2014) (26) collected over 22,500 adult mosquitoes of various species in a tularaemia-endemic region in Sweden, at four locations and on eight occasions from June until September. The mosquitoes were divided into three batches, which were then used for different experiments. In batch 1, about 13,500 adult mosquitoes were divided over 188 tubes, based on location and occasion of collection. Of these tubes with mixed mosquito species, 16 (9%) tested positive via PCR for the F. tularensis-specific lpnA gene. Most of the positive tubes were tubes of mosquitoes collected in one particular area and late in summer. Batch 2 consisted of approximately 9,000 mosquitoes that were morphologically identified on a species level and then sorted based on both sampling location, sampling occasion, and species. A subset of 89 single-species tubes (with, in total, almost 800 mosquitoes) from the sampling area and sampling occasions with the highest frequency of F. tularensis-positive tubes in the experiments with batch 1 was selected for further analysis. Twenty tubes of 11 different mosquito species (Ae. cinereus, Ae. sticticus, Ae. vexans, Ae. cantans, Aedes annulipes, Aedes intrudens, Aedes leucomelas, Anopheles claviger, Anopheles maculipennis, Coquillettidia richiardii, and Cx. pipiens) were found to be positive for the F. tularensis-specific lpnA gene, and, of these, 18 tubes were also found to be positive for a ssp. holarctica-specific deletion. Mosquitoes in batch 3 (number unknown) were homogenised and spread on different selective and non-selective plates and incubated, but this did not result in the growth of F. tularensis colonies. Duzlu et al. (2016) (30) collected over 6,000 adult mosquitoes of several species, mainly Ae. vexans and Cx. pipiens, (in total 95%), and small numbers of Culex hortensis, Culex theileri, Culiseta annulata, and An. maculipennis in a human tularaemia-endemic area in Anatolia, Turkey. Mosquitoes were pooled according to species in 2 × 599 pools each containing either the head and thorax or the abdomen of 1–17 mosquitoes. DNA from none of the pools was found to be positive for the F. tularensis lpnA gene with real-time PCR. In 2019, Dryselius et al. (2019) (31) collected 550 mosquitoes (68% Ae. cinereus and the others Ae. cantans, Ae. annulipes, Aedes communis, Culiseta morsitans, or An. maculipennis) in a tularaemia-endemic region in Sweden during a large outbreak of tularaemia among humans. When divided over 24 pools by species and collection site and analysed with molecular techniques, which were not described further, only one of the eight Ae. cinereus pools was found to be positive for F. tularensis ssp. holarctica.
Carvalho et al. (2012) (32) collected almost 5,000 mosquitoes in Portugal, where human tularaemia is not endemic (42). Almost 70% were captured in the Algarve region in 2007, during an outbreak of tularaemia in humans in the adjacent north-western part of Spain, and the others were captured from 2007 to 2011 elsewhere in Portugal. Collected species were ± 64% Culex (Cx. pipiens, Cx. theileri, Cx. perexiguus), ± 35% Ochlerotatus (O. caspius, O. detritus), and some An. maculipennis, Culiseta longiareolata, and Cs. annulata. A few Ae. aegypti specimens from Madeira were also included in the study. All samples were negative for the Francisella-specific tul4 gene when analysed with nested PCR.
Janse et al. (2017) (33) collected, in total, over 1,000 larvae and adults of unspecified local mosquito species in the Netherlands during the spring and summer of 2015 and 2016, in a region where F. tularensis was found to be the cause of death in some hares in 2015. No human cases were reported in the region during that period and the occurrence of tularaemia in the Netherlands is generally low (43). DNA extraction from the locally captured mosquitoes and subsequent qPCR did not result in the detection of F. tularensis DNA. In contrast, some water samples collected from the same region where the infected hares had been found were positive for the F. tularensis fopA gene. Formińska et al. (2020) (34) collected over 2,000 mosquitoes of unspecified species in residential areas in 2011 and 2012 in central Poland. However, none of the mosquitoes tested positive on analysis by PCR for the F. tularensis-specific tul4 gene. Tularaemia is not very common in Poland, with only six cases in both years, but the number of cases has increased since then (43, 44).
2.4.3 Criterion 3: direct contact
Although the most convincing way to demonstrate direct contact between a mosquito and a host is either by capturing mosquitoes during blood-feeding or by collecting mosquitoes and detecting blood from the host species inside the abdomen of the mosquito, such studies specifically associated with the transmission of F. tularensis were not found in the literature search. The only studies investigating possible contacts between mosquitoes and tularaemia patients were case–control studies on tularaemia outbreaks.
Eliasson et al. (2002) (35) published a case–control study on a tularaemia outbreak with over 460 cases that occurred in the year 2000 in Sweden. A questionnaire asking about symptoms, medication, housing conditions, outdoor activities, and any memories of contact with specific animals was sent to 270 reported patients (86% confirmed to be infected with F. tularensis via serology or culture) and 670 controls matched with the patients based on age, gender, and place of residence. Comparison of responses of 218 cases and 414 controls showed that a mosquito bite was the most important and independent risk factor for getting tularaemia. Two other statistically significant risk factors were owning a cat and doing farm work. Rossow et al. (2014) (36) published a similar case–control study on an outbreak of tularaemia in 2000 in Finland. A survey was sent to all laboratory-confirmed patients who were residents of a tularaemia-endemic region and whose first sample was collected between the 1 July and 6 October, and to four controls per case (matched with the patients based on age, sex, and place of residency) received a survey. The analyses of the 227 cases and 415 controls indicated that several risk factors were independently associated with acquiring tularaemia: farming activities, handling dead animals, and being bitten by mosquitoes. In the group of patients with ulceroglandular tularaemia, 93% reported that they had been bitten by a mosquito in the 2 weeks prior to the onset of the disease, compared to 69% of the control subjects.
Christenson (1984) (37) performed a case study during an epidemic with 529 human cases of tularaemia in central Sweden in the summer of 1981. A questionnaire to identify, among other things, risk factors such as contact with animals and insect bites prior to the disease was completed by 344 patients. The majority of the patients (83%), including all 31 patients who had been hospitalised because of the gravity of the disease, reported being bitten by mosquitoes prior to falling ill; about half (56%) even reported a specific mosquito bite related to their disease. Eliasson and Back (2007) (38) published a case study based on 234 tularaemia cases that had been reported in a specific area in Sweden between 2000 and 2004. Of these patients, 96% had fallen ill between July and September and 89% suffered from the ulceroglandular form of tularaemia (78% with primary lesions on the lower legs). In a follow-up study, which was partly based on the same patient cohort, Svensson et al. (2009) (39) found that nearly half of the 278 laboratory-confirmed tularaemia patients reported an arthropod vector bite as the cause of infection: 36% thought they had been infected through a mosquito bite, whereas 8% reported that it had been a tick, horse fly, or a mosquito bite. Dryselius et al. (2019) (31) investigated an outbreak of tularaemia (the ulceroglandular type in most patients) that occurred from July to early October in 2019 in central Sweden. There were almost 1,000 registered cases, which was four times more than usual during this period in the years 2000–2018. Seventy-three per cent of the patients specified insect bites as cause of infection. The article also reported “patients with inflamed and sometimes infected mosquito bites”. However, more detailed information, a methods section, or a reference to another more detailed publication were not provided, so it is unclear on what data this statement was based.
Hanke et al. (2009) (40) published about a case of ulceroglandular tularaemia in a German toddler. The child had a rash and a lesion on the face, and pus drained from the abscess that had developed around 5 weeks after the first symptoms, and was found to be positive for F. tularensis ssp. holarctica with real-time PCR. Diagnosis was confirmed by detection of anti-F. tularensis-specific antibodies. According to his caregiver, the child had been bitten by a mosquito at the site of the lesion. Other possible routes of infection were ruled out since, according to the caregiver, there had been no contact with pets or wild animals, nor bites from ticks, and the child did not travel in the period prior to the infection.
2.4.4 Criterion 4: sympatric association between mosquitoes and tularaemia patients
No studies were found that specifically examined overlap in space and time between mosquitoes and human tularaemia cases, or, in other words, whether or not human patients could have actually encountered infectious mosquitoes before becoming ill. However, although not primarily aimed at showing a sympatric association, all studies in which wild mosquitoes were collected in tularaemia-endemic regions during outbreaks in humans, as well as the case–control studies, indirectly also give information about such an association in space and time.
In the 1932 study by Philip et al. (11), larvae and pupae of various mosquito species were collected in Bitterroot Valley in Montana, USA. Cases of human tularaemia did occur in this region, but how many and how often were not reported. Olin (1942) (28) trapped the mosquitoes used in his investigation in a human tularaemia-endemic region in Sweden during the epidemic of 1938. Hubálek and Halouzka (1997) (29) collected mosquitoes in the spring and fall of 1995 and the spring of 1996 in a region in the Czechia where human tularaemia is endemic. Triebenbach et al. (2010) (25) captured mosquitoes from May to August 2006 in a region of Alaska where tularaemia is endemic in wildlife populations, but the incidence of human tularaemia is very low. Both Lundström et al. (2011) (27) and Thelaus et al. (2014) (26) collected mosquito larvae in human tularaemia-endemic regions in Sweden between the onset of summer and the beginning of fall (in August 2008 and June to September 2004, respectively), when most human cases was reported. Duzlu et al. (2016) (30) performed experiments on mosquitoes that were trapped during previous studies from 2006 to 2013 in an area of Turkey with human tularaemia cases, but the exact periods of collection (i.e., time in the year) were not mentioned. Dryselius et al. (2019) (31) trapped mosquitoes in a human tularaemia-endemic region in Sweden during a relatively large outbreak of tularaemia among humans during the summer of 2019. Formińska et al. (2020) (34) captured mosquitoes in 2011 and 2012 in central Poland, where the incidence of human tularaemia is low, with only six human patients in both of these years.
Ryden et al. (2011) (41) proposed a model to evaluate the role of mosquitoes in the transmission of tularaemia among humans in a region in central Sweden. Data on the time and place of human infections were combined with mosquito abundance as a function of environmental variables, and a correlation was found between the “predicted mosquito prevalence and the number of human tularaemia cases”. In addition, the occurrence of tularaemia outbreaks in humans could be predicted to some extent based on some environmental variables.
3 Discussion
Mosquitoes are responsible for the transmission of several serious infectious diseases caused by parasites or viruses. The aim of this study was to review the available evidence for the claim that mosquitoes play a role in the transmission of F. tularensis, the causal agent of tularaemia. To this end, primary articles describing research into multiple aspects of this possible transmission of F. tularensis by mosquitoes were analysed and evaluated based on four vector incrimination criteria: vector competence of mosquitoes, isolation of the bacteria from wild mosquitoes, proof of direct contact between the mosquitoes and humans, and proof of an association in space and time between mosquitoes and humans in human tularaemia-endemic regions. Before getting to the conclusions, some critical remarks should be made. These concern the vector incrimination criteria, as well as the research methods, the descriptions, and the conclusions of some of the publications.
In the vector competence studies, several general shortcomings can be distinguished as well as some weaknesses in some studies specifically. First, not all studies investigated the complete sequence of steps from (a) allowing uninfected mosquitoes to feed on infected vertebrate animals, to (b) allowing these mosquitoes to subsequently feed on uninfected animals and monitor if these animals develop disease and eventually to (c) performing experiments to detect the pathogen in the newly infected animals. Only Triebenbach et al. (2010) (25), Thelaus et al. (2014) (26), and Philip et al. (1932) (11) performed experiments that included all these steps. Second, the mosquitoes used in almost all these studies were laboratory strains of Ae. aegypti and/or An. gambiae; species that are not endemic in tularaemia-endemic regions. Only Philip et al. (1932) (11) used mosquitoes of locally captured species for some experiments. Preferably, vector competence studies are performed with local species suspected of being a vector because of differences between mosquito species, although Triebenbach et al. (2010) (25) argued that F. tularensis is unlikely to be adapted to only one certain mosquito species, because the bacterium has been found in over 200 different animal species from various phyla. Third, most experiments in all studies were performed with only a few individuals of both mosquitoes and host animals. Fourth, Triebenbach et al. (2010) (25) and Read et al. (2008) (24) used the non-human infectious F. tularensis ssp. novicida, and it is unclear which subspecies was used by Philip et al. (1932) (11), since in 1932 no subspecies were distinguished. Finally, there are some weaknesses in the different studies specifically: Philip et al. (1932) (11) considered adult mosquitoes that hatched in the laboratory from larvae and pupae collected in the field as uninfected, but they may have already carried F. tularensis; Read et al. (2008) (24) used a cell line instead of actual mosquitoes (although fitting their primary research question); Triebenbach et al. (2010) (25) allowed mosquitoes to take a blood meal on healthy hosts not earlier than 72 hours after the infective blood meal, which was quite a long period; Bäckman et al. (2015) (23) tested infection on mice only via injection of mosquito homogenate and not by blood-feeding by an infected mosquito; and, in the reporting of Thelaus et al. (2014) (26), it is not entirely clear what all the numbers in their discussion are based on. To summarise, the results of the studies described above suggest that the mosquito species used in the experiments can hardly or not at all be regarded as indisputable competent vectors of F. tularensis to the tested vertebrate animals via salivarian transmission in experimental settings. It is true that several studies indicated that various life stages of the tested mosquito species might pick up the bacteria (from water during the larval stage and from contaminated artificial blood meals or from infected rodents during the adult stage) and that sometimes transstadial transmission occurred, but it also appeared that there is no efficient infection nor replication of the bacteria within the larvae, pupae, or adult mosquitoes. Other studies showed that adult mosquitoes carried viable and virulent bacteria after exposure to the bacteria during their larval stage, as injection of the mosquito homogenates resulted in disease in the host animals, but normal blood-feeding by infected mosquitoes almost never resulted in tularaemia in host animals. In contrast, interrupted feeding by, and rubbing of, infected mosquitoes on damaged skin incidentally resulted in infection of host animals, which are tentative indications that there might be some form of mechanical transmission.
Studies on the isolation of F. tularensis from wild mosquitoes also had some shortcomings and weaknesses. First, some descriptions of methods and/or results by Olin (1942) (28), Hubálek and Halouzka (1997) (29), and Dryselius et al. (2019) (31) are quite limited, so it is difficult to rate the true value of their results. Second, Carvalho et al. (2012) (32), Janse et al. (2017) (33), and Formińska et al. (2020) (34) collected mosquitoes in regions with no or only a few human cases of tularaemia, and, as a result, these studies had a low probability of finding infected mosquitoes. Third, most studies did not report attempts to isolate and culture viable F. tularensis bacteria from the mosquitoes, but reported only approaches with molecular techniques to detect F. tularensis DNA in the collected mosquitoes. Finally, a few studies had specific shortcomings: it is not certain which subspecies was isolated by Olin (1942) (28) as no distinction had yet been made between subspecies (although it was probably ssp. holarctica because the mosquitoes were collected in Sweden); Dryselius et al. (2019) (31) reported human patients with infected mosquito bites, but no details were provided as to how that was determined nor a reference to another publication given; and Triebenbach et al. (2010) (25) determined the predominant mosquito species in a mixed species pools positive for F. tularensis, but the predominant species was not necessarily the one that caused the mixture to test positive for F. tularensis. To summarise, F. tularensis could not be cultured from wild-caught mosquitoes. F. tularensis DNA could only be detected in some mosquitoes (including species that are known to feed on humans) collected in tularaemia-endemic regions, and only in small percentages of the samples. F. tularensis DNA was not detected in mosquitoes from regions where tularaemia is not endemic. In some cases, water from the aquatic environment in the area where mosquito larvae or adults were captured was found to be positive for F. tularensis DNA, even when all mosquitoes tested negative, or vice versa.
Case–control studies, which suggested direct contact between mosquitoes possibly carrying F. tularensis and humans, also had shortcomings and weaknesses. In all these studies, patients were interviewed or had to fill in a questionnaire. In studies where results rely on the memory of participants, recall bias is an issue, unless correctly addressed. The time interval between disease and being interviewed or receiving a questionnaire was extremely long for some patients in some studies: up to 5 months in the study by Rossow et al. (2014) (36) and even up to 8 years in the study by Svensson et al. (2009) (39). In addition, most case–control studies [Christenson (1984) (37); Eliasson et al. (2002) (35); Eliasson & Back (2007) (38); Svensson et al. (2009) (39); Rossow et al. (2014) (36); and Dryselius et al. (2019) (31)] were probably subject to some extra bias because of the common belief that mosquitoes are the main culprit. The studies were performed in Sweden or Finland, where not only the density of mosquitoes is very high, but where it is also widely believed by the general public, as well as by many doctors, that mosquitoes can transmit tularaemia. Other shortcomings are the limited descriptions of methods by Svensson et al. (2009) (39) and Dryselius et al. (2019) (31), as well as the lack of controls in their studies and the studies from Christenson (1984) (37) and Eliasson and Back (2007) (38), as they all only conducted interviews or questionnaires with patients. Finally, the study by Hanke et al. (2009) (40) was about only one tularaemia case.
The weaknesses and shortcomings of the case–control studies and studies in which wild mosquitoes were collected, which are described above, also apply to their status as indicators of a sympatric association between mosquitoes and human tularaemia cases.
In addition to the comments on specific studies, there are some general issues that apply to almost all the studies. It is noteworthy that none of the studies mentioned one specific mosquito species that was suspected to spread F. tularensis: the studies report either about mosquitoes in general or about all local mosquitoes. Moreover, some articles end with a very firm conclusion that mosquitoes are responsible for transmission of F. tularensis, based on weak evidence.
4 Conclusion
The review of the experimental studies testing for aspects of vector competence did not provide convincing evidence that the tested mosquitoes are competent vectors for biological, salivarian transmission of F. tularensis to the vertebrate animals in experimental settings. Although various life stages of the tested mosquito species picked up the bacterium and indications of transstadial transmission were found in some studies, it also appeared that there was no replication of the bacteria within larvae, pupae, or adult mosquitoes. Moreover, while some studies showed disease in host animals after injection of infected-mosquito homogenate, transfer of bacteria from infected mosquitoes to blood vials and vice versa, and uptake of the bacteria by mosquitoes through blood-feeding on infected rodents, other studies showed that blood-feeding by infected mosquitoes on healthy host animals did not result in infection of the host. In contrast, rubbing of infected mosquitoes on damaged skin and blood-feeding by a mosquito that was interrupted during a blood meal taken from an infected animal moments before, incidentally resulted in infection of host animals. These findings are tentative indications that there might be some form of mechanical transmission.
From the studies that aimed to detect F. tularensis in wild mosquitoes, the picture emerges that the presence of the bacterium in wild mosquitoes is limited. Although F. tularensis DNA was detected in various species of mosquitoes that are known to feed on humans, this was quite rare and if it was detected, the percentage of positive samples was relatively low. Furthermore, although F. tularensis was not found in mosquitoes from regions where tularaemia is not endemic, it was also the case that all mosquito samples from some tularaemia-endemic areas tested negative. An attempt to cultivate F. tularensis bacteria from mosquitoes captured in the field on plate failed. In a few studies, both mosquitoes and water samples from the environment where mosquito larvae or adults were captured were positive for F. tularensis DNA. In other cases, the water was positive for F. tularensis DNA while all mosquitoes tested negative, or vice versa.
Case–control studies, together with studies in which wild mosquitoes were collected, demonstrated a certain level of sympatric association between the occurrence of mosquitoes and human tularaemia. Multiple case–control studies also suggested direct contact between mosquitoes and humans, and even a correlation between mosquito bites and human tularaemia.
Altogether, the studies suggest that mosquitoes might play a role in water-borne and/or zoonotic transmission of F. tularensis, but conclusive evidence for either is missing. This is an issue to be resolved before accepting tularaemia as (one of) the first mosquito-borne bacterial diseases.
5 Suggestions for future research
For conclusive and convincing evidence about vector competence, studies using larger numbers of mosquitoes and host animals are needed, as well as studies with mosquito species that live in the wild in regions where human tularaemia is endemic or species that have tested positive for F. tularensis DNA. Highly desirable are studies tracing the complete cycle, from uptake of bacteria from an infected animal or contaminated environment, via proof of survival, moving to salivary glands and replication within the mosquito, to transfer to healthy hosts during blood-feeding, and detection of viable bacteria in the latter. Future studies on isolation of F. tularensis from wild mosquitoes could try to show in which part of the mosquitoes the bacteria can be found, and might also consider testing for the presence of human blood inside the mosquitoes, as this would also confirm both direct contact and a sympatric association. Another useful direction of future research would be investigating other explanations for the correlation between mosquito bites and (ulceroglandular) tularaemia that was found in the case–control studies. One possible explanation could be that infection with the bacteria occurs through contact with contaminated water or other sources via minor lesions in the skin caused by mosquito bites (and possibly scratching). Another possible explanation could be that a form of mechanical transmission occurs, for example by crushing a contaminated mosquito and rubbing the remains in the lesion of the bite or a scratching wound. Finally, it might also be that transmission via mosquitoes is very unlikely but somehow possible, and that in areas where mosquitoes are very abundant it simply it occurs every now and then because mosquito bites are so common.
Author contributions
On request of MB, LJ did the literature review. LJ drafted the initial manuscript, after discussing perspectives with EF and MB. All authors reviewed drafts and approved the final version for submission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO (World Health Organization). Vector-borne diseases. Geneva: WHO (World Health Organization) (2020). Available at: https://www.who.int/en/news-room/fact-sheets/detail/vector-borne-diseases.
2. Braks M, Giglio G, Tomassone L, Sprong H, Leslie TE. Making vector-borne disease surveillance work: New opportunities from the SDG perspectives. Front Vet Sci (2019) 6(232):1–9. doi: 10.3389/fvets.2019.00232
3. WHO (World Health Organization). World Malaria Report 2019. Geneva: WHO (World Health Organization) (2019).
4. WHO (World Health Organization). Dengue and severe dengue. Geneva: WHO (World Health Organization) (2020). Available at: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
5. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature (2013) 496(7446):504–7. doi: 10.1038/nature12060
6. Godfray HCJ. Mosquito ecology and control of malaria. J Anim Ecol (2013) 82(1):15–25. doi: 10.1111/1365-2656.12003
7. Weaver SC, Barrett ADT. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol (2004) 2(10):789–801. doi: 10.1038/nrmicro1006
8. Santiago-Alarcon D, Palinauskas V, Schaefer HM. Diptera vectors of avian Haemosporidian parasites: Untangling parasite life cycles and their taxonomy. Biol Rev (2012) 87(4):928–64. doi: 10.1111/j.1469-185X.2012.00234.x
9. Maggi RG, Krämer F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasites Vectors (2019) 12(1):1–37. doi: 10.1186/s13071-019-3407-x
10. Laroche M, Raoult D, Parola P. Insects and the transmission of bacterial agents. Microbiol Spectr (2018) 6(5):1–6. doi: 10.1128/microbiolspec.MTBP-0017-2016
11. Philip CB, Davis GE, Parker RR. Experimental transmission of tularemia by mosquitoes. Public Health Rep (1932) 47(43):2077–88. doi: 10.2307/4580588
12. WHO (World Health Organization). WHO Guidelines on Tularemia. Tärnvik A, editor. Geneva: World Health Organization (WHO) (2007) p. 1–125.
13. Rijksinstituut voor Volksgezondheid en Milieu (RIVM). LCI Richtlijn Tularemie. Bilthoven: RIVM (2017) p. 1–15.
14. Tularemia MM. Hunter’s Tropical Medicine and Emerging Infectious Diseases. 10th. Ryan ET, Hill DR, Solomon T, Aronson NE, Endy TP, editors. Berkely: Elsevier Inc. p. 631–5.
15. Hennebique A, Boisset S, Maurin M. Tularemia as a waterborne disease: a review. Emerg Microbes Infect (2019) 8(1):1027–42. doi: 10.1080/22221751.2019.1638734
16. Petersen JM, Mead PS, Schriefer ME. Review article Francisella tularensis: an arthropod-borne pathogen. Vet Rev (2009) 40(7):1–9. doi: 10.1051/vetres:2008045
17. Telford SR, Goethert HK. Ecology of Francisella tularensis. Annu Rev Entomol (2020) 65:351–72. doi: 10.1146/annurev-ento-011019-025134
18. Clements AN. Host/parasite interactions. In: Cutts R, Head T, Chippendale F, editors. The biology of mosquitoes, Volume 3, Transmission of viruses and interactions with bacteria. Wallingford, England: Cabi publishing (2012). p. 1–8.
19. Eldridge BF. The epidemiology of arthropodborne diseases. In: Eldridge BF, Edman JD, editors. Medical entomology, vol. p . Dordrecht: Kluwer Academic Publishers (2004). p. 165–86.
20. Goddard J. Dynamics of arthropod-borne diseases. In: St. Georgiev V, editor. Infectious diseases and arthropods, 3rd. Cham, Switzerland: Springer International Publishing (2018). p. 27–36.
21. Agarwal A, Parida M, Dash PK. Impact of transmission cycles and vector competence on global expansion and emergence of arboviruses. Rev Med Virol (2017) 27(5):1–12. doi: 10.1002/rmv.1941
22. Beier JC. Vector incrimination and entomological inoculation rates. In: Doolan DL, editor. Malaria methods and protocols. New Jersey: Humana Press (2002). p. 3.
23. Bäckman S, Näslund J, Forsman M, Thelaus J. Transmission of tularemia from a water source by transstadial maintenance in a mosquito vector. Sci Rep (2015) 5:1–4. doi: 10.1038/srep07793
24. Read A, Vogl SJ, Hueffer K, Gallagher LA, Happ GM. Francisella genes required for replication in mosquito cells. J Med Entomol (2008) 45(6):1108–16. doi: 10.1093/jmedent/45.6.1108
25. Triebenbach AN, Vogl SJ, Lotspeich-Cole L, Sikes DS, Happ GM, Hueffer K. Detection of Francisella tularensis in alaskan mosquitoes (Diptera: Culicidae) and assessment of a laboratory model for transmission. J Med Entomol (2010) 47(4):639–48. doi: 10.1093/jmedent/47.4.639
26. Thelaus J, Andersson A, BrOman T, Bäckman S, Granberg M, Karlsson L, et al. Francisella tularensis Subspecies holarctica Occurs in Swedish Mosquitoes, Persists Through the Developmental Stages of Laboratory-Infected Mosquitoes and Is Transmissible During Blood Feeding. Microb Ecol (2014) 67(1):96–107. doi: 10.1007/s00248-013-0285-1
27. Lundström JO, Andersson AC, Bäckman S, Schäfer ML, Forsman M, Thelaus J. Transstadial transmission of Francisella tularensis holarctica in mosquitoes, Sweden. Emerg Infect Dis (2011) 17(5):794–9. doi: 10.3201/eid1705.100426
28. Olin G. The occurrence and mode of transmission of tularemia in Sweden. Acta Pathol Microbiol Scand (1942) 19:220–47. doi: 10.1111/j.1699-0463.1942.tb03345.x
29. Hubálek Z, Halouzka J. Mosquitoes (Diptera: Culicidae), in contrast to ticks (Acari: Ixodidae), do not carry Francisella tularensis in a natural focus of tularemia in the Czech Republic. J Med Entomol (1997) 34(6):660–3. doi: 10.1093/jmedent/34.6.660
30. Duzlu O, Yildirim A, Inci A, Gumussoy KS, Ciloglu A, Onder Z. Molecular investigation of francisella-like endosymbiont in ticks and Francisella tularensis in ixodid ticks and mosquitoes in Turkey. Vector-Borne Zoonotic Dis (2016) 16(1):26–32. doi: 10.1089/vbz.2015.1818
31. Dryselius R, Hjertqvist M, Mäkitalo S, Lindblom A, Lilja T, Eklöf D, et al. Large outbreak of tularemia, central Sweden, July to September 2019. Euro Surveill (2019) 24(42):1–5. doi: 10.2807/1560-7917.ES.2019.24.42.1900603
32. Carvalho CL, Zé-zé L, Leclerc Duarte E, Núncio MS. Screening of mosquitoes as vectors of Francisella tularensis in Portugal. 7th Int Conf Tularémia (2012) 1(4):185. Available at: https://www.rdpc.uevora.pt/bitstream/10174/8098/1/Poster_7th_Conf_Tul.pdf.
33. Janse I, Maas M, Rijks JM, Koene M, van der Plaats RQ, Engelsma M, et al. Environmental surveillance during an outbreak of tularemia in hares, the Netherlands, 2015. Euro Surveill (2017) 22(35):1–9. doi: 10.2807/1560-7917.ES.2017.22.35.30607
34. Formińska K, Wołkowicz T, Brodzik K, Stefanoff P, Gołąb E, Masny A, et al. Genetic diversity of Francisella tularensis in Poland with comments on MLVA genotyping and a proposition of a novel rapid v4-genotyping. Ticks Tick Borne Dis (2020) 11(2):1–6. doi: 10.1016/j.ttbdis.2019.101322
35. Eliasson H, Lindbäck J, Pekka Nuorti J, Arneborn M, Giesecke J, Tegnell A. The 2000 tularemia outbreak: A case-control study of risk factors in disease-endemic and emergent areas, Sweden. Emerg Infect Dis (2002) 8(9):956–60. doi: 10.3201/eid0809.020051
36. Rossow H, Ollgren J, Klemets P, Pietarinen I, Saikku J, Pekkanen E, et al. Risk factors for pneumonic and ulceroglandular tularemia in Finland: A population-based case-control study. Epidemiol Infect (2014) 142(10):2207–16. doi: 10.1017/S0950268813002999
37. Christenson B. An outbreak of tularemia in the Northern Part of Central Sweden. Scand J Infect Dis (1984) 16(3):285–90. doi: 10.3109/00365548409070402
38. Eliasson H, Bäck E. Tularemia in an emergent area in Sweden: An analysis of 234 cases in five years. Scand J Infect Dis (2007) 39(10):880–9. doi: 10.1080/00365540701402970
39. Svensson K, Bäck E, Eliasson H, Berglund L, Granberg M, Karlsson L, et al. Landscape epidemiology of tularemia outbreaks in Sweden. Emerg Infect Dis (2009) 15(12):1937–47. doi: 10.3201/eid1512.090487
40. Hanke CA, Otten JE, Berner R, Serr A, Splettstoesser W, Von Schnakenburg C. Ulceroglandular tularemia in a toddler in Germany after a mosquito bite. Eur J Pediatr (2009) 168(8):937–40. doi: 10.1007/s00431-008-0862-3
41. Rydén P, Björk R, Schäfer ML, Lundström JO, Petersén B, Lindblom A, et al. Outbreaks of tularemia in a boreal forest region depends on mosquito prevalence. J Infect Dis (2012) 205(2):297–304. doi: 10.1093/infdis/jir732
42. de Carvalho IL, Nascimento P, Núncio MS, Rico MT. First case of tularemia reported in Portugal: Probably of imported origin. Front Public Heal (2018) 6(NOV):10–3. doi: 10.3389/fpubh.2018.00325
43. European Centre for Disease Prevention and Control. Tularemia. ECDC. Annual epidemiological report for 2019. Stockholm: European Centre for Disease Prevention and Control (ECDC) (2021).
Keywords: mosquito, Francisella tularensis, transmission, vector-borne disease, bacteria
Citation: Jonckers Nieboer LFW, Fischer EAJ and Braks MAH (2023) Available evidence for mosquito-borne Francisella tularensis transmission is inconclusive. Front. Trop. Dis 4:1230903. doi: 10.3389/fitd.2023.1230903
Received: 29 May 2023; Accepted: 12 July 2023;
Published: 08 August 2023.
Edited by:
Nathan Daniel Burkett-Cadena, University of Florida, United StatesCopyright © 2023 Jonckers Nieboer, Fischer and Braks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. A. H. Braks, bWFyaWV0YS5icmFrc0ByaXZtLm5s
 L. F. W. Jonckers Nieboer1
L. F. W. Jonckers Nieboer1 E. A. J. Fischer
E. A. J. Fischer M. A. H. Braks
M. A. H. Braks