- 1Insect Pest Control Laboratory, Joint Food and Agriculture Organization (FAO)/International Atomic Energy Agency (IAEA) Centre of Nuclear Techniques in Food and Agriculture, Vienna, Austria
- 2Chinese Atomic Energy Agency Center of Excellence on Nuclear Technology Applications for Insect Control, Key Laboratory of Tropical Disease Control of the Ministry of Education, Sun Yat-sen University, Guangzhou, China
- 3SYSU Nuclear and Insect Biotechnology Co., Ltd., Dongguan, China
- 4International Atomic Energy Agency Collaborating Centre, Sun Yat-sen University, Guangzhou, China
- 5Guangdong Provincial Engineering Technology Research Center for Diseases-Vectors Control, Sun Yat-sen University, Guangzhou, China
Introduction: what does ionizing radiation have to do with insect pest control?
There are thousands of insect pest species on this planet that damage crops and contribute to severe food insecurity and hunger in this world (1). Other insect pests directly affect the health of livestock and humans, by transmitting parasites, bacteria, and viruses, and indirectly through the indiscriminate use of insecticides to manage these pests. These often very harmful chemicals leave residues in air, food, and water, causing significant health problems in humans, killing non-target and beneficial insects, and accelerate the development of insecticide resistance in the target insects and lead to outbreaks of secondary insects pests (2, 3). It is estimated that 3.5 million tons of pesticides were used worldwide in 2020, at a cost of more than 60 billion Euros, and this highlights the need for insect pest control strategies that are more friendly to the environment and hence, more sustainable (4).
In response to the global threat of insect pests and the harmful effects of insecticide use, the sterile insect technique (SIT) was conceptualized as early as the 1930’s. It is a pest control tactic that is attracting more attention in all regions of the world, be it for implementation at small or large scale. The SIT is an autocidal control tactic that requires the mass-rearing of the target pest, their reproductive sterilization using ionizing radiation and sequential release into the target area for the reduction of the population with each generation (5). Of crucial importance is the reproductive sterilization of the male insects as these must retain the ability to seek out and mate with wild females after being released, thereby inhibiting the development of viable offspring. Both high-energy particle and photon beams can be used to sterilize insects. Although particle beams (electrons, protons and neutrons) have been tested, photons (cobalt-60 and less often cesium-137) are more commonly used for insect sterilization. However, X-ray irradiators have become more popular in the last decade, especially for use in smaller, or start-up SIT projects. The reasons are obvious, i.e., irradiation capacity can be established at a lower capital cost, simplified procurement procedures and the absence of safety regulations in the importing country. On the other hand, there is little information available on the long-term reliability (in terms of durability) of X-ray irradiators, an important prerequisite for SIT programs. In addition, concern has been expressed on the relative biological effectiveness (RBE) of X-rays as compared with γ rays to obtain the desired sterility in the irradiated insects, as well as the insect volumes that can be processed, especially for medium sized and larger programs.
A literature search revealed that very few scientific publications are available that describe X irradiation of insects and compare it with γ irradiation. Most of these are very old and precise handling and irradiation protocols are poorly described, if at all. Most of these reports tend in general to prefer X irradiation above γ irradiation in terms of RBE, as in most cases, a lower dose was needed with X-rays as compared with γ rays to achieve the same target sterility. But does this also imply that more somatic damage is induced in the insect overall? During recent studies, the importance of a parameter that was overlooked by nearly all older studies has become obvious, i.e., the significance of dose rate.
In this paper, we summarize the available information on X and γ irradiation for insects, with a focus on mosquito vectors, and discuss the advantages and limitations of both types of irradiation and revisit the importance of dose rate.
What are the differences between X-ray irradiators and gamma-irradiators?
Gamma- and X-rays are both high energy photons with ionization properties, but with different origins. Gamma-rays are emitted as a result of nuclear processes within radioactive isotopes and are all similar in energy (or a few energies), whereas X-rays are principally created by the deceleration of high energy electrons when they strike a target (Bremsstrahlung), which creates a full spectrum of photons from a maximum at the energy of the incident electrons down to zero energy, with few of the high energy photons and increasing numbers with decreasing energy. Both conventional orthovoltage X-ray tubes (150 – 320 kV) and electron beam accelerators (3-7.5 MV) with a suitable target produce X-rays. Several self-shielded X irradiators are manufactured these days, that have sufficient power and processing volume to be suitable for insect irradiation (6–8).
In the past, isotopic irradiators were predominantly used for insect irradiation because of their high initial dose rates, unmatched high reliability and, after installation, requiring only a modest electricity supply to operate (some like the Gamma Cell 220, can even be operated manually, in case of a power cut). Whereas panoramic irradiators have a good dose uniformity, small self-shielded gamma irradiators often have high dose variation within the sample chamber. The higher energy photons of gamma irradiators have greater penetration ability than those of low energy X-irradiators, allowing larger loads to be processed. The most significant drawbacks of gamma irradiators, however, are the security and regulatory requirements of importing and housing high activity radioactive sources. Finally, the high and increasing cost of cobalt-60 make them prohibitively expensive for some insect pest control programs and they require reloading every 5-20 years.
Self-contained X irradiators have the big advantage of no security issues and minimal regulatory requirements. Moreover, they have become less expensive than isotopic irradiators. However, the dose rate is much lower (typically between 3 and 15 Gy/min, and the process volume often smaller than many isotopic irradiators (panoramic gamma irradiators have a processing capacity of several billion insects per week, whereas self-contained X-ray irradiators are currently limited to millions of insects per week). They also require a reliable power supply (often 400 V) with attendant continuing electricity costs and they are complex systems, with the inherent reliability issues that go with complexity. A summary of characteristics of various irradiator types can be found in Table 1.
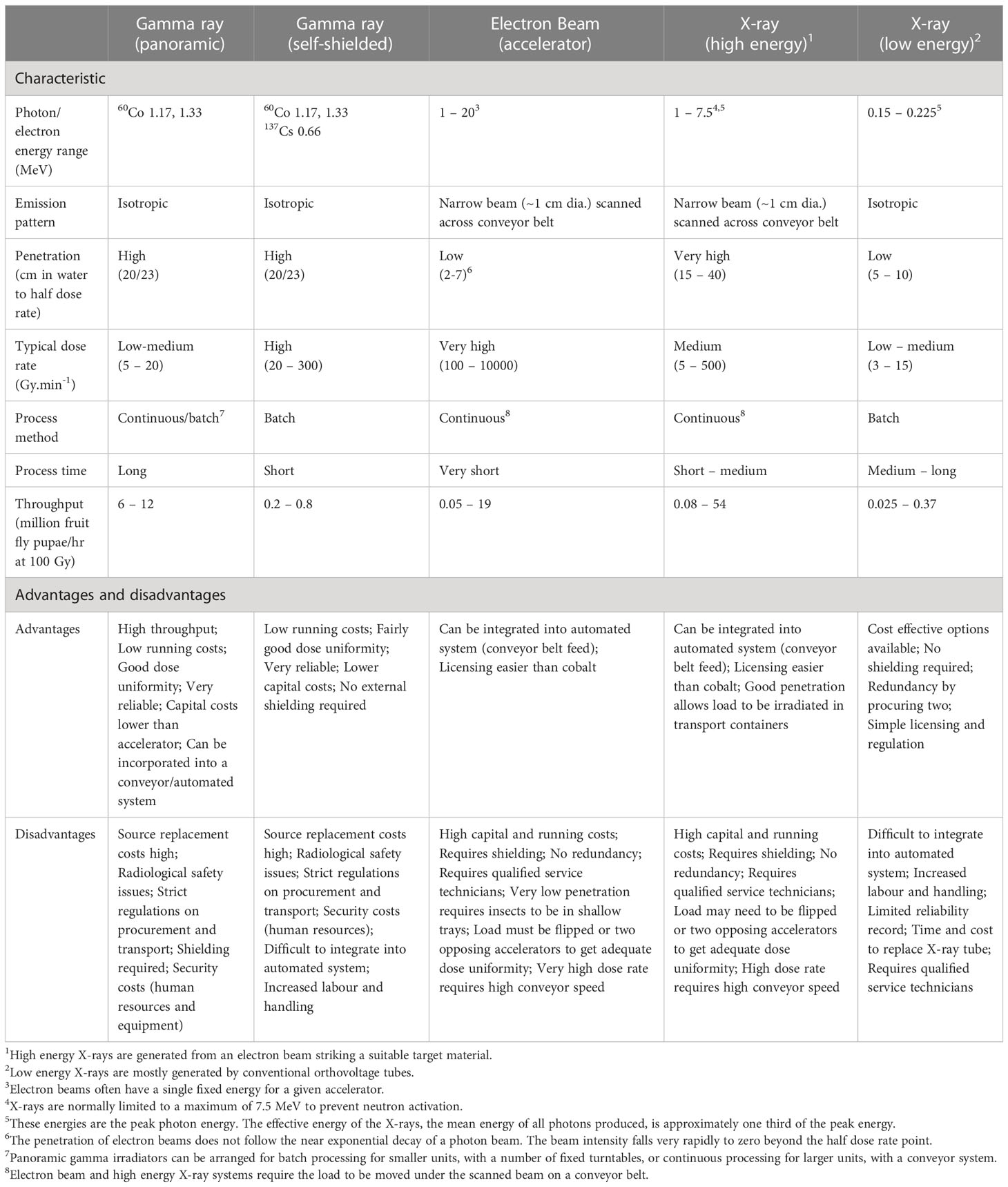
Table 1 Summary of typical characteristics of various irradiator types, their advantages and disadvantages.
What is the significance of dose-rate in inducing sterility?
The discrepancies in dose-response that have been observed in different SIT programs with the same insect species and sometimes even with the same irradiators, compelled the initiation of a recent study to better understand the effects of dose rate in mosquito models. The study revealed an interaction between dose rate and dose (9), but remarkably, the relationship proved to be non-linear, making the explanation of the mechanisms and causation of the biological effects very complex. Although the authors attempted a hypothesis to explain the interaction, the study concluded that dose rate is an important but neglected variable in insect irradiation and needs to be taken into account in SIT programs and reported in relevant publications. The data of the Yamada et al. study (9) showed that at higher radiation doses (in the mosquito models used, the threshold was between 30 and 40 Gy), increasing dose rate resulted in a decrease in induced sterility, i.e., a diminished RBE and thus a shift of the dose-response curve to the right. Contrarily, at lower radiation doses (< 30 Gy), increasing dose rates resulted in increased RBE. The study was carried out with Aedes aegypti and Anopheles arabiensis (Dongola strain), and all insects were irradiated under very stable and consistent conditions in several Gamma Cell 220 irradiators, that had dose rates ranging from 0.4 to 79 Gy/min. What has yet to be studied is the extent of off-target effects (somatic damage) following radiation exposure at varying dose rates. Typical dose rates and energies related to different radiation sources are summarized in Table 1.
A detailed review of the historic reports that dealt with irradiation studies of An. arabiensis and taken into account only those that adequately reported dose rate and dosimetry, two clear scenarios support our findings that dose rate is a driving factor in dose-responses in insect sterilization: Figure 1A shows dose-response curves of An. arabiensis (Dongola) irradiated in the same Gamma Cell 220 over a period of 12 years with dose rates of 16, 93, 84 and 74 Gy/min (low activity at the onset, high activity after reloading, and normal decay thereafter). A dose-response curve following X irradiation of the same strain is plotted for comparison and indicates that lower doses were required to reach the same target sterility. However, the X-ray irradiator dose rate is also the lowest in this comparison (Figure 1A, curve [a]). In Figure 1B, dose-response data for Ae. aegypti males irradiated in various X- and gamma irradiators show a decrease in biological effects with increasing dose rates, irrespective of radiation source, clearly indicating that dose rate is a more important factor than source type. Very few reports are published that compare the RBE of X- and γ-radiation directly in the same species, and in which other factors that affect dose response are controlled and accounted for. Even fewer adequately describe irradiator characteristics, especially the dose rate, and report dosimetry. The results of these studies are therefore ambiguous, confusing and questionable.

Figure 1 (A) Published dose-response curves (induced sterility against log (Dose) for Anopheles arabiensis (Dongola) irradiated with X-rays (Raycell, MK2), compared to γ irradiation with a GC220 with increasing dose rates. [a] (10); [b] (11); [c] (Ntoyi, unpublished data 1 ); [d] (9); [e] (9); (B) Dose-response curves of Aedes aegypti irradiated in various irradiators (X and γ) with increasing dose rates. [f] (10); [g] (Juarez, unpublished data 2 ); [h] (12); [i] (13); [j] (14); [k] (15).
Discussion: X-ray versus gamma-ray: which is preferred?
From a biological point of view, source type does not seem to influence irradiation outcome as much as dose rate and other potential biological and physical factors that play a role in dose-response (9, 16, 17). The debate should, therefore, not focus on which radiation source is better or worse, but rather be centered on the prerequisite to create awareness that dose rate is a very critical factor in dose-responses for insect sterilization, especially in operational action programs. These SIT programs should incorporate in their quality control protocols regular checks on the dose responses of the insects that are destined for release. Overdosing will reduce the quality of the released insects and underdosing will induce a lower sterility in the target female population. The outcome for both scenarios will be reduced efficiency of the program and success doubtful. This is especially crucial for programs that use gamma irradiators in view of the natural decay of the source and resultant change in dose rate.
The selection of X- or gamma ray will most like be dictated by the type or size of the program, i.e., economics rather than biology. For smaller, start-up programs, and where infrastructure and electricity supplies are suitable and reliable, the cheaper X-ray machines are probably the preferred choice. Industrial, panoramic or larger self-shielded gamma irradiators will remain indispensable for large operational SIT programs that require high output, utter reliability and sustainability.
Other options might become available in the future such as electron beams from linear accelerators. These potential competitors that are on the horizon are currently not commonly used in SIT programs. They are large, complex and expensive machines and the only option currently viable is to purchase the service from a commercial supplier. Smaller, compact accelerators with dose rates more suitable for insect irradiation are under development and the first prototypes might become available on the market in a couple of years. They are likely to still be relatively expensive but may be an alternative option for larger programs.
Author contributions
DZ proposed the discussion and topic. HY and DZ developed the discussion and drafted the initial manuscript. AP and MV contributed significantly to the discussion and the development of the paper and final draft. All authors contributed to the article and approved the submitted version.
Funding
DZ is supported by the NSFC-BMGF (82261128006 and 2022YFML1005), the IAEA Department of Technical Cooperation (RAS5095) and the IAEA Coordinated Research Project (D44005).
Conflict of interest
Author DZ was employed by the company SYSU Nuclear and Insect Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Nonhlanhla lindiwe Ntoyi <bGluZGl3ZS5udG95aUBnbWFpbC5jb20=>. The data presented was collected at the IPCL, IAEA.
- ^ Jose Juarez <amp1YXJlenZhbGRlekBnbWFpbC5jb20=>. The data presented was collected at the IPCL, IAEA
References
1. Vreysen MJB, Robinson AS. Ionising radiation and area-wide management of insect pests to promote sustainable agriculture. A review. Agron Sustain Dev (2011) 31:233–50. doi: 10.1051/agro/2010009
2. Pimentel D. Area-wide pest management: Environmental, economic, and food issues. In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-wide control of insect pests: from research to field implementation. Dordrecht, The Netherlands: Springer (2007). p. 35–47.
3. Vreysen MJB, Abd-Alla AMM, Bourtzis K, Bouyer J, Caceres C, de Beer C, et al. The Insect Pest Control Laboratory of the Joint FAO/IAEA Programme: Ten years (2010-2020) of research and development, achievements and challenges in support of the sterile insect technique. Insects (2021) 12:346. doi: 10.3390/insects12040346
4. Sharma A, Kumar V, Shahzad B, Tanveer M, Sidhu GPS, Handa N, et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci (2019) 1:1–16. doi: 10.1007/s42452-019-1485-1
5. Dyck VA, Hendrichs J, Robinson AS eds. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. 2nd ed. Boca Raton, FL: CRC Press (2021). p. xvii+1200. p. doi: 10.1201/9781003035572
6. Mastrangelo T, Parker AG, Jessup A, Pereira R, Orozco-Dávila D, Islam A, et al. A new generation of X ray irradiators for insect sterilization. J Econ Entomol (2010) 103:85–94. doi: 10.1603/EC09139
7. Mehta K, Parker A. Characterization and dosimetry of a practical x-ray alternative to self-shielded gamma irradiators. Radiat Phys Chem (2011) 80:107–13. doi: 10.1016/j.radphyschem.2010.08.011
8. Gómez-Simuta Y, Parker A, Cáceres C, Vreysen MJB, Yamada H. Characterization and dose-mapping of an X-ray blood irradiator to assess application potential for the sterile insect technique (SIT). Appl Radiat Isot (2021) 176:109859. doi: 10.1016/j.apradiso.2021.109859
9. Yamada H, Dias VS, Parker AG, Maiga H, Kraupa C, Vreysen MJB, et al. Radiation dose-rate is a neglected critical parameter in dose–response of insects. Sci Rep (2022) 12:6242. doi: 10.1038/s41598-022-10027-z
10. Yamada H, Kaboré BA, Bimbilé Somda NS, Ntoyi NL, de Beer CJ, Bouyer J, et al. Suitability of Raycell MK2 blood X-ray irradiator for the use in the sterile insect technique: Dose response in fruit flies, tsetse flies and mosquitoes. Insects (2023) 14:92. doi: 10.3390/insects14010092
11. Helinski MEH, Parker AG, Knols BG. Radiation-induced sterility for pupal and adult stages of the malaria mosquito. Anopheles arabiensis. Malar J (2006) 5:41. doi: 10.1186/1475-2875-5-41
12. Bimbilé Somda NS, Yamada H, Kraupa C, Mamai W, Maiga H, Kotla SS, et al. Response of male adult Aedes mosquitoes to gamma radiation in different nitrogen environments. Front Bioeng Biotechnol (2022) 10:942654. doi: 10.3389/fbioe.2022.942654
13. Yamada H, Maiga H, Bimbile-Somda NS, Carvalho DO, Mamai W, Kraupa C, et al. The role of oxygen depletion and subsequent radioprotective effects during irradiation of mosquito pupae in water. Parasit Vectors (2020) 13:198. doi: 10.1186/s13071-020-04069-3
14. Shetty V, Shetty NJ, Ananthanarayana SR, Jha SK, Chaubey RC. Evaluation of gamma radiation-induced DNA damage in Aedes aegypti using the comet assay. Toxicol Ind Health (2017) 33:930–7. doi: 10.1177/0748233717733599
15. Bond JG, Osorio AR, Avila N, Gómez-Simuta Y, Marina CF, Fernández-Salas I, et al. Optimization of irradiation dose to Aedes aegypti and Ae. albopictus in a sterile insect technique program. PLoS One (2019) 14:e0212520. doi: 10.1371/journal.pone.0212520
16. Yamada H, Maiga H, Juarez J, De Oliveira Carvalho D, Mamai W, Ali A, et al. Identification of critical factors that significantly affect the dose-response in mosquitoes irradiated as pupae. Parasit Vectors (2019) 12:435. doi: 10.1186/s13071-019-3698-y
Keywords: sterile insect technique (SIT), dose response, dose rate, gamma irradiator, X-ray irradiator
Citation: Yamada H, Zhang D, Parker AG and Vreysen MJB (2023) Sterilizing insects with X rays or gamma rays - which irradiator to select? Front. Trop. Dis 4:1224386. doi: 10.3389/fitd.2023.1224386
Received: 17 May 2023; Accepted: 12 July 2023;
Published: 28 July 2023.
Edited by:
Riccardo Moretti, Italian National Agency for New Technologies, Energy and Sustainable Economic Development (ENEA), ItalyReviewed by:
S. Noushin Emami, Stockholm University, SwedenCopyright © 2023 Yamada, Zhang, Parker and Vreysen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanano Yamada, aC55YW1hZGFAaWFlYS5vcmc=
†Present address: Andrew G. Parker, Roppersbergweg 15, 2381 Laab im Walde, Austria
‡These authors have contributed equally to this work and share first authorship
 Hanano Yamada
Hanano Yamada Dongjing Zhang
Dongjing Zhang Andrew G. Parker
Andrew G. Parker Marc J. B. Vreysen1
Marc J. B. Vreysen1