- Pediatric department, University Hospital of Liège, Liège, Belgium
Cystic echinococcosis is a zoonosis caused by the larval form of the tapeworm Echinococcus granulosus. It has a worldwide distribution and represents a particularly significant economic and health burden in endemic areas. The most affected organs are the liver and the lungs. Cerebral involvement is relatively rare. This is a case study of a clinical presentation of cerebral cystic echinococcosis in a 5-year-old female patient of Moroccan origin who had developed recurrent seizures. Currently, diagnosis is based on radiological imaging in the context of anamnestic suspicion. Beyond the conclusions that can be drawn from the various case reports, there are no guidelines on management methods or randomized controlled trials that have compared management methods, and their absence can be deleterious for children. We present this clinical case report to add to the existing ones and to assist clinicians in their therapeutic decision-making while they await guidelines.
Introduction
Echinococcosis is a zoonosis caused by the larval forms (metacestodes) of Echinococcus, a flatworm of the family Taeniae.
Among the Echinococcus genus, four species are pathogenic to humans: E. granulosus, E. multilocularis, E. vogeli, and E. oligarthrus; the first two of these are the most common and cause cystic echinococcosis (CE) and alveolar echinococcosis, respectively (1, 2). Most cases of CE present with a hepatic involvement and remain asymptomatic for years.
CE is mainly found in pastoral areas with sheep or other livestock (3). The endemic areas are found in the Mediterranean, North Africa, South America, and Central Asia.
The annual incidence of cystic echinococcosis in Europe varies between 1 and 8 cases per 100,000 inhabitants. In endemic areas, such as Morocco, the average annual prevalence is between 7,24 and 16 cases per 100,000 inhabitants depending on the region (4).
It is currently estimated that over one million people in the world are affected (5). The therapeutic management of echinococcosis and the loss of livestock is estimated to cost approximately 3 billion US dollars annually (5).
We present the case of cerebral CE in a child living in Belgium.
Case description
H.B. was a 5-year-old girl who had developed recurring seizures. During crisis, she presented with a rightward gaze lateralization, excessive chewing, and incoherent speech. Initial brain MRI revealed an upper-left parietal mass suggesting a neuroglial cyst. Treatment with valproic acid was initiated. There was no further occurrence of seizures for 5 months, until valproic acid had to be increased due to recurrence and an increase in the number of seizures. Subsequently, a switch to carbamazepine was made due to further recurrence.
During a viral infection, treated with ibuprofen, H.B. developed Lyell’s syndrome, probably due to antiepileptic and NSAID treatment. The current treatment was abandoned and levetiracetam introduced.
H.B. continued to have an average of one seizure per month for 6 months. In this situation, a brain MRI showed a cystic image with peri-lesional edema in the left parietal lobe, strongly suggestive of an echinococcal cyst (Figure 1).
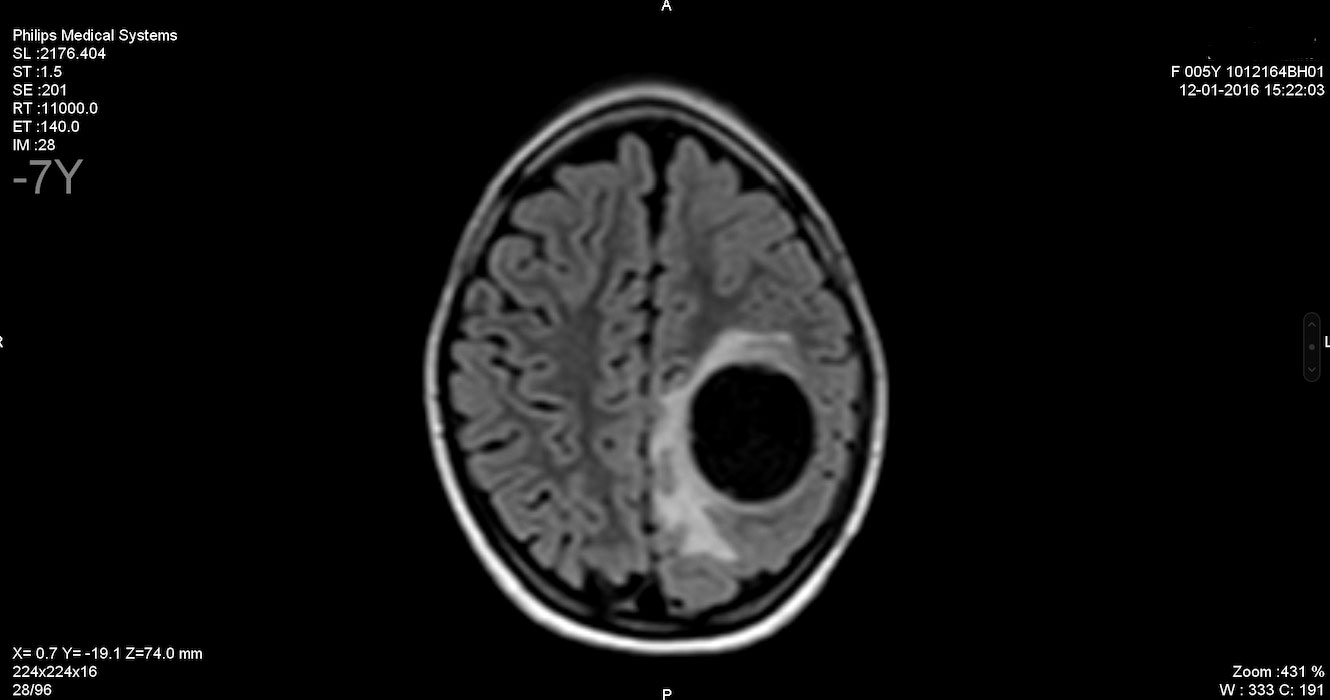
Figure 1 Brain MRI. T2 FLAIR sequence showing a round, unilocular, cystic image with peri-lesional edema in the left parietal brain, which is strongly suggestive of an echinococcal cyst. FLAIR, fluid-attenuated inversion recovery.
Although serology for echinococcosis was negative, the diagnosis of cerebral cystic echinococcosis was retained as anamnesis and imaging outputs were strongly suggestive of this. Indeed, H.B. lived on a farm in Morocco for several years. Owing to the risks of dissemination if the cyst ruptured and to the general risks associated with intracranial surgery, albendazole was started for 4 weeks, along with dexamethasone to counteract a possible inflammatory reaction induced by cystic fissure, which could occur in response to the antiparasitic treatment. Subsequently, serology for IgG antibodies against E. granulosus was positive.
The surgical procedure comprised puncture of the cyst, which yielded approximately 10 mL of pus, followed by complete removal of the capsule. Pathological examination revealed a morphological aspect consistent with a hydatid cyst caused by E. granulosus. The postoperative course was without complication. Albendazole was maintained for 2 months postoperatively to reduce the risk of intraoperative dissemination of echinococcus.
The patient was followed up by neuropediatric and infectious disease specialists.
Six years later, there had been no recurrence of seizures, but H.B. presents with some learning disabilities. She is currently still undergoing antiepileptic treatment with levetiracetam.
Discussion
The onset of infection is always asymptomatic and may remain so for several years due to the slow growth rate of hydatid cysts. Expansion occurs at a rate of 1–5 mm per year in 30% of cases, 6–15 mm per year in 43% of cases, and at an average of 31 mm per year in 11% of cases. However, 16% of cysts will decrease and then collapse (6).
The symptomatology associated with cyst extension will depend on several factors (including organ involvement, cyst size, cyst location within the organ, possible interaction between cyst expansion and the mass effect that it would have on neighboring organs, and possible rupture of the cyst) (2, 6, 7). Symptoms, therefore, usually appear when the cyst compresses the surrounding organs or when it ruptures.
In published cohorts of cerebral CE, the main symptoms are headache and vomiting, followed by muscle weakness. Seizures occur in 24% of patients, as they did in our patient (8).
To make the diagnosis, it is important to look at travel history and any contact with dogs, foxes, or cattle, or work in a pastoral area or slaughterhouse, during the 5–10 years prior to the onset of symptoms (6). Our patient had indeed been living on a farm in an endemic area for the last 5 years.
Beyond anamnestic and clinical suspicion, imaging is a key element and can strongly suggest or even confirm a diagnosis. CT scans and MRIs are the most reliable imaging methods for the diagnosis of cerebral cystic echinococcosis (6, 8). Both CT scan and MRI are indicated in subdiaphragmatic and extra-abdominal locations, disseminated involvement, cysts that have become complicated or preoperatively. The differential diagnosis of a hydatid cyst on brain imaging should be made with a cyst due to E. multilocularis or cysticercosis, a subarachnoid cyst, a neuroglial cyst, or a cystic tumor. A hydatid cyst appears round, well delimited, and unilocular (8). Most cases of CE are diagnosed by imaging (2), but biological diagnosis can be useful and relies on the detection of serum antibodies. The detection of antigens is less specific (3, 7) because of potential cross-reactions with other tapeworms (3), cancers, or liver cirrhosis (7).
The current gold standard in clinical biology is the detection of IgG antibodies against native or recombinant B antigen subunits from cystic fluid (7, 9). However, an absence of antibodies does not exclude echinococcosis, as was the case here. Antibody detection depends on the location, integrity, and viability of the cyst. Hepatic cysts are more likely to generate antibodies than pulmonary, splenic, or brain cysts. Similarly, a senescent, calcified, or dead cyst does not usually generate antibodies, nor does an intact cyst. The cracking of the cyst, on the other hand, can cause a rapid generation of antibodies (10), which explains the positivity of serology in our case after the antiparasitic treatment.
There is currently no therapeutic consensus concerning cerebral CE, due to the low rate of occurrence (1, 6, 7, 11). Management, therefore, is dependent on the experience of the clinician and the cases reported in the literature.
Benzimidazoles have become the gold-standard pharmacological option. Albendazole, however, has been found to be more effective than mebendazole due to a lower effective dose and better intestinal absorption. Albendazole is administered at a dosage of 10–15mg/kg/day in children.
The efficacy of albendazole varies according to the class of lesion: active cysts less than 6 cm in diameter respond well, but the treatment is less effective on cysts larger than 6 cm in diameter (2, 11, 12).
As an adjuvant treatment, albendazole is effective during the perioperative period to reduce the viability of cysts during surgery and the risk of recurrence. Preoperative use for 2 months significantly reduces cyst viability at the time of surgery, and its use postoperatively over 2 months significantly reduces the risk of recurrence (13). However, the long-term efficacy of benzimidazoles still needs to be assessed (11).
Praziquantel has a protoscolicidal activity (14). It has been shown that the combination of praziquantel and albendazole, compared with the use of albendazole alone, significantly reduces the viability of protoscoleces within the cyst, and, therefore, the risk of dissemination during surgery (11). Despite this recognized effect of combined treatment, there is no consensus for the systematic use of praziquantel (11, 15, 16). Our patient received combined therapy of albendazole and dexamethasone preoperatively and albendazole was continued for 2 months postoperatively to reduce the risk of dissemination.
In conclusion, cystic echinococcosis is a zoonosis caused by the larval form of E. granulosus that predominantly infests the liver and lungs. Cerebral involvement accounts for only a very small percentage of occurrences.
The infection represents an economic and human burden in highly endemic areas, such as North and Central Africa, Central Asia, and South America, specifically for E. granulosus, and European countries are affected by an increase in the prevalence of E. multilocularis.
This case initially raised no anamnestic suspicions and, therefore, the imaging was not suggestive. This was not the first diagnosis of cerebral echinococcosis in Belgium, and it was necessary to obtain anamnestic complements to raise suspicion, which explains the delay of diagnosis.
It is important to know that the diagnosis is made based on radiological imaging within the context of anamnestic suspicion. Serology may remain negative on initial diagnosis, and, therefore, should not delay treatment.
Currently, beyond from the conclusions that can be drawn from the various case reports, there are no guidelines for the treatment of cerebral CE in children, and their absence could be deleterious. We based our management on existing case reports and the multidisciplinary experience of physicians.
This type of case report will add to the existing ones and help clinicians in their future management of cerebral echinococcosis in children while they await guidelines.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor’s legal guardian for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Brunetti E, Kern P, Vuitton AD, Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and threatment of cystic and alveolar echinococcosis in humans. Acta Tropica (2010) 114:1–16. doi: 10.1016/j.actatropica.2009.11.001
2. McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet (2003) 362(9392):1295–304. doi: 10.1016/S0140-6736(03)14573-4
3. Craig PS, McManus DP, Lightowlers M, Chabalgoity J, Garcia H, Gavidia C, et al. Prevention and control of cystic echinococcosis. Lancet Infection (2007) 7:385–94. doi: 10.1016/S1473-3099(07)70134-2
4. Thompson RCA, McManus DP. Aetiology : parasites and life-cycles. WHO/OIE Manuel echinococcosis humans Anim A Public Health Problem Global Concern (2001), 1–15.
5. World Health Organisation. Echinococcosis (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/echinococcosis.
6. Pawlowski ZS, Eckert J, Vuitton DA, Ammann RW, Kern P, Craig PS, et al. Echinococcosis in humans : clinical aspects, diagnostis and treatment. WHO/OIE Manuel echinococcosis humans Anim A Public Health Problem Global Concern. (2001), 20–71.
7. McManus D, Gray D, Zhang W, Yang Y. Diagnostis, treatment, and management of echinococcis. BMJ (2012) 344:e3866. doi: 10.1136/bmj.e3866
8. Turgut M. Intracranial hydatosis in trukey : its clinical presentation, diagnostic studies, surgical management, and outcome. a review of 276 cases. Neurosurgical Rev (2001) 24:200–8. doi: 10.1007/s101430100168
9. Lorenzo C, Ferreira HB, Monteiro KM, Rosensvit M, Kamenetzky L, Garcia H, et al. Comparative analysis of the diagnostic performance of six major echinococcus granulosus antigens assessed in a double-blind, randomized multicenter study. J Clin Microbiol (2005) 43:2764–70. doi: 10.1128/JCM.43.6.2764-2770.2005
10. Center for Disease Control and Prevention. Center for disease control and prevention. In: Echinococcosis : Ressources for Health Professionnals (2020). Available at: https://www.cdc.gov/dpdx/echinococcosis/dx.html. (accessed on May 11 2023)
11. Mihmanli M, Idiz U, Kaya C, Demir U, Bostanci O, Omergoglu S, et al. Current status of diagnosis and treatment of hepatic echinococcosis. World J Hepatology (2016) 8:1169–81. doi: 10.4254/wjh.v8.i28.1169
12. Nazligul Y, Kucukazman M, Akbulut S. Role of chemotherapeutic agents in the management of cystic echinococcosis. Int Surgery (2015) 100:112–4. doi: 10.9738/INTSURG-D-14-00068.1
13. Arif SH, Shams-ul-Bari, Wani N, Zagar S, Wani M, Tabassum R, et al. Albendazole as an adjuvant to the standard surgical management of hydatid cyst liver. Int J Surgery (2008) 6:448–52. doi: 10.1016/j.ijsu.2008.08.003
14. Cobo F, Yarnoz C, Sesma B, Frale P, Aizcorbe M, Trujillo R, et al. Albendazole plus praziquantel versus albendazole alone as pre-operative treatment in intra-abdominal hydatosis caused by echinococcus granulosus. Trop Med Int Health (2998) 3:462–6. doi: 10.1046/j.1365-3156.1998.00257.x
15. Stojkovic M, Zwahlen M, Teggi A, Vutova K, Cretu CM, Virdone R, et al. Treatment response of cystic echinococcosis to benznimidazoles : a systematic review. PLoS – Negl Trop Diseases. (2009) 3:e524. doi: 10.1371/journal.pntd.0000524
Keywords: case-report, cystic echinococcosis, seizures, pediatrics, neglected tropical disease, Echinococcus granulosus
Citation: Menschaert D, Daron A and Frere J (2023) Case report of cerebral cystic echinococcosis in a 5-year-old child. Front. Trop. Dis 4:1090644. doi: 10.3389/fitd.2023.1090644
Received: 05 November 2022; Accepted: 18 May 2023;
Published: 08 June 2023.
Edited by:
Emmanuele Venanzi Rullo, University of Messina, ItalyReviewed by:
Joaquin Prada, University of Surrey, United KingdomMarkus Oertel, University Hospital Zurich, Switzerland
Copyright © 2023 Menschaert, Daron and Frere. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Denis Menschaert, ZGVuaXMubWVuc2NoYWVydEBnbWFpbC5jb20=
 Denis Menschaert
Denis Menschaert Aurore Daron
Aurore Daron Julie Frere
Julie Frere