
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 12 September 2022
Sec. Vaccines for Tropical Diseases
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.998353
This article is part of the Research Topic Rising Stars in Vaccines for Tropical Diseases Research View all 4 articles
 Valeria Melendez1†
Valeria Melendez1† Cheri Turner2†
Cheri Turner2† Vishal Khatri1
Vishal Khatri1 Jenn Davis2
Jenn Davis2 Nikhil Chauhan1
Nikhil Chauhan1 Divya Sree Nagalati Sudhakar1
Divya Sree Nagalati Sudhakar1 Richard Cabullos3
Richard Cabullos3 Darrick Carter2
Darrick Carter2 Sean A. Gray2
Sean A. Gray2 Ramaswamy Kalyanasundaram1*
Ramaswamy Kalyanasundaram1*This study was conducted to optimize a fusion protein vaccine for translational development as a vaccine against the human tropical parasitic infection, lymphatic filariasis (LF). The vaccine candidate, His-tagged rBmHAXT was developed previously in our laboratory and was tested in various animal models including mouse, gerbils and Rhesus macaque where it exhibited significant levels of vaccine-induced protection. However, for commercial manufacturing and for regulatory approval for human use, there was a need to modify the vaccine antigen and its production and analytical release methods. Therefore, the major focus of this study was to develop a process for manufacturing an affinity tag-free rBmHAXT and evaluate its immunogenicity, potency and protective efficacy in both inbred and outbred mouse models, as well as in outbred gerbil models. Our results demonstrate that the tag-free rBmHAXT vaccine produced with a process suitable for cGMP production had protective properties equivalent to the original His-tagged rBmHAXT.
Lymphatic filariasis (LF) is a neglected tropical parasitic disease that affects 120 million people in 49 countries worldwide and another 893 million people are at risk of acquiring the disease (1). Commonly known as elephantiasis, the disease impairs the lymphatic system and can cause severe edema of extremities, lowering quality of life (2, 3). LF is a mosquito-transmitted parasitic infection caused by one of three nematodes (Wuchereria bancrofti, Brugia malayi, and Brugia timori) with W. bancrofti being responsible for nearly 90% of the cases. Currently, the only tools we have to control LF are vector control and mass drug administration (MDA). Inadequate program budgeting for vector control has adversely affected the ability to control all vector borne infections including lymphatic filariasis (4). Similarly, the MDA approach, which is aimed at reducing microfilariae in the bloodstream, has little effect on killing the adult worms in patients leading to persistence of the infection once the drug effect wanes (5–7). After 20 years of MDA, it is becoming clear that there is little impact on infections globally for several reasons - including inadequacy of the scale and frequency of drug administration and non-compliance by subjects in adhering to the drug regimen (8, 9). A long-lasting vaccine that prevents or reduces infection – in conjunction with MDA – would be a strong weapon in the fight against this infection and could eliminate or eradicate LF (10). According to the World Health Organization (WHO) estimates, a vaccine that can reduce new helminth infections by at least 50% would reduce overall morbidity and mortality (11).
It has been shown that long-term protective immunity to LF can be induced in humans (12). Immunity against new infections due to antigens released by established parasites increases with age in populations repeatedly exposed to LF (13). A portion of the population in areas where LF is endemic show natural immunity with no existing infection (14). These endemic normal individuals have protective antibodies in their blood that can participate in the immune-mediated killing of the infective larvae (L3) of LF, which can prevent the establishment of the infection (15). Since the completion of the B. malayi genome project, many potential candidate vaccine antigens have been identified and screened for prophylactic efficacy (8). The most promising of these were a combination of four protein domains from B. malayi as a tetravalent fusion protein. The antigens identified included: Heat Shock Protein 12.6 (HSP12.6), Abundant Larval Transcript-2 (ALT-2), Thioredoxin PeroXidase-2 (TPX-2), and the Large Extracellular Loop of TetraSPanin-Large Extracellular Loop (TSP-LEL) (8, 16). Each of these proteins is highly conserved across parasitic nematodes resulting in cross-protection against all three parasites that cause LF. The homologs of the four vaccine antigens cloned from B. malayi and W. bancrofti showed significant antibody cross reactivity and cross protection (unpublished data). A his-tagged version of a fusion protein of these four protein domains - referred to as rBmHAXT - was expressed and purified from E. coli inclusion bodies and purified by immobilized metal ion affinity chromatography (IMAC) (16, 17). Vaccination using this rBmHAXT antigen combined with the AL019 adjuvant (a potent synthetic TLR-4 agonist, glucopyranosyl lipid adjuvant (GLA) adsorbed onto alum) (18), was tested in mouse, gerbil, and non-human primate models. This vaccine formulation demonstrated significant in vivo protection of 88% in mice (16), 65% in gerbils (17) and 57% in Rhesus macaques (18) after an infectious challenge.
A partnership between the University of Illinois and a for-profit company, PAI Life Sciences Inc., was formed to advance this promising vaccine through the initial steps required to file an Investigational New Drug Application (IND) with the US Food and Drug Administration (FDA). Pre-IND enabling activities included the re-design of the rBmHAXT antigen without tags, development of a process for purification of the untagged antigen suitable for transfer to a contract manufacturing organization (CMO) facility, and confirmation of biological potency in vivo. Thus, as reported here, we expressed rBmHAXT without the His-tag (tag-free rBmHAXT) and optimized expression to the 2-5 liter scale with potential for scaling to 30-100 liters. The purified tag-free rBmHAXT antigen was then tested for its ability to confer protection against an infectious challenge in BALB/c mice compared to his-tagged antigen generated previously. Immunogenicity and protection mediated by the tag-free rBmHAXT antigen were further confirmed in a dose escalation study using outbred CD1 mice. A final comparison of the two antigens was done in Mongolian Gerbils (17, 19). Here we also describe early-stage pre-clinical development activities for the tag-free rBmHAXT antigen as the first ever clinical vaccine candidate for human lymphatic filariasis.
To ensure traceability from clone development throughout process development, all cloning, expression, and purification reagents were carefully sourced and documented to ensure the absence of animal-derived products that could introduce adventitious agents into the drug product. The rBmHAXT DNA sequence (1557 nt) was codon optimized for expression in E. coli (Integrated DNA Technologies, Coralville, IA) and cloned into the pET29a(+) expression vector (Millipore Sigma, Burlington, MA). Sequence confirmed pBmHAXT/29a plasmid was transformed into E. coli expression strain HMS174(DE3) (Millipore Sigma, Burlington, MA). Subclones from each plasmid were screened for expression following induction with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) overnight at 37°C with 250 rpm shaking. The following day, 3 mL cells were pelleted by centrifugation and the pellet resuspended in 0.2 mL of B-PER bacterial lysis reagent (Thermo Fisher, Waltham, MA). The insoluble and soluble fractions were isolated separately per manufacturer’s instructions and analyzed on reducing 4-20% Tris-glycine SDS-PAGE gels for presence of a ~60 kDa protein corresponding to the rBmHAXT antigen.
Generation of a traceable seed stock
A research cell bank with 180 vials of the E. coli HMS174 (DE3) strain expressing rBmHAXT protein was laid down. The clone was grown in animal-product free LBK broth. The final cell bank was stored at -80°C. Confirmation of expression was performed, documented, and included in the cell bank production batch record.
Fermentation development was pursued at the 2L scale and a fermentation batch record was developed. Briefly, a cell bank vial was inoculated into 2 × 200 mL of LBK broth and grown overnight. Cultures were checked for purity and then used to inoculate. Culture was grown in a fermenter in Terrific Broth at 37°C until OD at 600 nm reached 4.0 ± 0.2, induced for 3 hours at a final concentration of 1 mM IPTG, harvested by centrifugation, and cell pellets frozen for future processing.
E. coli cell pellets were thawed and suspended in lysis buffer (50 mM tris/0.5% Triton X-100 pH 8) at 5 mL buffer per 1 g of wet pellet. The suspension was passed 3 times through a LM10 microfluidizer (Microfluidics Corp., Westwood, MA) at 15,000 psi. The lysate was centrifuged at 14,000 × g for 30 min to pellet insoluble material and IB. The IB pellet was washed with 1% CHAPS followed by 25% Isopropanol and centrifuged as before. The resulting pellet – highly enriched for rBmHAXT – was resuspended in 20 mL per g IB of solubilization buffer (50 mM Tris/8 M urea/50 mM DTT pH 8.0). After overnight solubilization at 2 – 8°C, the IB solution was centrifuged at 15,000 × g for 3 hrs at 4°C and the supernatant, containing solubilized tag-free rBmHAXT protein, was stored at 4°C until purification.
rBmHAXT IB solution was passed over a Capto Q ImpRes (Cytiva, Marlborough, MA) column and washed with Q wash buffer (50 mM Tris/8 M urea/180 mM NaCl/10 mM DTT pH 8.0). Bound rBmHAXT was eluted in Q elution buffer (50 mM Tris/8 M urea/300 mM NaCl/10 mM DTT pH 8.0). Eluted protein was then diluted 1/8 in SP loading buffer (20 mM acetate/8 M urea/10 mM DTT pH 4) and loaded onto a Capto SP ImpRes (Cytiva) resin column. After washing off the unbound protein with SP wash buffer (20 mM acetate/8 M urea/300 mM NaCl/10 mM DTT pH 4), tag-free rBmHAXT was eluted with SP elution buffer (20 mM acetate/8 M urea/1 M NaCl/10 mM DTT pH 4). Elution fraction was adjusted to pH 8.0 and urea was removed by buffer exchange with 50 mM Tris pH 8.0. The purified tag-free rBmHAXT was adjusted to 5% glycerol, filter-sterilized through 0.2 µM filters, and stored at -80°C at a final concentration of 1 mg/mL.
1µg each of rBmHAXT and bovine serum albumin (BSA) standard were separated on 4-20% Tris-glycine SDS-PAGE gel under reducing conditions and analyzed using ImageJ densitometry to confirm both concentration and purity. Specificity was confirmed by Western blot analysis using monkey anti-rBmHAXT antisera procured as part of a previous vaccination trial in our laboratory (17). The sera was used at 1:5000 and detected with Goat anti-monkey IgG (H+L) HRP antibody at a 1:10,000 dilution (Thermo Fisher Scientific, Waltham, MA). Presence of E. coli host cell proteins was determined by Western blot analysis using anti-E. coli HCP antisera (Rockland Immunochemicals, Inc., Limerick, PA). Residual endotoxin was measured using the Limulus Amoebocyte Lysate (PTS-LAL) assay (Charles River Laboratories, Worcester, MA).
Protein folding and structural studies were done using Jasco J1500 Circular Dichroism (CD) Spectroscopy (Jasco, Easton, MD) at the University of Chicago, IL. CD was used for determining the secondary structure of proteins and composition (% helix, sheet, turns, etc.). Then, 100 µl of both His-tag and Tag-free rBmHAXT protein at 1.5 mg/ml concentration were added separately to high transparency quartz cuvette having 1 mm path-length and placed in cuvette holder. Data was collected in a graph format. TBS buffer with 20% glycerol was used as a blank.
Two web server software was used to analyze the CD data. We implemented K2D3, a publicly available web server method to predict secondary structural content from a CD spectrum. The program accepts the CD spectrum of a protein as input and predicts percentages of alpha helix and beta‐strand content. The other bioinformatic tool we used for CD data analysis was CAPITO (CD Analysis and Plotting Tool), a novel web server-based tool for analyzing and plotting CD data. It allows reliable estimation of secondary structure content utilizing different approaches and gives a graph with curves pertaining to protein secondary structures.
Humane use of animals in this study was supervised by the IACUC committee of the University of Illinois Rockford following the National Institutes of Health guidelines for the care and use of laboratory animals. All animals used in this study were purchased from Charles River Laboratories. All animals were monitored for any adverse reactions including injection site reaction, fever, loss of appetite, allergy, hair loss, or weight loss. Animals showing distress were promptly removed from the study. All animal experiments were overseen by a licensed veterinarian. Infective larvae of B. malayi parasites were obtained from the NIAID/NIH Filariasis Research Reagent Resource Center (University of Georgia, Athens, GA) under NIAID supply contract AI#30022.
BALB/c mice. Thirty-seven 8-week-old male BALB/c mice were divided into 6 groups of 5 animals per group and one control group of 7 animals. Three groups received 200 µL injections of tag-free rBmHAXT (PAI) at either low (5 µg), medium (15 µg) or high (40 µg) doses of antigen each formulated with 10 µg AL019 adjuvant. Injections were performed subcutaneously (s.c.), split evenly between the two quadricep muscles. Another three groups of animals received identical doses of His-tagged rBmHAXT in AL019. The final control group (n=7) received AL019 adjuvant (10 µg) only. Each mouse was immunized three times at 15-day intervals with the vaccine plus adjuvant (experimental group) or adjuvant alone (control group). Blood was collected from the submandibular vein of each mouse before and 15 days after each immunization. Fifteen days after the last immunization, mice were challenged with 13-15 B. malayi infective L3 larvae using the micropore chamber method (16). At 72 hours post-challenge, the chambers were collected, and live and dead larvae were identified by microscopical examination. Percent larval death (represented as protection) was calculated. Serum samples were analyzed for anti-rBmHAXT IgG antibody titers and the levels of antibody isotypes (IgG1, IgG2a, IgG2b, IgG3, IgM and IgA) by standard ELISA as described previously (16).
CD1 mice. Twenty-eight 8-week-old male outbred CD1 mice were divided into 7 groups of 4 animals. Each group received an increasing dose of tag-free rBmHAXT ranging from 0 (adjuvant-only control), 0.5, 5, 10, 15, 25 or 40 µg in TBS pH 8.0 each adjuvanted with 10 µg AL019 adjuvant. Mice were injected with 200 µL volume s.c. three times at 15 days intervals. Blood was collected as described above with BALB/c mice before the prime injection and every 15 days thereafter as well as prior to challenge. Mice were challenged with B. malayi L3 larvae as described above (16).
Mongolian gerbils. Twenty-eight 12-week-old male outbred Mongolian Gerbils (Meriones unguiculatus) were divided into 7 groups of 4 animals per group and immunized with tag-free rBmHAXT or his-tagged rBmHAXT antigen with 3 doses of the antigen as described for the BALB/c mice above. Three doses of the antigen given s.c. were administered at 30-day intervals. Blood (0.5 mL) was collected from the retroorbital space before and every 30 days after immunization. Body weights were measured for the first 11 weeks during the immunizations. Antibody titers were measured 30 days after each immunization by ELISA as described previously (20). Ig antibody isotypes (IgG, IgG1, IgG2a, IgG2b, IgG3, IgM and IgA) were evaluated using an indirect ELISA as described previously (20). Thirty days after the final immunization, gerbils were challenged with 80-100 B. malayi L3 larvae and analyzed for worm establishment 180 days later as described previously (20).
As a surrogate to the challenge studies, an ADCC assay was performed as described previously (20, 21). Approximately 10 live B. malayi L3s were incubated at 37°C with 5% CO2 in triplicate wells together with 2×105 peritoneal cells and 50 μl of serum samples. After a 72 h incubation, the viability of the B. malayi L3 larvae were determined. The percentage of larval death was expressed as the ratio of the number of dead L3 larvae to the total number recovered from each well multiplied by 100.
GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA) was used to plot all the graphs and statistical analysis of the data was carried out using SPSS v 26.0 (IBM corporation, Armonk, NY). Data were analyzed for normality assumptions using the Shapiro-Wilk Test. The hypothesis of normality will be rejected when the p-value is less than or equal to 0.05. Thus, a probability “p value” of < 0.05 was considered statistically significant.
The original rBmHAXT sequence described by Chauhan et al. (16) was used as a starting template. First, the coding sequence was designed in silico by removing the 6 × His tag and codon optimizing the remaining sequence to optimize expression in E. coli. The resulting nucleic acid sequence was 76% identical to the parental sequence while the amino acid sequence remained unchanged. The synthesized gene was subcloned into pET29a(+) and transformed into the E. coli expression strain HMS174(DE3) cells. Subclones from sequence-confirmed plasmids were screened for expression and analyzed for presence of a ~60 kDa protein corresponding to the expected size of rBmHAXT. Western analysis of the fractions using monkey anti-rBmHAXT confirmed the identity of the ~60 kDa band. Clones produced protein at the expected MW in the insoluble fraction (data not shown). Additional experiments performed attempting to promote the protein folding and expression in the soluble fraction including reducing the concentration of IPTG and reducing the expression temperatures to as low as 14°C were unsuccessful (data not shown). Optimal expression of rBmHAXT was determined to be at 37°C with 1 mM IPTG and a 3 hour induction. An isolate with high expression levels was identified and prioritized for cell banking, fermentation, and process development.
Following fermentation, purification begins by resuspension and lysis of the bacterial pellet followed by isolation of the IB fraction (Figure 1A). Analysis of the IB fraction revealed that it is highly enriched for rBmHAXT, with various other E. coli protein and lipid contaminants present as well (data not shown). The IB pellet was washed in 1% CHAPS buffer followed by 25% isopropyl alcohol. These wash steps only minimally reduced the protein contaminants but significantly reduced the endotoxin levels prior to chromatographic purification. The washed IB pellet was solubilized in 8 M urea to denature the rBmHAXT protein and promote binding to the Capto Q ImpRes anion- and Capto SP ImpRes cation-exchange resins.
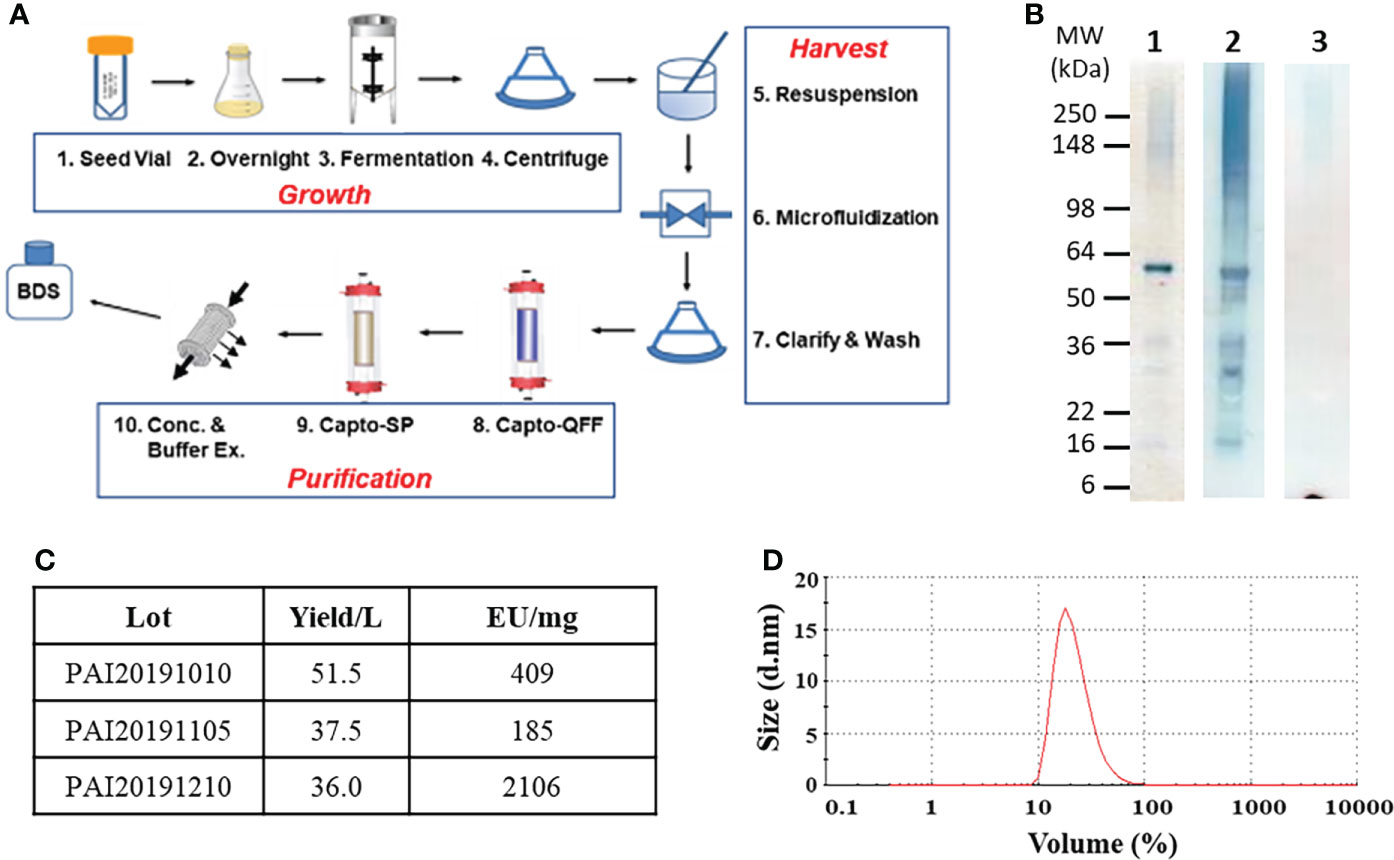
Figure 1 Process development of tag-free rBmHAXT. Panel (A) A flow diagram showing the steps required for production and purification of tag-free rBmHAXT. Panel (B) shows the quality analysis of a representative lot of rBmHAXT protein. Each lane contains 1μg antigen separated by SDS-PAGE and either stained by Coomassie (Lane 1) or transferred to PVDF membrane and probed with non-human primate anti-rBmHAXT polyclonal antiserum (Lane 2) or probed with anti-E. coli Host Cell Protein polyclonal antiserum (Lane 3). Panel (C) shows a table of tag-free rBmHAXT protein produced from three independent 2L fermentations indicating yield (average = 41.7 mg/L) and endotoxin levels from each batch. Panel (D) Dynamic light scattering scan of tag-free rBmHAXT Lot#PAI20191210 showing protein tends to form stable micro-aggregates with an average particle size of 36.1 nm. The figure was created using GIMP at https://www.gimp.org.
Protein was first bound to Capto Q Imp resin and contaminant proteins eluted by washing with 180 mM NaCl. Bound rBmHAXT was eluted at 300 mM NaCl. Elution fraction was then bound to a Capto SP ImpRes column. Removal of contaminants was performed at 300 mM NaCl, and elution of rBmHAXT at 1 M NaCl. The elution fraction was adjusted to pH 8, buffer exchanged into 50 mM Tris pH 8.0, and concentrated to ~1 mg/mL final concentration. Glycerol was added to 5% before storage at -80°C to promote stability and to reduce aggregation. Lane 1 of Figure 1B shows the final purified rBmHAXT protein resolved by reducing SDS-PAGE analysis. By Image J densitometry analysis, ~70% of rBmHAXT exists as a ~60 kDa monomer with the remaining protein forming aggregates at 100-300 kDa or existing as degradation products in the 16-36 kDa range. By non-reducing SDS-PAGE, the rBmHAXT tends to form small stable aggregates (data not shown). The monkey anti-rBmHAXT Western in Lane 2 and the anti-E. coli HCP Western in Lane 3 of Figure 1B confirm that nearly all of the protein seen in lane 1 is rBmHAXT derived and not E. coli contaminant proteins. Based on 3 independent runs starting with 2 L fermentation, the final process for rBmHAXT purification averages ~42 mg per liter of fermentation with varying levels of endotoxin in each batch (Figure 1C). Protein stability and quality was assessed by dynamic light scattering using a Malvern Zetasizer™ (Figure 1D). All lots were within the acceptable range for particle size (25-100 nm) averaging ~36.1 nm, including lot PAI20191105 which is shown in Figure 1D.
We also performed circular dichroism (CD) studies to determine if there are any structural differences between His-tagged rBmHAXT and tag-free rBmHAXT. These studies showed that the His-tagged rBmHAXT and tag-free rBmHAXT protein had similar percentages of α-helix and β strands (Supplementary Figures S1A, B). These findings suggested that the tag-free rBmHAXT protein is structurally similar to the parent His-tagged protein.
To confirm retention of biological activity, the untagged rBmHAXT was compared in vivo with the previously produced His-tagged protein. Figure 2 shows the titers induced in mice following immunization with 0 (adjuvant only), 5, 15, or 40 μg of either tag-free or His-tagged rBmHAXT adjuvanted with 10 μg AL019. None of the immunized animals developed significant titers of antibodies 15 days after the prime immunization (Figure 2A). After the 1st boost, a significant increase in titers was observed for both antigens (Figure 2B). After the 2nd boost, both antigens resulted in a dose-dependent increase in IgG titer with the 15 μg dose of tag-free rBmHAXT generating the highest titers, although not statistically significant (Figure 2C). Using immunoglobulin isotype-specific ELISAs, we measured the levels of IgG1, IgG2a, IgG2b, IgG3, IgA and IgM antibodies elicited by all 3 doses of both antigens after the third injection (Figure 3). Both antigens induced similar subtype profiles, with IgG1 levels being the highest at all three antigen doses. No significant differences were observed with any subtype elicited by either the tag-free or His-tagged rBmHAXT. To confirm protection, we tested the mouse sera in an antibody-dependent cell-mediated cytotoxic (ADCC) assay for their ability to participate in the killing of infective B. malayi L3 larvae. Larvae were considered dead if non-motile, limpid, or with several cells adhered to their surface. Both antigens elicited a strong, dose-dependent ADCC response resulting in the killing of L3 larvae, with the 5 μg dose resulting in ~52% killing and the 40 μg dose resulting in up to 90% killing (Figure 4). Again, there was no difference in killing between the tag-free and His-tagged rBmHAXT antigens, but a significant difference in killing was observed comparing the 5 μg dose to either the 15 or 40 μg doses (p<0.05). We conclude that both antigens generate statistically equivalent titers and that the 15 μg dose is ideal in BALB/c mice. We also noted elicitation of similar immunoglobulin isotypes and levels and nearly identical dose-dependent larvicidal activity with no significant difference noted in BALB/c mice comparing the tag-free or His-tagged rBmHAXT proteins.
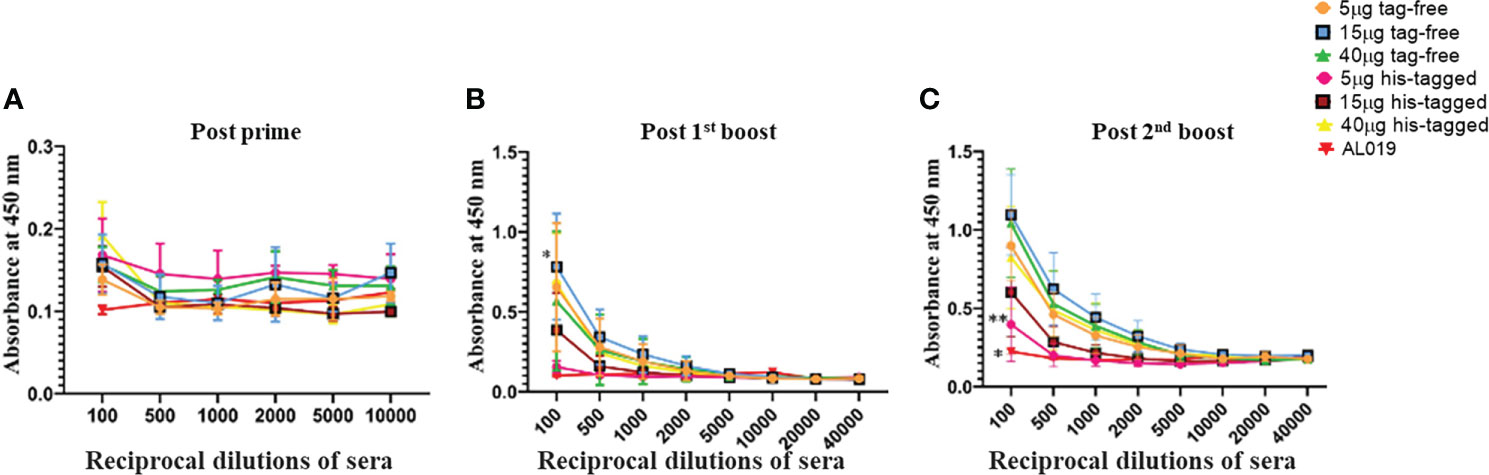
Figure 2 Bridging study showing generation of high titers of antibodies in the sera of BALB/c mice after immunization with varying doses of His-tagged and tag-free rBmHAXT antigen. Mice were primed with 5, 15, or 40 μg of either tag-free rBmHAXT or His-tagged rBmHAXT formulated with 10 μg AL019. Mice were primed on day 0 and boosted on days 15, 30 and 45. Panels (A–C) show induction of rBmHAXT-specific immune titers 15 days following the prime, 1st boost, and 2nd boost injections, respectively. Although titers do not begin to appear until after the first boost, there was no significant difference in titers between mice injected with tag-free rBmHAXT or His-tagged rBmHAXT proteins. There was a significant difference (p<0.05) observed between the AL019 control group and the rBmHAXT groups. N=5 for the experimental groups and n=7 for the controls; *Statistical significance P<0.001.
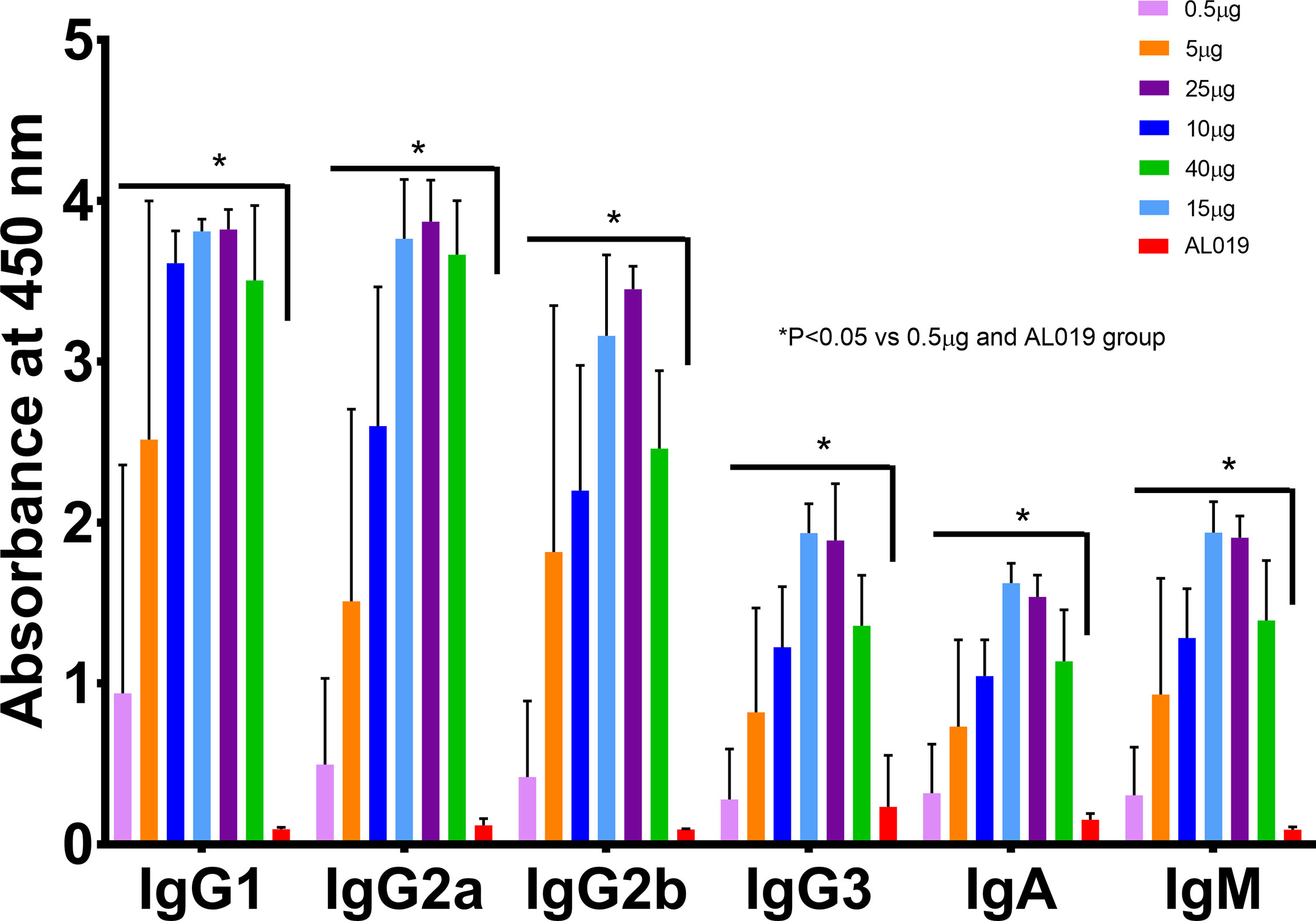
Figure 3 Immunization with tag-free and His-tagged rBmHAXT antigen induced similar levels of isotype-specific antibodies in the sera of BALB/c mice. Data presented here are values of IgG1, IgG2a, IgG2b, IgG3, IgA and IgM in the sera of BALB/c mice collected on day 45. Levels of all isotype-specific antibodies were significantly increased compared to the AL019 controls. There were no significant differences in the levels of isotype-specific antibodies in the sera samples collected from the tag-free and His-tagged rBmHAXT immunized mice. The maximum response was seen with the 15 and 40 ug dose in both the tag-free and His-tagged rBmHAXT groups. N=5 for the experimental groups and n=7 for the controls. *Statistically significant P<0.05 all groups vs. AL019.
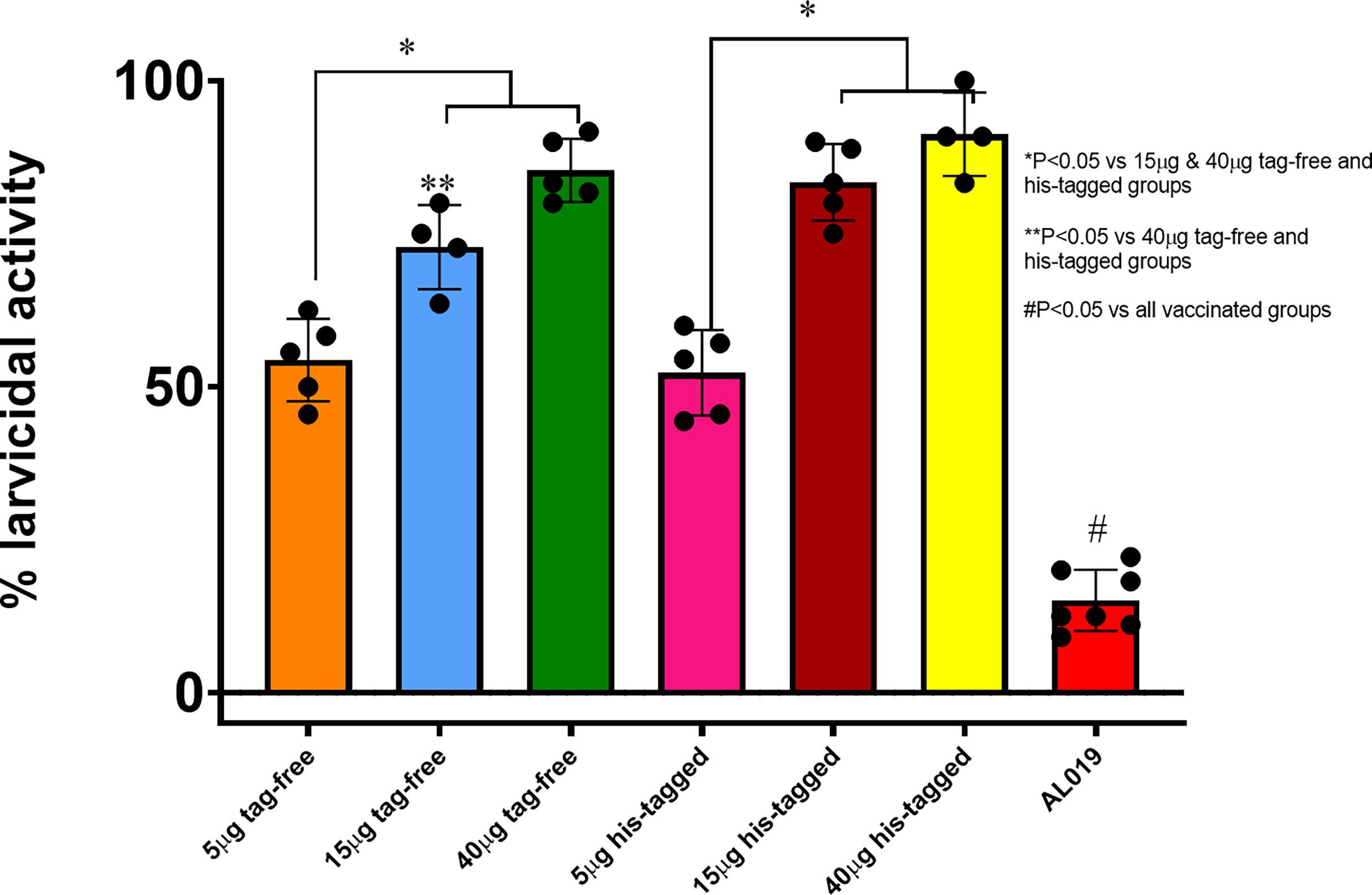
Figure 4 In vitro correlates of protection. Serum samples collected from mice that were immunized with varying doses (5, 15 and 40 ug) of tag-free or his-tagged rBmHAXT proteins were evaluated for their potential to kill infective larvae of B. malayi in an ADCC mechanism. About 100 µL of the serum samples were incubated with 10 larvae and 1x106 PBMCs for 72 hrs. Dead and live larvae were counted and percent dead calculated. Results show that compared to control serum samples, sera from all experimental groups showed significant killing. Sera from mice vaccinated with 40 ug of the proteins showed the highest larvicidal activity. There were no significant differences between the tag-free and his-tagged rBmHAXT immunized groups. N=5 *Statistical significance at p<0.05.
Given the known limited genetic diversity in BALB/c mice and seeking a more challenging model, we confirmed protection and dose in outbred male CD1 mice. For this study, we tested only the tag-free rBmHAXT antigen after each of 3 immunizations with 6 doses ranging from 0.5 μg to 40 μg with 10 μg AL019 adjuvant. In contrast to what we observed with BALB/c mice, modest titers were observed after the prime injection (data not shown). After the 3rd injection, we observed significantly higher titers with both the 15 and 25 μg doses (Figure 5A). These titers were statistically (p<0.05) higher than those observed with 40 μg of antigen, which generated titers similar to that of the 5 and 10 μg doses. As with BALB/c mice, we analyzed the immunoglobulin subtype profiles elicited by the different antigen doses (Figure 5B). We found significant dose-dependent increases in all isotypes, especially with IgG1, IgG2a and IgG2b. Again, the 15 and 25 μg doses generated the highest level of all isotypes and the 40 μg dose generated slightly lower levels with every isotype tested. Thirty days after the third injection, CD1 mice immunized with 0 (adjuvant only), 0.5, 25, or 40 ug doses were challenged with B. malayi L3 larvae and examined for ADCC killing 72 hours later (Figure 5C). Once again, the 25 μg dose resulted in ~82% killing of L3 larvae which was substantially higher (p<0.005 for 25 μg versus all other groups) than the ~15% and ~25% killing observed with the 0.5 μg and 40 μg groups, respectively, or the ~4% killing in the adjuvant only group. These studies with CD1 mice confirm the protective potential of the tag-free rBmHAXT antigen and confirm the optimal dose to be 25 μg in outbred mice.
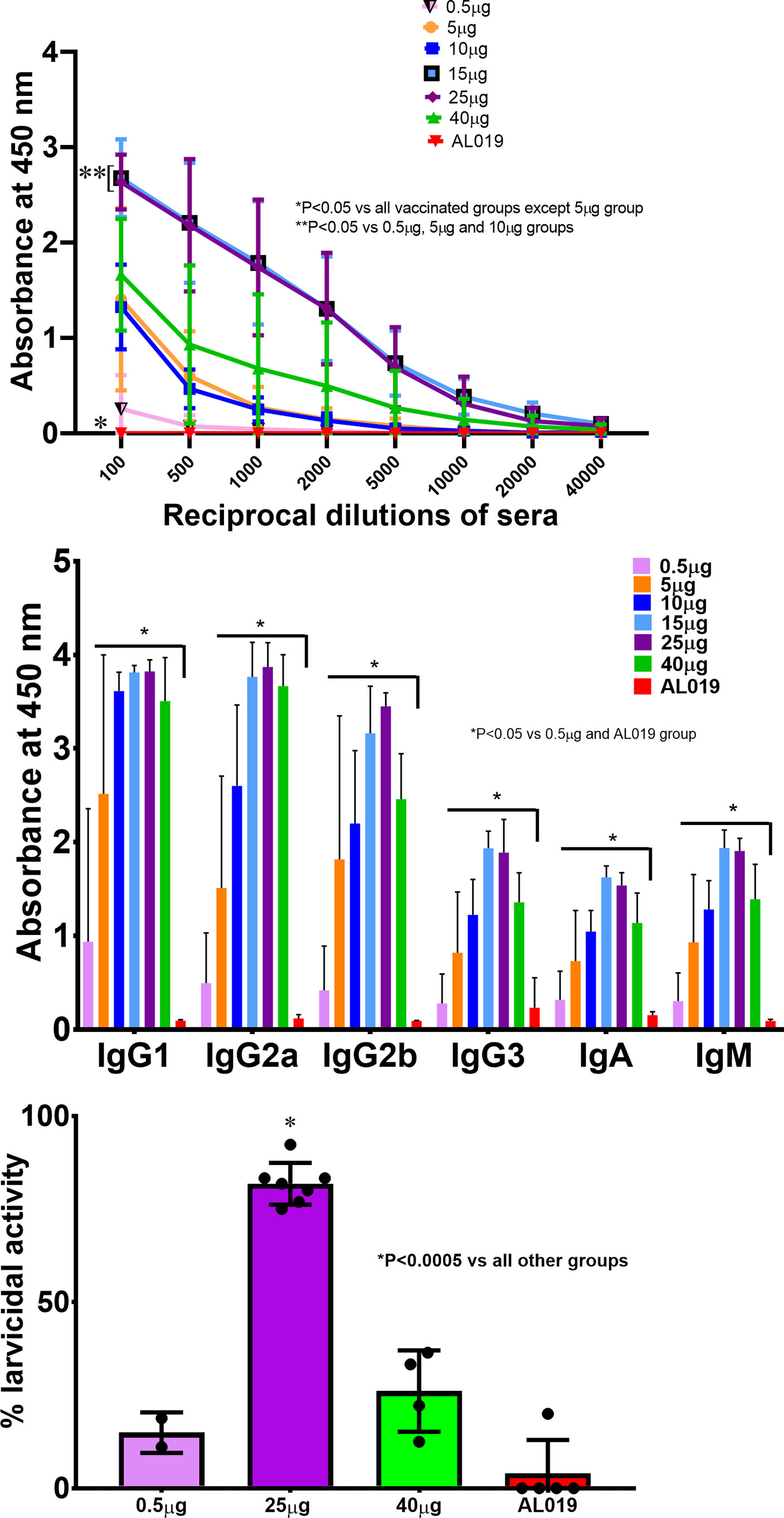
Figure 5 Dose escalation studies in outbred CD1 mice. CD1 mice were immunized with various doses of tag-free rBmHAXT ranging from 0.5 μg up to 40 μg in AL019. Panel (A) shows induction of rBmHAXT-specific IgG titers 30 days following the 3rd boost. There were significantly higher titers of IgG antibodies against rBmHAXT in all vaccinated mice. Mice immunized with the 15 μg dose showed the highest titers compared to all other doses and were even higher than the mice receiving 40 μg antigen. Panel (B) shows the isotype-specific antibody titers in the sera of immunized mice. All isotype-specific antibodies analyzed were significantly (P<0.05) increased compared to the control and 0.5 µg group. Panel (C) shows data from the ADCC assay using the sera samples from mice immunized with 0.5, 25 and 40 µg doses of tag-free rBmHAXT. Results showed that sera from mice immunized with 25 µg of rBmHAXT had the highest larvicidal activity compared to the other groups. N=5.
In a final bridging study, we compared the His-tagged rBmHAXT and tag-free antigen in a yet more rigorous and relevant animal model – specifically in Mongolian gerbils (Meriones unguiculatus). For B. malayi disease, gerbils are the preferred model for lymphatic filariasis due in part to their outbred nature and the fact that the parasites mature in the jirds and are able to maintain infection for several weeks (19, 20). Gerbils were immunized three times with 5 ug, 15 ug or 40 ug of the tag-free rBmHAXT adjuvanted with 10 μg AL019. Adjuvant only group remained as controls in these studies. All immunizations were given at 30 days interval.
As with mice, total IgG antibody titers were monitored and immunoglobulin isotypes were analyzed following the 3rd immunization dose. IgG titers at 30 days post the 3rd injection with His-tagged and tag-free rBmHAXT are shown in Figures 6A, B. With both antigens, the 40 μg dose generated the highest IgG titers with no statistically significant difference between the two antigens. Analysis of immunoglobulin isotypes showed a dose-dependent increase in all 6 isotypes tested - especially the levels of IgG3 were significantly high (Figure 6C).
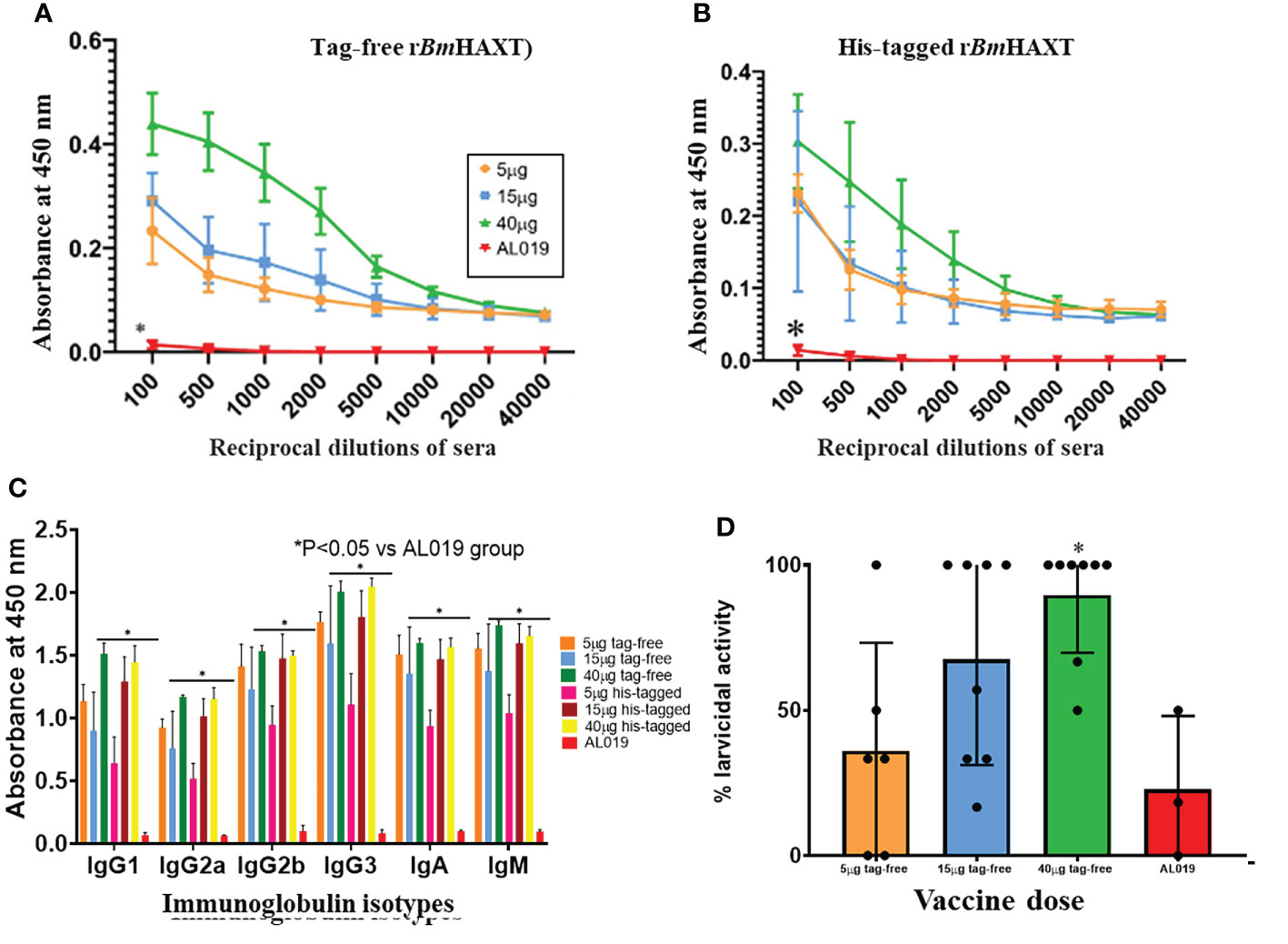
Figure 6 Correlates of protection studies in Mongolian gerbils. Outbred male Mongolian gerbils were immunized with 5, 15, or 40 μg of tag-free rBmHAXT. Panel (A) shows that significant titers of anti-rBmHAXT IgG antibodies were present in the sera of all tag-free rBmHAXT vaccinated gerbils 30-days after the 3rd immunization. Highest titers of anti-IgG rBmHAXT were obtained with the 40 μg dose compared to the 5 and 15 µg doses. Panel (B) shows the titers of anti-rBmHAXT IgG antibodies following immunization with His-tagged rBmHAXT. Similar to Panel (A), immunization with a 40 µg dose of His-tagged rBmHAXT gave the highest titer as with the tag-free rBmHAXT immunization. Panel (C) shows the levels of anti-rBmHAXT antibody isotypes in the sera after immunization with tag-free or His-tagged rBmHAXT. Results show that the antibody isotype profiles generated were similar after immunization with tag-free or His-tagged antigen. Panel (D) shows the ADCC-mediated larvicidal activity of sera 30-days after the 3rd immunization. A statistically significant (p < 0.05 vs AL019 and 40 ug group) dose-dependent increase in larvicidal activity was seen in the sera of tag-free rBmHAXT vaccinated animals compared to the AL019 control group. n=6-8 vaccinated and 3 controls.
We evaluated the potential of serum antibodies from immunized gerbils in killing the infective larvae of B. malayi in an ADCC assay as described previously (18). Briefly, 50 ul of immune sera from gerbils immunized with 5 ug, 15 ug or 40 ug of tag-free rBmHAXT along with 1x106 PBMCs from a normal gerbil were incubated with 10 infective larvae of B. malayi for 72 hrs at 37°C and 5% CO2. At 72 hrs, there was significant killing of the infective larvae (Figure 6D). Sera collected from gerbils immunized with 15 ug and 40 ug of tag-free rBmHAXT showed significant killing (67.54% and 89.58% respectively) of the infective larvae compared to adjuvant controls (22.77%). Sera from gerbils immunized with 5 ug of rBmHAXT showed only 36.1% killing, which was not significant compared to the control (Figure 6D).
Gerbils were immunized three times with 5 ug, 15 ug or 40 ug of the tag-free rBmHAXT at 30 days interval. Thirty (30) days following the last immunization, all gerbils were challenged with 50 L3 larvae of B. malayi. Weekly measurement of the body weight showed no significant differences between the control and vaccinated groups following immunization with any dose of either antigens suggesting that the vaccination had no adverse effect on the body weight (data not shown). Worm establishment was determined 90 days after the challenge. Our results showed that there was a significant decrease in the worm establishment in all vaccinated groups compared to controls (Figure 7). Fewer worms were recovered from gerbils immunized with 5 ug rBmHAXT, similarly no worms were recovered from gerbils immunized with 40 ug rBmHAXT, except for one gerbil that had 14 male and 14 female worms. In general, fewer male worms were recovered from vaccinated animals, compared to controls (Figure 7).
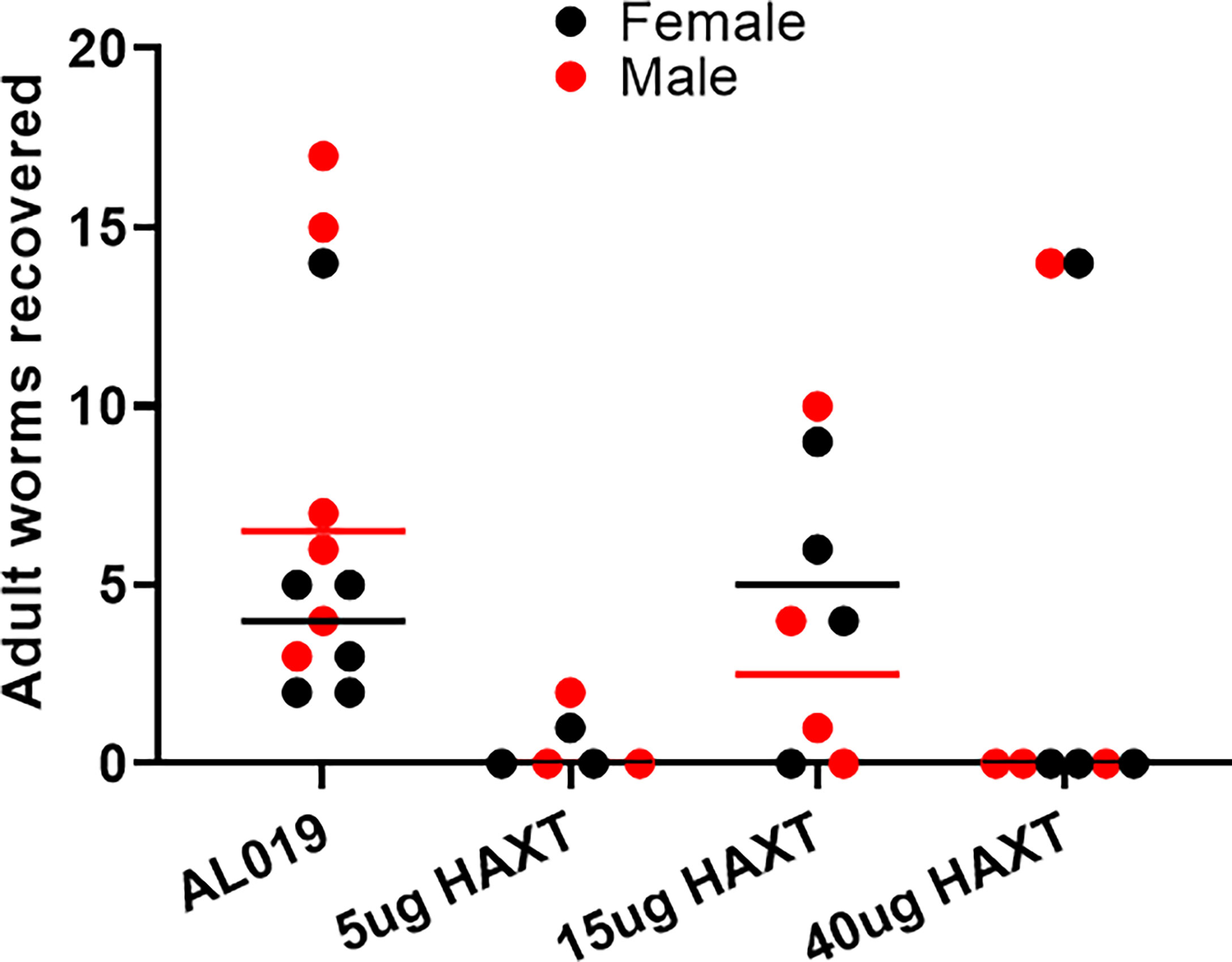
Figure 7 Worm establishment following a challenge in Mongolian gerbils. Outbred male Mongolian Gerbils (Meriones unguiculatus) were immunized three times s/c with 5, 15, or 40 μg of tag-free rBmHAXT. Control mice were given AL019 adjuvant and remained as the placebo group. Thirty (30) days after the last immunization, all gerbils were challenged with 50 infective larvae of B. malayi. Ninety (90) days after challenge, worms established in the internal organs and peritoneal cavity were counted. Average number of male and female worms recovered from each group of gerbils is shown in the figure. Mean values are represented with a bar for each group. Compared to the control group (AL019), fewer worms were recovered from all the vaccinated gerbils. .
The goal of this study was to take a promising research antigen, His-tagged rBmHAXT, which had previously been purified by immobilized metal ion chromatography, and redesign and optimize it for clinical translation and commercial manufacturing. The rBmHAXT was redesigned by removing the 6 × his tag, codon optimizing for E. coli, and cloning it into a commercially compliant plasmid vector and E. coli strain resulting in a research cell bank. A robust, scalable, and transferable process was developed for fermentation, IB isolation, and chromatographic purification of high-quality antigen. Master Batch Records for the production process were written to facilitate technology transfer to a good manufacturing practices (GMP)-compliant contract manufacturing organization. Initial bridging studies in mice comparing the research grade antigen with the redesigned tag-free antigen revealed equivalent antibody titers, immunoglobulin isotypes, and larvicidal killing by the tag-free antigen. Subsequently, the potency of the vaccine was tested in a gerbil model and we were able to demonstrate that the tag-free rBmHAXT antigen is immunogenic and induces protection similar to the original His-tagged rBmHAXT.
Previous vaccination trials in our laboratory in mouse, gerbil and rhesus macaque models demonstrated that rBmHAXT is an excellent vaccine candidate for human lymphatic filariasis (8). Vaccine-induced protection ranged from 57% (7 out of 10 macaques did not show infection) to 88% (in mice) following immunization with His-tagged rBmHAXT (16, 18). In order to get FDA approval and bring the vaccine to human use, there is a need to remove unneeded extraneous sequences like the His-tag from the vaccine antigen and to develop a scalable manufacturing process. Thus, our first goal was to generate tag-free rBmHAXT and optimize the expression of the protein for scale up for commercial manufacturing. Our initial vaccination trials in this study using the tag-free rBmHAXT protein in a mouse model showed that the immunogenicity and vaccine efficacy of tag-free rBmHAXT is not affected by removal of the His-tag. We also performed dose escalation studies to identify an optimal dose. Results from all these studies showed that at lower doses of tag-free rBmHAXT, vaccine efficacy was low, however, at higher doses, significant protection was achieved comparable to His-tagged rBmHAXT in the mouse model. Thus, our initial studies confirmed that tag-free rBmHAXT is as efficient as the original vaccine formulation. Unfortunately, there are no other multivalent vaccine against LF to compare at this stage.
To mimic the genetic diversity that is innate to humans (19, 21, 22) there is a need to test the vaccine antigen in an outbred strain. Numerous studies have suggested that inbred mouse strains - such as BALB/c - will typically result in highly reproducible results while outbred strains often tend to give variable results based on their individual genetic makeup (22). Similarly, the inbred and outbred mice differ in their ability to immunosense antigens that are offered to their immune system. The CD4 and CD8 T cells in outbred mice show equal and higher responses compared to the inbred mice, where CD8 T cell responses seem to predominate in inbred mice. Outbred mice showed peak responses later in the immune responses and these responses were prolonged compared to the inbred mice (23). Thus, immunity is long-lived in outbred mice. Another study by Tuttle et al. (24) demonstrated significant trait variability among inbred mice. Thus, inbred mice are probably not an accurate model for testing immune responses in the human (23, 24). Therefore, in this study we used an outbred strain of mice (CD1) and an outbred strain of gerbil to evaluate the potency and vaccine efficacy of the antigen in genetically diverse animals. Results from our studies confirm that the vaccine efficacy of our tag-free rBmHAXT antigen in CD1 mice and in gerbils are similar to that seen in inbred BALB/c mice. Thus, it can be safely concluded that the tag-free rBmHAXT vaccine has a broad spectrum of efficacy in genetically diverse rodent species. Dose escalation studies in CD1 mice showed that a dose of 15-25 ug gave the highest titer of IgG antibodies suggesting that the optimum range of the vaccine is between 15-25 ug. These findings also correlated with the protection studies after a challenge infection. A dose of 25 ug showed the highest percent of protection in CD1 mice confirming that the optimum dose of vaccine in an outbred strain of mice may be different than in inbred mice.
While mice are useful for early-stage validation, they have limitations for use with lymphatic filariasis due in part to phenotypic manifestations of disease and their short life span. However, they are useful to identify potential correlates of efficacy. It has been shown that while mice can be infected with W. bancrofti and will generate antibody titers, they do not maintain the infection (8). The B. malayi-infected gerbil serves as a laboratory model to mimic some of the aspects of the filarial infection of humans producing chronic parasitism characterized by microfilaremia and a Th2-mediated immune response (19, 20). Analysis of the vaccine in the gerbil model in this study showed that gerbils vaccinated with tag-free rBmHAXT generated significant titers of total IgG and various Ig isotypes. More importantly, the highest dose of antigen resulted in nearly 90% of killing of L3 larvae in an in vitro ADCC assay. This suggested that immunization with the tag-free rBmHAXT also generated protective antibodies as described previously with His-tagged rBmHAXT (20). Our challenge studies in gerbils also confirmed that worm recovery was significantly decreased in vaccinated animals compared to placebo control. Numbers of both male and female worms were substantially decreased. These findings confirm that tag-free rBmHAXT has the potential to be an excellent vaccine candidate for lymphatic filariasis. As such, we are moving the adjuvanted vaccine formulation termed “LFGuard™” forward to human testing.
Currently, there is no commercially licensed prophylactic vaccine available for LF (8). With over 880 million people at risk, there is a greater need for developing a vaccine for LF. An effective prophylactic vaccine should confer long-lasting and robust protective immune responses in the vaccinated individuals (8). Several previous attempts to develop a vaccine against LF resulted in the identification a number of potential vaccine candidates and approach to deliver the vaccines, such as cocktail vaccines (25), chimeric vaccines (26, 27), multi-subunit and multi-epitope vaccines (28–31), and multivalent vaccines (15, 16, 18). Among all these approaches, the multivalent vaccine approach gave slightly better results when tested in experimental rodent models. Subsequently, the best multivalent formulation, rBmHAXT was tested in a non-human primate model and showed great promise. In this manuscript we demonstrate that we could improve the final down selected multivalent formulation without losing its potency and immunogenicity.
From our studies in both inbred and outbred mice and Mongolian gerbils, we conclude that the newly redesigned tag-free rBmHAXT antigen results in equivalent protection compared to the original His-tagged antigen. These studies open the door for the next phase of developing this LFGuard™ vaccine as the first ever vaccine for human lymphatic filariasis. We are currently in the process of cGMP-compliant manufacturing of the vaccine. GLP toxicology studies in rabbits, developing an IND application for the FDA, and a human phase 1 clinical trial are currently being planned.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by IACUC committee at the University of Illinois College of Medicine at Rockford.
DC, RK, and SG planned the experiments. CT, JD, RC and SG prepared the tag-free vaccine antigens. VK, and NC prepared the His-tagged vaccine antigens. DN and VK performed the circular dichroism studies. VM, VK, NC, and RK performed the mouse and gerbil studies. DC, RK and SG drafted the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by an NIH grant (R43 AI140708 and R44 AI140708).
The infective larval stage (L3) of B. malayi was obtained from the NIH/NIAID Filariasis Research Reagent Resource Center, College of Veterinary Medicine, University of Georgia, Athens, GA under NIAID supply contract AI no. 30022.
DC is named inventor on the AL019 adjuvant formulation. RK is inventor of the rBmHAXT antigen. Authors CT, JD, DC and SG were employed by PAI Life Sciences, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.998353/full#supplementary-material
1. WHO. Available at: https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis.
2. Pisarski K. The global burden of disease of zoonotic parasitic diseases: Top 5 contenders for priority consideration. Trop Med Infect Dis (2019) 4:E44. doi: 10.3390/tropicalmed4010044
3. Klion A. Lymphatic filariasis: Epidemiology, clinical manifestations, and diagnosis. (2022). Available at: https://www.uptodate.com/contents/lymphatic-filariasis-epidemiology-clinical-manifestations-and-diagnosis (Accessed on August 30, 2022).
4. Davis EL, Reimer LJ, Pellis L, Hollingsworth TD. Evaluating the evidence for lymphatic filariasis elimination. Trends Parasitol (2019) 35:860–9. doi: 10.1016/j.pt.2019.08.003
5. de Souza DK, Otchere J, Ahorlu CS, Adu-Amankwah S, Larbi IA, Dumashie E, et al. Low microfilaremia levels in three districts in coastal Ghana with at least 16 years of mass drug administration and persistent transmission of lymphatic filariasis. Trop Med Infect Dis (2018) 3:105. doi: 10.3390/tropicalmed3040105
6. Kumar A, Bajpei A, Singh T, Masih SN. Mass drug administration (MDA) to eliminate lymphatic filariasis: Even 100% coverage would fail to achieve elimination of infection in community in 6 years. Int J Inf Dis (2019) 79:143. doi: 10.1016/j.ijid.2018.11.350
7. Dyson L, Stolk WA, Farrell SH, Hollingsworth TD. Measuring and modelling the effects of systematic non-adherence to mass drug administration. Epidemics (2017) 18:56–66. doi: 10.1016/j.epidem.2017.02.002
8. Kalyanasundaram R, Khatri V, Chauhan N. Advances in vaccine development for human lymphatic filariasis. Trends Parasitol (2020) 36(2):195–205. doi: 10.1016/j.pt.2019.11.005
9. Kalyanasundaram R. Lymphatic filariasis: Current status of elimination using chemotherapy and the need for a vaccine. In: Saxena AK, editor. Chapter 3. communicable diseases of the developing world. topics in medicinal chemistry, vol. vol 29. Cham: Springer (2016). p. pp 1–36. doi: 10.1007/7355_2015_5002
10. Kalyanasundaram R, Tyagi BK. Next step lymphatic filariasis eradication: Current status in the development of a vaccine against lymphatic filariasis, lymphatic filariasis. Singapore: Springer Nature (2018) p. 59–80.
11. Ahmad G1, Zhang W, Torben W, Ahrorov A, Damian RT, Wolf RF, et al. Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of Schistosoma mansoni infection and immunoglobulin G and e responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J Infect Dis (2011) 204:1437–49. doi: 10.1093/infdis/jir545
12. Steel C, Ottesen EA. Evolution of immunologic responsiveness of persons living in an area of endemic bancroftian filariasis: A 17-year follow-up. J Inf Dis (2001) 184:73–9. doi: 10.1086/321004
13. Brown SP, Grenfell BT. An unlikely partnership: parasites, concomitant immunity and host defense. Proc Biol Sci (2001) 268:2543–9. doi: 10.1098/rspb.2001.1821
14. Day KP. The endemic normal in lymphatic filariasis: A static concept. Parasitol Today (1991) 7(12):341–3. doi: 10.1016/0169-4758(91)90215-a
15. Dakshinamoorthy G, Samykutty AK, Munirathinam G, Reddy MV, Kalyanasundaram R. Multivalent fusion protein vaccine for lymphatic filariasis. Vaccine (2013) 31(12):1616–22. doi: 10.1016/j.vaccine.2012.09.055
16. Chauhan N, Khatri V, Banerjee P, Ramaswamy K. Evaluating the vaccine potential of a tetravalent fusion protein (rBmHAXT) vaccine antigen against lymphatic filariasis in a mouse model. Front Immunol Sec Vaccines Mol Ther (2018) 9. doi: 10.3389/fimmu.2018.01520
17. Khatri V, Chauhan N, Kalyanasundaram R. Fecundity of adult female worms were affected when Brugia malayi infected Mongolian gerbils were immunized with a multivalent vaccine (rBmHAXT) against human lymphatic filarial parasite. Acta Tropica (2020) 208:105487–97. doi: 10.1016/j.actatropica.2020.105487. ISSN 0001-706X.
18. Khatri V, Chauhan N, Vishnoi K, Gegerfelt. A, Gittens. C, Kalyanasundaram R. Prospects of developing a prophylactic vaccine against human lymphatic filariasis - evaluation of protection in non-human primates. Int J Parasitol (2018) 48:773–83. Corrigendum in Int. J. Parasitol. 2018; 48: 1071. doi: 10.1016/j.ijpara.2018.04.002
19. Arumugam S, Wei J, Liu Z, Abraham D, Bell A, Bottazzi ME, et al. Vaccination of gerbils with bm-103 and bm-RAL-2 concurrently or as a fusion protein confers consistent and improved protection against brugia malayi infection. PloS Negl Trop Dis (2016) 10:e0004586. doi: 10.1371/journal.pntd.0004586
20. Carter D, Fox CB, Day TA, Guderian JA, Liang H, Rolf T, et al. A structure- function approach to optimizing TLR4 ligands for human vaccines. Clin Transl Immunol (2016) 5:e108. doi: 10.1038/cti.2016.63
21. Dakshinamoorthy G, von Gegerfelt A, Andersen H, Lewis M, Kalyanasundaram R. Evaluation of a multivalent vaccine against lymphatic filariasis in Rhesus macaque model. PloS One (2014) 9(11):e112982. doi: 10.1371/journal.pone.0112982
22. Kurtz SL, Rossi AP, Beamer GL, Gatti DM, Kramnik I, Elkins KL. The diversity outbred mouse population is an improved animal model of vaccination against tuberculosis that reflects heterogeneity of protection. mSphere (2020) 5:e00097–20. doi: 10.1128/mSphere.00097-20
23. Shultz CL, Badowski M, Harris DT. The immune response in inbred and outbred strains of mice before and after bone marrow transplantation. Cell Tissue Transplant Ther (2013) 9. doi: 10.4137/CTTT.S10938
24. Tuttle AH, Philip VM, Chesler EJ, Mogil JS. Comparing phenotypic variation between inbred and outbred mice. Nat Methods (2018) 15:994–6. doi: 10.1038/s41592-018-0224-7
25. Anand SB, Murugan V, Prabhu PR, Anandharaman V, Reddy MVR, Kaliraj P. Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (ALT-2) and thioredoxin peroxidase (TPX) in mice. Acta Trop (2008) 107:106–12. doi: 10.1016/j.actatropica.2008.04.018
26. Anugraha G, Madhumathi J, Prince PR, JeyaPrita PJ, Khatri VK, Amdare NP, et al. Chimeric epitope vaccine from multistage antigens for lymphatic filariasis. Scand J Immunol (2015) 82:380–9. doi: 10.1111/sji.12340
27. Immanuel C, Ramanathan A, Balasubramanian M, Khatri V, Amdare N, Rao DN, et al. Immunoprophylaxis of multi-antigen peptide (MAP) vaccine for human lymphatic filariasis. Immunol Res (2017) 65:729–38. doi: 10.1007/s12026-017-8911-5
28. Shrivastava N, Singh PK, Nag JK, Kushwaha S, Misra-Bhattacharya S. Immunization with a multisubunit vaccine considerably reduces establishment of 639 infective larvae in a rodent model of Brugia malayi. Comp Immunol Microbiol Infect Dis (2013) 36:507–19. doi: 10.1016/j.cimid.2013.05.003
29. Madhumathi J, Prince PR, Rao DN, Karande AA, Reddy MVR, Kaliraj P. Epitope mapping of Brugia malayi ALT-2 and the development of a multi-epitope vaccine for lymphatic filariasis. J Helminthol (2017) 91:43–54. doi: 10.1017/S0022149X16000055
30. Prince PR, Madhumathi J, Anugraha G, Jeyaprita PJ, Reddy MVR, Kaliraj P. Tandem antioxidant enzymes confer synergistic protective responses in experimental filariasis. J Helminthol (2014) 88:402–10. doi: 10.1017/S0022149X13000333
31. Vanam U, Pandey V, Prabhu PR, Dakshinamurthy G, Reddy MVR, Kaliraj P. Evaluation of immunoprophylactic efficacy of Brugia malayi transglutaminase (BmTGA) in single and multiple antigen vaccination with BmALT-2 and BmTPX for human lymphatic filariasis. Am J Trop Med Hyg (2009) 80:319–24. doi: 10.4269/ajtmh.2009.80.319
Keywords: lymphatic filariasis, vaccine, process development, rBmHAXT, TLR4 agonist, alum
Citation: Melendez V, Turner C, Khatri V, Davis J, Chauhan N, Nagalati Sudhakar DS, Cabullos R, Carter D, Gray SA and Kalyanasundaram R (2022) Pre-clinical development of a vaccine for human lymphatic filariasis. Front. Trop. Dis 3:998353. doi: 10.3389/fitd.2022.998353
Received: 19 July 2022; Accepted: 22 August 2022;
Published: 12 September 2022.
Edited by:
Bin Zhan, Baylor College of Medicine, United StatesReviewed by:
Mehdi Mahdavi, Motamed Cancer Institute, IranCopyright © 2022 Melendez, Turner, Khatri, Davis, Chauhan, Nagalati Sudhakar, Cabullos, Carter, Gray and Kalyanasundaram. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramaswamy Kalyanasundaram, cmFtc3dhbXlAdWljLmVkdQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.