- 1Laboratório de Bioquímica de Insetos e Parasitos, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
- 2Instituto Nacional de Ciência e Tecnologia em Entomologia Molecular (INCT-EM), Rio de Janeiro, Brazil
- 3Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD, United States
- 4Laboratório de Bioquímica de Microrganismos, Instituto de Microbiologia Paulo de Góes, Departamento de Microbiologia Geral, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil
- 5Instituto de Medicina Molecular João Lobo Antunes, Faculdade de Medicina, Universidade de Lisboa, Lisboa, Portugal
Editorial on the Research Topic
Parasite interactions with insect hosts in tropical diseases
Vector-borne diseases such as malaria, dengue, schistosomiasis, Human African trypanosomiasis, leishmaniasis, Chagas disease, yellow fever, Japanese encephalitis, and onchocerciasis, are still a major health concern resulting in more than 700 thousand deaths worldwide (1). Over the years, numerous strategies have been implemented to control vector populations. However, vector-borne pathogen transmission still represents a major global health burden (2). Therefore, understanding insect vector-pathogen interactions is key to developing new and efficient strategies to control vector-borne diseases. The collection ‘Parasite interactions with insect hosts in tropical diseases’ received four submissions. Notably, all contributions come from labs with a long-standing interest in vector-pathogen interactions, and which are all located in regions where insect-borne diseases are endemic.
The paper by Barletta et al. explored the importance of sexual dimorphism in immune responses and infection resistance across various hematophagous insect vectors. This cross-species study revealed that unchallenged adult males have higher expression of immune-related genes than females and presented lower midgut basal microbiota in the most studied species, Aedes aegypti, Anopheles aquasalis and Lutzomyia longipalpis. Although divergence in immune gene expression and microbiota levels were not observed in A. aegypti during pupal stages or immediately after mosquito emergence, sexual dimorphism is still observed after antibiotic treatment. Sexual immune differences appear to be microbiota-independent, but might depend on midgut maturation and microbiome establishment. A more robust immune system in males would allow them to better overcome immune challenges. This can translate as an evolutionary strategy to remain fit, increasing the capacity of insemination of more females. Conversely, in Rhodnius prolixus, which transmits Trypanosoma cruzi, the expression of immune genes is more pronounced in unfed females than males. In R. prolixus, male and female adults share a restricted hematophagous diet. In contrast, among Aedes spp., Anopheles spp., and Luzomyia spp., the males feed solely on carbohydrate-rich solutions, while females additionally feed on blood. Therefore, feeding habits can contribute to more potent immune responses in males. The authors discuss that life-history strategies between sexes in a given species might be crucial to direct differences in the immune system and therefore affect pathogen transmission.
Next, in their primary work, Sousa et al. focused on how the kinetoplastid parasite T. cruzi regulates in its favor the lipid metabolism of its insect vector, R. prolixus. The uptake of the lipid component of R. prolixus lipophorin by T. cruzi affects the dietary lipid storage of the hemipteran. T. cruzi infection of R. prolixus led to downregulation of genes involved in metabolic pathways across different triatomine organs. Conversely, upregulation of lipid receptor transcripts suggests a compensation to take more lipids from lipoproteins of the hemolymph. Also, several lipid classes were involved in response to infection, despite modulating only the insect fat body. As T. cruzi is a gut-restricted parasite within its vector, the authors suggest that the association between the parasite and the vector tissues is possible through cell signaling molecules.
The issue also received two reviews which explored the interconnection of viruses and insect vectors from different points of view: one, on the epidemiological relevance of vector-borne viruses of public health importance; the other, on the role of the virome in vectors that act as parasite hosts, as is the case of R. prolixus.
Pereira et al. explored the entomo-epidemiological literature on vector-virus interactions and the transmission of two arboviruses: Mayaro virus (MAYV) and Oropouche orthobunyavirus (OROV). The authors discuss that although viruses such as Dengue, Zika, and Chikungunya have caused worrying epidemics over the last decades and are now the focus of major clinical and basic research investments, other viruses of the same family remain neglected despite their relevance to human health. The authors emphasize the relevance of environmental alterations (e.g., global warming) causing vectors to expand their geographical spread. Moreover, both viruses have unique genetic features that impact their spreading: MAYV has a remarkable genetic plasticity, which increases its capacity to adapt to multiple vertebrate and insect hosts, while OROV can interchange segments of RNA with other strains through genetic reassortment, allowing the development of novel viral strains. While MAYV and OROV have consistently appeared in the scientific literature since their discovery in the 1950s, it was only over the last decade that reports and research on these viruses significantly increased. This coincides with outbreaks in major cities of various Central and South American countries. The authors emphasize the importance of monitoring these arboviruses to avoid viral outbreaks similar to those recently faced with Dengue, Zika, and Chikungunya (3).
Finally, in the mini-review by Cardoso et al. the authors focus on the virome of R. prolixus, insect vectors of the parasite T. cruzi. Although the interaction between triatomes and their bacteriome has been explored to a large extent, viral species infecting triatomine insects remain largely unknown. In other insects, the vector’s virome has been shown to range from beneficial, symbiotic, or detrimental to the insect vector due to reduced survival, altered general homeostasis, and/or compromised reproduction capacities. The authors highlight the scientific knowledge on various Triatoma viruses (TrV) and explore their relevance in two contexts: a) some of these viruses have been detected in humans, raising the question on the role of the triatomine vector in viral transmission and the clinical relevance of TrVs, and b) TrV infections result in high mortality rates and a highly reduced oviposition capacity among the triatome population. The authors discuss the possibility of using TrVs as a biological tool against the triatomine vector population in the context of Chagas disease transmission-blocking. This strategy is not unheard of, as insect-infective pathogens have been previously explored as a tool for vector control of other infectious diseases (4–8). Although this work focuses solely on triatomine insects, it reminds the reader of the importance of considering a comprehensive toolkit in the fight against vector-borne parasitic diseases.
Together, the collection of articles in this Research Topic contemplates vector-pathogen interactions with significant momentum, including the relevance of the vector microbiome to vector survival and parasite transmission; the connection between sexual dimorphism and immune responses across different species; the effect of parasitism in the metabolism of the insect-vector; and emerging vector-borne arboviruses and their relevance to public health summarized in Figure 1.
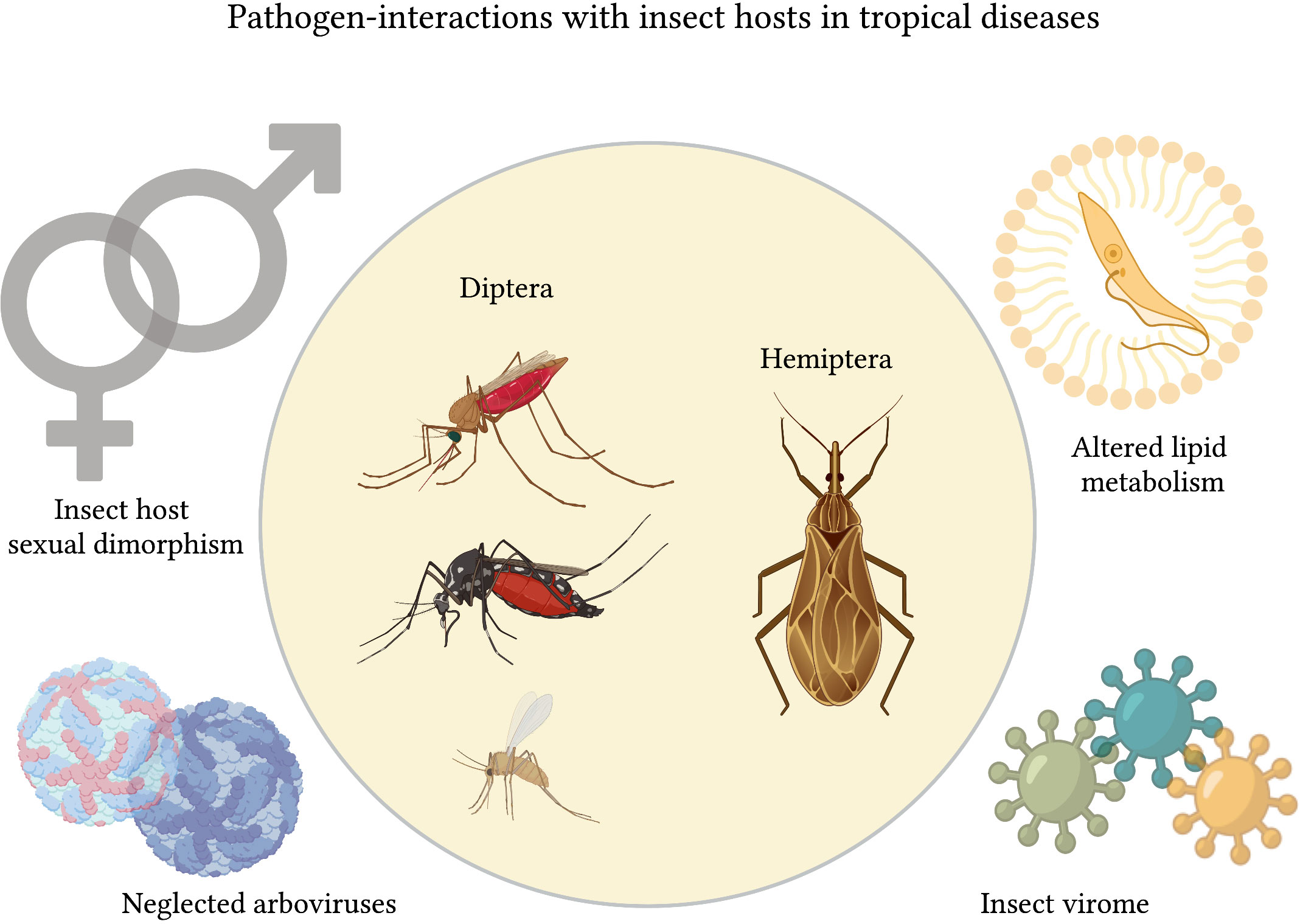
Figure 1 Collection overview. The collection ‘Parasite interactions with insect hosts in tropical diseases’ received submissions covering 4 main topics: immune sexual dimorphism among insect vectors; neglected arboviruses infecting mosquitoes; altered lipid metabolism in Rhodnius prolixus due to acute infection with T. cruzi; and the relevance of the R. prolixus virome for vector control. Figure created with BioRender.com.
Altogether, these topics represent the broader view under a ‘One Health’ approach (9, 10) guiding basic research, clinical research and public policy in the near and long-term future.
Author contributions
ACB, AFB, AL and MDN contributed equally to writing the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. Ethics and vector borne diseases (2020). Available at: https://apps.who.int/iris/handle/10665/336075 (Accessed July 2022).
2. WHO. Vector-borne diseases (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (Accessed July 2022).
3. Jones R, Kulkarni MA, Davidson TMV, Talbot B. Arbovirus vectors of epidemiological concern in the americas: A scoping review of entomological studies on zika, dengue and chikungunya virus vectors. PLoS One (2020) 15:e0220753. doi: 10.1371/journal.pone.0220753
4. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A wolbachia symbiont in aedes aegypti limits infection with dengue, chikungunya, and plasmodium. Cell (2009) 139:1268–78. doi: 10.1016/j.cell.2009.11.042
5. Andrew P, Yuemei D, Borhani DN, Anthony G, F ME, George D. Changes in the microbiota cause genetically modified anopheles to spread in a population. Sci (2017) 357:1396–9. doi: 10.1126/science.aak9691
6. Sibao W, Dos-Santos ALA, Wei H, Connie LK, Ali OM, Ge W, et al. Driving mosquito refractoriness to plasmodium falciparum with engineered symbiotic bacteria. Science (2017) 357:1399–402. doi: 10.1126/science.aan5478
7. Lovett B, Bilgo E, Millogo SA, Ouattara AK, Sare I, Gnambani EJ, et al. Transgenic metarhizium rapidly kills mosquitoes in a malaria-endemic region of Burkina Faso. Science (2019) 364:894–7. doi: 10.1126/science.aaw8737
8. Pinto SB, Riback TIS, Sylvestre G, Costa G, Peixoto J, Dias FBS, et al. Effectiveness of wolbachia-infected mosquito deployments in reducing the incidence of dengue and other aedes-borne diseases in niterói, Brazil: A quasi-experimental study. PLoS Negl Trop Dis (2021) 15:e0009556. doi: 10.1371/journal.pntd.0009556
9. CDC. One health (2022). Available at: https://www.cdc.gov/onehealth/index.html (Accessed July 2022).
10. WHO. One health global (2022). Available at: https://www.who.int/health-topics/one-health#tab=tab_1 (Accessed July 2022).
Keywords: vector, host, parasite, one health, virome, sexual dimorphism, arbovirus, infection
Citation: Bahia AC, Barletta ABF, Lopes AH and De Niz M (2022) Editorial: Parasite interactions with insect hosts in tropical diseases. Front. Trop. Dis 3:992277. doi: 10.3389/fitd.2022.992277
Received: 12 July 2022; Accepted: 25 July 2022;
Published: 17 August 2022.
Edited and Reviewed by:
Baldwyn Torto, International Centre of Insect Physiology and Ecology (ICIPE), KenyaCopyright © 2022 Bahia, Barletta, Lopes and De Niz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariana De Niz, bWFyaWFuYS5kZW5pemhAZ21haWwuY29t
 Ana C. Bahia
Ana C. Bahia Ana Beatriz F. Barletta
Ana Beatriz F. Barletta Angela H. Lopes
Angela H. Lopes Mariana De Niz
Mariana De Niz