
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis., 05 September 2022
Sec. Disease Prevention and Control Policy
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.987698
This article is part of the Research TopicPreventive Medicine System in Vietnam: Historical Development, Strategies, and Responses to COVID-19 PandemicView all 8 articles
 Xuan Thi Thanh Le*
Xuan Thi Thanh Le* Quan Long Hoang
Quan Long Hoang Nhung Thi Kim Ta
Nhung Thi Kim Ta Quan Thi Pham
Quan Thi Pham Thao Thanh Nguyen
Thao Thanh Nguyen Huong Thi Mai Phan
Huong Thi Mai Phan Thanh Van Nguyen
Thanh Van Nguyen Ha Thi Thanh Le
Ha Thi Thanh Le Nam Thuy Nguyen
Nam Thuy Nguyen Linh Dieu Hoang
Linh Dieu Hoang Phuong Thi Huyen Luong
Phuong Thi Huyen Luong Lien Hong An
Lien Hong An Thu Ha Nguyen
Thu Ha Nguyen Thinh Thi Nguyen
Thinh Thi Nguyen Hien Thuy Nguyen
Hien Thuy Nguyen Huong Thu Le
Huong Thu Le Doanh Quoc Nguyen
Doanh Quoc Nguyen Phuong Viet Nguyen
Phuong Viet Nguyen Tuan Xuan Nguyen
Tuan Xuan Nguyen Toan Thi Thanh Do
Toan Thi Thanh Do Thang Huu Nguyen
Thang Huu NguyenRationale: To prevent and control the COVID-19 pandemic, the biggest immunization campaign in history had been deployed worldwide. Therefore, it is important to inform the adverse events following immunization (AEFI) to populations.
Objectives: To prevent vaccine hesitancy, this study focused on finding the common AEFI with the COVID-19 Comirnaty vaccine (Pfizer-BioNTech) among participants aged 18 and above and related factors in Hanoi, Vietnam.
Methods: A cross-sectional study was carried out to collect participants’ data and AEFI after being vaccinated at Hanoi Medical University, Vietnam, in 2021. Logistic regression was utilized for analyzing the correlated factors of AEFI.
Results: We recruited a random sample of 820 participants who received both basic doses of Pfizer vaccine in September and October 2021. The proportion of AEFI after the first dose, second dose, and both doses of Pfizer vaccine was 24.4%, 64.2%, and 18.5%, respectively. AEFI mostly appeared within 1 day and lasted for 1 to 2 days. The AEFI were more common in females (OR=1.7; 95%CI=1.25–2.29) and younger age groups (OR=1.9; 95%CI=1.37–2.58). History of allergy, allergic diseases, chronic diseases, and occupations were not statistically significant with AEFI.
Conclusion: Our findings indicated that the COVID-19 Comirnaty vaccine is safe to be injected. Gender and age group are important factors influencing AEFI.
Up to 31 July 2022, COVID-19 pandemic has been affecting numerous areas and people worldwide. By the end of April 2022, Vietnam had 10,649,809 infections, with an average of 107,648 cases for every 1 million people (1). Although there is no specific treatment for the COVID-19 disease, the COVID-19 vaccines are developing and strongly recommended for effective prevention and control of the pandemic.
Vietnam started the COVID-19 vaccination campaign on March 8, 2021, originally targeting high-risk groups (1, 2). According to the Ministry of Health, to reach the target of 90% vaccine coverage among the over 18 population in the COVID-19 vaccination campaign, the Vietnamese Government has invested in the country’s budget and also received support from International Organization (COVAX) and foreign governments to ensure the COVID-19 vaccine supply. Moreover, Vietnam utilized diversified types of vaccines, including ChAdOx1 COVID-19 vaccine (AstraZeneca), Comirnaty vaccine (Pfizer-BioNTech), Spikevax (Moderna), Vero cell (Sinopharm), Gam-COVID-Vac (Sputnik), and Abdala (Abdala), so that the COVID-19 vaccination campaign can be conducted uninterruptedly as soon as possible. COVID-19 vaccines provide strong protection against serious illness, hospitalization, and death (3, 4). In Vietnam, as of May 4, 2022, the total number of vaccine doses administered was 214,774,198, among which the number of doses for people aged 18 years and above is 195,944,846 (including 71,457,483 first doses; 68,638,476 second doses; 1,505,935 third doses; 15,305,712 additional doses; 39,037,240 booster doses). The number of doses for children aged 12–17 years is 17,372,711 (including 8,906,086 first doses and 8,466,625 second doses). The number of doses for children aged 5–11 years is 1,456.641 (first doses) (5). The Comirnaty vaccine is also one of the COVID-19 vaccines utilized for immunization against COVID-19.
However, already before the pandemic, vaccine hesitancy was named as one of the top 10 threats to global health in 2019 by the World Health Organization (6). Many people based their hesitancy on the fact that the COVID-19 virus spread rapidly but had a low mortality rate so they refused to get vaccinated because of the adverse events after immunization (7).
Despite the benefits that the vaccine offers to global health, certain concerns about vaccine safety have increased significantly over the years. A considerable amount of the population hesitates or refuses to get vaccinated or has the tendency to be fastidious between different types of vaccine. Moreover, the vaccine’s adverse events following immunization (AEFI) have made healthcare workers become more conservative when it comes to vaccinating a new kind of vaccine, which results in vaccine hesitancy and refusal. Since the COVID-19 vaccine has only been introduced for 18 months, studies and research about AEFI are still limited, particularly for Comirnaty that was one of the common COVID-19 vaccines used for basic dose and booster in Vietnam.
Since COVID-19 vaccines are newly introduced, therefore, it is important to report adverse reaction following vaccination in Vietnam to inform the population. The present study aims to describe the common AEFI and analyze the factors influencing common adverse events among people aged 18 and above who were vaccinated with COVID-19 Comirnaty vaccine (Pfizer-BioNTech) at Hanoi Medical University in 2021.
The present study comprised people aged 18 and above at the time of vaccination, who got vaccinated with the COVID-19 Comirnaty vaccine (Pfizer-BioNTech) at the Hanoi Medical University vaccination site, Vietnam, in September and October, 2021. Inclusion criteria included participants who received both doses of the same vaccine at the HMU vaccination site. Written informed consent was obtained from the participants who agreed to participate in the research.
A cross-sectional descriptive study was carried out during the period from October 2021 to May 2022, in which data were collected in September (AEFI after first dose) and in October (AEFI after second dose) 2021.
Random sampling was conducted to select 820 participants who received both doses of Pfizer vaccine against COVID-19 from 2,147 participants in September and 1,112 participants in October 2021. The interval between two doses was 3 weeks according to the Ministry of Health guidance. Each member of the subset carries an equal opportunity of being chosen as a part of the sampling process for the sample to represent the population.
Firstly, the list of participants who were vaccinated with both basic doses of the vaccine was extracted from the immunization software. The list included ID immunization, full name, and telephone contact. Secondly, the list was alphabetically sorted by full name of participants. Finally, the participants were randomly selected by Stata software (using the command: sample 800, count).
We selected a random sample of 820 participants, but in reality, 813 adults agreed to participate in our study with a response rate of 99.1%.
The AEFI after the first dose were collected by medical doctors when the participants got the second dose (the interval was 3–4 weeks). The participants filled in the form with their personal information and adverse events after immunization of the first dose.
A phone-based interview was carried out within 1 month after the participants received the second dose of the vaccine. The participants being interviewed were the ones who also received the vaccine for their first dose. The same questionnaire was used for the data collection of the second dose.
The questionnaire was composed of two parts: demographic characteristics (age, gender, and occupation) and health history (allergic information, allergic diseases, and chronic diseases). The questionnaire was pretested before official use.
Data were entered and cleaned using Epidata 3.1 software and analyzed using STATA 14.0 software. Descriptive statistics of frequencies and percentages were utilized for describing the current status of adverse events after immunization with the COVID-19 Comirnaty vaccine (Pfizer-BioNTech), and logistic regression analysis was utilized for identifying the factors influencing the rate of adverse events after immunization of the COVID-19 Comirnaty vaccine (Pfizer-BioNTech) of people aged above 18 years. Independent variables were AEFI (yes/no).
Experiencing AEFI was defined if the participant had at least one symptom after immunization, either first dose or second dose. Experiencing AEFI in both doses was defined if the study participant had at least one symptom in the first dose and the second dose. The participants’ age was counted from their date of birth to 2021. The participants were divided into two groups according to their age as follows: 18 to 55 years old and >55 years old (8).
The research outline was approved by the Scientific Council of the School of Preventive Medicine and Public Health, Hanoi Medical University, in 2021, before official data collection. Written informed consent was obtained from all study participants.
Table 1 presents the demographic characteristics and health history of 813 study participants in the research. More than half of the study participants were 18 to 55 years old (69.7%). In terms of gender, the participation of females (55.2%) was more frequent than males (44.8%). The most prevalent occupation was others, including retirement and freelancers (57.1%), whereas the least prevalent belonged to medical students (0.9%). For health history, 2.1%, 2.0%, and 2.7% of the participants reported having allergy histories, allergic diseases, and chronic diseases, respectively.
The prevalence of people who experienced AEFI after both doses was 18.5%. The appearance of the AEFI after the second dose was more common than in the first dose (64.2% vs. 24.4%, respectively). The difference was statistically significant (Table 2). In particular, the frequency of AEFI after the second dose was 5.6 times higher than the first dose (OR=5.6; 95%CI: 4.49–6.91).
The most common AEFI was the local effect after both doses (14.8% after the first dose and 40.2% after the second dose). The most prevalent systemic effect was tiredness after both doses (10.3% after the first dose and 38.0% after the second dose). The prevalence of fever was 4.3% after the first dose and 24.4% after the second dose. The lowest effects after the first dose were throat allergic reactions, gastrointestinal effects, and other unusual effects (0.1%). The prevalence of skin allergic reactions and respiratory effects was 0.4% after the second dose. None of the participants experienced allergic effects around the mouth (Table 3).
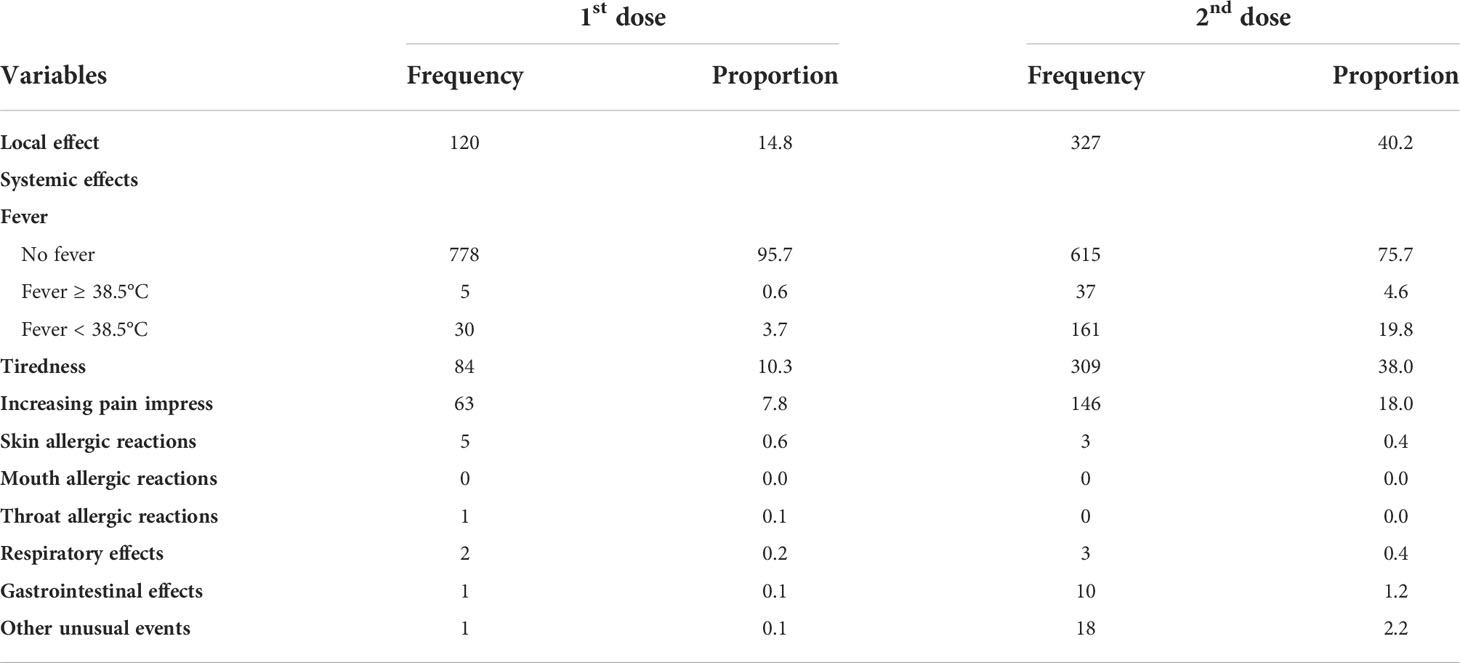
Table 3 Details of common adverse events after immunization of COVID-19 Comirnaty vaccine (Pfizer-BioNTech).
Table 4 shows the average time of the symptom onset. After the first dose, gastrointestinal effects and other unusual events appeared the earliest (within the first 2 h), whereas respiratory effects appeared the latest (after 38 h). After the second dose, local effects appeared the earliest (after 6 h), whereas other unusual events appeared the latest (after 21 h). Fever and tiredness were likely to appear at the same time.
Table 5 describes the average time that the symptom lasted. After the first dose, gastrointestinal and other unusual events disappeared the fastest (after 6 h), while skin allergic reactions lasted the longest (after 120 h). After the second dose, the fever disappeared the fastest (after 24 h), whereas other unusual events lasted the longest (after 215 h).
Table 6 displays the association between AEFI and demographic characteristics (age group, gender, and occupation) and health history (allergic disease, allergy history, and chronic diseases). Gender and age groups were significantly associated with AEFI (p-value<0.05). Participants aged from 18 to 55 years were more likely to experience AEFI compared to those aged above 55 years. The number of participants aged 18 to 55 years who experienced AEFI was 1.9 times higher than those aged above 55 years (OR=1.9; 95%CI=1.37–2.58). Females were more likely to experience AEFI than males. The number of females who experienced AEFI was 1.7 times higher than males (OR=1.7; 95%CI=1.25–2.29). However, the association between AEFI and occupation and health history was not statistically significant (Table 6).
The economic and social disruption caused by the COVID-19 pandemic is devastating. So far, vaccination is the best solution to protect people against the disease. In Vietnam, the biggest vaccination campaign had been deployed. The current study was conducted to describe the common adverse events among participants aged 18 and above vaccinated with COVID-19 Comirnaty vaccine (Pfizer-BioNTech) at Hanoi Medical University in 2021, as well as associated factors. A total of 813 participants who took part in this research had diverse occupations and health histories.
The present study is novel as it is one of the first safety data from Vietnam. It indicated that the most common AEFI of COVID-19 Comirnaty vaccine was localized pain in the injection site (14.8% after the first dose and 40.2% after the second dose). Moreover, the result showed that the appearance of the AEFI in the second dose was 5.6 times more than in the first dose. This result agreed with the result of the study conducted by Dr. Andrea Ossato and colleagues from 1 January to 28 February 2021 in Italy, where the number of subjects who experienced side effects after receiving the second dose (69.70%) was higher than those who reported side effects after the first dose (31.67%) (9). The explanation for this result is the response of the immune system to the COVID-19 mRNA vaccine (Pfizer-BioNTech Comirnaty vaccine). The adverse effects are more prevalent after the second dose because the immune system recognizes the virus spike protein from the first dose of the vaccine and responds stronger. To be specific, after the first dose, the virus protein that the vaccine causes the body’s cells to produce—known as the spike protein—is new to the body and the body recognizes the protein as an antigen, or something foreign, and starts reacting to it with inflammation at the injection site. This is also why the most common AEFI is localized pain at the injection site (10). It is similar in a cohort study in Hong Kong which pain at injection site was the most common adverse reactions reported for both doses of Comirnaty vaccine (11). After causing inflammation at the injection site, the cells send signals telling the body to create antibodies against the spike protein. This process can cause inflammation in other parts of the body, resulting in headache, fatigue, and fever (12). When receiving the second shot, the body already has some antibodies and some cells that remember the spike protein from the first dose. When these cells see it again, they recognize it and launch a very quick and stronger response. This response causes widespread inflammation that can lead to flu-like symptoms. The process also creates many more antibodies to help protect the vaccine receivers from the disease in the future (13).
The results also demonstrated that the period of symptoms onset was from 1 to 24 h after both doses and mostly lasted for 1 to 2 days. This result was similar to the result of the study conducted by Dr. Giancarlo Ripabelli and colleagues in Italy, which also indicated that adverse events generally occurred after 4–12 h from the first injection and resolved after 12 to 24 h (14). This finding indicated that participants were more likely to experience mild to moderate reactions because anaphylaxis usually occurs within the first hour after immunization. Furthermore, most symptoms lasted only for 1 or 2 days indicating that participants did not have to suffer from serious reactions after injection. Hence, the COVID-19 Comirnaty vaccine is safe to be injected because the AEFI are mostly mild and automatically wore off after 1 to 2 days.
Our study indicated that females were more likely to experience AEFI. The number of females who experienced AEFI was 1.7 times higher than that of males. This result was similar to the result of the study by Dr. Andrea Ossato and colleagues from 1 January to 28 February 2021 in Italy, in which the number of female participants who reported to have side effects after receiving both doses of the vaccine was significantly higher when compared to males. Among the population that suffered from some side effects of the COVID-19 vaccine, after the first dose, 24.73% were males and 75.27% were females, and after the second dose, 30.32% were males and 69.68% were females (9). Similar trend was also found in some previous studies in Vietnam, Italy, India, and Nepal (9, 15–18). This could be explained by the opposite role of estrogens and testosterone. Estrogens stimulate the immune system to produce a large number of antibodies, as demonstrated in response to the flu vaccine, while testosterone has an immunosuppressive action (19). Another possible explanation is the genetic factors. Based on the fact that several genes regulating the immune system are located on the X chromosome, it has been reported that 15% of genes are expressed at higher levels in women than in men because of escape from X inactivation. This could also concur to explain why some autoimmune diseases are more common in women (20). Furthermore, the reason that might be responsible for this situation is that females are usually more cautious about their health situation and, therefore, have a higher potential to detect and remember the AEFI.
In the current study, participants from the younger age group reported AEFI more frequently than the older age group. The number of participants from 18 to 55 years old who experienced AEFI was 1.9 times higher than those above 55 years old. This result was similar to a study conducted by Dr. Giancarlo Ripabelli and colleagues in Italy, which showed that the number of participants aged ≤ 55 years who experienced AEFI was 2.9 times higher than those aged above 55 years after both the first and second dose, and the number of participants aged ≤ 55 years who experienced AEFI was 2.8 times higher compared to those aged above 55 years (14). Immunosenescence could explain this result. The term immunosenescence describes the decreasing functional capacity and reduction in the efficiency of the immune system as the age increases. The consequence is the reduction of antibody production in the older age (21). In addition, the immune memory repertoire in elderly responders is larger compared with younger people with more naive T and B lymphocytes, which result to respond actively to the vaccine (22). Hence, younger participants react to the vaccine more frequently because their immune systems are stronger.
In the present research, compared to medical staff/students, people who worked as officers were more likely to experience AEFI (OR=1.2; 95%CI=0.46–2.94), as well as businessmen (OR=1.6; 95%CI=0.63–3.82) and others (OR=1.6; 95%CI=0.56–3.15). This result was different from the research conducted by Giacomo Pietro Vigezzi and colleagues in Italy in 2021, in which there were 52.7% who experienced AEFI and defined themselves as healthcare workers and 47.3% as non-healthcare workers (23). One possible cause that can lead to this result is that in the healthcare workers group, most of the participants were medical students aged 18 to 24 years who studied at Hanoi Medical University, Vietnam. Combined with our prior result about the association between age and AEFI rate, the difference might be affected by the young population in the healthcare workers group. However, the difference was not statistically significant and may need further investigation.
In the current study, the association between AEFI and medical history was not statistically significant. This result was similar to the study conducted by Ryuta Urakawa and colleagues in Japan in 2022, indicating that there was no evidence of the relationship between comorbidities and AEFI (24).
The study had some limitations. First, it was a cross-sectional study so we could not conclude the causal–effect relationship for AEFI and some factors. Second, the population receiving the COVID-19 Pfizer vaccine were not representative for some other occupations or rural areas of Vietnam. At the time of the research, COVID-19 vaccine was a new vaccine to prevent COVID-19 pandemic, and the study about the COVID-19 vaccine safety in reality was still rare in Vietnam and worldwide. Therefore, the comparison between our study results and other studies is limited. Lastly, this study was conducted from September 2021. At that point, Vietnam had just carried out the vaccination campaign for 6 months, and the number of COVID-19 cases was still moderately low. Therefore, this study was not able to detect AEFI patterns in participants who had COVID-19 before the vaccination.
Our findings indicated that the COVID-19 Comirnaty vaccine is safe to be injected because the AEFI are mostly mild and automatically wore off after 1 to 2 days. This study also provided a detailed assessment of factors related to AEFI in Vietnamese people who were vaccinated with the COVID-19 Comirnaty vaccine. Gender and age are major factors affecting AEFI of the vaccine.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The research outline was approved by the Scientific Council of the School of Preventive Medicine and Public Health, Hanoi Medical University in 2021 before official data collection. All study participants were written informed consent. The patients/participants provided their written informed consent to participate in this study.
XL, QH, NT, QP, TThanhN, HP, TVN, NN, LH, LA, THaN, HN, HTL, DN, TD, and THuN were responsible for conceptualization. XL, QH, and NT conducted data curation. XL, QH, QP, LA, and THaN performed formal analysis. XL, QH, NT, QP, TThanhN, HP, TVN, HTTL, NN, LH, PL, LA, THaN, TThiN, HN, HTL, DN, PN, TXN, TD, and THuN carried out investigation. XL, QH, NT, and LA were responsible for methodology. XL, QP, TD, and THuN contributed to project administration. XL, QP, TThanhN, TVN, THaN, TThiN, HN, HTL, DN, PN, TXN, TD, and THuN supervised the project. XL, QH, LA, QP, and TVN wrote the original draft. XL, QH, NT, QP, TThanhN, HP, TVN, HTTL, NN, LH, PL, LA, THaN, TThiN, HN, HTL, DN, PN, TXN, TD, and THuN reviewed and edited the paper. All authors contributed to the article and approved the submitted version.
The authors are grateful to the Vaccination Department Team, Hanoi Medical University, who supported this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO. Middle East respiratory syndrome coronavirus (MERS-CoV) (2019). Available at: https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov).
2. WHO. Coronavirus disease (COVID-19) (2020). Available at: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19.
3. WHO. Coronavirus disease (COVID-19): Vaccines (2022). Available at: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines.
4. WHO. WHO coronavirus (COVID-19) dashboard (2022). Available at: https://covid19.who.int/.
6. WHO. Ten threats to global health in 2019 (2019). Available at: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
7. Azarpanah H, Farhadloo M, Vahidov R, Pilote L. Vaccine hesitancy: Evidence from an adverse events following immunization database, and the role of cognitive biases. BMC Public Health (2021) 21(1):1686. doi: 10.1186/s12889-021-11745-1
8. CDC. Pfizer-BioNTech COVID-19 vaccine reactions & adverse events (2022). Available at: https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html.
9. Ossato A, Tessari R, Trabucchi C, Zuppini T, Realdon N, Marchesini F, et al. Comparison of medium-term adverse reactions induced by the first and second dose of mRNA BNT162b2 (Comirnaty, pfizer-BioNTech) vaccine: A post-marketing Italian study conducted between 1 January and 28 February 2021. Eur J Hosp Pharm (2021) 1–6. doi: 10.1136/ejhpharm-2021-002933
10. Manuela AH, Helmut JW, Peter E, Hans-Georg B, Bodo P. Age- and sex-graded data evaluation of vaccination reactions after initial injection of the BNT162b2 mRNA vaccine in a local vaccination center in Germany. Vaccines (2021) 9(8):911. doi: 10.3390/vaccines9080911
11. Lai FTT, Leung MTY, Chan EWW, Huang L, Lau LKW, Peng K, et al. Self-reported reactogenicity of CoronaVac (Sinovac) compared with comirnaty (Pfizer-BioNTech): A prospective cohort study with intensive monitoring. Vaccine (2022) 40(10):1390–6. doi: 10.1016/j.vaccine.2022.01.062
12. Renuka AKK, Ravali J, Sharanya P, Srikrishna VM. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int J Infect Dis (2021) 106(2021):376–81. doi: 10.1016/j.ijid.2021.04.047
13. Elterman K. Why are pfizer and moderna vaccines’ side effects worse after the second shot? (2021). Available at: https://www.goodrx.com/conditions/covid-19/why-side-effects-worse-after-second-covid-19-shot.
14. Ripabelli G, Tamburro G, Buccieri N, Adesso C, Caggiano V, Cannizzaro F, et al. Active surveillance of adverse events in healthcare workers recipients after vaccination with COVID-19 BNT162b2 vaccine (Pfizer-BioNTech, comirnaty): A cross-sectional study. J Community Health (2022) 47(2):211–25. doi: 10.1007/s10900-021-01039-3
15. WHO. Adverse events following immunization (AEFI) (2021). Available at: https://www.who.int/teams/regulation-prequalification/regulation-and-safety/pharmacovigilance/health-professionals-info/aefi.
16. Yoon D, Kim JH, Lee H, Shin Y. Updates on vaccine safety and post-licensure surveillance for adverse events following immunization in south Korea, 2005-2017. Yonsei Med J (2020) 61(7):623–30. doi: 10.3349/ymj.2020.61.7.623
17. (MHRA), T.M.a.H.p.R.A. Coronavirus vaccine - summary of yellow card reporting (2022). Available at: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting.
18. Ponticelli D, Madotto F, Conti S, Antonazzo IC, Vitale A, Ragione GD, et al. Response to BNT162b2 mRNA COVID-19 vaccine among healthcare workers in Italy: A 3-month follow-up. Intern Emerg Med (2022) 17(2):481–6. doi: 10.1007/s11739-021-02857-y
19. Potluri T, Fink AL, Sylvia KE, Dhakal S, Vermillion MS, Steeg L, et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines (2019) 4:29. doi: 10.1038/s41541-019-0124-6
20. Carrel L, Willard HF. X-Inactivation profile reveals extensive variability in X-linked gene expression in females. Nature (2005) 434(7031):400–4. doi: 10.1038/nature03479
21. Aw D, Silva AB, Palmer DB. Immunosenescence: Emerging challenges for an ageing population. Immunology (2007) 120(4):435–46. doi: 10.1111/j.1365-2567.2007.02555.x
22. Bajaj V, Gadi N, Spihlman AP, Wu SC, Choi CH, Moulton VR. Aging, immunity, and COVID-19: How age influences the host immune response to coronavirus infections? Front Physiol (2020) 11. doi: 10.3389/fphys.2020.571416
23. Vigezzi GP, Lume A, Minerva M, Nizzero P, Biancardi A, Gianfredi V, et al. Safety surveillance after BNT162b2 mRNA COVID-19 vaccination: Results from a cross-sectional survey among staff of a large Italian teaching hospital. Acta BioMed (2021) 92(S6):e2021450. doi: 10.23750/abm.v92iS6.12217
Keywords: adverse events following immunization, COVID-19 vaccine, adult, associated factors, Vietnam
Citation: Le XTT, Hoang QL, Ta NTK, Pham QT, Nguyen TT, Phan HTM, Nguyen TV, Le HTT, Nguyen NT, Hoang LD, Luong PTH, An LH, Nguyen TH, Nguyen TT, Nguyen HT, Le HT, Nguyen DQ, Nguyen PV, Nguyen TX, Do TTT and Nguyen TH (2022) Common adverse events following immunization with the COVID-19 comirnaty vaccine (Pfizer-BioNTech) among adult population in Hanoi, Vietnam, 2021. Front. Trop. Dis 3:987698. doi: 10.3389/fitd.2022.987698
Received: 09 July 2022; Accepted: 15 August 2022;
Published: 05 September 2022.
Edited by:
Tomabu Adjobimey, University Hospital Bonn, GermanyReviewed by:
Celine SL Chui, The University of Hong Kong, Hong Kong SAR ChinaCopyright © 2022 Le, Hoang, Ta, Pham, Nguyen, Phan, Nguyen, Le, Nguyen, Hoang, Luong, An, Nguyen, Nguyen, Nguyen, Le, Nguyen, Nguyen, Nguyen, Do and Nguyen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuan Thi Thanh Le, bGV0aGl0aGFuaHh1YW5AaG11LmVkdS52bg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.