
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Trop. Dis. , 21 September 2022
Sec. Major Tropical Diseases
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.985890
Background: Malaria remains one of the deadliest diseases in the tropic. Its severe form represents a major public health concern in sub-Saharan Africa. The study aimed to describe and analyze clinical features and outcomes of severe malaria in children from Libreville.
Methods: Medical records (March 2018- to December 2019) from the emergency ward of the “Mother and Child University Hospital” were analyzed. Children hospitalized for malaria who met one or more criteria of the severe form rating according to the WHO guideline were included in the study.
Results: One hundred thirty-four children (134) children were included in the study. All children were anemic with 44% of children showing severe anemia. Thirty-three percent (33%) of admitted children were comatose or agonizing. The most frequent form of severe malaria was cerebral malaria with 101 cases (75.4%). The death rate was 18.6% (25/134). Twenty-one (21) children (84% of the deceased) died within the first 48 hours of hospitalization. In the subgroup of the deceased children, hepatomegaly was significantly more frequent (88%) than in the subgroup of those who survived (2.8%) (χ2 = 97.38; p<0.0001); Leukocytosis was more pronounced in the subgroup of the children under one year p<0.0001). Deep acidotic breathing was more frequent in cerebral malaria (χ2 = 5.4; p = 0.02).
Conclusions: Data revealed a high malaria-associated fatality rate. Cerebral malaria was the most frequent severe form of malaria. The relatively high frequency of comatose and/or agonizing children on admission raises the question of parents’ awareness and poor initial assessment of children’s clinical state.
Malaria caused by Plasmodium falciparum is one of the most deadly, infectious diseases in Africa. In 2020 96% of all malaria cases and 98% of malaria associated death occurred in Africa (1). In the WHO African region, malaria cases rose from 211 million to 228 million cases between 2018 and 2020 (2). The number of deaths also increased from 533 000 to 602 000 deaths during the same period (2). It has been shown that younger children under the age of 5 years old are more susceptible to severe or life-threatening malaria regardless of transmission intensity (3). Eighty percent of Africans who died from malaria in 2020 were children under the age of 5 years old (1). High parasitemia, the subsequent anemia, hypoglycemia, acidosis and are conditions that characterize and worsen severe malaria outcomes (4).
In Gabon, the number of confirmed malaria cases decreased from 264 676 to 80 266 cases, and the number of deaths from 591 to 224 cases between 2018 and 2020 (2). Previous reports revealed in the Gabonese context, that severe malaria represents 18.4% to 32% of malaria cases, and that the malaria-associated death rate ranged between 3.1% and 9% (5, 6).
More than fifteen (15) years ago, Dzeing-Ella and colleagues reported that most children (92.3%) presenting with severe falciparum malaria were less than 5 years old (6). In their study anemia was the most frequent feature of severe malaria (67.8% of cases), followed by respiratory distress (31%), cerebral malaria (24%), hyperlactatemia, and hypoglycemia (16%) [5]. Seventeen (17) years later, has the frequency or weight of these characteristics changed?
We investigated the epidemiologic aspects, clinical, paraclinical, and outcomes of severe forms of malaria caused by Plasmodium falciparum in children attending the emergency room (ER) of the Mother and Child University Hospital in Libreville, Gabon.
We retrospectively analyzed medical records (between March 2018- to December 2019) from the emergency ward of the “Mother and Child University Hospital in Libreville, Gabon”. Children hospitalized with a Plasmodium falciparum positive smear test (complemented by a rapid diagnostic test (RDT)) and who met one or more criteria of the severe form grading according to the WHO guideline were included in the study (7). We analyzed patients’ parasitemia, hemoglobin concentration, full blood count, glycemia, urea, creatinine, transaminases, bilirubin, ionogram, blood gas, alkaline plasma reserves (and pH), plasma lactate concentration, and chest x-ray.
Blood smear were grades based on the number of parasites by μl (<1 000parasites (p)/μl, 1 000–9 999p/μl, 10 000–99 999 p/μl, 100 000–249 999 p/μl and ≥ 250.000 p/μl). The diagnosis of hyperparasitaemia was made when the parasite density exceeded 250000p/µl of blood.
Anemia was characterized as severe if the level of Hb was below 5g/dL (Hb < 5 g/dL); moderate if the concentration was between 5 and 8 g/dL: (5 <Hb <8 g/dL) and mild if the concentration was between 8 and 11 g/dL (8 ≤ Hb <11 g/dL).
Hypoglycemia was considered when the glycemia was below 2.2 mmol/L.
Impaired kidney function was considered when creatinine concentration was above 265 µmol/L.
Acidosis and hyperlactatemia were established when sanguine pH was below 7.35 or bicarbonates below15mmol/L. Hyperlactatemia was considered when plasma lactations were above 4 mmol/L.
Cerebral malaria was defined in children whose comatose state progressed in less than 30 minutes and or had at least two episodes of generalized seizure within 24 hours and/or were in a convulsive status.
The treatment aimed to ensure parasitic clearance, correct the electrolyte imbalance, normalize the level of hemoglobin and blood sugar, and alleviate hyperthermia. The doses of the antimalarial (Artesunate) were 3 mg/kg/dose every 12 hours in children under 20 kg and 2.4 mg/kg/dose when it exceeded the mass of 20 kg (a total of 5 doses administered intravenously (IV)) with a positive effect. To correct electrolyte imbalance, intravenous electrolytes infusions were administered. To normalize of hemoglobin in children with the level of hemoglobin above 8 g children were given 2 to 3 mg of iron per day for 3 months. For children with their hemoglobin levels below 8 g blood transfusion was considered. Hypoglycemia was corrected by administering 7 g of glucose/kg (monitored every hour).
The patient’s demographic clinic and medical records were extracted from a hospital information system. Data were analyzed with Stata 9.2 (Stata Corporation, College Station, TX USA). The differences between the groups were evaluated using Pearson χ (2). In case of non-compliance with the confirmed restrictions, the exact Fisher test, Student’s t-test, and analysis of variants (ANOVA) or Kruskal-Wallis test were calculated as appropriate, the relative risk with confidence intervals being equal to 95% (95% CI). A p-value of less than 0.05 was considered statistically significant.
A total of 134 patients (71 women (F) and 63 men (M)) aged 0 and 15 years old were included in the study (Table 1). Data analysis showed a total of 64% of patients were brought first to the hospital emergency service or room (ER). Thirty-six percent (36%) of patients were referral patients from other services or healthcare facilities (Table 2).
All severe malaria cases had anemia, 59 (44%) had severe anemia, 57 (42.5%) had moderate anemia and 18 (13.4%) had mild anemia.
Based on neurological manifestations, 101 (75.4%) children were diagnosed with cerebral malaria (Table 3), a consequence of brain damage during marsh access. Forty-four (44) of cerebral malaria cases (43.6%) had hyperparasitaemia. Details on patients’ symptomatology are described Table 3. Seizures were most commonly noted in infants in their first year of life (63.6%) and children under five (5) years of age (11.4%). Twenty percent (20%) of children with cerebral malaria fell into a coma. The coma was characterized by very discreet occipital stiffness, unaccompanied by photophobia or the abolition of corneal reflexes. In 4.5% of coma cases, opisthosoma redness was found, which had an unfavorable prognosis (rapid death) (p <0.05). Deep acidic breathing was more prevalent in cerebral malaria cases than non-cerebral malaria (24.8% vs 6.1%; RR = 1.304; p = 0.02). Signs of dehydration in cerebral malaria occurred in 60.4% of cases. Hemorrhagic syndrome was more frequent in non-cerebral malaria cases (27.3% vs 9.9%; RR = 0.665; p = 0.013).
Hyperparasitaemia was observed in 58 patients (76% had cerebral malaria). However, hyperparasitaemia was not associated with cerebral malaria (RR = 1.2; p > 0.05)
Comatose on admission was observed in 37 (27.6%) cases and was predominant in males (36.5%) than female (19.7%) (RR = 2; p = 0.03). Seven (7) children (5.2%) were agonizing at the time of hospitalization. Seven (7) children (5%) were agonizing on admission. Thus, 33% of admitted children were comatose or agonizing.
Twenty-five patients (19%) died. Nine (9) died in the first 24 hours, 12 between 24 hours and 48 hours, and 4 (3%) later on during their ER care. Overall, 21 children (15.6% of all admitted children and 48% of children who died) died within 48 hours of their ER care. Children with cerebral malaria had higher fever than those who showed no apparent signs of central nervous system impairment (Unpaired t-test p = 0.008) (Figure 1). Deceased children experienced higher fever compared to children that survived (Unpaired t-test p = 0.002) (Figure 1B). Hepatomegaly was significantly more frequent (88%) in deceased children than in the subgroup of those who survived (2.8%) (RR = 31. 97; p<0.0001). Two (II) and three (III)-degree splenomegaly (Hackett’s system) was the most frequently found in the subgroups of the children who lived (Table 3).
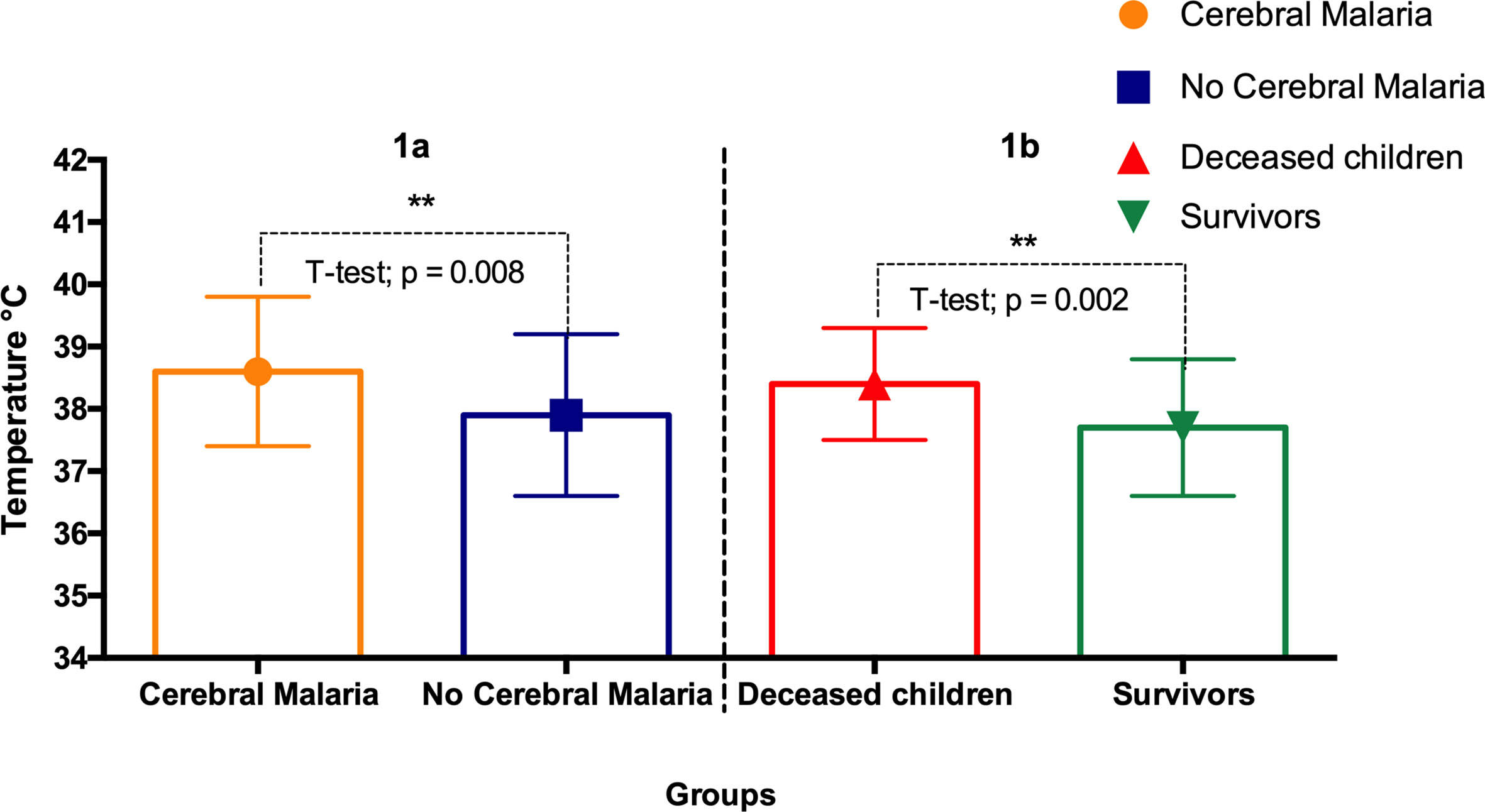
Figure 1 Body temperatures of: (A) cerebral malaria vs non- cerebral malaria; (B) deceased children vs survivors. The star (**) indicates statistical significance (p < 0.01, Unpaired t-test).
Leukocytosis was more pronounced in the subgroup of the children under a year and those between the ages of 5 - 15 years (Unpaired t-test p<0.0001). Thrombocytopenia (less than 150 x 109) was established in 106 (79.1%) cases, with no predictive value in the course of the disease and no statistically significant differences between age and sex. Thrombocytosis, a value considered greater than 400x109 was found in 13 (9.7%) without predictive value and significant differences between study groups.
Malaria is still one of the most deadly, infectious diseases in Africa, with children paying the greatest tribute (1, 2). Our data showed that all patients were anemic, which was expected, as anemia is a general feature of severe malaria (6, 8). Forty-six percent (46%) of patients had severe anemia (hemoglobin < 5 g/dL), which is higher than what was reported in Uganda (9) and Vietnam (10), but similar to what was reported in a Nigerian (11) and Ghanaian study (12). In our study, 19% of patients with severe malaria died, which is not too different from what, was previously reported in the Gambia and Malawi (13, 14), but higher than what was observed in Uganda (9). Also, the fatality rate observed in our study (19%) is higher than what was observed by Dzeing-Ella et al., (9%) between the years 2000 and 2002 in the setting of Libreville’s largest public hospital (6). About 7% and 16% of admitted patients died respectively in the first 24 hours and 48 hours. The 24 hours death rate was low when compared to a previous study done in Libreville (6). Factors that were associated with death included high fever, and hepatomegaly (13).
With 33% of children in the state of comatose or agonizing on admission, and 64% of children brought directly from home to the ER by family members, we believe that poor assessment of the clinical state of patients by parents most probably increases the time between disease onset and admission to the ER which in return, impacted the chance of survival (15, 16). Indeed, severe malaria can progress rapidly to death, and immediate access to pre-transfer treatment can save lives. Parents’ awareness is key to limiting malaria-associated morbidity and death. Today self-testing or home testing for a number of conditions, including HIV is encouraged. This could also be done for malaria. The malaria fatality rate remains high due to limited healthcare access, the delay in soliciting professional healthcare, and the weakness of preventive measures (15, 17).
Data presented and described here covers outcomes of severe malaria and its clinical features in an urban, tertiary hospital settings. This may constitute a limit because to fully grasp the extend of severe malaria consequences data from primary and secondary care level hospital would have been necessary. Also, further studies are required to establish the rate of sever malaria in different settings.
Cerebral malaria was the most common form of severe malaria, caused by Plasmodium falciparum. Anemia and high parasitemia were the principal features of severe malaria. Hepatomegaly appears to have a negative predictive value. More important malaria programs should address parents’ delay in soliciting professional healthcare through an awareness campaign.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Mother and Child University Hospital. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
AV and JFDS participated equally in creating the concept, collecting data, supervising the quality of laboratory tests, records analysis, statistical analysis, and manuscript writing. EKK and SA provided medical records and participated in manuscript writing. All authors contributed to the article and approved the submitted version.
We would like to acknowledge and give our thanks to the hospital’s administrative and medical staff.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
3. Paton RS, Kamau A, Akech S, Agweyu A, Ogero M, Mwandawiro C, et al. Malaria infection and severe disease risks in Africa. Science (2021) 373:926–31. doi: 10.1126/science.abj0089
4. Trampuz A, Jereb M, Muzlovic I, Prabhu RM. Clinical review: Severe malaria. Crit Care (2003) 7:315–23. doi: 10.1186/cc2183
5. Koko J, Dufillot D, Zima-Ebeyard A, Duong Minh T, Gahouma D, Kombila M, et al. Aspects of malaria in the hospitalized child in Gabon. Médecine tropicale Rev du Corps santé colonial (1997) 57:177–80.
6. Dzeing-Ella A, Nze Obiang PC, Tchoua R, Planche T, Mboza B, Mbounja M, et al. Severe falciparum malaria in gabonese children: clinical and laboratory features. Malaria J (2005) 4:1. doi: 10.1186/1475-2875-4-1
7. World Health Organization. (2012). Management of severe malaria: A practical handbook, 3rd. https://apps.who.int/iris/handle/10665/79317.
9. Olupot-Olupot P, Engoru C, Nteziyaremye J, Chebet M, Ssenyondo T, Muhindo R, et al. The clinical spectrum of severe childhood +malaria in Eastern Uganda. Malar J (2020) 19:322. doi: 10.1186/s12936-020-03390-7
10. Ha MT, Ho TAT, Nguyen AN, Nguyen TA. Characteristics of severe malaria in hospitalized children in ho chi minh city from 2012 to 2019. Trop biomedicine (2021) 38:371–6. doi: 10.47665/tb.38.3.082
11. Edelu BO, Ndu IK, Igbokwe OO, Iloh ON. Severe falciparum malaria in children in enugu, south East Nigeria. Nigerian J Clin Pract (2018) 21:1349–55.
12. Orish VN, Ilechie A, Combey T, Onyeabor OS, Okorie C, Sanyaolu AO. Evaluation of blood transfusions in anemic children in effia nkwanta regional hospital, sekondi-takoradi, Ghana. Am J Trop Med hygiene (2016) 94:691–4. doi: 10.4269/ajtmh.15-0310
13. Waller D, Krishna S, Crawley J, Miller K, Nosten F, Chapman D, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis an Off Publ Infect Dis Soc America (1995) 21:577–87. doi: 10.1093/clinids/21.3.577
14. Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med (1989) 71:441–59.
15. Mousa A, Al-Taiar A, Anstey NM, Badaut C, Barber BE, Bassat Q, et al. The impact of delayed treatment of uncomplicated p. falciparum malaria on progression to severe malaria: A systematic review and a pooled multicentre individual-patient meta-analysis. PloS Med (2020) 17:e1003359.
16. Christian U, Angelique CK, Lisine T, Peter C. Caregiver delay in seeking healthcare during the acute phase of pediatric illness, Kigali, Rwanda. PAMJ (2018) 30. doi: 10.11604/pamj.2018.30.160.15286
Keywords: Severe malaria, cerebral malaria, anemia, parasitemia, children, falciparum, gabon
Citation: Voloc A, Kuissi Kamgaing E, Ategbo S and Djoba Siawaya JF (2022) Outcomes of severe malaria and its clinical features in Gabonese children. Front. Trop. Dis 3:985890. doi: 10.3389/fitd.2022.985890
Received: 04 July 2022; Accepted: 01 September 2022;
Published: 21 September 2022.
Edited by:
Vanderson de Souza Sampaio, Fundação de Medicina Tropical Doutor Heitor Vieira Dourado (FMT-HVD), BrazilReviewed by:
Adekunle Sanyaolu, Federal Ministry of Health, NigeriaCopyright © 2022 Voloc, Kuissi Kamgaing, Ategbo and Djoba Siawaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joel Fleury Djoba Siawaya, am9lbC5kam9iYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.