- Department of Infectious Diseases, Leiden University Medical Center, Leiden, Netherlands
Leprosy is an infectious disease caused by Mycobacterium leprae (M. leprae) that mainly involves the skin and peripheral nerves, causing lifelong deformities and social stigma. As evident from the practically stable number of new cases reported worldwide during the past decade, transmission is still ongoing. On route to leprosy elimination, an appropriate tool is needed to monitor M. leprae transmission. M. leprae-specific antibodies indicate infection with M. leprae, but do not differentiate between present and past infection. Nevertheless, detection of M. leprae infection in young children per definition indicates recent infection. Hence, seroprevalence in young children can be used to monitor recent M. leprae transmission. Despite having eliminated leprosy in most parts of the country, studies on transmission conducted in China are not sufficiently reported in the English literature. Therefore, we performed a systematic review of Chinese literature describing serological studies in healthy children in (former) leprosy endemic areas in China, available in the Chinese databases: China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP), and Wanfang Database. From the 710 articles identified in these three Chinese databases, only four full-text articles fulfilled all inclusion criteria regarding sufficiently detailed descriptions of anti-M. leprae antibodies in healthy children. Two additional papers were identified through snowballing, resulting in a total of six articles considered for this review reporting quantitative serological data from three Chinese provinces between 1987 and 2003. All studies used ELISAs to quantify antibody levels. Seroprevalence in healthy children ranged from 7.93% (Yunnan) to 32.35% (Jiangsu). If the same method was used (in Jiangsu), direct comparison of studies at different time points indicated that decrease in disease prevalence (0.28 to 0.16 per 100,000) or new case detection rate (2.6 to 1.0 per 100,000) from 1987-1991 corresponded to decrease in anti-M. leprae antibody seroprevalence (30.86% to 22.61%) in healthy children. Thus, these findings are consistent with the previous finding that anti-M. leprae antibody seroprevalence in young children represents a surrogate indicator to monitor transmission.
1 Introduction
Leprosy is a chronic infectious disease caused by Mycobacterium leprae (M. leprae) or M. lepromatosis that mainly damages the skin and the peripheral nerves, causing skin lesions, loss of sensation, deformities, and social stigma (1–3). After tuberculosis, leprosy ranks second in severity as a human mycobacterial disease. The majority of new leprosy cases occur in low- and middle-income countries, with India, Brazil, and Indonesia accounting for 80% (4). Currently, millions of people bear long-term consequences of this neglected tropical disease (NTD), even after treatment when they are not included in WHO records as patients anymore. During the past decade, around 200,000 new cases were diagnosed annually, of whom 14,981 (7.4%) were under the age of 15 years in 2019 (4). This indicates that M. leprae transmission is still ongoing (4), despite the fact that multi-drug treatment (MDT) is globally available.
The clinical spectrum of leprosy, based on Ridley Jopling Classification (5), is characterized by tuberculoid leprosy (TT) on one end, and lepromatous leprosy (LL) on the other end. The latter, is a more severe type involving disseminated infection (6, 7) and associated with a high bacterial load (8–10). Between these two ends, the leprosy spectrum is further subdivided into borderline tuberculoid (BT), borderline borderline (BB), and borderline lepromatous (BL). Since it is not always feasible to apply the RJ classification as it requires histological analysis, the WHO classification system is often used in the field. This simply classifies leprosy as multibacillary (MB) or paucibacillary (PB) based on the total number of leprosy lesions in an individual (PB ≤5 lesions or MB >5 lesions).
In China, leprosy has been prevalent for thousands of years (11). In the early days of the People’s Republic of China, the high disease burden seriously affected people’s health and hindered social and economic development (12). Through several effective interventions, the incidence of leprosy in China has been declining in the past seven decades (12–15). China has eliminated leprosy as a public health problem in 1981 at the national level and in 1991 at the provincial level based on the definition of WHO (a prevalence rate of <1/10,000 residents) (12, 16). However, leprosy still occurs in China and is particularly prevalent in some difficult-to-access mountainous areas in southwest China (17): a total of 406 new cases of leprosy were reported in 2020, ranking China as the 21st country worldwide with respect to the number of new cases detected (18).
M. leprae is assumed to be transmitted via aerosol droplets by the respiratory route during close and frequent contact with untreated patients (19). It has been estimated that only 5% of the individuals exposed to M. leprae will become infected, of whom 20% will eventually develop the disease (20–22). On average, the disease incubation period is 2-5 years. However, it may take more than 20 years before leprosy becomes clinically apparent (23).
In order to achieve leprosy elimination, interventions such as intensified active case finding and effective contact tracing combined with a single-dose of rifampicin as post-exposure prophylaxis (PEP) are recommended in the WHO guidelines for diagnosis, treatment, and prevention of leprosy (24–26). LL patients display well detectable antibody levels, in particular IgM directed against M. leprae phenolic glycolipid-I (PGL-I), a cell wall component unique to M. leprae (27), whereas IgG and IgA are detected mostly in lower levels. Various studies have demonstrated that the levels of IgM antibodies against PGL-I correlate well with the bacterial load within individuals (28, 29) and thus can be used to indicate M. leprae infection in humans (30–33) as well as animals (34–37).
However, anti-PGL-I IgM antibodies can neither discriminate between past and present M. leprae infection nor indicate the time elapsed since the beginning of the infection. In young children, infection is recent by definition, which allows assessment of more current state of transmission in an area using seroprevalence. Therefore the WHO “Task force on definition, criteria and indicators for interruption of transmission and elimination of leprosy” advises using seroprevalence of anti-M. leprae PGL-I IgM in children of 5-7 years old as an indicator of recent transmission to monitor interruption of transmission in areas where leprosy is not endemic anymore (38).
Recently, Pierneef et al. conducted a systematic literature review of anti-PGL-I serology in young children without leprosy or known contact with leprosy patients (39). This review demonstrated that quantitative detection of anti-PGL-I antibodies is a promising tool to assess M. leprae infection and thereby provides a proxy for measuring transmission and monitoring the effect of interventions focused on the elimination of leprosy. However, Pierneef et al. only included English, Spanish, and Portuguese literature, and lacked similar research from Chinese literature.
Among leprosy studies published in English in the past two decades, Chinese studies mostly focused on the relationship between leprosy and genes, compared to the top five most productive countries (Brazil, India, the United States, the United Kingdom, and the Netherlands) that have focused on early diagnosis and post exposure prophylaxis (40). Data on leprosy serology in healthy Chinese children is not available in English literature (39).
Here, we conducted a comparable systematic review of Chinese literature to identify studies describing data of anti-M. leprae antibodies in Chinese children without leprosy and known contact with leprosy patients.
2 Methods
2.1 Literature search and search strategy
This systematic review was conducted according to the guidelines of the Preferred Reporting Items for Systematic reviews (41), targeting Chinese literature describing leprosy serology in children using the following databases as sources: China National Knowledge Infrastructure (CNKI; https://www.cnki.net), China Science and Technology Journal Database (VIP; http://www.cqvip.com) and Wanfang Database (Wanfang; https://www.wanfangdata.com.cn/index.html). In order to expand the search scope, the search terms were searched in all available fields (such as Title, Author, Publication, Abstract, Keyword, etc.) in VIP and Wanfang databases, while searched in subjects (Title, Abstract, Keyword) in CNKI (since all fields search not applicable). The “Chinese article” filter was used when available (Wangfang and CNKI). The search strategy is shown in the flowchart (Figure 1) using the search strings listed in Table 1, and included all available peer-reviewed publications until January 11, 2022. If no full-text was available in the databases described above, full-texts were found in one of the following online libraries: Duxiu scholar (https://www.duxiu.com); Aixueshu (https://www.ixueshu.com); Zhangqiao research (https://www.zhangqiaokeyan.com). Articles retrieved from Chinese databases were screened by one of the authors (ZZ) based on the title and abstract, followed by a full-text assessment of the original articles for eligibility. Data were translated from Chinese to English (ZZ) and summarized in a table which was reviewed by the co-authors and used for discussion and analysis in this paper. To maximize the finding and inclusion of eligible articles, the reference lists of the included articles were investigated (“snowballing”). The authors were not blinded to the names of the study authors, journals, or institutions.
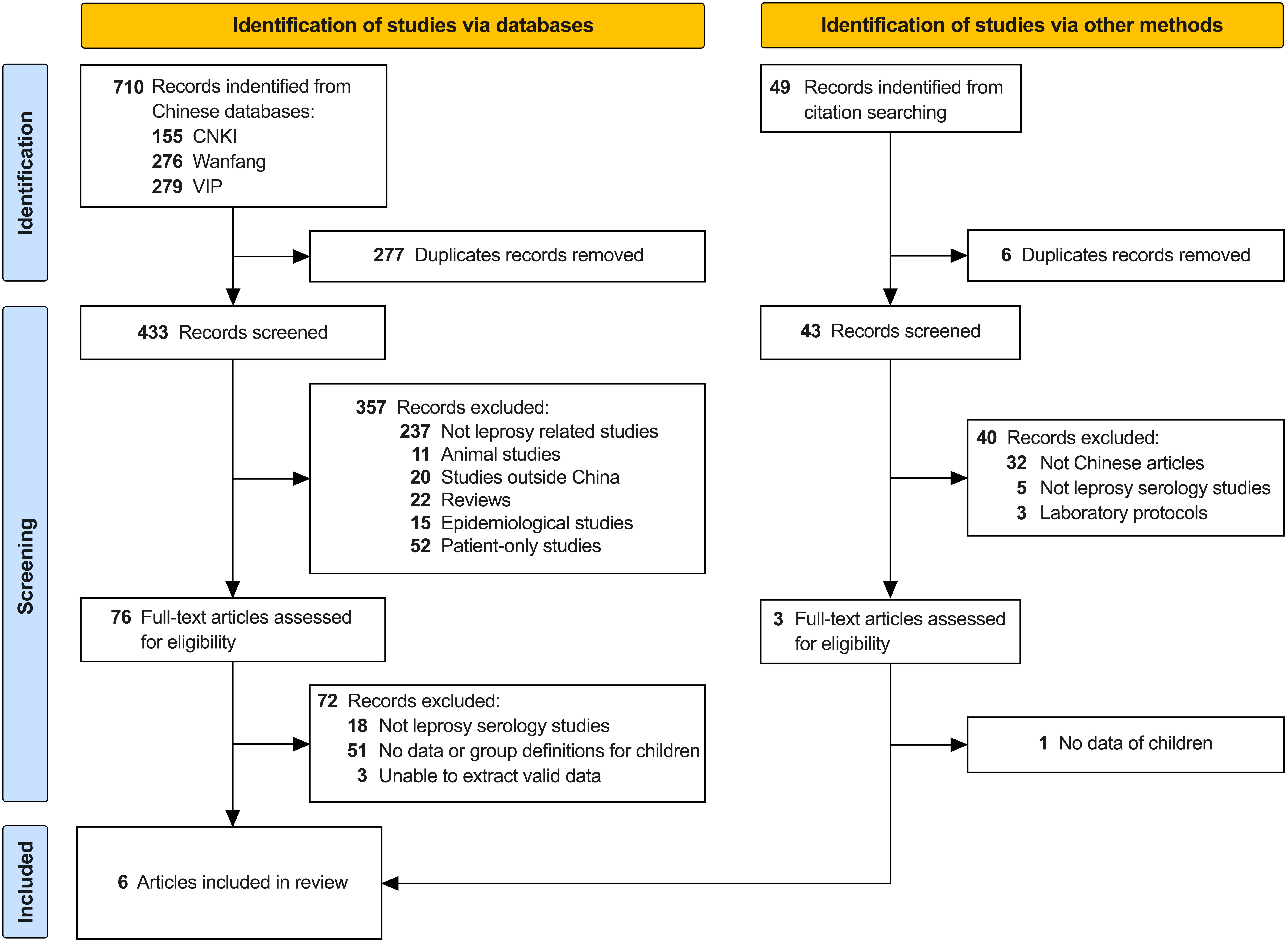
Figure 1 PRISMA flow chart. Overview of the selection procedure of six full-text articles included in the review. Studies were identified via three Chinese databases: China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP) and Wanfang Database (Wanfang). The search strings are shown in Table 1. Additional studies were included via investigation of the reference lists of the included articles (“snowballing”).
2.2 Inclusion and exclusion criteria
Articles were included if written in Chinese and described original studies on leprosy serology in Chinese children below 15 years of age who were not affected by leprosy. Articles were excluded if they primarily discussed studies on skin diseases other than leprosy, reported studies on serology related only to leprosy disease or to leprosy reactions, or if they lacked critical information (seroprevalence in children or the age definition of children).
2.3 Data extraction and analysis
After inclusion of eligible papers, information about the study design, the research site location, the year of study conducted and article published, the sample type, the types of serological test used (including the laboratory protocol and target antigen), the study population, the age group, gender, the sample size of children tested and the seroprevalence(s) reported were extracted into Microsoft Excel 2016. The geographic distribution map of the study was produced using Microsoft PowerPoint 2016.
Quantitative data was copied from the full texts. In case percentages for seroprevalence in children could not be derived from the text (42), they were estimated from figures in the articles using Microsoft Paint 11.2203.2.0 software. Differences in seroprevalence were determined by Fisher’s exact test using GraphPad Prism 9.0.2.
2.4 Epidemiological data retrieval
As the WHO website (https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/leprosy) only provides leprosy prevalence per country, an additional literature search on the leprosy prevalence and new case detection rate (NCDR) in the counties, cities, and provinces of experimental sites in the included articles was performed. The following search string: “Place (counties, cities, provinces) name” and “leprosy” and “Epidemiology” was applied to CNKI, VIP, and Wanfang databases as well as PubMed (https://pubmed.ncbi.nlm.nih.gov/).
3 Results
Applying the search strategy described above, a total of 710 articles [CNKI (n=155), Wanfang (n=276), VIP (n=279)] were identified (Figure 1). After removing duplicates, 433 articles were screened for the inclusion criteria (title and abstract) of this review. Articles were excluded because they were not related to leprosy research (n=237). Among them, 148 articles covered other skin diseases that were published in the China Journal of Leprosy and Skin Diseases, and 55 articles on measles-mumps-rubella were retrieved after using the keyword of our search because the first and last characters in the Chinese abbreviations for these three diseases (麻腮风) are the same as the Chinese characters for leprosy (麻风). In addition, articles in Chinese language describing only animal experiments (n=11), covering studies outside China (n=20), analyzing the epidemiology of leprosy (n=15), involving only leprosy patients without healthy individuals from that area (n=52), or reviews of leprosy literature (n=22), were excluded.
The remaining 76 full-text articles were assessed for eligibility, and 18 articles were subsequently excluded because they did not include leprosy serology. Of the remaining 58 serology studies (including either healthy contacts or endemic controls without known contact to leprosy patients), 51 studies were excluded based on the fact that they lacked serology data of children below the age of 15 years or did not include any age-definition of the children. Three articles were excluded due to the inability to extract valid data: one did not specify the location of sampling; one (43) included data derived from another study that was already included in our review (44); one study included selection bias as it aimed at recruiting anti-PGL-I antibodies positive contacts for follow-up and matched the numbers with anti-PGL-I antibody negative individuals. To maximize the identification of eligible articles, the reference lists of the included articles were investigated (“snowballing”), which led to the inclusion of two additional articles. Thus, the final selection included in this review consisted of six full-text articles in the Chinese language.
3.1 Study characteristics
At the date of the literature search (January 11, 2022), a total of six population-wide studies on leprosy serology of healthy contacts or endemic controls with detailed data for children as a subgroup were included in this review.
Two out of six articles included in the review did not identify the number of children, but the seroprevalence of anti-M. leprae antibodies in the children group were reported. One article included 1,632 endemic controls (EC) and 723 household contacts (HC) (45), and the other included 360 HC (42). The remaining four articles covered more than 586 children in total (Table 2).
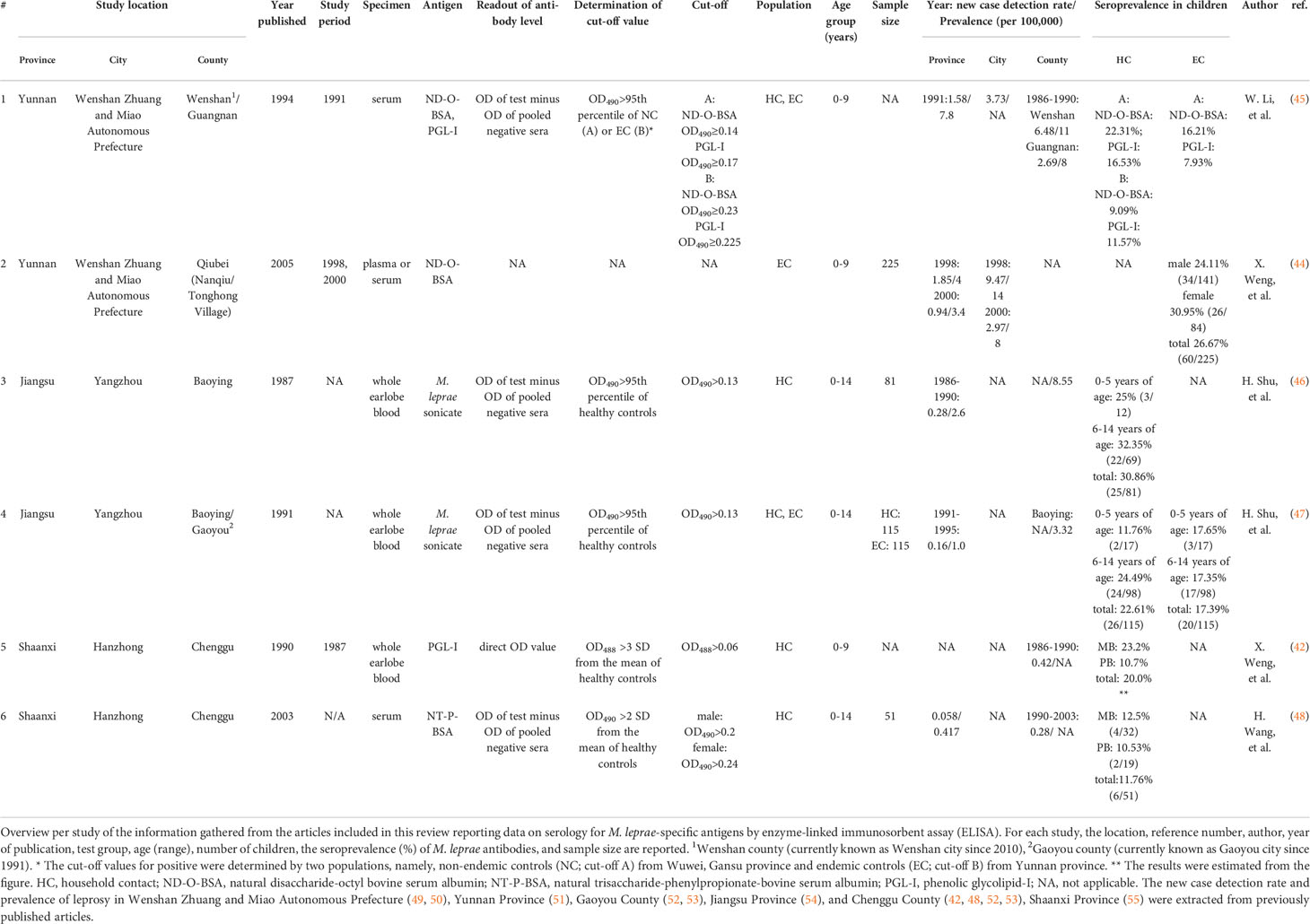
The studies involved data from 1987 to 2003 in three provinces of China (Table 2; Figure 2): Yunnan, Shaanxi, and Jiangsu. Two articles from each province were included in the analysis. All studies used enzyme-linked immunosorbent assay (ELISA) for IgM antibody detection, but the types of samples tested were diverse. Earlobe blood was tested in three studies [n=311 (46, 47), of which one study did not specify the number of samples (42)]. Serum was tested in two studies [n=51 (48) one study did not specify the number of samples (45)]. Plasma or serum was tested in one study [n=225, proportions per sample type were unknown (44)]. The composition of target antigens used for the detection ranged from complex and undefined to well-defined single, synthetic components: In Jiangsu, two articles were published (46, 47) using M. leprae sonicate as antigen; in Yunnan, two articles were published using both native PGL-I and disaccharide-octyl bovine serum albumin (ND-O-BSA) in 1994 (45), and only ND-O-BSA in 2005 (44) as antigen; in Shaanxi, two articles were published using native PGL-I (42) in 1990, and natural trisaccharide-phenylpropionate-bovine serum albumin (NT-P-BSA) in 2003 (48) as antigen.
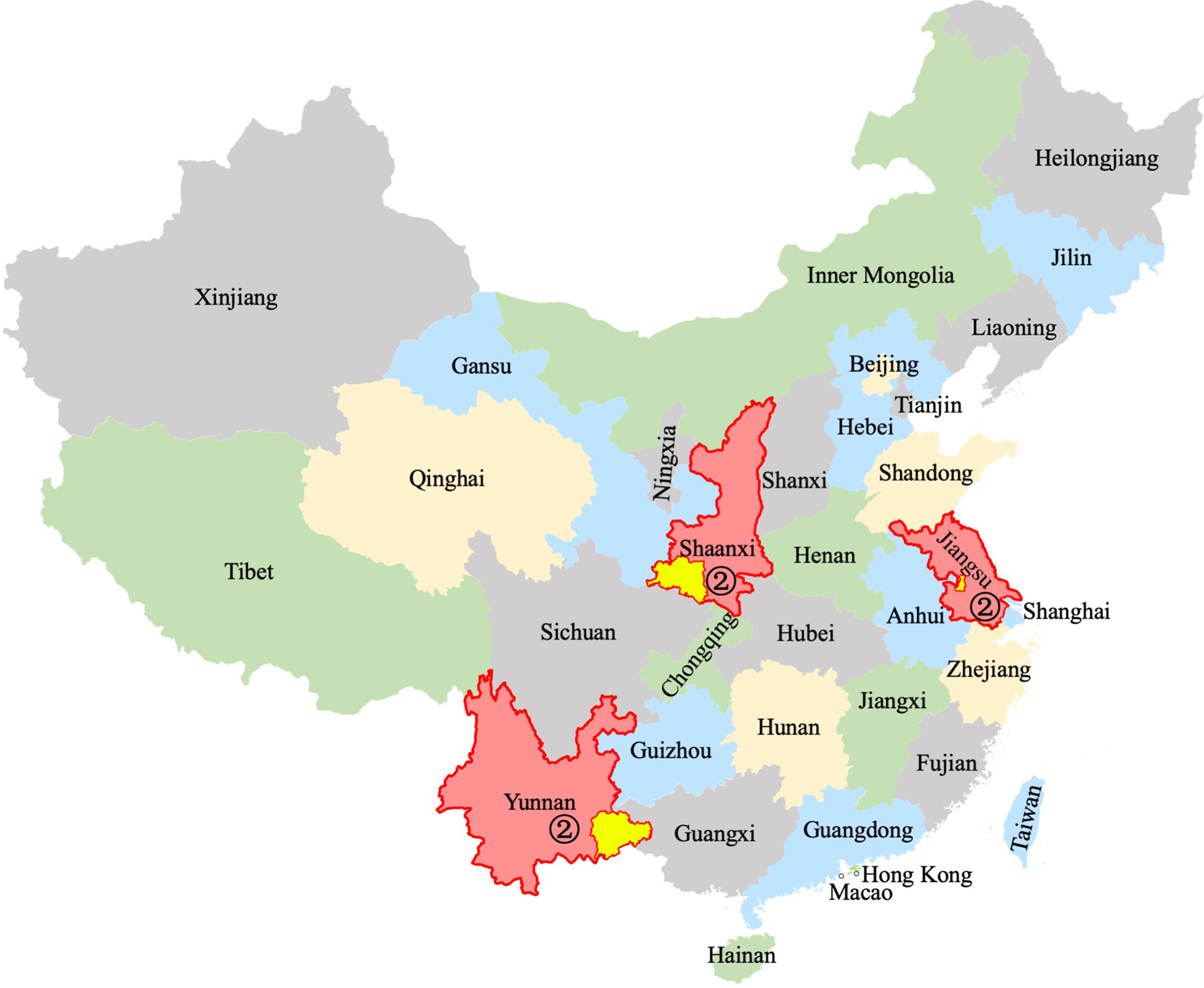
Figure 2 Serology studies in children without leprosy in China. Schematic representation of the cities (yellow shade with red border) in the provinces (red shade/border) where the selected studies in this review were located. The digital label refers to the number of studies in the province. The map templates of China and provinces were retrieved from: https://www.renrendoc.com/paper/189168283.html and https://www.1ppt.com/article/71782.html, and synthesized according to location. Province names, colors and numbers were added.
The levels of antibodies were expressed as the optical density (OD) of pooled negative samples subtracted from the OD of test samples in four articles (45–48), whereas one article reported direct OD values of test samples (42) (Table 2).
3.2 Yunnan province
In Yunnan Province, two studies were conducted in Wenshan County and Guangnan County in 1991 and Qiubei County in 1998-2000 in Wenshan (i.e., Wenshan Zhuang and Miao Autonomous Prefecture).
In 1991, Li et al. compared seroprevalence of two target antigens, namely ND-O-BSA and native PGL-I, in sera from the same population. The total number of HC and EC were 723 and 1,632, respectively, but the total number of children (0-9 years of age) was not specified in the text (45). The cut-off values for positivity were determined using the 95th percentile of non-endemic controls (NC) from a non-endemic area (Wuwei, Gansu province) in China. The cut-off values were OD490 > 0.14 for ND-O-BSA, and OD490 > 0.17 for PGL-I. Among children (0-9 years old), the seroprevalence of anti-ND-O-BSA IgM was higher when anti-PGL-I IgM was measured in HC (22.32% vs 16.53%) and EC (16.21% vs 7.93%), but no raw data were available for statistical analysis (Table 2).
Weng et al. collected sera or plasma from EC (n=919) in two leprosy-endemic villages, namely Nanqiu and Tonghong Village, in Qiubei County in 1998 and 2000 (44). The author did not specify the cut-off for positivity in the ELISA. The seroprevalence of anti-ND-O-BSA IgM in healthy children (0-9 years of age, n=225) was 26.67% (60/225). There were no differences in seroprevalence between female- and male children (30.95% vs 24.11%, p= 0.278), as well as seroprevalence in children from Tonghong Village and Nanqiu Village (33.7% vs 20.7%, p=0.282) (Table 2).
The leprosy prevalence and NCDR in the counties (Guangnan County and Wenshan County (45), city (49, 56–58), and province (51) were summarized and shown in Table 2, Figure S1. However, since these two studies (44, 45) were conducted in different areas (counties), a direct, longitudinal comparison of seroprevalence could not be performed. Also, direct comparison of seroprevalence between the two studies conducted in 1991 and 1998 was hindered due to the fact that a large number of leprosy patients was identified in Wenshan during the Leprosy Elimination Campaign in 1998 (43, 59). This led to a peak in leprosy prevalence and NCDR in that year due to active case finding where the cases detected in 1991 mainly resulted from self-reporting.
3.3 Jiangsu province
Shu et al. conducted two studies detecting M. leprae-specific antibodies in Baoying County and Gaoyou County, Yangzhou, Jiangsu Province, in HC and random people around the patient’s house (defined as EC). The two articles were published in 1987 (46) and 1991 (47), but the time of sample collection was not mentioned. Antigen (M. leprae sonicate), age group (0-14 years of age), sample type (whole blood from earlobe), and cut-off value for positive determined using the 95th percentile of NC (OD490 > 0.13) were the same for both studies, allowing direct comparison.
In their first study in Baoying County, the subjects included HC of both currently treated (n=167) and former (n=124) leprosy patients (n=291; of which 81 children). The seroprevalence of antibodies in young children was not different from that in older children [0-5 years old, 25% (3/12) vs 6-14 years old, 32.35% (22/69); p=0.746] in child HC (Table 2 and Figure S2).
Shu et al. then expanded the research scope to include Gaoyou County in the 1991 study, focusing on child HC of currently treated leprosy patients only (n=115), and additionally introduced the same number of random people (n=115) around the house based on age matching (EC). Antibody seroprevalence did not differ between HC and EC, either among younger children [0-5 years of age: 11.76% (2/17) vs 17.65% (3/17), p>0.999], older children [6-14 years of age: 24.49% (24/98) vs 17.35% (17/98), p=0.292], or all children [22.61% (26/115) vs 17.36% (20/115), p=0.410].
The anti-M. leprae antibody seroprevalence in child HC has decreased in 1991 compared to the data published in 1987 (from 30.86% to 22.62%, p=0.247), especially in the young children (from 25% to 11.76%, p=0.622), suggesting that the transmission in the region decreased. This trend was consistent with the rapid decline of leprosy prevalence and NCDR (42, 48, 52, 53) from 1987 to 1991 in Baoying County (from 8.55/100,000 to 3.32/100,000 for NCDR) and Jiangsu Province (from 0.28/100,000 to 0.16/100,000 for NCDR, and 2.6/100,000 to 1.0/100,000 for prevalence) (Table 2 and Figure S2).
3.4 Shaanxi province
In 1987, Weng et al. used anti-PGL-I IgM ELISA to detect M. leprae infection in 360 HC (unknown number of children age 0 to 9) in an endemic leprosy area named Chenggu County (42), Hanzhong, Shaanxi Province. Sixteen years later, Wang et al. used NT-P-BSA as an antigen to detect 416 HC (including 51 children age 0 to 14) in the same county again (48).
In the 1987 study, the seroprevalence in HC exposed to MB patients (23.2%) was higher than HC of PB patients (10.7%) among children, but raw data were unavailable for statistical analysis. In a study from 2003, the seroprevalence in children in HC of MB patients was 12.5% (4/32) and 10.53% (2/19) for HC of PB patients (Table 2). Longitudinal comparison was not possible due to differences in antigen, sample type, and the cut-off values for positivity used in the two studies (Table 2).
4 Discussion
Leprosy is a neglected tropical disease with a relatively small number of papers compared to other diseases, and even fewer studies of leprosy serology in healthy children in China that are written in Chinese. Although China is not among the 23 global priority countries identified by WHO, there were still 464 new leprosy cases reported in 2019. The number of children, MB cases, and grade-2 disability (G2D) cases were 6 (1.3%), 419 (90.3%) and 100 (21.6%), respectively (4, 60). In order to control leprosy, China applies a prevalence rate of less than 1 case per 100,000 at the county or city level (15, 61) instead of the WHO standard of less than 1 per 10,000 as an indicator for leprosy elimination. However, due to the unequal burden of disease, access and distribution of health resources, as well as socio-economic status across the whole country (15), there were still 56 counties with a prevalence rate of more than 1/100,000 in 2019, mainly distributed in Yunnan, Sichuan, and Hunan provinces (60).
Previously, we showed that in Eurasian red squirrel (Sciurus vulgaris) naturally infected with M. leprae, anti-PGL-I antibodies were present before the onset of disease and that armadillos experimentally infected with M. leprae became seropositive as early as 140 days after M. leprae inoculation (34). However, in humans, the often long-incubation period of leprosy impedes the determination of the exact timepoint of M. leprae infection. As infection in young children, per definition represents recent infection, we previously performed a systematic review of English, Spanish, and Portuguese literature on leprosy serology in healthy children to identify the potential of seroprevalence in children to monitor transmission (39). However, six papers covering data from China identified were excluded from that review since: two papers were not about leprosy; three papers described no data on healthy children; one paper was not about serology. A systematic review of the Chinese literature can provide information on serological data in Chinese children, insight into the M. leprae infection rates and transmission in China to explore the correlation between the seroprevalence of anti-PGL-I IgM in children and the prevalence of leprosy in non-endemic areas. Therefore, we searched three Chinese databases and evaluated 710 records.
All studies included in this review were conducted in provinces with (historically) high leprosy prevalence (14). Yunnan Province, consists of 16 prefecture-level divisions (eight prefecture-level cities and eight autonomous prefectures), located in the southwest of China and bordering Myanmar, Laos, and Vietnam, and has the highest leprosy burden in China (14, 62, 63). The detected cases and prevalence of leprosy ranked first and second in China for a long time, respectively (60, 62, 64). In 2018, the standard for leprosy elimination was met at the provincial level for the first time in Yunnan, but still 17 counties remained leprosy endemic areas by the end of 2019 (65). Shaanxi Province is located in the northwest of China, and the leprosy NCDR and prevalence have dropped significantly over the past 20 years, and as of 2018, only one county did not meet the leprosy elimination standard (55). Jiangsu Province, China’s eastern coastal province, has historically been an area highly endemic for leprosy. All counties and cities in Jiangsu have been endemic for leprosy, but reached the standard of eradication of leprosy in 1998 (66).
In view of the long incubation time of leprosy, the WHO recommends using child cases (below 15 years of age) as an epidemiological indicator of ongoing transmission in the community (4). However, the new child leprosy cases do not accurately reflect recent M. leprae transmission since most individuals infected with M. leprae do not develop the disease. Especially in non-leprosy-endemic areas, such as Baoying and Chenggu county in this review, no child cases have been reported since the late 1980s (53, 67), and they have reached the WHO standard of elimination of transmission. However, in the articles published in Baoying and Chenggu county in 1991 and 2003, still anti-M. leprae IgM seropositive children (0-14 years old) were detected, indicating that transmission of M. leprae was continued but was masked by the zero NCDR in children.
Although no statistical analysis could be performed, interestingly, in the study of Wenshan and Guangnan counties, Yunnan, the seroprevalence of anti-M. leprae antibodies (both ND-O-BSA and PGL-I) in all age groups (HC+EC; the children group was not analyzed) in Guangnan County seemed to be higher in percentage than that in Wenshan County (ND-O-BSA: 24.95% vs 10.86%, PGL-I: 17.15% vs 6.95%), but the prevalence seemed to be lower (11/100,000 vs 8/100,000; Table 2 and Figure S3). It shows that the anti-M. leprae antibody seroprevalence covering all age groups cannot be used to evaluate leprosy elimination. However, in the cross-comparison of the two villages in the study in Qiubei County, the seropositivity in children (0-9 years old) corresponded with the local leprosy prevalence and NCDR. Moreover, in the studies conducted in Gaoyou County, a trend could be observed that the anti-M. leprae IgM seroprevalence of children aged 6-14 is higher than that of children aged 0-5. This could reflect a longer exposure time in an endemic area in the higher age group, thus having more chances of getting infected. Therefore, M. leprae infection rates in children, especially in young children, may provide a good indicator of recent transmission.
It is worth mentioning that in the study in Yunnan in 1998-2000 (44), the seroprevalence of anti-PGL-I IgM in female- was similar to that of male children (30.95% vs 24.11%), indicating that the infection rate of females is not lower than that of males. However, among the 792 newly reported cases of leprosy in the city from 1997 to 2001, 570 (71.97%) were male and 222 female (68). This suggests that there may still be undetected female leprosy patients. In the study in Shaanxi published in 2003 (48), the cut-off value for positivity was equal to the mean OD plus twice the standard deviation of the OD detected in healthy individuals in the same area. Since this was calculated separately for males and females a higher cut-off value for females (OD492>0.24) than for males (OD492>0.20) was applied, resulting in similar seroprevalence rates in both genders. Using the female population to calculate the cut-off for seropositivity will thus lead to false negatives among females.
Even though both Yunnan and Jiangsu Province introduced MDT in 1983 (69, 70), the progress of leprosy elimination in the two provinces differs significantly, as reflected in the prevalence: leprosy prevalence in Yunnan (7.8/100,000) Province was higher than that in Jiangsu Province (1/100,000), but seroprevalence in HC and EC in Yunnan Province (22.31% in HC, 16.21% in EC) were similar to that in Jiangsu Province (22.61% in HC, 17.39% in EC) (Table 2). This may be due to the difference in antigens (M. leprae sonicate vs ND-O-BSA) or cut-off values for positivity. In Yunnan, the seroprevalence in children detected using synthetic antigen (ND-O-BSA) is higher than when native PGL-I (22.32% vs 16.53%) was used in the same population (HC). Moreover, when performing leprosy serological screening, the 95th quantile of the local healthy population (EC) was used as the cut-off value for positive, which was higher than that of the NC as the control, resulting in a lower seroprevalence (Table 2). This indicates that cross-sectional comparisons can only be made if the methods used to assess seroprevalence are consistent.
However, except for two studies in Jiangsu, different sample types, antigens, and cut-off values for positivity were used in the studies in the other regions, limiting the comparability of results across studies and impeding pooled analysis. A uniform test is required to allow direct comparisons. As cut-off values are negatively correlated with seropositivity (Figure S3), application of a unified detection method with an exact cut-off, such as low-complexity lateral flow assays (71–73), is vital to apply serology data to monitor leprosy elimination in future leprosy research and surveillance.
4.1 Limitations of this study
This review focused on Chinese literature as an addition to the previous review on the same subject in English, Spanish, and Portuguese literature (39), to review M. leprae infection and transmission in China in relation to serology in children. Data extraction was done by one author (ZZ) whose native language is Chinese. After literature search and eligibility scanning, six articles met the inclusion criteria and were included for analysis. The number of articles and the lack of availability of information on the number of samples or year of sampling limited the analyses that could be performed in this review. In addition, the diversity of sample types, antigens, and cut-off values for positivity used in the articles impeded pooled analysis and comparison of studies or regions directly. However, we believe that also “negative” findings or differences that do not reach statistical significance are of importance as it provides insight into what information is lacking and which topics should be addressed in future leprosy research on route to elimination of leprosy.
5 Conclusion
In order to achieve the elimination of leprosy, it is vital to find suitable indicators to reflect the intensity of transmission. This review of leprosy research reported in Chinese literature is in line with previous findings that anti-M. leprae antibody seroprevalence in healthy children corresponded to local leprosy prevalence and NCDR. To ensure applicability in difficult-to-access mountainous areas, globally-applicable, low-complexity tests that can quantitatively detect M. leprae-specific antibodies are required.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization: AG. Data collection: ZZ. Data curation and analysis: ZZ, AG. Writing – original draft: ZZ, AG. Writing – review & editing: ZZ, AG, AH, LP. Agree with manuscript results and conclusions: all authors. All authors contributed to the article and approved the submitted version.
Funding
This study was supported with grants from the Q.M. Gastmann-Wichers Foundation (to AG), ZZ was supported with grant from the China Scholarship Council.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.963674/full#supplementary-material
References
1. Britton WJ, Lockwood DN. Leprosy. Lancet (London England) (2004) 363(9416):1209–19. doi: 10.1016/s0140-6736(04)15952-7
2. Geluk A. Correlates of immune exacerbations in leprosy. Semin Immunol (2018) 39:111–8. doi: 10.1016/j.smim.2018.06.003
3. Haas CJ, Zink A, Pálfi G, Szeimies U, Nerlich AG. Detection of leprosy in ancient human skeletal remains by molecular identification of mycobacterium leprae. Am J Clin Pathol (2000) 114(3):428–36. doi: 10.1093/ajcp/114.3.428
4. WHO. Global leprosy (Hansen disease) update, 2019: Time to step-up prevention initiatives. Wkly Epidemiol Rec (2020) 95(36):417–40.
5. Ridley D, Jopling W. Classification of leprosy according to immunity. Int J leprosy other mycobact. Dis Off Organ Int Leprosy Assoc (1966) 34:255–73.
6. Hungria EM, Bührer-Sékula S, Oliveira RM, Aderaldo LC, Pontes MAA, Cruz R, et al. Mycobacterium leprae-Specific Antibodies in Multibacillary Leprosy Patients 433 Decrease During and after Treatment with Either the Regular 12 Doses Multidrug 434 Therapy (Mdt) or the Uniform 6 Doses Mdt. Front Immunol (2018) 9:915. doi: 10.3389/fimmu.2018.00915
7. van Hooij A, Geluk A. In Search of Biomarkers for Leprosy by Unraveling the 437 Host Immune Response to Mycobacterium Leprae. Immunol Rev (2021) 301(1):175–92. doi: 10.1111/imr.12966
8. Aarão TL, de Sousa JR, Botelho BS, Fuzii HT, Quaresma JA. Correlation 440 between Nerve Growth Factor and Tissue Expression of Il-17 in Lepros. Microbial pathogenesis (2016) 90:64–8. doi: 10.1016/j.micpath.2015.11.019
9. Quaresma JA, Aarão TL, Sousa JR, Botelho BS, Barros LF, Araujo RS, et al. T-Helper 17 cytokines expression in leprosy skin lesions. Br J Dermatol (2015) 173(2):565–7. doi: 10.1111/bjd.13608
10. Salgame P, Abrams J, Clayberger C, Goldstein H, Convit J, Modlin R, et al. Differing lymphokine profiles of functional subsets of human Cd4 and Cd8 T cell clones. Science (1991) 254(5029):279–82. doi: 10.1126/science.1681588
11. Tayles N, Buckley HR. Leprosy and tuberculosis in iron age southeast Asia? Am J Phys Anthropol.: Off Publ Am Assoc Phys Anthropol. (2004) 125(3):239–56. doi: 10.1002/ajpa.10378
12. Chen X-S, Li W-Z, Jiang C, Ye G-Y. Leprosy in China: Epidemiological trends between 1949 and 1998. Bull World Health Organ (2001) 79:306–12.
13. Shen JP, Gupte MD, Jiang C, Manickam P, Yu MW, Li WZ. Trends of case detection and other indicators of leprosy in China during 1985-2002 [in Chinese]. Chin Med Sci J (2005) 20(2):77–82.
14. Jiang Y, Dou X, Wan K. Epidemiological characteristics and trends of registered leprosy cases in China from 2004 to 2016. The American journal of tropical medicine and hygiene (2021) 105(1):31–6. doi: 10.4269/ajtmh.20-0178
15. Yu M, Sun P, Wang L, Wang H, Gu H, Chen X. Towards a leprosy-free country - China, 2011-2018. China CDC Wkly (2020) 2(4):50–3. doi: 10.46234/ccdcw2020.014
16. Chen XS, Li WZ, Jiang C, Zhu ZL, Ye G. Computerization of leprosy records: National leprosy recording and reporting system in China. Lepr Rev (2000) 71(1):47–56. doi: 10.5935/0305-7518.20000007
17. Shen J, Zhou M, Li W, Yang R, Wang J. Features of leprosy transmission in pocket villages at low endemic situation in China. Indian J Lepr (2010) 82(2):73–8.
18. WHO. Leprosy - number of new leprosy cases data by country (2021). Available at: https://apps.who.int/gho/data/node.main.A1639?lang=en.
19. Bratschi MW, Steinmann P, Wickenden A, Gillis TP. Current knowledge on mycobacterium leprae transmission: A systematic literature review. Lepr Rev (2015) 86(2):142–55. doi: 10.47276/lr.86.2.142
21. Scollard DM, Adams L, Gillis T, Krahenbuhl J, Truman R, Williams D. The continuing challenges of leprosy. Clin Microbiol Rev (2006) 19(2):338–81. doi: 10.1128/CMR.19.2.338-381.2006
22. Quilter EE, Butlin CR, Singh S, Alam K, Lockwood DN. Patients with skin smear positive leprosy in Bangladesh are the main risk factor for leprosy development: 21-year follow-up in the household contact study (Cocoa). PLoS neglected tropical diseases (2020) 14(10):e0008687. doi: 10.1371/journal.pntd.0008687
23. Richardus JH, Ignotti E, Smith WCS. Epidemiology of leprosy. In: International textbook of leprosy. International textbook of leprosy. (2016). Available at: https://internationaltextbookofleprosy.org/chapter/epidemiologyleprosy.
24. WHOGuidelines for the diagnosis, treatment and prevention of leprosy. (2018). Available at: https://www.who.int/publications/i/item/9789290226383.
25. Hambridge T, Nanjan Chandran SL, Geluk A, Saunderson P, Richardus JH. Mycobacterium leprae transmission characteristics during the declining stages of leprosy incidence: A systematic review. PLoS neglected tropical diseases (2021) 15(5):e0009436. doi: 10.1371/journal.pntd.0009436
26. Blok DJ, de Vlas SJ, Geluk A, Richardus JH. Minimum requirements and optimal testing strategies of a diagnostic test for leprosy as a tool towards zero transmission: A modeling study. PloS Negl Trop Dis (2018) 12(5):e0006529. doi: 10.1371/journal.pntd.0006529
27. Hunter SW, Brennan PJ. A novel phenolic glycolipid from mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J bacteriol. (1981) 147(3):728–35. doi: 10.1128/jb.147.3.728-735.1981
28. van Hooij A, Tjon Kon Fat EM, van den Eeden SJF, Wilson L, Batista da Silva M, Salgado CG, et al. Field-friendly serological tests for determination of M. leprae-499 specific antibodies. Sci Rep (2017) 7(1):8868. doi: 10.1038/s41598-017-07803-7
29. Tió-Coma M, Avanzi C, Verhard EM, Pierneef L, van Hooij A, Benjak A, et al. Genomic characterization of Mycobacterium leprae to explore transmission patterns identifies new subtype in Bangladesh. Front Microbiol (2020) 11:1220. doi: 10.3389/fmicb.2020.01220
30. Young DB, Buchanan TM. A serological test for leprosy with a glycolipid specific for mycobacterium leprae. Science (1983) 221(4615):1057–9. doi: 10.1126/science.6348948
31. Brett SJ, Draper P, Payne SN, Rees R. Serological activity of a characteristic phenolic glycolipid from mycobacterium leprae in sera from patients with leprosy and tuberculosis. Clin Exp Immunol (1983) 52(2):271.
32. Cho SN, Yanagihara DL, Hunter SW, Gelber RH, Brennan PJ. Serological specificity of phenolic glycolipid I from mycobacterium leprae and use in serodiagnosis of leprosy. Infect Immun (1983) 41(3):1077–83. doi: 10.1128/iai.41.3.1077-1083.1983
33. Fujiwara T, Aspinall GO, Hunter SW, Brennan PJ. Chemical synthesis of the trisaccharide unit of the species-specific phenolic glycolipid from mycobacterium leprae. Carbohydr Res (1987) 163(1):41–52. doi: 10.1016/0008-6215(87)80163-5
34. Zhou Z, Pena M, van Hooij A, Pierneef L, de Jong D, Stevenson R, et al. Detection and monitoring of mycobacterium leprae infection in nine banded armadillos (Dasypus novemcinctus) using a quantitative rapid test. Front Microbiol (2021) 12:763289(3257). doi: 10.3389/fmicb.2021.763289
35. Schilling A-K, van Hooij A, Corstjens P, Lurz PWW, DelPozo J, Stevenson K, et al. Detection of humoral immunity to mycobacteria causing leprosy in Eurasian red squirrels (Sciurus vulgaris) using a quantitative rapid test. Eur J Wildl Res (2019) 65(3). doi: 10.1007/s10344-019-1287-1
36. Schilling AK, McCurdy K, Fish A, Lurz PWW, Geluk A, Van Hooij A, et al. Diagnosing and categorizing leprosy in live Eurasian red squirrels (Sciurus vulgaris) for management, surveillance, and translocation purposes. J Zoo wildlife Med Off Publ Am Assoc Zoo Vet. (2021) 52(2):648–59. doi: 10.1638/2020-0066
37. Schilling AK, van Hooij A, Lurz PWW, Shaw DJS, Geluk A, Corstjens PLAM, et al. Clinical progression of leprosy in Eurasian red squirrels (Sciurus vulgaris) in a naturally infected wild population. J Zoo wildlife Med Off Publ Am Assoc Zoo Vet. (2021) 52(4). doi: 10.1638/2020-0067
38. World Health Organization. Regional office for south-East a. In: Task force on definitions, criteria and indicators for interruption of transmission and elimination of leprosy: Report of the final meeting, vol. 2021. . New Delhi: World Health Organization. Regional Office for South-East Asia (2021).
39. Pierneef L, van Hooij A, Taal A, Rumbaut R, Nobre ML, van Brakel W, et al. Detection of anti-M. leprae antibodies in children in leprosy-endemic areas: A systematic review. PloS Negl Trop Dis (2021) 15(8):e0009667. doi: 10.1371/journal.pntd.0009667
40. Li X, Yang J, Zhang L, Jin G, Xu L, Fang F, et al. A bibliometric analysis of leprosy during 2000–2021 from web of science database. Int J Environ Res Public Health (2022) 19(14):8234. doi: 10.3390/ijerph19148234
41. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
42. Weng X, Deng Y, Wu Q, Wang Z, Yin Z, Wang W. A preliminary study on subclinical infection of leprosy and its seroepidemiology. Chin J Epidemiol (1990) 11(1):16–9.
43. Weng X, Li K, Wen Y, Xing Y, Liu J, Hong B, et al. Study on the factors influencing steady transmission of leprosy in qiubei county, China. Chin J Epidemiol (2011) 32(6):559–64.
44. Weng X, Wen Y, Yuan L, Yang R, Long H, Lu S, et al. The detection of pgl-igm and M. leprae in nasal secretion in the application of leprosy epidemiology. China J Lepr Skin Dis (2005) 21(6):425–30.
45. Li W, Ye G, Chen X, Shen J, Wu Q, Jiang C, et al. Seroepidemiological study of leprosy in household contacts using Nd-O-Bsa and pgl-I as antigens. China J Lepr (1994) 10(4):207–12.
46. Shu H, Wu Q, Li X, Liu Q, Xue Z, Ye G, et al. Study on the subclinical infection of leprosy–—I. preliminary study on the infection rate of household contacts and the discovery of high-risk population. China J Lepr (1987) 3(4):197–200.
47. Shu H, Wu Q, Li X, Liu Q, Xue Z, Ye G, et al. Study on the subclinical infection of leprosy: (II) a comparative analysis of the subclinical infection of leprosy contacts. China J Lepr (1991) 7(1):12–6.
48. Wang H, Wang T, Luo X, Wang M, Ding H, Wang W, et al. Anti-phenolic glycolipid I antibody test results for different populations in leprosy-endemic areas. China J Lepr Skin Dis (2003) 19(3):242–3. doi: 10.3969/j.issn.1009-1157.2003.03.024
49. Long H, Wang J, Yang R. Epidemiological analysis of leprosy in wenshan prefecture, yunnan province, 1994-2008. China J Lepr Skin Dis (2011) 27(002):144–5. doi: 10.3969/j.issn.1009-1157.2011.02.039
50. He Q, Zhang C, Long H, Yu X, Yin L, Shen L, et al. Analysis on the effect of leprosy control during the "Thirteenth five-year plan" period in wenshan prefecture. Pi Fu Bing Yu Xing Bing Za Zhi (2021) 43(5):4. doi: 10.3969/j.issn.1002-1310.2021.05.017
51. Li HY, Weng XM, Li T, Zheng DY, Mao ZM, Ran SP, et al. Long-term effect of leprosy control in two prefectures of China, 1955-1993. Int J leprosy other mycobact. Dis Off Organ Int Leprosy Assoc (1995) 63(2):213–21.
52. Li H, Weng X, Li T. Long-term effect of leprosy control in both highly endemic prefectures, weifang of Shandong and wenshan of yunnan. Natl Med J Chin (1995) 75(6):333–7,81-2.
53. Zhang Z, Zhou Y, Li Q, Liu H. Analysis on the trend of leprosy epidemic in yunnan province in recent 20 years. Pi Fu Bing Yu Xing Bing Za Zhi (2012) 34(5):1. doi: 10.3969/j.issn.1002-1310.2012.05.035
54. Li H, Yang R, Long H, Zhang D, Hong B. Assessment on effectiveness of leprosy elimation campaign in wenshan prefecture, yunnan province. China J Lepr Skin Dis (2003) 19(4):3. doi: 10.3969/j.issn.1009-1157.2003.04.003
55. Zhou D, Chen L, Jiang C, Shu H, Ye G. Theoretical epidemiological study on regional distribution and epidemic prediction of leprosy. China J Lepr (1984) 3(2):84–9.
56. Bian J, Zhu X, Sun S, Chen J. Analysis on the effect of leprosy control in baoying county, jiangsu province. China J Lepr (1987) 3(1):16–8.
57. Wang L, Sun P-W, Yu M-W, Gu H, Wang H-S, Chen X-S. Leprosy update in China, 2019. Int J Dermatol Venereol. (2022) 5(1):15–9. doi: 10.1097/jd9.0000000000000178
59. Wang L, Sun P, Yu M, Gu H, Wang H, Chen X. Epidemiological characteristics of leprosy in China, 2018. Int J Dermatol Venereol. (2020) 3(1):27–30. doi: 10.1097/jd9.0000000000000065
60. Shen JP, Zhang GC, Chen XS, Zhou M, Yu MW, Yan LB. A long-term evolution on the epidemiological characteristics of leprosy, towards the goal of its elimination in 1949 - 2007 in China. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi (2008) 29(11):1095–100. doi: 10.3321/j.issn:0254-6450.2008.11.010.
61. Sun P, Wang L, Yu M, Gu H, Shen J, Yan L, et al. Leprosy statistics in China, 2017. Int J Dermatol Venereol. (2019) 2(1):1–5. doi: 10.3760/cma.j.issn.2096-5540.2019.01.001
62. Shui TJ, Long H, Xiong L, Zhang XH, He J, Chen X. Towards the elimination of leprosy in yunnan, China: A time-series analysis of surveillance data. PloS Negl Trop Dis (2021) 15(3):e0009201. doi: 10.1371/journal.pntd.0009201
63. Zhang QP, Li G, Li C, Lin ZX, Chen P. Epidemiological situation of leprosy in a province in China: A long time to diagnosis and a high rate of deformity. BMC Public Health (2020) 20(1):1790. doi: 10.1186/s12889-020-09933-6
64. Chen N, Zhao S, Yu H. Epidemiological analysis of leprosy in jiangsu province. China J Dermatol (2000) (S1):3. doi: 10.3760/j.issn:0412-4030.2000.z1.009
65. Wang H, Wang T, Luo X, Cui J, Wang M, Zhang Z, et al. Analysis of 18-year effect of combined chemotherapy for comprehensive prevention and treatment of leprosy in chenggu county. China J Lepr Skin Dis (2005) 21(5):2. doi: 10.3969/j.issn.1009-1157.2005.05.063
66. Long H, Li H. Epidemiological analysis of 792 newly registered leprosy patients in wenshan from 1997 to 2001. China J Lepr Skin Dis (2003) 19(3):298–9. doi: 10.3969/j.issn.1002-1310.2003.03.062
67. Zhou Y, Zhou L. Effect of mdt on leprosy epidemic in yunnan province. Chin J Lepr Skin Dis (1995) 11(4):2.
68. Tao Y. Epidemiological status of leprosy before and after combined chemotherapy in gaoyou city. Jiangsu J Prev Med (2017) 28(1):90–1. doi: 10.13668/j.issn.1006-9070.2017.01.34
69. van Hooij A, van den Eeden S, Richardus R, Fat ETK, Wilson L, Franken K, et al. Application of new host biomarker profiles in quantitative point-of-Care tests facilitates leprosy diagnosis in the field. Ebiomedicine (2019) 47:301–8. doi: 10.1016/j.ebiom.2019.08.009
70. van Hooij A, Tjon Kon Fat EM, Batista da Silva M, Carvalho Bouth R, Cunha Messias AC, Gobbo AR, et al. Evaluation of immunodiagnostic tests for leprosy in Brazil, China and Ethiopia. Sci Rep (2018) 8(1):17920. doi: 10.1038/s41598-018-36323-1
71. van Hooij A, Tió-Coma M, Verhard EM, Khatun M, Alam K, Tjon Kon Fat E, et al. Household contacts of leprosy patients in endemic areas display a specific innate immunity profile. Front Immunol (2020) 11:1811. doi: 10.3389/fimmu.2020.01811
72. Long H. Epidemiological analysis of leprosy in wenshan from 1994 to 2003. China J Lepr Skin Dis (2006) 22(3):2.
Keywords: antibodies, children, M. leprae infection, leprosy, PGL-I, transmission, serology, serum
Citation: Zhou Z, Pierneef L, van Hooij A and Geluk A (2022) Detection of anti-M. leprae antibodies in healthy children in China: A systematic review of Chinese literature. Front. Trop. Dis 3:963674. doi: 10.3389/fitd.2022.963674
Received: 07 June 2022; Accepted: 12 September 2022;
Published: 07 October 2022.
Edited by:
Pushpendra Singh, National Institute for Research in Tribal Health (ICMR), IndiaReviewed by:
Anamaria Mello Miranda Paniago, Federal University of Mato Grosso do Sul, BrazilVoahangy R. l. Rasolofo, Institut Pasteur de Madagascar, Madagascar
Hongsheng Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2022 Zhou, Pierneef, van Hooij and Geluk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annemieke Geluk, YS5nZWx1a0BsdW1jLm5s
 Zijie Zhou
Zijie Zhou Louise Pierneef
Louise Pierneef Anouk van Hooij
Anouk van Hooij Annemieke Geluk
Annemieke Geluk