- 1Rollins School of Public Health, Emory University, Atlanta, GA, United States
- 2School of Medicine, Emory University, Atlanta, GA, United States
- 3Department of Medical Microbiology, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 4School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: Despite extensive control measures and a declining number of human reservoirs, the incidence of leprosy in excess of 200,000 new cases each year suggests that alternative pathways of transmission may play a role in continued endemicity. Parasitic coinfection and limited water, sanitation, and hygiene (WASH) have been suggested to predispose individuals to Mycobacterium leprae infection and were further explored in this study.
Methods: Leprosy cases and uninfected controls were recruited from areas around North Gondar, Ethiopia throughout 2019. Participants completed dietary and WASH surveys in addition to providing stool for helminth microscopic diagnosis and urine for Schistosoma mansoni Point-of-care circulating cathodic antigen (POC-CCA)™ rapid diagnostic testing. A similar methodology was employed for a case–control study of leprosy previously conducted by our research team in North Gondar from May to October of 2018. To more comprehensively evaluate associations between the above exposures and leprosy, data from the present 2019 study and the previous 2018 study were combined in select multivariate logistic regression analyses.
Results: A total of 47 men (59%) and 33 women (41%) participated in this study with an average age of 40 (SD 15.0 years). Most leprosy cases were multibacillary (93%). There was a high prevalence of parasitic coinfection among both cases (71%) and controls (60%). WASH insecurities were also widespread. On multivariate analysis, lack of soap for handwashing [aOR= 2.53, 95% CI (1.17, 5.47)] and the lack of toilet facilities [Adjusted odds ratio (aOR)= 2.32, 95% CI (1.05, 5.12)] were significantly associated with leprosy. Positive directionality was identified for a number of other inputs, including helminth infection [aOR= 3.23, 95% CI (0.85, 12.35)].
Conclusions: Taken together, these findings strengthen previous research conducted in 2018 implicating poor WASH conditions as a driver of leprosy infection. Leprosy remains the leading infectious cause of disability in the world. As such, future research should explore the above susceptibilities in more depth to curtail the global burden of disease.
Introduction
Neglected tropical diseases (NTDs) infect over 2.7 billion people worldwide, a disproportionate number of whom reside in low- and middle-income countries (LMICs) (1). Leprosy, also known as Hansen’s disease, is one such NTD that contributes to significant morbidity and disability in certain areas of the world. In 2018 alone, over 208,619 incident cases of leprosy were reported globally, and an estimated 2–3 million people were living with disease-related disabilities (2, 3).
Mycobacterium leprae, a rod-shaped bacillus and the causative agent of disease, is believed to be transmitted via droplets from the nose or mouth during sustained contact with an infected individual. Incubation lasts an average of 1–5 years but may extend up to 20 years in some cases (2). A special affinity for keratinocytes in the skin and Schwann cells in the peripheral nervous system gives rise to some of the more characteristic symptoms of disease including skin discoloration, nodules, lesions, sensory loss, blindness, and deformity (4).
Leprosy is generally categorized according to immune response and disease burden. Tuberculoid (TT) leprosy falls on the less severe end of the spectrum with lower bacillary loads, while lepromatous (LL) leprosy is more severe with notably higher bacillary loads (5). Effective cellular immunity against TT leprosy is characterized by a T helper type 1 (Th1) response that allows for complete destruction of the pathogen, although this strong Th1 activation is not without consequence and often leads to neurologic compromise. In contrast, the response against LL is characterized by malfunctioning cytotoxic T cells, or Th2 immunity, which allows the pathogen to survive and multiply (6, 7).
Since the advent of targeted multidrug therapy and aggressive control measures to counteract the human-to-human proliferation of M. leprae, the overall prevalence of leprosy has decreased, but incidence in endemic areas continues to persist. The majority of these new cases also cannot be linked to a household contact (8). Taken together, these factors strongly suggest the presence of alternative mechanisms of leprosy transmission. The potential for secondary routes of infection or environmental reservoirs opens the door for the consideration of risk factors that may facilitate infection. A few susceptibility factors proposed in the literature include parasitic coinfection and water, sanitation, and hygiene (WASH) (6, 9–15).
Recent research implicates parasitic coinfection along the pathway of leprosy infection and disease. Intestinal helminths have been found to invoke a strong Th2 immune response and weaken Th1 immunity, which serves as a critical defensive system against mycobacteria (6). Individuals harboring intestinal helminths are also known to exhibit defective immune cell signaling and a greater stimulation of regulatory T cells, which may further suppress Th1 activation (6, 16). Thus, the cumulative immune response to a parasitic infection may physiologically predispose colonization by M. leprae, trigger active leprosy from latent infection, and favor the more severe lepromatous end of the disease spectrum (6, 9–11, 16, 17).
In much the same way, poor WASH conditions may facilitate leprosy transmission in addition to cultivating an environment favorable for parasitic disease (18–20). Reliance on an unimproved water source, open defecation, and limited handwashing have previously been explored as risk factors for leprosy (12, 13). Water has also been investigated as a potential reservoir of M. leprae, with proximity to water bodies implicated in leprosy onset (8, 14, 21). Potentially viable bacilli have been detected in communal water sources as well as soil samples in areas used for bathing (15, 21–23). Further, laboratory studies have demonstrated the survival of the pathogen outside of the human body, with viability maintained for up to 5 months in shaded soil samples and 8 months when phagocytized by common free-living amoebas (24, 25).
In Ethiopia, approximately 5,000 new cases of leprosy are diagnosed each year (1). This number is much higher when accounting for existing cases of leprosy, those who have recovered with disability, and probable underreporting due to stigma and lack of access to healthcare. Ethiopia is also endemic for parasitic diseases, many related to poor WASH conditions, making this region uniquely suited for studying the overlap between these susceptibility factors. Schistosomiasis, for example, affects close to 5 million Ethiopians each year (1). Hookworm, a common soil-transmitted helminth, affects over 11 million people in Ethiopia, amounting to 5.6% of the hookworm burden in sub-Saharan Africa. Ascariasis affects over 26 million Ethiopians, or 15% of the overall burden in sub-Saharan Africa. Ethiopia ranks within the top 10 countries for the highest counts of both leishmaniasis and lymphatic filariasis, and over 12 million Ethiopians are at risk of onchocerciasis and subsequent blindness (1).
Given the potential secondary mechanisms of leprosy transmission detailed above, the primary objective of this case–control study was to better understand coinfection and environmental factors in the context of leprosy infection. As previously suggested in the literature, patients with leprosy are hypothesized to lack appropriate WASH resources and exhibit higher rates of parasitic coinfection compared to healthy controls.
Materials and methods
Study site and population
This case–control study was conducted in Gondar, Ethiopia with a focus on North Gondar and surrounding woredas (administrative districts). North Gondar has a population of 3,225,022 individuals, most of whom reside in rural or agricultural areas (84.21%) (26). An average of 5,000 new cases of M. leprae are diagnosed at health facilities in Ethiopia each year, resulting in one of the highest burdens in sub-Saharan Africa (1). Approximately 30% of these cases occur among those living in the Amhara Region, which encapsulates the densely populated zone of North Gondar (27).
Data were collected from June to December of 2019. Potential subjects were identified from a leprosy registry and recruited from local dermatology and health clinics. Cases were defined as adults over the age of 18 diagnosed in the prior 12 months with multibacillary or paucibacillary leprosy by trained dermatologists. Unconfirmed cases, pregnant women with leprosy, and patients who have completed multidrug therapy (MDT) were excluded from evaluation. Controls were sampled from the adult members of surrounding communities with no current or previous leprosy diagnosis and no known leprosy exposures. Controls were excluded if there was a history of an unconfirmed neurological or dermatological disease or known proximal contact with a leprosy case.
A previous case–control study of leprosy was conducted by our study team in North Gondar from May to October of 2018 (12). The case definition, exclusion criteria, recruitment protocols, and methods of obtaining WASH and schistosoma data were consistent between the 2018 and 2019 collection periods. The only addition to the protocol for the present 2019 study was the collection and testing of stool samples using the Kato–Katz technique, which allowed for a more comprehensive investigation of parasitic infection beyond the singular S. mansoni datapoint collected via urine sample in 2018. Thus, in select analyses, both years of schistosoma infection and WASH data were combined to allow for a more comprehensive exploration of susceptibilities.
Data collected
Participants were asked to complete a questionnaire and provide stool and urine samples after consenting to participate in the study. On-site medical personnel assisted with the administration of surveys in Amharic and the collection of biological specimens. Demographic and other key health information from medical records supplemented data obtained directly from patients.
Field procedures
Cases were diagnosed at local dermatology and health centers by experienced physicians on the basis of clinical and bacillary index findings per current World Health Organization (WHO) guidelines. The WHO differentiates leprosy into two types based on physical symptoms and the presence or absence of bacilli in slit-smear tests. Paucibacillary (PB) leprosy is characterized by five or fewer skin patches and no bacilli on a slit-smear test, while multibacillary (MB) leprosy applies to cases with greater than five skin patches and visible bacilli on a slit-smear test (2).
Secondary data in the form of demographic information, medical charts, and survey responses were collected with written informed consent from participants. This information was utilized to determine leprosy classification and the degree of disability.
Evaluation of parasitic coinfection
The Schisto POC-CCA™ rapid diagnostic test was used to detect Schistosoma mansoni infection. Schisto POC-CCA™ rapid diagnostic tests identify active infections in urine specimens with burdens as low as 50 worms. The sensitivity reaches 100% at higher burdens of 400 or more eggs per gram of feces (28). The presence of other helminths (including eggs for S. mansoni) was determined by Kato–Katz stool exam using one sample per individual. Protozoa were assessed by direct microscopy. Participants who tested positive for a parasitic infection were immediately referred to providers at local health centers for treatment
Evaluation of water, sanitation, and hygiene conditions
A survey adapted from the WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation (JMP) core questions on WASH for household surveys was completed by all participants to better understand water usage, contact, treatment, soap accessibility, handwashing, and other general sanitation practices (29).
Information collected regarding drinking water source, cooking water source, and toilet type was classified according to improved and unimproved categories for analysis. Per the WHO, improved water sources are located on premises, available when needed, and free of fecal and priority chemical contamination (30). Improved water sources adequately protect water from outside contamination through avenues such as a household connection, public standpipe, borehole, protected dug well, protected spring, and rainwater collection. Unimproved sources include unprotected dug wells, unprotected springs, surface water, vendor-provided water, and tanker truck water. Because the majority of participants in this study did not have access to a water source on premises, the time it took participants to collect water was further subcategorized from improved and unimproved into basic and limited-service groupings. Water collection involving a round trip of 30 min or less merited a classification of basic service, while a round trip to collect water exceeding this cutoff was considered limited water service in accordance with WHO guidelines (31).
In terms of sanitation, improved facilities are defined by the hygienic separation of human excreta from human contact through mechanisms such as a sewer connection, septic system connection, pour-flush latrines, ventilated improved pit latrines, and pit latrines with a slab or covered pit (30). Unimproved toilet facilities include pit latrines without slabs or platforms, hanging latrines, bucket latrines, and open defecation. Handwashing data, a key indicator of hygiene, was dichotomized into limited-service and no-service groupings based on the presence or absence of functional hand hygiene stations (32). Finally, previous studies have linked proximity to bodies of water as a potential susceptibility factor to leprosy (12, 20, 21). The local water source in Gondar, Lake Tana, is also a known reservoir for S. mansoni (12). Thus, village residency reported by each participant was recategorized on the basis of proximity to Lake Tana with individuals assigned to “on the lake” or “not on the lake” groupings as was done in previous studies (12).
Statistical analysis
Drawing on previous publications, an alpha of 0.05 and power of 0.8 coupled with an estimated helminth prevalence in the North Gondar region between 20% and 25% and a predicted odds ratio of 3–4 for the association of helminth infection with leprosy yielded a total goal sample size of 80 split evenly between cases and controls (26).
Following data cleaning, all analyses were conducted using Statistical Analysis Software (SAS) 9.4. Descriptive statistics, univariate comparisons, and logistic regression were the primary mathematical outputs. Independent two-sample t-test and chi-square tests were completed during univariate procedures to describe differences between cases and controls. Odds ratios were calculated to indicate associations between the exposures of interest (parasitic coinfection and WASH status) and the outcome (leprosy). A p-value of 0.05 or below was employed as the threshold for significance.
Helminth infection, select WASH variables, and confounders significantly associated with the outcome on univariate analyses were included in a multivariate logistic regression model. Potential confounders included age, sex, and socioeconomic status. Socioeconomic status was evaluated on the basis of monthly income, education, and household size. Monthly income was dichotomized into two groups after reviewing the frequency and distribution of the data. A large right skew influenced the selection of the first quartile as the cutoff value with participants separated into “above Q1” and “below Q1” groups. Education was categorized on the basis of junior secondary school (grade 8) (33). Thus, participants were divided into “below grade 8” and “grade 8 and above” categories. Household size was dichotomized into “crowded” and “normal” living conditions in accordance with a systematic review and meta-analysis of socioeconomic risk markers of leprosy, which linked crowded living conditions, characterized by five or more individuals residing in a single household, to leprosy infection (34).
Given the exploratory nature of the study, variables of fundamental importance to the research question were evaluated at each phase of the analysis. A detailed report of findings is provided in the following tables. A Hosmer–Lemeshow goodness-of-fit test was conducted at the conclusion of the analysis to investigate the suitability of the final logistic regression models. A p-value of 0.05 was again employed as the cutoff with any value below this point prompting the rejection of the model. Collinearity was also examined, and there was no evidence to suggest correlations between variables included in the final models.
Ethical approval
This study was approved by the Institutional Review Boards of Emory University and the University of Gondar in Ethiopia. Involvement in the study was voluntary, and informed written consent was obtained from all participants. Data collection presented very little risk to patients given the minimal invasiveness of testing procedures. All data containing private or identifying information was stored in a locked room at the University of Gondar or on a password-protected computer. Data utilized for the purpose of this secondary analysis were obtained with permission from the principal investigator and deidentified before being accessed in the US.
Results
Descriptive statistics
A total of 80 participants, 31 cases and 49 controls, were enrolled in the study. The average age of enrollees was 40 with a standard deviation of 15 years. Approximately 59% of participants were men and 41% were women. The majority of cases were diagnosed with MB leprosy (93.3%) and had a positive bacillary index (70.4%). Participants varied in terms of their disability grade, but a total of 61% experienced some degree of chronic impairment due to leprosy. Although cases enrolled in the present study were diagnosed within the past year, it is possible that symptom onset began well before healthcare services were sought out. This delay in diagnosis may occur for many reasons, but perhaps the most important being the continued stigma surrounding leprosy that often deters individuals from pursuing treatment until after symptoms are severe and chronic disability is more likely (35).
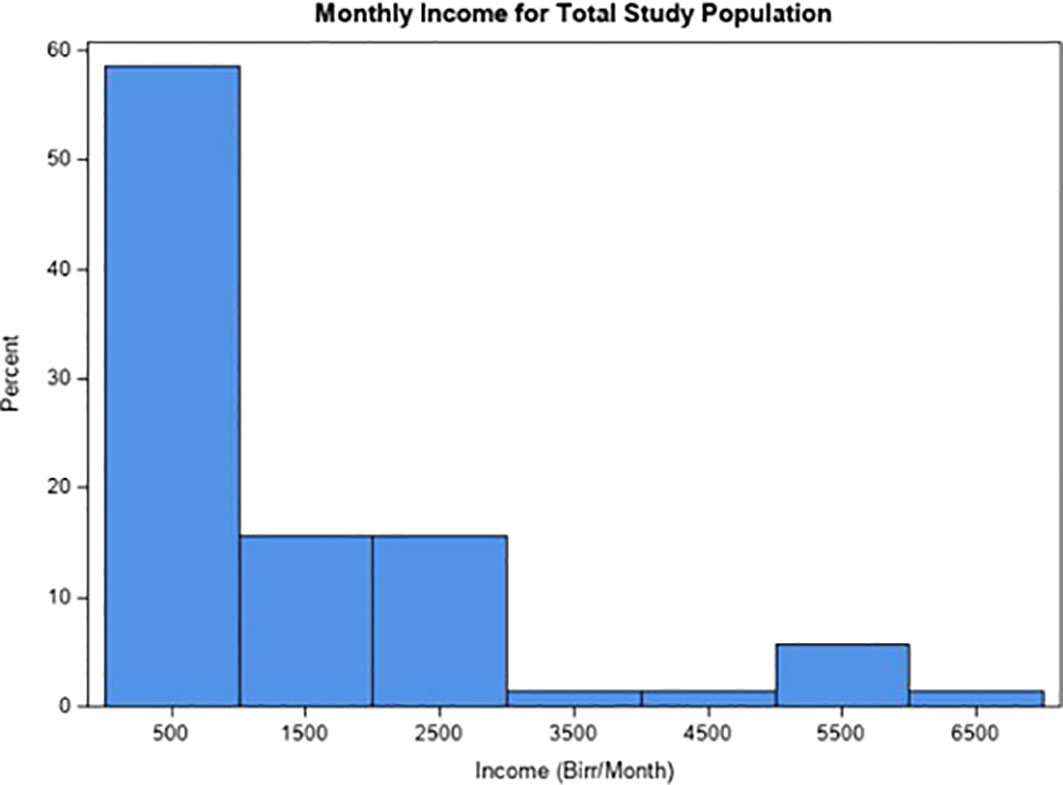
Additionally, over 87% of participants did not complete junior secondary school, and 30% of the population reported a monthly income below 300 Birr. The mean monthly income was 500 Birr (Figure 1). For reference, the national absolute poverty line in Ethiopia is set at 3,781 Birr per adult equivalent per year, or 315 Birr per adult equivalent per month (36). Given that 63.8% of participants reported living in households of five or more individuals, poverty is likely even more pervasive than reflected by these numbers. Further demographic, socioeconomic, and clinical markers are presented in Table 1.
Univariate analysis
Parasitic coinfection
Most participants (64.1%) tested positive for one or more helminths (Table 2). A total of 41 participants (52.6%) were infected with S. mansoni, making this parasite the primary driver of the total helminth prevalence in the study population. Approximately 16 (22.5%) participants were infected with a protozoan. The most frequently identified protozoan was E. histolytica, infecting 11.3% of participants. Although univariate analysis did not yield significant differences in helminth or protozoa infection between cases and controls, these findings underscore the frequency of parasitic infection within the general study population. Of the 31 cases, 22 (71.0%) tested positive for a helminth and 7 (22.6%) were infected with a protozoan. Similarly, 28 controls (59.6%) were identified as having a helminth infection and 9 (22.5%) tested positive for a protozoan out of the entire group of 49 people. Thus, the majority of cases and controls tested positive for a helminth, protozoa, or both. Additional outcomes relating parasitic infection to leprosy are presented in Table 2.
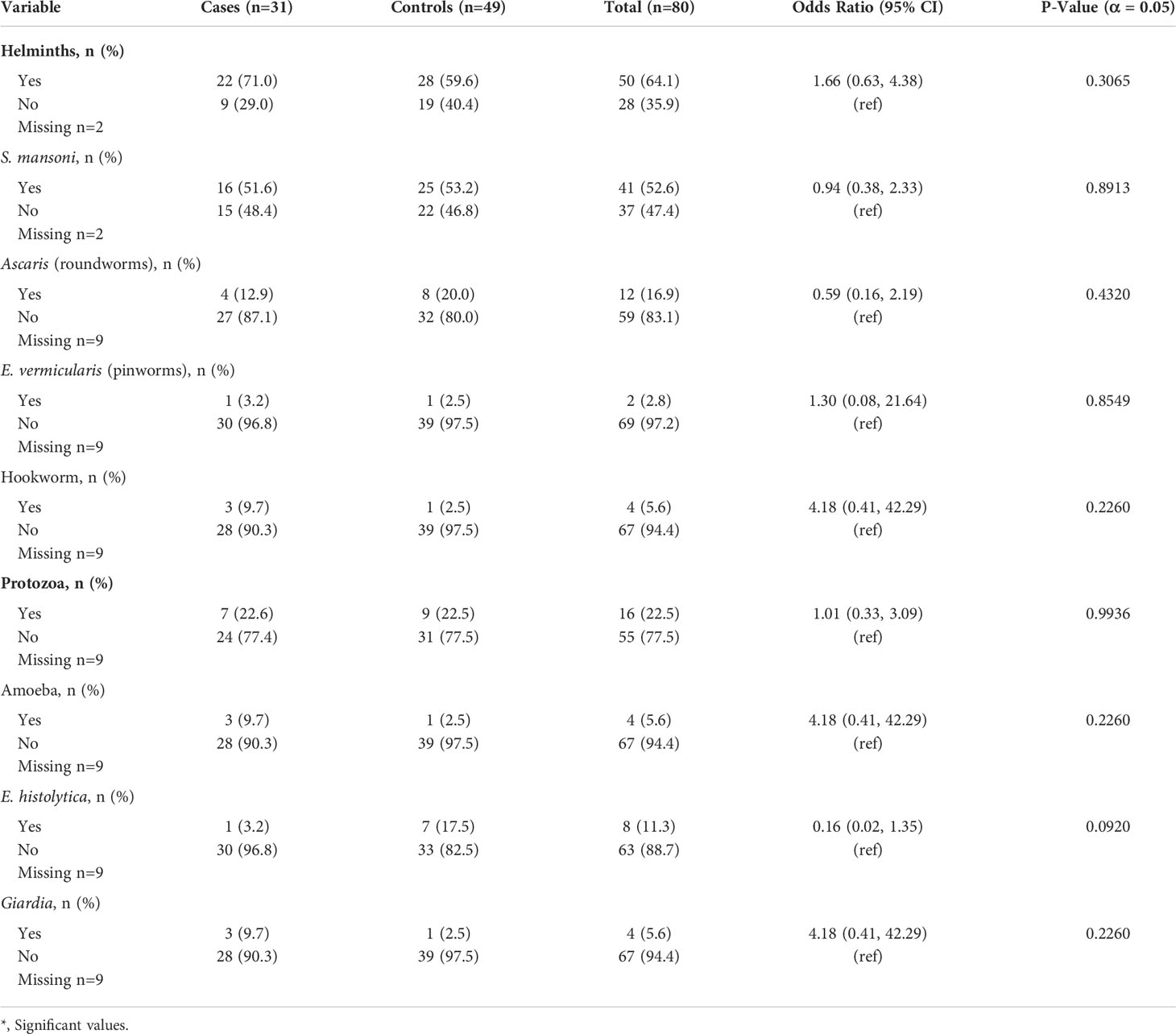
Table 2 Univariate analysis of parasitic coinfection data collected over 2019 among leprosy cases and controls.
As detailed in the methods, a subset of parasitic coinfection data (S. mansoni POC-CCA data without stool samples) was available for both the 2018 and 2019 study periods. Thus, additional univariate analysis was conducted with this combined dataset. Of the 161 total individuals tested, 24 cases (33.8%) and 38 controls (43.2%) were positive for S. mansoni. Additional results are recorded in Table 3.
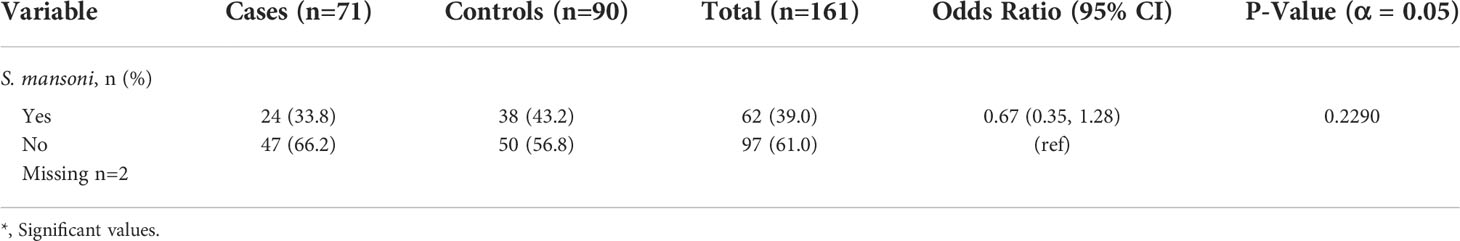
Table 3 Univariate analysis of parasitic coinfection data collected over 2018 and 2019 among leprosy cases and controls.
Water, sanitation, and hygiene conditions
As illustrated in Table 4, many participants did not have access to improved WASH services. A total of 14 participants (17.7%) collected water from an unimproved drinking water source, while 15 (18.8%) obtained cooking water from an unimproved source. Close to 95% of participants did not have access to water on premises. The majority of individuals with access to water off premises reported a roundtrip time to collect water under 30 min, with only 6.9% exceeding this demarcation. In terms of water treatment, 34 (41.3%) participants consistently treated their water while 43 (55.8%) did not. Access to handwashing facilities, a key metric of hygiene, was only noted for nine (13.0%) participants with the remaining 87% of the population lacking functional handwashing stations. Only 38 (48.7%) participants reported consistent access to soap. Although univariate analysis did not yield significant differences between cases and controls for the above WASH conditions, these findings underscore the prevalence of WASH insecurity within the general study population.
Similarly, proximity to a body of water was associated with leprosy [OR= 2.74, 95% CI (0.93, 8.01)]. These findings build on the results of a previous study conducted in Ethiopia showing increased odds of schistosomiasis among leprosy cases living near Lake Tana compared to those geographically distanced from this water source (12). The type of toilet facilities available to participants was also found to differ between cases and controls. Approximately 37 (49.3%) participants exclusively relied upon unimproved toilet facilities that did not appropriately segregate waste. A total of 31 (41.3%) people practiced open defecation, a statistically significant number of whom were also diagnosed with leprosy. In fact, the odds of open defecation were 2.6 times higher in leprosy cases compared to controls [OR= 2.6, 95% CI (1.01, 6.73)]. Additional outcomes relating WASH to leprosy are presented in Table 4.
As previously detailed, WASH data were available for both the 2018 and 2019 study. Thus, additional univariate analyses were conducted with this combined dataset and statistically significant findings were demonstrated overall. Results, again, illustrate widespread WASH insecurity within the study population. Close to 18% of participants relied on an unimproved source for drinking water, 85.9% traveled off premises for all water needs, and 77.4% lacked access to handwashing facilities. As illustrated in Table 5, a total of 57% of the study population were reliant on untreated water. Consequently, individuals with leprosy had greater odds of not treating their water compared to controls [OR= 2.24, 95% CI (1.10, 4.55)]. Lack of soap for handwashing was also significantly associated with leprosy [OR= 2.19, 95% CI (1.16, 4.15)]. Consistent with findings from the 2019 data, 32.9% of the population did not have access to toilet facilities, a disproportionate number of whom were also diagnosed with leprosy. Among leprosy cases, the odds of open defecation were 2.81 times greater than the odds of this exposure in controls [OR= 2.81, 95% CI (1.40, 5.61)]. Additional outcomes relating WASH to leprosy are presented in Table 5.
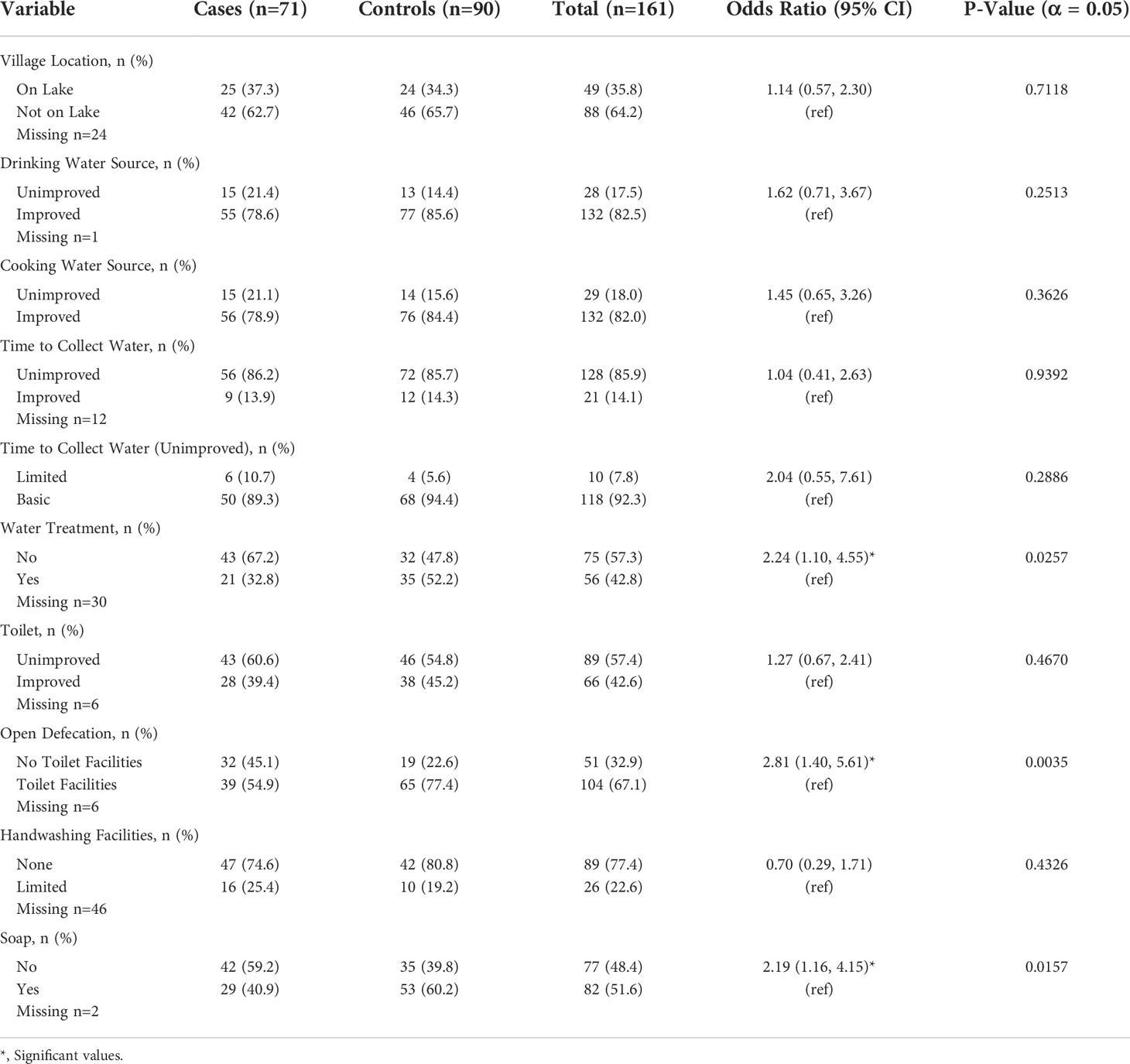
Table 5 Univariate analysis of WASH data collected over 2018 and 2019 among leprosy cases and controls.
Multivariate analysis
Model 1: 2019 alone
On the multivariate analysis of the primary study exposures collected in 2019 (Table 6), several susceptibilities maintained a connection to leprosy. Controlling for monthly income (indicator of SES), leprosy was significantly associated with lack of soap [aOR= 6.09, 95% CI (1.53, 24.24)]. Associations were also sustained between leprosy and lack of water treatment [aOR= 1.43, 95% CI (0.45, 4.53)] as well as open defecation [aOR= 1.22, 95% CI (0.38, 3.89)], although not statistically significant. Thus, markers of WASH all exhibited a positive relationship with the outcome. Other WASH indicators, such as village location, were dropped from the model due to a number of missing data points. Although not statistically significant, positive directionality persisted for the association between helminth infection and leprosy [aOR= 3.23, 95% CI (0.85, 12.35)].

Table 6 Model 1: Multivariate logistic regression model of data collected during 2019 featuring leprosy as the outcome and parasitic coinfection, lack of toilet facilities, lack of water treatment, and lack of soap as the exposure variables.
The small study size and necessary inclusion of an SES marker constrained the total number of variables able to be incorporated in the model. Thus, age and sex were removed from the final 2019 multivariate results after confirming these variables did not act as significant confounders. Given the exploratory nature of the study and the limited size of the dataset, interaction terms were not investigated but may be considered as this line of research moves forward.
Model 2: 2018 and 2019 combined
On the multivariate analysis of S. mansoni infection and WASH data collected over 2018 and 2019 (Table 7), open defecation [aOR= 2.32, 95% CI (1.05, 5.12)] and lack of soap [aOR= 2.53, 95% CI (1.17, 5.47)] were significantly associated with leprosy when controlling for age, sex, and education (indicators of SES) (Table 7). Drinking water was included as a metric of water quality but did not prove significant in this particular model. Other WASH variables, such as water treatment, contained incomplete data from 2018 and were unable to be evaluated in the combined model. In terms of parasitic coinfection, schistosomiasis was not significantly associated with leprosy in the present study. However, education below 8th grade was strongly related to the outcome, suggesting that a low socioeconomic status may increase risk of disease [aOR= 3.02, 95% CI (1.02, 8.98)]. Older age (60 years and above) also exhibited positive directionality, which may be explained on the basis of declining immunity with advancing age [aOR= 1.28, 95% CI (0.41, 3.98)].

Table 7 Model 2: Multivariate logistic regression model of data collected over 2018 and 2019 featuring leprosy as the outcome and schistosomiasis infection, lack of toilet facilities, unimproved water, and lack of soap as the exposure variables.
Discussion
The elimination of leprosy as a public health threat, defined as a prevalence of less than 1 case per 10,000 individuals, was achieved globally in 2000, yet there were still over 208,619 incident cases of leprosy reported worldwide in 2018 alone (2). Interestingly, an estimated 95% of the human population is not genetically susceptible to leprosy and most infected persons are not contagious (5). Taken together, these factors depress the explanatory power of human-to-human transmission as the sole driver of continued leprosy incidence. The recent identification of the nine-banded armadillo as an animal reservoir of leprosy in both the US and Brazil further suggests that other secondary routes of infection may be at play (37).
In order to cast light on alternative pathways that perpetuate transmission, the present study set out to explore the contributions of potential susceptibilities. Findings suggest that host and environmental considerations play a significant role. In terms of coinfection, the abundance of parasitic disease was very high among both cases and controls. Helminth infection was particularly prevalent with 64.1% of the total study population testing positive for an intestinal parasite. S. mansoni was identified as the primary driver of these infections which aligns with current schistosomiasis prevalence estimates in Ethiopia in the amount of 5 million cases per year (38). As observed in the course of univariate analysis, multivariate logistic regression modeling revealed a positive relationship between helminths and leprosy when controlling for potential confounders [aOR 3.23, 95% CI (0.85, 12.35)]. Although this finding was not sustained after combining the 2018 and 2019 datasets, prior literature affirms the connection between parasitic coinfection and leprosy as well as other mycobacterial diseases such as tuberculosis (6, 10, 17). A previous study conducted in Brazil, for example, identified a 6.80 increased chance of contracting leprosy in a community with known schistosomiasis cases compared to a community without [RR= 6.80, 95% CI (1.46, 31.64)] (9).
A related study in Brazil also demonstrated an association between leprosy and schistosomiasis with the odds of S. mansoni infection 8.69 times higher in leprosy cases compared to household contacts [aOR= 8.69, 95% CI (1.50, 50.51)]. However, this relationship was not sustained when the comparison group was made up of non-contact controls [aOR= 1.27, 95% CI (0.38, 4.26)] (11). Given that households contacts more stringently control for SES and other common exposures, this finding suggests that helminth infection may drive susceptibility to leprosy infection under conditions in which all other exposures are equivalent. It is likely, then, that the present study would have benefited from a more robust recruitment and matching scheme involving the household contacts of leprosy cases. Results from the 2-year model may have been further attenuated by the availability of only S. mansoni infection data rather than information regarding all helminth exposures in 2018.
Helminth infection is a product of both the host response and the environment. Thus, the true nature of the relationship between parasitic coinfection and leprosy may have been blurred by the inability to include all possible, relevant confounders. Going forward, a deeper examination of the epidemiologic triangle and the dynamic relationships between host, pathogen, and environment may be needed to fully elucidate the impact of helminth infection on leprosy.
Parasitic coinfection is also known to occur in settings characterized by poor WASH conditions and high rates of leprosy. In the present study, open defecation was positively associated with leprosy in the 2-year model [aOR= 2.32, 95% CI (1.05, 5.12)]. This finding strengthens previous research conducted by our study team in 2018 which identified a connection between open defecation and leprosy [aOR= 19.9, 95% CI (2.2, 176.3)] (12). Additionally, lack of water treatment [aOR= 2.24, 95% CI 1.10, 4.55)] and lack of soap [aOR 2.53, 95% CI (1.17, 5.47)] were significantly associated with the outcome. These findings build on work from our study group conducted in 2018 linking lack of soap to leprosy [aOR = 7.3, 95% CI (1.1, 49.9)] (12). In combination with previous literature connecting unimproved water, lack of water access on premises, and the lack of handwashing with an infectious disease risk, it is likely that these conditions meaningfully contribute to leprosy susceptibility and/or transmission (12, 13).
It is also important to note that these risk factors are a product of a low socioeconomic status. In order to truly achieve leprosy control, improvements in poverty must occur tangentially with other risk reduction measures. The fact that 30% of the participants in this study were found to live on less than 300 Birr ($6.47 USD) per month substantiates the prevalence of poverty in Ethiopia and underscores the need for multifactorial public health interventions that address both physical and socioeconomic contributions to leprosy infection. Future research involving larger sample sizes may generate more confidence in these findings and advance understanding of the interplay between poverty, WASH, and the transmission of M. leprae.
That said, the persistence of significant relationships between WASH conditions after controlling for socioeconomic status lends credence to the proposed existence of environmental reservoirs of M. leprae and the role of poor WASH in driving continued transmission. Research has identified the presence of M. leprae in communal water sources as well as soil samples in highly frequented areas (15, 39). The survivability and viability of the pathogen outside of a human host has also been demonstrated in excess of 8 months under certain circumstances (24). The prevalence of open defecation in this study population coupled with lack of water treatment and soap for handwashing is strongly suggestive of an environmental pathway beginning with M. leprae exposure through limited WASH and ending in clinically symptomatic disease. As Ethiopia continues to net a high incidence of leprosy each year despite extensive control measures, additional research to elucidate this pathway must be carried out.
Methodologies that more effectively capture the potential for repeat parasitic exposures may also be warranted. Open defecation, for example, is a surrogate marker of both poor WASH and exposure to soil-transmitted helminths (12, 13). Unfortunately, the one-time sampling of helminths in the present study only captured individuals currently infected with a parasite. It is possible that other cases harbored a helminth at the time of their leprosy diagnosis but did not test positive for a persistent or repeat helminth infection when enrolled in this study. While helminth infections may be transient depending on the life cycle of the infectious agent and the availability of curative treatments, the practice of open defecation due to a lack of sanitation resources is likely more enduring. Approaches that capture a more comprehensive history of parasitic coinfection may clarify the relationship between this exposure and leprosy and improve our understanding of the risks associated with open defecation.
In addition to sustained leprosy endemicity, many areas of Ethiopia also grapple with a high prevalence of NTDs and insecure WASH. This study is unique in that it combines both host and environmental risk factors in the examination of leprosy in this setting. A major strength of the above investigation lies in the utilization of a variety of different data streams including self-report questionnaires, information from medical records, and biological specimens. Further, this study builds on previous research in the realm of parasitic coinfection by investigating a number of helminths and protozoa in addition to S. mansoni. However, as with all case–control studies, establishing temporality between exposure and disease can prove challenging. This study was no exception, leaving many lingering uncertainties regarding the prospective relationship between parasitic coinfection, high WASH insecurity, and leprosy onset. The long incubation period of M. leprae further complicates the situation by extending the window between transmission and the manifestation of clinical symptoms, thus opening the door to extraneous variables that may obscure the true nature of this relationship.
Fortunately, existing literature speaks to the presence of underlying biological mechanisms that support the proposed forward directionality of the above exposures and leprosy onset. For example, parasitic infection is known to downregulate cellular and humoral responsiveness to all pathogens, paving the way for the development of active leprosy after exposure to the disease-causing bacterium (6). Perhaps even more compellingly, laboratory studies have confirmed the survivability of M. leprae outside of a human host for many months (7, 25). The existence of potentially viable M. leprae in highly frequented areas, such as community water sources and open areas where individuals defecate, has been detected through DNA sampling and may serve as a common environmental exposure driving sustained leprosy transmission in endemic areas (38). Finally, the collection of physiological data in real time, such as the stool and urine samples, provides greater confidence in the prospective nature of these relationships.
Despite the above assurances, the small sample size of the study poses another possible limitation, especially as the number of cases did not quite reach the target sample size derived from the power calculations due to challenges surrounding case enrollment and dropout. Individuals with leprosy continue to face tremendous stigma and may have declined to participate in the present study despite assurances of confidentiality. Additionally, a diagnosis of leprosy has previously been associated with unemployment, the loss of income, poverty, and reduced overall wellbeing (40, 41). In combination with physical disability from the disease process itself, those diagnosed with leprosy may have been unable to seek healthcare services at the same rate as their well counterparts. Concerns regarding the resulting discrepancy in group size and impact on power were somewhat attenuated by the supplemental S. mansoni and WASH data obtained in 2018 which allowed for a larger database to explore exposure and outcome relationships as well as the opportunity to build on previous findings. Nevertheless, future investigations should emphasize the recruitment of additional cases in order to maximize statistical power. Focus should also be directed toward the collection of complete data given that certain variables in the present analysis, such as village location, were unable to be examined in depth due to the quantity of missing values.
Finally, while this study measured a number of parasitic coinfection and WASH variables, the impact of concurrent susceptibilities in a single individual was not explored in the present analysis and should be examined in future research. Similarly, other host and environmental conditions, like nutritional status, are known to affect the immune response and may further alter the risk profile for leprosy infection. An investigation of these variables in the context of leprosy transmission is currently being conducted by our research group.
It is also possible that exposure to parasitic coinfection and limited WASH not only facilitates transmission but also accelerates the progression of disease from latent to clinically symptomatic in previously infected individuals. Alternatively, one or more of these exposures may predispose individuals to the multibacillary form of leprosy which is associated with less cell-mediated immunity and a more severe disease course. Poor WASH conditions, such as open defecation and lack of soap identified in the present analysis, may promote increased reservoirs of multibacillary M. leprae in the environment, thereby heightening the chance of human exposure to this subtype of the pathogen. The fact that 93.3% of cases in the present study were diagnosed with multibacillary leprosy substantiates this theory. Future research should explore these potentialities in more depth in order to gain a better understanding of leprosy transmission and inform prevention efforts.
Data availability statement
Due to the stigmatized nature of the infection and the small sample size, we have not made the dataset readily available. Requests for data can be sent to Dr. Kassahun Alemu Gelaye, Director of the Institute of Public Health at the University of Gondar, Ethiopia at a2Fzc2FsZW11QGdtYWlsLmNvbQ==.
Ethics statement
This study involving human participants was reviewed and approved by the Emory University Institutional Review Board and the University of Gondar Institutional Review Board. The participants provided their written informed consent prior to enrollment in the study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was funded through generous donations of the Order of St. Lazarus, US Priory, as well as Emory University.
Acknowledgments
We are incredibly grateful for Yawyewsew Alemu’s work on this project including coordination of visits and administration of questionnaires. We also appreciate the assistance of health center staff and thank the participants without which the study could not have been done.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Deribe K, Meribo K, Gebre T, Hailu A, Ali A, Aseffa A, et al. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasites Vectors (2012) 5(1):240. doi: 10.1186/1756-3305-5-240
2. World Health Organization (WHO). Leprosy. (Geneva: WHO) (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/leprosy.
3. Centers for Disease Control and Prevention National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of High-Consequence Pathogens and Pathology (DHCPP). World leprosy day. In: Bust the myths, learn the facts. (Atlanta: CDC) (2021). Available at: https://www.cdc.gov/leprosy/world-leprosy-day/index.html.
4. Centers for Disease Control and Prevention (CDC). Signs and symptoms. (Atlanta: CDC) (2017). Available at: https://www.cdc.gov/leprosy/symptoms/index.html.
5. White C, Franco-Paredes C. Leprosy in the 21st century. Clin Microbiol Rev (2015) 28(1):80–94. doi: 10.1128/CMR.00079-13
6. Diniz LM, Magalhães EF, Pereira FE, Dietze R, Ribeiro-Rodrigues R. Presence of intestinal helminths decreases T helper type 1 responses in tuberculoid leprosy patients and may increase the risk for multi-bacillary leprosy. Clin Exp Immunol (2010) 161(1):142–50. doi: 10.1111/j.1365-2249.2010.04164.x
7. Wambier CG, Wambier S, Furini RB, Simão JCL, Frade MAC, Foss NT. Factors associated with seropositivity for APGL-iamong household contacts of leprosy patients. Rev da Sociedade Bras Medicina Tropical (2016) 49(1):83–9. doi: 10.1590/0037-8682-0325-2015
8. Kerr-Pontes LR, Barreto ML, Evangelista CM, Rodrigues LC, Heukelbach J, Feldmeier H. Socioeconomic, environmental, and behavioural risk factors for leprosy in north-east Brazil: Results of a case–control study. Int J Epidemiol (2006) 35(4):994–1000. doi: 10.1093/ije/dyl072
9. Phillips DA, Ferreira JA, Ansah D, Teixeira HS, Kitron U, Filippis Td, et al. A tale of two neglected tropical infections: Using GIS to assess the spatial and temporal overlap of schistosomiasis and leprosy in a region of minas gerais, Brazil. Memórias Do Instituto Oswaldo Cruz (2017) 112(4):275–80. doi: 10.1590/0074-02760160395
10. Prost A, Nebout M, Rougemont A. Lepromatous leprosy and onchocerciasis. Br Med J (1979) 1(6163):589. doi: 10.1136/bmj.1.6163.589-a
11. Dennison CL, de Oliveira LB, Fraga LAO, Lima RS, Ferreira JA, Clennon JA, et al. Mycobacterium leprae–helminth co-infections and vitamin d deficiency as potential risk factors for leprosy: A case–control study in south-eastern Brazil. Int J Infect Diseases (2021) 105:261–6. doi: 10.1016/j.ijid.2021.02.048
12. Emerson LE, Anantharam P, Yehuala FM, Bilcha KD, Tesfaye AB, Fairley JK, et al. (Water, sanitation, and hygiene) conditions are associated with leprosy in north gondar, Ethiopia. Int J Environ Res Public Health (2020) 17(17):6061. doi: 10.3390/ijerph17176061
13. Freitas LRS, Duarte CE, Garcia LP. Leprosy in b razil and its association with characteristics of municipalities: ecological study, 2009–2011. Trop Med Int Health (2014) 19(10):1216–25. doi: 10.1111/tmi.12362
14. Feenstra SG, Nahar Q, Pahan D, Oskam L, Richardus JH. Recent food shortage is associated with leprosy disease in Bangladesh: A case-control study. PloS Negl Trop Dis (2011) 5(5):e1029. doi: 10.1371/journal.pntd.0001029
15. Arraes MLB, Holanda MV, Lima LNGC, Sabadia JAB, Duarte CR, Almeida RLF, et al. Natural environmental water sources in endemic regions of northeastern Brazil are potential reservoirs of viable mycobacterium leprae. Memórias do Instituto Oswaldo Cruz (2017) 112(12):805–11. doi: 10.1590/0074-02760170117
16. Borkow G, Leng Q, Weisman Z, Stein M, Galai N, Kalinkovich A, et al. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J Clin Invest (2000) 106(8):1053–60. doi: 10.1172/JCI10182
17. Babu S, Bhat SQ, Kumar NP, Jayantasri S, Rukmani S, Kumaran P, et al. Human type 1 and 17 responses in latent tuberculosis are modulated by coincident filarial infection through cytotoxic T lymphocyte antigen-4 and programmed death-1. J Infect Dis (2009) 200(2):288–98. doi: 10.1086/599797
18. Greenland K, Dixon R, Khan SA, Gunawardena K, Kihara JH, Smith JL, et al. The epidemiology of soil-transmitted helminths in bihar state, India. PloS Negl Trop Dis (2015) 9(5):e0003790. doi: 10.1371/journal.pntd.0003790
19. Kattula D, Sarkar R, Ajjampur SSR, Minz S, Levecke B, Muliyil J, et al. Prevalence & risk factors for soil transmitted helminth infection among school children in south India. Indian J Med Res (2014) 139(1):76.
20. Couto LD, Tibiriça SH, Pinheiro IO, Mitterofhe A, Lima AC, Castro MF, et al. Neglected tropical diseases: Prevalence and risk factors for schistosomiasis and soil-transmitted helminthiasis in a region of minas gerais state, Brazil. Trans R Soc Trop Med Hygiene (2014) 108(6):363–71. doi: 10.1093/trstmh/tru054
21. Sterne JA, Pönnighaus JM, Fine PE, Malema SS. Geographic determinants of leprosy in karonga district, northern Malawi. Int J Epidemiol (1995) 24(6):1211–22. doi: 10.1093/ije/24.6.1211
22. Mohanty PS, Naaz F, Katara D, Misba L, Kumar D, Dwivedi DK, et al. Viability of mycobacterium leprae in the environment and its role in leprosy dissemination. Indian J Dermatol Venereol Leprol (2016) 82(1):23. doi: 10.4103/0378-6323.168935
23. Lavania M, Katoch K, Katoch VM, Gupta AK, Chauhan DS, Sharma R, et al. Detection of viable mycobacterium leprae in soil samples: Insights into possible sources of transmission of leprosy. Infect Genet Evolution (2008) 8(5):627–31. doi: 10.1016/j.meegid.2008.05.007
24. Wheat WH, Casali AL, Thomas V, Spencer JS, Lahiri R, Williams DL, et al. Long-term survival and virulence of mycobacterium leprae in amoebal cysts. PloS Negl Trop Dis (2014) 8(12):e3405. doi: 10.1371/journal.pntd.0003405
25. Desikan K. Extended studies on the viability of mycobacterium leprae outside the human body. Leprosy Rev (1995) 66(4):287.
26. Central Statistical Agency of Ethiopia. North gondar zone. Central Statistical Agency of Ethiopia [Addis Ababa: Core questions on drinking water, sanitation and hygiene for household sEthiopian Statistics Service (ESS)] (2019). Available at: http://www.csa.gov.et/.
27. Sori E. Review on the burden of leprosy in Ethiopia. J Trop Dis (2019) 7(297):2. doi: 10.4172/2329-891X.1000297
28. Rapid Diagnostics. Rapid medical diagnostics schisto POC-CCA rapid test for qualitative detection of: Bilharzia (Schistosomiasis) (2020). Available at: https://www.rapid-diagnostics.com/#:~:text=The%20urine%2DCCA%20(Circulating%20Cathodic,S.&text=This%20test%20should%20be%20used,consistent%20with%20a%20Bilharzia%20infection.
29. United Nations Children’s Fund (UNICEF), World Health Organization (WHO). Core questions on drinking water, sanitation and hygiene for household surveys. (New York: United Nations Children’s Fund (UNICEF) and World Health Organization) (2018) WHO/UNICEF JMP (2018). Available at: https://washdata.org/monitoring/methods/core-questions.
30. United Nations Children’s Fund (UNICEF) and World Health Organization (WHO). Key terms: WHO/UNICEF joint monitoring report 2012. (New York: United Nations Children’s Fund (UNICEF) and World Health Organization (WHO) (2012). Available at: https://www.who.int/water_sanitation_health/monitoring/jmp2012/key_terms/en/#:~:text=Improved%20drinking%20water%20source%20is,public%20standpipe.
31. World Health Organization (WHO). Monitoring drinking-water. (Geneva: WHO) (2021). Available at: https://www.who.int/water_sanitation_health/monitoring/coverage/monitoring-dwater/en/.
32. WHO/UNICEF JMP. Health care facilities. (Geneva: WHO/UNICEF) (2019). Available at: https://washdata.org/monitoring/health-care-facilities.
33. Bishaw A, Lasser J. Education in Ethiopia: Past, present and future prospects. Afr Nebula (2012) (5):53–69. Available at: link.gale.com/apps/doc/A367546040/AONE?u=anon~a5284d9c&sid=googleScholar&xid=a9e5ba02.
34. Pescarini JM, Strina A, Nery JS, Skalinski LM, de Andrade KVF, Penna MLF, et al. Socioeconomic risk markers of leprosy in high-burden countries: A systematic review and meta-analysis. PloS Negl Trop Diseases (2018) 12(7):e0006622. doi: 10.1371/journal.pntd.0006622
35. Rodrigues LC, Lockwood DN. Leprosy now: Epidemiology, progress, challenges, and research gaps. Lancet Infect Diseases (2011) 11(6):464–70. doi: 10.1016/S1473-3099(11)70006-8
36. World Bank Group. Ethiopia Poverty assessment 2014. (Washington, DC: World Bank) (2015), Report No.: AUS6744. Available at: http://hdl.handle.net/10986/21323.
37. Bhat RM, Prakash C. Leprosy: An overview of pathophysiology. Interdiscip Perspect Infect Diseases (2012) 2012:1–6. doi: 10.1155/2012/181089
38. World Health Organization (WHO). Global health observatory. Ethiopia: WHO (2020). Available at: http://apps.who.int/gho/data/node.country.country-ETH.
39. Matsuoka M, Izumi S, Budiawan T, Nakata N, Saeki K. Mycobacterium leprae DNA in daily using water as a possible source of leprosy infection. Indian J Leprosy (1999) 71(1):61.
40. Diffey B, Vaz M, Soares M, Jacob A, Piers L. The effect of leprosy-induced deformity on the nutritional status of index cases and their household members in rural south India: A socio-economic perspective. Eur J Clin Nutr (2000) 54(8):643–9. doi: 10.1038/sj.ejcn.1601068
Keywords: neglected tropical disease (NTD), helminths, coinfection, water, sanitation, hygiene, WASH (water sanitation and hygiene), leprosy
Citation: Wasson MK, Whitson C, Miller B, Abebe W, Tessema B, Emerson LE, Anantharam P, Tesfaye AB and Fairley JK (2022) Potential drivers of leprosy infection: A case–control study of parasitic coinfection and water, sanitation, and hygiene in North Gondar, Ethiopia. Front. Trop. Dis 3:934030. doi: 10.3389/fitd.2022.934030
Received: 02 May 2022; Accepted: 26 July 2022;
Published: 19 August 2022.
Edited by:
Malcolm Scott Duthie, HDT Biotech Corporation, United StatesReviewed by:
David J. Blok, Erasmus Medical Center, NetherlandsCollins Okoyo, Kenya Medical Research Institute (KEMRI), Kenya
Copyright © 2022 Wasson, Whitson, Miller, Abebe, Tessema, Emerson, Anantharam, Tesfaye and Fairley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jessica K. Fairley, amVzc2ljYS5mYWlybGV5QGVtb3J5LmVkdQ==
 Megan K. Wasson
Megan K. Wasson Cassidy Whitson1
Cassidy Whitson1 Bridget Miller
Bridget Miller Wondwossen Abebe
Wondwossen Abebe Belay Tessema
Belay Tessema Lisa E. Emerson
Lisa E. Emerson Annisa Befekadu Tesfaye
Annisa Befekadu Tesfaye Jessica K. Fairley
Jessica K. Fairley