- 1Intensive care unit, Department of Neurology, Fujian Medical University Union Hospital, Fuzhou, China
- 2Department of Neurology, Fujian Medical University Union Hospital, Fuzhou, China
- 3Department of Parasitic Disease, Fujian Center for Disease Control and Prevention, Fuzhou, China
- 4Department of Imaging, Fujian Medical University Union Hospital, Fuzhou, China
- 5Department of Geriatrics, Fujian Medical University Union Hospital, Fujian Key Laboratory of Molecular Neurology, Fuzhou, China
Introduction: Primary amoebic meningoencephalitis (PAM) caused by Naegleria fowleri is seldom reported in mainland China.
Methods: One case from South China was presented, and the clinical features of the PAM, especially the early CT features, were compared to those in the literatures from PubMed/Web of Science/China National Knowledge internet (CNKI).
Case Presentation and Results: A 47-year-old man with a high fever came to the fever clinic. Twelve hours later, the man lost consciousness and exhibited generalized tonic-clonic seizures and needed ventilator-controlled ventilation. Then, he was admitted to the neurology intensive care unit (NICU). The opening pressure of his cerebrospinal fluid (CSF) was over 500 mm H2O with highly increased leukocyte/protein levels and very low glucose levels. Three days after admission, high copy numbers of Naegleria fowleri amoebae were detected by metagenomics next-generation sequencing (mNGS) and cysts were visible with wet mount microscopy. Four days after admission, the patient experienced brain death. However, the relatives of the patient did not want to give up, and he received amphotericin B (AmB). During hospitalization, he suffered from severe damage to the liver and kidneys and electrolyte disorders that required continuous renal replacement therapy (CRRT).
Review: All 20 included PAM patients suffered from fever. Seventeen of them had headache and neck stiffness. Ten of them showed generalized brain edema. To date, 7 cases of PAM have been reported in China. Only one patient survived. Most of the patients showed generalized brain edema. Only the surviving patient showed focal edema. He died three months later.
Conclusion: Rapidly progressive meningoencephalitis in which the CSF results are similar to those suffered from a bacterial infection should be considered a possible case of PAM. It can be rapidly detected with microscopy in CSF wet mounts but needs further molecular investigation for confirmation, and mNGS should be a new method used for rapid and precise identification. Moreover, CRRT may prolong the survival time of PAM patients with multiple organ failure.
Introduction
Free-living amoebae were first reported as causing severe central nervous system (CNS) infection in the 1960s (1, 2). There are various types of pathogenic free-living amoebae (FLA), including Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, Sappinia spp. and Vahlkampfia spp. The first three types are common, and Naegleria fowleri usually results in primary amoebic meningoencephalitis (PAM). Over 420 cases of PAM have been reported (3, 4), most PAM cases are from the USA and Australia (4) and rarely from China. To date, only four Chinese cases have been reported in English (from Hong Kong (5), Taiwan and the mainland (6, 7)). The other three cases were reported in Chinese journals (6). There are multiple difficulties in handling PAM. First, traditional detection methods are too slow to diagnose this condition because of the rapid progression of the disease. Second, it is difficult to distinguish PAM from bacterial meningoencephalitis. Third, autopsies are rarely performed, so the incidence of this disease may be underreported. In this study, metagenomics next-generation sequencing (mNGS) contributed to the prompt diagnosis of N. fowleri-related PAM. Moreover, we reviewed the clinical features (especially the imaging spectrum) of PAM from China and other countries. Finally, brain functional evaluation contributes to the prognosis of PAM, and continuous renal replacement therapy (CRRT) may prolong the survival time of PAM patients with multiple organ failure.
Methods
One case from South China was presented. Then, all of the PAM cases reported in China, including Taiwan and Hong Kong, were reviewed by searching PubMed, Web of Science, and China National Knowledge Infrastructure (CNKI) databases.
Moreover, because of the rapid progression of PAM, most patients fail to undergo magnetic resonance imaging(MRI). Therefore, we tried to look for evidence implying PAM from the computerized tomography(CT) imaging data. All the cases were searched by primary amoebic meningoencephalitis [Title] and case report [article type] from PubMed, Web of Science, and CNKI. Those articles that had detailed clinical data, including CT imaging, were included for further review.
The mNGS procedure was as follows:
[1] Plasma Sample Processing and DNA Extraction: A volume of 3 mL of blood was drawn from the patient, placed in a blood collection tube, stored at room temperature for 3-5 min before plasma separation and centrifuged at 4,000 rpm for 10 min at 4°C within 8 h of collection. Then, the plasma sample was transferred to a new sterile tube. DNA was extracted from 300 µL of plasma using the TIANamp Micro DNA Kit (DP316, TIANGEN BIOTECH, Beijing, China) following the manufacturer’s operational manual. The extracted DNA specimens were used for the generation of DNA libraries.
[2] Generation of DNA libraries and sequencing: DNA libraries were generated through DNA fragmentation, end repair, adapter-ligation and PCR amplification. An Agilent 2100 was used for quality control of the DNA libraries. The libraries meeting quality standards were pooled, and DNA nanoballs (DNBs) were made and sequenced by the BGISEQ-50/MGISEQ-2000 platform.
[3] Bioinformatics analysis: High-quality sequencing data were generated by removing low-quality reads, followed by computational subtraction of human host sequences mapped to the human reference genome (hg19) using Burrows–Wheeler Alignment. The remaining data obtained by removal of low-complexity reads were classified by simultaneous alignment to the Pathogens Metagenomics Database (PMDB), which comprises data regarding bacteria, fungi, viruses and parasites. The classification reference databases were downloaded from NCBI (ftp://ftp.ncbi.nlm.nih.gov/genomes/). Reference Sequence Database contains 4,945 whole genome sequences of viral taxa, 6,350 bacterial genomes or scaffolds, 1064 fungi related to human infection, and 234 parasites associated with human diseases.
Results
Case Presentation
On September 10, 2020, a 47-year-old man with a high fever (39.8°C) came to the fever clinic at Fujian Medical University Union Hospital. He lived in Fuzhou and worked at a construction company. His working environment commonly included ponds and sewage. His disease began with weakness, headache, and a backache 2 weeks earlier and a high fever 2 days prior to presentation. After excluding the possible diagnosis of COVID-19, he was sent to the Emergency Internal Medical Care Unit.
A computed tomography (CT) scan of the brain showed diffuse cerebral edema (Figures 1A, B). The midbrain ventricles had almost disappeared. After 12 h, the man lost consciousness, his right pupil dilated (5 mm), and there was no light reflection. Sixteen hours later, he developed generalized tonic-clonic seizures and experienced dyspnea. He was immediately given endotracheal intubation, requiring ventilator-controlled ventilation. Then, he was admitted to the neurology intensive care unit (NICU).
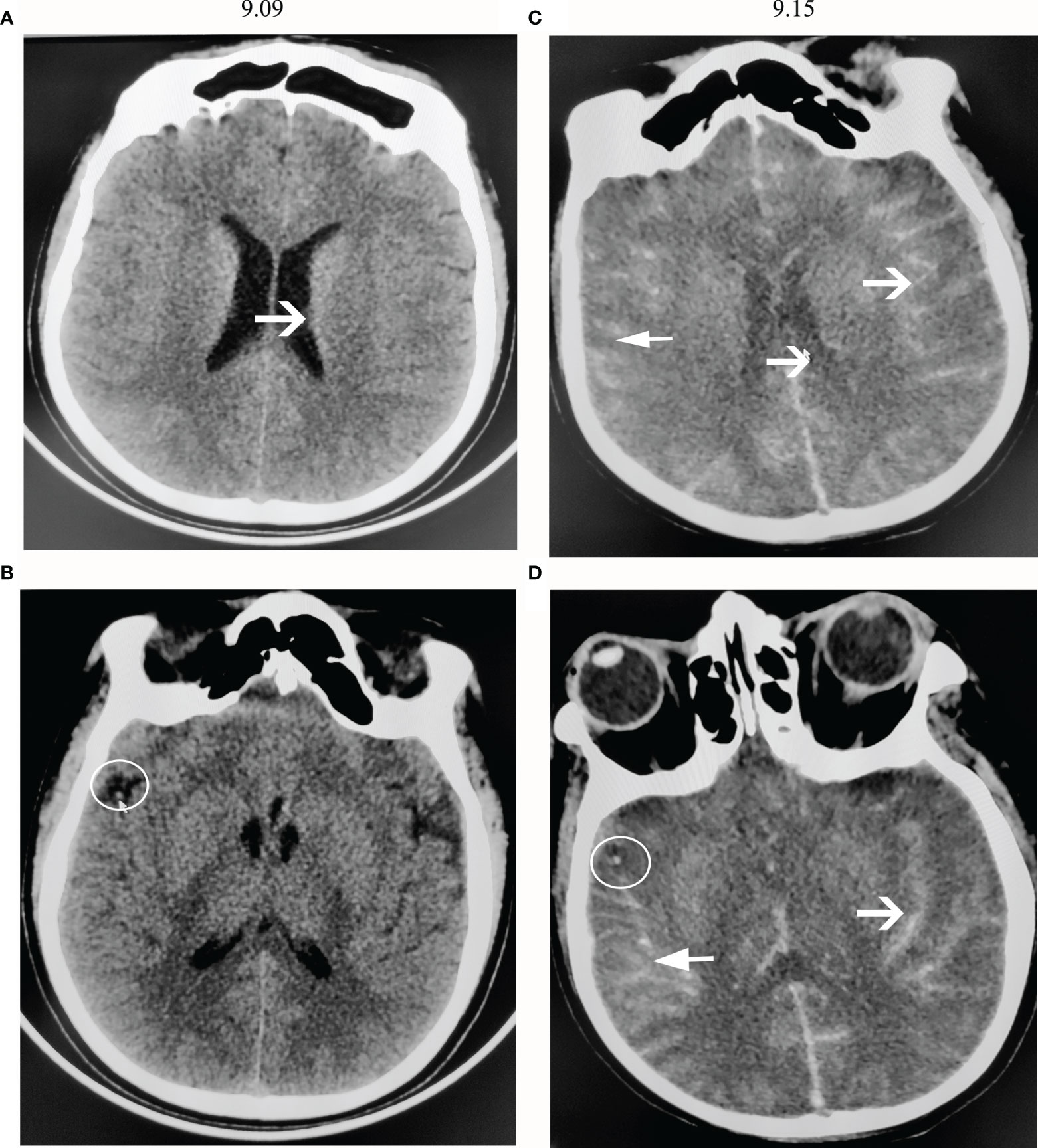
Figure 1 CT scans of the brain. (A, B) show CT images on the first day of admission (9.09). (C, D) show CT images 6 days after admission (9.15). (A) The brain sulci became obscure, showing diffuse cerebral edema. (B) There was a high-density shadow on the right lateral fissure, as shown by the arrow. (C) The brain sulci showed diffuse subarachnoid hemorrhage, and the lateral ventricles narrowed. (D) A high-density shadow on the right lateral fissure was still present.
Lumbar puncture was performed immediately after admission, and the opening pressure exceeded 500 mm H2O. The yellow and cloudy cerebrospinal fluid (CSF) samples showed very high levels of white blood cells and red blood cells (WBCs, 8700 x 106 cells/mL, 87% neutrophils; RBCs 3+), with a high protein concentration of 2250 mg/L and a very low glucose concentration of 0.01mmol/L. His CSF sample was immediately cultured for bacterial and fungal detection. Moreover, his CSF sample was sent to BGI Clinical Laboratories (Shenzhen) Co., Ltd. for pathogen detection by mNGS.
He was administered 6.0 g/d meropenem (2.0 q8 h iv-vp), 2.0 g/d vancomycin (0.5 q6 h iv-vp) and 10 mg/d dexamethasone. However, his condition remained deteriorated. On the night of admission, his other pupil was also enlarged (5 mm) without light reflection, and his other brain stem reflexes disappeared. Two days after admission, the electroencephalogram (EEG) showed a low wave (Figure 2A), and the TCD showed a sharp small wave in the right middle cerebral artery (RCMA)/left middle cerebral artery (LCMA)/basilar artery (BA) (Figures 2D–F). Moreover, his blood pressure dropped below 60/30 mmHg, and his sodium levels increased from 132 to 195 mmol/L in 24 h. The patient began to produce urine at 300 ml/hour, and as the sodium level increased, the serum osmolality increased sharply, and the urine volume dropped from 7200 ml/day to less than 1000 ml/day. Then, his creatinine(CREA) level gradually rose to 350 µmol/L. He experienced hyperpotassemia. In addition, continuous renal replacement therapy (CRRT) was applied discontinuously, after which his sodium levels decreased from 195 to 145 mmol/L and his urine output was maintained at approximately 2000-3000 ml/day. The potassium ion level dropped from 8.1 mmol/L to a normal level.
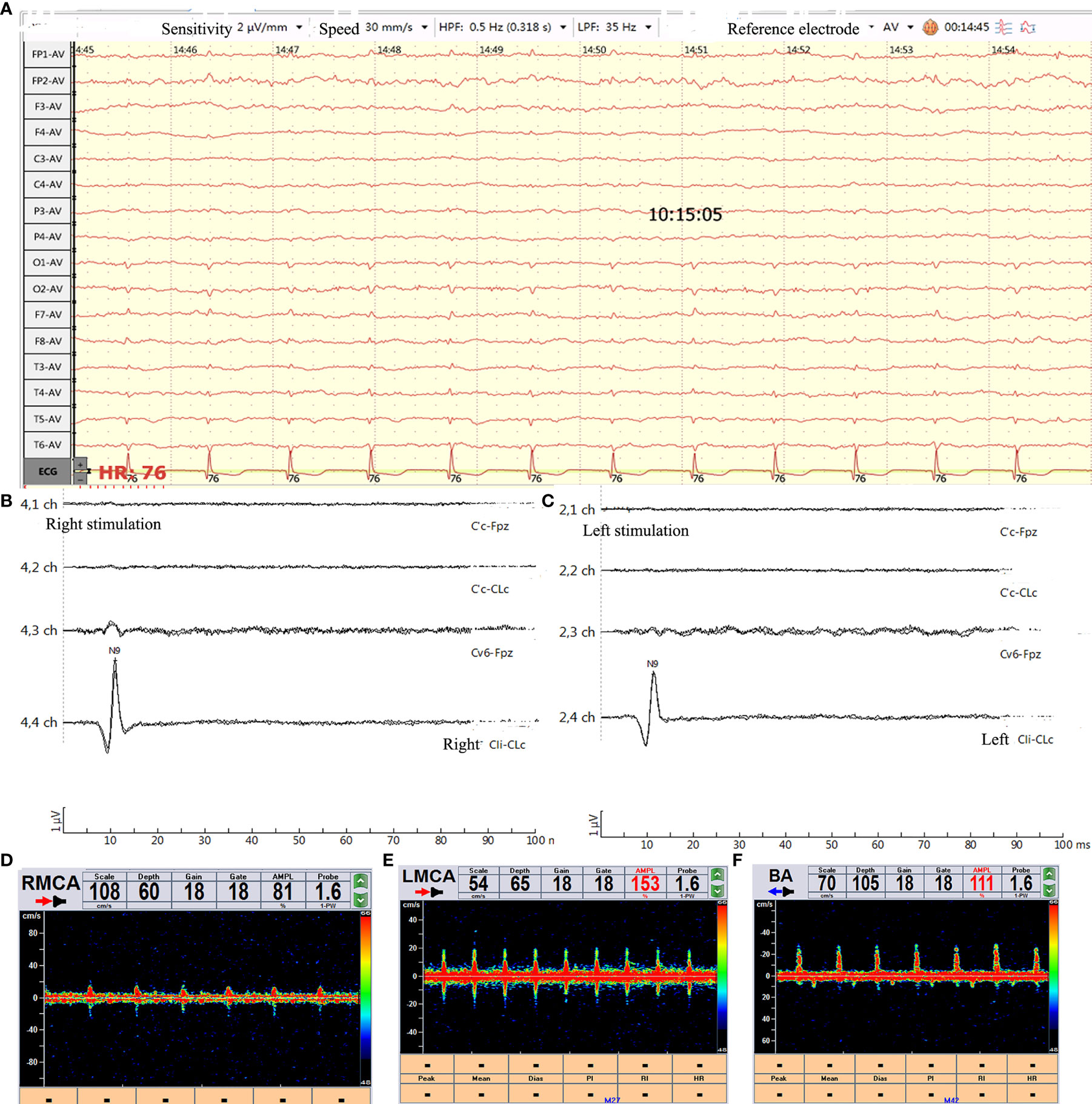
Figure 2 Brain function evaluation. (A) Video-EEG recording (international 10-20 system using the average reference electrode, over half an hour) showed that the wave amplitude was lower than 2 µv. (B, C) SSEP showed bilateral N9 normal and N13/P14/N18/N20 disappeared. (D–F) TCD showed sharp small waves or nail-like waves in RCMA/LCMA/BA. RCMA, Right middle cerebral artery; LCMA, Left middle cerebral artery; BA, basilar artery.
On the first day of admission, as mentioned, the CSF sample was sent to BGI Clinical Laboratories for mNGS detection. As in previous reports (8), the samples underwent preprocessing, nucleic acid extraction, library generation, and 50 bp single-end read sequencing on the BGISEQ-500 platform. A total of 34,798,232 reads were sequenced, and 12,752 reads that were uniquely aligned with the N. fowleri genome were identified, accounting for 0.017% of the total sequencing reads (Figure 3B) and covering 0.986% of the N. fowleri genome (Figure 3A). After excluding the reads from the human host, N. fowleri had the most number of reads of the microbial species detected, accounting for 0.602% of the total microbial readings, and the number of unknown or unclassified reads (Figure 3B) exceeded the read number of bacteria or fungi. The genome ID was ATCC 30863, and the version GCA_000499105.1 was used to map the reads.
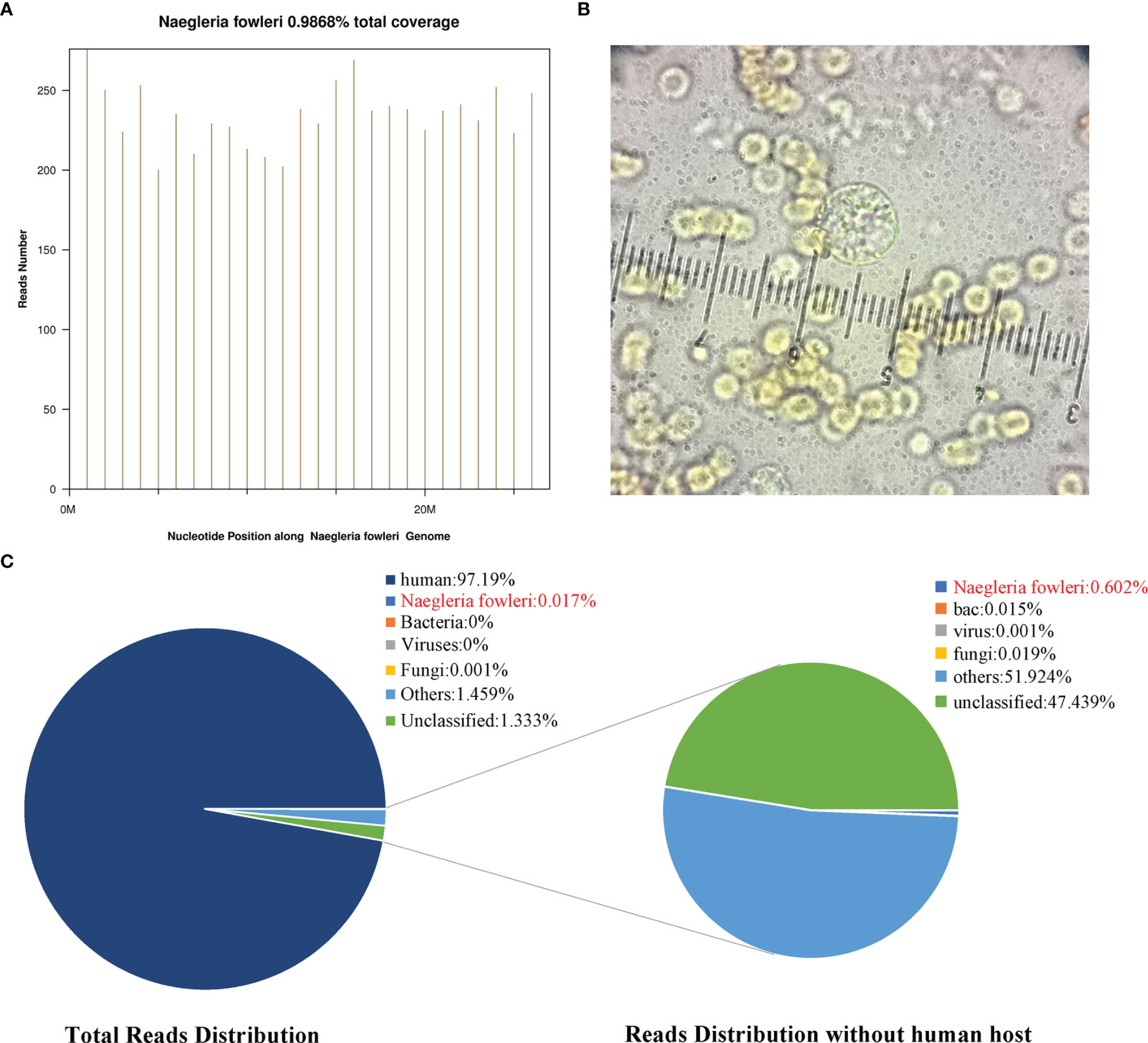
Figure 3 The detection of N. fowleri. (A) The distribution of reads in the Nigeria fowleri genome, covering 0.986% of the genomic region. (B) The number of reads uniquely corresponding to the N. fowleri genome accounted for 0.017% of the total reads and 0.602% of the nonhuman reads. (C) The amoebic cyst is visible in the CSF wet mounts with microscopy. It is in the middle of the figure and was approximately 9 mm. There were many red blood cells on the CSF wet mounts.
To verify this result, CSF was collected again on the 5th day after admission. The CSF had become orange, and the opening pressure was lower than 80 mmH2O. The fresh CSF sample at room temperature was immediately sent to the Fujian Centers for Disease Control and Prevention (CDC) for rapid detection. One hour later, the CDC found that amoebic cysts were visible in the CSF wet mounts with microscopy (as shown in Figure 3C). They were approximately 8 µm and were smaller than amoebic trophozoites, which ranged from 10-25 µm. Therefore, they may have been cysts or at the pre-cyst stage.
All results indicated that the patient was infected with N. fowleri. In addition, the medical history collected from his wife showed that the man was a building worker who was splashed by wastewater from an old water pipe while working shortly before the onset of his illness.
When his Na+ concentration returned to normal, somatosensory evoked potential (SSEP) analysis showed that the bilateral N13/P14/N18/N20 disappeared (Figures 2B, C). A computed tomography (CT) scan of the brain showed a diffuse subarachnoid hemorrhage (Figures 1C, D). His clinical features indicated brain death. However, the relatives of the patient did not want to give up, and he was administered amphotericin B liposomes (10 mg-20 mg-40 mg-60 mg/d). During hospitalization, he suffered from severe damage to the liver and kidneys and electrolyte disorders that required CRRT. He died after 3 months. The timeline with relevant data from the episode of care was showed as Figure 4 (supplement).
Review
The patient in the present case showed a rapid worsening and generalized brain edema with very high brain pressure and very high protein and blood cells in CSF. Although he had been brain dead before being treated with anti-amoebae therapies, he sustained his cardiac for 3 months via life support system including CRRT. over a long time. This condition seems to be seldom reported in China. The PAM cases reported in China, including Taiwan and Hong Kong, were reviewed by Wang, Q. et al. in 2018 (6). There were 6 patients in that review. All of them were male. From 2018 to the present, there has only been one other Chinese patient with this condition who was only 8 years old. Up to now, there were 8 PAM cases reported in China, including our present case. The clinical characteristics are listed in the Supplementary Materials as shown in the Table 1-supplement, which was based on the review by Wang, Q. The first case was reported in 1978. All of the patients showed definite contact with fresh, contaminated water before their illnesses. The available data showed that intracranial pressure in these patients was high, usually over 300 mmH2O. The white cell count in the CSF was very high and was usually over 300x106/mL, and neutrophil granulocytes accounted for over 70%. The concentration of protein contained in the CSF was higher than 2 g/L in most patients. The glucose level was lower than 3.0 mmol/L and lower than 0.2 mmol/L in two patients. Before 1990, the patients were all diagnosed after death via autopsy. Two patients were diagnosed via an abscess biopsy. The other patient was diagnosed via microscopy, and the more recent patients were found by metagenomic next-generation sequencing (mNGS). Three of them were found by mNGS. It was interesting that one of the three patients also exhibited N. fowleri DNA detected in his blood. Three out of the 8 patients were treated with Amb. Only one survived. Our present patient with multiple organ failure was treated with continuous renal replacement therapy (CRRT).
Our present patient showed generalized brain edema and diffuse subarachnoid hemorrhage on CT scanning. These findings seem to be common. However, due to the rapid progression of PAM and the failure of most patients to undergo MRI, we were looking forward to finding some evidence of early features in CT imaging. Thirty-five cases were identified from PubMed, Web of Science and CNKI that met the criteria. Nineteen of them had detailed clinical data, including CT imaging, as shown in the Table 1-Supplement. The other was our patient. The clinical data and CT features were calculated as shown in Figure 4. The clinical data were as follows: the first symptoms of PAM included headache, fever, and neck stiffness. Sixteen of them experienced headache as their first symptom. All 20 PAM patients suffered from fever. Seventeen of them experienced headache during illness. Additionally, 17 of them had neck stiffness. The CT features were as follows: generalized brain edema or focal edema, hydrocephalus, small or absent ventricle, subarachnoid hemorrhage and erosion of the sphenoid sinus. Most PAM patients suffer from generalized brain edema. Notably, only the patient with focal brain edema survived. Considering the methods of invasion of Naegleria fowleri, the erosion of the sphenoid sinus may theoretically be an early marker of PAM. However, the Neuroimaging staff paid attention to this feature in only one case.
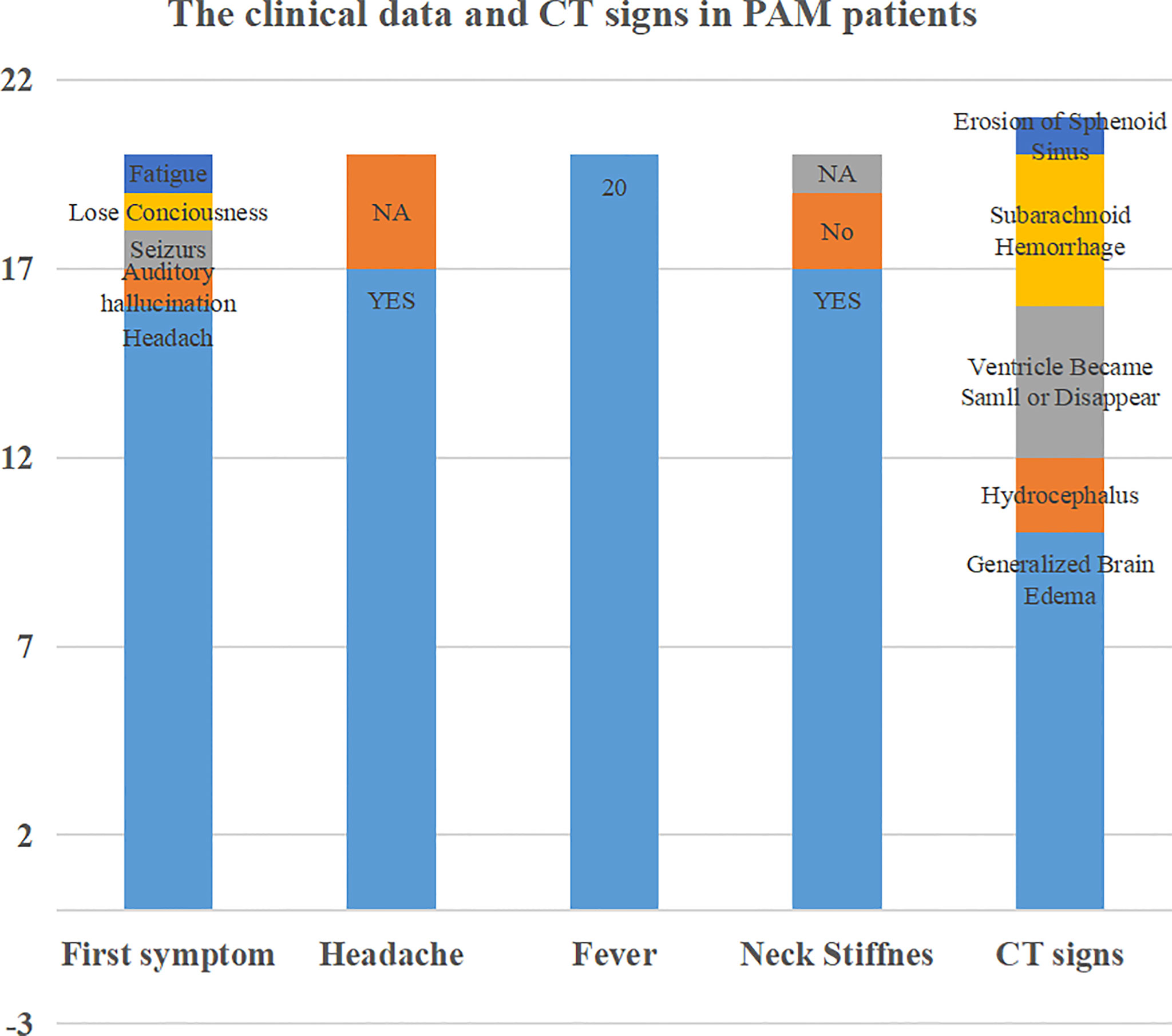
Figure 4 Review of the clinical data and CT features in PAM patients. Sixteen of the patients experienced headache as their first symptom. All 20 PAM patients suffered from fever. Seventeen of them experienced headache during illness. Additionally, 17 of them had neck stiffness. The CT features were as follows: generalized brain edema or focal edema, hydrocephalus, small or absent ventricle, subarachnoid hemorrhage and erosion of the sphenoid sinus.
Discussion
There are several kinds of pathogenic free-living amoebae. Naegleria fowleri is one of them that causes primary amoebic meningoencephalitis (PAM). PAM is an acute infection that lasts only a few days. From the present reports, over 95% of patients with PAMs caused by Naegleria fowleri died within several days of exposure (9). The rapid progression of PAM results in it being diagnosed too late to treat. Moreover, most patients died before they were diagnosed, and were only diagnosed via autopsy. Our present patients were diagnosed via both CSF mNGS AND microscopy in time. However, he had experienced brain death when he was diagnosed. It is difficult to diagnose in time due to a lack of awareness [the early symptoms lack specificity, so history taking is very important (10)] and a lack of available diagnostic tools.
There are three morphological forms of Naegleria fowleri: trophozoite (amoeba), flagellate, and cyst. Generally, trophozoites are thought to be infectious forms of N. fowleri. In the cyst phase, even though it is unable to promote tissue degradation, this resistant form can enter the host and revert to the trophozoite form. If the patients were considered infected by amoebae, the fastest diagnostic tool seems to be microscopic examination of the CSF, but its positivity depends on the following factors: (1) fresh CSF samples at room temperature, which means that the CSF samples should be examined immediately after collection; (2) wet mounts of the CSF, which can improve the percentage of positivity; and (3) experienced specialists. Thus, it is not a definitive diagnosis; other approaches, such as molecular investigation, should be used to confirm the identification. Another method for the definitive diagnosis of N. fowleri infection is the use of immunohistochemical staining of the brain tissue. This method requires a biopsy, which can be performed in few patients. The third method is polymerase chain reaction (PCR) testing, which has been applied to some American patients. However, if you are not aware of the method to find pathogen. another possibility is mNGS. There are few reports of PAM cases detected via mNGS in China (6, 7). Our present case is another PAM case detected via mNGS and verified by microscopy detection.
Naegleria fowleri is a ubiquitous, thermophilic, free-living amoeba that is found in many warm freshwater ponds, lakes, streams, and canals. Trophozoites may be inhaled into the nasal cavity, penetrate the nasal mucosa and migrate via the cribriform plate into the central nervous system (CNS) through the olfactory nerves. Then, the mobile trophozoites spread quickly throughout the CNS. Then, this infection causes severe cerebral edema and necrosis and prompts brain herniation. In review articles, all patients who showed generalized brain edema via CT scanning promptly developed brain herniation and died. Our present patient showed the same course. In Chinese cases, only a patient from Hong Kong whose CT scanning showed focal edema (in the right parieto-occipital region) survived (5). Therefore, if the CT images illustrate focal edema, the outcome of PAM may be good. From the reported American cases, the CT or MRI images showed no special features (9). In our review articles, one patient from India showed erosion of the sphenoid sinus (11). The staff in the other cases ignored this feature. Considering the unique invasive route of the organisms causing PAM, whether the sphenoid sinus was destroyed should be noted. Second, most PAM patients showed diffuse subarachnoid hemorrhage. The CSF cytological tests showed dramatically increased numbers of white blood cells with many red blood cells. In our present patient, the CSF sample showed many red blood cells, and CT scanning showed high-density puncta in the right lateral fissure on the day of admission. Then, 5 days after admission, the patient showed diffuse subarachnoid hemorrhage, and the CSF showed many red blood cells around the trophozoites. The above data illustrated that if the CSF sample showed changes similar to those of a bacterial infection and there was subarachnoid hemorrhage at the same time, PAM should be considered.
It is well known that the progression of PAM is rapid, and over 95% of patients die (12). There are several risk factors related to the outcome of PAM. The first factor is whether the lesion is generalized or focal. Second, the duration from admission to diagnosis is related to the outcome. Third, whether anti-amoeba treatment is given in time is another key factor. Moreover, whether anti-amoeba drugs are effective for PAM is important (13).
In this report, we reviewed the clinical features and CT images of PAM patients reported in PubMed and our local publications. There were 20 cases included in the review. Most of the patients had headache, and all of the patients had fever (100%). Seventeen out of 20 patients suffered from headache at onset, and 16 out of 18 had neck stiffness. Our present patient also experienced headache, fever and generalized seizures. The above data showed intracranial hypertension and meningeal targeting. The CSF sample showed a dramatic increase in the number of white blood cells (up to 52860/µl) and in protein concentration (up to 49.96 g/L). As showed in our present patient, the glucose level dropped to lower than 0.11 mmol/L. These changes are similar to those in bacterial infection. However, it is not common for abundant red cells to be detected in bacterial meningoencephalitis (14). Moreover, the progression of PAM is more rapid than that of bacterial meningoencephalitis. In most of our local patients, the time from onset to admission was less than 7 days and peaked at 10 days. Therefore, if patients show a dramatic increase in the number of white blood cells with red blood cells and rapid progression of the disease, PAM should be considered.
It is well known that the treatment of PAM is limited and that the outcome is poor (13). The key drugs used for this specific condition include amphotericin-B and rifampicin (3, 15). However, there are several patients that did not respond to the above drugs. Recent studies have been devoted to new drugs targeting (16) fatty acid oxidation, such as etomoxir, orlistat, perhexiline, thioridazine, and valproic acid. Moreover, it is inspiring that miltefosine is considered to decrease the usual mortality rate of both PAM and GAE (12). Another factor related to the outcome is the timing of treatment initiation. Regarding patients with obvious brain edema in PAM, it is inconclusive whether they benefit from decompressed craniotomy or ventricular drainage. The Hong Kong patient who survived underwent ventricular drainage on the day of admission and then underwent right parietal lobe decompression craniotomy. In our case, the patient rapidly developed multiple organ failure, including hypernatremia, hyperpotassemia, liver failure, renal failure, and circulatory failure. CRRT prolonged his survival time even though the patient progressed to brain death. It may be worthwhile to perform decompression in the early stages of PAM. It is argued that CRRT not only contributes to the clearance of inflammatory factors but also alleviates brain edema (17). Moreover, the evaluation of brain function contributes to the prediction of PAM. The early onset of brain death is characteristic of the rapid prognosis of PAM (18). Hence, it is important to intervene as early as possible.
There are few case reports of PAM recovery, and prevention is the key point. Naegleria fowleri is a free-living amoeba in the natural environment. It is a public health security issue around the world. To date, it is not completely understood why millions of people are exposed to fresh water with amoebae, but only a few people develop PAM due to Naegleria fowleri infection. Among cases from China, most patients had a history of fresh water exposure (10). Some patients swam in warm freshwater ponds or lakes. Some patients were splashed with dirty water. Our present patient was a building worker who was splashed by wastewater from an old water pipe while working shortly before the onset of his illness. Considering the unique invasive route of Naegleria fowleri, wearing a mask when working or applying protection to prevent dirty or fresh water from being inhaled into the nose during swimming is important.
Conclusion
Rapid progressive meningoencephalitis in which the results of CSF analysis are similar to those during bacterial infection should be considered as a possible case of PAM. It can be rapidly detected with microscopy in CSF wet mounts by an experienced parasite specialist. mNGS should be applied as a new method for the rapid and precise identification of N. fowleri. Brain functional evaluation contributes to the prognosis of PAM, and CRRT may prolong the prediction of PAM patients with multiple organ failure.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI, accession ID: SUB11332407, PRJNA826739.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SC and CC collected the clinical data. BC and XH were contributed to the CSF examination and imaging process. WL and HH were contributed to the design and writing of the article. CL was contributed to collect the review articles. CC performed the neuroelectrophysiological tests and TCD. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant number: 2017Y9058) and Funds for the Orientative Project of Science and Technology, Fujian Province (Grant number: 2019Y0025).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.899700/full#supplementary-material
Supplementary Figure 4 | The timeline with relevant data from the episode care. Before the patient was sent to the Emergency clinic, he had got headache, weakness and backache for two weeks. In Emergency, he lost consciousness and experienced generalized seizures with respiratory failure. 3-4 Days after admission, his CSF mNGS and microscopy tests showed that he got PAM. He experienced brain death, electrolyte disturbance and multiple organ failure. Amphotericin B liposomes and CRRT was treated for over two months. Finally, he died after 3months.
References
2. Symmers WC. Primary Amoebic Meningoencephalitis in Britain. Br Med J (1969) 4(5681):449–54. doi: 10.1136/bmj.4.5681.449
3. Cooper AM, Aouthmany S, Shah K, Rega PP. Killer Amoebas: Primary Amoebic Meningoencephalitis in a Changing Climate. JAAPA (2019) 32(6):30–5. doi: 10.1097/01.JAA.0000558238.99250.4a
4. Maciver SK, Pinero JE, Lorenzo-Morales J. Is Naegleria Fowleri an Emerging Parasite? Trends Parasitol (2020) 36(1):19–28. doi: 10.1016/j.pt.2019.10.008
5. Wang A, Kay R, Poon WS, Ng HK. Successful Treatment of Amoebic Meningoencephalitis in a Chinese Living in Hong Kong. Clin Neurol Neurosurg (1993) 95(3):249–52. doi: 10.1016/0303-8467(93)90132-Z
6. Wang Q, Li J, Ji J, Yang L, Chen L, Zhou R, et al. A Case of Naegleria Fowleri Related Primary Amoebic Meningoencephalitis in China Diagnosed by Next-Generation Sequencing. BMC Infect Dis (2018) 18(1):349. doi: 10.1186/s12879-018-3261-z
7. Huang S, Liang X, Han Y, Zhang Y, Li X, Yang Z. A Pediatric Case of Primary Amoebic Meningoencephalitis Due to Naegleria Fowleri Diagnosed by Next-Generation Sequencing of Cerebrospinal Fluid and Blood Samples. BMC Infect Dis (2021) 21(1):1251. doi: 10.1186/s12879-021-06932-9
8. Xing XW, Zhang JT, Ma YB, He MW, Yao GE, Wang W, et al. Metagenomic Next-Generation Sequencing for Diagnosis of Infectious Encephalitis and Meningitis: A Large, Prospective Case Series of 213 Patients. Front Cell infect Microbiol (2020) 10:88. doi: 10.3389/fcimb.2020.00088
9. Capewell LG, Harris AM, Yoder JS, Cope JR, Eddy BA, Roy SL, et al. Diagnosis, Clinical Course, and Treatment of Primary Amoebic Meningoencephalitis in the United States, 1937-2013. J Pediatr Infect Dis Soc (2015) 4(4):e68–75. doi: 10.1093/jpids/piu103
10. Sharma A, Sharma A, Guleria S. Successful Treatment of a Case of Primary Amoebic Meningoencephalitis: How Important is History Taking. Indian J Crit Care Med (2015) 19(2):126–7. doi: 10.4103/0972-5229.151024
11. Gupta N, Bhaskar H, Duggal S, Ghalaut PS, Kundra S, Arora DR. Primary Amoebic Meningoencephalitis: First Reported Case From Rohtak, North India. Braz J Infect Dis (2009) 13(3):236–7. doi: 10.1590/S1413-86702009000300016
12. Alli A, Ortiz JF, Morillo Cox A, Armas M, Orellana VA. Miltefosine: A Miracle Drug for Meningoencephalitis Caused by Free-Living Amoebas. Cureus (2021) 13(3):e13698. doi: 10.7759/cureus.13698
13. Ong TYY, Khan NA, Siddiqui R. Brain-Eating Amoebae: Predilection Sites in the Brain and Disease Outcome. J Clin Microbiol (2017) 55(7):1989–97. doi: 10.1128/JCM.02300-16
14. Zahid MF, Saad Shaukat MH, Ahmed B, Beg MA, Kadir MM, Mahmood SF. Comparison of the Clinical Presentations of Naegleria Fowleri Primary Amoebic Meningoencephalitis With Pneumococcal Meningitis: A Case-Control Study. Infection (2016) 44(4):505–11. doi: 10.1007/s15010-016-0878-y
15. Shrestha GS, Parajuli NP, Shrestha PS, Acharya SP, Hamal R, Gajurel B, et al. Primary Amoebic Meningoencephalitis. J Neurosci Rural Pract (2015) 6(2):284–6. doi: 10.4103/0976-3147.153244
16. Sarink MJ, Tielens AGM, Verbon A, Sutak R, van Hellemond JJ. Inhibition of Fatty Acid Oxidation as a New Target To Treat Primary Amoebic Meningoencephalitis. Antimicrob Agents Chemother (2020) 64(8):1–8. doi: 10.1128/AAC.00344-20
17. Honore PM, Barreto Gutierrez L, Kugener L, Redant S, Attou R, Gallerani A, et al. Hepcidin Is Described as the Master Regulator of Iron: Could its Removal by CRRT Lead to Iron Dysmetabolism in the Critically Ill? Crit Care (2020) 24(1):570. doi: 10.1186/s13054-020-03295-6
Keywords: primary amoebic meningoencephalitis (PAM), hypernatremia, brain death, CT, CRRT, metagenomics Next-generation Sequencing (mNGS)
Citation: Chen S, Che C, Lin W, Chen B, Huang X, Liu C and Huang H (2022) Case Report: Recognition of Devastating Primary Amoebic Meningoencephalitis (PAM) Caused by Naegleria fowleri: Another Case in South China Detected via Metagenomics Next-Generation Sequencing Combined With Microscopy and a Review. Front. Trop. Dis 3:899700. doi: 10.3389/fitd.2022.899700
Received: 24 March 2022; Accepted: 12 May 2022;
Published: 18 August 2022.
Edited by:
Jacob Lorenzo-Morales, University of La Laguna, SpainReviewed by:
Natália Karla Bellini, Federal University of Minas Gerais, BrazilSutherland Kester Maciver, University of Edinburgh, United Kingdom
María Reyes-Batlle, University of La Laguna, Spain
Copyright © 2022 Chen, Che, Lin, Chen, Huang, Liu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huapin Huang, aGgtcEAxNjMuY29t; Wanhui Lin, eGlhb2ZlaWdlOTk5MDMzOTlAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Shenggen Chen
Shenggen Chen Chunhui Che2†
Chunhui Che2† Wanhui Lin
Wanhui Lin Xinming Huang
Xinming Huang Huapin Huang
Huapin Huang