- Indian Council of Medical Research-National Institute of Research in Tribal Health, Jabalpur, India
Leprosy is a public health issue, and early detection is critical to avert disability. Despite the global attempt to eradicate this disease as a public health problem, it remains an important cause of global neurological disability. India, Brazil and Indonesia share more than 70% of the cases. The reduction of new cases is a priority in the WHO global strategy 2021-2030 which aims to reduce disease transmission in the community by diagnosing cases and identifying subclinical infection. The clinical manifestations of leprosy range from a few to several lesions. The identification remains difficult due to the limited sensitivity of traditional approaches based on bacillary counts of skin smears and histology. To aid in the diagnosis of this disease, molecular biology, and biotechnological technologies have been applied, each with its own set of benefits and downsides despite providing an essential tool to validate the clinical diagnosis of leprosy. Because of this, it is strongly recognized that specific, inexpensive point of care technologies should be developed, particularly to identify asymptomatic M. leprae infections or leprosy nearer to the suspected cases seeking medical attention. Thus, this review will provide an overview of the advancements in leprosy diagnosis over the world. The purpose of this review is to improve our understanding of the outcomes of current tests and technologies used in leprosy diagnosis and to emphasize critical aspects concerning the detection of leprosy bacilli.
Introduction
Leprosy is caused by an uncultivated pathogen, Mycobacterium leprae and Mycobacterium lepromatosis which primarily affect the skin, mucosal surface of the upper respiratory tract, the peripheral nerves, eyes, and internal organs (1, 2). It has a long incubation period which can take as long as 20 years or more. Leprosy occurs in several clinical manifestations that extend from the spectrum that extends from tuberculoid leprosy (TT), through borderline tuberculoid (BT), mid-borderline (BB), borderline lepromatous (BL), to the poorly resistant lepromatous leprosy (LL) (3). The clinical diagnosis of pure neural leprosy (PNL) also remains a public health concern, owing to the absence of skin lesions, which are the cardinal symptoms of leprosy. Operationally leprosy is classified into Paucibacillary and Multibacillary leprosy (4). A broad range of intermediate types also exists commonly known as borderline leprosy. Even though leprosy is claimed to be eliminated in most countries, according to WHO, 213535 new leprosy cases were detected globally in 2020, with ~85% of those detected mainly in India, Brazil, and Indonesia (WHO, 2020) (5). With over 1.25 lack new leprosy cases detected in 2019, India accounts for >60% of the total cases reported globally indicating an active transmission. Leprosy diagnosis is mostly based on clinical presentations, Acid-fast bacilli (AFB) staining methods for slit skin in smears, and lymph or histological examination of biopsy samples are useful for confirming the diagnosis of leprosy. However, these tests are less productive in the early diagnosis when clinical symptoms are often not clearly defined, for example, in some paucibacillary cases (PB) (6). Moreover, since expertise is important in the practical application of histological or molecular biological assays, these methods of detection are mostly used in reference labs and not in peripheral labs or field settings. It is the same for testing the medication sensitivity with higher labor and time use in mouse footpads since M. leprae is not yet successfully cultivated despite several decades of attempts for in-vitro cultivation. Therefore, the phenotypic drug susceptibility must be done in animals, for example, mouse footpad (MFP) testing. However, a one-year-long experiment in the MFP experiments for phenotypic sensitivity testing is not very popular (7). Moreover, the low capacity to distinguish M. leprae from other mycobacteria by microscopy is an important concern due to the poor precision and sensitivity of these experiments (8). In the last twenty years, many experiments in clinical and molecular settings have been conducted which have advanced the area of leprosy diagnostics (9–11). However, a variety of diagnostics tests have been proposed based on the detection of pathogen’s DNA, RNA, protein or host antibodies, cytokines and other host biomarkers such as gene expression profiles (12). Based on the principle, these diagnostic tests can be broadly divided into three types: Immunological tests (Based on antigen/antibody detection), Nucleic acid-based tests, and tests based on host biomarkers (13).
Diagnostic Tests Based on Antigen-Antibody Detection
These rapid diagnostic assays detect the presence of leprosy bacilli proteins or host antibodies against them. If adequate quantities of the target antigen are present in the sample, it will bind to specific antibodies attached to a paper strip which will produce a visually detectable signal within 30 minutes. The observed antigen(s)/antibodies, which can only be expressed if bacteria are present in the host and have a high BI index, are thus usually used for acute or early infection detection (14, 15). Antibody detection is a more general form of leprosy diagnosis test, which measures the presence of antibodies in the blood of people suspected to be afflicted with Mycobacterium leprae. Specific antibodies are made within days or weeks after initial exposure. The intensity of the antibody response relies on a variety of factors such as bacillary load, type of immune response as well as other host factors such as age, nutritional status, disease severity and certain medications or infections such as HIV suppresses the immune response (16). Though antibody levels provide some insight into the status of infection, but they are insufficient to distinguish between recent and past infections. However, finding the evidence of infection would suggest a recent transmission (17).
Diagnostic Tests Based on Nucleic Acid Amplification Tests (NAAT)
M. leprae can be specifically detected by PCR or qPCR techniques in a very sensitive manner i.e. for detecting a very small number of genomic templates in a clinical sample. Several specific genomic regions have been targeted such as 16S rRNA (7) rpoT and sodA (18, 19) 36-kDa antigen (20), Complex 85 (21) and the repetitive sequences (LEPRPT, LEPREP, REPLEP and RLEP) (22) among other M. leprae genes (23–25) of which the most sensitive and specific sequence was found to be RLEP region (26). Other repeated loci can also be targeted that are present in fewer copies. Leprosy is often diagnosed by molecular testing either from PCR or qPCR (7, 8, 27). NAAT evaluations are focused on amplifying the M. leprae’s targeted sequence of the genetic material (DNA or RNA) with PCR. NAATs are more rapid than conventional mycobacterial detection technologies and are also available at different healthcare levels, therefore allowing them to perform drug susceptibility testing, such as rifampicin (RIF), for the detection of leprosy (28). In this way, they simplify leprosy diagnostics and help improve the quality of leprosy treatment.
Diagnostics Tests Based on Host Biomarkers
The immunological spectrum of leprosy, which ranges from widespread infection to a self-limited type of disease, often complicates the diagnostic procedure. The intricate interaction between innate and adaptive immune responses, which is controlled by host genetic background and environmental variables, determines M. leprae infection and subsequent disease development. Biomarkers based on the host immune response to M. leprae are thus a topic of interest as detecting M. leprae in the preclinical phases of the disease is challenging. Many research groups have recognized the potential of M. leprae specific antigens in serologic and T cell tests since the disclosure of the M. leprae genome. Despite the screening process to identify the early candidate that triggers M. leprae specific T lymphocytes, suitable candidate antigens are yet to be identified that would cover the entire immunological leprosy spectrum.
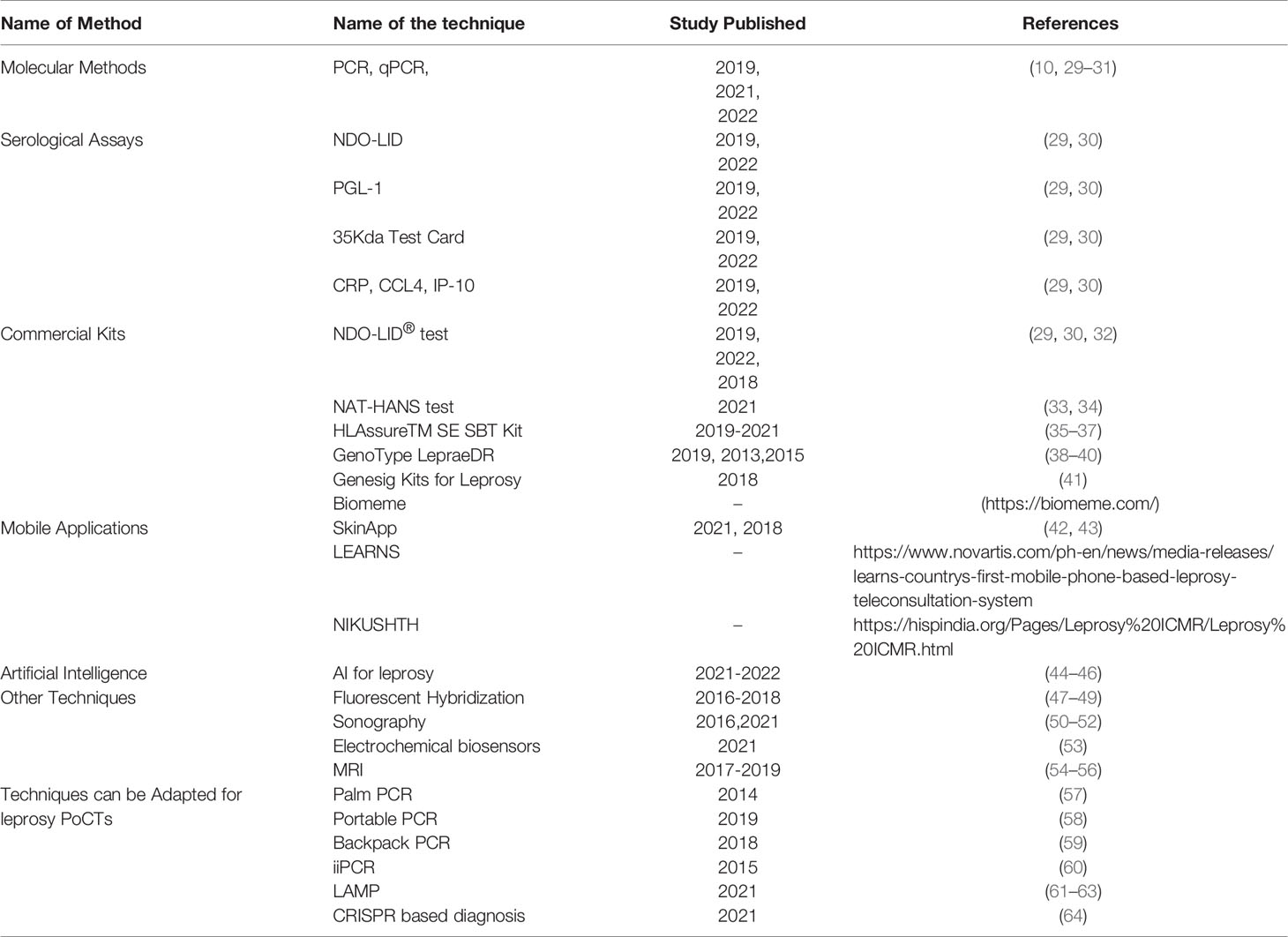
In this review, we have tried to compile all the information available in the literature regarding the recent developments for leprosy diagnosis (Table 1). We refer readers to other reviews to know about the recent advancements in biomarker-based research for leprosy detection (65–68) and meta-analysis for sensitivity and specificity studies of laboratory tests (10, 29–31).
Different Diagnostics Approaches for the Detection of M. leprae
Clark-Curtiss and Docherty provided the first paper of 1989 on the use of nuclear acid-based identification of M. leprae (69). A 2.2-kb M. leprae DNA fragment was used to facilitate the precise identification of bacilli in a hybridization-based technique in materials of multibacillary (MB) patients. During the same year, Woods and Cole described the use of PCR to selectively identify the M. leprae specific repetitive sequence RLEP in M. leprae, explaining agarose gel-based visualization of approximately 100 M. leprae cells in the armadillo liver, mouse footpads and human biopsies (70). Several other PCR systems were subsequently developed for the detection of M. leprae (6, 24, 71, 72), which showed the detection limits of 1 to 1000 bacilli. Observations of amplicons dependent on agarose gel were mainly reported. Later, with the advent of new sensitive techniques like NGS, various new targets were identified (27, 73–79). Several electrophysiological, ultrasonographic, Electroneuromyography (ENMG) and histological techniques are also developed and adapted to diagnose PNL (80, 81). Subsequently, many other sensitive techniques have been used previously or in development that can be updated and further used as a point of care tools in the laboratory as well as field settings as diagnostic tools such as variants of PCR (8, 24, 71, 72, 82), colorimetric assays (83), Biomarkers (66), Filter paper (84), peptide-nucleic-acid-ELISA (85, 86), reverse line probe (87), synthetic peptide reagents (88–90), Ultrasonography (50–52), Fingerstick test (91), Electrochemical biosensors (53), LAMP (61–63), MRI (54–56), LF Assays (92, 93), Fluorescent staining (47–49) and Artificial Intelligence to combat leprosy (44, 45). Here we will be discussing budding techniques that have the potential to be used in the field settings easily.
Polymerase Chain Reaction
Following the publication of the genome sequence of the M. leprae in 2001, species-specific genetic sequences have been sought to standardize the diagnostic procedures based on DNA analysis. These sequences were amplified using the PCR method, allowing the identification of bacillus DNA from modest numbers of M. leprae cells. PCR and quantitative PCR (qPCR) are extremely informative and responsive and guarantee to be diagnosed and treated early enough to guarantee the timely medication required to avoid disability and to mitigate leprosy spread (7, 71). The PCR-based approaches used to detect pathogenic DNA and RNA can also assess the viability of leprosy bacilli and be used in touch screening and monitoring programs. Several tissue origins, including skin biopsy tests, swabs of the nasal or dental, and entire blood, can be extended with PCR for M. leprae DNA. However, the use of skin biopsies instead of conveniently obtained samples (blood, skin scrapings, saliva etc) provides maximal performance (24).
Although M. leprae was considered the exclusive causative agent of leprosy, recently, M. lepromatosis was discovered and found in an unusual type of leprosy known as diffuse lepromatous leprosy (DLL) (94). A unique repetitive factor, RLPM, was defined by genomic analysis of M. lepromatosis strain (NHDP-385) on which a real-time quantitative PCR (qPCR) assay was established and validated in compliance with the guidelines for Clinical Laboratory Improvement Amendments as clinical diagnostic assays (27). Similarly, a new assay was developed that can simultaneously detect both the leprosy bacilli through PCR in a single reaction by amplifying a part of rpoT gene (19). Therefore, in clinical diagnosis and monitoring of leprosy in the field settings, the RLPM (M. leprae), RLEP (M. lepromatosis) and rpoT based PCR, 16SrRNA, sodA, RLEP based PCR (18) and qPCR assays can support healthcare providers. The availability of a rapid, specific and sensitive, and field-deployable PCR assay would support local decision-making during the surveillance and screening period (95). Few PCR examples are already reported such as Portable PCR (58), Palm PCR (57), insulated isothermal PCR (iiPCR) (60), RT-insulated isothermal PCR (96), Backpack PCR (59), etc. These assays have been successfully used for several pathogens detection and can also be adapted for the detection of leprosy bacilli.
Upconverting Reporter Particle-Lateral Flow Assay (UCP-LFA)
Endemic areas of leprosy are often lacking specialized labs that highlight the need for low-complexity diagnostics (97–99). A test was developed and field-assessed known as the lateral flow test (100). Recently, Upconverting phosphor (UCP) reporter technology was used in all LFAs to increase sensitivity and quantitation (101). The test consists of the UCP technology in conjunction with low-cost immune chromatography (i.e. lateral flow). It performs exceptionally well for the identification of cytokines and anti-M. leprae PGL-I IgM Ab (αPGL-I) in serum. A quick test using the visual detection of immunogold particles has been used to detect antibodies against M. leprae (93). No sophisticated analytical laboratory equipment is required for the user-friendly UCP-LFAs. This low-cost, lightweight portable reader offers complete, instrument-assisted analysis and prevents operator interference. Using UCP-LFA, the quantity of every biomarker type present in biological samples may also be quantified, which will enable the measurement of variations in the quantities of biomarkers and adjustable cut-off values to fulfill exposure and specificity criteria for areas where the leprosy is endemic (92, 102).
Loop-Mediated Isothermal Amplification LAMP
Isothermal amplification is a simple process that rapidly amplifies nucleic acid sequences at constant temperature (103). It is favored over typical PCR approaches to get rid of the specifications/complications of a costly thermocycler system and is cost-effective. The loop-mediated isothermal amplification (LAMP) technique has many applications in the area of point-of-care (POC) research across many isothermal techniques (104). It uses four to six primers to determine particular temperature regions which can be multiplied at a constant temperature using high strand displacement DNA polymerase. Gene amplification and detection can be achieved in one step, using the basic heat block or water bath, by the incubation of the polymerase primer and template mix in a reaction buffer. It can obtain outcomes in less than 30 minutes at a single temperature with a high yield and specificity. LAMP is considered to be useful for many molecular diagnostic applications i.e. from laboratory infectious agents to different pathogens, food processing, environmental examinations etc. (105, 106). Several LAMP-based diagnostic kits and approaches have been developed to identify a variety of challenging pathogenic agents for highly infectious illnesses, including SARS-CoV-2 (107). More importantly, the advent of LAMP has led to improved diagnosis of neglected tropical diseases all over the world. Esmatabadi et Al checked different methods for identification that can be paired with the LAMP system to interpret results quickly, such as colorimetry, turbidometry, hybridization samples, lateral flow dipsticks, ELISA, gold nano-particles (108). M. leprae is uncultivated in vitro and has a highly reduced genome that has a high degree of identification with the MTB genome. Considering its unique advantages that provide a rapid and low-cost diagnostic tool for disease detection in poor and remote parts of the world, recently LAMP technology for M. leprae detection has been developed using RLEP sequence as a molecular target for this assay (61–63). However, no isothermal amplification assay has been developed for M. lepromatosis yet.
Detection of M. leprae or Associated Biomarkers by Commercially Available Products
NDO-LID® Test
The NDO-LID® test, a new ELISA-based diagnostic test, was developed for the diagnosis of MB leprosy. The test is based on the detection of antibodies against the unique protein-glycolipid complex. The NDO-LID® immunochromatographic test takes only a tiny sample of serum or whole blood. This test includes both the LID-1 and PGL-I antigens. These antigens are immobilised on nitrocellulose membranes, which allow for the transfer and detection of the specific antibodies against these antigens in patient sera. This test, when combined with a new mobile based platform (Smart Reader® application), can give measurable and reliable data to aid in leprosy diagnosis. It detects MB patients and, perhaps, HHC at a higher risk of acquiring MB leprosy, much as the other leprosy serological tests (32, 109–112).
NAT-HANS Test
It is the first PCR-based diagnostic test for leprosy that follows Good Manufacturing Practice (GMP). This multiplex real-time PCR test has been developed to detect M. leprae 16S rRNA and RLEP genes, as well as one mammalian target (18S rRNA gene) that acts as a control. This detection occurs by the increase, at each reaction cycle, of the fluorescence signal emitted by two molecular probes specific when the target DNA is present in the sample. The success of the reaction is monitored through a fluorescence signal, emitted by a third probe in the same reaction, which increases the amplification of DNA. The Brazilian Health Regulatory Agency (Anvisa) approved the NAT-HANS test for people suspected of leprosy in 2021. The test showed high sensitivity and specificity values comparable to Lateral Flow Assays with a limit of detection of 2.29 copies of the M. leprae genome (33, 34).
HLAssure™ SE SBT Kit (Personalised Medicine)
Dapsone is an anti-inflammatory and antibacterial medication used in dermatology to prevent and treat infectious and chronic inflammatory conditions (113). Dapsone is used to treat leprosy, malaria, and diseases as an antibiotic and as an anti-inflammatory medication but is most commonly utilised for its anti-leprotic action. However, there have been reports of Dapsone Hypersensitivity Syndrome (DHS) which poses serious concern in certain populations. HLA-B*13:01 is associated with DHS and prior screening is essential to significantly minimize the chances of occurrence of DHS. A single DNA polymorphism termed HLA-B*13:01 located in the human leukocyte antigen locus is more common in those who developed DHS (35–37). Individuals with one copy of HLA-B*13:01 risk variant are 34 times more likely to develop DHS than individuals without it. Two copies of HLA-B*13:01 make individuals times more susceptible to the syndrome (75). Using TBG Biotechnology Corp’s HLA-B*13:01 assay kit, Clinicians and researchers are finally able to screen the patients for DHS. Recently, HLA-B*13:01 is validated as a biomarker for DHS in leprosy patients and Screening for HLA-B*13:01 has shown promising results in reducing DHS incidence significantly in the Indonesian population (114). The kit also provides the typing results of HLA-A, B, C, DRB1, DRB3, DRB4, DRB5, DQB1 and DPB1 with high resolution by a DNA-based method (http://www.tbgbio.com/en/product/product_detail/12).
GenoType LepraeDR (Drug Resistance)
A test was developed in 2012 as a GenoType LepraeDR kit using DNA strip technology (detection of amplified DNA or RNA sample via hybridization and alkaline phosphatase reaction on a membrane strip) to overcome this and extended to the molecular diagnosis of antibiotic resistance in leprosy (https://www.hain-lifescience.de/en/products/microbiology/mycobacteria/lepra/genotype-lepraedr.html). The GenoType LepraeDR enables the simultaneous identification of M. leprae and its susceptibility to first and second-line drugs within 5 hours. Initially, 120 M. leprae strains analyzed for genotypic and phenotypic characters of resistance were routinely screened for evaluation. The assay findings were found to be 100% compatible with those of the in vivo susceptibility test, while the PCR sequencing results were 98.3% for rifampicin, 100% for Dapsone, and 100% for fluoroquinolones (28). Later many publications have described this assay to identify the drug resistance in M. leprae (29, 38–40).
Genesig Kits for Leprosy (qPCR and Whole Genome Sequencing)
The Primerdesign genesig Kit® for Leprosy is intended for quantification of M. leprae genomes in vitro. The kit is intended to provide a wide detection profile. Their primers show 100 percent homology with more than 95 percent of the NCBI database reference sequences available at the time of creation. Because of the dynamic nature of genetic diversity, additional sequence information may become accessible after the initial design. The method employs a TaqMan probe to perform real-time PCR of the non-coding repetitive element RLEP, which is unique to the M. leprae genome. The excellent test specificity was demonstrated utilizing pathogenic and opportunistic pathogenic mycobacterium reference DNA samples, as well as PCR detection of single-copy genes of M. leprae such as fbp, MntH and rrs. The application of the developed approach increased the sensitivity of a commercially available test system based on single-copy rpoB gene detection to 96.8%, while the use of a commercially available test system based on single-copy rpoB gene detection provided 59.4 percent sensitivity to the detection of M. leprae in clinical material (41).
Biomeme
American Leprosy Mission and its network of research partners have collaborated with Biomeme, Inc. to develop and commercialize hand-held point-of-need PCR testing solutions for combatting leprosy diagnostics for use in distant locations. Their FranklinTM mobile qPCR thermocycler and field-prepared, shelf-stable reagents are easy-to-use reagents for field-friendly operations (https://biomeme.com/). Biomeme uses a polymerase chain reaction (PCR) methodology that turns a smartphone into a powerful mobile lab capable of performing PCR, RT-PCR, qPCR, and isothermal assays in real-time. A Biomeme mobile qPCR thermocycler, which is smaller than a box of tissues, is at the core of the solution. The lightweight, internally battery-powered gadget produces results in 30-45 minutes without the need for electricity, centrifugation, or cold chain logistics (https://www.ccih.org/technology-in-global-health-an-instrument-of-impact/). The use of the Biomeme platform as a field-friendly PCR-based early diagnostic tool to detect subclinical infection among household contacts and the general population has been rolled out in some parts of the world (India, Nepal and Ghana).
Diagnosis of Leprosy Through Digital Application
SkinApp
Leprosy in endemic regions has been diagnosed based on the presence of one of 3 cardinal signs: skin lesion with loss of sensation associated or not with thickened peripheral nerves and microscopic detection of acid-fast bacilli in skin lesions/slit skin smears. Keeping in mind, WHO released a manual on neglected tropical diseases by skin changes in 2018 (115). This serves as a training guide for the front-line health professionals, a detailed guide to recognizing skin NTDs employing signs and skin symptoms offering guidance about how to detect and cope with serious skin conditions that are faced by unspecialized front-line health care personnel. This pictorial training guide on neglected tropical diseases of the skin (including leprosy) has been recently converted into an interactive mobile phone application. The program allows easy access to the knowledge about skin conditions – such as its clinical symptoms, management and geographical distribution – which provides a list of possible indications to health care professionals and the general public. The software also facilitates rapid knowledge exchanging through a chatbox, which will promptly answer general questions. In collaboration with national dermatological federations, the SkinApp has been evaluated in field settings in Ethiopia, Mozambique and Tanzania under the PEP4LEP initiative in 2019. During their daily clinical activity, the primary health staff found it very helpful (42, 43).
Leprosy Alert and Response Network System (LEARNS)
LEARNS is a mobile-based leprosy identification system that enables primary health providers to send an SMS to a specialist with photos of suspected leprosy and symptoms. This helps in a dramatic reduction in the time required for testing and treatment times. Beginning in the Philippines, where nearly 2,000 new leprosy patients have been identified in leprosy annually, LEARNS was initiated with the support of a Leprosy Task Force as part of the Philippines Department of Health (DOH)-Novartis Foundation. In 2015, A diagnostic compatibility between LEARNS and the clinician-guided diagnosis was determined. LEARNS has proven to be a successful screening method for the proper detection of suspected lesions as 83 percent leprosy and 77 percent leprosy except for specifics, where the suspected lesions have been presented in the picture. About 3,500 healthcare providers have been qualified nationally in LEARNS to date (https://www.novartisfoundation.org/past-programs/accelerating-leprosy-and-malaria-elimination/leprosy-alert-and-response-network-system-learns). In line with LEARNS, similar computer-based software application like NIKUSHTHA which is developed to report leprosy in India has been started working. (https://dghs.gov.in/content/1349_3_ NationalLeprosyEradicationProgramme.aspx) whereas a Portuguese mobile application system is in development for detecting leprosy suspects (116).
Future Directions
Artificial Intelligence (AI) Powered Diagnostic Tool
Artificial intelligence (AI) has become a hot topic among doctors in recent years with the evolution of information technology and interdisciplinary studies have become more common. Accurate identification of leprosy lesions is often a challenge, especially for clinicians with little experience such as those in low-burden settings. To address this problem, the convolutional neural networks (CNN) project was developed using DermnetNz datasets in 2016, achieving 91.6 percent accuracy (117). In 2018, researchers used the Kohonen Self-Organizing Maps algorithm to evaluate data from patients and their household connections using Artificial Intelligence approaches to study the epidemiology of leprosy (118). The results show a significant frequency of late diagnosis, as well as Anti PGL-1 levels in clusters, indicating a higher bacillary load and consequently a high risk of disease progression. Microsoft and Novartis Foundation have also teamed up to develop an AI-powered digital tool for the early detection of leprosy (https://www.novartisfoundation.org/news/ai-powered-diagnostic-tool-aid-early-detection-leprosy). They have named it AI4Leprosy project which aims to develop an easy-to-use AI-powered solution to accelerate the diagnosis of leprosy with skin images. Recently, a cross-platform app for leprosy screening based on artificial intelligence has been developed which can recognize patterns of leprosy cases in a database with ~94% sensitivity and 87% specificity (45). Recently, Barbieri et al. described that AI models have the potential to become a complementary diagnosis tool, especially for the remote areas where very few clinicians are available known as AI4Leprosy (46). It is an AI-enabled image-based diagnosis tool for leprosy that is based on a mix of skin images and clinical data collected using a defined method. For leprosy diagnosis, it had a high classification accuracy (90%) and an area under curve (AUC) of 96.46 percent.
Discussion
The current COVID-19 pandemic has raised the awareness and preparedness levels towards the rapid and in-time diagnosis of infectious agents to reduce their transmission. It has been anticipated that financial markets will remain volatile as the virus continues to disrupt economic activity which is associated with worse infectious diseases outcomes. According to expert opinion and some guidelines, COVID-19 may influence the occurrence and severity of lepra reactions as an economic crisis may contribute to the increase in the incidence of leprosy (119). The epidemic threatened to undermine any progress that had been made in eliminating leprosy. For example, loss of financial and human resources from national leprosy programs when leprosy funding and employees are redirected to the fight against Covid-19, resulting in a significant drop in leprosy-related projects and experts. However, amid the challenging condition of lockdown, very precise and effective diagnostics for SARS-CoV-2 were developed in a record time. To this date, several commercially available tests such as CBNAAT and TrueNat were adapted for POCs for SARS-CoV-2 detection addressing several challenges such as instability of RNA targets and Biohazard issues. However, a similar focus on leprosy and other NTDs has yet to be attempted or prioritized. Real time PCR and CBNAAT/TrueNat machines/kits are more available at district levels in many places. The development of suitable primers and probes compatible with CBNAAT/TrueNat and other systems would give an additional advantage towards the rapid detection of leprosy bacilli. These assays have the high potential to be used as POC for leprosy diagnostics. With the decline in leprosy cases, clinical expertise is bound to further decrease which may result in diagnostic delays. As a result, the period between clinical symptoms, diagnoses and treatment of patients is longer and the transmission of stays almost constant. Hence, efforts to get rid of this disease are then hindered. An optimal diagnostic test will recognize M. leprae infected persons at risk of developing diseases and/or leading to their spread, hence there is a need to also switch from leprosy management to prevention of infection. A screening test for verifying leprosy disease will be appropriate and probably effective in the early stages for patients and are asymptomatic. At the same time, stakeholders must strive to support the idea that zero transmission is feasible only when the assessment of M. leprae infection is feasible, and that biomarkers to detect asymptomatic infection, and risk of contracting the disease, are sufficiently invested in clinical studies.
Author Contributions
PS and MS contributed to the conceptualization and design. Review of literature, data mining and analysis were performed by MS. The first draft of the manuscript was written by MS. Both authors read and approved the final manuscript.
Funding
Research work of PS is funded in part by R2STOP (effect:hope TLM) Canada, Turing Foundation and Leprosy Research Initiative (LRI) Netherlands, (Grant No. 704.16.59) the Department of Biotechnology (Government of India, Grant No. DBTRLF/Re-entry/10/2014) and Indian Council of Medical Research (ICMR). MS is the recipient of ICMR-Research Associateship. The manuscript has been approved by the Publication Screening Committee of ICMR-NIRTH, Jabalpur and assigned with the number ICMR-NIRTH/PSC/09/2022.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Avanzi C, Singh P, Truman RW, Suffys PN. Molecular Epidemiology of Leprosy: An Update. Infect Genet Evol (2020), 86:104581. doi: 10.1016/j.meegid.2020.104581
2. Deps P, Collin SM. Mycobacterium Lepromatosis as a Second Agent of Hansen's Disease. Front Microbiol (2021) 12:698588. doi: 10.3389/fmicb.2021.698588
3. Massone C, Brunasso AMG. Classification. In: Nunzi E, Massone C, editors. Leprosy: A Practical Guide. Milano: Springer Milan (2012)ISBN: 978-88-470-2376-5. p. 43–7.
4. Parkash O. Classification of Leprosy Into Multibacillary and Paucibacillary Groups: An Analysis. FEMS Immunol Med Microbiol (2009) 55(1):1–5. doi: 10.1111/j.1574-695X.2008.00491.x
5. World Health Organisation. Weekly Epidemiological Record (WER), Vol. 96(36). (USA: World Health Organisation)(2021). pp. 421–44. Available at: https://reliefweb.int/report/world/weekly-epidemiological-record-wer-10-september-2021-vol-96-no-36-pp-421-444-enfr.
6. Barbieri RR, Manta FSN, Moreira SJM, Sales AM, Nery JAC, Nascimento LPR, et al. Quantitative Polymerase Chain Reaction in Paucibacillary Leprosy Diagnosis: A Follow-Up Study. PloS Negl Trop Dis (2019) 13(3):e0007147. doi: 10.1371/journal.pntd.0007147
7. Martinez AN, Lahiri R, Pittman TL, Scollard D, Truman R, Moraes MO, et al. Molecular Determination of Mycobacterium Leprae Viability by Use of Real-Time PCR. J Clin Microbiol (2009) 47(7):2124–30. doi: 10.1128/JCM.00512-09
8. Siwakoti S, Rai K, Bhattarai NR, Agarwal S, Khanal B. Evaluation of Polymerase Chain Reaction (PCR) With Slit Skin Smear Examination (SSS) to Confirm Clinical Diagnosis of Leprosy in Eastern Nepal. PloS Negl Trop Dis (2016) 10(12):e0005220. doi: 10.1371/journal.pntd.0005220
9. Acosta L, Torres P. Leprosy Diagnosis: An Update on the Use of Molecular Tools. JMB (2015) 4(139):2. doi: 10.4172/2168-9547.1000139
10. Andrade ESN, Brandão JG, da Silva JS, Kurizky PS, Rosa PS, de Araújo WN, et al. A Systematic Review and Meta-Analysis of Studies on the Diagnostic Accuracy and Screening of Tests to Detect Antimicrobial Resistance in Leprosy. Diagn Microbiol Infect Dis (2021) 100(1):115325. doi: 10.1016/j.diagmicrobio.2021.115325
11. Tió-Coma M, Kiełbasa SM, van den Eeden SJ, Mei H, Roy JC, Wallinga J, et al. Blood RNA Signature RISK4LEP Predicts Leprosy Years Before Clinical Onset. EBioMedicine (2021) 68:103379. doi: 10.1016/j.ebiom.2021.103379
12. Dwivedi S, Purohit P, Misra R, Pareek P, Goel A, Khattri S, et al. Diseases and Molecular Diagnostics: A Step Closer to Precision Medicine. Indian J Clin Biochem (2017) 32(4):374–98. doi: 10.1007/s12291-017-0688-8
13. Bharadwaj M, Bengtson M, Golverdingen M, Waling L, Dekker C. Diagnosing Point-of-Care Diagnostics for Neglected Tropical Diseases. PloS Negl Trop Dis (2021) 15(6):e0009405. doi: 10.1371/journal.pntd.0009405
14. Geluk A, Duthie MS, Spencer JS. Postgenomic Mycobacterium Leprae Antigens for Cellular and Serological Diagnosis of M. Leprae Exposure, Infection and Leprosy Disease. Lepr Rev (2011) 82(4):402–21. doi: 10.47276/lr.82.4.402
15. Brennan PJ, WW B. Evidence for Species-Specific Lipid Antigens in Mycobacterium Leprae. JIjol Assoc omdoootIL (1980) 48(4):382–7.
16. Spencer JS, Kim HJ, Wheat WH, Chatterjee D, Balagon MV, Cellona RV, et al. Analysis of Antibody Responses to Mycobacterium Leprae Phenolic Glycolipid I, Lipoarabinomannan, and Recombinant Proteins to Define Disease Subtype-Specific Antigenic Profiles in Leprosy. Clin Vaccine Immunol (2011) 18(2):260–7. doi: 10.1128/CVI.00472-10
17. Pierneef L, van Hooij A, Taal A, Rumbaut R, Nobre ML, van Brakel W, et al. Detection of Anti-M. Leprae Antibodies in Children in Leprosy-Endemic Areas: A Systematic Review. PloS Negl Trop Dis (2021) 15(8):e0009667. doi: 10.1371/journal.pntd.0009667
18. Turankar RP, Pandey S, Lavania M, Singh I, Nigam A, Darlong J, et al. Comparative Evaluation of PCR Amplification of RLEP, 16s rRNA, rpoT and Sod A Gene Targets for Detection of M. Leprae DNA From Clinical and Environmental Samples. Int J Mycobact (2015) 4(1):54–9. doi: 10.1016/j.ijmyco.2014.11.062
19. Dwivedi P, Sharma M, Patel P, Singh P. Simultaneous Detection and Differentiation Between Mycobacterium Leprae and Mycobacterium Lepromatosis Using Novel Polymerase Chain Reaction Primers. J Dermatol (2021) 48(12):1936–9. doi: 10.1111/1346-8138.16165
20. Kampirapap K, Singtham N, Klatser PR, Wiriyawipart S. DNA Amplification for Detection of Leprosy and Assessment of Efficacy of Leprosy Chemotherapy. International Journal of Leprosy and Other Mycobacterial Diseases. Int J Lepr Other Mycobact Dis (1998) 66(1):16–21.
21. Martinez AN, Britto CF, Nery JA, Sampaio EP, Jardim MR, Sarno EN, et al. Evaluation of Real-Time and Conventional PCR Targeting Complex 85 Genes for Detection of Mycobacterium Leprae DNA in Skin Biopsy Samples From Patients Diagnosed With Leprosy. J Clin Microbiol (2006) 44(9):3154–9. doi: 10.1128/JCM.02250-05
22. Cole ST, Supply P, Honoré N. Repetitive Sequences in Mycobacterium Leprae and Their Impact on Genome Plasticity. Lepr Rev (2001) 72(4):449–61.
23. Custodio LA, Saito A, Amarante MK, Fujita TC, Perim A, Costa IC, et al. Detection of Lsr2 Gene of Mycobacterium Leprae in Nasal Mucus. Braz Arch Biol Technol (2012) 55(3):375–80. doi: 10.1590/S1516-89132012000300007
24. Manta FSN, Leal-Calvo T, Moreira SJM, Marques BLC, Ribeiro-Alves M, Rosa PS, et al. Ultra-Sensitive Detection of Mycobacterium Leprae: DNA Extraction and PCR Assays. PloS Negl Trop Dis (2020) 14(5):e0008325. doi: 10.1371/journal.pntd.0008325
25. Banerjee S, Sarkar K, Gupta S, Mahapatra PS, Gupta S, Guha S, et al. Multiplex PCR Technique Could be An Alternative Approach for Early Detection of Leprosy Among Close Contacts–a Pilot Study From India. BMC Infect Dis (2010) 10:252. doi: 10.1186/1471-2334-10-252
26. Braet S, Vandelannoote K, Meehan CJ, Brum Fontes AN, Hasker E, Rosa PS, et al. The Repetitive Element RLEP Is a Highly Specific Target for Detection of Mycobacterium Leprae. J Clin Microbiol (2018) 56(3):e01924–17. doi: 10.1128/JCM.01924-17
27. Sharma R, Singh P, McCoy RC, Lenz SM, Donovan K, Ochoa MT, et al. Isolation of Mycobacterium Lepromatosis and Development of Molecular Diagnostic Assays to Distinguish Mycobacterium Leprae and M. Lepromatosis. Clin Infect Dis (2020) 71(8):e262-e9. doi: 10.1093/cid/ciz1121
28. Cambau E, Chauffour-Nevejans A, Tejmar-Kolar L, Matsuoka M, Jarlier V. Detection of Antibiotic Resistance in Leprosy Using GenoType LepraeDR, a Novel Ready-to-Use Molecular Test. PloS Negl Trop Dis (2012) 6(7):e1739. doi: 10.1371/journal.pntd.0001739
29. Gurung P, Gomes C, Vernal S, Leeflang M. Diagnostic Accuracy of Tests for Leprosy: A Systematic Review and Meta-Analysis. JCM Infect (2019) 25(11):1315–27. doi: 10.1016/j.cmi.2019.05.020
30. Romero CP, Castro R, do Brasil PEA, Pereira DR, Pinheiro RO, Toscano CM, et al. Accuracy of Rapid Point-of-Care Serological Tests for Leprosy Diagnosis: A Systematic Review and Meta-Analysis. Memórias do Instituto Oswaldo Cruz (2022) 117:. doi: 10.1590/0074-02760220317
31. Torres RT, Fachi MM, Böger B, Marson BM, Ferreira VL, Pontarolo R, et al. Sensitivity and Specificity of Multibacillary and Paucibacillary Leprosy Laboratory Tests: A Systematic Review and Meta-Analysis. Diagn Microbiol Infect Dis (2021) 100(2):115337. doi: 10.1016/j.diagmicrobio.2021.115337
32. Devides AC, Rosa PS, Belone d, Coelho NMB, Ura S, Silva EA. Can Anti-PGL-1 and Anti-NDO-LID-1 Antibody Titers Be Used to Predict the Risk of Reactions in Leprosy Patients? Diagn Microbiol Infect Dis (2018) 91(3):260–5. doi: 10.1016/j.diagmicrobio.2018.03.002
33. Moraes M. NAT-HANS: The First PCR-Based Diagnostic Test for Hansen’s Disease Using Good Manufacturing Practice (GMP) (2021). Available at: https://en.infohansen.org/resources/blog/nat-hans-the-first-pcr-based-test-with-good-manufacturing-practices-for-ha.
34. Manta FSN, Jacomasso T, Rampazzo RCP, Moreira SJM, Zahra NM, Cole ST, et al. Development and Validation of a Multiplex Real-Time qPCR Assay Using GMP-Grade Reagents for Leprosy Diagnosis. PloS Negl Trop Dis (2022) 16(2):e0009850. doi: 10.1371/journal.pntd.0009850
35. Park HJ, Park JW, Kim SH, Choi SY, Kim HK, Jung CG, et al. The HLA-B*13:01 and the Dapsone Hypersensitivity Syndrome in Korean and Asian Populations: Genotype- and Meta-Analyses. Expert Opin Drug Saf (2020) 19(10):1349–56. doi: 10.1080/14740338.2020.1796965
36. Satapornpong P, Pratoomwun J, Rerknimitr P, Klaewsongkram J, Nakkam N, Rungrotmongkol T, et al. HLA-B*13 :01 Is a Predictive Marker of Dapsone-Induced Severe Cutaneous Adverse Reactions in Thai Patients. Front Immunol (2021) 12:661135. doi: 10.3389/fimmu.2021.661135
37. Liu H, Wang Z, Bao F, Wang C, Sun L, Zhang H, et al. Evaluation of Prospective HLA-B*13:01 Screening to Prevent Dapsone Hypersensitivity Syndrome in Patients With Leprosy. JAMA Dermatol (2019) 155(6):666–72. doi: 10.1001/jamadermatol.2018.5360
38. Tan YE, Yeo YW, Ang DJQ, Chan MMF, Pang SM, Sng L-H. Report of a Leprosy Case in Singapore: An Age-Old Disease Not to be Forgotten in Developed Countries With Low-Prevalence Settings. JAM (2019) 1(3):e000014. doi: 10.1099/acmi.0.000014
39. Miller D, Girgis D, Karp C, Alfonso EC. New Aspects in the Diagnosis and Therapy of Mycobacterial Keratitis. In: Corneal Disease. Springer (2013). p. 1–18. doi: 10.1007/978-3-642-28747-3_1
40. Pfyffer GE. Mycobacterium: General Characteristics, Laboratory Detection, and Staining Procedures. JMocm (2015), 536–69. doi: 10.1128/9781555817381.ch30
41. Obraztsova OA, Verbenko DA, Karamova AE, Semenova VG, Kubanov AA, Deryabin DG. [The Refinement of Leprosy PCR Diagnostics by the Amplification of Specie-Specific Repeated Fragment of the Mycobacterium Leprae Genome.]. Klin Lab Diagn (2018) 63(8):511–6. doi: 10.18821/0869-2084-2018-63-8-511-516
42. Schoenmakers A, Hambridge T, van Wijk R, Kasang C, Richardus JH, Bobosha K, et al. PEP4LEP Study Protocol: Integrated Skin Screening and SDR-PEP Administration for Leprosy Prevention: Comparing the Effectiveness and Feasibility of a Community-Based Intervention to a Health Centre-Based Intervention in Ethiopia, Mozambique and Tanzania. BMJ Open (2021) 11(8):e046125. doi: 10.1136/bmjopen-2020-046125
43. Mieras LF, Taal AT, Post EB, Ndeve AGZ, van Hees CLM. The Development of a Mobile Application to Support Peripheral Health Workers to Diagnose and Treat People With Skin Diseases in Resource-Poor Settings. Trop Med Infect Dis (2018) 3(3):102. doi: 10.3390/tropicalmed3030102
44. Sharma M, Singh P. Use of Artificial Intelligence in Research and Clinical Decision Making for Combating Mycobacterial Diseases. In: Saxena A, Chandra S, editors. Artificial Intelligence and Machine Learning in Healthcare. Singapore: Springer Singapore (2021). p. 183–215. doi: 10.1007/978-981-16-0811-7_9
45. De Souza MLM, Lopes GA, Branco AC, Fairley JK, Fraga LAO. Leprosy Screening Based on Artificial Intelligence: Development of a Cross-Platform App. JMIR Mhealth Uhealth (2021) 9(4):e23718. doi: 10.2196/23718
46. Barbieri RR, Xu Y, Setian L, Souza-Santos PT, Trivedi A, Cristofono J, et al. Reimagining Leprosy Elimination With AI Analysis of a Combination of Skin Lesion Images With Demographic and Clinical Data. JLancet Regional Health- Americas (2022) 9:100192. doi: 10.1016/j.lana.2022.100192
47. Díaz Acosta CC, Dias AA, Rosa T, Batista-Silva LR, Rosa PS, Toledo-Pinto TG, et al. PGL I Expression in Live Bacteria Allows Activation of a CD206/Pparγ Cross-Talk That may Contribute to Successful Mycobacterium Leprae Colonization of Peripheral Nerves. PloS Pathog (2018) 14(7):e1007151. doi: 10.1371/journal.ppat.1007151
48. Adiga DS, Hippargi SB, Rao G, Saha D, Yelikar BR, Karigoudar M. Evaluation of Fluorescent Staining for Diagnosis of Leprosy and its Impact on Grading of the Disease: Comparison With Conventional Staining. J Clin Diagn Res (2016) 10(10):Ec23-ec6. doi: 10.7860/JCDR/2016/22470.8739
49. Girma S, Avanzi C, Bobosha K, Desta K, Idriss MH, Busso P, et al. Evaluation of Auramine O Staining and Conventional PCR for Leprosy Diagnosis: A Comparative Cross-Sectional Study From Ethiopia. PloS Negl Trop Dis (2018) 12(9):e0006706. doi: 10.1371/journal.pntd.0006706
50. Lugão HB, Frade MA, Marques W Jr., Foss NT, Nogueira-Barbosa MH. Ultrasonography of Leprosy Neuropathy: A Longitudinal Prospective Study. PloS Negl Trop Dis (2016) 10(11):e0005111. doi: 10.1371/journal.pntd.0005111
51. Suneetha S, Rao P. High-Resolution Ultrasonography in Leprosy: Value and Applications. Indian Dermatol Online J (2021) 12(4):497–9. doi: 10.4103/idoj.IDOJ_111_21
52. Zhao H, Nepal P, Alam SI. Sonographic Evaluation of Leprosy of Ulnar Nerve. Radiol Case Rep (2021) 16(5):1057–60. doi: 10.1016/j.radcr.2021.02.003
53. Afonso AS, Madurro JM, Brito-Madurro AG. Electrochemical DNA Biosensor for Mycobacterium Leprae Identification. Brazilian Archives of Biology and Technology. Braz Arch Biol Technol (2021) 64:e21210030. doi: 10.1590/1678-4324-2021210030
54. Bagga B, Das CJ. MRI of Radial Cutaneous Nerve Abscess in Recurrent Neural Leprosy. BMJ Case Rep (2018) 11(1):e228704. doi: 10.1136/bcr-2018-228704
55. Beltrame A, Barabino G, Cicciò C, Badona Monteiro G, Cavalchini A, Carbognin G, et al. Magnetic Resonance Imaging in Pure Neural Leprosy. Int J Infect Dis (2017) 60:42–3. doi: 10.1016/j.ijid.2017.04.022
56. Polavarapu K, Preethish-Kumar V, Vengalil S, Nashi S, Lavania M, Bhattacharya K, et al. Brain and Spinal Cord Lesions in Leprosy: A Magnetic Resonance Imaging-Based Study. Am J Trop Med Hyg (2019) 100(4):921–31. doi: 10.4269/ajtmh.17-0945
57. Lim S, Nan H, Lee MJ, Kang SH. Fast on-Site Diagnosis of Influenza A Virus by Palm PCR and Portable Capillary Electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci (2014) 963:134–9. doi: 10.1016/j.jchromb.2014.06.004
58. Lim J, Jeong S, Kim M, Lee JH. Battery-Operated Portable PCR System With Enhanced Stability of Pt RTD. PloS One (2019) 14(6):e0218571. doi: 10.1371/journal.pone.0218571
59. Zaky WI, Tomaino FR, Pilotte N, Laney SJ, Williams SA. Backpack PCR: A Point-of-Collection Diagnostic Platform for the Rapid Detection of Brugia Parasites in Mosquitoes. PloS Negl Trop Dis (2018) 12(11):e0006962. doi: 10.1371/journal.pntd.0006962
60. Wilkes RP, Lee PY, Tsai YL, Tsai CF, Chang HH, Chang HF, et al. An Insulated Isothermal PCR Method on a Field-Deployable Device for Rapid and Sensitive Detection of Canine Parvovirus Type 2 at Points of Need. J Virol Methods (2015) 220:35–8. doi: 10.1016/j.jviromet.2015.04.007
61. Garg N, Sahu U, Kar S, Ahmad FJ. Development of a Loop-Mediated Isothermal Amplification (LAMP) Technique for Specific and Early Detection of Mycobacterium Leprae in Clinical Samples. Sci Rep (2021) 11(1):9859. doi: 10.1038/s41598-021-89304-2
62. Jiang H, Tsang L, Wang H, Liu C. Loop-Mediated Isothermal Amplification (LAMP) Assay Targeting RLEP for Detection of Mycobacterium Leprae in Leprosy Patients. Int J Infect Dis (2021) 107:145–52. doi: 10.1016/j.ijid.2021.04.041
63. Joshi S, Dixit KK, Sharma V, Ramesh V, Singh R, Salotra P. Rapid Multiplex Loop-Mediated Isothermal Amplification (M-LAMP) Assay for Differential Diagnosis of Leprosy and Post-Kala-Azar Dermal Leishmaniasis. Am J Trop Med Hyg (2021) 104(6):2085–90. doi: 10.4269/ajtmh.19-0313
64. Kaminski MM, Abudayyeh OO, Gootenberg JS, Zhang F, Collins JJ. CRISPR-Based Diagnostics. Nat Biomed Eng (2021) 5(7):643–56. doi: 10.1038/s41551-021-00760-7
65. van Hooij A, Geluk A. In Search of Biomarkers for Leprosy by Unraveling the Host Immune Response to Mycobacterium Leprae. Immunol Rev (2021) 301(1):175–92. doi: 10.1111/imr.12966
66. Gautam S, Sharma D, Goel A, Patil SA, Bisht D. Insights Into Mycobacterium Leprae Proteomics and Biomarkers-An Overview. Proteomes (2021) 9(1):7. doi: 10.3390/proteomes9010007
67. Geluk A. Correlates of Immune Exacerbations in Leprosy. Semin Immunol (2018) 39:111–8. doi: 10.1016/j.smim.2018.06.003
68. Luo Y, Kiriya M, Tanigawa K, Kawashima A, Nakamura Y, Ishii N, et al. Host-Related Laboratory Parameters for Leprosy Reactions. Front Med (2021) 8:694376. doi: 10.3389/fmed.2021.694376
69. Clark-Curtiss JE, Docherty MA. A Species-Specific Repetitive Sequence in Mycobacterium Leprae DNA. JJoID (1989) 159(1):7–15. doi: 10.1093/infdis/159.1.7
70. Woods SA, Cole ST. A Rapid Method for the Detection of Potentially Viable Mycobacterium Leprae in Human Biopsies: A Novel Application of PCR. FEMS Microbiol Lett (1989) 53(3):305–9. doi: 10.1111/j.1574-6968.1989.tb03678.x
71. Martinez AN, Talhari C, Moraes MO, Talhari S. PCR-Based Techniques for Leprosy Diagnosis: From the Laboratory to the Clinic. PloS Negl Trop Dis (2014) 8(4):e2655. doi: 10.1371/journal.pntd.0002655
72. Manta FSN, Barbieri RR, Moreira SJM, Santos PTS, Nery JAC, Duppre NC, et al. Quantitative PCR for Leprosy Diagnosis and Monitoring in Household Contacts: A Follow-Up Study, 2011–2018. Sci Rep (2019) 9(1):16675. doi: 10.1038/s41598-019-52640-5
73. Wang D, Fan Y, Malhi M, Bi R, Wu Y, Xu M, et al. Missense Variants in HIF1A and LACC1 Contribute to Leprosy Risk in Han Chinese. Am J Hum Genet (2018) 102(5):794–805. doi: 10.1016/j.ajhg.2018.03.006
74. Wang D, Zhang DF, Feng JQ, Li GD, Li XA, Yu XF, et al. Common Variants in the PARL and PINK1 Genes Increase the Risk to Leprosy in Han Chinese From South China. Sci Rep (2016) 6:37086. doi: 10.1038/srep37086
75. Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, et al. HLA-B*13:01 and the Dapsone Hypersensitivity Syndrome. N Engl J Med (2013) 369(17):1620–8. doi: 10.1056/NEJMoa1213096
76. Tió-Coma M, Avanzi C, Verhard EM, Pierneef L, van Hooij A, Benjak A, et al. Genomic Characterization of Mycobacterium Leprae to Explore Transmission Patterns Identifies New Subtype in Bangladesh. Front Microbiol (2020) 11:1220. doi: 10.3389/fmicb.2020.01220
77. Avanzi C, Lécorché E, Rakotomalala FA, Benjak A, Rapelanoro Rabenja F, Ramarozatovo LS, et al. Population Genomics of Mycobacterium Leprae Reveals a New Genotype in Madagascar and the Comoros. Front Microbiol (2020) 11:711. doi: 10.3389/fmicb.2020.00711
78. Benjak A, Avanzi C, Singh P, Loiseau C, Girma S, Busso P, et al. Phylogenomics and Antimicrobial Resistance of the Leprosy Bacillus Mycobacterium Leprae. Nat Commun (2018) 9(1):352. doi: 10.1038/s41467-017-02576-z
79. Singh P, Benjak A, Schuenemann VJ, Herbig A, Avanzi C, Busso P, et al. Insight Into the Evolution and Origin of Leprosy Bacilli From the Genome Sequence of Mycobacterium Lepromatosis. Proc Natl Acad Sci U S A (2015) 112(14):4459–64. doi: 10.1073/pnas.1421504112
80. Santos DFD, Mendonça MR, Antunes DE, Sabino EFP, Pereira RC, Goulart LR, et al. Revisiting Primary Neural Leprosy: Clinical, Serological, Molecular, and Neurophysiological Aspects. PloS Negl Trop Dis (2017) 11(11):e0006086-e. doi: 10.1371/journal.pntd.0006086
81. Shukla B, Verma R, Kumar V, Kumar M, Malhotra KP, Garg RK, et al. Pathological, Ultrasonographic, and Electrophysiological Characterization of Clinically Diagnosed Cases of Pure Neuritic Leprosy. J Peripher Nerv Syst (2020) 25(2):191–203. doi: 10.1111/jns.12372
82. Iwao Y, Mori S, Ato M, Nakata N. Simultaneous Determination of Mycobacterium Leprae Drug Resistance and Single-Nucleotide Polymorphism Genotype by Use of Nested Multiplex PCR With Amplicon Sequencing. JJoCM (2021) 59(10):e00814-21. doi: 10.1128/JCM.00814-21
83. Haile Y, Ryon JJ. Colorimetric Microtitre Plate Hybridization Assay for the Detection of Mycobacterium Leprae 16S rRNA in Clinical Specimens. JLr (2004) 75(1):40. doi: 10.47276/lr.75.1.40
84. Sekar B, Anandan D. Evaluation of Mycobacterium Leprae Particle Agglutination Test, Using Eluates of Filter Paper Blood Spots. Lepr Rev (1992) 63(2):117–24.
85. Beyene D, Aseffa A, Harboe M, Kidane D, Macdonald M, Klatser PR, et al. Nasal Carriage of Mycobacterium Leprae DNA in Healthy Individuals in Lega Robi Village, Ethiopia. Epidemiol Infect (2003) 131(2):841–8. doi: 10.1017/S0950268803001079
86. Jadhav RS, Macdonald M, Bjune G, Oskam L. Simplified PCR Detection Method for Nasal Mycobacterium Leprae. Int J Lepr Other Mycobact Dis (2001) 69(4):299–307.
87. Sapkota BR, Ranjit C, Macdonald M. Reverse Line Probe Assay for the Rapid Detection of Rifampicin Resistance in Mycobacterium Leprae. Nepal Med Coll J (2006) 8(2):122–7.
88. Alban SM, de Moura JF, Minozzo JC, Mira MT, Soccol VT. Identification of Mimotopes of Mycobacterium Leprae as Potential Diagnostic Reagents. BMC Infect Dis (2013) 13(1):42. doi: 10.1186/1471-2334-13-42
89. Alban SM, de Moura JF, Thomaz-Soccol V, Bührer Sékula S, Alvarenga LM, Mira MT, et al. Phage Display and Synthetic Peptides as Promising Biotechnological Tools for the Serological Diagnosis of Leprosy. PloS One (2014) 9(8):e106222. doi: 10.1371/journal.pone.0106222
90. Barbosa M, de Sousa IBA, Simionatto S, Borsuk S, Marchioro SB. Recombinant Polypeptide of Mycobacterium Leprae as a Potential Tool for Serological Detection of Leprosy. AMB Express (2019) 9(1):201. doi: 10.1186/s13568-019-0928-9
91. Corstjens P, van Hooij A, Tjon Kon Fat EM, Alam K, Vrolijk LB, Dlamini S, et al. Fingerstick Test Quantifying Humoral and Cellular Biomarkers Indicative for M. Leprae Infection. Clin Biochem (2019) 66:76–82. doi: 10.1016/j.clinbiochem.2019.01.007
92. Bobosha K, Tjon Kon Fat EM, van den Eeden SJ, Bekele Y, van der Ploeg-van Schip JJ, de Dood CJ, et al. Field-Evaluation of a New Lateral Flow Assay for Detection of Cellular and Humoral Immunity Against Mycobacterium Leprae. PloS Negl Trop Dis (2014) 8(5):e2845. doi: 10.1371/journal.pntd.0002845
93. van Hooij A, Tjon Kon Fat EM, Richardus R, van den Eeden SJ, Wilson L, de Dood CJ, et al. Quantitative Lateral Flow Strip Assays as User-Friendly Tools To Detect Biomarker Profiles For Leprosy. Sci Rep (2016) 6:34260. doi: 10.1038/srep34260
94. Han XY, Seo Y-H, Sizer KC, Schoberle T, May GS, Spencer JS, et al. A New Mycobacterium Species Causing Diffuse Lepromatous Leprosy. Am J Clin Pathol (2008) 130(6):856–64. doi: 10.1309/AJCPP72FJZZRRVMM
96. Ambagala A, Fisher M, Goolia M, Nfon C, Furukawa-Stoffer T, Ortega Polo R, et al. Field-Deployable Reverse Transcription-Insulated Isothermal PCR (RT-iiPCR) Assay for Rapid and Sensitive Detection of Foot-And-Mouth Disease Virus. Transbound Emerg Dis (2017) 64(5):1610–23. doi: 10.1111/tbed.12554
97. Santos VS, de Souza CDF, Martins-Filho PRS, Cuevas LE. Leprosy: Why Does it Persist Among Us? Expert Rev Anti Infect Ther (2020) 18(7):613–5. doi: 10.1080/14787210.2020.1752194
98. Li Y-Y, Shakya S, Long H, Shen L-F, Kuang Y-Q. Factors Influencing Leprosy Incidence: A Comprehensive Analysis of Observations in Wenshan of China, Nepal, and Other Global Epidemic Areas. Front Public Health (2021) 9(681):666307. doi: 10.3389/fpubh.2021.666307
99. Rao PN. Leprosy: The Challenges Ahead for India. J Skin Sex Transmitted Dis (2021) 3(2):106–10. doi: 10.25259/JSSTD_42_2021
100. Bührer-Sékula S, Smits HL, Gussenhoven GC, van Leeuwen J, Amador S, Fujiwara T, et al. Simple and Fast Lateral Flow Test for Classification of Leprosy Patients and Identification of Contacts With High Risk of Developing Leprosy. J Clin Microbiol (2003) 41(5):1991–5. doi: 10.1128/JCM.41.5.1991-1995.2003
101. Tjon Kon Fat EM, Abrams WR, Niedbala RS, Corstjens PLAM. Chapter 11 - Lateral Flow Sandwich Assay Utilizing Upconverting Phosphor (UCP) Reporters. In: Conn PM, editor. Methods in Cell Biology, vol. 112 . Academic Press (2012). p. 203–34. doi: 10.1016/B978-0-12-405914-6.00011-1
102. Tjon Kon Fat EM, Abrams WR, Niedbala RS, Corstjens PLAM. Chapter 11 - Lateral Flow Sandwich Assay Utilizing Upconverting Phosphor (UCP) Reporters. In: Conn PM, editor. Methods in Cell Biology, vol. 7 (2017). p. 8868. doi: 10.1016/B978-0-12-405914-6.00011-1
103. Zhao Y, Chen F, Li Q, Wang L, Fan C. Isothermal Amplification of Nucleic Acids. Chem Rev (2015) 115(22):12491–545. doi: 10.1021/acs.chemrev.5b00428
104. Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, et al. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res (2000) 28(12):E63. doi: 10.1093/nar/28.12.e63
105. Wong YP, Othman S, Lau YL, Radu S, Chee HY. Loop-Mediated Isothermal Amplification (LAMP): A Versatile Technique for Detection of Micro-Organisms. J Appl Microbiol (2018) 124(3):626–43. doi: 10.1111/jam.13647
106. Li Y, Fan P, Zhou S, Zhang L. Loop-Mediated Isothermal Amplification (LAMP): A Novel Rapid Detection Platform for Pathogens. Microb Pathog (2017) 107:54–61. doi: 10.1016/j.micpath.2017.03.016
107. Chaouch M. Loop-Mediated Isothermal Amplification (LAMP): An Effective Molecular Point-of-Care Technique for the Rapid Diagnosis of Coronavirus SARS-CoV-2. Rev Med Virol (2021) 31(6):e2215. doi: 10.1002/rmv.2215
108. Dehghan Esmatabadi MJ, Bozorgmehr A, Motalebzadeh H, Bodaghabadi N, Farhangi B, Babashah S, et al. Techniques for Evaluation of LAMP Amplicons and Their Applications in Molecular Biology. Asian Pac J Cancer Prev (2015) 16(17):7409–14. doi: 10.7314/APJCP.2015.16.17.7409
109. Duthie MS, Balagon MF, Maghanoy A, Orcullo FM, Cang M, Dias RF, et al. Rapid Quantitative Serological Test for Detection of Infection With Mycobacterium Leprae, the Causative Agent of Leprosy. J Clin Microbiol (2014) 52(2):613–9. doi: 10.1128/JCM.02085-13
110. Jian L, Xiujian S, Yuangang Y, Yan X, Lianchao Y, Duthie MS, et al. Evaluation of Antibody Detection Against the NDO-BSA, LID-1 and NDO-LID Antigens as Confirmatory Tests to Support the Diagnosis of Leprosy in Yunnan Province, Southwest China. Trans R Soc Trop Med Hyg (2019) 114(3):193–9. doi: 10.1093/trstmh/trz089
111. Leturiondo AL, Noronha AB, do Nascimento MOO, Ferreira CO, Rodrigues FDC, Moraes MO, et al. Performance of Serological Tests PGL1 and NDO-LID in the Diagnosis of Leprosy in a Reference Center in Brazil. BMC Infect Dis (2019) 19(1):22. doi: 10.1186/s12879-018-3653-0
112. Rumondor BB, Prakoeswa AC, Trianita MN, Iswahyudi I, Herwanto N, Listiawan MY, et al. Immunoglobulin AMG Anti Natural Disaccharide Octyl - Leprosy IDRI Diagnostic (NDO-LID) Serologic Test for Leprosy Diagnosis: A Pilot Study. Dermatol Rep (2019) 11(s1):8025. doi: 10.4081/dr.2019.8025
113. Grunwald MH, Amichai B. Dapsone - The Treatment of Infectious and Inflammatory Diseases in Dermatology. Int J Antimicrob Agents (1996) 7(3):187–92. doi: 10.1016/S0924-8579(96)00321-4
114. Krismawati H, Irwanto A, Pongtiku A, Irwan ID, Maladan Y, Sitanggang YA, et al. Validation Study of HLA-B*13:01 as a Biomarker of Dapsone Hypersensitivity Syndrome in Leprosy Patients in Indonesia. PloS Negl Trop Dis (2020) 14(10):e0008746. doi: 10.1371/journal.pntd.0008746
115. World Health Organisation. Recognizing Neglected Tropical Diseases Through Changes on the Skin: A Training Guide for Front-Line Health Workers. (USA:World Health Organisation) (2018).
116. Canci B, Pereira EG, Sakata-So K, Nichiata L. The Development of a Portuguese Mobile Application for Clinical Support in Detecting Leprosy Suspects. Lepr Rev (2021) 92:141–51. doi: 10.47276/lr.92.2.141
117. Baweja HS, Parhar T eds. (2016). Leprosy Lesion Recognition Using Convolutional Neural Networks, in: 2016 International Conference on Machine Learning and Cybernetics (ICMLC), 10-13 July 2016. Proceedings of International Conference on Machine Learning and Cybernetics (ICMLC '16).USA:Institute of Electrical and Electronics Engineers). doi: 10.1109/ICMLC.2016.7860891
118. da Silva YED, Salgado CG, Conde VMG, Conde GAB eds. (2018). Application of Clustering Technique With Kohonen Self-Organizing Maps for the Epidemiological Analysis of Leprosy, in: Proceedings of SAI Intelligent Systems Conference. Cham:Springer. doi: 10.1007/978-3-030-01057-7_24
Keywords: leprosy, diagnostics, PCR, emerging techniques, nucleic acid tests (NAT), serological tests
Citation: Sharma M and Singh P (2022) Advances in the Diagnosis of Leprosy. Front. Trop. Dis 3:893653. doi: 10.3389/fitd.2022.893653
Received: 10 March 2022; Accepted: 11 April 2022;
Published: 07 July 2022.
Edited by:
Manoel Barral-Netto, Gonçalo Moniz Institute (IGM), BrazilReviewed by:
Roberta Olmo Pinheiro, Instituto Oswaldo Cruz (Fiocruz), BrazilGerson Oliveira Penna, Oswaldo Cruz Foundation (Fiocruz), Brazil
Copyright © 2022 Sharma and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pushpendra Singh, UHVzaHBlbmRyYS5TQGljbXIuZ292Lmlu; Mukul Sharma, c211a3VsMjBAZ21haWwuY29t
 Mukul Sharma
Mukul Sharma Pushpendra Singh
Pushpendra Singh