
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Trop. Dis., 18 March 2022
Sec. Vaccines for Tropical Diseases
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.857844
 Taiwo Opeyemi Aremu1,2*
Taiwo Opeyemi Aremu1,2* Oluwafemi Augustine Ajibola3
Oluwafemi Augustine Ajibola3 Oluwatosin Esther Oluwole4
Oluwatosin Esther Oluwole4 Kehinde Oluwatosin Adeyinka5
Kehinde Oluwatosin Adeyinka5 Stephen Oreoluwa Dada6
Stephen Oreoluwa Dada6 Olihe Nnenna Okoro7
Olihe Nnenna Okoro7Growing up in the Malaria-endemic region of sub-Saharan Africa, one becomes inescapably aware of the devastating effect of malaria. From the common experiences of pyrexia, hospital admissions, and daily intramuscular injections to the early childhood trauma of learning that a classmate will not be returning to school because they did not recover from a severe bout the illness, to the sudden, painful loss of loved ones. In many instances of illness, there is an emergency demand for blood transfusion due to severe anemia secondary to malarial infection. Malaria remains a public health threat to sub-Saharan regions of Africa, with enormous health and economic implications, hence the global attention.
Malaria is a vector-borne infectious disease that is caused by Plasmodium species (1). Five species of Plasmodium can infect and be spread by humans - P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi (1). Of the five species, P. falciparum is the protozoan parasite associated with severe disease and accounts for most malaria deaths. Malaria spreads to humans through the bites of infected hosts, the female Anopheles mosquitoes. The sporozoites, which are a motile infective form, are transmitted into the host human. The sporozoites spread through the bloodstream into the liver, where the sporozoites mature into schizonts. The schizonts form in the liver, rupture, and release merozoites into the bloodstream, infecting new red blood cells (blood stage). The parasite in the red blood cells matures into schizonts, which rupture and release merozoites. The erythrocyte/blood stage of the parasites is responsible for the symptoms in individuals infected with malaria. Of note, plasmodium ovale and plasmodium vivax can remain dormant in the liver as hypnozoites which can persist and cause relapses if left untreated.
The transmission/distribution of malaria is mainly dependent on climatic factors, especially in the warmer regions of the tropics (2, 3). Lesser malaria transmission has been established in some tropical and subtropical areas of Africa, including the desserts (except the oases), due to the prevalence of colder seasons, very high altitudes, and successful control/elimination of malaria (3).
Malarial infection can present as asymptomatic or symptomatic, with low to high-grade intermittent fever, headache, chills, and fatigue. Severe cases can result in renal failure, pulmonary edema, acute respiratory distress syndrome (ARDS), jaundice, seizures, coma, or death (4). Children and the elderly are more prone to the complications of malarial infection. Pregnant mothers with malaria can have complications including premature delivery (before 37 weeks of gestation), miscarriages, intrauterine growth restriction (IUGR) of the baby, and low birth weight (LBW) (4).
Sub-Saharan Africa accounts for more than 90% of the world’s deaths due to malaria (5). According to the latest World malaria report, in 2019, there were 229 million malaria cases globally that led to 409,000 deaths (6). Of these deaths, 67 percent (274,000) were children under five years of age, which translates into a daily toll of nearly 750 children under age 5 (6). The under-five mortality rate is devastating. Every two minutes, a child under five dies of malaria. Many of these deaths are preventable. The cost associated with the treatment of malaria and lost labor, in sub-Saharan Africa, is estimated at 12 billion USD every year (7).
The diagnosis of malaria can be made through several means, including clinical diagnosis using symptoms, microscopic examinations in the medical laboratory, and antigen-based rapid diagnostic tests (RDTs) (8). Over the decades, several measures have been implemented for malaria prevention, including vector control with insecticide-treated nets and indoor residual spraying and seasonal malaria prophylactics. Pharmacotherapy, such as the Artemisinin-based combination therapies (ACTs), has been the mainstay for treating uncomplicated and severe P. falciparum malaria, including the symptomatic management of malaria complications. Pharmacotherapy mainly targets the blood stage of the life cycle (9). Despite these management strategies, resistance to the available anti-malaria medications has continued to grow, resulting in more deaths (10).
Due to the unrelenting morbidity and mortality from malaria infections, researchers have sought for more robust preventative measures. Years of clinical trials have led up to the World Health Organization’s (WHO) recent approval of the groundbreaking four-dose malaria vaccine, RTS, S/AS01 (Mosquirix), on October 6, 2021, designed to target the sporozoite (pre-blood) stage of P. falciparum before they infect liver cells (10–12). Preceding this approval, a phase 3 efficacy study in 7 sub-Saharan African countries revealed that the 4-dose schedule vaccine efficacy was 25.9% (95% CI, 19.9 to 31.5) among a population of 6537 children between the ages of 6-12 weeks and 36.3% (95% CI, 31.8 to 40.5) among a population of 8922 children between the ages of 5-17 months with clinical malaria. The vaccine efficacy against severe malaria was 17.3% (95% CI, -9.4 to 37.5) among a population of 6537 children between age of 6 to 12 weeks and 32.2% (95% CI, 13.7 to 46.9) among a population of 8922 children 5 to 17 months of age (8). The newly approved malaria vaccine will be administered intramuscularly. The first three doses have some flexibility in administration, starting at five months and must be completed at nine months, while the fourth dose is expected to be administered at 15-18 months of age.
As a sequel to the varied degree of malaria endemicity and the differing needs, we may expect differing implications of the malaria vaccine across the sub-Saharan Africa regions. Considering the vaccine’s efficacy was tested in seven sub-Saharan African countries (Burkina Faso, Gabon, Ghana, Kenya, Malawi, Mozambique, and the United Republic of Tanzania) while excluding other endemic regions poses some limitations (13). Nigeria, which contributes to about 31.9% of the global malaria deaths, being the highest malaria disease burden, was excluded from the vaccine efficacy testing (14). This narrative, therefore, limits the generalizability of the malaria vaccine efficacy.
The malaria vaccine is undoubtedly a giant innovation with a promising outlook. However, we know that history is replete with examples of novel vaccines met with vaccine hesitancy due to low confidence in the safety and efficacy of the vaccines (15). In the wake of the recent approval, public awareness and perception about the malaria vaccine are scant. For example, the online search for the keyword “Malaria vaccine,” as expressed on google trends, has remained scanty in the last 12 months, with pockets of search in only 8 of the 36 states in Nigeria (Figure 1).
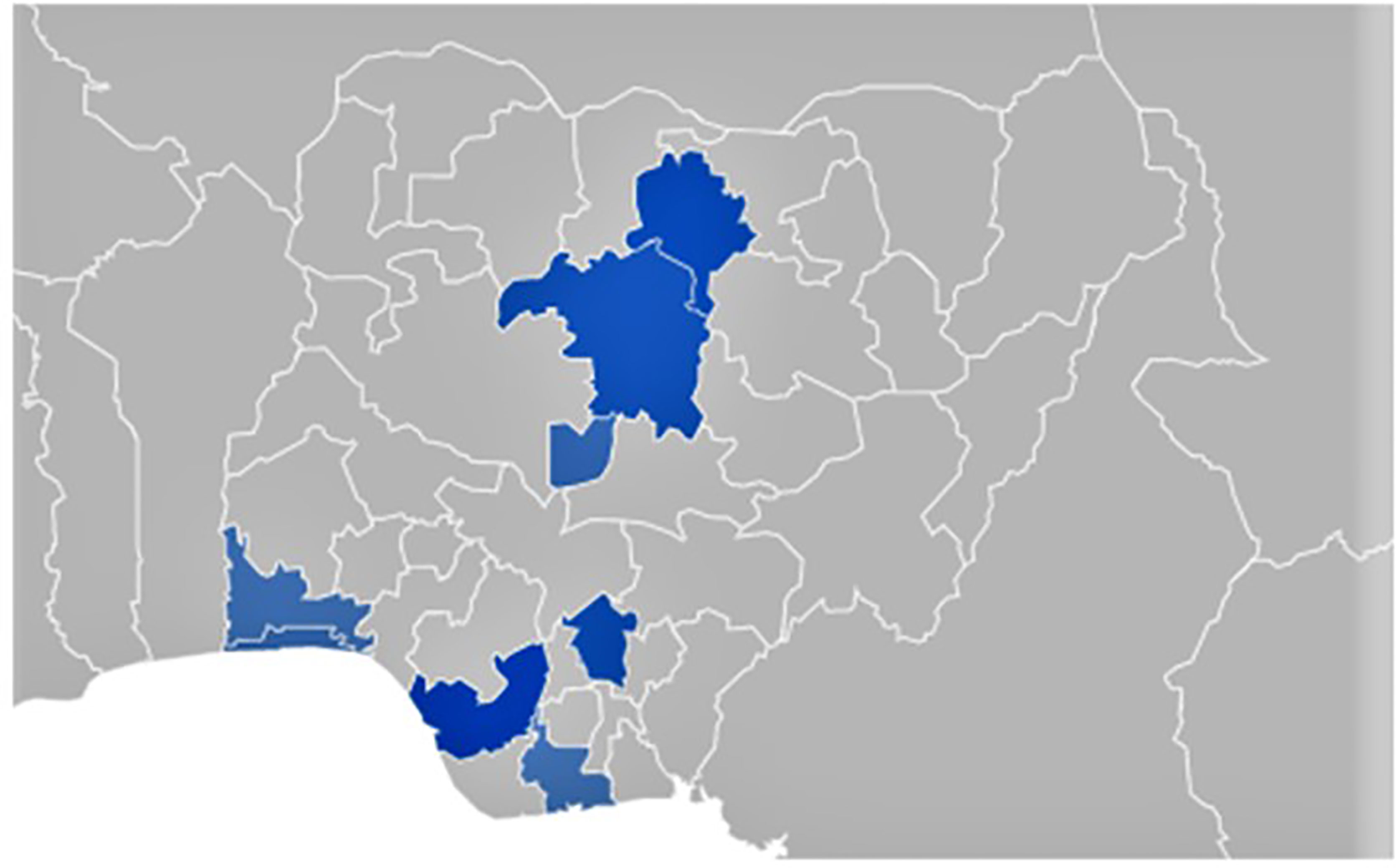
Figure 1 Google trend showed that the keyword “malaria vaccine” was searched online in 8 of 36 states in Nigeria (November 14, 2020 – November 14, 2021).
Considering the knowledge gaps, we recommend initiating studies that assess public awareness and explore perceptions of the malaria vaccine especially around its acceptability. This will inform public health and policy measures targeted at maximizing the health benefit of the malaria vaccine, including health education and promotion. We also recommend that studies be initiated to assess the safety and efficacy of the malaria vaccine in other vulnerable populations like the elderly, pregnant mothers, persons living with sickle cell disease, and immunocompromised individuals. While there are studies that have accessed the prevalence of malaria and challenges with the acceptance of the malaria vaccine before its approval, no study has examined these variables after the vaccine’s approval, further necessitating the need to initiate awareness and acceptability studies.
To address the scourging effect of malaria, we hope that every willing resident of malaria-endemic regions (not only in the tropical areas of Africa but also in other endemic parts of the world), will eventually have the option of being a recipient of the malaria vaccine. Structured public health education and massive campaigns, in collaboration with stakeholders, including community and faith-based leaders, may be resourceful in achieving this feat. The potential to reduce and possibly eliminate the burden of malaria in endemic regions will translate to significant economic gains. Considering that malaria-endemic areas are mainly developing countries, the malaria vaccine, if proven to have sustained efficacy across the life span, will free up resources that can be channeled towards addressing other challenges. The malaria vaccine is a welcome development and efforts must be sustained to ensure that its approval culminates in actual adoption and administration.
Conceptualization, TA, OA, and OEO. Methodology, TA, OA, OEO, and ONO. Writing—original draft preparation, TA and OA. Writing—review and editing, TA, OA, OEO, KA, SD, and ONO. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
P, Plasmodium; ARDS, acute respiratory distress syndrome; IUGR, Intrauterine growth restriction; LBW, Low birth weight; RDT, Rapid diagnostic tests; ACTs, Artemisinin-based combination therapies; WHO, World Health Organization.
1. Siwal N, Singh US, Dash M, Kar S, Rani S, Rawal C, et al. Malaria Diagnosis by PCR Revealed Differential Distribution of Mono and Mixed Species Infections by Plasmodium Falciparum and P. Vivax in India. PloS One (2018) 13(3):e0193046. doi: 10.1371/journal.pone.0193046
2. Caminade C, Kovats S, Rocklov J, Tompkins AM, Morse AP, Colón-González FJ, et al. Impact of Climate Change on Global Malaria Distribution. Proc Natl Acad Sci USA (2014) 111(9):3286–91. doi: 10.1073/pnas.1302089111
3. Centers for Disease Control and Prevention – CDC. Malaria - About Malaria - Where Malaria Occurs (2020). Available at: https://www.cdc.gov/malaria/about/distribution.html (Accessed on 30 January 2022).
4. Malaria - Complications (2018). Available at: https://www.nhs.uk/conditions/malaria/complications/ (Accessed on 16 January 2022). nhs.uk.
5. Centers for Disease Control and Prevention – CDC. Malaria - Malaria Worldwide - Cdc’s Global Malaria Activities - Sub-Saharan Africa (2019). Available at: https://www.cdc.gov/malaria/malaria_worldwide/cdc_activities/sub-saharan_africa.html (Accessed on 16 January 2022).
6. Malaria in Africa - UNICEF DATA. Available at: https://data.unicef.org/topic/child-health/malaria/ (Accessed on 17 January 2022).
7. Greenwood BM, Bojang K, Whitty CJ, Targett GA. Malaria. Lancet (2005) 365(9469):1487–98. doi: 10.1016/S0140-6736(05)66420-3
8. Gitta B, Kilian N. Diagnosis of Malaria Parasites Plasmodium Spp. In Endemic Areas: Current Strategies for an Ancient Disease. BioEssays (2020) 42(1):1900138. doi: 10.1002/bies.201900138
9. Burns AL, Dans MG, Balbin JM, de Koning-Ward TF, Gilson PR, Beeson JG, et al. Targeting Malaria Parasite Invasion of Red Blood Cells as an Antimalarial Strategy. FEMS Microbiol Rev (2019) 43(3):223–38. doi: 10.1093/femsre/fuz005
10. Daily JP. Malaria 2017: Update on the Clinical Literature and Management. Curr Infect Dis Rep (2017) 19(8):28. doi: 10.1007/s11908-017-0583-8
11. World Health Organization – WHO. WHO Recommends Groundbreaking Malaria Vaccine for Children at Risk. Available at: https://www.who.int/news/item/06-10-2021-who-recommends-groundbreaking-malaria-vaccine-for-children-at-risk (Accessed on 14 November, 2021).
12. Laurens MB. RTS,S/AS01 Vaccine (MosquirixTM): An Overview. Hum Vaccin Immunother (2019) 16(3):480–9. doi: 10.1080/21645515.2019.1669415
13. World Health Organization – WHO. Malaria: Phase 3 Trial Results for Vaccine RTS,S/As01. Available at: https://www.who.int/news-room/questions-and-answers/item/phase-3-trial-results-for-malaria-vaccine-rtss-as01 (Accessed on 01 February 2022).
14. World Health Organization – WHO. Available at: https://www.who.int/news-room/fact-sheets/detail/malaria (Accessed on 01 February 2022).
Keywords: malaria, malaria vaccine, sub-Saharan Africa, antimalaria, infectious disease, RTS,S/AS01 vaccine vaccine, vaccine efficacy
Citation: Aremu TO, Ajibola OA, Oluwole OE, Adeyinka KO, Dada SO and Okoro ON (2022) Looking Beyond the Malaria Vaccine Approval to Acceptance and Adoption in Sub-Saharan Africa. Front. Trop. Dis 3:857844. doi: 10.3389/fitd.2022.857844
Received: 19 January 2022; Accepted: 10 February 2022;
Published: 18 March 2022.
Edited by:
Matthew B. Laurens, University of Maryland, United StatesReviewed by:
Collins Ouma, Maseno University, KenyaCopyright © 2022 Aremu, Ajibola, Oluwole, Adeyinka, Dada and Okoro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taiwo Opeyemi Aremu, YXJlbXUwMDZAdW1uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.