
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 11 March 2022
Sec. Tropical Disease Epidemiology and Ecology
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.846955
This article is part of the Research Topic Insights In Tropical Diseases: 2021 View all 6 articles
 Guillermo A. García1*
Guillermo A. García1* Godwin Fuseini1
Godwin Fuseini1 Jose Antonio Mba Nlang1
Jose Antonio Mba Nlang1 Valeriano Olo Nsue Maye1
Valeriano Olo Nsue Maye1 Nestor Rivas Bela1
Nestor Rivas Bela1 Rachel N. Wofford2
Rachel N. Wofford2 Thomas A. Weppelmann3
Thomas A. Weppelmann3 Graham Matulis2
Graham Matulis2 Prudencio Bibang Efiri1
Prudencio Bibang Efiri1 Jordan M. Smith1
Jordan M. Smith1 Matilde Riloha Rivas4
Matilde Riloha Rivas4 Wonder Philip Phiri1
Wonder Philip Phiri1 Michael E. von Fricken2
Michael E. von Fricken2Background: In 2015 and 2016, the Bioko Island Malaria Control Project (BIMCP) introduced a pilot larvicide program, which recruited local volunteers to assess the sustainability and effectiveness of community-led larval source management. This study evaluates the effectiveness of the community-led LSM program to determine if this type of intervention could be used as a sustainable malaria control method on Bioko Island.
Methods: The pilot program was split into two phases, both taking place between February and December, with phase I in 2015 and phase II in 2016. During phase I, the BIMCP team assisted in identifying and treating Anopheles species mosquito breeding habitats. During phase II, community volunteers, with supervision from designated community leaders, identified and treated breeding habitats. Larval source management took place at thirteen locations around the Island during both phases. Human landing catches were conducted at seven sentinel sites once every month for the duration of the study period to determine average nightly biting rates.
Results: During phase I, 1,033 breeding sites were identified with a 100% treatment coverage rate. Only 970 breeding sites were identified in phase II with a 75% treatment coverage rate, a significant decrease from phase I (p<0.001). Between phase I and phase II, larvicide usage also decreased by 45% (95% CI: 32, 59%, p=0.003). However, excluding the sentinel site Balboa, vector density showed a nonsignificant (p=0.272) relationship between phase I and phase II.
Conclusion: Overall, community-based larval source management can be effective with strong operational management and oversight. However, repeated training and evaluation will be necessary to monitor the effectiveness and sustainability of such interventions.
Over the past decade, malaria control programs have made significant strides in reducing malaria morbidity and mortality (1) through the use of indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) (2–4). These control measures have prevented an estimated 663 million malaria cases between 2000 and 2015 (5, 6). Since 2004, the Bioko Island Malaria Control Project (BIMCP) has substantially reduced the burden of malaria on Bioko Island, Equatorial Guinea (7–10). The BIMCP has relied on IRS campaigns and mass distributions of LLINs, with IRS coverage rates between 80% and 92% in recent spray campaigns (10, 11). Despite significant success in decreasing the parasite prevalence in 2-14-year-old children from 45% in 2004 to 11.1% in 2016, at the time of this study, malaria remains a significant health concern with year-round transmission (7, 8).
Through sustained vector control, both An. funestus and S form An. gambiae s.s. populations were eliminated from the Island, leaving An. coluzzii and An. melas as the primary vector species (8, 12). Outdoor host-seeking behaviors within these mosquito populations have increased from 58.8% in 2009 to 70% in 2014 (12). Insecticide resistance has also posed a threat to the efficacy of IRS and LLINs on Bioko Island, where mosquito mortality rates from deltamethrin dropped from 97% in 2013 to 38% in 2016 (10, 13–15). The increasing threat of insecticide resistance and changes in biting behavior of malaria mosquito vectors have led to a renewed interest in larval source management (LSM) as a supplement to core vector control strategies (4, 8, 12, 16–19). The primary goal of LSM is to decrease the number of pupae and larval instars that can develop into adult mosquitoes through habitat modification, biological control, and applying larvicides like Bacillus thuringiensis israeliensis (Bti) (4). Deploying Bti as a larvicide costs roughly one-third of the annual per capita cost for anti-malaria drugs and LLINs while also reducing densities of indoor and outdoor biting mosquitoes and other secondary vectors (4, 20).
In 2015, the BIMCP introduced a pilot Bti larviciding initiative that trained local communities to identify breeding sites and deploy LSM treatments. The objective of this study was to measure the effectiveness of the community-led LSM program compared to the BIMCP managed LSM program on Bioko Island.
This study was conducted on Bioko Island, Equatorial Guinea, located 32 km off the coast of Cameroon, with a population of approximately 270,000 individuals. Between 2015 and 2016, the BIMCP conducted a two-phase pilot community-based larviciding program. This study took place at thirteen locations across Bioko Island: Arena Blanca, Balboa, Basacato del Oeste, Basupu, Bayong, Biabia, Baloco, Chechenia, Fortuni, Long Street, Luba Ciudad, Mongola, and Cacahual (Figure 1). Twelve of the locations were located along the North to Northwestern coast of Bioko Island, Balboa was the only location situated on the Eastern coastline. Due to the BIMCP’s established presence within the communities, these sites were selected. Phase I occurred between February to December in 2015 and phase II between the same months in 2016.
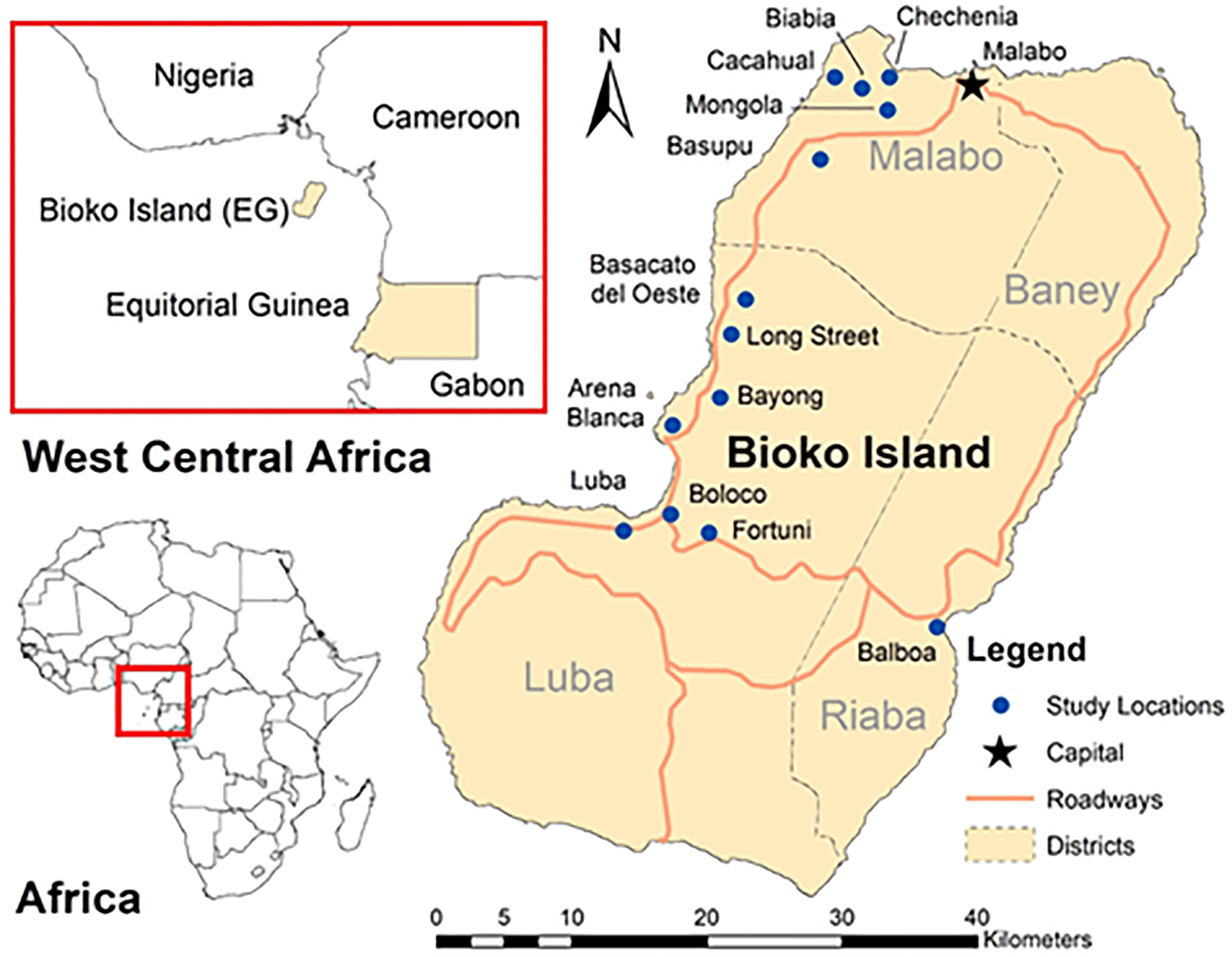
Figure 1 Map of Bioko Island with study locations. A map of Bioko Island, Equatorial Guinea is presented with the geographic relation to the region of West Central Africa and African continent along with the capital city of Malabo, major roadways, and administrative districts. The areas with larval source management are presented in blue circles.
A total of ninety-two volunteers were selected to participate in the pilot larviciding program. BIMPC team members took regular attendance of participates during both phases of the pilot program. In phase I of the program, the vector control/entomology team from BIMCP assisted community volunteers in properly deploying LSM. The BIMCP team provided training on proper breeding site identification and treatment and worked alongside volunteers weekly throughout phase I. Additionally, breeding sites missed by volunteers were treated by the BIMCP team. In phase II, volunteers were solely responsible for identifying and treating the breeding sites under the supervision of community leaders. The BIMCP team members, during phase II, continued to monitor the proportion of breeding sites that were correctly identified and treated, marking sites that volunteers missed. The larvicide used during both phases was Bti, which was applied at an application rate of 1 g/m2 (VectoBac® GS, Valent Biosciences, USA).
To measure the program’s impact on vector density, human landing catches (HLC) were conducted in seven communities once every month for the duration of the study period. The seven communities included were Arena Blanca, Balboa, Basupu, Biabia, Luba Ciudad, Mongola, and Basacato del Oeste, which have established HLC sentinel surveillance programs managed by the BIMCP. During phase I, 56 collectors (eight per community) gathered data on HLC. In phase II, 84 collectors (12 per community) were used monthly between February and December for HLC. On each collection night, pairs of trained volunteers would place themselves at a household site within each community. Between 7:00 pm and 6:00 am, one collector would be situated inside a residence, and one collector would be outside the same residence, switching positions at midnight. Collectors exposed their arms and legs and collected any mosquitoes that landed on them. For each collection, the same field supervisors and locations were used. The risks involved with the study were conveyed to HLC collectors, and free diagnosis and treatment was provided to any individual who showed symptoms. Ethical approval for this study was granted by the National Malaria Control Programme (NMCP) of the Ministry of Health and Social Welfare, Equatorial Guinea.
Statistical comparisons between phase I and phase II were conducted for all key variables. The number of breeding sites identified and treated and the proportion of volunteers who attended each month were compared using tests for two proportions. The average quantity of larvicide applied each month was compared using linear regression. The number of mosquitoes that landed on each volunteer per night was calculated as the number of mosquitoes divided by the number of volunteers on that night and expressed as a human biting rate per person. Poisson regressions were used to compare human bites per person between periods, with additional regressions run excluding Balboa data to identify changes in statistical significance without that site. An alpha value of 0.05 was used to denote statistical significance; all analyses were completed in STATA (StataCorp, College Station, Texas, USA).
During phase I of the program, with the assistance of the BIMCP team, a total of 1,033 potential breeding sites were identified and treated with larvicide. Of these sites, 78% (805/1033) were identified and treated by the trained volunteers, with an additional 228 sites identified and treated by the BIMCP team. During phase II of the program, with only the supervision of community leaders, volunteers identified and treated 75% (970/1297) of potential breeding sites, with an additional 327 sites observed by the BIMCP team that were not identified or treated by the volunteers. Compared to phase I, where the treatment of potential breeding sites was 100% (1033/1033), there was a significant decrease (p <0.001) in the treatment of potential breeding sites during phase II to 75% (970/1297). There was also a statistically significant difference between the proportion of potential breeding sites identified by the trained volunteers without the help of the BIMCP team (78% in phase I vs. 75% in phase II; p =0.038) (Table 1).
The average monthly attendance of volunteers during phase I with BIMCP supervision was 87% (80/92), with a slight decrease in attendance to 82% (75/92) of volunteers during phase II. Despite no significant changes in attendance, there was a large difference in the amount of larvicide applied to potential breeding sites throughout the year (Figure 2). Under the supervision of BIMCP during phase I, an average of 580 kg (sd =233 kg) of larvicide was administered per month across the 13 study locations, ranging from 185 to 963 kg. Without the supervision of the BIMCP in phase II, an average of 317 kg (sd =109.2 kg) of larvicide was applied monthly, with a range from 149 to 470 kg per month. This translated to an average difference of 263 kg (95% CI: 185, 339 kg) of larvicide per month, representing a statistically significant (p=0.003) 45% (95% CI: 32, 59%) decrease from phase I. A summary of all variables and test statistics can be found in Table 1.

Figure 2 Quantification of the amount of larvicide used with and without supervision. The amount of larvicide use (kgs) is depicted above by study month capturing differences in community participation and volume of larvicide used between phases. The gray bars represent data collected during phase I in 2015 when direct supervision from the BIMCP was present; the green bars represent data collected during phase II when BIMCP observed but did not aid in the administration of larvicide.
During the first phase with supervised identification and treatment of potential breeding sites, the average human biting rate for each of the volunteers was 3.0 per night, with a standard deviation of 3.5, a minimum of zero, and a maximum of 13.5. During the second phase without BIMCP supervision, the average human biting rate was 4.6 per night, with a standard deviation of 6.2, a minimum of zero, and a maximum of 25.3. Across most sentinel sites, the trends in bites per night were relatively even, except at Balboa and Basacato Oeste (Figure 3). At Balboa, the number of average bites per night was significantly higher (IRR= 2.35, p<0.001) during phase II than in phase I. Because of this discrepancy and the geographic differences associated with Balboa being located on the Southeastern coast, Poisson regression models were run with and without Balboa. Across all sentinel sites, a significant (p<0.001) 55% increase in human biting rates occurred during phase II when compared to phase I (IRR= 1.55; 95% CI IRR: 1.31, 1.83). This relationship remained significant and unchanged (IRR= 1.54; 95% CI IRR: 1.30, 1.82) after adjusting for the effect of the month, a variable which also had an association with the observed human biting rate (p<0.001). However, after removing Balboa from the analysis, a nonsignificant association (p=0.272) between average bites per night during phase I and phase II was observed.

Figure 3 HLC results with and without BIMCP supervision. The average human biting rate per night is presented by study week. Weeks 1-12 represent phase I where supervision and support by the BIMCP team was available to community volunteers. Phase II is represented by weeks 13-24 where community volunteer led LSM efforts with no assistance from the BIMCP team. Poisson regressions were used to determine the relationship between average bites per night during phase I and phase II at each sentinel site and across all seven sites. As Balboa was a clear outlier in the relationship between phase I and phase II, an additional regression was run to determine significance excluding this site.
A correlation was seen between average bites per night and the amount of Bti applied (Figure 4). As the amount of Bti increased, average bites per night decreased across all sentinel sites. Balboa was again an exception to this observation with an observed increase in bites per night with a seemly indifferent amount of Bti applied between phase I and phase II. Figure 4 also demonstrates how, at the majority of sites, a larger amount of Bti applied was seen during phase I with the supervision of the BIMCP team, even though a nonsignificant increase in average bites per night was not experienced with the exclusion of Balboa.

Figure 4 Bti Consumption by sentinel site for both phase I and phase II. The correlation between average bites per night and the amount of Bti applied for the seven sentinel sites individually and together is visualized above. Gray dots correspond to phase I data where volunteers had supervision from the BIMPC team. Green dots correspond to phase II where volunteers alone were responsible for identifying and treating breeding sites.
This study demonstrates the feasibility of community-led LSM as a supplemental vector control strategy on Bioko Island. After one season of supervision, community volunteers were able to identify and treat 78% of breeding habitats, which is commendable. Although there was a decrease in attendance, a lower amount of larvicide applied, and a corresponding increase in the observed biting rate, we believe there is a role for community-based larval source management on Bioko Island. In addition, community awareness of malaria and mosquito breeding sites likely increased through direct participation, and the intervention was effective at identifying a large number of sites.
In a study by Ingabire et al., community acceptance and participation in LSM programs was related to their knowledge of malaria and Bti safety (21). Although our study did not collect volunteer knowledge of malaria or LSM, further education and training about malaria transmission and the use of Bti may result in higher acceptance and participation in a community-based LSM program. Additionally, adequate training has been linked to a community’s ability to successfully manage malaria vector populations through LSM. However, community volunteers were less effective in monitoring and managing breeding habitats (22), which was also observed in our study. In general, rural areas implementing LSM strategies will face more challenges given the volume of breeding habitats, with LSM most effective when most habitats can be tracked and treated (4).
LSM has shown to be an effective measure in controlling the malaria vector mosquitoes in sub-Saharan Africa (4), many of which have implemented community-based LSM with varying degrees of success in decreasing vector prevalence (17, 18, 21, 23, 24). Based on this pilot study, community-based LSM showed no significant difference from the control efforts provided by BIMCP when Balboa was excluded. Further research is needed to identify why the discrepancy occurred at Balboa to understand what factors influence the increase in the average bite per night rate during phase II. In this study, we were unable to compare community-led LSM efforts to a no-intervention control due to ethical concerns, resulting in a limitation of our ability to quantify the overall impact of LSM on biting rate and mosquito density. However, based on the nonsignificant relationship between average bites per night during phase I and phase II, community-led LSM can result in similar prevention efforts compared to BIMCP-led LSM efforts.
Although they were less effective at identifying sites without the assistance of the BIMCP, community members were eager to participate in managing larval sources and agreeable to future interventions. While perhaps one season of training and direct supervision is not enough time to become as reliable at locating sites as trained professionals, we are hopeful that such efforts will empower communities to continue towards a sustainable larval source management program. Therefore, future Bioko Island community-based LSM programs will require period training and continued supervision while still allowing communities to engage and take ownership of local vector control initiatives.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
GG, GF, and MV contributed conceptualization, study design, and wrote original draft. GG, GF, JM, VN, NB, PE, JS, MR, and WP contributed to study implementation, data management, and oversight. RW, TW, GM, and MV conducted statistical analysis, data visualization, and editing. All authors contributed to writing and of final submission.
Funding for this study was provided by the Bioko Island Malaria Control Project (BIMCP), now the Bioko Island Malaria Elimination Project, a private-public partnership between oil companies (Marathon Oil, Noble Energy, AMPCO, SONAGAS) and the Government of Equatorial Guinea.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a shared affiliation with one of the authors, TW, at time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This work was supported by the National Malaria Control Program and the Ministry of Health and Social Welfare of Equatorial Guinea, and Medical Care Development International (MCDI) through the Bioko Island Malaria Control Project (BIMEP). In particular, we thank the BIMCP staff and community members for their efforts in implementing larval source management activities on Bioko Island. Finally, we would like to thank Marathon Oil, Noble Energy, AMPCO (Atlantic Methanol Production Company), and the Ministry of Mines and Energy of Equatorial Guinea for their continued efforts and support for malaria control on Bioko Island.
2. Fillinger U, Lindsay SW. Larval Source Management for Malaria Control in Africa: Myths and Reality. Malar J (2011) 10:353. doi: 10.1186/1475-2875-10-353
3. Sherrard-Smith E, Skarp JE, Beale AD, Fornadel C, Norris LC, Moore SJ, et al. Mosquito Feeding Behavior and How It Influences Residual Malaria Transmission Across Africa. Proc Natl Acad Sci USA (2019) 116(30):15086–95. doi: 10.1073/pnas.1820646116
4. Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito Larval Source Management for Controlling Malaria. Cochrane Database Syst Rev (2013) 2013(8):Cd008923. doi: 10.1002/14651858.CD008923.pub2
5. Kleinschmidt I, Sharp B, Benavente LE, Schwabe C, Torrez M, Kuklinski J, et al. Reduction in Infection With Plasmodium Falciparum One Year After the Introduction of Malaria Control Interventions on Bioko Island, Equatorial Guinea. Am J Trop Med Hyg (2006) 74(6):972–8. doi: 10.4269/ajtmh.2006.74.972
6. Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The Effect of Malaria Control on Plasmodium Falciparum in Africa Between 2000 and 2015. Nature (2015) 526(7572):207–11. doi: 10.1038/nature15535
7. Cook J, Hergott D, Phiri W, Rivas MR, Bradley J, Segura L, et al. Trends in Parasite Prevalence Following 13 Years of Malaria Interventions on Bioko Island, Equatorial Guinea: 2004-2016. Malar J (2018) 17(1):62. doi: 10.1186/s12936-018-2213-9
8. Overgaard HJ, Reddy VP, Abaga S, Matias A, Reddy MR, Kulkarni V, et al. Malaria Transmission After Five Years of Vector Control on Bioko Island, Equatorial Guinea. Parasit Vectors (2012) 5:253. doi: 10.1186/1756-3305-5-253
9. Kleinschmidt I, Schwabe C, Benavente L, Torrez M, Ridl FC, Segura JL, et al. Marked Increase in Child Survival After Four Years of Intensive Malaria Control. Am J Trop Med Hyg (2009) 80(6):882–8. doi: 10.4269/ajtmh.2009.80.882
10. Fuseini G, Nguema RN, Phiri WP, Donfack OT, Cortes C, Von Fricken ME, et al. Increased Biting Rate of Insecticide-Resistant Culex Mosquitoes and Community Adherence to IRS for Malaria Control in Urban Malabo, Bioko Island, Equatorial Guinea. J Med Entomol (2019) 56(4):1071–7. doi: 10.1093/jme/tjz025
11. Fuseini G, Ismail HM, von Fricken ME, Weppelmann TA, Smith J, Ellis Logan RA, et al. Improving the Performance of Spray Operators Through Monitoring and Evaluation of Insecticide Concentrations of Pirimiphos-Methyl During Indoor Residual Spraying for Malaria Control on Bioko Island. Malar J (2020) 19(1):35. doi: 10.1186/s12936-020-3118-y
12. Meyers JI, Pathikonda S, Popkin-Hall ZR, Medeiros MC, Fuseini G, Matias A, et al. Increasing Outdoor Host-Seeking in Anopheles Gambiae Over 6 Years of Vector Control on Bioko Island. Malar J (2016) 15:239. doi: 10.1186/s12936-016-1286-6
13. N'Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced Efficacy of Insecticide-Treated Nets and Indoor Residual Spraying for Malaria Control in Pyrethroid Resistance Area, Benin. Emerg Infect Dis (2007) 13(2):199–206. doi: 10.3201/eid1302.060631
14. Coleman M, Hemingway J, Gleave KA, Wiebe A, Gething PW, Moyes CL. Developing Global Maps of Insecticide Resistance Risk to Improve Vector Control. Malar J (2017) 16(1):86. doi: 10.1186/s12936-017-1733-z
15. Fuseini G, Phiri WP, von Fricken ME, Smith J, Garcia GA. Evaluation of the Residual Effectiveness of Fludora Fusion WP-SB, a Combination of Clothianidin and Deltamethrin, for the Control of Pyrethroid-Resistant Malaria Vectors on Bioko Island, Equatorial Guinea. Acta Trop (2019) 196:42–7. doi: 10.1016/j.actatropica.2019.05.006
16. Dambach P, Baernighausen T, Traoré I, Ouedraogo S, Sié A, Sauerborn R, et al. Reduction of Malaria Vector Mosquitoes in a Large-Scale Intervention Trial in Rural Burkina Faso Using Bti Based Larval Source Management. Malar J (2019) 18(1):311. doi: 10.1186/s12936-019-2951-3
17. Fillinger U, Lindsay SW. Suppression of Exposure to Malaria Vectors by an Order of Magnitude Using Microbial Larvicides in Rural Kenya. Trop Med Int Health (2006) 11(11):1629–42. doi: 10.1111/j.1365-3156.2006.01733.x
18. Geissbuhler Y, Kannady K, Chaki PP, Emidi B, Govella NJ, Mayagaya V, et al. Microbial Larvicide Application by a Large-Scale, Community-Based Program Reduces Malaria Infection Prevalence in Urban Dar Es Salaam, Tanzania. PloS One (2009) 4(3):e5107. doi: 10.1371/journal.pone.0005107
19. Govella NJ, Ferguson H. Why Use of Interventions Targeting Outdoor Biting Mosquitoes Will be Necessary to Achieve Malaria Elimination. Front Physiol (2012) 3:199. doi: 10.3389/fphys.2012.00199
20. Dambach P, Schleicher M, Stahl HC, Traore I, Becker N, Kaiser A, et al. Routine Implementation Costs of Larviciding With Bacillus Thuringiensis Israelensis Against Malaria Vectors in a District in Rural Burkina Faso. Malar J (2016) 15(1):380. doi: 10.1186/s12936-016-1438-8
21. Ingabire CM, Hakizimana E, Rulisa A, Kateera F, Van Den Borne B, Muvunyi CM, et al. Community-Based Biological Control of Malaria Mosquitoes Using Bacillus Thuringiensis Var. Israelensis (Bti) in Rwanda: Community Awareness, Acceptance and Participation. Malar J (2017) 16(1):399. doi: 10.1186/s12936-017-2046-y
22. Vanek MJ, Shoo B, Mtasiwa D, Kiama M, Lindsay SW, Fillinger U, et al. Community-Based Surveillance of Malaria Vector Larval Habitats: A Baseline Study in Urban Dar Es Salaam, Tanzania. BMC Public Health (2006) 6:154. doi: 10.1186/1471-2458-6-154
23. Chaki PP, Kannady K, Mtasiwa D, Tanner M, Mshinda H, Kelly AH, et al. Institutional Evolution of a Community-Based Programme for Malaria Control Through Larval Source Management in Dar Es Salaam, United Republic of Tanzania. Malar J (2014) 13:245. doi: 10.1186/1475-2875-13-245
Keywords: larval source management, vector control, community-based program, entomological monitoring, Bioko island, Anopheles
Citation: García GA, Fuseini G, Mba Nlang JA, Nsue Maye VO, Bela NR, Wofford RN, Weppelmann TA, Matulis G, Efiri PB, Smith JM, Rivas MR, Phiri WP and von Fricken ME (2022) Evaluation of a Multi-Season, Community-Based Larval Source Management Program on Bioko Island, Equatorial Guinea. Front. Trop. Dis 3:846955. doi: 10.3389/fitd.2022.846955
Received: 31 December 2021; Accepted: 28 January 2022;
Published: 11 March 2022.
Edited by:
Edwin Michael, University of South Florida, United StatesReviewed by:
William Hawley, Centers for Disease Control and Prevention (CDC), United StatesCopyright © 2022 García, Fuseini, Mba Nlang, Nsue Maye, Bela, Wofford, Weppelmann, Matulis, Efiri, Smith, Rivas, Phiri and von Fricken. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guillermo A. García, Z2dhcmNpYUBtY2Qub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.