- 1Infectious Disease Unit, Directorate of Child Health, Komfo Anokye Teaching Hospital, Kumasi, Ghana
- 2Department of Child Health, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana
- 3Directorate of Surgery, Komfo Anokye Teaching Hospital, Kumasi, Ghana
- 4Directorate of Obstetrics and Gynaecology, Komfo Anokye Teaching Hospital, Kumasi, Ghana
- 5Laboratory of Medical Microbiology, Faculty of Medicine and Health Science, University of Antwerp, Antwerp, Belgium
- 6Public Health Unit, Komfo Anokye Teaching Hospital, Kumasi, Ghana
Background: The Global Point Prevalence Study (PPS) provides a platform for institutions to register and add clinical information on antimicrobial usage and determine variables related to proper antimicrobial stewardship.
Objective: To assess the trends in antimicrobial usage and quality indicators in antimicrobial prescriptions at our hospital.
Method: We retrospectively compared data collected at Komfo Anokye Teaching Hospital (KATH) during the Global PPS in 2015 and 2019. Both surveys took place on a day in September of the respective year. Medical records of all in-patients on admission at 0800 hours on a specific day were reviewed for antimicrobial use in the survey. Data on antibiotic use, including indications for use and the presence of quality indicators, were recorded.
Results: The total number of patients on admission in 2015 and 2019 were 386 and 630, respectively. The proportion of patients on at least one antimicrobial was 64% (247/386) and 58.4% (368/630) in 2015 and 2019, respectively. Pneumonia was the most common medical condition for which antimicrobial was prescribed for 2015, 30(16.9%) and 2019, 44(23.0%), respectively. There was a decrease in Hospital-acquired infections from 2015, 6.2% (24/386) to 4.8% (30/630) in 2019. The use of biomarkers increased from 4.9% (12/247) to 7.6% (28/368).
Conclusion: Over 50% of hospitalised patients were on antimicrobials for both years. The inauguration of the antimicrobial stewardship committee at KATH will improve these quality indicators.
Introduction
The Global Point Prevalence Survey (Global-PPS) on antimicrobial consumption and resistance provides institutions with clinical information on antimicrobial usage and quality indicators on appropriate antimicrobial prescribing.
The platform has allowed individual institutions to benchmark their antimicrobial use with merged results for the country, their region and Europe.
The irrational use of antimicrobials and lack of development of new drug molecules have precipitated microorganisms’ antimicrobial resistance (AMR) (1). Therefore, to tackle this rise in AMR, the World Health Organization (WHO) advocates the adoption of antimicrobial stewardship by healthcare providers to monitor and reduce the burden of AMR (2). It is important to note that the unavailability of antibiotics in a country can also play a role in appropriate prescribing behaviour (3).
Using antimicrobials at the right time, for the proper purpose, and at the appropriate doses reduces mortality and morbidity. Antimicrobials have been widely abused for viral conditions such as covid19 and respiratory viral infections, marked by seasonal changes (4).
The decision to give antimicrobial is often clinical, but the willingness to stop after more evidence against their use is lacking. Factors influencing the prescribing of antimicrobials include clinicians’ personal experience with medications, evidence-based scientific publication, recent influence by pharmaceutical presentation, and locally or internationally validated antimicrobial sensitivity patterns (5).
Biomarkers to determine when to start or stop antimicrobial is increasingly relevant in countries where poorly regulated drug authorities make it easy for anyone to get prescription-only medications at pharmacy shops without proper authorisation. Some patients reporting to the health facility might have had access to multiple medications over-the-counter (OTC). Similarly, patients reporting to community facilities are prescribed antimicrobials without prior investigations. When expected responses are suboptimal, patients are referred to a higher level where clinicians are in a dilemma on what to do next without some baseline investigations. When investigations for cultures are done after prior antimicrobials use, it negatively affects the microbiological yield.
The affordability and availability of biomarkers make it difficult for those in need to benefit. Biomarkers such as procalcitonin and C-reactive proteins, vital biomarkers for infections, cost 50-60 dollars per test in Ghana. With an average daily minimum wage of 2 dollars, most patients cannot afford it (6). There are instances where families’ ability to mobilise resources delays getting the test done. This situation drives clinicians to prescribe second-and third-line antimicrobials blindly. Aside from adding to cost, there is also prolonged hospital stay, exposure to healthcare-associated infections and adverse effects of medication.
Before the introduction of antimicrobial stewardship, Komfo Anokye Teaching Hospital (KATH), with the child health directorate, had participated in the “Antibiotic Resistance and Prescribing in European Children Point Prevalence Survey” (ARPEC-PPS) (7). Next, two full-hospital Global-PPS were conducted in 2015 (8) and 2019. This study aimed to review the trends in hospital-wide antimicrobial usage and quality indicators on antimicrobial prescribing habits, using survey data collected in 2015 and 2019 at KATH.
Methodology
Study Setting
The studies took place at Komfo Anokye Teaching hospital (KATH). KATH is a 1,200 bed-capacity hospital located in the Ashanti Region of Ghana. It is a tertiary health care facility serving Ghana’s middle and northern zones.
Komfo Anokye Teaching Hospital took part in the Global-PPS in 2015 and 2019, involving the whole hospital, including all adults, children and neonates.
Study Procedure
The Global-PPS protocol and paper data collection templates were freely available at www.global-pps.com. Both surveys took place on a day in September of the respective year. All in-patients occupying a bed at 8 am on the survey day were included. They were counted at ward level and served as denominators noted down on the ward paper form. Medical records of patients admitted to the ward and on antimicrobials at 8 am on the survey day were extracted and recorded on the patient paper form. Variables in this study included gender, age, type of antimicrobial, route of administration, reasons for use, presence of active community- or healthcare-associated infections (HAI) versus prophylactic prescribing, and results of routine microbiology tests performed. According to the Global-PPS protocol, HAI were defined as infections whereby symptoms started 48 hours after admission to the hospital. Variables included further a set of antimicrobial quality indicators such as whether the reason for the prescription and a stop/review date was written in the patient notes, whether local antibiotic prescribing guidelines existed, and compliance with these guidelines. Full details of the methodology employed are available elsewhere (8).
KATH has log-in details for accessing the Global-PPS web-based data entry repository. Information was then entered in a freely available web-based Global-PPS tool designed explicitly for data entry, validation and reporting and available from www.global-pps.com.
Data Analysis and Statistics
After extracting the data in Excel from the Global-PPS web-based data repository for KATH during the 2015 and 2019 surveys, variables of interest for the respective years were analysed.
Variables were represented as absolute numbers using Pivot Tables in Excel 2010 and percentages of total numbers of patients on antibiotics in respective years.
Ethical Approval
The study received approval from Komfo Anokye Teaching Hospital Institutional review board (IRB) number CHRPE/AP/523/19.
Results
The total number of patients on admission in 2015 and 2019 on the survey day were 386 and 630, respectively. The proportion of patients on at least one antimicrobial was 64% (247/386) and 58.4% (368/630) in 2015 and 2019.
Table 1 shows the Directorate’s data on bedspace, admissions, and antimicrobial uses during the 2015 and 2019 surveys. Aside from the neonatal unit, there was an increase in bedspace, patients admissions, and patients treated with antimicrobials in all other directorates. Whilst the children’s wards saw an increase in antimicrobial use, there was a downwards trend in the other directorates between the two surveys. The neonatal medical unit had the highest antimicrobial use, 68.4%.
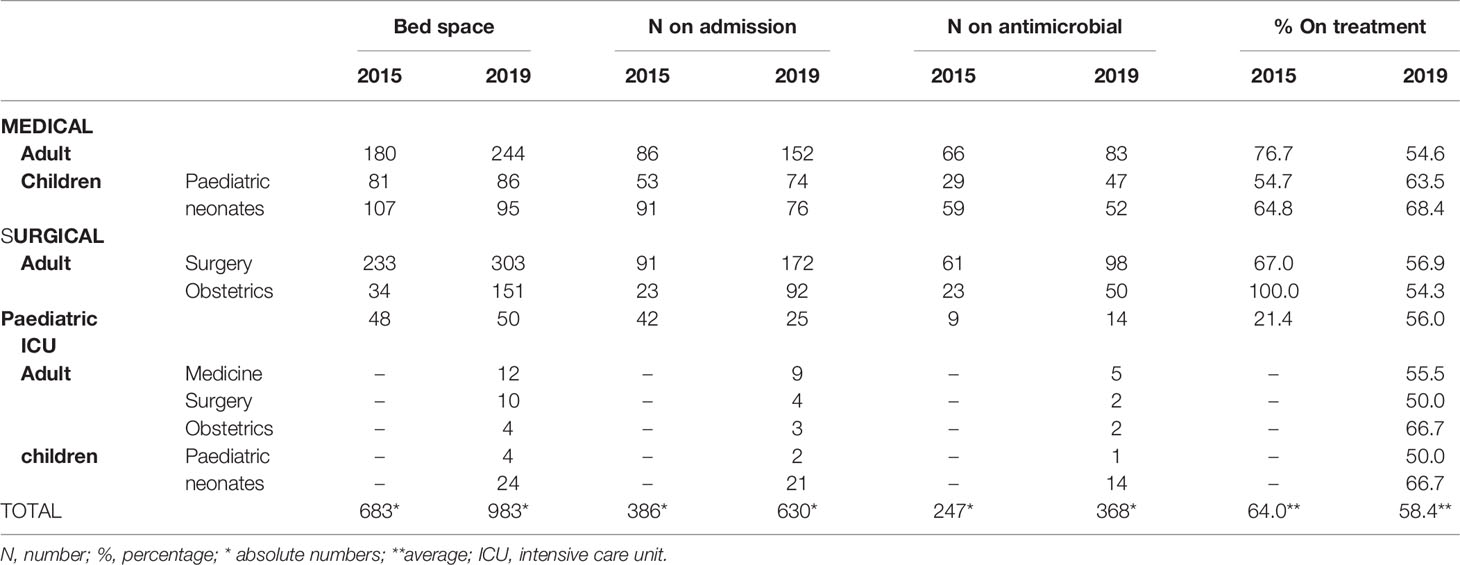
Table 1 Comparing Directorates data on bedspace, admissions, and antimicrobial uses during the 2015 and 2019 survey.
Table 2 shows the distribution of the top ten (10) medical conditions for which antimicrobials were prescribed in 2015 and 2019 at KATH. Pneumonia was the most common medical condition for which antimicrobial was prescribed in 2015, 30 (16.9%) and 2019, 44 (23.0%), respectively. Tuberculosis was the seventh and third medical condition with prescribed antimicrobial in 2015 and 2019. HIV was not amongst the top ten (10) in the 2015 survey.
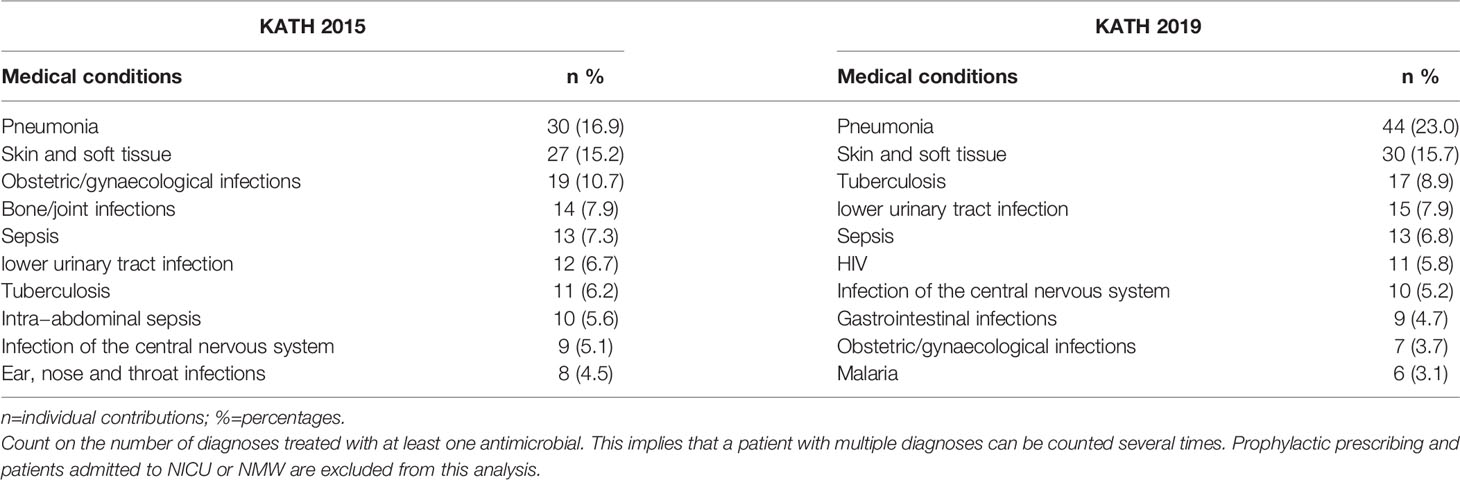
Table 2 Top ten (10) in-hospital medical conditions treated with antimicrobials during the 2015 and 2019 survey.
Of all antimicrobials prescribed in 2015 and 2019 at KATH, 81.0% and 88.1% were antibacterials for systemic use. Amongst all antibacterials for systemic use, other beta-lactams remain the commonest antibiotics prescribed during both study years (40.6% and 36.8%, respectively), represented by the 2nd generation cephalosporin cefuroxime and the 3rd generation cephalosporin ceftriaxone. Quinolones (mainly ciprofloxacin) represented 8.1% and 10.8% in 2015 and 2019. Also, parenteral metronidazole was frequently prescribed (18.6% and 15.1%, respectively), mostly combined with another antibacterial for systemic use (Tables 3, 4). The ratio of Access/Watch prescribing antibiotics (J01) remained the same over time (47% access versus 53% Watch antibiotics).
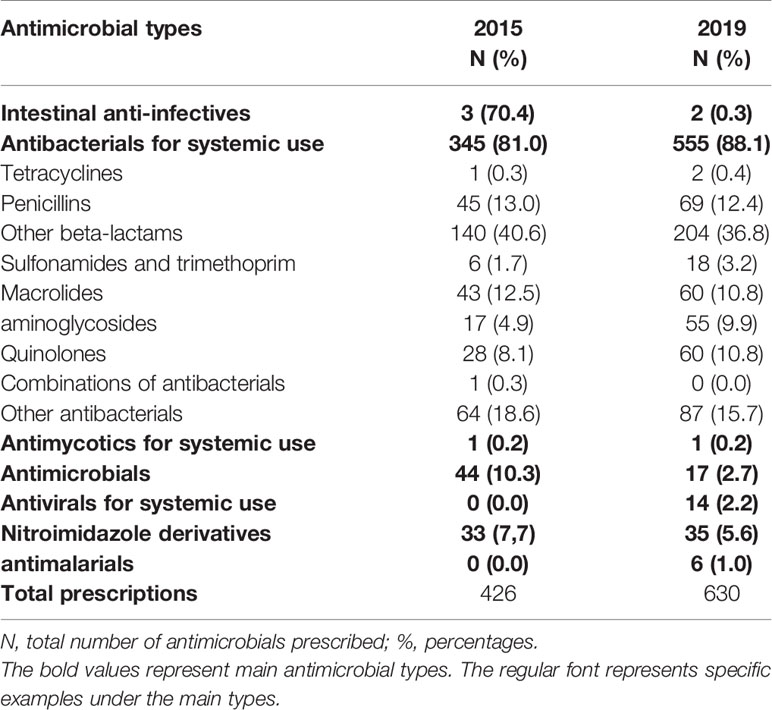
Table 3 Overview of antimicrobials prescribed by antimicrobial therapeutic subgroup in 2015 and 2019.
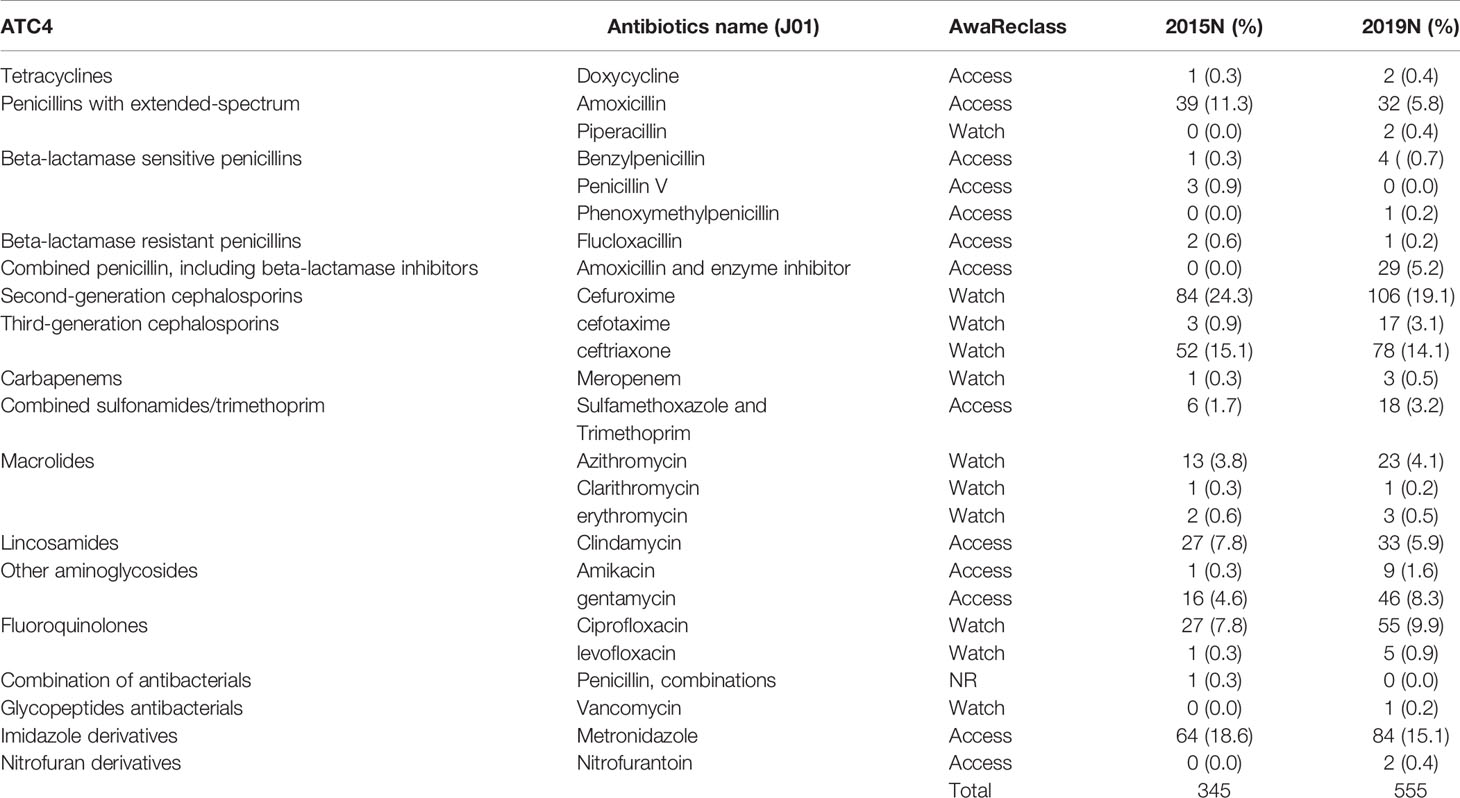
Table 4 Overview antibacterials for systemic use (J01) prescribed by chemical subgroup (ATC4) and the WHO AWaRe classification in 2015 and 2019.
Table 5 shows the quality indicators for antimicrobial prescriptions. Most of the parameters improved from 2015 to 2019. We also observed a decrease in Hospital-acquired infections from 2015, 6.2% (24/386) to 4.8% (30/630) in 2019. Biomarkers in supporting diagnosis increased from 4.9% (12/247) to 7.6% (28/368). Data on cultures, sensitivity or resistance results were not available for extraction.

Table 5 Comparing Quality indicators for antimicrobial prescriptions during the 2015 and 2019 surveys.
Discussion
This study reviewed antimicrobial usage and quality indicators in a single tertiary care facility during two different time points, 2015 and 2019.
Komfo Anokye Teaching hospital had seen a significant expansion between 2015 and 2019, as observed by the increase in bedspace between the two surveys (from 683 to 983). The estimated bed capacity of the hospital, however, is around 1,200. There was also a difference in the supply of ward specialities. Intensive care (ICU) beds were only included in the 2019 survey because ICU beds were not yet available in the hospital in 2015. The bedspace for the neonatal medical unit was reduced in 2019 because space was created out of the total medical bedspace for the neonatal ICU. The KATH neonatal unit serves not only the Ashanti region but ten (10) out of 16 other Regions in Ghana.
For each time point, more than 50% of patients on admission were on at least one antimicrobial, even though the percentage was lower in 2019 (58.4%) compared to 2015 (64.0%). A similar trend was found in a study in the Ho Teaching Hospital in Ghana, where the prevalence of patients on at least one antimicrobial had reduced from 66.7% in July 2019 to 54.9% in January 2020, which they attributed to the introduction of antimicrobial stewardship interventions within the six months (9). Over the four years in our study, AMS interventions were not introduced. We estimate that the difference in the supply of ward specialities over time partly contributed to the different overall antimicrobial use prevalence in KATH.
The neonatal unit had the highest antimicrobial usage because of the unit’s high referrals of critically ill patients. On the other hand, we saw a halving of antimicrobial use (AMU) prevalence among women admitted for obstetric reasons (from 100% in 2015 to 54.3% in 2019). Unlike neonatal units exclusively found only at KATH, many hospitals (public and public) are dotted all over the region with expertise in managing obstetric cases.
The most prescribed antibacterials for systemic use over time were “other beta-lactams” cefuroxime and ceftriaxone. Our study over time showed similar results as compared to pooled results of a multi-centre study in Ghana at seven sentinel sites where the top-five antibiotics used consisted of metronidazole (20.6%), cefuroxime (12.9%), ceftriaxone (11.8%), amoxicillin/clavulanic acid (8.8%) and ciprofloxacin (7.8%). This study consisted of 4 teaching hospitals (including the current study site), two regional Hospitals, and one district hospital (10). In another study done in 2019 in 3 district hospitals in the same region as this tertiary facility, antibiotic agents used included a strikingly higher use of amoxicillin (36.5%), penicillin with extended-spectrum belonging to the WHO Access class (beside ciprofloxacin (17.4%), ceftriaxone (11.3%), cefuroxime (9.6%) and ampicillin (7.8%) (11). In our study, amoxicillin use decreased considerably over time from 11.3% in 2015 to 5.8% in 2019. KATH serves as the only major referral hospital for district hospitals. Therefore, we expect to observe a more broad spectrum of antibiotics prescriptions compared to the referenced publications from district hospitals. The similarities in antimicrobial use may be attributed to KATH serving as a primary care facility for adjourning communities.
Among the medical conditions for which antibacterials were prescribed was pneumonia, followed by skin and soft tissue infections for the 2015 and 2019 surveys. Tuberculosis and HIV were significantly represented in the top ten medical conditions in 2019 compared to 2015. The availability and quality of antiretrovirals in 2019 were better than in 2015. HIV positive persons on antiretrovirals are considered stable individuals living with a chronic medical condition and subject to every environmental condition like HIV negative individuals. HIV positive patients have a significant risk of developing tuberculosis than non-HIV positives (12). This might explain why in 2019, both HIV and TB pooled up among the top ten.
There was an increase in two “Watch” medications on the World Health Organization (WHO) AWaRe classification between 2015 and 2019. Ceftriaxone and ciprofloxacin are “Watch” medications that have high resistance potential (13). Such antibiotics should therefore be selected guided by culture and sensitivity results. However, there was no documentation of positive bacterial growth on cultures in this study. No documentation on culture results might be due to the point prevalence study. The antibiotics prescription based on biomarkers increased from 4.9% in 2015 to 7.6% in 2019. In a multi-centre sentinel study in 2019, 5.2% of antibiotics prescriptions were based on biomarkers (10). The proportion is generally low supporting the use of antibiotics empirically than targeted. The survey results showed that the empiric treatment was 85.4% (356/426) in 2015. This had increased to 98.3% (619/630) in 2019. Antimicrobial stewardship is vital to highlight specimen collection for culture and sensitivity before issuing prescriptions.
There was an increase in parenteral use of antimicrobials from 55.9% in 2015 to 70.5% in 2019, which deserves a deeper investigation. In a National tertiary hospital in Nigeria, a Global PPS study found an increase in parenteral antimicrobials from 70% in 2015 to 82% in 2017 (14). Even though the exact reasons why parenteral antimicrobials increased in the institutions are not clear; both hospitals had not implemented antimicrobial stewardship programme.
There was a decrease in Healthcare-Associated infections (HAI) between the two-time points, from 6.2% in 2015 to 4.8% in 2019. In a multicenter study conducted in Ghana in 2016, the overall HAI prevalence was 8.2%, ranging from 3.5% to 14.4%, with higher proportions of infections in secondary and tertiary care facilities (15). The decrease in trend at KATH could be attributed to a robust infection control and prevention committee which proactively oversees the establishment of systems to control infection spread.
In conclusion, the trends in antimicrobial usage over the four years (2015-2019) remained high, with AMU prevalence over 50%. Encouraging was to observe the increase in the use of biomarkers. We hope to further increase the diagnostic capacity in future and also move towards the possibility of antimicrobial susceptibility testing. The inauguration of the antimicrobial stewardship committee in July 2021 at KATH has been mandated to improve clinical outcomes, optimise patient safety, reduce antimicrobial resistance, and reduce cost by reducing hospital stay through strategic stewardship activities such as stewardship rounds and monitoring of quality indicators for antimicrobial use. After implementing the AMS interventions in February 2022, a new PPS will be performed to investigate the effectiveness of imposed interventions and define adjustments as needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Komfo Anokye Teaching Hospital Institutional review board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
AE, DA, IP, HG and AV developed the concept and contributed to the manuscript write-up. MY, KA, and NM collected the data and contributed to the manuscript write-up. All authors read through and accepted the final version of the manuscript.
Funding
bioMérieux is the sole private sponsor (funder) of the Global Point Prevalence Survey. bioMérieux has no role in study design, data collection, data analysis, data interpretation or writing of the report, which is done under the responsibility of the University of Antwerp. Data are strictly confidential and stored anonymously at the coordinating centre of the University of Antwerp.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge the management of Komfo Anokye Teaching Hospital for this hospital-wide study. We also recognise all health workers who supported data collection on the day of the records review.
References
1. Gould IM, Bal AM. New Antibiotic Agents in the Pipeline and How They can Help Overcome Microbial Resistance. Virulence (2013) 4(2):185. doi: 10.4161/viru.22507
2. No-Time-to-Wait-Securing-the-Future-From-Drug-Resistant-Infections-En.Pdf. Available at: https://www.who.int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.pdf?sfvrsn=5b424d7_6.
3. Tängdén T, Pulcini C, Aagaard H, Balasegaram M, Hara GL, Nathwani D, et al. Unavailability of Old Antibiotics Threatens Effective Treatment for Common Bacterial Infections. Lancet Infect Dis (2018) 18(3):242–4. doi: 10.1016/S1473-3099(18)30075-6
4. Garg SK. Antibiotic Misuse During COVID-19 Pandemic: A Recipe for Disaster. Indian J Crit Care Med Peer-Rev Off Publ Indian Soc Crit Care Med (2021) 25(6):617–9. doi: 10.5005/jp-journals-10071-23862
5. Calbo E, Alvarez-Rocha L, Gudiol F, Pasquau J. A Review of the Factors Influencing Antimicrobial Prescribing. Enferm Infecc Microbiol Clin (2013) 31 Suppl 4:12–5. doi: 10.1016/S0213-005X(13)70127-7
6. Ghana Increases Minimum Wage for 2021 and 2022 . Available at: https://news.bloombergtax.com/payroll/ghana-increases-minimum-wage-for-2021-and-2022.
7. Versporten A, Bielicki J, Drapier N, Sharland M, Goossens H, ARPEC project group. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) Point Prevalence Survey: Developing Hospital-Quality Indicators of Antibiotic Prescribing for Children. J Antimicrob Chemother (2016) 71(4):1106–17. doi: 10.1093/jac/dkv418
8. Versporten A, Zarb P, Caniaux I, Gros M-F, Drapier N, Miller M, et al. Antimicrobial Consumption and Resistance in Adult Hospital in-Patients in 53 Countries: Results of an Internet-Based Global Point Prevalence Survey. Lancet Glob Health (2018) 6(6):e619–29. doi: 10.1016/S2214-109X(18)30186-4
9. Dodoo CC, Orman E, Alalbila T, Mensah A, Jato J, Mfoafo KA, et al. Antimicrobial Prescription Pattern in Ho Teaching Hospital, Ghana: Seasonal Determination Using a Point Prevalence Survey. Antibiotics (2021) 10(2):199. doi: 10.3390/antibiotics10020199
10. Labi A-K, Obeng-Nkrumah N, Dayie NTKD, Egyir B, Sampane-Donkor E, Newman MJ, et al. Antimicrobial Use in Hospitalised Patients: A Multi-Centre Point Prevalence Survey Across Seven Hospitals in Ghana. JAC-Antimicrob Resist (2021) 3(3). doi: 10.1093/jacamr/dlab087
11. Amponsah OKO, Buabeng KO, Owusu-Ofori A, Ayisi-Boateng NK, Hämeen-Anttila K, Enlund H. Point Prevalence Survey of Antibiotic Consumption Across Three Hospitals in Ghana. JAC-Antimicrob Resist (2021) 3(1). doi: 10.1093/jacamr/dlab008
12. Moore D, Liechty C, Ekwaru P, Were W, Mwima G, Solberg P, et al. Prevalence, Incidence and Mortality Associated With Tuberculosis in HIV-Infected Patients Initiating Antiretroviral Therapy in Rural Uganda. AIDS Lond Engl (2007) 21(6):713–9. doi: 10.1097/QAD.0b013e328013f632
13. WHO Releases the 2019 AWaRe Classification Antibiotics . Available at: https://www.who.int/news/item/01-10-2019-who-releases-the-2019-aware-classification-antibiotics.
14. Nwajiobi-Princewill P, Medugu N, Gobel M, Aigbe A, Versporten A, Pauwels I, et al. Using Longitudinal Antibiotic Point Prevalence Survey (PPS) to Drive Antimicrobial Stewardship Programmes in a Nigerian Tertiary Hospital. Afr J Clin Exp Microbiol (2021) 22(2):284–9. doi: 10.4314/ajcem.v22i2.22
Keywords: comparison, antimicrobial, stewardship, global study, tertiary, WHO AWaRe Classification
Citation: Enimil A, Agbedinu K, Yeboah M, Pauwels I, Goossens H, Ansong D, Mensah N and Vesporten A (2022) Comparing Patterns in Antimicrobial Use During Global Point Prevalence Study at a Single Tertiary Hospital in Ghana: Implications for Antimicrobial Stewardship Programme. Front. Trop. Dis 3:843509. doi: 10.3389/fitd.2022.843509
Received: 26 December 2021; Accepted: 27 April 2022;
Published: 07 July 2022.
Edited by:
Anou M. Somboro, University of KwaZulu-Natal, South AfricaReviewed by:
Susan Coffin, University of Pennsylvania, United StatesLuqman Satti, PNS Shifa Teaching Hospital, Pakistan
Copyright © 2022 Enimil, Agbedinu, Yeboah, Pauwels, Goossens, Ansong, Mensah and Vesporten. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony Enimil, dGVuaW1pbEBsaXZlLmNvbQ==
 Anthony Enimil
Anthony Enimil Kwabena Agbedinu
Kwabena Agbedinu Michael Yeboah4
Michael Yeboah4 Nicholas Mensah
Nicholas Mensah Ann Vesporten
Ann Vesporten