- 1Department of Medicine, University of California, San Diego, San Diego, CA, United States
- 2Karius, Inc., Redwood City, CA, United States
- 3Division of Infectious Diseases and Global Public Health, University of California, San Diego, San Diego, CA, United States
- 4Infectious Diseases Section, Veterans Affairs (VA) San Diego Healthcare System, La Jolla, CA, United States
- 5Division of Cardiothoracic Surgery, Sulpizio Cardiovascular Center at the University of California, San Diego, La Jolla, CA, United States
Background: Detection and sequencing of circulating microbial cell-free DNA (mcfDNA) in plasma is an increasingly popular tool for diagnosing many infectious diseases, but could also be used to monitor the progress of infection. However, the decay of this microbial cell-free DNA in blood following treatment has not been previously characterized.
Case Presentation: A 53 year-old male was diagnosed with Bartonella quintana bioprosthetic aortic valve endocarditis by sequencing of the mcfDNA in the blood (Karius, Redwood City, CA). We then monitored the kinetics of decay of mcfDNA after parenteral antibiotics and valve resection in this individual. We measured plasma mcfDNA (Karius) in serial samples obtained in the operating room to calculate mcfDNA half-life after valve resection. After four weeks of parenteral antibiotics, Bartonella mcfDNA signal decreased by 78%. The signal subsequently rose during operative manipulation of the infected valve but dropped 81-fold over four hours following valve resection. The half-life of mcfDNA between the time shortly following resection of the infected valve and 24 to 48 hours post-operatively was between 35 and 115 minutes. The trend in mcfDNA signal was characterized by rapid and then slower phases of decay within 24 hours, and little change between 24 and 48 hours.
Conclusions: This study is one of the first to characterize decay kinetics of mcfDNA and highlights the potential of monitoring mcfDNA in addressing major challenges in infective endocarditis management, including monitoring the response to therapy, and as an early screen for recurrence.
Introduction
Detection and identification of circulating microbial cell-free DNA (mcfDNA) by next-generation sequencing (NGS) in plasma has shown utility in diagnosing multiple types of infections, including endocarditis and other endovascular infections (1–4). Infective endocarditis (IE) is a serious, even life-threatening disease that accounts for up to 50,000 cases per year and with an average inpatient cost per patient of $120,000 in the US alone (5). More than one-third of people with IE die within a year of diagnosis (6). Culture negative endocarditis (CNE), defined as IE without a positive blood culture from at least 3 independent blood samples (7), represents ~20%-70% of cases of IE (8–10). Endocarditis can be culture-negative either due to prior or concomitant antibiotic therapy that inhibits growth of bacteria in blood cultures or because the pathogen does not grow in standard blood culture systems. In both of these cases, circulating mcfDNA sequencing is rapidly becoming a useful method to identify the pathogens responsible for CNE (11). While many advances have been made in diagnostic testing, determining the appropriate duration of antibiotic therapy, timing of surgery, and response to treatment of CNE remain challenges for clinicians (6).
It is difficult to know when a valve has been sterilized in cases of culture-negative endocarditis, and, as seen with certain malignancies, serial measurements of tumor cfDNA offer a potential, objective measure of response to therapy (either surgical or medical) (12–14). However, in the setting of infection, correctly interpreting changes in microbial cfDNA first requires an understanding of its rate of decay. While the half-life of cell-free DNA from human-derived tissues has been established (15–22), there have been no definitive assessments of the rate of decay of microbial cfDNA in plasma. Monitoring the changes of mcfDNA after the debridement of a discrete, high-burden, focal infection would afford the opportunity to measure mcfDNA decay.
In a case of Bartonella quintana aortic valve endocarditis, we describe a unique use of mcfDNA to monitor the response to antibiotic treatment, and to calculate the rate of plasma mcfDNA decay after valve resection through serial mcfDNA measurements after removal of the infected valve.
Case Presentation
In 2016, a 50-year-old man with a bioprosthetic aortic valve (AV) (23mm pericardial tissue) and HIV infection (CD4 nadir 348, viral load undetectable), underwent bioprosthetic AV replacement (23mm Carpentier-Edwards Magna Ease pericardial tissue) for severe aortic stenosis and aortic insufficiency associated with a congenital bicuspid aortic valve. Work up at the time for endocarditis was negative and the pathology showed only myxomatous degeneration. Following valve replacement, he was asymptomatic with normal valve and ventricular function [by transthoracic echocardiogram (TTE)] until a month prior to presentation in September of 2019 when he presented to the emergency department because of progressive shortness of breath over one month and more recent centralized chest pressure with exertion.
On the day of this presentation, he had a repeat TTE that demonstrated a valve area of 0.8 cm2, an AV gradient of 64 mmHg and maximum AV flow velocity of 5.23 m/sec, compared to 15 mmHg and 2.7 m/sec measured after his valve replacement. He had normal dentition. He had no history of intravenous drug use, recent dental procedures, or recent international travel. He was homeless previously but currently he was studying to become a social worker, with active involvement in outreach programs for the homeless in his urban San Diego community. In the emergency department his vital signs were normal. Physical examination revealed a harsh, 4/6 holosystolic murmur with radiation to the carotids and mild bibasilar crackles, but no peripheral edema, splenomegaly, peripheral embolic lesions, large-vessel emboli, cutaneous lesions, or abnormal retinal/conjunctival findings. Laboratory studies revealed a leukocyte count of 5.2 x 109 cells/L, hemoglobin of 11.6 g/dL, MCV of 82.7, and serum creatinine of 0.77 mg/dL. C-reactive protein was 5.89 mg/dL [upper limit of normal 0.8] and erythrocyte sedimentation rate was 74 mm/hr [normal range 0-15] compared with 0.3 and 22, respectively, 3 years prior at the time of his valve replacement. Electrocardiogram showed normal sinus rhythm and a PR interval of 182. A focal area of abnormal soft tissue was visualized along the valve annulus on cardiac CT and transesophageal echocardiography revealed a paravalvular leak abutting the interatrial septum, but no abscess (Figures 1A, B). Three sets of blood cultures separated by ≥ 24 hours failed to isolate an organism. Fungal and mycobacterial blood cultures were also done and were eventually negative.
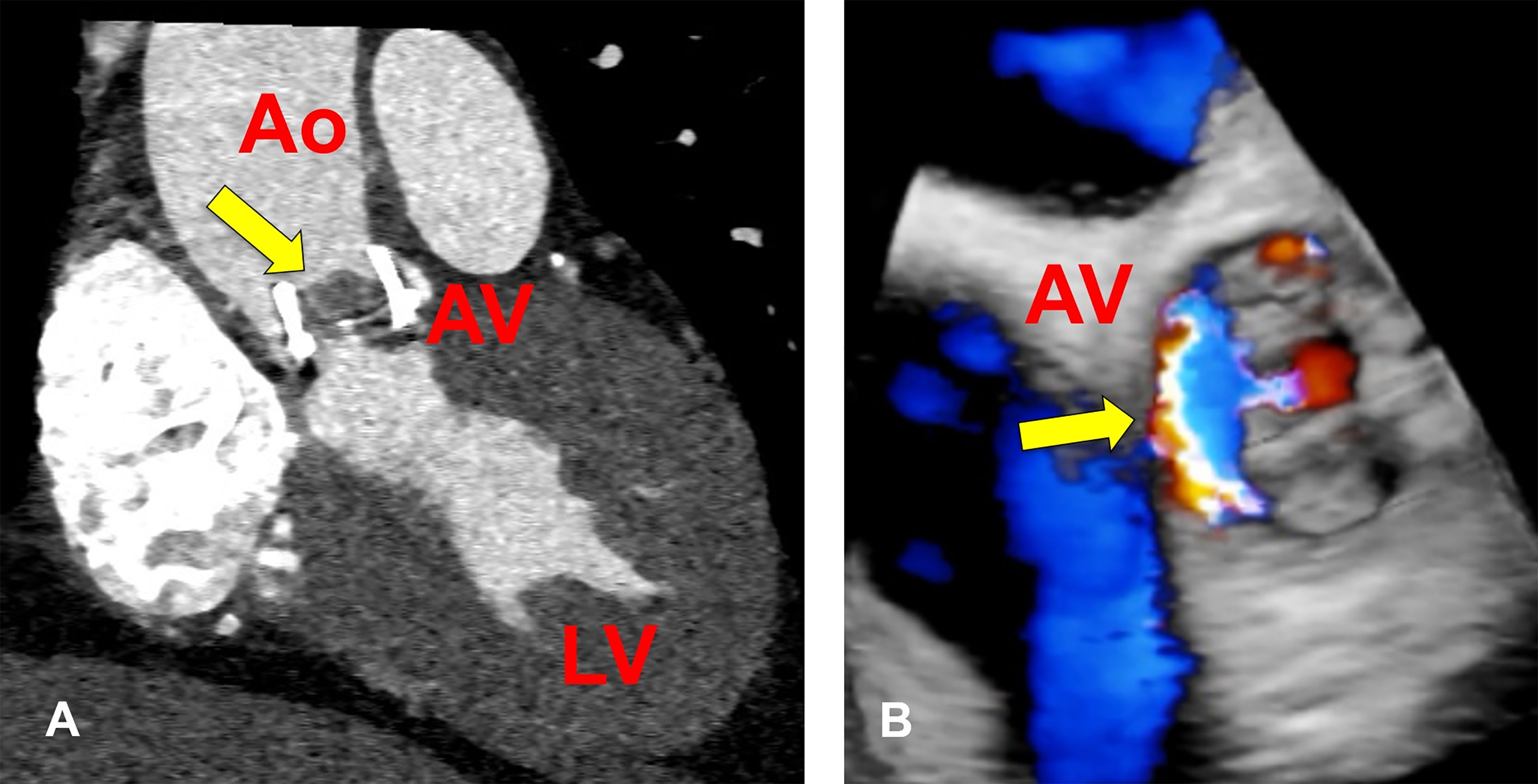
Figure 1 Bioprosthetic Aortic Valve Abnormalities in a Patient with Bartonella quintana Endocarditis. (A) Cardiac CT showing soft tissue mass on the bioprosthetic valve measuring 11 x 8mm (arrow). (B) Three-dimensional rendition of bioprosthetic aortic valve visualized on transesophageal echocardiogram showing regurgitation and paravalvular leak (arrow) at the time of diagnosis of B quintana endocarditis. Ao, aorta; AV, aortic valve; LV, left ventricle.
Suspecting IE, empiric treatment was started with ceftriaxone and doxycycline. Serologic work-up for CNE was notable for Bartonella henselae IgG titer of 1:1024, Coxiella IgM titer of 1:64 (without IgG seroconversion), Chlamydia pneumoniae IgG titer of 1:64, and a positive Brucella IgG. Fungal serologies and serologies for Bartonella quintana, Legionella, and other Chlamydia species were negative. Nucleic acid amplification testing for Tropheryma whipplei was also negative (ARUP Laboratories, Salt Lake City, Utah). Rifampin was added when Brucella serologies returned positive. Karius mcfDNA NGS of plasma detected Bartonella quintana, so ceftriaxone and rifampin were discontinued and gentamicin was added. Blood cultures eventually grew Bartonella quintana. Following three weeks of doxycycline and gentamicin the patient underwent infected valve resection and re-do open bioprosthetic aortic valve replacement. He was continued on doxycycline postoperatively for three months. He ultimately recovered well, returning to his former performance status, and with a normal follow-up TTE (normal left ventricular function, AV area of 1.0 cm2).
Materials and Methods
Serial blood samples were obtained prior to aortic valve replacement, intra-operatively, and post-valve replacement in this individual with culture negative endocarditis. Microbial cfDNA was extracted from plasma and NGS was performed by Karius, Inc. (Redwood City, California) in Karius’s CLIA/CAP-accredited laboratory. Intact bacteria as well as human cells and cellular debris are separated out by spin centrifugation prior to extraction of soluble DNA in the cell-free plasma fraction. Within this fraction, human sequences were removed and remaining sequences were aligned to a curated database of over 1,400 pathogens. DNA from organisms present above a predefined statistical significance threshold were reported and quantified in DNA molecules per microliter (MPM). Each measurement of MPM was done in duplicate. Detailed description of the methodology for sample processing, NGS and associated software is included in the Supplementary Material, adapted from Blauwkamp et al. (4). Following IRB review and approval, chart review was performed by physicians for clinical correlation.
Results
Microbial Cell-Free DNA Signal Prior to Infected Valve Resection
Bartonella quintana mcfDNA signal at the time of his initial diagnosis was 40,185 +/-1,173 DNA molecules per microliter. After 4 weeks of parenteral gentamicin plus doxycycline, Karius testing demonstrated a 78% (4.6-fold) decrease in the Bartonella quintana mcfDNA signal to 8,813 MPM (Table 1). However, his valve malfunction required surgery. With the patient’s consent serial sampling was done in the operating room for Karius testing.
Microbial Cell-Free DNA Signal Decay Intra- and Post-Operatively
Bartonella quintana mcfDNA rose dramatically after the operative manipulation of the infected valve (timepoint 1, Figure 2). The signal then dropped 81-fold in the first 4 hours following valve resection. The half-life of mcfDNA over this time period ranged from 35 minutes (calculated based on timepoints 3-7) to 115 minutes (timepoints 4-8, Figure 2). Following valve resection, Karius testing showed further decay of the Bartonella quintana mcfDNA signal to 103 MPM after 24 hours and effectively maintained a basal amount of 123 MPM at 48 hours (Figure 2). Antibiotics were continued intra- and post-operatively.
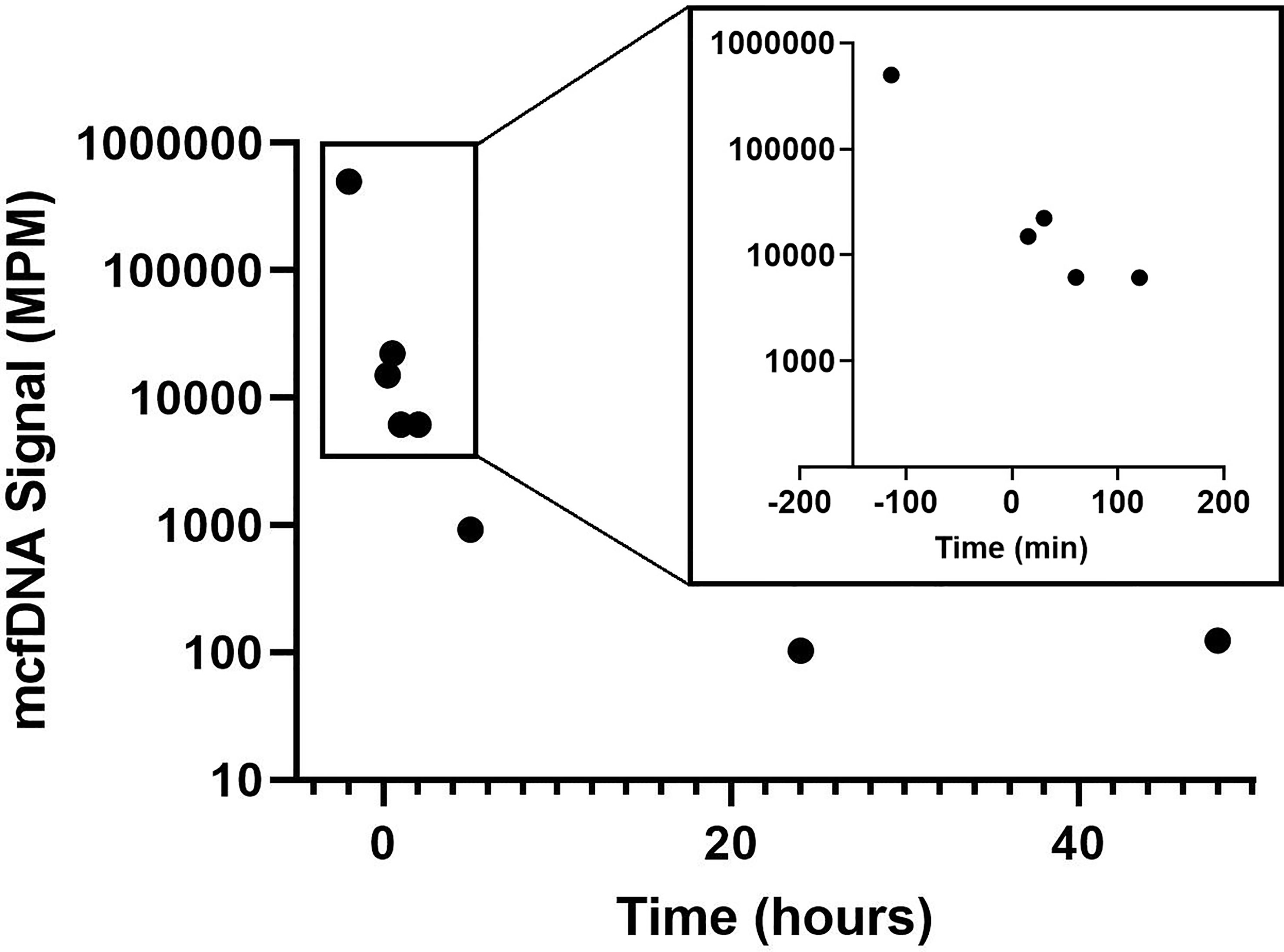
Figure 2 Bartonella quintana mcfDNA Decay Following Valve Resection. Decay of (B) quintana mcfDNA following removal of the infected valve. Time 0 min represents time after patient was removed from cardiopulmonary bypass used for valve replacement surgery. The first timepoint (-114 min) represents time of valve resection. mcfDNA, microbial cell-free DNA; MPM, molecules per microliter; min, minutes.
Discussion
Here we describe the successful use of mcfDNA sequencing of plasma to not only identify the species of Bartonella causing prosthetic aortic valve endocarditis and monitor treatment response, but to also characterize mcfDNA decay kinetics following surgical resection of the involved valve, which presumably removed the bulk of the infected tissue. Following valve resection, we observed an overall decrease in mcfDNA signal that can be characterized by three phases: 1) a rapid decline in mcfDNA over the first 2 hours, 2) a slower decline in the subsequent 22 hours, and 3) a sustained basal amount of mcfDNA in the final 24 hours of monitoring. Since we stopped measuring mcfDNA levels at the 48-hour timepoint, the duration of the sustained basal level remains to be determined.
Serologic testing is used for the presumptive identification of the causative organisms in many cases of CNE, because isolation of the majority of these organisms from the blood requires specialized techniques and media that are not available in most clinical labs (23). However, the interpretation of serological results, particularly in Bartonella endocarditis, can be complicated because of significant cross-reactivity not only among Bartonella species themselves, but also between Bartonella spp. and Chlamydophila spp., Coxiella burnetii, and Brucella as seen in this case (24, 25). There have also been reports of serologic cross-reactivity between Bartonella spp. and pathogens such as Epstein-Barr virus, cytomegalovirus, Toxoplasma gondii and Streptococcus pyogenes (26).
Molecular nucleic acid-based diagnostics offer an approach that can be more specific than serology. The sensitivity of PCR and sequencing assays on excised valvular tissue is generally greater than PCR on whole blood or serum as organisms are more abundant in valve tissue than in blood (11). Blood samples in patients with CNE are presumed to contain ~10 viable bacteria per mL, a concentration that is thought to remain constant during infection (27), but standard blood cultures rarely are positive in part because the organisms are so slow-growing or will not grow at all in standard blood culture media. The sensitivity of detection of organisms by PCR is not established, but one study of Bartonella endocarditis reported a sensitivity for a Bartonella-specific PCR assay of 92% for valvular tissue compared to only 33% and 36% using whole blood and serum, respectively (28). Even the sensitivity and specificity of 16S rDNA PCR-based assays on excised valve tissue varies widely, ranging from 41% to 96% and 95% to 100% (29–32),. Additionally, efforts to increase the sensitivity of PCR based diagnosis using blood samples are constrained by a decrease in specificity due to background bacterial DNA contamination (33).
The mcfDNA NGS assay used in this study minimizes the effect of background bacterial DNA contamination by custom-curating and sourcing reagents with minimal basal levels of background and establishing quality control monitoring procedures to detect DNA contamination in the molecular biology procedural pipeline. Theoretically, this assay has the capacity for greater sensitivity than analogous PCR-based assays given the larger set of molecular targets (potentially encompassing the entire genome of a pathogen) that NGS affords over the limited number of PCR-targeted sequences used in most diagnostic laboratories. Continued refinement in methods and more widespread use of mcfDNA as described here can and will further improve the diagnosis of CNE, enabling a higher yield of microbiological diagnosis and as a result, faster time to institution of definitive targeted therapy.
Despite the advances in diagnostics for CNE ushered in by molecular testing, determining the duration of antibiotic therapy, the timing of surgery, and whether there has been an adequate response to antibiotic therapy remain significant challenges in CNE management. Microbial cfDNA sequencing offers a promising rapid, non-invasive method to not only diagnose infections caused by fastidious and difficult to culture organisms, but to also monitor the load of organisms in a given infection, aiding in assessing the response to therapy. Currently, the duration of antibiotic therapy for CNE is based largely on expert opinion and case series, but has not been rigorously studied (34). Antibody titers can be useful for monitoring treatment response if they are positive, but antibodies will persist for weeks even after bacteria have been eradicated. Further, cut-offs for what is considered a positive serology are subject to assay-dependent limitations such as the type of antigen used, pre-determined thresholds, and processing error (27, 35). Potentially, using serial mcfDNA measurements in patients with CNE on antibiotic therapy will offer a more sensitive and specific alternative for assessing response to treatment. Measurements of mcfDNA may also have a role in monitoring response to antibiotic cessation and identifying those at risk for residual disease and recrudescence in individuals on chronic suppressive therapy. In theory, the mcfDNA signals may initially increase with treatment as pathogens lyse and release their DNA. If so, serial measurement may be needed to interpret the adequacy of the therapy.
The use of mcfDNA to quantify microbial DNA has the potential to improve the management of CNE. The American Association of Thoracic Surgery 2016 Consensus Guidelines on endocarditis management recommend surgical intervention for uncomplicated PVE for persistent bacteremia or relapsing infection (36), but for CNE that determination is not feasible so clinicians rely on changes in antibody titers to determine response, which provides only an indirect indication of response and one that likely lags behind actual microbial eradication by weeks to months. Therefore, in the setting of CNE, mcfDNA assessment could serve as a direct measure of response to therapy and a way to detect recurrence that may indicate the need for surgical intervention, before an individual develops symptoms due to valvular complications that could potentially complicate a surgery.
This study is one of the first to asses decay kinetics of mcfDNA. The kinetics of human cfDNA has been previously characterized, primarily from studies of tumor-derived (17, 21, 22) and fetal (15, 16, 18–20) cfDNA. Half-life calculations in such studies have ranged from several minutes to approximately 2 hours (12). For example, Lo et al. investigated the clearance of circulating fetal DNA following delivery and found that the mean half-life was 16.3 minutes (range 4 – 30 minutes) (20). Serial analysis of circulating tumor cfDNA from people with colorectal cancer demonstrated a half-life of 114 minutes following complete tumor resection (17). Further, circulating viral cfDNA from EBV in patients with nasopharyngeal carcinoma demonstrated a median half-life of 139 minutes (37) following surgical removal of their tumor. Human cfDNA not associated with malignancy has also been assessed in patients on hemodialysis and was found to have a significantly shorter half-life, approximately 4 minutes (38). Elimination of cfDNA appears to occur in the liver, spleen and kidney (39, 40), with the liver removing approximately 70-85% of nucleosomes from the circulation within 10 minutes (41). Using serial mcfDNA sequencing analogous to the experiments that initially established the half-life of human cfDNA (20), our study estimated the half-life of Bartonella mcfDNA after valve replacement in this case to be between 35 and 115 minutes, which is within the range of the decay of other cfDNA. We based our half-life calculations on samples taken 15 and +30 minutes after the clamps used for cardiac bypass were removed, assuming that by then the equilibrium of the mcfDNA in the systemic circulation had been achieved. The slower phase of decay observed 24-48 hours following infected valve removal, though limited by only two timepoints, possibly represents an effect of antibiotics that were continued post-operatively on bacteria from extra-cardiac tissues. The decay characteristics in different patients with different etiologies will need to be determined in order to establish the range of expected results, which could be influenced by changes in the volume of distribution secondary to cross-clamping in cardiac bypass surgery, blood loss, fluid replacement, and the rate of redistribution of mcfDNA in the systemic circulation after the relief of cross-clamping.
A formal cost analysis was not performed in this case. However, we propose that the sensitivity, non-invasive sampling, and capacity for broad testing afforded by NGS of plasma microbial cell-free DNA – both in this case and in the additional clinical applications described – would in many cases outweigh the limitations of sequencing cost and potentially avoid the need for invasive procedures that may incur additional expenses and morbidity.
Although the kinetics of circulating eukaryotic and viral cfDNA in humans have been described, prior to this study, reports of the decay kinetics of bacterial cfDNA were virtually non-existent. Ultimately, additional studies will have to be performed in a variety of infections with different pathogens to better understand the characteristics and range of decay of mcfDNA. The future is bright for pathogen agnostic nucleic acid technologies, but there is still much to learn.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by UCSD Human Research Protections Program. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
Conceptualization: AA, JF, and SM. Methodology: DS, AA, and SM. Investigation: DS, AA, EG, MJ, and SM. Visualization: DS, AA, and SM. Funding acquisition: AA and SM. Project administration: DS and SM. Supervision: SM. Writing – original draft: DS and AA. Writing – review and editing: DS, AA, JF, EG, and SM. All authors have read and approved this manuscript.
Funding
Support for laboratory analysis was provided by Karius, Inc. SRM is supported by NIH Grants AI106039, MH124590, AI135992, DA049644, and the PIMSA foundation.
Conflict of Interest
AA is Senior Medical Director at Karius, Inc. The authors also declare that this study received funding from Karius, Inc. The funder had the following involvement with the study: running the assay and laboratory analysis.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.842100/full#supplementary-material
Abbreviations
AV, aortic valve; cfDNA, cell-free DNA; CNE, culture-negative endocarditis; IE, infective endocarditis; McfDNA, microbial cell-free DNA; MPM, molecules per microliter; NGS, next generation sequencing; TTE, trans-thoracic echocardiogram.
References
1. Kondo M, Dalai SC, Venkatasubrahmanyam S, Eisenberg N, Robinson BD, Westblade LF, et al. Diagnosis and Genotyping of Coxiella Burnetii Endocarditis in a Patient With Prosthetic Pulmonary Valve Replacement Using Next-Generation Sequencing of Plasma Microbial Cell-Free DNA. Open Forum Infect Dis (2019) 6:ofz242. doi: 10.1093/ofid/ofz242
2. Vudatha V, Ranson M, Blair L, Ahmed AA. Rapid Detection of Bacille Calmette-Guérin-Associated Mycotic Aortic Aneurysm Using Novel Cell-Free DNA Assay. J Vasc Surg Cases Innov Tech (2019) 5:143–8. doi: 10.1016/j.jvscit.2018.11.006
3. Downey RD, Russo SM, Hauger SB, Murphey DK, Marx G, Huynh T, et al. Identification of an Emergent Pathogen, Bartonella Vinsonii, Using Next-Generation Sequencing in a Patient With Culture-Negative Endocarditis. J Pediatr Infect Dis Soc (2021) 10:213–6. doi: 10.1093/jpids/piaa014
4. Blauwkamp TA, Thair S, Rosen MJ, Blair L, Lindner MS, Vilfan ID, et al. Analytical and Clinical Validation of a Microbial Cell-Free DNA Sequencing Test for Infectious Disease. Nat Microbiol (2019) 4:663–74. doi: 10.1038/s41564-018-0349-6
5. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective Endocarditis in the U.S., 1998-2009: A Nationwide Study. PloS One (2013) 8:e60033–3. doi: 10.1371/journal.pone.0060033
6. Cahill TJ, Baddour LM, Habib G, Hoen B, Salaun E, Pettersson GB, et al. Challenges in Infective Endocarditis. J Am Coll Cardiol (2017) 69:325. doi: 10.1016/j.jacc.2016.10.066
7. Raoult D, Casalta JP, Richet H, Khan M, Bernit E, Rovery C, et al. Contribution of Systematic Serological Testing in Diagnosis of Infective Endocarditis. J Clin Microbiol (2005) 43:5238–42. doi: 10.1128/JCM.43.10.5238-5242.2005
8. Fournier PE, Gouriet F, Casalta JP, Lepidi H, Chaudet H, Thuny F, et al. Blood Culture-Negative Endocarditis: Improving the Diagnostic Yield Using New Diagnostic Tools. Med (Baltimore) (2017) 96:e8392. doi: 10.1097/MD.0000000000008392
9. Werner M, Andersson R, Olaison L, Hogevik H. A Clinical Study of Culture-Negative Endocarditis. Med (Baltimore) (2003) 82:263–73. doi: 10.1097/01.md.0000085056.63483.d2
10. Zamorano J, Sanz J, Almería C, Rodrigo JL, Samedi M, Herrera D, et al. Differences Between Endocarditis With True Negative Blood Cultures and Those With Previous Antibiotic Treatment. J Heart Valve Dis (2003) 12:256–60.
11. Liesman RM, Pritt BS, Maleszewski JJ, Patel R. Laboratory Diagnosis of Infective Endocarditis. J Clin Microbiol (2017) 55:2599–608. doi: 10.1128/JCM.00635-17
12. Kustanovich A, Schwartz R, Peretz T, Grinshpun A. Life and Death of Circulating Cell-Free DNA. Cancer Biol Ther (2019) 20:1057–67. doi: 10.1080/15384047.2019.1598759
13. Zhou J, Wang C, Lin G, Xiao Y, Jia W, Xiao G, et al. Serial Circulating Tumor DNA in Predicting and Monitoring the Effect of Neoadjuvant Chemoradiotherapy in Patients With Rectal Cancer: A Prospective Multicenter Study. Clin Cancer Res (2021) 27:301–10. doi: 10.1158/1078-0432.CCR-20-2299
14. Oellerich M, Schütz E, Beck J, Kanzow P, Plowman PN, Weiss GJ, et al. Using Circulating Cell-Free DNA to Monitor Personalized Cancer Therapy. Crit Rev Clin Lab Sci (2017) 54:205–18. doi: 10.1080/10408363.2017.1299683
15. Ariga H, Ohto H, Busch MP, Imamura S, Watson R, Reed W, et al. Kinetics of Fetal Cellular and Cell-Free DNA in the Maternal Circulation During and After Pregnancy: Implications for Noninvasive Prenatal Diagnosis. Transfusion (2001) 41:1524–30. doi: 10.1046/j.1537-2995.2001.41121524.x
16. Chan LY, Leung TN, Chan KC, Tai HL, Lau TK, Wong EM, et al. Serial Analysis of Fetal DNA Concentrations in Maternal Plasma in Late Pregnancy. Clin Chem (2003) 49:678–80. doi: 10.1373/49.4.678
17. Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating Mutant DNA to Assess Tumor Dynamics. Nat Med (2008) 14:985–90. doi: 10.1038/nm.1789
18. Jimenez DF, Tarantal AF. Quantitative Analysis of Male Fetal DNA in Maternal Serum of Gravid Rhesus Monkeys (Macaca Mulatta). Pediatr Res (2003) 53:18–23. doi: 10.1203/00006450-200301000-00007
19. Lo YM, Tein MS, Lau TK, Haines CJ, Leung TN, Poon PM, et al. Quantitative Analysis of Fetal DNA in Maternal Plasma and Serum: Implications for Noninvasive Prenatal Diagnosis. Am J Hum Genet (1998) 62:768–75. doi: 10.1086/301800
20. Lo YM, Zhang J, Leung TN, Lau TK, Chang AM, Hjelm NM. Rapid Clearance of Fetal DNA From Maternal Plasma. Am J Hum Genet (1999) 64:218–24. doi: 10.1086/302205
21. Taback B, O’Day SJ, Hoon DS. Quantification of Circulating DNA in the Plasma and Serum of Cancer Patients. Ann NY Acad Sci (2004) 1022:17–24. doi: 10.1196/annals.1318.004
22. Wu TL, Zhang D, Chia JH, Tsao K, Sun CF, Wu JT. Cell-Free DNA: Measurement in Various Carcinomas and Establishment of Normal Reference Range. Clin Chim Acta (2002) 321:77–87. doi: 10.1016/S0009-8981(02)00091-8
23. Anderson BE, Neuman MA. Bartonella Spp. As Emerging Human Pathogens. Clin Microbiol Rev (1997) 10:203–19. doi: 10.1128/CMR.10.2.203
24. La Scola B, Raoult D. Serological Cross-Reactions Between Bartonella Quintana, Bartonella Henselae, and Coxiella Burnetii. J Clin Microbiol (1996) 34:2270–4. doi: 10.1128/jcm.34.9.2270-2274.1996
25. Maurin M, Eb F, Etienne J, Raoult D. Serological Cross-Reactions Between Bartonella and Chlamydia Species: Implications for Diagnosis. J Clin Microbiol (1997) 35:2283–7. doi: 10.1128/jcm.35.9.2283-2287.1997
26. Vermeulen MJ, Verbakel H, Notermans DW, Reimerink J, Peeters MF. Evaluation of Sensitivity, Specificity and Cross-Reactivity in Bartonella Henselae Serology. J Med Microbiol (2010) 59:743–5. doi: 10.1099/jmm.0.015248-0
27. Brouqui P, Raoult D. New Insight Into the Diagnosis of Fastidious Bacterial Endocarditis. FEMS Immunol Med Microbiol (2006) 47:1–13. doi: 10.1111/j.1574-695X.2006.00054.x
28. Edouard S, Nabet C, Lepidi H, Fournier PE, Raoult D. Bartonella, a Common Cause of Endocarditis: A Report on 106 Cases and Review. J Clin Microbiol (2015) 53:824–9. doi: 10.1128/JCM.02827-14
29. Voldstedlund M, Nørum Pedersen L, Baandrup U, Klaaborg KE, Fuursted K. Broad-Range PCR and Sequencing in Routine Diagnosis of Infective Endocarditis. Apmis (2008) 116:190–8. doi: 10.1111/j.1600-0463.2008.00942.x
30. Marín M, Muñoz P, Sánchez M, Del Rosal M, Alcalá L, Rodríguez-Créixems M, et al. Molecular Diagnosis of Infective Endocarditis by Real-Time Broad-Range Polymerase Chain Reaction (PCR) and Sequencing Directly From Heart Valve Tissue. Med (Baltimore) (2007) 86:195–202. doi: 10.1097/MD.0b013e31811f44ec
31. Breitkopf C, Hammel D, Scheld HH, Peters G, Becker K. Impact of a Molecular Approach to Improve the Microbiological Diagnosis of Infective Heart Valve Endocarditis. Circulation (2005) 111:1415–21. doi: 10.1161/01.CIR.0000158481.07569.8D
32. Goldenberger D, Künzli A, Vogt P, Zbinden R, Altwegg M. Molecular Diagnosis of Bacterial Endocarditis by Broad-Range PCR Amplification and Direct Sequencing. J Clin Microbiol (1997) 35:2733–9. doi: 10.1128/jcm.35.11.2733-2739.1997
33. Madico GE, Rice PA. 16S-Ribosomal DNA to Diagnose Culture-Negative Endocarditis. Curr Infect Dis Rep (2008) 10:280–6. doi: 10.1007/s11908-008-0046-3
34. Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr., Tleyjeh IM, Rybak MJ, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation (2015) 132:1435–86. doi: 10.1161/CIR.0000000000000296
35. Favacho ARM, Roger I, Akemi AK, Pessoa AA Jr., Varon AG, Gomes R, et al. Molecular Identification of Bartonella Henselae in a Seronegative Cat Scratch Disease Patient With AIDS in Rio De Janeiro, Brazil. Rev Do Inst Med Trop Sao Paulo (2014) 56:363–5. doi: 10.1590/S0036-46652014000400017
36. Pettersson GB, Coselli JS, Pettersson GB, Coselli JS, Hussain ST, Griffin B, et al. 2016 The American Association for Thoracic Surgery (AATS) Consensus Guidelines: Surgical Treatment of Infective Endocarditis: Executive Summary. J Thorac Cardiovasc Surg (2017) 153:1241–1258.e1229. doi: 10.1016/j.jtcvs.2016.09.093
37. To EW, Chan KC, Leung SF, Chan LY, To KF, Chan AT, et al. Rapid Clearance of Plasma Epstein-Barr Virus DNA After Surgical Treatment of Nasopharyngeal Carcinoma. Clin Cancer Res (2003) 9:3254–9.
38. Rumore P, Muralidhar B, Lin M, Lai C, Steinman CR. Haemodialysis as a Model for Studying Endogenous Plasma DNA: Oligonucleosome-Like Structure and Clearance. Clin Exp Immunol (1992) 90:56–62. doi: 10.1111/j.1365-2249.1992.tb05831.x
39. Yu SC, Lee SW, Jiang P, Leung TY, Chan KC, Chiu RW, et al. High-Resolution Profiling of Fetal DNA Clearance From Maternal Plasma by Massively Parallel Sequencing. Clin Chem (2013) 59:1228–37. doi: 10.1373/clinchem.2013.203679
40. Butler TM, Spellman PT, Gray J. Circulating-Tumor DNA as an Early Detection and Diagnostic Tool. Curr Opin Genet Dev (2017) 42:14–21. doi: 10.1016/j.gde.2016.12.003
Keywords: microbial cell-free DNA, DNA decay kinetics, Bartonella, therapeutic response, culture-negative endocarditis
Citation: Solanky D, Ahmed AA, Fierer J, Golts E, Jones M and Mehta SR (2022) Utility of Plasma Microbial Cell-Free DNA Decay Kinetics After Aortic Valve Replacement for Bartonella Endocarditis: Case Report. Front. Trop. Dis 3:842100. doi: 10.3389/fitd.2022.842100
Received: 26 December 2021; Accepted: 24 February 2022;
Published: 24 March 2022.
Edited by:
Ricardo Khouri, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Luciane Amorim Santos, Bahiana School of Medicine and Public Health, BrazilPedro Milet Meirelles, Federal University of Bahia, Brazil
Copyright © 2022 Solanky, Ahmed, Fierer, Golts, Jones and Mehta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanjay R. Mehta, c3JtZWh0YUBoZWFsdGgudWNzZC5lZHU=
 Dipesh Solanky
Dipesh Solanky Asim A. Ahmed2
Asim A. Ahmed2 Joshua Fierer
Joshua Fierer Sanjay R. Mehta
Sanjay R. Mehta