- 1Institute of Biodiversity, Animal Health and Comparative Medicine, University of Glasgow, Glasgow, United Kingdom
- 2Royal (Dick) School of Veterinary Studies and the Roslin Institute, University of Edinburgh, Easter Bush Campus, Roslin, United Kingdom
- 3Institute of Health and Wellbeing, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, United Kingdom
As part of the ‘Zero by 30’ strategy to end human deaths from dog-mediated rabies by 2030, international organizations recommend a One Health framework that includes Integrated Bite Case Management (IBCM). However, little is understood about the implementation of IBCM in practice. This study aims to understand how IBCM is conceptualized, exploring how IBCM has been operationalized in different contexts, as well as barriers and facilitators to implementation. Semi-structured interviews were conducted with seventeen practitioners and researchers with international, national, and local expertise across Africa, Asia, and the Americas. Thematic analysis was undertaken using both inductive and deductive approaches. Four main themes were identified: 1) stakeholders’ and practitioners’ conceptualization of IBCM and its role in rabies elimination; 2) variation in how IBCM operates across different contexts; 3) barriers and facilitators of IBCM implementation in relation to risk assessment, PEP provisioning, animal investigation, One Health collaboration, and data reporting; and 4) the impact of the COVID-19 pandemic on IBCM programs. This study highlights the diversity within experts’ conceptualization of IBCM, and its operationalization. The range of perspectives revealed that there are different ways of organizing IBCM within health systems and it is not a one-size-fits-all approach. The issue of sustainability remains the greatest challenge to implementation. Contextual features of each location influenced the delivery and the potential impact of IBCM. Programs spanned from highly endemic settings with limited access to PEP charged to the patient, to low endemicity settings with a large patient load associated with free PEP policies and sensitization. In practice, IBCM was tailored to meet the demands of the local context and level of rabies control. Thus, experts’ experiences did not necessarily translate across contexts, affecting perceptions about the function, motivation for, and implementation of IBCM. To design and implement future and current programs, guidance should be provided for health workers receiving patients on assessing the history and signs of rabies in the biting animal. The study findings provide insights in relation to implementation of IBCM and how it can support programs aiming to reach the Zero by 30 goal.
Introduction
Effective rabies vaccines for humans and animals have been available for over a century, providing means to eliminate this fatal and incurable zoonotic disease (1). Through mass dog vaccinations starting in the 1920s, rabies has been eliminated from domestic dog populations in high-income countries across western Europe, North America, and parts of Asia, such as Japan and Taiwan (2, 3). Additionally, low- and middle-income countries (LMICs) in Latin America have successfully reduced dog-mediated rabies cases by over 98% through large-scale coordinated dog vaccination programs (4, 5). Despite this progress, an estimated 59,000 people still die from rabies every year, with the vast majority in resource-poor countries in Africa and Asia (2, 6).
There are several challenges for LMICs in reducing the burden of rabies within their populations. First, to control and eliminate rabies a high vaccination coverage, typically around 70% of the susceptible dog population, must be sustained for 3-7 years via recurrent annual campaigns (2). Yet many LMICs have not initiated routine dog vaccination (7). Furthermore, countries face the threat of reintroductions if endemic rabies circulates at their borders (8). Second, post-exposure prophylaxis (PEP)—which is needed immediately after a bite from a rabid dog to prevent the fatal onset of rabies—is often expensive for both bite victims and governments. As a result, PEP availability is frequently limited, especially in rural areas (9, 10). PEP costs can even drain the finite budget available for rabies, without impacting the incidence of rabies in dog populations that are the source of exposures (11, 12). Third, surveillance in LMICs is typically weak and does not capture accurate data on either human or animal rabies cases (13). This significant under-reporting leads to lack of awareness and understanding of the burden of rabies, which further results in limited community/stakeholder engagement and inadequate funding. Thus, the absence of robust surveillance gives rise to a cycle of underestimating the disease burden and consequently neglecting control measures, such as dog vaccination and PEP provisioning (12, 14).
To overcome these challenges, the Tripartite [World Health Organization (WHO), World Organisation for Animal Health (OIE), Food and Agriculture Organization (FAO)] along with the Global Alliance for Rabies Control (GARC) developed the ‘Zero by 30’ global strategic plan to end human deaths from dog-mediated rabies by 2030. Within this strategy, international organizations jointly recommend a One Health framework, recognizing the interconnections between the health of humans, animals, and their shared environment (11). To control and eliminate rabies, the strategy advocates Integrated Bite Case Management (IBCM) (15). WHO describes IBCM as an advanced surveillance method involving “investigations of suspected rabid animals and sharing information with both animal and human health investigators for appropriate risk assessments” (2). Through multisectoral collaboration and communication, this One Health approach aims to enhance surveillance by increasing detection of animal cases and human exposures, as well as to improve PEP allocation and compliance (4, 13, 16).
While the objectives, aims, and benefits of IBCM are becoming better known by international organizations and experts in the field, this approach is relatively new and has only been implemented within the last decade. Official guidelines for IBCM and risk assessments in relation to biting animals were first mentioned in WHO guidance in 2018 (2). Peer-reviewed empirical evidence about the impact or implementation of IBCM remains limited. A PubMed search using keywords “novel surveillance” OR “integrated bite case management” AND “rabies” identified only eleven studies from four countries: Chad (10, 17), Haiti (4, 14, 16, 18–20), the Philippines (12, 21), and Tanzania (13). Although several IBCM programs have been implemented within the last decade, the approaches undertaken and the lessons learned from these programs have not yet been synthesized. This study aims to understand how IBCM is conceptualized and practiced by stakeholders involved in rabies prevention and control programs and barriers and facilitators to its implementation.
Materials and Methods
This qualitative study was conducted among experts with experience designing, implementing, and/or managing IBCM programs in different epidemiological and geographical contexts (Table 1). Purposive sampling of known professional networks was used to identify and recruit experts. All participants were contacted by email and provided with a participant information sheet. Participants provided consent prior to interviews, which were all conducted in English.
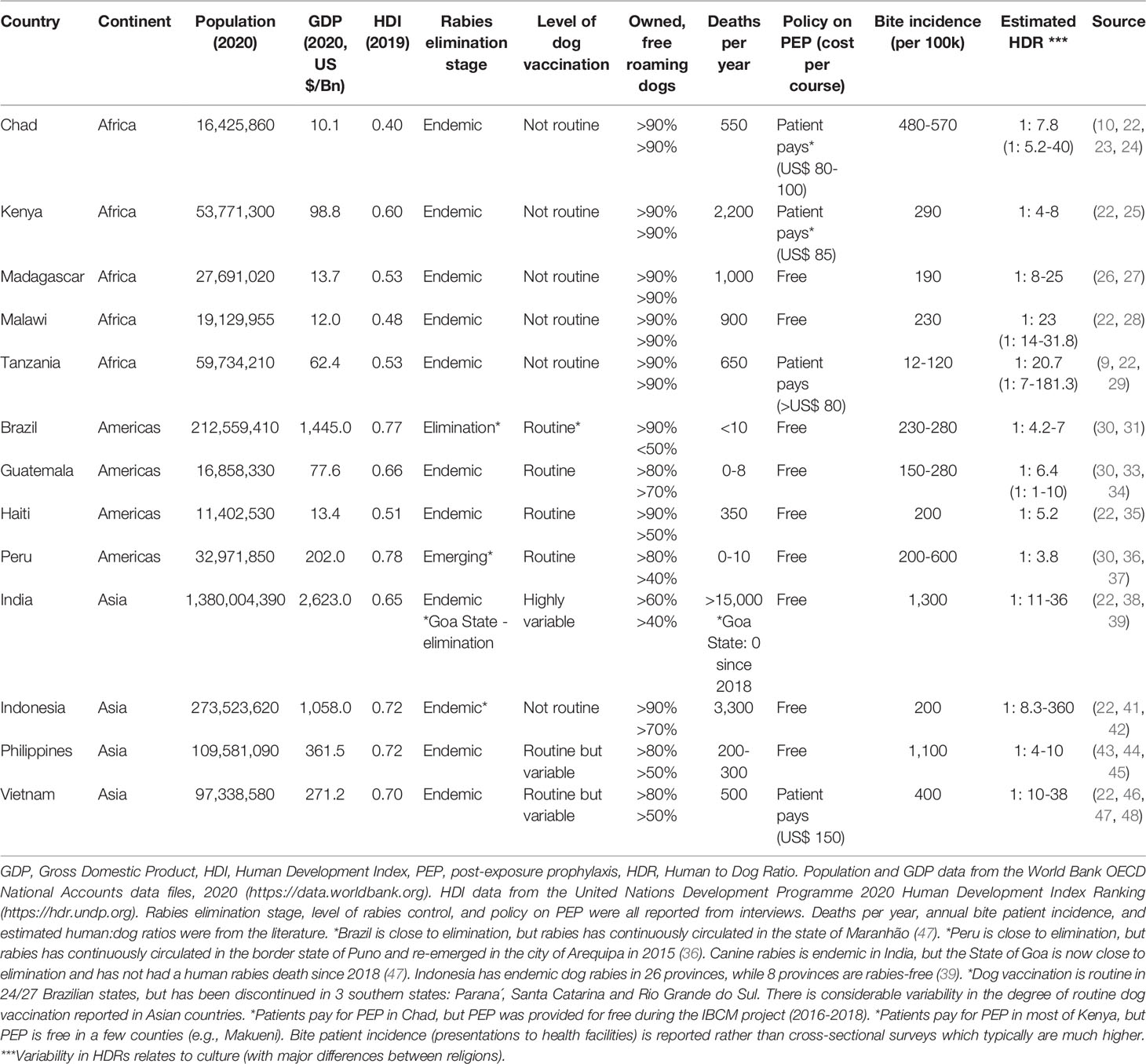
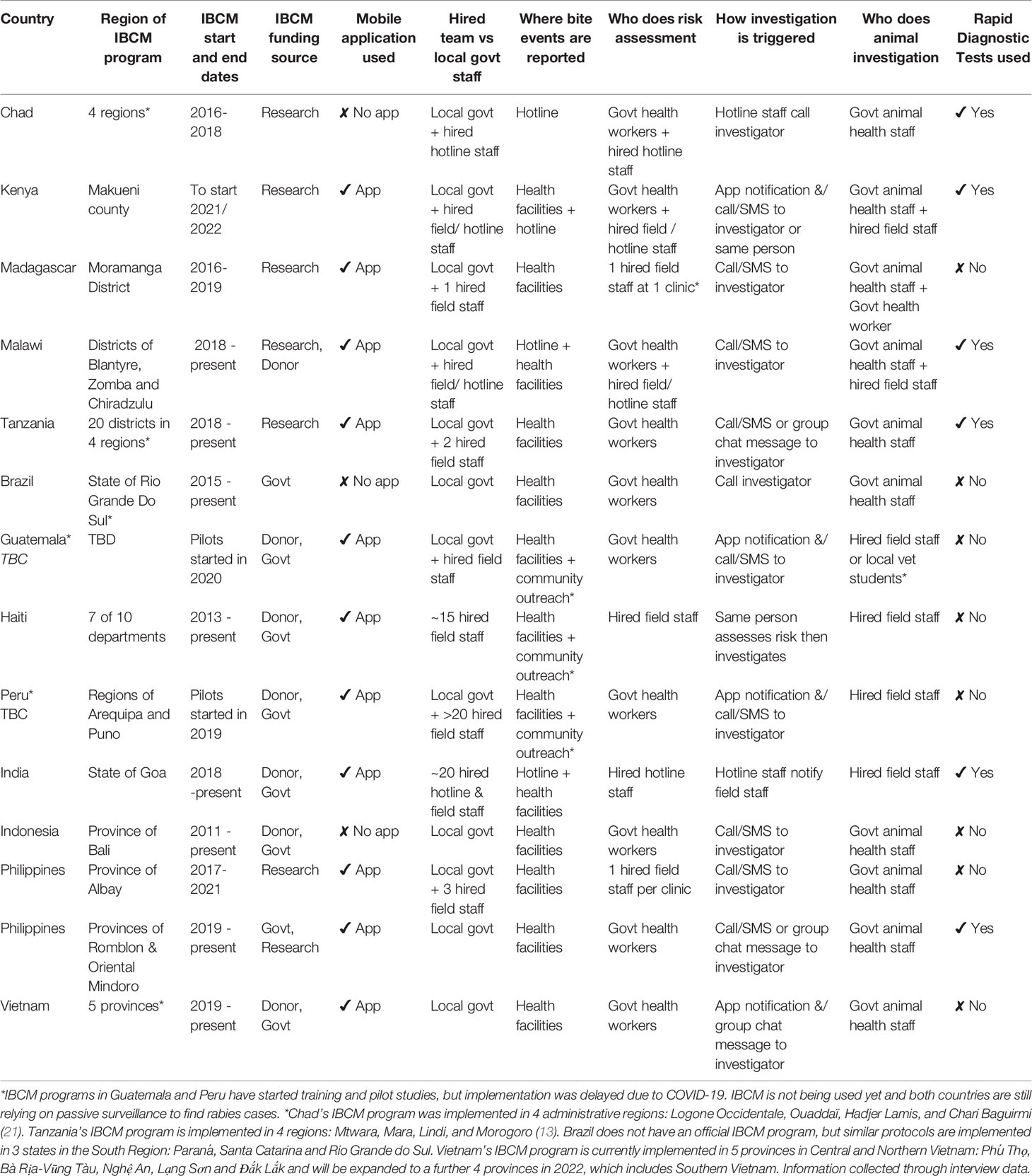
Seventeen participants were interviewed: five international-level, six national-level, and six local-level experts. The majority of participants had a degree in veterinary medicine (twelve); a doctoral research degree (nine); or both (five); and one had a MSc in medical statistics. All participants had some educational/experiential background in epidemiology. Their work experience ranged from academic, government, non-profit, and international organization, with most having experience with more than one. Fourteen IBCM programs were included in the study representing thirteen countries in the Americas, Africa, and Asia (Table 2). All IBCM programs were/are being implemented in countries with endemic dog rabies, with the exception of Rio Grande Do Sul, a state in Brazil which has not reported a dog or human case since the 1980s.
Semi-structured one-to-one interviews (40-65 minutes) were conducted between January 2020 and August 2021. One interview was conducted face-to-face before COVID-19 restrictions and the other sixteen were conducted over the videoconferencing platform, Zoom (49). Interview topic guides were generated for each level of expertise: international, national, and local. The questions were designed to be open-ended and encourage experts to share their experiences and elaborate on the issues they felt to be important. Interviews were audio-recorded, transcribed verbatim, pseudonymized, then uploaded into NVivo 12 Pro software (50).
Data analysis was conducted by the first author and supervised by the last author, an experienced qualitative researcher. The data was analyzed using a six-step thematic analysis (51). All transcripts were read for familiarization to develop initial codes. An inductive approach was used to develop descriptive codes identified from similar patterns, topics, and elements of the intervention, which were then collated into themes, categories, and subcategories. Transcripts were also coded deductively using assumptions underlying a logic model of IBCM, depicting the relationship between program activities and the intended impact of IBCM. Themes were developed and reviewed iteratively and checked for consistency and appropriateness, amending where necessary. Themes included: inputs, activities, outputs, outcomes, and aims. Transcripts were compared for differences and similarities in how IBCM was operationalized, barriers and facilitators encountered during implementation, and the desired outcomes and aims of each program. Interviewees were sent a copy of the manuscript to validate accurate representation of their IBCM program and their feedback was incorporated.
Results
Conceptualization of IBCM
Description of IBCM
Several experts first heard the term ‘Integrated Bite Case Management’ in relation to rabies control in Bali, Indonesia in 2011 (52). However, most learned of IBCM from a program in Haiti (14) or through their own experience. All programs used the term IBCM, except for three where their intervention was referred to as either ‘a One Health approach to bite management’ (Chad) (10); ‘clinic-based surveillance’ (Madagascar) (27); or a ‘One Health approach’ to guide PEP recommendations (Brazil) (31).
Experts described IBCM in a way that combined its key components (activities) with the role it plays (outputs/outcomes):
“IBCM at its simplest is the ability to provide a proper risk assessment, usually in the context of the exposing animal, in a way in which the outcomes of the risk assessment can impact the human treatment decision.” (Expert #1, International-level)
These reported components (activities) were mostly consistent and aligned with the WHO definition of an IBCM program. They included: 1) reporting a bite or exposure event, 2) performing a risk assessment, 3) triggering an investigation for any bite deemed high-risk, 4) conducting an animal investigation, 5) observing animal for 10-14 days (to confirm a healthy animal) or collecting samples and diagnostic testing (from dead/euthanized animals), and 6) sharing feedback and investigation results across sectors (Figure 1).
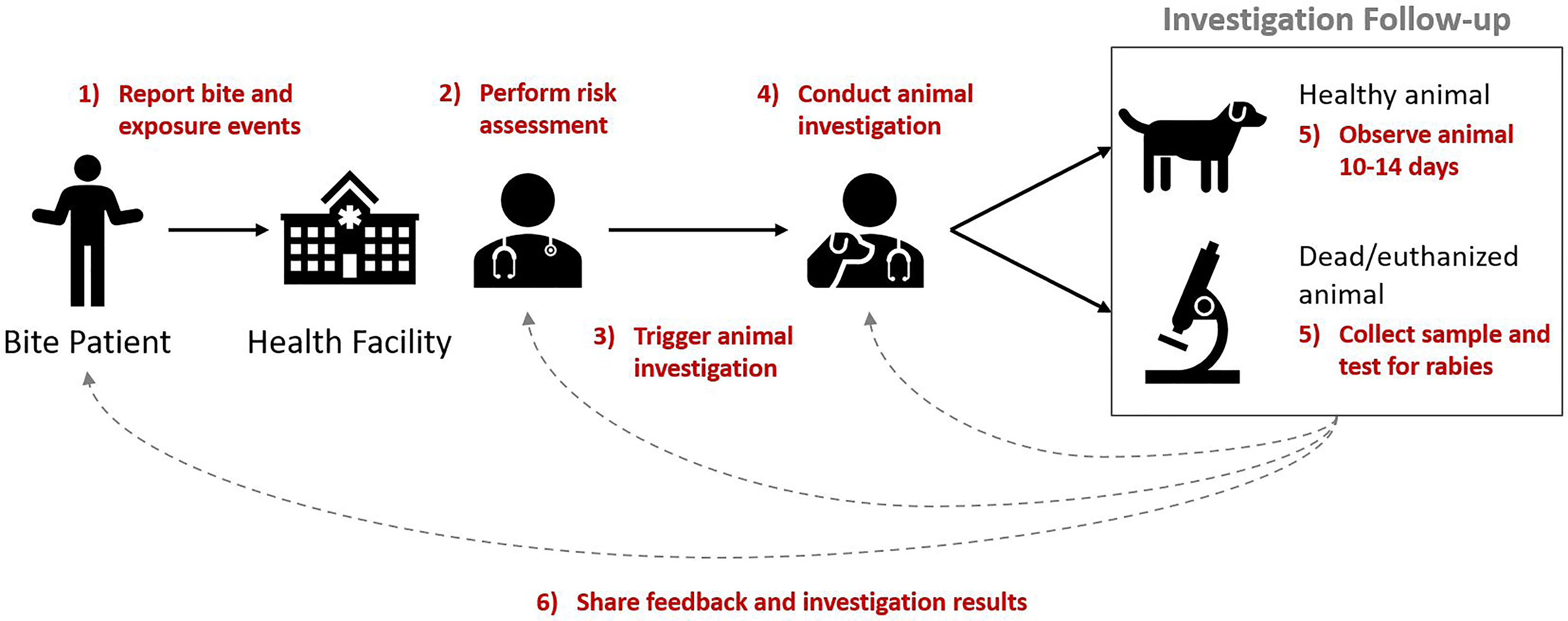
Figure 1 Key components of IBCM. Annotated in red are the six components (activities) that comprise the IBCM approach with arrows and numbering indicating the sequential order of these components.
Although a consensus emerged about the required components of IBCM, there was still some uncertainty about the definition. Specifically, experts had varied opinions about whether IBCM must always be initiated by a bite event, or determine treatment decisions or be used to specifically enhance surveillance. Therefore, while most interviewees perceived their work as being IBCM or similar to IBCM, some did not consider their program to formally be IBCM (for example, in Southern Brazil where the objective was not to strengthen surveillance, but to better manage PEP).
Participant’s concept of IBCM evolved over time and with experience. Most international-level experts viewed this approach as “passive public health surveillance” initiated by any suspect rabid animal – not only from bites:
“I used to think it was integrated BITE case management. Whereas, my attitude now is it’s the full investigation of a suspect animal, whether it’s for a bite or just a dog in the community behaving strangely.” (Expert #3, International-level)
Purpose of IBCM
Participants consistently identified several key roles of IBCM (outlined in the outcomes section of our conceptualized logic model, Figure 2). These roles were emphasized differently for each program and not all roles were relevant for every program. These roles were to: a) enhance surveillance through improved case detection, thereby enabling evaluation of control and prevention measures; b) directly and formally connect the health sector to the veterinary sector; c) inform PEP administration, aiming to improve patient care and increase adherence; d) better manage limited resources through judicious use of PEP; and e) advocate for community and stakeholder support and funding for rabies programs, and guide their implementation.
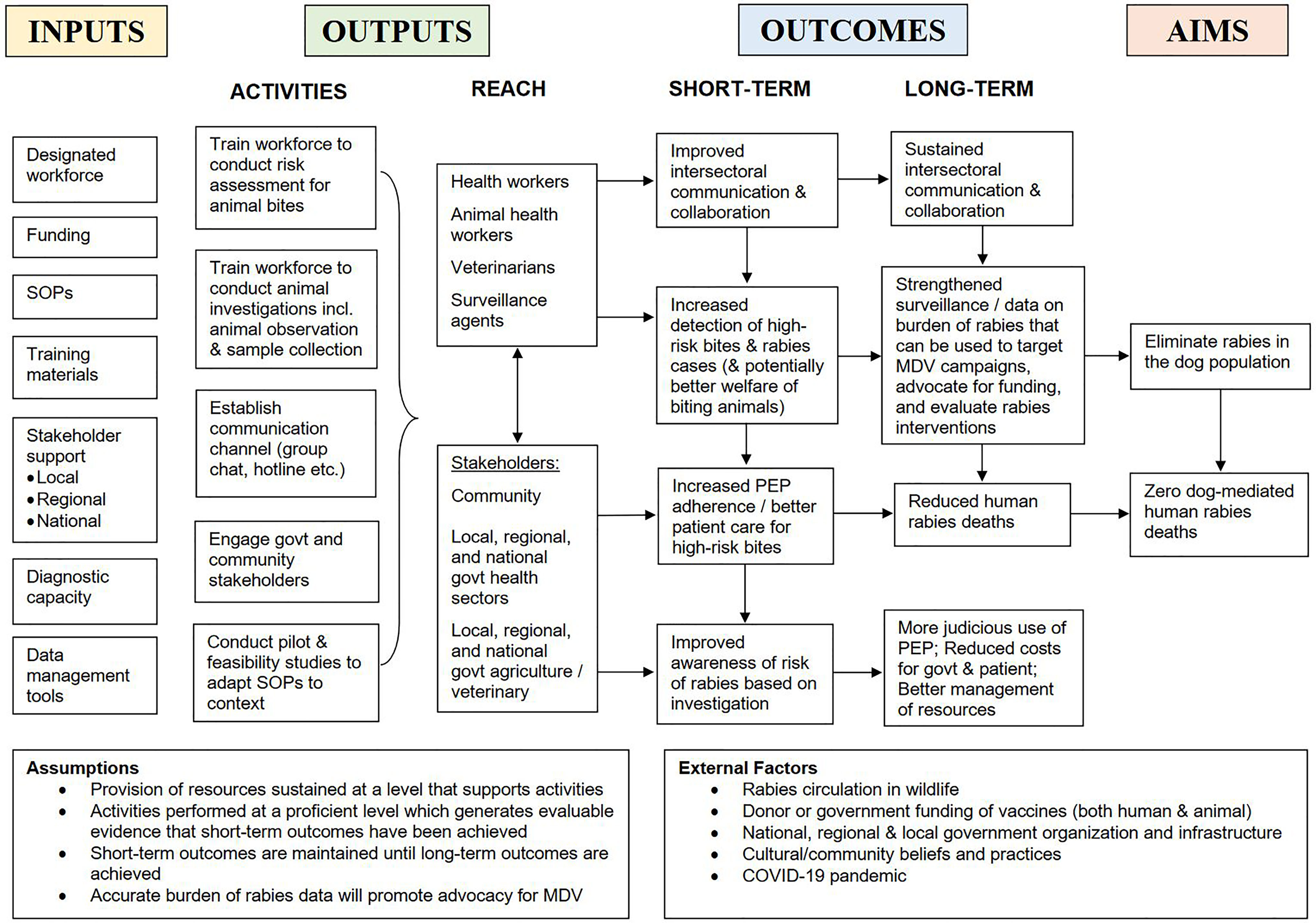
Figure 2 Logic model of IBCM. Representation of the relationship between resources (inputs), activities, outputs, short- and long-term outcomes, and aims of an IBCM program.
Experts agreed that countries with endemic dog rabies should all have a surveillance program, but opinions differed on when IBCM should be incorporated. A few experts viewed IBCM as an advanced surveillance strategy specifically meant for countries with a well-established control program who were close to elimination. Others argued that IBCM is fundamental and required at all stages (e.g., endemic, emerging, elimination and post-elimination) within routine surveillance:
“IBCM is needed as a country scales up its rabies elimination efforts and as an early intervention to try to bring down human deaths. It’s needed in countries where 1) you have a lot of human deaths and you need to do a better job getting PEP to the people at risk and 2) as you really start to take elimination seriously, it’s needed as a foundational system for evaluating the efforts that are going into vaccinating dogs. Then it’s important in the end-game, post-elimination phase to continue to evaluate the risk to people bitten.” (Expert #3, International-level)
Operationalization of IBCM
There was considerable variation in how IBCM was operationalized across settings, which were diverse in terms of their economic and epidemiological contexts (Table 1).
The most important prerequisites considered necessary for IBCM were the identification of a designated person/team responsible for investigating animals; and health facilities (hospitals, clinics, etc.) where bites are reported and PEP is administered. Several experts further mentioned the importance of stakeholder and community engagement prior to implementation.
The key input that differed between programs was who was identified and trained to carry out activities (Table 2). Three categories of workforce were identified: fully hired, a combination of hired and local government, and fully local government. Other inputs that varied were the use of rapid diagnostic tests (RDTs) and mobile applications for reporting/data management.
Program activities were similar amongst each workforce category. IBCM programs with a fully hired workforce—meaning their primary job responsibility was rabies/IBCM and their salary was paid by external funding—relied on the same team or person to conduct most or all of the IBCM components:
“We have trained surveillance agents … They all have the app on a tablet and go to the sentinel hospitals in their area weekly to check for bites … also through word of mouth. Then they go out and investigate the dog bites and report them.” (Expert #10, National-level)
In contrast, programs with a fully local government workforce trained existing capacity to complete IBCM activities. This involved health workers (nurses, doctors, etc.) conducting risk assessments and alerting their animal health counterparts (animal health workers, veterinarians, etc.) to investigate biting animals. Programs with a combined workforce trained a hired team and used local government staff—sometimes volunteer medical/veterinary students—to conduct risk assessments or animal investigations.
Almost all IBCM programs used paper-based or electronic registers from health facilities to collect bite data. These were typically from district and regional-level hospitals supplying PEP, but in some countries from rural community-level clinics with PEP access (e.g., Philippines). In addition to registers from health facilities, some programs used hotlines and/or trained local community health workers to report bite events. This was done particularly to enhance surveillance in rural areas with low PEP-seeking behaviors or limited access to PEP.
The mechanism to trigger animal investigations varied from calling/messaging the investigators, using hotline staff to notify them, and/or group chats or submitting data into mobile apps that send notifications to investigators.
Barriers and Facilitators to IBCM Implementation
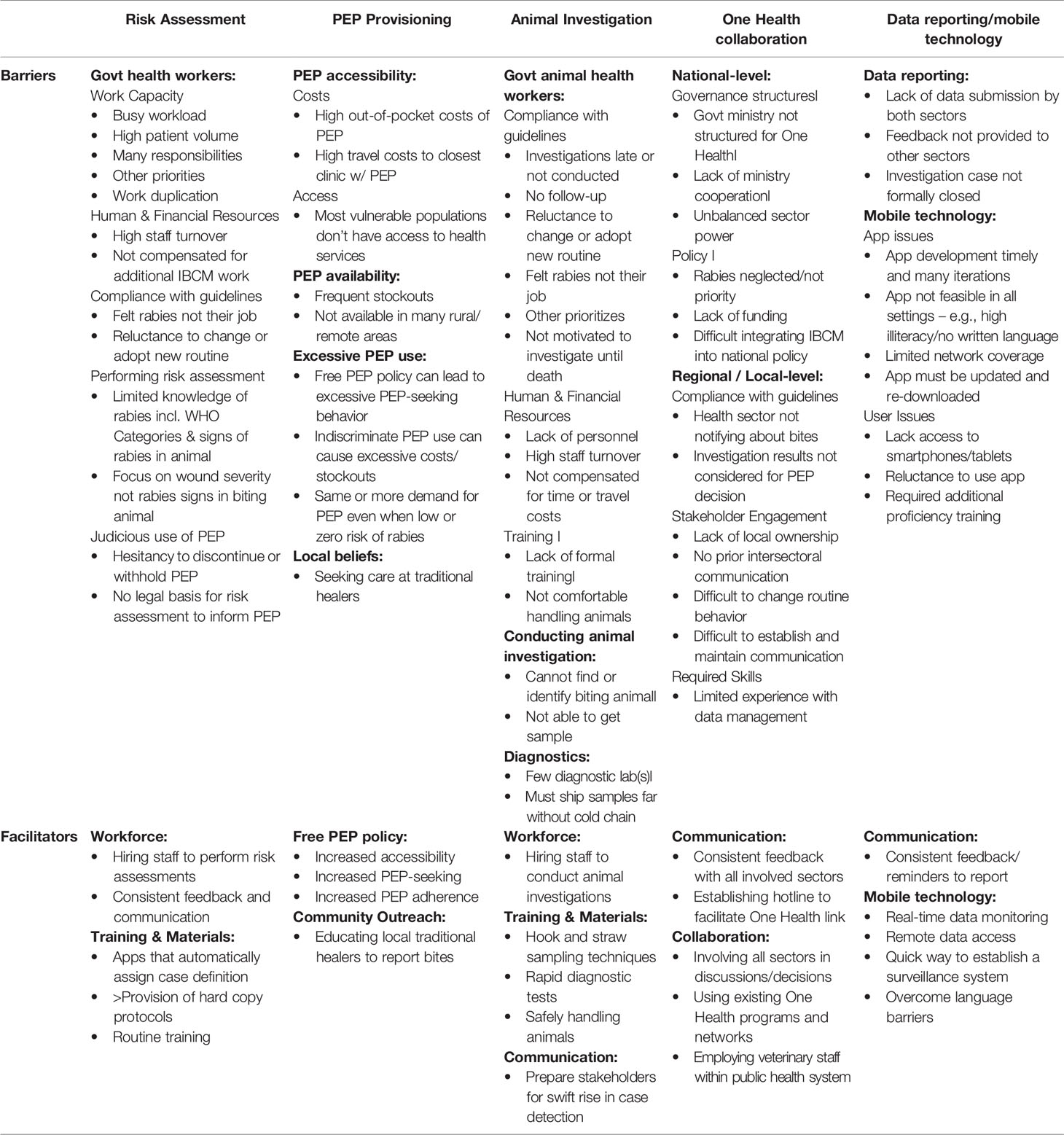
Reported barriers and facilitators could be placed under five main categories: risk assessment; PEP provisioning; animal investigation; the use of IBCM to facilitate One Health collaboration; and data reporting and mobile technology (Table 3).
Risk Assessment
IBCM programs with a hired workforce designated to perform risk assessments generally experienced fewer challenges than those with a local government workforce. Health workers were often stretched by busy workloads and other responsibilities/priorities. Some commonly reported barriers were: high volume of patients; added workload without compensation; high staff turnover; feeling that IBCM is not their responsibility or lacking interest; frustrations from work duplication (already have a reporting system); no accountability or lack of supervisory support; and reluctance to change/adopt a new way of working. Hiring staff addressed many of these barriers since rabies was their primary responsibility. Though this usually required additional funding from research grants or donors, challenging sustainability.
Some programs aimed to use risk assessments for more judicious use of PEP, typically in locations with frequent shortages or high expenditure on rabies biologics. These programs often experienced the challenge of ensuring health workers could perform risk assessments to a sufficiently reliable and effective level for PEP to be withheld or discontinued. While some health workers were proficient using WHO protocols to assess wound severity (Category I, II, and III), others required multiple training sessions. The majority were not familiar with the clinical signs of rabies in animals or the importance of assessing risk via the status of the biting animal:
“The main issue is that it’s been difficult for the nurses to perform high-risk assessments … a lot of the cases they post in the peer support chat are not high-risk based on our definition. And when you look at the protocols of the Department of Health, they actually have a lot more criteria on what is considered high-risk, which concentrates on the nature of the wound rather than the animal status.” (Expert #15, Local-level)
Other significant challenges regarding judicious use of PEP included there being no legal basis for determining PEP decisions from risk assessments; and health workers’ hesitancy to withhold or discontinue PEP, even where there was no apparent risk. Hard copy protocols, routine training and communication, and use of apps that automatically assign the animal case definition, supported health workers’ ability to more accurately determine low-risk vs high-risk bites and make treatment decisions.
PEP Provisioning
Most experts expressed that the level of PEP provisioning influenced health-seeking behavior, adherence, and the number of bite patients that present to health facilities. Free PEP policies reportedly increased accessibility and adherence to vaccine regimens. However, the provision of free PEP could also lead to much higher patient throughput and sometimes an excessive workload for those performing risk assessments. Furthermore, demand for PEP frequently remained high even when the risk of rabies was very low or even zero.
Typically, health-seeking and PEP adherence is much lower where patients pay for PEP or where travel costs are high due to limited PEP accessibility and availability. These settings often have a lower throughput of patients, with a larger proportion of those presenting as very high risk, as patients make their own assessment of risk. A few experts said that despite limited access to PEP being an issue, IBCM was often easier to implement in these settings:
“Of the people presenting for post-exposure prophylaxis, we’re probably looking at about half of them being bitten by dogs that we would consider to be high-risk, and likely to be rabid. We don’t see a lot of patients, but the patients that we see have a high chance of being really at risk for rabies.” (Expert #5, International-level)
Experts reported that the most-at-risk regions for rabies were rural areas where people had poor access to health services and may seek out traditional healers after being bitten. This also may affect the performance of health facilities supplying PEP as sentinels for high-risk bites. Some experts stated that improving PEP access could use up substantive rabies funding with diminishing returns. Alternatively, they felt that investment in mass dog vaccination, could decrease the incidence of rabies in source populations.
Animal Investigation
Barriers to conducting animal investigations using a government workforce were similar to those reported for risk assessments. Oftentimes, when an investigation was triggered, they were not conducted, were conducted too late, or there was no follow-up after the 10-14 day observation period. Factors included: required time/travel/resources (fuel, transport etc.); lack of personnel; high staff turnover; prioritizing diseases considered more economically important in livestock and poultry (e.g., foot-and-mouth disease, avian influenza, lumpy skin disease); lacking formal training as a veterinarian; and/or not feeling comfortable handling animals. Commonly, the person designated to investigate the animal had a background and responsibilities unrelated to animal rabies control and did not feel rabies was their job:
“The problem is that these inspectors can be veterinarians, biologists, environmental engineers or what they call ‘technicians’ without formal training … When they’re biologists, they spend most of their time doing vector surveillance … If they’re environmental scientists and engineers, they’re more interested in water or restaurant inspection … When there is a veterinarian, usually there is more focus on rabies.” (Expert #9, National-level)
Hiring program staff resolved many of these issues, but was not always an option due to funding constraints. Hired investigators also reportedly felt more confident handling animals and collecting samples, although hands-on training improved these skills for government investigators. Both hired and government staff experienced similar challenges during investigations, including not being able to find or identify the biting animal or collect a sample because the animal was already killed, buried, or decomposing on investigation.
A few countries had only one diagnostic laboratory with limited capacity in terms of equipment, staff, and quality control. Samples sometimes had to be shipped long distances, without cold chain or costs covered, often limiting sample submission to nearby areas. IBCM programs implemented in locations with established diagnostic capacity had a significant advantage. Furthermore, programs that trained field staff to use hook and straw sampling techniques simplified procedures and facilitated the collection, storage, and shipping of animal samples.
Some experts stated that rapid diagnostic tests (RDTs) are not reliable and pose complications to protocols for treatment decisions in the case of false negative results. Moreover, RDTs are not yet recommended by WHO and OIE, thus there is no guidance available for practitioners. Other experts found RDTs to be a facilitator for implementing IBCM, encouraging investigators to collect samples by providing immediate results to report to the health sector and communities:
“The vets applied rapid test kits, which are not validated yet, but it was very good to give the vets something at hand to empower them. The veterinary system is usually less financed than the human health services. If you want to apply ‘One Health’ you should empower them to bring them closer to the human health services. Otherwise, it’s a mismatch between roles … This test balanced it out a bit by giving the vets something to motivate the human health services to communicate with them.” (Expert #7, National-level)
Protocols usually stipulated that all samples tested with RDTs should be confirmed with laboratory diagnostics and that PEP decisions should not depend on RDT results. Experts stated that RDT results generally matched laboratory results.
Experts noted that motivation to investigate commonly increased only after a human death or several animal cases in their area. Moreover, IBCM typically increased detection resulting in a swift rise in cases, which could be a disincentive, especially when approaching elimination. Sometimes animal investigators would be blamed for rising cases, which discouraged their future reporting. Experts articulated the importance of preparing leadership to expect increased case numbers on introduction of IBCM and explain that this provides better guidance for control.
The Use of IBCM to Facilitate One Health Collaborations
Barriers to collaboration between sectors were found at the national, regional, and local level. Government ministries were rarely structured to facilitate intersectoral collaboration and faced challenges getting sectors to work together. Typically, sectors had unbalanced power, with the human health sector having more resources in terms of funding and influence. The priority placed on national rabies programs varied between countries, and was often neglected by both sectors. Using pre-existing One Health programs as a resource for IBCM activities was reported as a significant advantage. Also, programs that involved all relevant sectors in joint discussions, decisions, and training experienced more success:
“You need to have the buy-in of both the health and veterinary sectors, from the national- to the local-level. Because if they don’t think it’s important then we cannot force them to implement IBCM. They have to have a better understanding of why it is important so that it translates to actual work. If the National Program doesn’t believe in it, then it is pointless to push it further. But if they recommend IBCM as a policy, then it should go down the line … from national, regional, to local government.” (Expert #2, International-level)
Experts talked about the difficulty of creating local ownership and changing routine behaviors for information sharing at the individual level up to the ministerial level. There were barriers to getting the health sector to report high-risk bites to their animal health counterparts and to consider investigation results when making treatment decisions. Establishing and maintaining a line of communication between sectors was challenging in many settings. In a few instances, local government staff had limited data management skills which made it hard to link human bite cases to animal investigations.
To overcome these challenges, experts emphasized the importance of providing regular feedback and establishing a rapport between human and animal health workers, IBCM staff, and other stakeholders. Additionally, experts reported the need to consider local context and adapt protocols accordingly. Innovations, such as using hotlines as the link between sectors and the community, or developing different protocols for urban and rural settings, encouraged participation. Lastly, a few countries facilitated One Health collaboration by employing veterinary staff within the public health system (e.g., Brazil) - a strategy used in many high-income countries (e.g., United States).
Data Reporting and Mobile Technology
Experts reported a common barrier was lack of data submission from all involved sectors. Many times, the protocols for the risk assessment and all steps of the investigation (e.g., quarantine, follow-up, sample collection) were completed, but results were not reported, nor was feedback provided to other sectors.
The use of mobile applications for data reporting and management created both challenges and opportunities. App development initially took a great deal of time and many iterations. Yet once finalized, apps enabled IBCM protocols to be standardized by providing a template for questions and procedures for the risk assessment and animal investigation. Experts said the app allowed real-time data to be accessed remotely by program managers. This facilitated rapid identification and response to high-risk cases, as well as the timely provisioning of information to stakeholders and communities:
“The apps allow the technical expertise to sit anywhere in the world and real-time monitor cases, [human] vaccinations, dog vaccination programs … In the past it would take 2-3 years to get all of this paper data, enter it, and start to learn anything from your system. This ability to real-time evaluate and monitor what’s going on and make adjustments is invaluable.” (Expert #1, International-level)
While programs using mobile apps experienced issues with network coverage and internet access, these barriers were overcome with a feature allowing data to be saved. Not all program staff had access to smartphones where the app could be downloaded, which required some programs to purchase tablets or work phones. In addition, experts mentioned there was reluctance from some staff to use the app. Older staff in particular experienced difficulties using this technology and often required additional proficiency training. Changes in technology meant apps frequently needed to be re-downloaded/updated or risked becoming obsolete.
Most experts felt apps were a solution to many issues, while others saw them as an added complexity to training and implementation. In many settings, mobile apps helped overcome language barriers and could be tailored to local contexts (language, geo-hierarchy etc.). Yet one expert said using a mobile app would be difficult in their setting due to high illiteracy in official and local languages.
Impact of COVID-19
IBCM programs encountered COVID-19-related obstacles on several levels. Some program start dates were postponed, and most training for ongoing programs were postponed or canceled in 2020 and 2021. Moreover, allocated time and resources (personnel, diagnostics, vaccine storage, surveillance efforts, supply chains, funding) were re-focused on COVID-19:
“I think the main impact that COVID-19 is going to have on rabies in [country] is that it’s going to hide the problem. Because the surveillance system stops - it’s now focused on COVID … most of the resources for the lab go to COVID. And let’s say on a regular basis rabies is really neglected - now it’s going to be even more neglected.” (Expert #11, National-level)
Increased pressure on the health sector meant many health workers were extremely busy and did not report high-risk bites as frequently. Local travel restrictions limited in-person animal investigations or prevented them entirely. Experts in some countries observed drops in animals tested due to decreased surveillance efforts. Several IBCM programs experienced declines in bite patient presentations, typically for 2-5 months at the start of 2020 lockdowns. Experts similarly described the cancellation or disruption of dog vaccination campaigns in 2020 and/or 2021 resulting in lower vaccination coverages. Some areas are already seeing rising human and dog cases, further necessitating the need for IBCM.
Lastly, the pandemic affected peoples’ livelihoods and caused income losses, making healthcare less affordable. Experts speculated that people are not feeding community dogs as frequently, leading them to roam farther for food and creating a more favorable environment for rabies transmission. People were reported to have also been abandoning pets due to costs and fear of COVID-19 transmission, potentially increasing stray dog numbers. In order to accurately measure the impact of the pandemic, enhanced surveillance such as IBCM is needed.
Discussion
The main finding from this study was the variation between IBCM programs reported across epidemiological and geographical settings. Specifically, the interviews highlighted the diversity within experts’ conceptualization of the definition and roles of IBCM. This range of perspectives demonstrates that there are different ways of organizing IBCM within health systems and that it is not a one-size-fits-all approach. While there was consensus among experts and the wider literature about the required components (inputs and activities) of IBCM (Figure 1) which aligned with WHO guidance (2), these components were operationalized differently between programs. Moreover, experts’ perspective of the purpose of IBCM often differed and by implication, so did the desired outcomes of each program. In practice, IBCM was tailored to meet the demands of the local context and level of rabies control in place. Experts were well versed about how IBCM operated in their settings and what outcomes they wished to achieve by implementing this One Health approach. But experiences did not necessarily translate across contexts, affecting perceptions about the function, motivation for, and implementation of IBCM. Nonetheless, despite differences in operationalization and desired outcomes, programs shared many similar experiences with the challenges they faced and progress in overcoming them.
Contextual features of each location—which can be described broadly as epidemiological or non-epidemiological (53)—contributed to differences in desired outcomes and barriers and facilitators to implementation. The epidemiological context (e.g., human deaths, incidence in dog population) partially influenced how much rabies was prioritized at the national and local level. However, most variation in terms of the success of IBCM and the impact achieved was due to the non-epidemiological context. This includes features such as: social and economic (e.g., health-seeking behaviors, GDP, HDI); cultural (beliefs, attitudes, and practices among policymakers, practitioners, communities); geographical (e.g., urban vs. rural); service and organization (e.g., motivation, willingness to change); policy (PEP provisioning); financial (e.g., funding); political (e.g., level of decentralization, distribution of power among sectors/stakeholders); and historical (e.g., presence of rabies). The issue of sustainability was at the core of many barriers to implementation, which the literature acknowledges as a major hindrance for other evidence-based interventions (54). Using an existing government workforce for program activities was typically seen as more sustainable. Yet, experts found it difficult to incentivize or motivate human and animal health workers to change their way of working and complete extra work without supplemental pay. The overall success of programs appeared to be influenced by practitioners’ and stakeholders’ viewpoint of the added value of IBCM relative to the extra time and effort required from them and ultimately the degree to which funding was allocated.
Most programs operated in settings with endemic dog rabies, weak or nonexistent surveillance, and scarce funding. Thus, the primary desired outcome was to establish a cost-effective surveillance system to rapidly identify high-risk human exposures and potential rabid animals. One exception was the IBCM-like approach used in three southern states in Brazil, where dog rabies has been eliminated and strong surveillance already exists. Instead of surveillance, their aim was to reduce indiscriminate use of PEP following a shortage. Judicious use of PEP was considered a pivotal role for some IBCM programs (e.g., Bali, Haiti, Philippines), while being of minimal importance to others (e.g., Goa, India). In general, countries where frequent PEP shortages occur and/or governments pay for PEP placed more emphasis on its judicious use. Though, that was not always the case. Both India and the Philippines have high numbers of patients receiving government supplied PEP for healthy animal bites. Yet, in India, where they affordably manufacture human rabies biologics, there was no aim to reduce unnecessary PEP. Furthermore, certain country contexts made implementing IBCM challenging. For example, in India where unowned and fairly homogeneous-looking dogs limited the ability to trace animals. Program success was also hindered in extremely resource-poor countries (e.g., Chad, Madagascar) due to inadequate infrastructure and more pressing priorities. Alternatively, some contexts made implementing IBCM more straightforward, such as countries in Latin America (e.g., Brazil, Peru) with substantial budgets for mass dog vaccination, and a strong history of One Health in action facilitating successful rabies control.
This study underscores the complexity of IBCM which stems from the interaction of its many components as they relate to the context or system where they are implemented (55). These findings demonstrate the importance of transferring the evidence base of the IBCM approach to inform adaptations when implemented in a new context to ensure effectiveness (56). Tools such as the ADAPT guidance provide a framework and checklist, facilitating the streamlining of these processes and reducing research waste (57). To prevent misunderstanding of the concept of IBCM, future guidance should include an explicit program theory (58), articulating how IBCM is expected to contribute to a chain of intermediate results and ultimately to expected outcomes (via a Theory of Change, similar to Figure 2). This has the potential to illustrate to stakeholders how implementing IBCM can be useful in their own context and might help overcome challenges specific to their setting. Furthermore, guidance should be expanded to include clear examples of how IBCM has been operationalized in various settings and how activities, outcomes, and aims might differ accordingly. As well as, guidance should be provided for practitioners on the use of RDTs, and for health workers receiving patients to cover assessment of the history and signs of rabies in biting animals, which is not integrated into WHO guidance on post-exposure prophylaxis. Lastly, IBCM programs should use standardized WHO tools and practices (2)—tailored to local understandings—for interpreting rabies risk to improve data quality and comparability of the burden of rabies and transmission pathways between settings.
As a relatively new approach, IBCM exemplifies the challenges faced when implementing One Health (59), with lessons that could be applied to other complex zoonotic diseases. Integrated One Health approaches are vital for tackling both endemic and emerging zoonoses, and for antimicrobial resistance (60, 61). Strengthening surveillance systems in LMICs for endemic diseases, such as rabies, builds foundations to address emerging zoonotic diseases like COVID-19 (62). Formalizing One Health approaches through intersectoral government bodies, including joint budgets and policies, can help to overcome institutionalized/structural barriers (61, 62). Yet, like IBCM, varying perceptions about the concept of ‘One Health’ make it difficult to standardize the operationalization of these approaches in both high-income countries and LMICs (63, 64). These challenges are amplified by the lack of sustainability of funding and infrastructure that are exacerbated in LMICs. This study emphasizes the need for more implementation research to improve and understand IBCM program delivery and policies (65). In recent years, such studies have helped strengthen the gap between knowledge and real-world action for a variety of neglected tropical diseases (65, 66). Future research exploring the knowledge-practice gaps of implementing IBCM could improve the cost-effective roll out of IBCM and provide a potential example for other One Health interventions.
There are some limitations in this study. One-hour interviews provided limited time to discuss perspectives of and experiences with IBCM. For broader comprehension about the implementation and adaptation of IBCM, more in-depth qualitative research is required (e.g., ethnographic participant observations, development of program theory) (54). Caution is required regarding interpretation over the study’s reliability, as it was not designed to be representative for IBCM programs or specific regions/countries. Most participants had a background in veterinary medicine and/or research, and representation from the medical sector was lacking. Future research including the perspectives of clinicians and public health experts would be beneficial, however, it should be noted that there is relatively little involvement of medical professionals leading One Health programs for rabies. The logic model of IBCM (Figure 2) serves as a template, but does not fully describe the complexity of IBCM and how that relates to different contexts. Moreover, the programs included varied in maturity, from a decade in Bali, Indonesia (2011) to programs still in development, limiting direct comparison. Nevertheless, this study covered a wide scope of perspectives, work experience, epidemiological contexts (from elimination to high endemicity), and geographies. Hence, the study is a valuable step towards discovering lessons about this approach and for understanding how One Health can be operationalized to achieve dog-mediated rabies elimination.
We conclude with preliminary recommendations to support the design and implementation of IBCM programs keeping sustainability in mind. As the only endemic zoonosis with an official elimination goal set by the WHO, rabies should be prioritized and funded to support hiring of staff and implementation of control programs (15). A One Health approach to surveillance should be implemented in all endemic countries at any stage of their control program as this is the most targeted way to identify rabid animals. Many existing reporting systems for rabies are not fit-for-purpose for surveillance and provisioning of PEP. Surveillance systems are often siloed and do not consider the risk of the biting animal or recognize the value of risk assessments, leading to uninformed administration of PEP. In response, IBCM has been developed as a cost-effective approach to address these weaknesses (18). However, current structures within governments, policies, and ways of working pose barriers to introducing IBCM. To successfully and sustainably implement IBCM, there needs to be consideration for how governments and policy can be updated to better facilitate multisectoral, interdisciplinary approaches generally (67). It is imperative that each program is tailored to the context of the country/region where it will be implemented, with careful consideration of context during development, implementation, and evaluation (53). Lastly, the joint involvement and ownership of government authorities from all relevant sectors, local stakeholders, and the community is essential for the effective development and adoption of IBCM protocols (68, 69).
Data Availability Statement
Data supporting the conclusions of this article will be made available on request due to privacy/ethical restrictions.
Ethics Statement
The studies involving human participants were reviewed and approved by the College of Medical, Veterinary & Life Sciences Ethics Committee at the University of Glasgow (Ref No. 200190081). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CS collected, analyzed and interpreted the data, and drafted the initial manuscript. KH and NRC supervised data collection and methodology, contributed to the analysis and interpretation, and revision of the manuscript. SM and RM contributed to the interpretation of the results and oversaw the writing and revision of the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
funding
CS is funded by the joint University of Glasgow and University of Edinburgh doctoral training programme in One Health. KH is funded by Wellcome (207569/Z/17/Z). NC is funded by UKRI MRC (MR/R025649/1).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are extremely grateful to our participants for their time and invaluable expertise including feedback that greatly improved the manuscript.
Abbreviations
FAO, Food and Agriculture Organization; GARC, Global Alliance for Rabies Control; IBCM, Integrated Bite Case Management; LMIC, low- and middle-income countries; OIE, World Organisation for Animal Health; PEP, post-exposure prophylaxis; RDT, rapid diagnostic test; WHO, World Health Organization.
References
1. Cleaveland S, Hampson K. Rabies Elimination Research: Juxtaposing Optimism, Pragmatism and Realism. Proc R Soc B (2017) 284(1869):20171880. doi: 10.1098/rspb.2017.1880
2. World Health Organization. WHO Expert Consultation on Rabies: Third Report. Geneva, Switzerland: World Health Organization (2018). p. 183. (WHO Technical Report Series).
3. Hampson K, Dushoff J, Cleaveland S, Haydon DT, Kaare M, Packer C, et al. Transmission Dynamics and Prospects for the Elimination of Canine Rabies. PloS Biol (2009) 7(3):e1000053. doi: 10.1371/journal.pbio.1000053
4. Wallace R, Etheart M, Ludder F, Augustin P, Fenelon N, Franka R, et al. The Health Impact of Rabies in Haiti and Recent Developments on the Path Toward Elimination, 2010–2015. Am J Trop Med Hyg (2017) 97(4_Suppl):76–83. doi: 10.4269/ajtmh.16-0647
5. Velasco-Villa A, Escobar LE, Sanchez A, Shi M, Streicker DG, Gallardo-Romero NF, et al. Successful Strategies Implemented Towards the Elimination of Canine Rabies in the Western Hemisphere. Antiviral Res (2017) 143:1–12. doi: 10.1016/j.antiviral.2017.03.023
6. Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, et al. Estimating the Global Burden of Endemic Canine Rabies. PloS Negl Trop Dis (2015) 9(4):e0003709. doi: 10.1371/journal.pntd.0003709
7. Wallace RM, Undurraga EA, Blanton JD, Cleaton J, Franka R. Elimination of Dog-Mediated Human Rabies Deaths by 2030: Needs Assessment and Alternatives for Progress Based on Dog Vaccination. Front Vet Sci (2017) 4:9. doi: 10.3389/fvets.2017.00009
8. Undurraga EA, Blanton JD, Thumbi SM, Mwatondo A, Muturi M, Wallace RM. Tool for Eliminating Dog-Mediated Human Rabies Through Mass Dog Vaccination Campaigns. Emerg Infect Dis (2017) 23(12):2114–6. doi: 10.3201/eid2312.171148
9. Changalucha J, Steenson R, Grieve E, Cleaveland S, Lembo T, Lushasi K, et al. The Need to Improve Access to Rabies Post-Exposure Vaccines: Lessons From Tanzania. Vaccine (2019) 37:A45–53. doi: 10.1016/j.vaccine.2018.08.086
10. Lechenne M, Mindekem R, Madjadinan S, Oussiguéré A, Moto DD, Naissengar K, et al. The Importance of a Participatory and Integrated One Health Approach for Rabies Control: The Case of N’djaména, Chad. Trop Med (2017) 2(3):43. doi: 10.3390/tropicalmed2030043
11. Lavan RP, King A, Sutton DJ, Tunceli K. Rationale and Support for a One Health Program for Canine Vaccination as the Most Cost-Effective Means of Controlling Zoonotic Rabies in Endemic Settings. Vaccine (2017) 35(13):1668–74. doi: 10.1016/j.vaccine.2017.02.014
12. Rysava K, Miranda ME, Zapatos R, Lapiz S, Rances P, Miranda LM, et al. On the Path to Rabies Elimination: The Need for Risk Assessments to Improve Administration of Post-Exposure Prophylaxis. Vaccine (2019) 37:A64–72. doi: 10.1016/j.vaccine.2018.11.066
13. Lushasi K, Steenson R, Bernard J, Changalucha JJ, Govella NJ, Haydon DT, et al. One Health in Practice: Using Integrated Bite Case Management to Increase Detection of Rabid Animals in Tanzania. Front Public Health (2020) 8:13. doi: 10.3389/fpubh.2020.00013
14. Wallace RM, Reses H, Franka R, Dilius P, Fenelon N, Orciari L, et al. Establishment of a Canine Rabies Burden in Haiti Through the Implementation of a Novel Surveillance Program. PloS Negl Trop Dis (2015) 9(11):e0004245. doi: 10.1371/journal.pntd.0004245
15. Zero by 30: The Global Strategic Plan to End Human Deaths From Dog-Mediated Rabies by 2030. World Health Organization (WHO), Food and Agriculture Organization (FAO) of the United Nations, World Organisation for Animal Health (OIE), and the Global Alliance for Rabies Control (GARC), Geneva, Switzerland (2018).
16. Etheart MD, Kligerman M, Augustin PD, Blanton JD, Monroe B, Fleurinord L, et al. Effect of Counselling on Health-Care-Seeking Behaviours and Rabies Vaccination Adherence After Dog Bites in Haiti, 2014–15: A Retrospective Follow-Up Survey. Lancet Global Health (2017) 5(10):e1017–25. doi: 10.1016/S2214-109X(17)30321-2
17. Mbaipago N, Mindekem R, Madjiadinan A, Moyengar R, Oussigueré A, Naissengar K, et al. Short Communication on the Use of a Free Rabies Hotline Service in Chad. Acta Trop (2020) 206:105446. doi: 10.1016/j.actatropica.2020.105446
18. Undurraga EA, Meltzer MI, Tran CH, Atkins CY, Etheart MD, Millien MF, et al. Cost-Effectiveness Evaluation of a Novel Integrated Bite Case Management Program for the Control of Human Rabies, Haiti 2014–2015. Am J Trop Med Hyg (2017) 96(6):1307–17. doi: 10.4269/ajtmh.16-0785
19. Medley A, Millien M, Blanton J, Ma X, Augustin P, Crowdis K, et al. Retrospective Cohort Study to Assess the Risk of Rabies in Biting Dogs, 2013–2015, Republic of Haiti. Trop Med (2017) 2(2):14. doi: 10.3390/tropicalmed2020014
20. Ma X, Blanton JD, Millien MF, Medley AM, Etheart MD, Fénelon N, et al. Quantifying the Risk of Rabies in Biting Dogs in Haiti. Sci Rep (2020) 10(1):1062. doi: 10.1038/s41598-020-57908-9
21. Lapiz SMD, Miranda MEG, Garcia RG, Daguro LI, Paman MD, Madrinan FP, et al. Implementation of an Intersectoral Program to Eliminate Human and Canine Rabies: The Bohol Rabies Prevention and Elimination Project. PloS Negl Trop Dis (2012) 6(12):e1891. doi: 10.1371/journal.pntd.0001891
22. Hampson K, Ventura F, Steenson R, Mancy R, Trotter C, Cooper L, et al. The Potential Effect of Improved Provision of Rabies Post-Exposure Prophylaxis in Gavi-Eligible Countries: A Modelling Study. Lancet Infect Dis (2019) 19(1):102–11. doi: 10.1016/S1473-3099(18)30512-7
23. Madjadinan A, Hattendorf J, Mindekem R, Mbaipago N, Moyengar R, Gerber F, et al. Identification of Risk Factors for Rabies Exposure and Access to Post-Exposure Prophylaxis in Chad. Acta Trop (2020) 209:105484. doi: 10.1016/j.actatropica.2020.105484
24. Mbilo C, Léchenne M, Hattendorf J, Madjadinan S, Anyiam F, Zinsstag J. Rabies Awareness and Dog Ownership Among Rural Northern and Southern Chadian Communities—Analysis of a Community-Based, Cross-Sectional Household Survey. Acta Trop (2017) 175:100–11. doi: 10.1016/j.actatropica.2016.06.003
25. Ngugi JN, Maza AK, Omolo OJ, Obonyo M. Epidemiology and Surveillance of Human Animal-Bite Injuries and Rabies Post-Exposure Prophylaxis, in Selected Counties in Kenya, 2011–2016. BMC Public Health (2018) 18(1):996. doi: 10.1186/s12889-018-5888-5
26. Filla C, Rajeev M, Randriana Z, Hanitriniana C, Rafaliarison RR, Edosoa GT, et al. Lessons Learned and Paths Forward for Rabies Dog Vaccination in Madagascar: A Case Study of Pilot Vaccination Campaigns in Moramanga District. Trop Med (2021) 6(2):48. doi: 10.3390/tropicalmed6020048
27. Rajeev M, Edosoa G, Hanitriniaina C, Andriamandimby SF, Guis H, Ramiandrasoa R, et al. Healthcare Utilization, Provisioning of Post-Exposure Prophylaxis, and Estimation of Human Rabies Burden in Madagascar. Vaccine (2019) 37:A35–44. doi: 10.1016/j.vaccine.2018.11.011
28. Sánchez-Soriano C, Gibson AD, Gamble L, Bailey JLB, Mayer D, Lohr F, et al. Implementation of a Mass Canine Rabies Vaccination Campaign in Both Rural and Urban Regions in Southern Malawi. PloS Negl Trop Dis (2020) 14(1):e0008004. doi: 10.1371/journal.pntd.0008004
29. Sambo M, Hampson K, Changalucha J, Cleaveland S, Lembo T, Lushasi K, et al. Estimating the Size of Dog Populations in Tanzania to Inform Rabies Control. Vet Sci (2018) 5(3):77. doi: 10.3390/vetsci5030077
30. Pan American Health Organization (PAHO/WHO). The Regional Information System for The Epidemiological Surveillance of Rabies (SIRVERA). Available at: https://sirvera.panaftosa.org.br/ (Accessed 11 Nov 2021).
31. Benavides JA, Megid J, Campos A, Rocha S, Vigilato MAN, Hampson K. An Evaluation of Brazil’s Surveillance and Prophylaxis of Canine Rabies Between 2008 and 2017. PloS Negl Trop Dis (2019) 13(8):e0007564. doi: 10.1371/journal.pntd.0007564
32. Centro Nacional De Epidemiología, MSPAS Guatemala . Available at: http://epidemiologia.mspas.gob.gt/ (Accessed November 11, 2021).
33. Lunney M, Jones A, Stiles E, Waltner-Toews D. Assessing Human–Dog Conflicts in Todos Santos, Guatemala: Bite Incidences and Public Perception. Prev Vet Med (2011) 102(4):315–20. doi: 10.1016/j.prevetmed.2011.07.017
34. Barbosa Costa G, Ludder F, Monroe B, Dilius P, Crowdis K, Blanton JD, et al. Barriers to Attendance of Canine Rabies Vaccination Campaigns in Haiti, 2017. Transbound Emerg Dis (2020) 67(6):2679–91. doi: 10.1111/tbed.13622
35. De la Puente-León M, Levy MZ, Toledo AM, Recuenco S, Shinnick J, Castillo-Neyra R. Spatial Inequality Hides the Burden of Dog Bites and the Risk of Dog-Mediated Human Rabies. Am J Trop Med Hyg (2020) 103(3):1247–57. doi: 10.4269/ajtmh.20-0180
36. Castillo-Neyra R, Toledo AM, Arevalo-Nieto C, MacDonald H, de la Puente-León M, Naquira-Velarde C, et al. Socio-Spatial Heterogeneity in Participation in Mass Dog Rabies Vaccination Campaigns, Arequipa, Peru. PloS Negl Trop Dis (2019) 13(8):e0007600. doi: 10.1371/journal.pntd.0007600
37. Sudarshan MK, Narayana DH. Appraisal of Surveillance of Human Rabies and Animal Bites in Seven States of India. Indian J Public Health (2019) 63:S3–8. doi: 10.4103/ijph.IJPH_377_19
39. Putra AAG, Hampson K, Girardi J, Hiby E, Knobel D, Mardiana W, et al. Response to a Rabies Epidemic, Bali, Indonesia, 2008–2011. Emerg Infect Dis (2013) 19(4):648–51. doi: 10.3201/eid1904.120380
40. Wismandanu O, Balia RL, Aprianti W. The Effectiveness of Rabies Control Program in West Bandung Regency, West Java, Indonesia. Population (2019) 29:9. doi: 10.2991/isessah-19.2019.30
41. Quiambao B, Varghese L, Demarteau N, Sengson RF, Javier J, Mukherjee P, et al. Health Economic Assessment of a Rabies Pre-Exposure Prophylaxis Program Compared With Post-Exposure Prophylaxis Alone in High-Risk Age Groups in the Philippines. Int J Infect Dis (2020) 97:38–46. doi: 10.1016/j.ijid.2020.05.062
42. Republic of the Philippines, Department of Health. Rabies Prevention and Control Program. Rabies Prevention and Control Program | Department of Health website (doh.gov.ph (Accessed Aug 11, 2021).
43. Lachica ZPT, Evangelio SA, Diamante EO, Clemente AJ, Peralta JM, Murao LAE, et al. Trends of Canine Rabies Lyssavirus and Impact of the Intensified Rabies Control Program in Davao City, Philippines: 2006–2017. Philipp J Sci (2019) 148.4:751–63.
44. Nguyen HTT, Tran CH, Dang AD, Tran HGT, Vu TD, Pham TN, et al. Rabies Vaccine Hesitancy and Deaths Among Pregnant and Breastfeeding Women — Vietnam, 2015–2016. MMWR Morb Mortal Wkly Rep (2018) 67(8):250–2. doi: 10.15585/mmwr.mm6708a4
45. Overview of the ASEAN Rabies Elimination Strategy and its Application in Vietnam (2018). Available at: https://rabiesalliance.org/resource/overview-asean-rabies-elimination-strategy-and-its-application-vietnam (Accessed Nov 11, 2021).
46. Pham QD, Phan LT, Nguyen TPT, Doan QMN, Nguyen HD, Luong QC, et al. An Evaluation of the Rabies Surveillance in Southern Vietnam. Front Public Health (2021) 9:610905. doi: 10.3389/fpubh.2021.610905
47. Rysava K, Mancero T, Caldas E, de Carvalho MF, Castro APB, Gutiérrez V, et al. Towards the Elimination of Dog-Mediated Rabies: Development and Application of an Evidence-Based Management Tool. BMC Infect Dis (2020) 20(1):778. doi: 10.1186/s12879-020-05457-x
48. Acharya KP, Subedi D, Wilson RT. Rabies Control in South Asia Requires a One Health Approach. One Health (2021) 12:100215. doi: 10.1016/j.onehlt.2021.100215
49. Zoom Video Communications Inc. Security Guide. Zoom Video Communications Inc. San Jose, California (2016). Available at: https://d24cgw3uvb9a9h.cloudfront.net/static/81625/doc/Zoom-Security-White-Paper.pdf.
50. QSR International Pty Ltd. NVivo (Version 12) (2018). Available at: https://www.qsrinternational.com/nvivo-qualitative-data-analysis-software/home.
51. Braun V, Clarke V. Using Thematic Analysis in Psychology. Qual Res Pyschol (2006) 3(2):77–101. doi: 10.1191/1478088706qp063oa
52. Food and Agriculture Organizatin (FAO). Emergency Centre for Transboundary Animal Diseases (ECTAD): Annual Report. Jakarta, Indonesia: FAO Indonesia (2013) p. 22–3. Available at: https://coin.fao.org/coin-static/cms/media/20/14012461211060/2014_ar2013_final_lr_comp.pdf.
53. Craig P, Di Ruggiero E, Frohlich KL, Mykhalovskiy E, White M, on behalf of the Canadian Institutes of Health Research (CIHR)–National Institute for Health Research (NIHR) Context Guidance Authors Group (listed alphabetically), et al. Taking Account of Context in Population Health Intervention Research: Guidance for Producers, Users and Funders of Research FAO Indonesia (2018). Available at: https://www.journalslibrary.nihr.ac.uk/nihr-research/canadian-institutes-of-health-research-cihr-and-nihr-collaboration.htm.
54. Hailemariam M, Bustos T, Montgomery B, Barajas R, Evans LB, Drahota A. Evidence-Based Intervention Sustainability Strategies: A Systematic Review. Implement Sci (2019) 14(1):57. doi: 10.1186/s13012-019-0910-6
55. Hawe P. Lessons From Complex Interventions to Improve Health. Annu Rev Public Health (2015) 36(1):307–23. doi: 10.1146/annurev-publhealth-031912-114421
56. Copeland L, Littlecott H, Couturiaux D, Hoddinott P, Segrott J, Murphy S, et al. The What, Why and When of Adapting Interventions for New Contexts: A Qualitative Study of Researchers, Funders, Journal Editors and Practitioners’ Understandings. PloS One (2021) 16(7):e0254020. doi: 10.1371/journal.pone.0254020
57. Moore G, Campbell M, Copeland L, Craig P, Movsisyan A, Hoddinott P, et al. Adapting Interventions to New Contexts—The ADAPT Guidance. BMJ (2021) 374:n1679. doi: 10.1136/bmj.n1679
58. Rogers PJ, Funnell SC. Purposeful Program Theory: Effective Use of Theories of Change and Logic Models. San Francisco, Calif: Jossey-Bass (2013). Available at: http://rbdigital.oneclickdigital.com.
59. Hampson K, De Balogh K, Mcgrane J. Lessons for Rabies Control and Elimination Programmes: A Decade of One Health Experience From Bali, Indonesia: -EN- -FR- Les Enseignements Des Programmes De Contrôle Et D’élimination De La Rage: Une Décennie D’expérience Une Seule Santé À Bali (Indonésie) -ES- Un Decenio De Experiencia En Bali (Indonesia) Con Una Sola Salud Y Sus Enseñanzas Para Los Programas De Control Y Eliminación De La Rabia. Rev Sci Tech OIE (2019) 38(1):213–24. doi: 10.20506/rst.38.1.2954
60. Destoumieux-Garzón D, Mavingui P, Boetsch G, Boissier J, Darriet F, Duboz P, et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front Vet Sci (2018) 5:14. doi: 10.3389/fvets.2018.00014
61. Mackenzie JS, Jeggo M. The One Health Approach—Why Is It So Important? TropicalMed (2019) 4(2):88. doi: 10.3390/tropicalmed4020088
62. Halliday J, Daborn C, Auty H, Mtema Z, Lembo T, Bronsvoort BMd, et al. Bringing Together Emerging and Endemic Zoonoses Surveillance: Shared Challenges and a Common Solution. Philos Trans R Soc B (2012) 367(1604):2872–80. doi: 10.1098/rstb.2011.0362
63. Lerner H, Berg C. The Concept of Health in One Health and Some Practical Implications for Research and Education: What Is One Health? Infect Ecol Epidemiol (2015) 5(1):25300. doi: 10.3402/iee.v5.25300
64. Evans BR, Leighton FA. A History of One Health: -EN- A History of One Health -FR- Histoire Du Concept « Une Seule Santé » -ES- Historia De «Una Sola Salud». Rev Sci Tech OIE (2014) 33(2):413–20. doi: 10.20506/rst.33.2.2298
65. Theobald S, Brandes N, Gyapong M, El-Saharty S, Proctor E, Diaz T, et al. Implementation Research: New Imperatives and Opportunities in Global Health. Lancet (2018) 392(10160):2214–28. doi: 10.1016/S0140-6736(18)32205-0
66. Bardosh KL. Towards a Science of Global Health Delivery: A Socio-Anthropological Framework to Improve the Effectiveness of Neglected Tropical Disease Interventions. PloS Negl Trop Dis (2018) 12(7):e0006537. doi: 10.1371/journal.pntd.0006537
67. UNICEF, UNDP, World Bank, and WHO. Special Programme for Research and Training in Tropical Diseases (TDR). Available at: https://tdr.who.int/home/our-work (Accessed December 1, 2021).
68. Masefield SC, Msosa A, Chinguwo FK, Grugel J. Stakeholder Engagement in the Health Policy Process in a Low Income Country: A Qualitative Study of Stakeholder Perceptions of the Challenges to Effective Inclusion in Malawi. BMC Health Serv Res (2021) 21(1):98. doi: 10.1186/s12913-021-07016-9
Keywords: dog-mediated rabies, rabies elimination, rapid diagnostic tests, mobile health, post-exposure prophylaxis, zoonosis, implementation research
Citation: Swedberg C, Mazeri S, Mellanby RJ, Hampson K and Chng NR (2022) Implementing a One Health Approach to Rabies Surveillance: Lessons From Integrated Bite Case Management. Front. Trop. Dis 3:829132. doi: 10.3389/fitd.2022.829132
Received: 05 December 2021; Accepted: 12 April 2022;
Published: 20 June 2022.
Edited by:
Anna Sophie Fahrion, Friedrich Loeffler Institute, GermanyReviewed by:
Semeeh Omoleke, World Health Organization - Regional Office for Africa, Republic of CongoTerence Peter Scott, Global Alliance for Rabies Control, United States
Kathrin Heitz Tokpa, Swiss Centre for Scientific Research, Côte d’Ivoire
Copyright © 2022 Swedberg, Mazeri, Mellanby, Hampson and Chng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Catherine Swedberg, Yy5zd2VkYmVyZy4xQHJlc2VhcmNoLmdsYS5hYy51aw==
†These authors have contributed equally to this work
 Catherine Swedberg
Catherine Swedberg Stella Mazeri
Stella Mazeri Richard J. Mellanby
Richard J. Mellanby Katie Hampson
Katie Hampson Nai Rui Chng3†
Nai Rui Chng3†