
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 02 August 2022
Sec. Neglected Tropical Diseases
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.824484
This article is part of the Research Topic Insights in Neglected Tropical Diseases: 2021 View all 7 articles
 Peter Nambala1,2
Peter Nambala1,2 Julius Mulindwa1
Julius Mulindwa1 Priscilla Chammudzi2
Priscilla Chammudzi2 Edward Senga2
Edward Senga2 Marshal Lemelani3
Marshal Lemelani3 Drifton Zgambo4
Drifton Zgambo4 Enock Matovu5
Enock Matovu5 Annette MacLeod6
Annette MacLeod6 Janelisa Musaya2,7* on behalf of the TrypanoGEN+ Research Group as Members of the H3Africa Consortium
Janelisa Musaya2,7* on behalf of the TrypanoGEN+ Research Group as Members of the H3Africa ConsortiumBackground: Human African trypanosomiasis (HAT) has caused social–economic burden in remote rural communities mostly in sub-Saharan Africa for over a century. The World Health Organization had targeted the year 2020 for the elimination of HAT caused by Trypanosoma brucei rhodesiense, which is mainly endemic in Malawi, Uganda, Tanzania, and Zambia. Significant progress has been made in reducing reported HAT cases in some countries. Area-specific updated epidemiological and clinical data may facilitate in understanding the progress of such efforts as well as the development of new intervention strategies.
Methods: We analyzed HAT prevalence and demographics from epidemiological surveys carried out from 2012 to 2020 obtained from the Ministry of Health, Malawi. In addition, we analyzed blood samples and clinical profiles of HAT patients surveyed between 2016 and 2020 from Rumphi and Nkhotakota districts. From the blood samples, parasite observations and speciation were carried out, whereas disease staging and severity were ascertained from the clinical profiles.
Results: Malawi reported 315 HAT cases from 2012 to 2020. The majority of HAT cases were men (70.2%), and the mean age was 29.9 ± 15.3 with all HAT fatalities resulting from stage 2 disease. Clinical symptoms were not significantly associated with disease outcome; however, swollen lymph nodes (p = 0.004), weight loss (p = 0.010), headache (p = 0.019), and sleep disturbance (p = 0.032) were significantly associated with the HAT stage of patients. About 50% of all HAT patients were reported within 2 years from 2019 to 2020, suggesting a HAT outbreak in Malawi.
Conclusion: This study has highlighted the current epidemiological insights of the rHAT trend in Malawi. We have shown that rHAT clinical phenotypes in Malawi are focus-dependent and that there has been a steady increase in rHAT cases compared to all countries with incidences of rHAT. We have also highlighted an outbreak of rHAT that occurred in Malawi from 2019 to 2020 with almost 50% of the total rHAT cases that we have presented in this study reported within 2 years of the outbreak. These should call for a review of Malawi’s rHAT control and elimination strategies. A One-Health approach with the inclusion of key stakeholders such as the department of parks and wildlife may also be considered.
Human African trypanosomiasis (HAT), also known as sleeping sickness, is a neglected tropical disease that has negatively impacted the economic and health status of the majority of rural African communities, and about 70 million people are at different levels of risk of getting the disease (1). HAT is caused by Trypanosoma brucei gambiense (Tbg) and Trypanosoma brucei rhodesiense (Tbr) parasites that are transmitted by a tsetse fly vector of the genus Glossina which are distributed between latitudes of 14°N and 29°S in sub-Saharan Africa (1). Tbg causes HAT (gHAT) in Western and Central Africa, whereas Tbr disease (rHAT) is restricted in Eastern and Southern Africa. HAT is fatal if not treated on time and has two disease stages, namely, early stage 1 disease, where the trypanosomes are in circulation in the blood only, and late stage 2, which progresses to a meningoencephalitic disease (2).
Tbr infections are characterized by an acute onset of disease that also progresses rapidly to a stage 2 disease, although the clinical phenotypes of rHAT may vary in each disease focus. For instance, rHAT in Malawi presents with a prolonged period of stage 1 disease compared to rHAT cases in Uganda (3). Such clinical phenomenon of rHAT is associated with i) host inflammatory cytokine response, ii) dominant protection against Tbr in individuals with apolipoprotein-L1 (APOL1) G2 variant, and iii) Tbr parasite genotype (4–6). Indeed, there is diversity and clonality in Tbr population genetics with Malawian Tbr isolates demonstrating a greater diversity and evidence of genetic exchange (7). Despite such progress in understanding the biology of Tbr and the clinical presentation of rHAT in Malawi, previous studies had focused on HAT cases in Nkhotakota district although there are two other rHAT foci in Malawi, namely, Rumphi and Kasungu districts. The clinical phenotypes of rHAT may differ within the same country that has many rHAT foci such as the example of rHAT in Tororo and Soroti foci in Uganda (8). In the current effort to control rHAT championed by the WHO (9), country-specific updated epidemiological and clinical rHAT data may facilitate in understanding the progress and challenges faced by such control strategy efforts.
In this study, we determined the trend and demographic attributes of rHAT in Malawi from 2012 to 2020 and compared the clinical presentation of rHAT in two endemic foci during a HAT outbreak from 2019 to 2020.
Ethical approval was obtained from the Malawi National Health Sciences Research Committee under the TrypanoGEN plus project “The Genetic Determinants of Two Neglected Tropical Diseases” (Protocol Number: 19/03/2248). All blood samples used in this study were collected upon signed consent by the participant or a guardian if the participant was below 18 years of age. HAT surveillance by the Malawi National Trypanosomiasis Program is done as part of the routine control programs by the Ministry of Health.
The study was conducted in Rumphi, Nkhotakota, and Kasungu districts of Malawi (Figure 1A), which previously had reported cases of HAT in Malawi (10). Retrospective HAT data were provided by the Ministry of Health’s National Trypanosomiasis Control Program, and all HAT data from January 2012 to August 2020 were included in this study. The standard diagnosis of HAT in Malawi is by microscopic identification of trypanosome parasites in the blood either through the hematocrit centrifugation technique (11) or microscopic examination of blood smears (12). Following the identification of trypanosomes in the blood, cerebrospinal fluid (CSF) was taken through lumbar puncture from each patient for HAT staging. Microscopic examination of CSF pellet for trypanosome parasites and white blood cell (WBC) count was done after a single centrifugation at 6,000 rpm for 10 min. Stage 2 patients were confirmed and treated for second stage when trypanosome parasites were identified in the CSF regardless of WBC count. Patients with no parasitemia in the CSF were treated for stage 1.
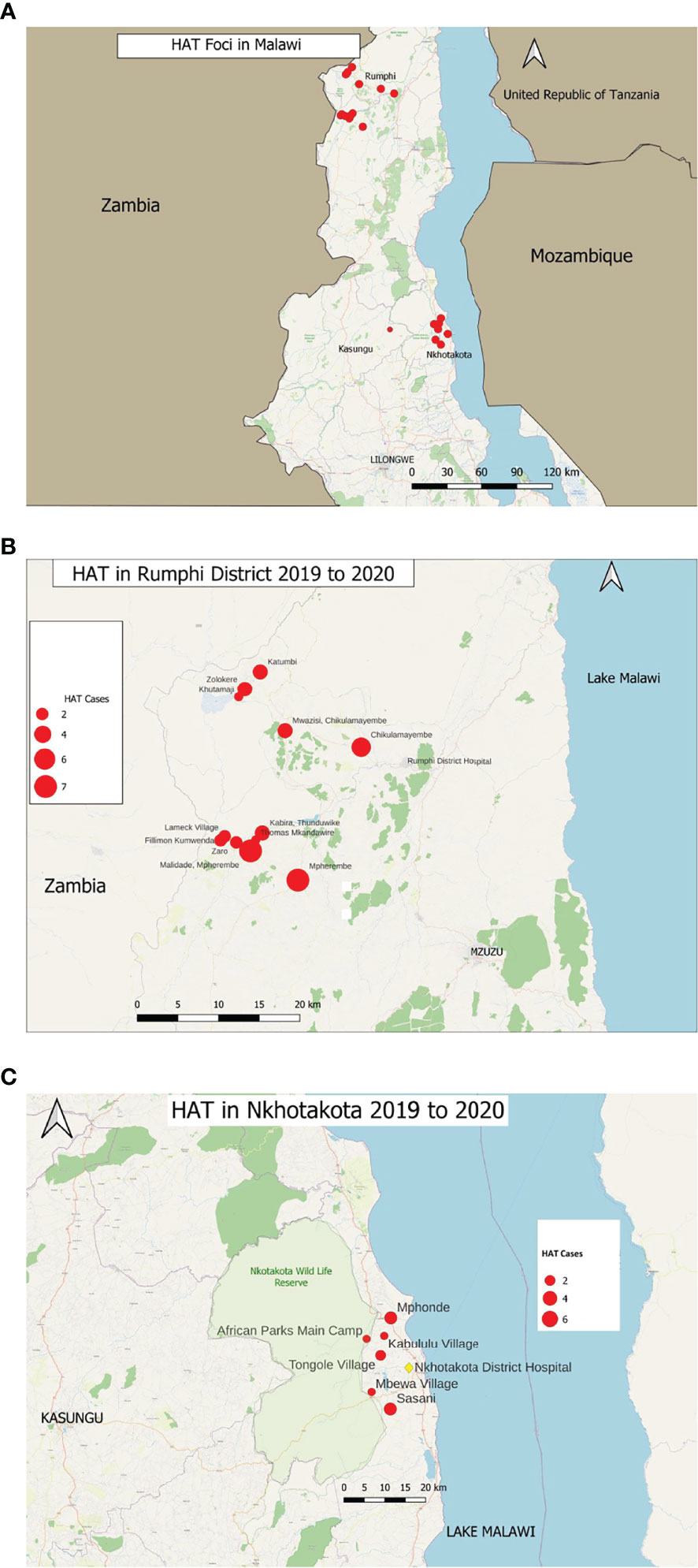
Figure 1 Map of Malawi showing the (A) rHAT foci in Malawi, that is Nkhotakota, Kasungu, and Rumphi districts that reported HAT cases. (B) HAT cases in Rumphi district reported from 2019 to 2020. Red circles represent the number of HAT cases in each indicated village. (C) HAT cases in Nkhotakota district reported during a HAT outbreak from 2019 to 2020. Red circles represent the number of cases in each respective village as indicated.
Blood samples from rHAT patients (n = 78) were obtained between May 2016 and December 2020 during active and passive surveillance conducted by H3Africa Consortium’s TrypanoGEN Project (13) in Nkhotakota and Rumphi districts. Whole blood and examination of clinical symptoms related to HAT for the study were also obtained from the patients after signing a consent form. All clinical examinations were carried out by a trained clinician or a nurse. Whole blood samples were collected on Whatman FTA cards (Sigma-Aldrich, USA) and in heparin tubes. Heparinized blood was kept at −20°C in the respective district hospital laboratories before being transferred to the TrypanoGEN Plus Laboratory at Kamuzu University of Health Sciences for DNA extraction to be used for PCR analysis. Whole blood on FTA cards was also directly used for PCR. Additionally, two thin blood films from HAT patients were made to be used for parasite count.
A 1-mm disk was obtained by punching spotted blood on the FTA card on a pad into a clean 1.5-ml microcentrifuge tube containing 50 µl of nuclease-free water. The pad and the puncher were cleaned before using them on the next sample. The tubes were incubated at 52°C for 7 min followed by another incubation of 5 min in fresh nuclease-free water. Thereafter, the disk was air-dried for immediate use in PCR.
A 200-µl aliquot of whole blood in heparin tubes was used for DNA extraction using the Qiagen DNeasy Blood and Tissue kit (Qiagen, USA) according to the manufacturer’s protocol. Thereafter, DNA was eluted in a 100-µl final volume and kept at −20°C for use in downstream analysis.
Trypanosome parasite count was obtained by first fixing the thick blood smears with ethanol and then staining with Giemsa (Merck, Darmstadt, Germany) for 30 min. Trypanosomes were counted per 100 microscopic fields on each slide using a ×100 oil objective. An average count was obtained for the two thick blood smear slides that were examined for each participant.
A nested PCR was used to confirm the trypanosome species by amplifying a serum resistance-associated (SRA) gene of T. brucei rhodesiense (14). All primers used in this study are shown in Supplementary Table 1.
The first PCR was done in a 25-µl reaction volume containing 2.5 µl of 10 µM each of the CDC F′ and CDD R′ primers, 2.5 µl of 10× Custom PCR Master Mix (Thermo Scientific, USA), 0.25 µl of 5 U/µl Taq polymerase (New England Biolabs, UK), and either 4 µl of DNA template or a washed FTA disk. The thermocycling conditions were as follows: 95°C for 2 min; 35 cycles of 95°C for 50 s, 55°C for 50 s, and 72°C for 90 s; and final extension at 72°C for 5 min.
The primary PCR products were diluted 1/100 in nuclease-free water and 2 µl was used as template for nested PCR in a 25-µl reaction volume. All the reaction cocktail and conditions were the same as the first PCR except for the CDC F′ primer which was replaced with LPA F′ primer and the annealing temperature was changed to 57°C for 50 s. The nested PCR products were run on 1.2% agarose gel stained with ethidium bromide (Invitrogen, USA). DNA bands were visualized on a transilluminator with an expected PCR product size of 1,280 bp for the SRA in T. brucei rhodesiense-positive samples.
All statistical analyses were done using SPSS Statistics software version 22 (IBM, USA) and Microsoft Excel software. The death rate and frequency of each HAT stage were calculated against the total number of cases reported in each district. Linear regression and Student’s t-test were used to compare the HAT stage and treatment outcome with clinical symptoms. The maps were created using Quantum Geographic Information System (QGIS) software version 3.20.1.
First, we sought to understand the current documented trend of HAT cases in Malawi. A total of 315 HAT cases were reported in three endemic HAT districts of Malawi, namely, Nkhotakota, Kasungu, and Rumphi, from January 2012 to August 2020 with an annual mean of 35 ± 25 HAT cases (Figures 1A–C). No HAT case was reported outside of the three endemic districts as per national trend (Figure 2A) most of the cases reported were in stage 2. All HAT cases were treated for stage 1 when trypanosome parasites were identified in the bloodstream only or for stage 2 when parasites were identified in both blood and CSF regardless of WBC count. Stage 1 patients were treated with suramin at 4–5 mg/kg body weight on day 1 and then every 7 days at 20 mg/kg body weight for five times. On the other hand, stage 2 patients were treated with melarsoprol at 2.2 mg/kg body weight for 10 consecutive days. Some stage 1 and stage 2 patients were optionally treated with fexinidazole (15), three tablets (1,800 mg) once a day for 4 days and two tablets (1,200 mg) once a day for the next 6 days in an ongoing clinical trial. Treatment of all HAT cases was done through government HAT treatment centers following the Ministry of Health guidelines.
Rumphi district had the highest number of HAT cases (80.3%) followed by Nkhotakota (19.4%) and Kasungu (0.32%) districts (Figure 2B). From 2019 to 2020, Rumphi and Nkhotakota districts had a surge in rHAT (157 total cases) which represents 49.5% of all cases reported from 2012 to 2020. Rumphi had a total of 108 (34.3%) HAT cases and Nkhotakota had 48 (15.2%) cases within 2 years (Figure 2B and Supplementary Table 2). Most of the HAT cases were identified through passive HAT surveillance at district hospitals.
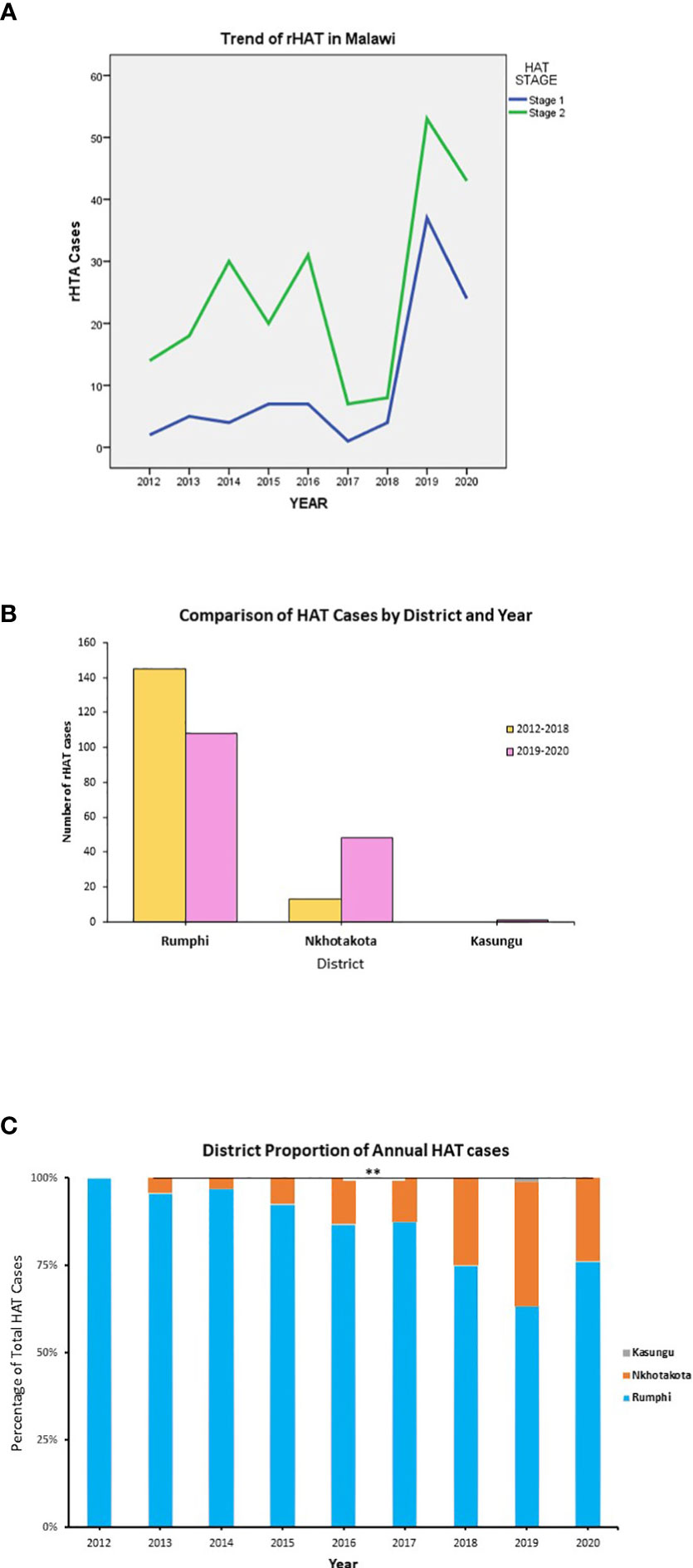
Figure 2 Reported HAT cases in Malawi from 2012 to 2020. (A) The cumulative number of HAT cases in each endemic district of Malawi. (B) The cumulative trend of HAT cases by disease stage in each year. (C) The proportion of HAT cases in each endemic district in comparison to cumulative national HAT cases. **p < 0.01.
From the year 2012 to 2020, Nkhotakota district showed a significant increase in HAT cases in comparison to the national total HAT cases (p < 0.01), whereas Rumphi district had a decrease in the percentage of HAT cases though not statistically significant (p < 0.5) (Figure 2C). The factors influencing these changes are not known, although other factors such as the training of clinicians on HAT, increased HAT surveillance, community engagement, and environmental factors might also contribute to increases in HAT detection; nonetheless, the contribution of such factors entails further investigation in future studies.
We also observed that the clinical stage of HAT was dependent on the focus. Rumphi district recorded a higher number of cases with stage 2 disease (84.6%), whereas in Nkhotakota, most of the cases (82.0%) were early stage 1 disease (Supplementary Figure 1A and Table 1).
The prevalence of a disease in a community may be influenced by several factors such as gender, age, and ethnicity among others (5). These factors could also have a reciprocal impact, for example, on the risks of exposure to tsetse fly bites infected with human infective trypanosomes in the case of HAT. In this study, we found that the mean age of all HAT cases was 29.91 ± 15.3 years and there were no remarkable differences in the mean age between HAT stages (p = 0.761) as well as the patient’s gender (p = 0.524) (Figure 3A). Interestingly, there were remarkable differences in the frequency of infected men compared to women as more men were infected with both stage 1 and stage 2 HAT, except in Nkhotakota district where more women (1 female to 0.571 male ratio) were reported with stage 2 disease (Table 1). The proportion of female HAT cases detected compared to male HAT cases was also improving through the years with an astounding proportion of female HAT cases (60.6% of the total female cases) detected in 2019 and 2020 (Figure 3B).
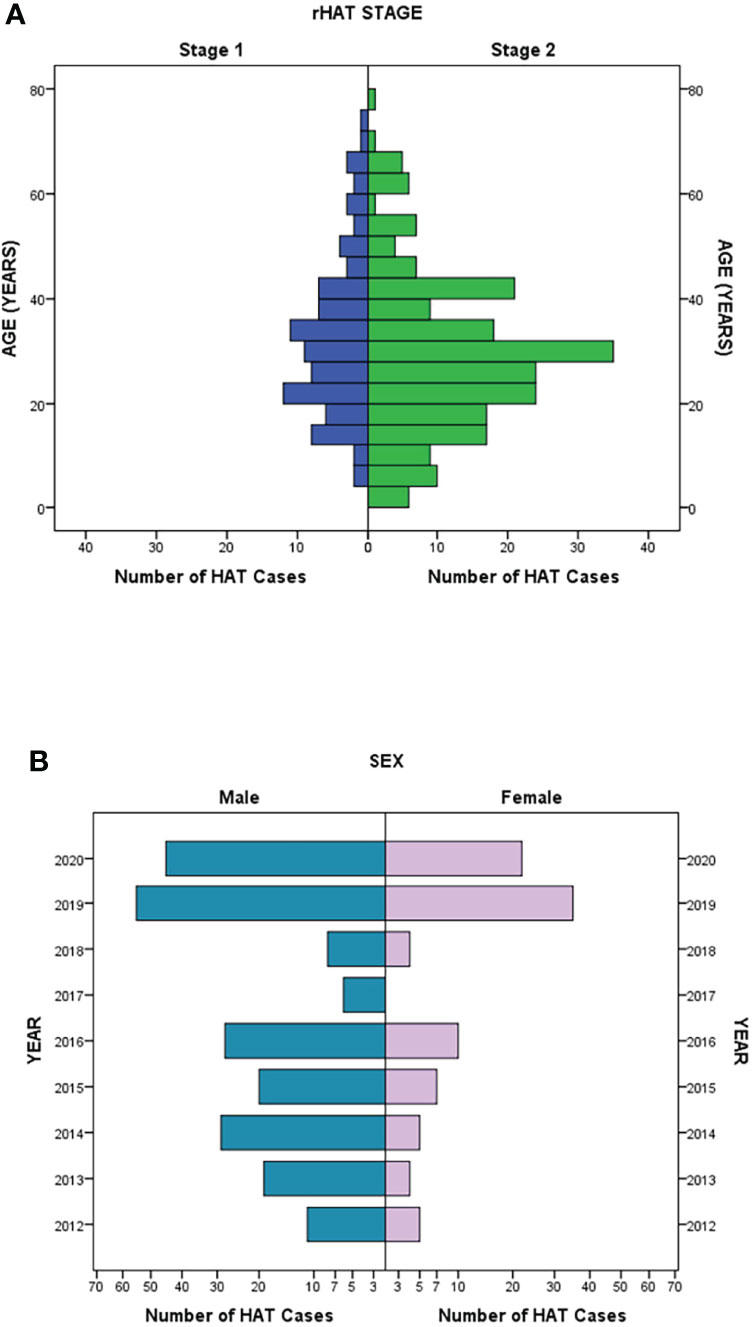
Figure 3 Demographic trend of rHAT cases. (A) Distribution of stage 1 and stage 2 rHAT in comparison to age. (B) The reported number of rHAT in men and women per year.
The distribution of HAT cases among different ethnic groups was also analyzed as ethnic background might also have an impact on one’s susceptibility to diseases due to inherited genetic polymorphisms of resistance to a particular disease. For example, α-thalassemia and apolipoprotein L1 (APOL1) variants provide innate immunity against malaria and HAT, respectively (5, 16, 17). Most HAT cases in Rumphi were from the Tumbuka tribe (80.0%) and those in Nkhotakota were from the Chewa tribe (17.8%). Few cases from the Ngoni tribe (three HAT cases), which borders Rumphi, were also identified (Supplementary Figure 1B).
All HAT patients who died during treatment (35 cases) were late stage 2 cases with more male deaths (p < 0.0001) compared to female deaths although HAT treatment outcome was not associated with gender (p < 0.573) (Supplementary Figures 2A, B). The age group that reported most HAT deaths was from 16 to 30 years with a median age of 27.5 ± 15.7 years in both men and women (Supplementary Figure 2C). Overall, the total death among individuals diagnosed with HAT was 11.1%, whereas treatment success was 88.9% (Table 1). Some individuals aged <5 years were successfully treated for HAT although some died during treatment within the same age group. In Nkhotakota district, the percentage of deaths in patients with stage 2 disease during treatment was 54.5% compared to 13.6% in Rumphi district (Table 1).
Trypanosome parasite count was verified from thin blood smears of 38 patients whose slides were stored. The majority of HAT cases (84.4%) had low parasitemia regardless of the clinical stage with only one patient having over 50 trypanosomes per 100 microscopic fields (Table 1). The patient with high parasitemia was an early clinical stage 1 case from Nkhotakota.
Clinical symptoms were recorded from 48 HAT patients diagnosed between 2019 and 2020 using a standardized case report form for rHAT symptoms as previously described (18). The common clinical symptoms were headache (97.9%), body or joint pain, fever over 1 week, weight loss, and sleep disturbance as summarized in Table 2. There was no significant relationship between treatment outcome and clinical symptoms. However, swollen lymph nodes (p = 0.004), weight loss (p = 0.010), headache (p = 0.019), and sleep disturbance (p = 0.032) were significantly associated with the HAT stage of patients. Malaria treatment within a month before HAT diagnosis was reported in 47.7% of the patients in this study with recorded clinical symptoms (Table 2).
Lastly, confirmation of trypanosome species by SRA PCR was done on 78 HAT patients whose blood was collected between 2016 and 2020, and all samples were confirmed as T. brucei rhodesiense parasites (Supplementary Figure 3).
The annual reported cases of rHAT in Malawi have comparably remained similar with no significant reduction in rHAT cases since the year 2000 (1). Other countries such as Uganda and Tanzania have had a noticeable reduction in annual rHAT cases; for example, Tanzania and Uganda had 0 and 4 rHAT cases, respectively, in 2018, compared to 350 and 300 cases, respectively, in the year 2000 (19). Achieving control and elimination of rHAT in a country may be attributed to several factors such as intensive active surveillance that is guided by updated clinical and epidemiological data (9).
In this study, we have presented updated epidemiological rHAT data for Malawi from 2012 to 2020. During this period, most rHAT cases (99.7% of the total cases) were reported in Nkhotakota and Rumphi districts which were similar to previous study findings suggesting that there have not been enough efforts to control HAT in Malawi endemic areas in the past decade (3, 10, 20). Notwithstanding, rHAT cases in Nkhotakota increased from 0% of the national total cases in 2012 to 36% in 2019, whereas in Rumphi, the cases decreased from 100% of the national cases in 2012 to 63% in 2019 (Figure 2C). Interestingly, from 2003, a similar inverse proportion was also observed in a previous study that showed that HAT cases in Rumphi were increasing, while in Nkhotakota, HAT cases were decreasing (10). Several factors such as intensified or reduced active HAT surveillance might influence increased or reduced HAT case detection, respectively.
We also found that most rHAT cases were from the Tumbuka and Chewa tribes in Rumphi and Nkhotakota districts, respectively. Although there might not be a direct association between ethnic groups and susceptibility to HAT in Malawi as the majority of the affected tribes live closer to wildlife reserves, there are increased chances of exposure to infected tsetse fly bites, and this opens an avenue for future HAT association studies.
In both occurrences discussed above, it may be apparent that either i) not enough control effort or vice versa has been made at the national level to put rHAT under control in Malawi or ii) environmental and social factors may be driving the increases in rHAT cases in Malawi. For example, from 2016 to 2017, the Nkhotakota Wildlife Reserve was repopulated with 486 elephants which previously had less than 100 elephants, and the elephant population has been increasing ever since (21), and informal interviews with the communities around the Nkhotakota Wildlife Reserve attributed the increase in HAT cases to an increased population of elephants in the wildlife reserve which sometimes trespassed into the communities. Previous studies have shown that elephants may form an integral part of HAT transmission as they are one of the sources of blood meal for tsetse flies that might end up transmitting the trypanosome parasite to humans as well (22, 23). Nonetheless, a One-Health approach in the control of rHAT may facilitate in determining the influence of ecological factors in rHAT transmission in Malawi.
We further observed that the clinical stage of rHAT was focus-dependent with Nkhotakota district mostly reporting cases of early stage 1 rHAT (82.0%) and Rumphi district with common cases of late stage 2 rHAT (84.6%). This raises the question of whether Tbr parasite strains circulating in Nkhotakota (Central Malawi) are different from Tbr strains circulating in Rumphi (Northern Malawi) or whether genetic differences in the human host population are influencing the clinical phenotypes in Malawi. Genotyping of trypanosome isolates in Malawi has only been done with Nkhotakota isolates which were reported to have a distinguished polymorphism in the SRA gene, and very few patients progressed to stage 2 HAT compared to Ugandan Tbr isolates and rHAT disease, respectively (4). At the moment, genotyping of Tbr isolates circulating in Rumphi district has not been elucidated, and future studies should consider unraveling such fundamental data that may facilitate in the development of new control strategies against rHAT in Malawi that might later be adopted universally. On the other hand, a considerable proportion of rHAT patients (41.7%) that had been prescribed antimalarial treatment at least within a month before being diagnosed with rHAT might suggest misdiagnosis of rHAT. Diagnosis of rHAT might be missed during the early stage of the disease until the disease progresses to late stage 2. This might be due to the similarity of symptoms associated with Plasmodium infections or a co-infection of Plasmodium and Tbr parasites which in this study we did not investigate in detail, hence opening an avenue for future research consideration.
Additionally, we found that most rHAT patients had low blood parasitemia (1–10 trypanosomes/100 microscopic fields) irrespective of the clinical phenotypes, which might suggest that progression to stage 2 rHAT disease in Malawi might not be influenced by trypanosome density in the blood (24), and such phenotypes should be considered for further investigation in future studies.
We also found that the treatment outcome of stage 2 rHAT in Malawi (11.1% deaths and 88.9% successful treatment) was comparable to that described in a study from Tanzania and Uganda (18), and no clinical symptoms in our study were significantly associated with treatment outcome. There was a significant association between the rHAT stage and the presence of swollen lymph nodes (p = 0.004), weight loss (p = 0.010), headache (p = 0.019), and sleep disturbance (p = 0.032), which were not present in a retrospective study that analyzed rHAT data in Rumphi district from 2000 to 2006 (20). Overall, more rHAT deaths occurred in men (77.1%) than in women (Supplementary Figure 2A). More deaths in men might be due to the high prevalence of HAT in men in the affected communities as mainly men are involved in farming activities along wildlife reserves, hence the high risk of exposure to HAT. Nonetheless, other contributing factors such as health-seeking behavior should be explored in future research.
In conclusion, this study has updated the epidemiological insight of the current rHAT trend in Malawi. We have shown that rHAT clinical phenotypes in Malawi are focus-dependent and that there has been a steady increase in rHAT cases compared to all countries with incidences of rHAT. Although the number of patients in this study with description of clinical presentation was very low, clinical data have provided us an overview of the current trend in HAT clinical presentation in Malawi. Also, in this study, we did not exhaust all HAT clinical signs and complications such as cardiac complications as we only focused on visible clinical symptoms. We have also highlighted an outbreak of rHAT that occurred in Malawi from 2019 to 2020 with almost 50% of the total rHAT we have presented in this study reported within 2 years of the outbreak. We recommend regular active surveillance to be done in all rHAT hotspots in order to isolate cases as early as possible to reduce the transmission cycle. Community health education on the transmission dynamics of rHAT and engagement with other key stakeholders such as the department of parks and wildlife which has an oversight of wildlife reserves in Malawi should also be considered.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The National Health Sciences Research Committee (NHSRC), National Science Commission Malawi. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
PN: conceptualization, methodology, investigation, formal analysis, and writing—original draft. JuM: conceptualization, writing—review and editing, methodology, and formal analysis. PC: investigation. DZ: investigation. ML: investigation. AM: conceptualization and methodology. EM: conceptualization. JaM: conceptualization, writing—review and editing, methodology, supervision, and formal analysis. All authors contributed to the article and approved the submitted version.
This study was funded by a grant to the TrypanoGEN+ project from the African Academy of Sciences (grant number H3A/18/004 to EM). This work was supported through H3Africa (H3A-18-004). The Human Heredity and Health in Africa (H3Africa) is a program of the Alliance for Accelerating Excellence in Science in Africa (AESA) platform. AESA is a funding, agenda-setting, and program management initiative of the African Academy of Sciences (AAS), the African Union Development Agency (AUDA-NEPAD), founding and funding global partners, and through a resolution of the summit of the African Union.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to acknowledge the Malawi Ministry of Health National Trypanosomiasis Control Program for providing some of the data used in this study. We also thank Nkhotakota and Rumphi district health offices for their assistance with data collection.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.824484/full#supplementary-material
1. Franco JR, Simarro PP, Diarra A, Jannin JG. Epidemiology of Human African Trypanosomiasis. Clin Epidemiol (2014) 6:257–75. doi: 10.2147/CLEP.S39728
2. Kennedy PG. Clinical Features, Diagnosis, and Treatment of Human African Trypanosomiasis (Sleeping Sickness). Lancet Neurol (2013) 12(2):186–94. doi: 10.1016/S1474-4422(12)70296-X
3. MacLean LM, Odiit M, Chisi JE, Kennedy PG, Sternberg JM. Focus-Specific Clinical Profiles in Human African Trypanosomiasis Caused by Trypanosoma Brucei Rhodesiense. PLoS Negl Trop dis (2010) 4(12):e906. doi: 10.1371/journal.pntd.0000906
4. MacLean L, Chisi JE, Odiit M, Gibson WC, Ferris V, Picozzi K, et al. Severity of Human African Trypanosomiasis in East Africa is Associated With Geographic Location, Parasite Genotype, and Host Inflammatory Cytokine Response Profile. Infect immunity (2004) 72(12):7040–4. doi: 10.1128/IAI.72.12.7040-7044.2004
5. Kamoto K, Noyes H, Nambala P, Senga E, Musaya-Mwalija J, Kumwenda B, et al. Association of APOL1 Renal Disease Risk Alleles With Trypanosoma Brucei Rhodesiense Infection Outcomes in the Northern Part of Malawi. PLoS Negl Trop dis (2019) 13(8):e0007603. doi: 10.1371/journal.pntd.0007603
6. Cooper A, Ilboudo H, Alibu VP, Ravel S, Enyaru J, Weir W, et al. APOL1 Renal Risk Variants Have Contrasting Resistance and Susceptibility Associations With African Trypanosomiasis. eLife (2017) 6:e25461. doi: 10.7554/eLife.25461
7. Duffy CW, MacLean L, Sweeney L, Cooper A, Turner CM, Tait A, et al. Population Genetics of Trypanosoma Brucei Rhodesiense: Clonality and Diversity Within and Between Foci. PLoS Negl Trop dis (2013) 7(11):e2526. doi: 10.1371/journal.pntd.0002526
8. Maclean L, Odiit M, Macleod A, Morrison L, Sweeney L, Cooper A, et al. Spatially and Genetically Distinct African Trypanosome Virulence Variants Defined by Host Interferon-Gamma Response. J Infect dis (2007) 196(11):1620–8. doi: 10.1086/522011
9. WHO. Control and Surveillance of Human African Trypanosomiasis. World Health Organ Tech Rep Ser (2013) 984):1–237.
10. Chisi JE, Muula AS, Ngwira B, Kabuluzi S. A Retrospective Study of Human African Trypanosomiasis in Three Malawian Districts. Tanzan J Health Res (2011) 13(1):62–8. doi: 10.4314/thrb.v13i1.61014
11. Woo PT. The Haematocrit Centrifuge for the Detection of Trypanosomes in Blood. Can Z zool (1969) 47(5):921–3. doi: 10.1139/z69-150
12. Chappuis F, Loutan L, Simarro P, Lejon V, Buscher P. Options for Field Diagnosis of Human African Trypanosomiasis. Clin Microbiol Rev (2005) 18(1):133–46. doi: 10.1128/CMR.18.1.133-146.2005
13. Ilboudo H, Noyes H, Mulindwa J, Kimuda MP, Koffi M, Kabore JW, et al. Introducing the TrypanoGEN Biobank: A Valuable Resource for the Elimination of Human African Trypanosomiasis. PLoS Negl Trop dis (2017) 11(6):e0005438. doi: 10.1371/journal.pntd.0005438
14. Radwanska M, Chamekh M, Vanhamme L, Claes F, Magez S, Magnus E, et al. The Serum Resistance-Associated Gene as a Diagnostic Tool for the Detection of Trypanosoma Brucei Rhodesiense. Am J Trop Med hyg (2002) 67(6):684–90. doi: 10.4269/ajtmh.2002.67.684
15. Fairlamb AH. Fexinidazole for the Treatment of Human African Trypanosomiasis. Drugs Today (Barc) (2019) 55(11):705–12. doi: 10.1358/dot.2019.55.11.3068795
16. Cooper A, Capewell P, Clucas C, Veitch N, Weir W, Thomson R, et al. A Primate APOL1 Variant That Kills Trypanosoma Brucei Gambiense. PloS Negl Trop dis (2016) 10(8):e0004903. doi: 10.1371/journal.pntd.0004903
17. Kariuki SN, Williams TN. Human Genetics and Malaria Resistance. Hum Genet (2020) 139(6-7):801–11. doi: 10.1007/s00439-020-02142-6
18. Kuepfer I, Hhary EP, Allan M, Edielu A, Burri C, Blum JA. Clinical Presentation of T.b. Rhodesiense Sleeping Sickness in Second Stage Patients From Tanzania and Uganda. PloS Negl Trop Dis (2011) 5(3):e968. doi: 10.1371/journal.pntd.0000968
19. WHO. Report of the Third WHO Stakeholders Meeting on Rhodesiense Human African Trypanosomiasis. Geneva, Switzerland: WHO (2020) p. 10–1. Available at: https://www.who.int/publications/i/item/9789240012936.
20. Madanitsa M, Chisi J, Ngwira B. The Epidemiology of Trypanosomiasis in Rumphi District, Malawi: A Ten Year Retrospective Study. Malawi Med J J Med Assoc Malawi (2009) 21(1):22–7. doi: 10.4314/mmj.v21i1.10985
21. AfricanParks. Largest Elephant Translocation in History Concludes in Malawi 2017. Available at: https://www.africanparks.org/largest-elephant-translocation-history-concludes-malawi.
22. Muturi CN, Ouma JO, Malele II, Ngure RM, Rutto JJ, Mithöfer KM, et al. Tracking the Feeding Patterns of Tsetse Flies (Glossina Genus) by Analysis of Bloodmeals Using Mitochondrial Cytochromes Genes. PLoS One (2011) 6(2):e17284. doi: 10.1371/journal.pone.0017284
23. Makhulu EE, Villinger J, Adunga VO, Jeneby MM, Kimathi EM, Mararo E, et al. Tsetse Blood-Meal Sources, Endosymbionts and Trypanosome-Associations in the Maasai Mara National Reserve, a Wildlife-Human-Livestock Interface. PLoS Negl Trop dis (2021) 15(1):e0008267. doi: 10.1371/journal.pntd.0008267
Keywords: trypanosomiasis, Malawi, SRA PCR, incidence, endemic
Citation: Nambala P, Mulindwa J, Chammudzi P, Senga E, Lemelani M, Zgambo D, Matovu E, MacLeod A and Musaya J (2022) Persistently High Incidences of Trypanosoma brucei rhodesiense Sleeping Sickness With Contrasting Focus-Dependent Clinical Phenotypes in Malawi. Front. Trop. Dis 3:824484. doi: 10.3389/fitd.2022.824484
Received: 29 November 2021; Accepted: 17 June 2022;
Published: 02 August 2022.
Edited by:
Luc E. Coffeng, Erasmus Medical Center, NetherlandsReviewed by:
Johannes Blum, Swiss Tropical and Public Health Institute (Swiss TPH), SwitzerlandCopyright © 2022 Nambala, Mulindwa, Chammudzi, Senga, Lemelani, Zgambo, Matovu, MacLeod and Musaya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janelisa Musaya, am11c2F5YUBrdWhlcy5hYy5tdw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.