- 1Discipline of Public Health Medicine, School of Nursing and Public Health, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
- 2Communicable Disease Control Directorate, National Department of Health, Pretoria, South Africa
- 3Norwegian Centre for Imported and Tropical Diseases, Department of Infectious Diseases Ullevaal, Oslo University Hospital, Oslo, Norway
- 4Department of Parasitology, Leiden University Medical Center, Leiden, Netherlands
- 5Department of Cell and Chemical Biology, Leiden University Medical Center, Leiden, Netherlands
- 6Department of Biomedical and Clinical Technology, Durban University of Technology, Durban, South Africa
- 7Institute for Global Development and Planning, University of Agder, Kristiansand, Norway
- 8Section for Parasitology and Aquatic Pathobiology, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark
- 9BRIGHT Academy, Ugu District, Durban, South Africa
Background: In areas where reinfection with schistosomiasis is rampant, it is not known if the lesions of Female Genital Schistosomaisis are a consequence of live worms, or caused by dead ova. Live schistosome worms regurgitate Circulating Anodic Antigen (CAA). We sought to explore the association between the different lesions of FGS (grainy sandy patches, homogenous yellow patches, rubbery papules and abnormal blood vessels) and the presence of live worms as indicated by S. haematobium-derived CAA in blood.
Materials and Methods: In this cross-sectional study, rural high schools were randomly selected from Ilembe, uThungulu and Ugu Districts on the East Coast of South Africa, KwaZulu-Natal Province. Serum samples for CAA analysis were collected from 246 female learners aged 16 - 23 years. Uncorrected chi-square and odds ratio with 95% confidence interval (CI) were used to evaluate the null hypothesis.
Results: CAA was positive in 82/246 (33%) of the participants. Sandy patches were found in 123 (50%) of the study population. Grainy sandy patches were significantly associated with CAA even after controlling for age (Adjusted Odds Ratio (AOR) 4.2, 95% CI 2.3 - 7.9, p < 0.001). Likewise, abnormal blood vessels were associated with CAA (AOR 3.0, 95% CI 1.5-4.5, p = 0.001) whereas homogenous yellow patches were not associated with CAA (p = 0.57). Rubbery papules were not found in this study population.
Conclusion: Grainy sandy patches and abnormal blood vessels are found more commonly in women who harbour live Schistosoma haematobium worms whilst homogenous yellow patches may indicate chronic tissue damage due to dead ova.
Introduction
Female Genital Schistosomiasis (FGS) is a common complication of schistosomiasis caused by Schistosoma (S.) haematobium ova deposited in genital tissue (1). Two different morphologic subtypes of genital sandy patches have been described, namely: grainy sandy patches and homogenous yellow patches at 15 times magnification (1). In addition, women with FGS may have abnormal blood vessels, contact bleeding and rubbery papules. Diagnosis is made by visual inspection of the characteristic genital sandy patches (also called FGS lesions) on the cervix and vaginal wall (2).
Millions of women have domestic, commercial and recreational contact with infested waterbodies and it is estimated that 56 million women in sub-Saharan Africa have FGS (3), and almost 20 million more cases will occur in the next decade unless girls are treated (4). FGS has been linked to abnormal discharge, a burning sensation in the genitals, sub-fertility, ectopic pregnancy, and increased transmission of HIV (3). The control of neglected tropical diseases is gaining momentum; however FGS is underdiagnosed, its burden is significantly underestimated and there is a “huge gap in epidemiological assessment” (3, 5–8).
Worldwide, only one-quarter of the schistosomiasis infected people have been treated and the optimal timing for prevention of morbidity, HIV susceptibility and infertility is unexplored (3). People may receive several rounds of treatment and yet continue to excrete ova, probably due to suboptimal efficacy of praziquantel and reinfection (9). One study found that genital lesions remained unchanged after treatment (9). However, it is not known if these lesions remained because patients were re-infected immediately or, alternatively, if some forms of FGS are irreversible.
Women and children have been found to have FGS from Egypt to Southern Africa and Madagascar (5, 10), and in some areas FGS may be more common than the sexually transmitted infections. Furthermore, a study in South African schools showed that more than 20% of adolescent girls and young women had FGS (11). Another study showed that children, before menstrual and sexual debut, have bloody and malodorous discharge (12), indicating that intra-vaginal morbidity starts in childhood. However, it is not known if FGS lesions are caused by recently laid ova from live worms (13).
The Circulating Anodic Antigen (CAA) is a regurgitate from live schistosome worms (14) and the Lateral Flow test utilizing Up-Converting reporter Particles is a highly sensitive and 100% specific test for this unique carbohydrate structure (15–17). CAA is measurable in serum and urine but would not be present if the worms are already dead.
In this study of adolescent girls and young women of KwaZulu-Natal Province of South Africa, we sought to explore the association between the different lesions of FGS and the presence of live worms as indicated by CAA in blood.
Materials and Methods
Ethical Considerations
The study was approved by the Biomedical Research Ethics Committee (BREC), University of KwaZulu-Natal (Ref BF029/07), KwaZulu-Natal Department of Health (Reference HRKM010-08) and the Regional Committee for Medical and Health Research Ethics (REC), South Eastern Norway (Ref 46907066a1.2007.535). The ethical committees, BREC (annual renewal) and REC, were aware that minors (aged 16 and 17 years) were participating in the study and specifically approved independent minor consent without parental consent. According to South African legislation, persons over the age of 12 may consent independently to participate in research. Each participant received a detailed explanation of the gynaecological examination procedure for identifying lesions, and questions they had were answered. All study participants were offered anti-schistosomal and, if applicable, sexually transmitted diseases’ treatment, and/or referral to the local health system for treatment of HIV when needed. STI treatment was offered to participants with clinical signs and symptoms, and their partners in accordance with the South African syndromic treatment protocol (18).
Study Subjects and Area
The study was conducted between 2011 and 2013 in the KwaZulu-Natal Province of South Africa. The participants were female learners, aged 16 - 23 years, from randomly selected high schools in Ilembe, uThungulu and Ugu Districts on the East Coast of South Africa that had not undergone anti-schistosomal mass-treatment the last year before investigation. The participants were recruited from schools that were classified as rural by the Department of Education and were below the altitude of 400 meters above sea level, with an estimated prevalence of S. haematobium of 10% or more based on an initial show of hands for red urine in Ugu District and a haematuria dipstick survey in Ilembe and uThungulu districts (11). Only those who completed the gynaecological examination were included; virgins, pregnant, and severely ill females were excluded. Schools with prevalence below 10% were excluded.
Questionnaires and Clinical Examinations
The investigation has been described previously but briefly, a questionnaire on water contact, reproductive history, genital and abdominal symptoms was administered individually to participants in isiZulu prior to gynaecological examination (11). The clinician performing the exams was blinded to the childhood origin schistosomal status. Examination was commenced by cervico-vaginal lavage. Saline (10 ml) was sprayed on the vaginal wall and cervix twice, whereupon it was drawn back into a syringe and deposited into four tubes. This was followed by photocolposcopic examination (Leisegang Photocolposcope, Germany, Magnifications 7.5; 15; 30 or Olympus OSC 500 photocolposcope, Olympus America Inc., Center Valley, PA, USA with a mounted camera Olympus E420, 10.0 megapixels) using an autoclaved metal speculum after which Pap (Papanicolau) smears were collected from all consenting women. The cervix, the fornices, the entire vaginal wall and vulval surfaces were inspected section by section according to a predefined protocol (1). Acetic acid and/or iodine application for colposcopic examination was always done last. Typical lesions of FGS are shown in Figure 1.
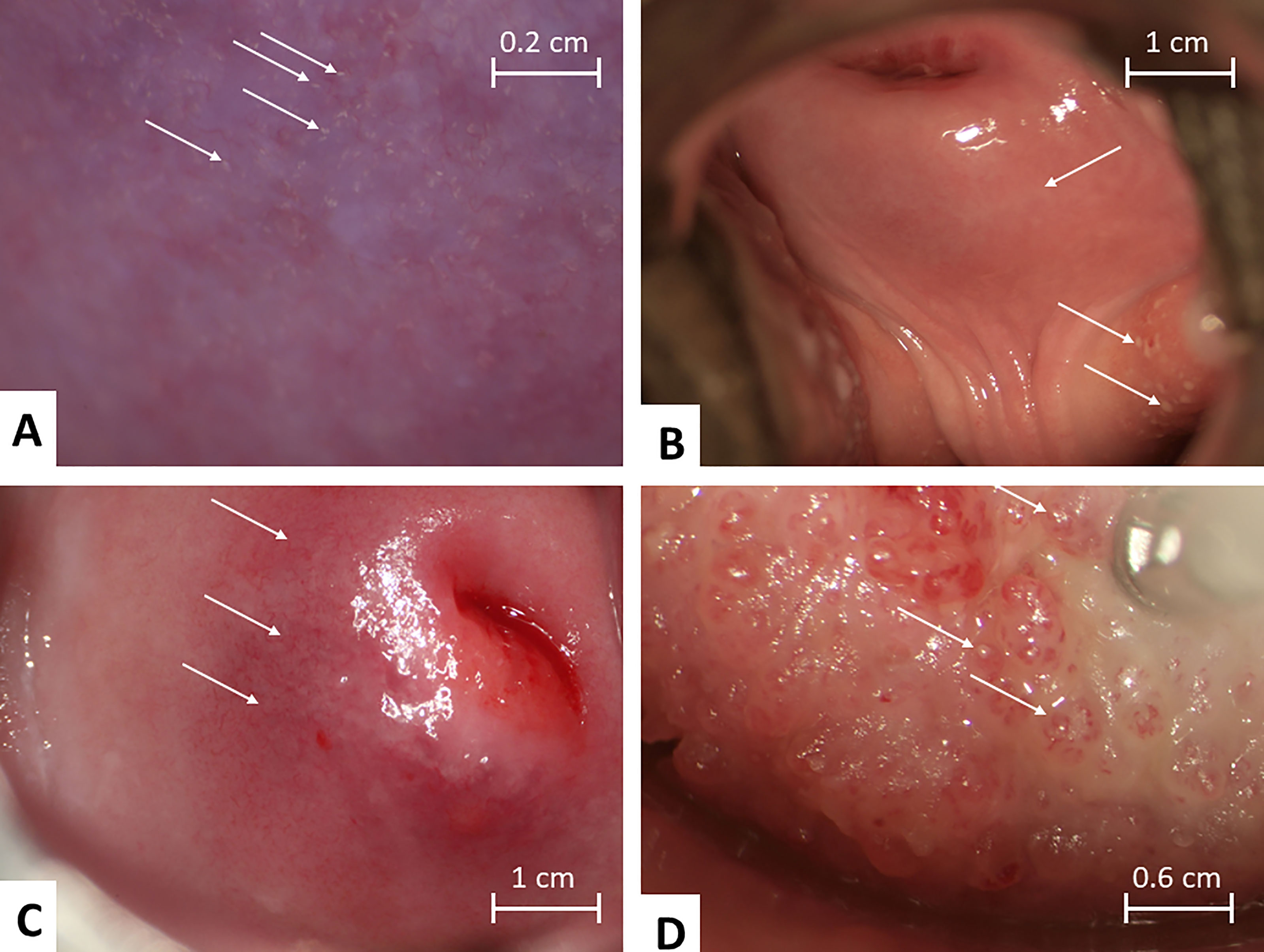
Figure 1 Mucosal colposcopic findings of clinical manifestations of Female Genital Schistosomiasis. (A) Grainy sandy patches appearing as single grains and clusters of grains. (B) Rubbery papules in both fornices (left and right), abnormal blood vessels (circular) are seen on cervix 6 -7 o’clock. (C) Homogenous sandy patch on the cervix (clearly seen on left side of image), abnormal blood vessels are seen especially clearly 7 - 10 o’clock outside the transformation zone, mottled yellowish areas can be seen in the background. (D) Rubber papules on cervix found in a 22 year old women in Madagascar. Photos by: Elisabeth Kleppa, Bodo Raniandrasolo and Eyrun Kjetland.
Laboratory Analyses
Serum samples were collected as has been described elsewhere and stored at minus 80 degrees celcius (19). The samples were analysed for CAA (14). After thawing, 0.5 mL 4% trichloro-acetic acid (TCA) was added to 0.5 mL of serum samples in 1.5mL microfuge tubes and mixed well using a vortex. The TCA/serum mixture was centrifuged for 15 minutes (13,000 rpm) using a Biofuge Pico 21 Heraeus and Biofuge Pico Heraeus centrifuges. 0.5mL clear TCA-supernatant was transferred to a concentration device (Amicon® Ultra 0.5 mL Centrifugal Filters; Merck-Millipore) and centrifuged for 30 minutes at 13,000 rpm. Assay buffer was dissolved and 80 µL added to hydrate dry UCP conjugate in microtiter wells. A 20 µL of the concentrated TCA-supernatant was mixed with hydrated UCP conjugate and incubated on thermos-shakers (60 min). LF was initiated by placing a UCP-LF CAA strip in the microtiter wells and left overnight. The UCP-LF CAA strips were read using the UCP-Quant reader (ESE Quant) and analysed with matched software, LateralFlowStudio version 3.3.7 (QIageN Lake Constance GmbH, Stockach, Germany). A sub-sample of the population (the early participants, before full sample was reached) had been investigated by Schistosoma PCR, as described previously (20).
Sample Size Calculation and Statistical Analyses
We planned a study of independent CAA positive and negative females. Prior data was not available for CAA, however as a proxy, in urine microscopy negative the probability of sandy patches is 40 percent. If the true probability of sandy patches among CAA positive is 60 percent, we needed to study 97 CAA positive and 97 CAA negative patients to be able to reject the null hypothesis that the sandy patch prevalence for CAA positive and CAA negative are equal with probability (power) 80%. To cater for uncertainties in the calculations we added 52 cases. The Type I error probability associated with the test of this null hypothesis is 0.05. We used an uncorrected chi-square statistic and odds ratio (OR) with 95% confidence interval (CI) to evaluate this null hypothesis; to study the association between CAA and different lesions of FGS (after controlling for age and treatment). Adjusted odds ratio was calculated using logistic regression to control for age and treatment. The SPSS version 27 (IBM) was used.
Results
A total of 246 young females were included; 82 (33%) of these were positive for CAA. The mean age of the study participants was 19 years (standard deviation (SD) 1.8). Sandy patches were found in 123 (50%) of the study population, grainy sandy patches were found in 63 (26%) (Figure 1). Homogenous yellow patches were found in 75 (30.5%) females, and 118 (48%) had current risk water contact. Urine microscopy results were available for 243 participants, 39 (16%) were positive by microscopy of one urine sample, geometric mean 12.1 eggs/10mL (SD 43.9). A sub-sample of 178 study participants were tested by schistosoma PCR. Table 1 shows the association between demographic variables, the lesions and the CAA in univariate analysis.
A total of 126 people reported that they had never been treated and 68 did not know. Of the 52 who had been treated, 26 reported their age at treatment was 10 years (SD 4). The time between previous treatment and the clinical investigation was mean 9 years (SD 4.8).
FGS Lesions: Grainy Sandy Patches and the Presence of Live Worms
In the sub-group of people with grainy sandy patches, 59% (37/63) were positive for CAA; the association was significant even after controlling for age (Adjusted Odds Ratio (AOR) 4.2, 95% Confidence Interval (CI) 2.3 - 7.9, p < 0.001), indicating the presence of live worms. Of those with grainy sandy patches, 42% (16/38) were schistosomiasis PCR positive (p < 0.001). Many had both sandy patch types, however, in a sub-analysis of those with grainy sandy patches only, 56% (27/48) were CAA positive (AOR 4.4, 95% CI 2.1 - 9.1, p < 0.001). Amongst those who had grainy sandy patches but were CAA negative, 5/26 (19%) had eggs in their urine upon microscopy.
FGS Lesions: Homogenous Yellow Patches and the Presence of Live Worms
In the sub-group of people who had homogenous yellow patches only (excluding the ones with grainy sandy patches), 30% (18/60) were positive for CAA (AOR 1.7, 95% CI 0.8 - 3.5, p = 0.15). Of those who had homogenous yellow patches only, 20% (9/45) were positive by PCR (p < 0.001).
FGS Lesions: Abnormal Blood Vessels and the Presence of Live Worms
Abnormal blood vessels were strongly associated with CAA (p = 0.001). Amongst those with abnormal blood vessels, 21% (15/73) were positive by Schistosoma PCR (p = 0.061).
CAA as a Continuous Variable
Confirming the finding above, CAA as a continuous variable was found to be significantly associated with grainy sandy patches after controlling for age (AOR 4.2, 95% CI 2.3 – 7.8, P <0.001), and abnormal blood vessels (AOR 2.7, CI 1.5 – 4.7, p = 0.001). However, CAA was not associated with homogenous yellow patches (AOR 1.4, CI 0.8 – 2.5, p = 0.26). CAA concentrations were not significantly associated with urinary egg count (p = 0.17).
Other Lesions, Symptoms and Live Worms
Red urine and thick and lumpy discharge were the only symptoms that were associated with CAA, indicating the presence of live worms (Table 2). No association was observed between CAA and other symptoms and lesions such as warts, polyps or ulcers (p > 0.4 for all).
Prior Treatments and Current Water Contact
Unexpectedly, CAA was positive significantly more often in those who had been treated (Table 2). The associations between CAA and grainy sandy patches or abnormal blood vessels were not influenced by prior treatment (Adjusted OR 3.0, 95% CI 1.4 - 6.6, p = 0.004 and Adjusted OR 2.8, 95% CI 1.4 - 5.5, p = 0.004, respectively). Likewise, CAA was not associated with homogenous yellow patches after controlling for prior treatment and age (Adjusted OR 1.2, 95% CI 0.6 - 2.6, p = 0.57).
Current risk water contact was not associated with grainy sandy patches (OR 1.2, 95% CI 0.7 - 2.1, p = 0.60), abnormal blood vessels (OR 1.6, 95% CI 0.9 - 2.6, p = 0.076), and homogenous yellow patches (OR 0.2, 95% CI 0.9 - 2.5, p = 0.17).
Discussion
The current study shows, for the first time, that genital schistosomiasis - grainy sandy patches and abnormal blood vessels - are found in women who harbour live Schistosoma haematobium worms. However, homogenous yellow patches in the genitals, although associated with urinary egg excretion, seem not to be associated with live worms; indicating that homogenous yellow patches may be a result of a long-standing infection or chronic tissue damage caused by constant irritation from dead ova. We found that the presence of CAA was associated with having received anti-schistosomal treatment previously. This was expected as study participants live in an endemic area with continuous risk of exposure and developing morbidity, it may be an indication of continuous re-infection (21).
Current exposure to schistosomiasis infested water was, however, not associated with any of the lesions, indicating that lesions may have been established in childhood and possibly maintained by a continuous flow of new ova (21). Laboratory technicians did not differentiate between calcified and viable-looking ova during microscopy of urine and therefore we cannot preclude that S. haematobium positive women with homogenous yellow patches lesions may have had dead ova. Schistosoma PCR may also be an indication of recent egg deposition, however, only a sub-sample had been tested by PCR, therefore results should be interpreted with caution. A lack of association between homogenous yellow patches and CAA in this study could also represent a Type 2 error. Therefore, further studies are needed to confirm the chronicity of homogenous yellow patches.
In this study area, mass-treatment had not yet been rolled out (22). Therefore, participants had only been treated upon presentation at the local clinics. Such individual therapy is often not repeated, the surrounding community members are still infesting the waters, and infection control is not sustained, resulting in new live worms and genital lesions in these young women. South Africa should implement mass-treatment in endemic areas (23). Furthermore, as recommended by UNAIDS, WHO, many scientists, and programme managers, prevention of FGS lesions and screening should be integrated into sexual and reproductive health programmes, such as HPV vaccination, cervical cancer screening, antiretroviral therapy, and pre-exposure prophylaxis (PrEP) for HIV/AIDS (3, 7).
The findings indicate that some lesions may be untreatable and further studies are needed to explore the significance of the different lesions and the living worms on symptoms, cervical cancer and HIV susceptibility. Furthermore, it is still not clear for how long homogenous yellow patches, symptoms, and risks persist after the worms are dead. CAA may be effective to do this research but CAA is not yet available as a diagnostic tool, it may not be affordable in routine health care.
For now, patients should be informed that lesions and symptoms may be refractory to treatment. Nevertheless, they should be treated to kill the living worms and prevent further morbidity. Further investigations and a follow-up study using CAA and PCR, with a larger sample size, several urine samples and biopsies, differentiating between viable-looking and dead ova by microscopy, are needed.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Biomedical Research Ethics Committee (BREC), University of KwaZulu-Natal (Ref BF029/07), KwaZulu-Natal Department of Health (Reference HRKM010-08) and the Regional Committee for Medical and Health Research Ethics (REC), South Eastern Norway (Ref 46907066a1.2007.535). The ethical committees, BREC and REC, were aware that learners who are 16 - 17 years old were participating in the study and specifically approved the consent procedure (independent minor consent, no parental consent). According to South African legislation, persons over the age of 12 may consent independently to participate in research. STI treatment was offered to participants with clinical signs and symptoms, and their partners in accordance with the South African syndromic treatment protocol. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
TN conceived the presented idea, carried out the laboratory and statistical analysis, contributed to the writing and finalization of the manuscript. EFK helped conceive the presented idea, supervised the project, carried out statistical analysis, contributed to the writing and finalization of the manuscript. SN helped supervise the project and contributed to writing. GD and PC supplied laboratory test kits and contributed to writing. PP contributed to writing. EK, HG-A, SG, BJV, PN, and MT contributed to conceptualization and writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the University of KwaZulu-Natal College of Health Sciences PhD Scholarship (student number 216073797). The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement no. PIRSES-GA-2010-269245, University of Copenhagen with the support from the Bill and Melinda Gates Foundation, Grant # OPPGH5344, and South-Eastern Regional Health Authority, Norway project no. 2016055.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the girls and young women who participated in the study. We are appreciative of the data support from Roy Manyaira, and other staff at BRIGHT Research in KwaZulu-Natal, South Africa. Bodo Raniadrasolo and Peter Leutscher are thanked for the images on Rubbery papules. Claudia J. de Dood from Leiden University Medical Center is acknowledged for the production and quality control of the UCP-LF CAA test materials. Cathy van Rooyen and Zani Ansel from Ampath Laboratory, Pretoria are thanked for their assistance in CAA training and analysis.
References
1. Kjetland EF, Gwanzura F, Ndhlovu PD, Mduluza T, Gomo E, Mason PR, et al. Simple Clinical Manifestations of Genital Schistosoma Haematobium Infection in Rural Zimbabwean Women. Am J Trop Med Hyg (2005) 72(3):311–9. doi: 10.4269/ajtmh.2005.72.311
2. Mbabazi PS, Vwalika B, Randrianasolo BS, Roald B, Ledzinski D, Olowookorun F, et al. World Health Organisation Female Genital Schistosomiasis. A Pocket Atlas for Clinical Health-Care Professionals . Vol. 2015. Geneva: WHO (2015). p. 49. Available at: http://apps.who.int/iris/bitstream/10665/180863/1/9789241509299_eng.pdf. WHO/HTM/NTD/2015.4.
3. UNAIDS. No More Neglect. Female Genital Schistosomiasis and HIV. Integrating Reproductive Health Interventions to Improve Women’s Lives. Geneva, Switzerland (2019). Available at: https://www.unaids.org/sites/default/files/media_asset/female_genital_schistosomiasis_and_hiv_en.pdf.
4. Freer JB, Bourke CD, Durhuus GH, Kjetland EF, Prendergast AJ. Schistosomiasis in the First 1000 Days. Lancet Infect Dis (2018) 18(6):e193–203. doi: 10.1016/S1473-3099(17)30490-5
5. Christinet V, Lazdins-Helds JK, Stothard JR, Reinhard-Rupp J. Female Genital Schistosomiasis (FGS): From Case Reports to a Call for Concerted Action Against This Neglected Gynaecological Disease. Int J Parasitol (2016) 46(7):395–404. doi: 10.1016/j.ijpara.2016.02.006
6. Engels D, Hotez PJ, Ducker C, Gyapong M, Bustinduy AL, Secor WE, et al. Integration of Prevention and Control Measures for Female Genital Schistosomiasis, HIV and Cervical Cancer. Bull World Health Organ (2020) 98(9):615–24. doi: 10.2471/BLT.20.252270
7. Hotez PJ, Engels D, Gyapong M, Ducker C, Malecela MN. Female Genital Schistosomiasis. N Engl J Med (2019) 381:2493–5. doi: 10.1056/NEJMp1914709
8. Kukula VA, MacPherson EE, Tsey IH, Stothard JR, Theobald S, Gyapong M. A Major Hurdle in the Elimination of Urogenital Schistosomiasis Revealed: Identifying Key Gaps in Knowledge and Understanding of Female Genital Schistosomiasis Within Communities and Local Health Workers. Hsieh MH, Editor. PloS Negl Trop Dis (2019) 13(3):e0007207. doi: 10.1371/journal.pntd.0007207
9. Kjetland EF, Mduluza T, Ndhlovu PD, Gomo E, Gwanzura L, Midzi N, et al. Genital Schistosomiasis in Women: A Clinical 12-Month In Vivo Study Following Treatment With Praziquantel. Trans R Soc Trop Med Hyg (2006) 100(8):740–52. doi: 10.1016/j.trstmh.2005.09.010
10. Norseth HM, Ndhlovu PD, Kleppa E, Randrianasolo BS, Jourdan PM, Roald B, et al. The Colposcopic Atlas of Schistosomiasis in the Lower Female Genital Tract Based on Studies in Malawi, Zimbabwe, Madagascar and South Africa. PloS Neglect Trop Dis (2014) 8(11):e3229. doi: 10.1371/journal.pntd.0003229
11. Galappaththi-Arachchige HN, Holmen S, Koukounari A, Kleppa E, Pillay P, Sebitloane M, et al. Evaluating Diagnostic Indicators of Urogenital Schistosoma Haematobium Infection in Young Women: A Cross Sectional Study in Rural South Africa. PloS One (2018) 13(2):1–15. doi: 10.1371/journal.pone.0191459
12. Hegertun IEA, Sulheim Gundersen KM, Kleppa E, Zulu SG, Gundersen SG, Taylor M, et al. S. Haematobium as a Common Cause of Genital Morbidity in Girls: A Cross-Sectional Study of Children in South Africa. PloS Negl Trop Dis (2013) 7(3):e2104. doi: 10.1371/journal.pntd.0002104
13. Kjetland EF, Leutscher PDC, Ndhlovu PD. A Review of Female Genital Schistosomiasis. Trends Parasitol (2012) 28(2):58–65. doi: 10.1016/j.pt.2011.10.008
14. Corstjens PL, De Dood CJ, Kornelis D, Fat EM, Wilson RA, Kariuki TM, et al. Tools for Diagnosis, Monitoring and Screening of Schistosoma Infections Utilizing Lateral-Flow Based Assays and Upconverting Phosphor Labels. Parasitology (2014) 141(14):1841–55. doi: 10.1017/S0031182014000626
15. Corstjens P, de Dood CJ, Knopp S, Clements MN, Ortu G, Umulisa I, et al. Circulating Anodic Antigen (CAA): A Highly Sensitive Diagnostic Biomarker to Detect Active Schistosoma Infections-Improvement and Use During SCORE. Am J Trop Med Hyg (2020) 103(1_Suppl):50–7. doi: 10.4269/ajtmh.19-0819
16. Langenberg MCC, Hoogerwerf MA, Koopman JPR, Janse JJ, Kos-van Oosterhoud J, Feijt C, et al. A Controlled Human Schistosoma Mansoni Infection Model to Advance Novel Drugs, Vaccines and Diagnostics. Nat Med (2020) 26(3):326–32. doi: 10.1038/s41591-020-0759-x
17. Sousa MS, van Dam GJ, Pinheiro MCC, de Dood CJ, Peralta JM, Peralta RHS, et al. Performance of an Ultra-Sensitive Assay Targeting the Circulating Anodic Antigen (CAA) for Detection of Schistosoma Mansoni Infection in a Low Endemic Area in Brazil. Front Immunol (2019) 10:682. doi: 10.3389/fimmu.2019.00682
18. National Department of Health. Standard Treatment Guidelines And Essential Medicines List for South Africa: Primary Health Care Level. Pretoria, South Africa: The South African National Department of Health (2008) p. 1–407. Available at: http://www.kznhealth.gov.za/edlphc2008.pdf.
19. Galappaththi-Arachchige HN, Zulu SG, Kleppa E, Lillebo K, Qvigstad E, Ndhlovu P, et al. Reproductive Health Problems in Rural South African Young Women: Risk Behaviour and Risk Factors. Reprod Heal (2018) 15(1):138. doi: 10.1186/s12978-018-0581-9
20. Pillay P, Taylor M, Zulu SG, Gundersen SG, Verweij JJ, Hoekstra P, et al. Real-Time Polymerase Chain Reaction for Detection of Schistosoma DNA in Small-Volume Urine Samples Reflects Focal Distribution of Urogenital Schistosomiasis in Primary School Girls in KwaZulu Natal, South Africa. Am J Trop Med Hyg (2014) 90(3):546–52. doi: 10.4269/ajtmh.13-0406
21. Kjetland EF, Ndhlovu PD, Kurewa EN, Midzi N, Gomo E, Mduluza T, et al. Prevention of Gynecologic Contact Bleeding and Genital Sandy Patches by Childhood Anti-Schistosomal Treatment. Am J Trop Med Hyg (2008) 79(1):79–83. doi: 10.4269/ajtmh.2008.79.79
22. Magaisa K, Taylor M, Kjetland EF, Naidoo PJ. A Review of the Control of Schistosomiasis in South Africa. South Afr J Sci (2015) 111:1–6. doi: 10.17159/sajs.2015/20140427
Keywords: female genital schistosomiasis, FGS, CAA, homogenous yellow patch, grainy sandy patch
Citation: Nemungadi TG, Kleppa E, van Dam GJ, Corstjens PLAM, Galappaththi-Arachchige HN, Pillay P, Gundersen SG, Vennervald BJ, Ndhlovu P, Taylor M, Naidoo S and Kjetland EF (2022) Female Genital Schistosomiasis Lesions Explored Using Circulating Anodic Antigen as an Indicator for Live Schistosoma Worms. Front. Trop. Dis 3:821463. doi: 10.3389/fitd.2022.821463
Received: 24 November 2021; Accepted: 23 February 2022;
Published: 24 March 2022.
Edited by:
Mark Taylor, Liverpool School of Tropical Medicine, United KingdomReviewed by:
Paul Ogongo, Institute of Primate Research, KenyaAbdel Jelil Njouendou, University of Buea, Cameroon
Copyright © 2022 Nemungadi, Kleppa, van Dam, Corstjens, Galappaththi-Arachchige, Pillay, Gundersen, Vennervald, Ndhlovu, Taylor, Naidoo and Kjetland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takalani Girly Nemungadi, dGFrYWxhbmluZW11bmdhZGlAZ21haWwuY29t
 Takalani Girly Nemungadi
Takalani Girly Nemungadi Elisabeth Kleppa3
Elisabeth Kleppa3 Paul L. A. M. Corstjens
Paul L. A. M. Corstjens Pavitra Pillay
Pavitra Pillay Birgitte J. Vennervald
Birgitte J. Vennervald