- 1Tangerang District Hospital, Tangerang, Indonesia
- 2Indonesia Research Partnership on Infectious Disease (INA-RESPOND), Jakarta, Indonesia
- 3HIV Dynamics and Replication Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, United States
- 4National Institute of Health Research and Development (NIHRD), Ministry of Health, Jakarta, Indonesia
Background: Since its emergence in China, SARS-CoV-2 has infected more than 240 million people worldwide, including in regions where dengue virus (DENV) is hyperendemic such as Latin America and Southeast Asia, including Indonesia. Diagnosis of COVID-19 in dengue endemic regions as well as DENV and SARS-CoV-2 co-infection can be challenging.
Case Presentation: We describe a 68-year-old woman with diabetes mellitus type II who was admitted to the Tangerang District Hospital on 14 April 2020. She lived in a neighborhood where a few people were contracting dengue fever. She presented with five days of fever, malaise, anorexia, nausea, myalgia, and arthralgia. Hematology revealed anemia, thrombocytopenia, normal leukocyte count, increased neutrophil proportion, and decreased lymphocyte proportion and absolute lymphocytes. Her chest X-ray showed right pericardial infiltrates. Although dengue was clinically suspected, she was also tested for SARS-CoV-2 infection as she met screening criteria. After being confirmed SARS-CoV-2 positive by RT-PCR, she was treated with ceftriaxone, paracetamol, azithromycin, oseltamivir, and chloroquine. She was clinically improved four days later and discharged from the hospital on 25 April 2020 after SARS-CoV-2 RT-PCR was negative on two consecutive samples. Dengue was diagnosed retrospectively based on sero-conversion of dengue IgM and a very high dengue IgG index (ELISA, Focus Diagnostics®, Cypress, CA, USA), and sero-conversion of dengue IgM and positive IgG (Rapid test, PanBio ®Dengue duo cassette, Inverness Medical Innovations, QLD, AU), which was equivalent to high Hemagglutination Inhibition (HI) antibody titer (≥1280) found in secondary dengue infection.
Conclusion: The overlapping clinical presentations of COVID-19 and dengue; limited diagnostic capacity of laboratories in resource constrained settings; and complexities of interpreting results make identification of COVID-19 in the dengue endemic setting challenging. Clinicians in endemic areas must be aware of diagnostic challenges and maintain a high index of suspicion for COVID-19 coinfection with DENV and other tropical pathogens.
Background
Since its emergence at the end of December 2019 in Wuhan, China, SARS-CoV-2 has infected more than 240 million people worldwide, including in regions where dengue virus (DENV) is hyperendemic such as Southeast Asia and Latin America (1). Challenges associated with diagnosis of COVID-19 while DENV and other tropical infections are circulating and simultaneous infection by DENV and SARS-CoV-2 have been anticipated (2–7) as Indonesia is highly affected by both viruses. Since Indonesia’s first reported COVID-19 case in early March 2020, the number of confirmed cases has risen to >4.2 million with an estimated 3.4% mortality rate (8) Dengue, despite having a lower mortality rate (0.7%) than COVID-19, caused 108,000 cases in 2020 (9). We report the identification of DENV and SARS-CoV-2 coinfection in Indonesia to highlight the challenge of diagnosis when multiple tropical pathogens are circulating.
Case Presentation
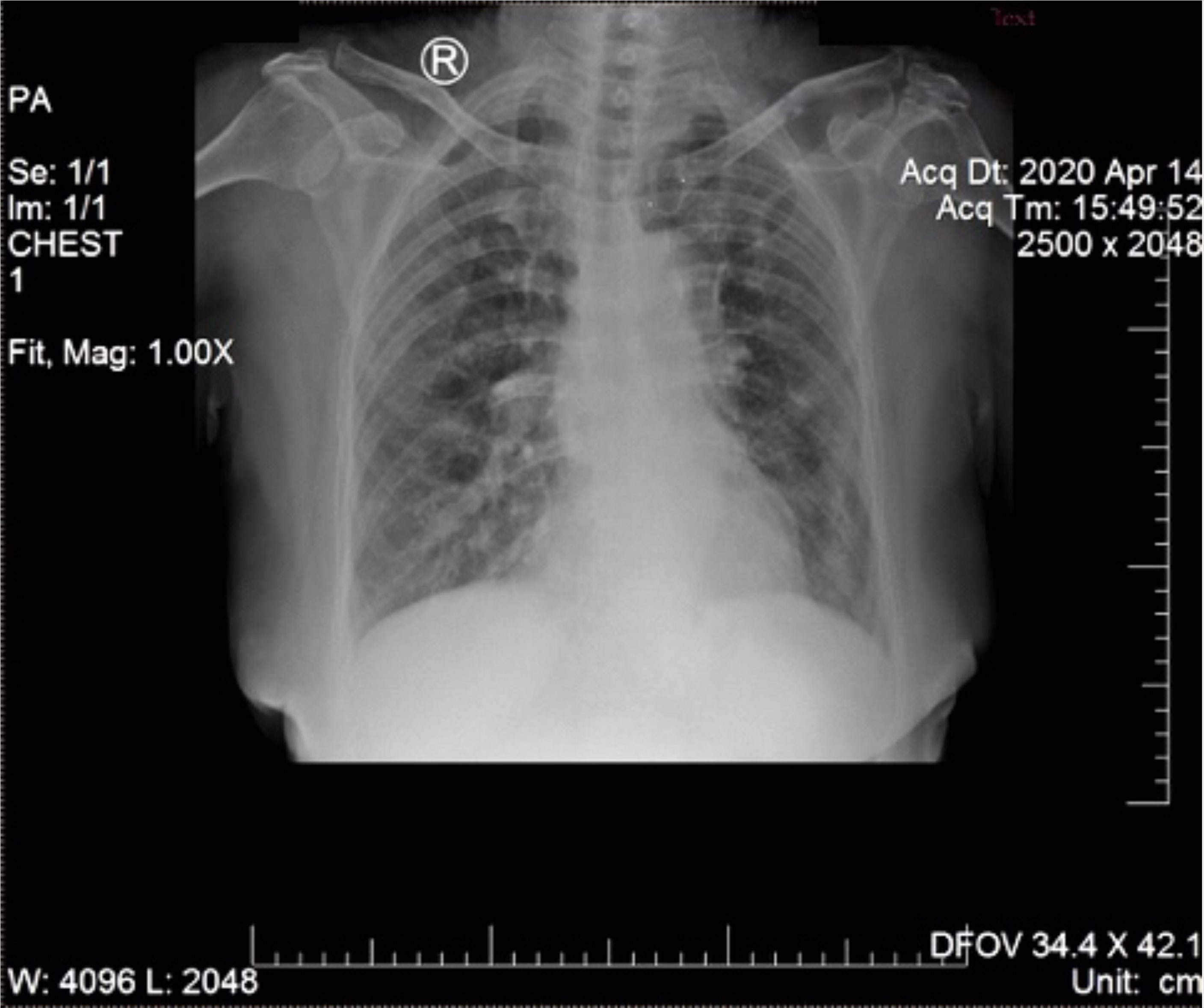
A 68-year-old woman with chronic diabetes mellitus type II was referred to Tangerang District Hospital on 14 April 2020 by a general practitioner with a clinical diagnosis of dengue fever. She presented with five days of fever to 39°C managed with oral antipyretics, accompanied by malaise, anorexia, nausea, myalgia, and arthralgia. Cough and diarrhea had appeared one day later. She reported living in a neighborhood where a few people were contracting dengue fever and participating in a religious gathering four days prior to symptom onset. On exam, vital signs were within normal limits, except for mildly elevated blood pressure (150/90 mmHg). No rash or bleeding was observed. Chest X-ray (CXR) showed right pericardial infiltrates, suggesting bronchopneumonia (Figure 1). Hematology results revealed anemia (hemoglobin 10.9 mg/dL), thrombocytopenia (99,000/mm3), normal leukocyte count (8,000/mm3), increased neutrophil proportion (78%), decreased lymphocyte proportion (13%), and decreased absolute lymphocytes (1040/mm3). Her random blood sugar, ureum and creatinine levels were normal (110 mg/dL, 24 mg/dL, and 0.8 mg/dL, respectively). She was taking glibenclamide, and had no history of heart or kidney disease.
Despite agreement that the patient had dengue based on clinical presentation, she still underwent COVID-19 screening. Per national regulations, hospitals screen all patients for COVID-19 at admission and isolate them if the virus is suspected. Consultations with the internist and pulmonologist occurred because the patient met COVID-19 screening criteria (fever, pericardial infiltrates on CXR, and exposure history). A COVID-19 serologic rapid diagnostic test (RDT) was ordered and returned faintly positive for IgM and IgG antibody combined (Wondfo®, Wondfo Biotech, Guangzhou, China). The patient was then admitted to the isolation ward for supportive care.
Pending SARS-CoV-2 rRT-PCR results (10) from mixed nasopharyngeal and oropharyngeal specimens, the patient was empirically treated with ceftriaxone (1g IV q12hr for seven days) and paracetamol (500mg PO q8hr). SARS-CoV-2 infection was confirmed two days later based on the 15 April 2020 specimen, which showed Ct values of 29.9 and 31.1 for N1 and N2 genes, respectively. The pulmonologist treated her as moderate COVID-19 and administered azithromycin (500mg PO q24hr PO for three days), oseltamivir (75mg PO q12hr for five days), and chloroquine (250mg PO q12hr for five days) per the COVID-19 management guidelines from the Indonesian Society of Respirology and available evidence at the time (11, 12). As COVID-19 had been confirmed, laboratory work up for dengue (DENV IgM/IgG RDT) was not performed. The patient clinically improved (resolution of fever, improvement of constitutional symptoms and normal platelet count (189,000/mm3) by 18 April 2020. Follow-up SARS-CoV-2 PCR was negative on two consecutive tests 24 hours apart (21 and 22 April 2020). She was discharged from the hospital on 25 April 2020.
Retrospective serology for SARS-CoV-2 IgM and IgG antibodies using RDT SD Biosensor, Korea) was performed in addition to PCR testing. The IgM and IgG bands appeared faint in acute serum (day 6 of illness) and were strongly positive in the convalescent serum (day 11). Dengue diagnostic tests were also performed retrospectively. The diagnosis of DENV infection was based on seroconversion of IgM (index values of 0.85 in acute to 2.03 in convalescent sera) by ELISA (Focus Diagnostics®, Cypress, CA, USA) and by rapid test (from negative to positive, PanBio ®Dengue duo cassette, Inverness Medical Innovations, QLD, AU) and the positive IgG in acute and convalescent sera by rapid test (PanBio ®Dengue duo cassette, Inverness Medical Innovations, QLD, AU). This was confirmed by high index (11.3) values of IgG ELISA (Focus Diagnostics®, Cypress, CA, USA). The IgG detection threshold for this rapid immunochromatography test is set at a high IgG titer, equivalent to HI titer of ≥1280. Thus, positive rapid IgG test in both acute and convalescent sera was considered indicative of acute secondary dengue infection. DENV NS1 (PanBio ®Dengue early, Inverness Medical Innovations, QLD, AU) and DENV rRT-PCR from acute serum were negative. All serologic assays were performed in the Reference Laboratory of INA-RESPOND. Clinical course and laboratory results are shown in Figure 2.
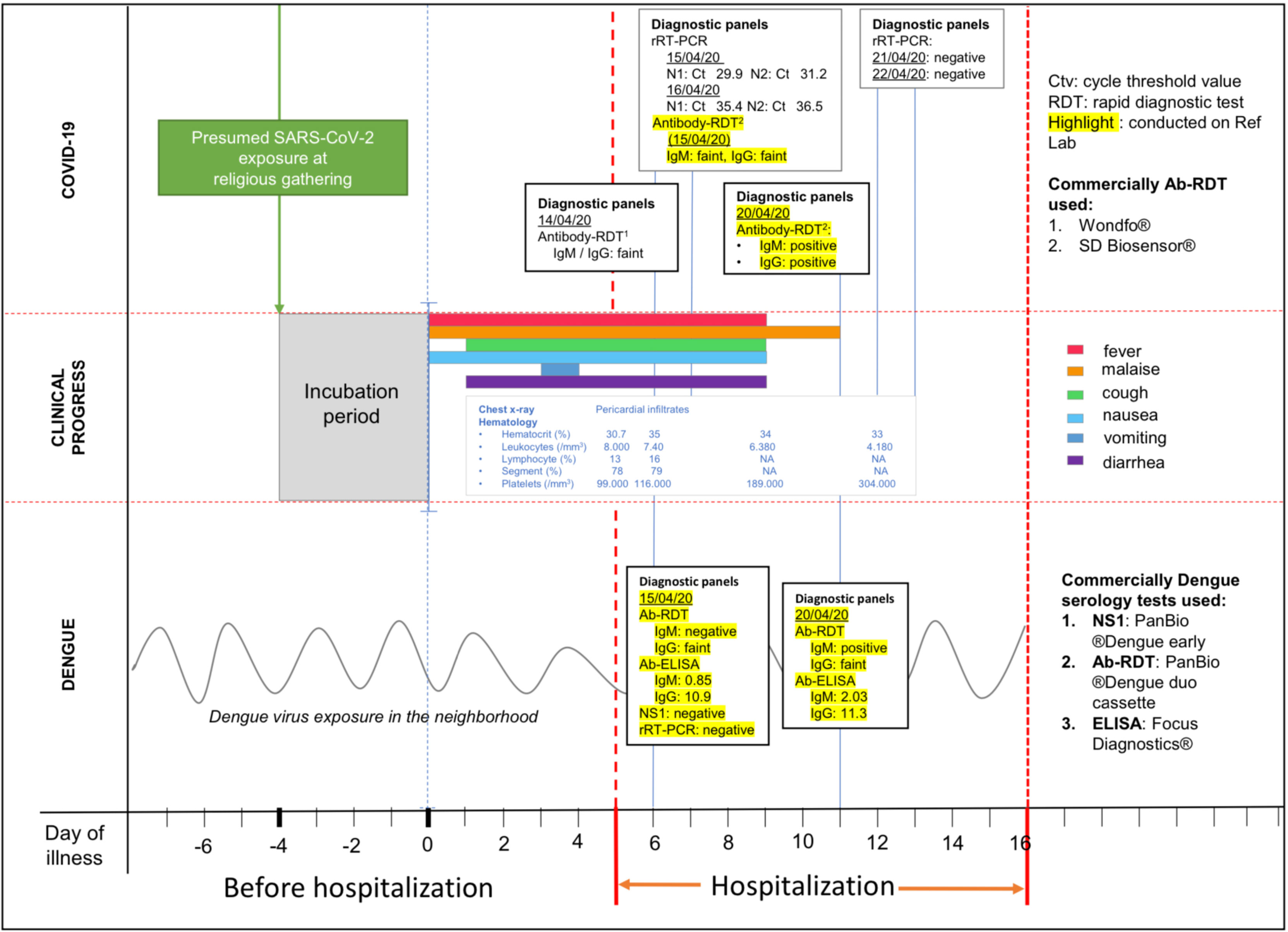
Figure 2 Time course of clinical and laboratory findings. COVID-19 data is shown in the top panel; clinical course is shown in the middle panel; dengue data is shown in the bottom panel. Procedures highlighted in yellow were performed retrospectively for research purposes.
Discussion and Conclusion
We report a SARS-CoV-2 and DENV co-infection that occurred in April 2020, at the beginning of the pandemic in Indonesia. Since then, several additional co-infections have been recognized (13, 14). Our patient’s clinical manifestations were moderate despite having dual DENV and SARS-CoV-2 infections on the backdrop of DM type II. This may be attributable to well-controlled blood sugar, the absence of DM related complications such as heart or kidney diseases (15), and possibly the rapid clearance of DENV in blood during recurrent infection. In recurrent DENV infection, IgG antibodies rise quickly during acute illness, often in the absence or with low titers of IgM antibodies (16). This may explain the negative DENV RT-PCR and NS1. It has been reported that DENV in secondary infection peaks on day 2 of illness and then decreases rapidly to undetectable on day 5 (17). Similarly, NS1 in secondary infection disappears earlier than in primary infection and is often undetected (17–19). Although potential cross-reactivity between SARS-CoV-2 and DENV antibodies in rapid serological tests has been suggested (13, 20–23), recent dengue virus infection in our case was confirmed by seroconversion in paired acute-convalescent sera using both RDT and ELISA to exclude a false-positive dengue serology. Our patient’s conversion to SARS-CoV-2 PCR negativity by day 12 of illness is within the range expected (24, 25). However, we do not know precisely when SARS-CoV-2 cleared as we did not have swabs between day seven (positive) and day 12 (negative).
The overlapping clinical presentations of COVID-19, dengue and typhoid fever; limited diagnostic capacity of laboratories in resource constrained settings; and complexities of interpreting results make identification of COVID-19 in the dengue endemic setting challenging. Constitutional symptoms, such as fever, headache, and myalgia are often reported in both symptomatic dengue infection and during COVID-19 (26, 27). Both diseases can present with thrombocytopenia associated with depressed platelet synthesis due to virus-induced bone marrow suppression and immune-mediated clearance of platelets (26). However, clinical manifestations diverge as the COVID and dengue progress. The hallmark of dengue infection is progression to plasma leakage, contracted intravascular volume, and hypovolemic shock in the critical phase (28). COVID-19 can progress to severe pneumonia with acute respiratory distress syndrome, which can lead to respiratory failure, septic shock, and/or multiple organ dysfunction (29).
Dengue can be clinically diagnosed and confirmed by assays including anti-DENV antibodies, non-structural protein 1 (NS1) antigen, or DENV-specific nucleic acid detection (30). Dengue NS1 and IgM/IgG RDTs are useful in the emergency room to guide clinicians. However, several studies in Indonesia have reported detection of dengue antibodies in confirmed COVID-19 patients (13, 21, 23, 31), and cross-reactivity between DENV and COVID-19 antibody RDTs has been suggested based on similarities between epitopes in the HR2 domain of the SARS-CoV-2 spike protein and the dengue envelope protein from in-silico analysis, though false positives and alternate antigen cross-reactivity cannot be ruled out (32). Cross-reactivity between Covid-19 antibodies and other co-circulating infections is also possible (33). Continued development of diagnostic tests, such as reverse transcriptase loop-mediated isothermal amplification (RT-LAMP), CRISPR, and antigen-based assay RDTs, is essential for identification of COVID-19 cases (34, 35).
When our case occurred, RDTs for the SARS-CoV-2 antigen were not available, as reflected by the assessments of the general practitioner and emergency room physician, both of whom suspected dengue based on clinical presentation and ongoing local transmission of DENV. Along with a high index of suspicion, epidemiology, clinical symptoms, laboratory testing, and imaging enable diagnosis of COVID-19 (27). Radiography, including CXR, is performed to screen suspected cases according to the COVID-19 diagnosis and management guidelines from Indonesian Society of Respirology. CXR findings of multiple bilateral ground-glass opacities in the peripheral lower lung zone and pericardial infiltrate may help distinguish COVID-19 from dengue, the latter of which may present with pleural effusions (34, 36). Subsequently, the pulmonologist suspected COVID-19 despite the existing DENV clinical diagnosis in light of the consistent respiratory, laboratory, and imaging findings. It was then discovered that the patient had contact with a confirmed COVID-19 case 4 days prior to emergence of symptoms.
Despite reports of low sensitivity of the COVID-19 antibody RDT during acute illness (37), use in this patient was vital as it facilitated her management as a COVID-19 patient. Difficulty in distinguishing COVID-19 and dengue, particularly when diagnostics perform suboptimally, has been reported in other countries including Singapore (20) and Thailand (38). Findings from a tertiary hospital in Singapore indicate that routine screening of patients with viral prodromes during a dual outbreak of COVID-19 and dengue can enable containment of COVID-19 cases masquerading as dengue (39). Nowadays, presence of SARS-CoV-2 antigen based RDTs with excellent performance within the first week of illness has largely replaced rapid antibody tests for early detection of COVID-19, especially in resource-limited settings (34). Furthermore, positive dengue infection should not prevent evaluation for COVID-19, particularly when patients have a history of contact with suspected or confirmed COVID-19 cases.
At the time of this co-infection, the standard of care for both dengue and COVID-19 in Indonesia was supportive care. No effective specific antivirals for Dengue or COVID were yet available. Thus, clinical management of the patient would not have been altered by knowledge of the diagnosis. However, unrecognized COVID-19 presents epidemiological risks, particularly the possibility of transmission to hospital staff and other patients. This patient’s household contacts were tested for SARS-CoV-2 IgM and IgG using RDT and found to be negative. Ideally molecular diagnosis and appropriate isolation precautions would be employed at presentation for patients at risk for COVID-19. With improving COVID-19 treatment options ranging from antivirals, passive immunotherapy, anti-inflammatory drugs, and cell-based therapy (40), early diagnosis can facilitate better outcomes. Furthermore, clinicians must be cognizant of the possibility of SARS-CoV-2 infections amongst those who are vaccinated or have been previously infected.
Diagnostic strategies specifically for resource limited settings in which health care systems are already overburdened are needed. Point-of-care RDT antigen tests will expedite triage of patients due to sensitivity in detecting high pathogen load associated with the early phase of infection (41). Combination RDT antigen and RDT antibody tests could improve COVID-19 detection in patients presenting to the emergency department (42). Continued development of preventive and therapeutic approaches for both DENV and COVID-19 will also be helpful for alleviating risks to patients as well as burden on the healthcare system. In the meantime, clinicians in endemic areas must maintain a high index of suspicion for the concurrent infection with COVID-19, DENV, and other tropical pathogens.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval were not required for this publication per local legislation and institutional requirements. Written informed consent was obtained from the patient for publication of this case report.
Author Contributions
Conceptualization and Methodology, PH, DL, AN, NL, HK, C-YL, and MK. Software, YM and AP. Laboratory Work, DL, NL, GS, and DB. Formal Analysis, PH, DL, and HK. Investigation and Data Curation, PH, DL, AN, GA, and IP. Writing – Original Draft Preparation, HK, AN, YM, and C-YL. Writing – Review & Editing, all authors. Visualization, YM, HK, RS, and AP. Supervision, PH, DL, HK, and MK. All authors contributed to the article and approved the submitted version.
Funding
This manuscript has been funded in whole or in part with MoH Indonesia and Federal funds from the NIAID, NIH, under contract Nos. HHSN261200800001E and HHSN261201500003I, through the Indonesia Research Partnership on Infectious Disease (INA- RESPOND) and from the Intramural Research Program, National Institutes of Health, National Cancer Institute, Center for Cancer Research, National Institutes of Health. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Indonesia Research Partnership on Infectious Disease (INA-RESPOND) for their support. We are also grateful to the laboratory team (Wahyu Nawang Wulan, Yuanita Jayadi, and Rizki Amalia Sari) at Tangerang District Hospital, Tangerang, Indonesia. We also would like to thank Aly Diana for technical assistance with the manuscript.
References
1. WHO. Weekly Epidemiological Update on Covid-19 19 October 2021. (2021). Available at:. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---19-october-2021. (Accessed on 20 October 2021)
2. Saavedra-Velasco M, Chiara-Chilet C, Pichardo-Rodriguez R, Grandez-Urbina A, Inga-Berrospi F. Coinfection Between Dengue and Covid-19: Need for Approach in Endemic Zones. Rev Fac Cien Med Univ Nac Cordoba (2020) 77(1):52–4. doi: 10.31053/1853.0605.v77.n1.28031
3. Lam LTM, Chua YX, Tan DHY. Roles and Challenges of Primary Care Physicians Facing a Dual Outbreak of COVID-19 and Dengue in Singapore. Fam Pract (2020) 37(4):578–9. doi: 10.1093/fampra/cmaa047
4. Wu D, Lu J, Liu Q, Ma X, He W. To Alert Coinfection of COVID-19 and Dengue Virus in Developing Countries in the Dengue-Endemic Area. Infect Control Hosp Epidemiol (2020) 41(12):1482. doi: 10.1017/ice.2020.187
5. Lorenz C, Azevedo TS, Chiaravalloti-Neto F. COVID-19 and Dengue Fever: A Dangerous Combination for the Health System in Brazil. Travel Med Infect Dis (2020) 35:101659. doi: 10.1016/j.tmaid.2020.101659
6. Harapan H, Ryan M, Yohan B, Abidin RS, Nainu F, Rakib A, et al. Covid-19 and Dengue: Double Punches for Dengue-Endemic Countries in Asia. Rev Med Virol (2021) 31(2):e2161. doi: 10.1002/rmv.2161
7. Lokida D, Lukman N, Salim G, Butar-Butar DP, Kosasih H, Wulan WN, et al. Diagnosis of COVID-19 in a Dengue-Endemic Area. Am J Trop Med Hyg (2020) 103(3):1220–2. doi: 10.4269/ajtmh.20-0676
8. WHO. Coronavirus Disease 2019 (COVID-19): Indonesia Situation Report-76. Geneva, Switzerland: World Health Organization (2021).
9. Primadi O, Ma’ruf A, Hardhana B, Sibuea F, Widiantini W, Indrayani YA, et al. Kementerian Kesehatan Republik Indonesia. Profil Kesehatan Indonesia 2020. Jakarta: Kementerian Kesehatan Republik Indonesia (2021).
10. US-CDC. Real-Time Rt-Pcr Panel for Detection 2019-Novel Coronavirus Georgia, US: Centers for Disease Control and Prevention (2020).
11. Yunus F, Andarini S. Letter From Indonesia. Respirology (2020) 25(12):1328–9. doi: 10.1111/resp.13953
12. Varghese GM, John R, Manesh A, Karthik R, Abraham OC. Clinical Management of COVID-19. Indian J Med Res (2020) 151(5):401–10. doi: 10.4103/ijmr.IJMR_957_20
13. Masyeni S, Santoso MS, Widyaningsih PD, Asmara DW, Nainu F, Harapan H, et al. Serological Cross-Reaction and Coinfection of Dengue and COVID-19 in Asia: Experience From Indonesia. Int J Infect Dis (2021) 102:152–4. doi: 10.1016/j.ijid.2020.10.043
14. Alam A, Sudarwati S, Hakim DDL, Mahdiani S. Case Report: Severe COVID-19 and Dengue in an Indonesian Infant. Am J Trop Med Hyg (2021) 104:1456–60. doi: 10.4269/ajtmh.20-1244
15. Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of Blood Glucose Control and Outcomes in Patients With COVID-19 and Pre-Existing Type 2 Diabetes. Cell Metab (2020) 31(6):1068–77.e3. doi: 10.1016/j.cmet.2020.04.021
16. Kosasih H, Alisjahbana B, Nurhayati, de Mast Q, Rudiman IF, Widjaja S, et al. The Epidemiology, Virology and Clinical Findings of Dengue Virus Infections in a Cohort of Indonesian Adults in Western Java. PloS Negl Trop Dis (2016) 10(2):e0004390. doi: 10.1371/journal.pntd.0004390
17. Muller DA, Depelsenaire AC, Young PR. Clinical and Laboratory Diagnosis of Dengue Virus Infection. J Infect Dis (2017) 215(suppl_2):S89–95. doi: 10.1093/infdis/jiw649
18. Tricou V, Minh NN, Farrar J, Tran HT, Simmons CP. Kinetics of Viremia and NS1 Antigenemia Are Shaped by Immune Status and Virus Serotype in Adults With Dengue. PloS Negl Trop Dis (2011) 5(9):e1309. doi: 10.1371/journal.pntd.0001309
19. Kosasih H, Alisjahbana B, Widjaja S, Nurhayati, de Mast Q, Parwati I, et al. The Diagnostic and Prognostic Value of Dengue Non-Structural 1 Antigen Detection in a Hyper-Endemic Region in Indonesia. PloS One (2013) 8(11):e80891. doi: 10.1371/journal.pone.0080891
20. Yan G, Lee CK, Lam LTM, Yan B, Chua YX, Lim AYN, et al. Covert COVID-19 and False-Positive Dengue Serology in Singapore. Lancet Infect Dis (2020) 20(5):536. doi: 10.1016/S1473-3099(20)30158-4
21. Kembuan GJ. Dengue Serology in Indonesian COVID-19 Patients: Coinfection or Serological Overlap? IDCases (2020) 22:e00927. doi: 10.1016/j.idcr.2020.e00927
22. Prasitsirikul W, Pongpirul K, Pongpirul WA, Panitantum N, Ratnarathon AC, Hemachudha T. Nurse Infected With Covid-19 From a Provisional Dengue Patient. Emerg Microbes Infect (2020) 9(1):1354–5. doi: 10.1080/22221751.2020.1775131
23. Santoso MS, Masyeni S, Haryanto S, Yohan B, Hibberd ML, Sasmono RT. Assessment of Dengue and COVID-19 Antibody Rapid Diagnostic Tests Cross-Reactivity in Indonesia. Virol J (2021) 18(1):54. doi: 10.1186/s12985-021-01522-2
24. Xiao AT, Tong YX, Zhang S. Profile of RT-PCR for SARS-Cov-2: A Preliminary Study From 56 COVID-19 Patients. Clin Infect Dis (2020) 71(16):2249–51. doi: 10.1093/cid/ciaa460
25. Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-Cov-2. JAMA (2020) 323(22):2249–51. doi: 10.1001/jama.2020.8259
26. Tsheten T, Clements ACA, Gray DJ, Adhikary RK, Wangdi K. Clinical Features and Outcomes of COVID-19 and Dengue Co-Infection: A Systematic Review. BMC Infect Dis (2021) 21(1):729. doi: 10.1186/s12879-021-06409-9
27. Sanyaolu A, Okorie C, Marinkovic A, Ayodele O, Abbasi AF, Prakash S, et al. Navigating the Diagnostics of COVID-19. SN Compr Clin Med (2020) 2:1393–400. doi: 10.1007/s42399-020-00408-8
28. Wang WH, Urbina AN, Chang MR, Assavalapsakul W, Lu PL, Chen YH, et al. Dengue Hemorrhagic Fever - A Systemic Literature Review of Current Perspectives on Pathogenesis, Prevention and Control. J Microbiol Immunol Infect (2020) 53(6):963–78. doi: 10.1016/j.jmii.2020.03.007
29. Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and Multiorgan Response. Curr Probl Cardiol (2020) 45(8):100618. doi: 10.1016/j.cpcardiol.2020.100618
30. Raafat N, Blacksell SD, Maude RJ. A Review of Dengue Diagnostics and Implications for Surveillance and Control. Trans R Soc Trop Med Hyg (2019) 113(11):653–60. doi: 10.1093/trstmh/trz068
31. Khairunisa SQ, Amarullah IH, Churrotin S, Fitria AL, Amin M, Lusida MI, et al. Potential Misdiagnosis Between COVID-19 and Dengue Infection Using Rapid Serological Test. Infect Dis Rep (2021) 13(2):540–51. doi: 10.3390/idr13020050
32. Lustig Y, Keler S, Kolodny R, Ben-Tal N, Atias-Varon D, Shlush E, et al. Potential Antigenic Cross-Reactivity Between Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Cov-2) and Dengue Viruses. Clin Infect Dis (2021) 73(7):e2444–e9. doi: 10.1093/cid/ciaa1207
33. Vanroye F, Bossche DVD, Brosius I, Tack B, Esbroeck MV, Jacobs J. COVID-19 Antibody Detecting Rapid Diagnostic Tests Show High Cross-Reactivity When Challenged With Pre-Pandemic Malaria, Schistosomiasis and Dengue Samples. Diagnostics (Basel) (2021) 11(7):1163–75. doi: 10.3390/diagnostics11071163
34. Mardian Y, Kosasih H, Karyana M, Neal A, Lau CY. Review of Current COVID-19 Diagnostics and Opportunities for Further Development. Front Med (Lausanne) (2021) 8:615099. doi: 10.3389/fmed.2021.615099
35. Jamshaid H, Zahid F, Din IU, Zeb A, Choi HG, Khan GM, et al. Diagnostic and Treatment Strategies for COVID-19. AAPS PharmSciTech (2020) 21(6):222. doi: 10.1208/s12249-020-01756-3
36. Kalayanarooj S. Clinical Manifestations and Management of Dengue/DHF/DSS. Trop Med Health (2011) 39(4 Suppl):83–7. doi: 10.2149/tmh.2011-S10
37. Cassaniti I, Novazzi F, Giardina F, Salinaro F, Sachs M, Perlini S, et al. Performance of Vivadiag COVID-19 Igm/Igg Rapid Test Is Inadequate for Diagnosis of COVID-19 in Acute Patients Referring to Emergency Room Department. J Med Virol (2020) 92(10):1724–7. doi: 10.1002/jmv.25800
38. Joob B WV. COVID-19 Can Present With a Rash and Be Mistaken for Dengue. J Am Acad Dermatol (2020) 82(5):e177. doi: 10.1016/j.jaad.2020.03.036
39. Wee LE, Cherng BPZ, Conceicao EP, Goh KC, Wan WY, Ko KKK, et al. Experience of a Tertiary Hospital in Singapore With Management of a Dual Outbreak of COVID-19 and Dengue. Am J Trop Med Hyg (2020) 103(5):2005–11. doi: 10.4269/ajtmh.20-0703
40. Lythgoe MP, Middleton P. Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol Sci (2020) 41(6):363–82. doi: 10.1016/j.tips.2020.03.006
41. Wilde H, Suankratay C. There Is Need for Antigen-Based Rapid Diagnostic Tests to Identify Common Acute Tropical Illnesses. J Travel Med (2007) 14(4):254–8. doi: 10.1111/j.1708-8305.2006.00094.x
Keywords: coinfection, dengue virus, SARS-CoV-2, serology, diagnostic challenge
Citation: Hariadi P, Lokida D, Menur Naysilla A, Lukman N, Kosasih H, Mardian Y, Andru G, Pertiwi I, Sugiyono RI, Pradana AA, Salim G, Butar-butar DP, Lau C-Y and Karyana M (2022) Coinfection With SARS-CoV-2 and Dengue Virus: A Case Report Highlighting Diagnostic Challenges. Front. Trop. Dis 3:801276. doi: 10.3389/fitd.2022.801276
Received: 25 October 2021; Accepted: 04 January 2022;
Published: 15 February 2022.
Edited by:
Yodi Mahendradhata, Gadjah Mada University, IndonesiaReviewed by:
Adekunle Sanyaolu, Federal Ministry of Health, NigeriaViravarn Luvira, Mahidol University, Thailand
Copyright © 2022 Hariadi, Lokida, Menur Naysilla, Lukman, Kosasih, Mardian, Andru, Pertiwi, Sugiyono, Pradana, Salim, Butar-butar, Lau and Karyana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Mardian, eW1hcmRpYW5AaW5hLXJlc3BvbmQubmV0
 Prasetyo Hariadi
Prasetyo Hariadi Dewi Lokida
Dewi Lokida Adhella Menur Naysilla
Adhella Menur Naysilla Nurhayati Lukman
Nurhayati Lukman Herman Kosasih
Herman Kosasih Yan Mardian
Yan Mardian Gestana Andru
Gestana Andru Inggar Pertiwi1
Inggar Pertiwi1 Retna I. Sugiyono
Retna I. Sugiyono Antonius A. Pradana
Antonius A. Pradana Gustiani Salim
Gustiani Salim Deni P. Butar-butar
Deni P. Butar-butar Chuen-Yen Lau
Chuen-Yen Lau Muhammad Karyana
Muhammad Karyana