
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 28 February 2022
Sec. Antimicrobial Resistance
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.779084
This article is part of the Research Topic Insights in Antimicrobial Resistance: 2021 View all 5 articles
Background: Multidrug-resistant tuberculosis (MDR-TB) represents a significant clinical and public health challenge worldwide. Out of concern for possible resistance, some providers prescribe first- and second-line tuberculosis treatment together before completing drug susceptibility testing (DST), which may increase emergent resistance.
Methods: MDR-TB patients at an Indian referral center were enrolled in an observational cohort. Participants with drug susceptibility test (DST) results were categorized as prescribed fluoroquinolones, streptomycin, both, or neither with first-line treatment before DST. Odds of additional resistance to fluoroquinolones and aminoglycosides (XDR-TB) were calculated in association with empiric combined first- and second-line treatment before DST.
Results: Of 494 participants, 130 (26.3%) received a fluoroquinolone or streptomycin with first-line drugs before DST. Odds of XDR-TB were associated with fluoroquinolone prescription before DST [odds ratio (OR): 2.19, 95% confidence interval (CI): 1.26–3.76). The association with XDR-TB persisted in multivariable analysis (adjusted OR: 2.43, 95% CI: 1.19-4.91). Combined empiric first- and second-line treatment before DST was not associated with eventual outcomes.
Conclusion: Many participants received empiric combined first- and second-line drugs before DST, which was associated with XDR-TB. To minimize emerging resistance, treatment-associated side effects, and provide the best possible care, this approach should be discouraged in favor of early DST and DST-guided TB treatment.
India is home to over a quarter of global tuberculosis, including multidrug resistant tuberculosis (MDR-TB, resistant to isoniazid and rifampin) (1). Each year, India has 2.2 million new cases, over 300,000 deaths, and economic losses of $23 billion from tuberculosis, making it India’s biggest health crisis prior to the COVID-19 pandemic (2). While many are infected directly by drug-resistant strains without ever taking tuberculosis treatment, MDR-TB can also emerge during treatment with ineffective drugs, including those prescribed before completing drug susceptibility testing (DST). To circumvent this problem, some clinicians add additional tuberculosis drugs to standard first-line therapy (rifampin, isoniazid, pyrazinamide, and ethambutol, “HRZE”) to hedge against possible drug resistance. In Mumbai, it is not uncommon to see prescriptions of first-line tuberculosis therapy along with a fluoroquinolone or streptomycin (“HRZE+FQ”, or “HRZE+S”), which risks “adding a single drug to a failing regimen” in a setting with high rates of circulating drug resistance to both drug classes (3, 4). Particularly as fluoroquinolones see rising importance as part of WHO-recommended MDR-TB treatment regimens and within so-called “universal regimens,” it will be important to ensure that fluoroquinolone resistance is limited as much as possible (5, 6). While less frequently employed, streptomycin and amikacin remain fallback drugs for MDR-TB treatment so prevention of resistance, as well as toxicity, continues to be important as well (6). In order to determine the extent to which empiric simultaneous prescription of fluoroquinolones and streptomycin with first-line tuberculosis treatment increases rates of drug resistance and limits future treatment options, we analyzed data from an ongoing observational cohort of MDR-TB patients at a referral center in Mumbai.
From October 20, 2015–October 20, 2020, adults and adolescents seeking tuberculosis care at the outpatient chest clinic of the P.D. Hinduja National Hospital and Medical Research Centre (Hinduja Hospital) in Mumbai, India were enrolled in a longitudinal observational cohort study approved by the institutional review boards at the PD Hinduja National Hospital and Medical Research Centre (884-14-ZFU) and the Johns Hopkins University School of Medicine (IRB00076738). As a referral center, many of these patients had been tested or treated for tuberculosis prior to arriving at Hinduja Hospital, including treatment with fluoroquinolones or streptomycin (HRZE+FQ, HRZE+S, or HRZE+FQ+S). Several participants started these regimens without completing DST to confirm their MDR-TB or starting MDR-TB-specific treatment regimens. Clinic patients aged 15 or older with confirmed MDR-TB were referred by clinicians to study staff for enrolment in this cohort. After providing written informed consent (or assent with written consent from a legal guardian in the case of minors), participants completed structured interviews at each clinic visit and had their medical histories abstracted for this cohort study. The current analysis compares those MDR-TB patients who received HRZE+FQ, HRZE+S, or HRZE+FQ+S prior to the identification of their MDR-TB at Hinduja Hospital to those who only received HRZE prior to the identification of their MDR-TB.
Data collected included participant demographic information (age, sex, ethnicity, education level, marital status), contact history (known contact with drug susceptible or MDR-TB), clinical history including months between symptom and diagnosis, months between diagnosis and identification of MDR-TB, history of tuberculosis treatment (drugs prescribed and dates of prescription), site of tuberculosis (defined as pulmonary, extrapulmonary, or both), height, and weight. In addition, medical records were abstracted to collect laboratory testing data (HIV status, hemoglobin A1c), smear, culture, Xpert MTB/RIF, line probe assay, pyrosequencing results (7), and DST results from mycobacteria growth indicator tubes (MGIT, using published concentrations (3)). Chest X-rays were scored according to a validated, standardized method evaluating percent lung involvement and the presence or absence of a cavity, represented as a numeric score from 0-140 (8). Body mass index (BMI) was calculated as kg/m2, and underweight was defined as enrolment BMI <18.5kg/m2. Diabetes mellitus was defined as hemoglobin A1c >6.5%. Drug resistance to fluoroquinolones and second-line injectable drugs (amikacin, kanamycin, and capreomycin) was primarily assessed by phenotypic testing in MGIT according to previously published methods (ofloxacin at 2 µg/mL and moxifloxacin at both 0.5 and 2 µg/mL) (3), though some participants completed DST for these drugs by line probe assay and pyrosequencing. Resistance to fluoroquinolones and injectable drugs was considered equivalent when identified by either phenotypic or molecular DST methods, with discordance between methods classified as the most resistant result obtained. While some participants completed DST more than once, only the first DST completed for each participant was considered for this analysis. Duration of treatment prior to DST was measured in months between prescription and sample collection for DST. Adherence was not measured directly in this study.
Cohort participants were selected for analysis if they had both a documented prescription history and DST results available. Each participant was classified as receiving a later generation fluoroquinolone, streptomycin, or both in combination with first line therapy (HRZE) before DST. The primary outcome of interest was identification of fluoroquinolone or injectable drug resistance when DST was first performed. Additional outcomes of interest included resistance to other drugs and final treatment outcome among participants who had completed treatment at the time of analysis. Outcome was defined as good (completion of treatment with or without confirmation that no relapse occurred within the subsequent 12 months) or bad (death, relapse, default, or loss to follow-up). Each variable was stratified by the exposure of interest, and univariable logistic and linear regressions were constructed for each categorical and continuous variable, respectively. Variables with clinical or statistical significance in univariable models were included in multivariable models. Collinearity was assessed by excluding variables with variance inflation factors ≥2, and the variables representing extent of pulmonary infection (pulmonary TB, % lung involvement on X-ray, and cavitary lung disease) were combined into a single variable of chest X-ray score (8). Analysis was performed in R (version 3.5.3, R Core Team, Vienna, Austria).
Of 602 total study participants, 494 had both full prescription records and DST available for analysis (Figure 1). Overall, 130 (26.3%) received either a fluoroquinolone, streptomycin, or both along with first-line drugs prior to their first DST. This includes 112 (22.7%) participants receiving fluoroquinolones for a median of 5.6 months before DST (interquartile range (IQR): 2.1–14.2 months), 48 (9.7%) receiving streptomycin (median 2.8 months, IQR: 1.4–7.1 months), and 30 (6.1%) receiving both a fluoroquinolone and streptomycin (median 2.0 months, IQR: 0.5–3.8). Of 112 participants prescribed a fluoroquinolone with first-line tuberculosis treatment, 57 (51%) were prescribed moxifloxacin and 55 received levofloxacin.
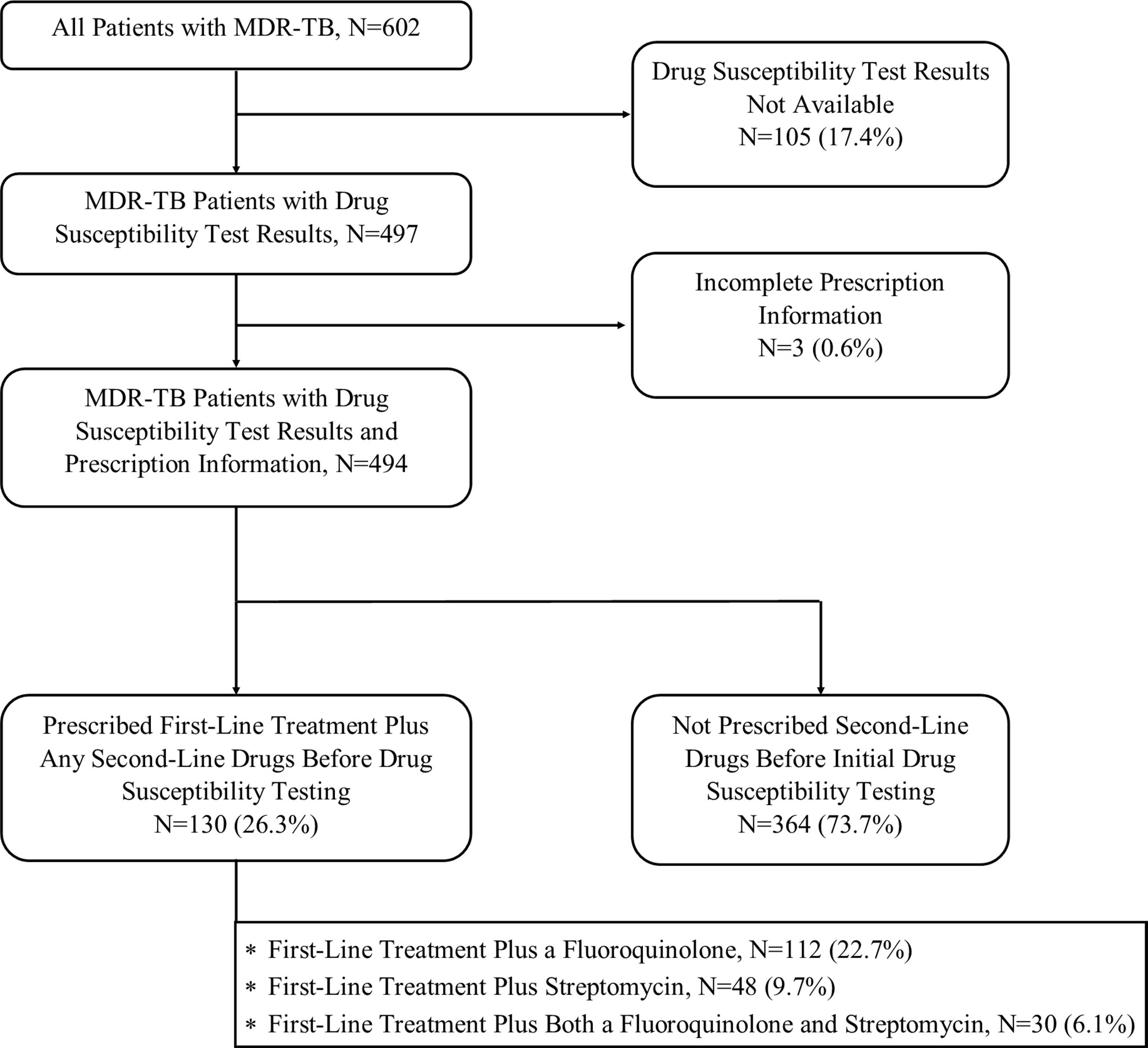
Figure 1 Participant Inclusion and Analytic Schema. Study flow indicating the number of participants included at each stage of analysis.
Of the 494 included participants 472 (95.5%) of initial DST results performed testing for isoniazid resistance, 470 (95.1%) for rifampin, 456 (90.5%) for pyrazinamide, 467 (94.5%) for ethambutol, 476 (96.4%) for fluoroquinolones, 477 (96.6%) for second-line injectables, 456 (92.3%) for ethionamide, 452 (91.5%) for para-aminosalicylic acid (PAS), 420 (85%) for clofazimine, and 274 (55.4%) for linezolid. Most participants completed DST only once (median 1 test, IQR 1-2), which did not differ according to whether the participant received either a fluoroquinolone or streptomycin before their first DST (p=0.865 and p=0.739, respectively).
Diabetes and second-line injectable drug resistance were more frequent among participants prescribed fluoroquinolones prior to DST compared to those who were not (p=0.019 and p=0.042, respectively), as was longer time to confirmed MDR-TB (p<0.001) and extent of radiographic lung involvement (p=0.005), while prior tuberculosis was less frequent among those prescribed fluoroquinolones before DST (p=0.001, Table 1). Similarly, those prescribed streptomycin before DST had longer time to confirmed MDR-TB (p=0.009), more extensive radiographic disease (p=0.031), and more frequent PAS resistance identified at first DST (p=0.041) than those not prescribed streptomycin before DST. Those prescribed both fluoroquinolones and streptomycin before DST had more extensive radiographic involvement (p=0.021) and more frequent ethambutol resistance (p=0.004) than those not prescribed those drugs before DST. Cavitary lung disease, household contacts with tuberculosis, and resistance to capreomycin and clofazimine at first DST were more frequent among those prescribed fluoroquinolones before DST, though these differences were not significant. Time to confirmation of MDR-TB was longer and PAS resistance more frequent among those prescribed both fluoroquinolones and streptomycin before DST, though these results did not reach significance. No other variables were associated with significant differences by prescription status.
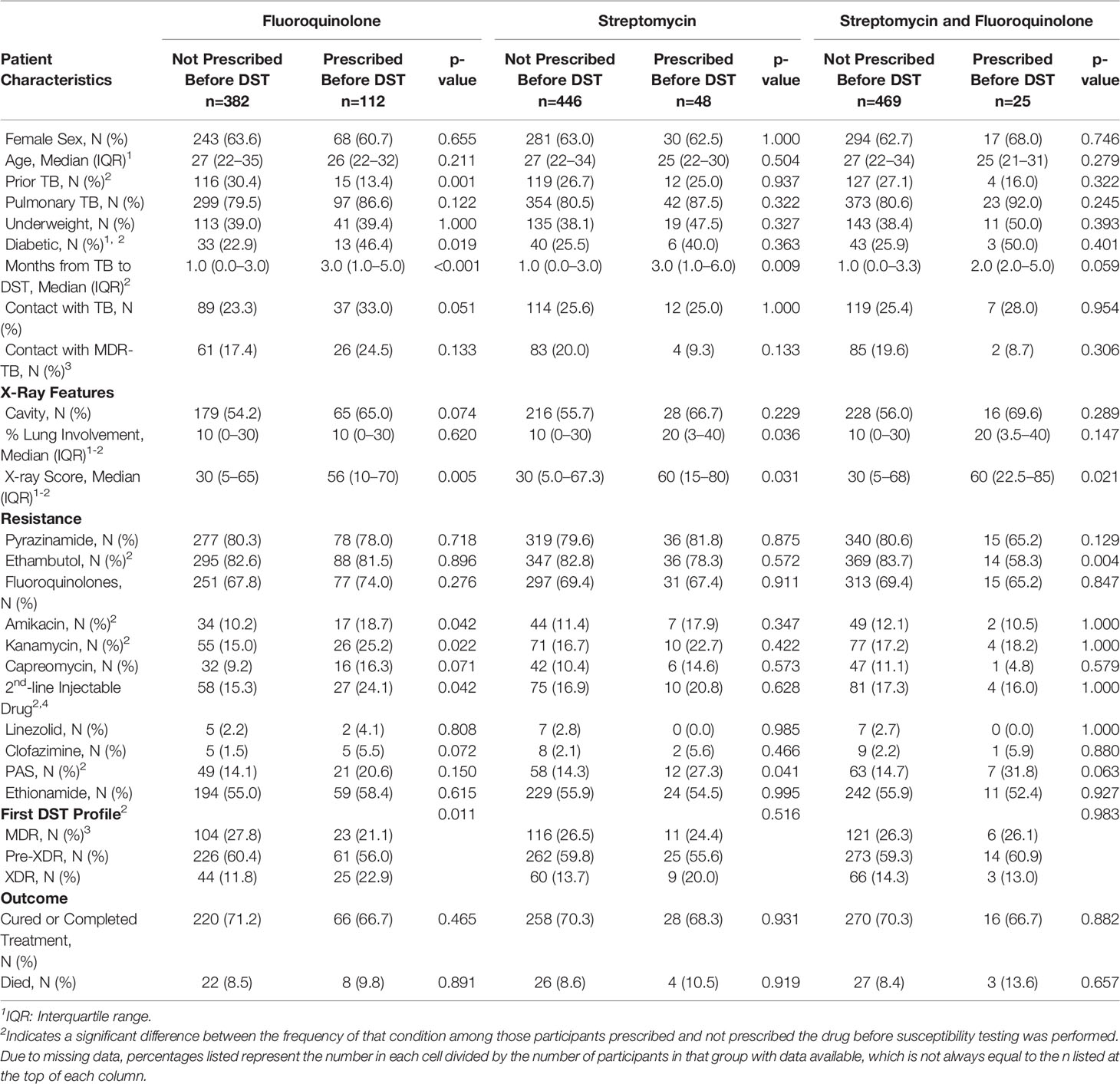
Table 1 Participant characteristics, by prescription of fluoroquinolones, streptomycin, both, or neither prior to drug susceptibility testing.
Univariable analysis found that odds of fluoroquinolone resistance was associated with underweight [odds ratio (OR): 1.61, 95% confidence interval (CI): 1.02–2.59], known contact with MDR-TB (OR: 2.29, 95% CI: 1.29–4.32), cavitary lung disease (OR: 1.64, 95% CI: 1.08–2.49), and less radiographic involvement (OR for 10% increments:0.13, 95% CI: 0.03–0.25, Table 2). Although not statistically significant, diabetes was associated with fluoroquinolone resistance at first DST (OR: 1.86, 95% CI: 0.89–4.06), and history of prior tuberculosis was associated with lower odds of fluoroquinolone resistance (OR: 0.69, 95% CI: 0.45–1.06). Importantly, neither fluoroquinolone prescription (OR: 1.35, 95% CI: 0.84–2.24) nor duration of fluoroquinolone prescription were associated with fluoroquinolone resistance (OR: -0.002, 95% CI: -0.009–0.005).
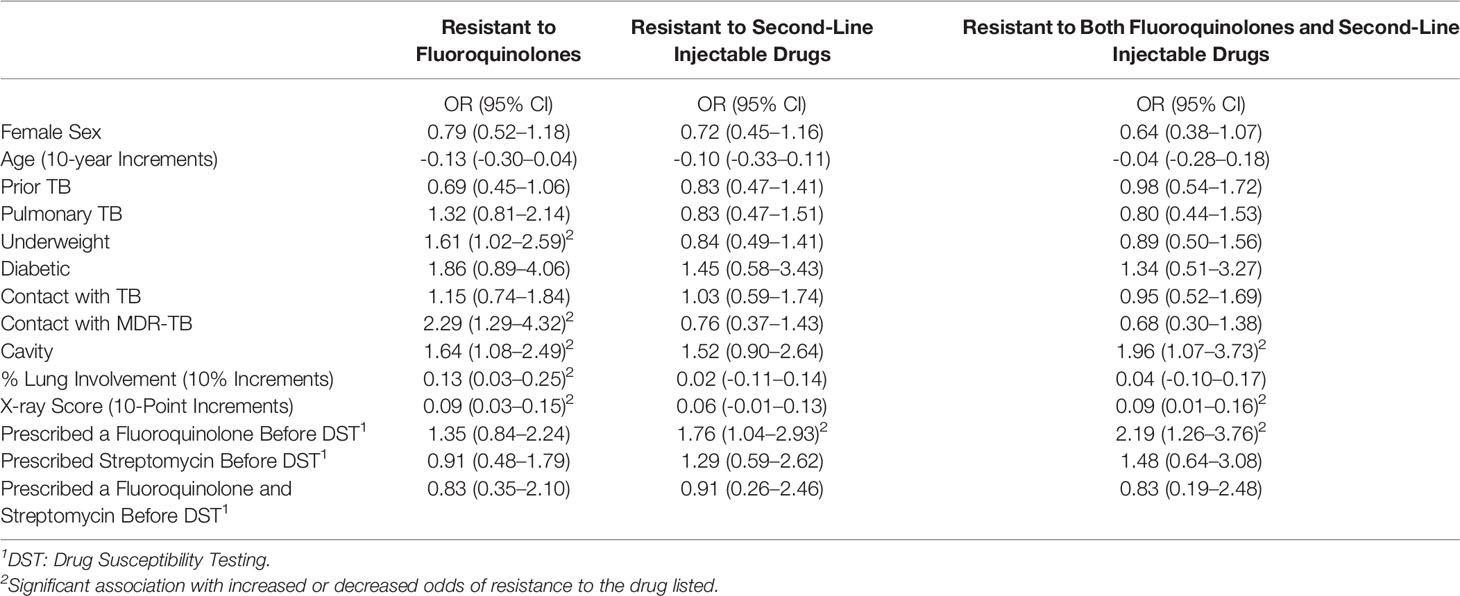
Table 2 Odds of resistance to fluoroquinolones and injectable drugs at the time of first drug susceptibility testing.
Univariable odds of injectable resistance at first DST were associated with fluoroquinolone use prior to DST (OR: 1.76, 95% CI: 1.04–2.93). While not significant, cavitary lung disease was associated increased odds of fluoroquinolone resistance (OR: 1.52, 95% CI: 0.90–2.64). Likewise, increased X-ray involvement (OR for 10% increments of total lung field: 0.06 95% CI: -0.01–0.13) and months of streptomycin use (OR per month: 0.013, 95% CI: -0.001-0.027) were associated with large, but non-significant odds of injectable drug resistance (Table 2).
Greater odds of resistance to both a fluoroquinolone and a second-line injectable drug (XDR-TB) at first DST were associated with fluoroquinolone use before DST (OR:2.19, 95% CI:1.26–3.76) and cavitary lung disease (OR: 1.96, 95% CI: 1.07–3.73). Lower odds of XDR-TB were associated with months of streptomycin use before DST (OR: 0.01, 95% CI: 0.00-0.03) and extent of X-ray involvement (OR for 10-point increments: 0.09, 95% CI: 0.01–0.16). A non-significant association was found between XDR-TB and female sex (OR: 0.64, 95% CI: 0.38–1.07). Exploratory analysis to identify impact on alternative outcomes found no significant associations between prescription of either fluoroquinolones, streptomycin, or both with odds of streptomycin resistance, good treatment outcome, or death (Table 3), though prescription of both drugs before DST was associated with lower odds of ethambutol resistance (OR: 0.78, 95% CI: 0.66-0.91), and higher odds of PAS resistance (OR: 1.19, 95% CI: 1.02-1.39). A similar nonsignificant reduction of odds was noticed between fluoroquinolone and streptomycin use and pyrazinamide resistance (OR: 0.86, 95% CI: 0.72-1.01, p=0.075).
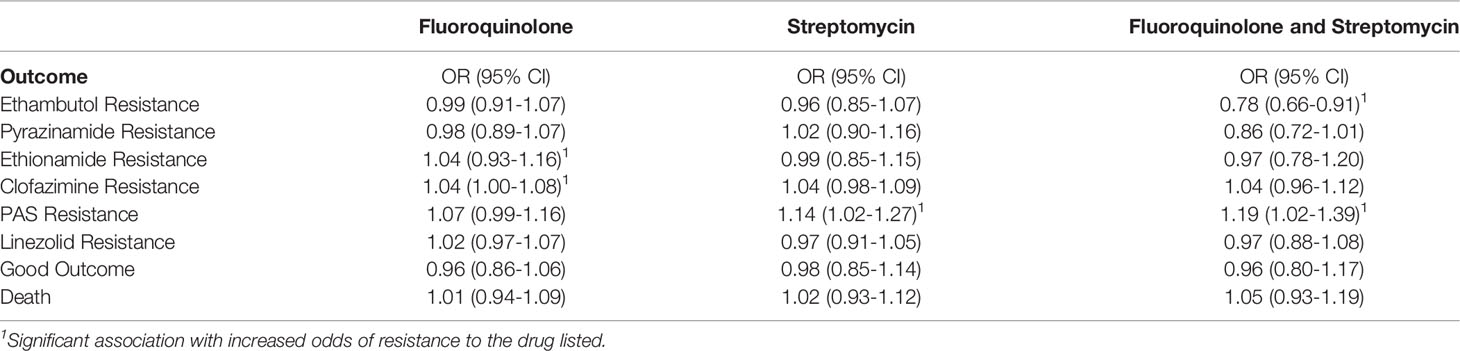
Table 3 Odds of other outcomes associated with empiric prescription of fluoroquinolone, streptomycin, or both prior to drug susceptibility testing.
Multivariable regression analysis including age, sex, underweight, contact with MDR-TB, X-ray score, and treatment before DST found that fluoroquinolone prescription before DST was associated with increased adjusted odds ratios (aOR) of XDR-TB (aOR: 2.43, 95% CI: 1.19-4.91), as was higher X-ray score (aOR: 1.11, 95% CI: 1.01-1.22), while female sex was associated with decreased odds (aOR: 0.49, 95% CI: 0.25-0.96, Table 4 and Figure 2). Having a known contact with MDR-TB was also associated with a nonsignificant reduction in odds of XDR-TB (aOR: 0.38, 95% CI: 0.12-0.98).
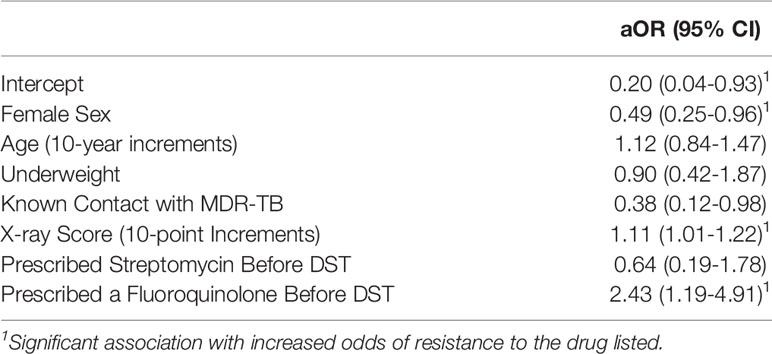
Table 4 Impact of fluoroquinolone and streptomycin prescription on adjusted odds of XDR-TB at the time of first drug susceptibility testing.
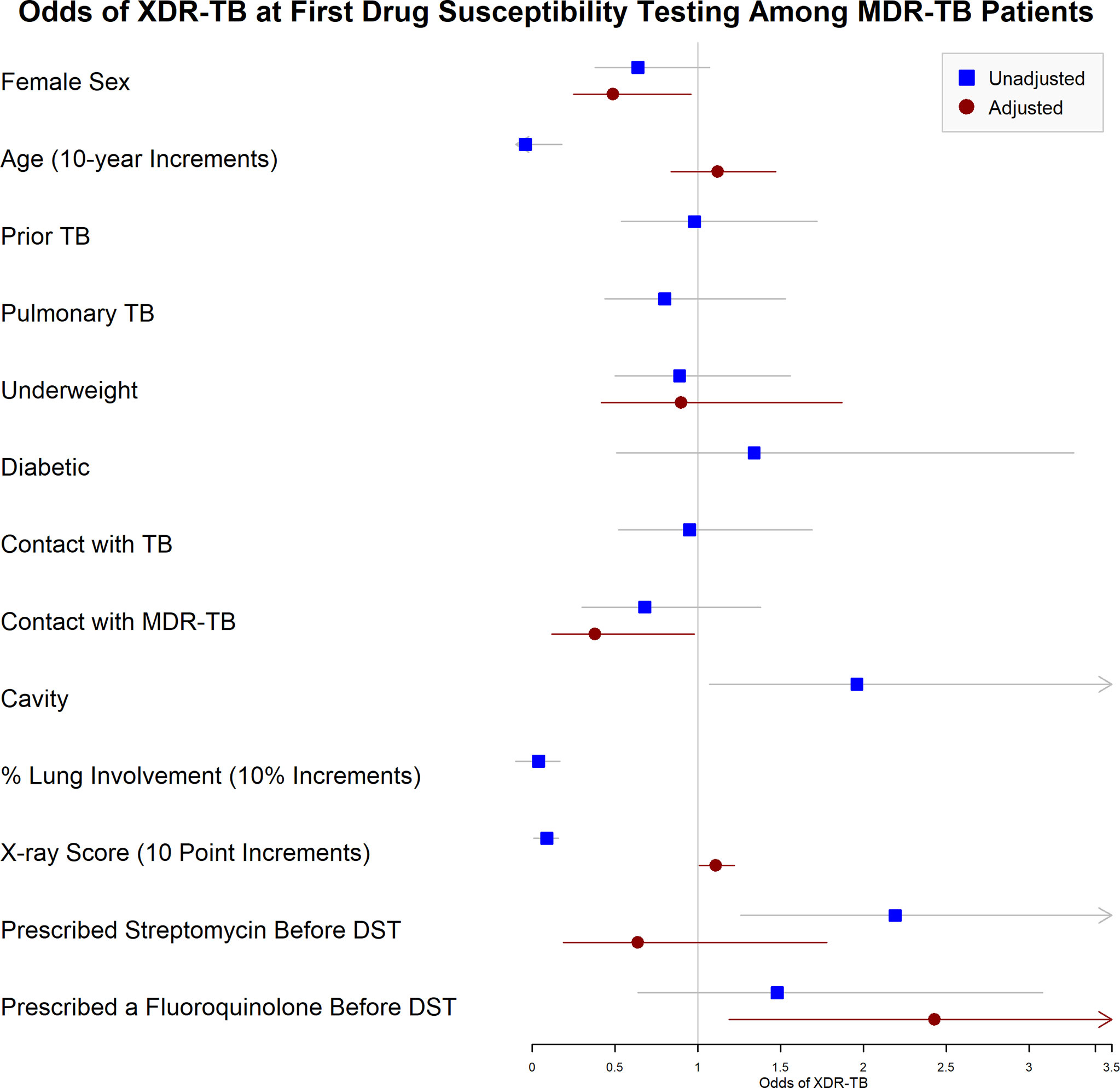
Figure 2 Unadjusted and Adjusted Odds of Resistance to both Fluoroquinolones and Injectable Drugs at the Time of First Drug Susceptibility Testing among MDR-TB Patients. Forest plot indicating the unadjusted and adjusted odds of XDR-TB identified when drug susceptibility testing (DST) was first performed. XDR-TB was defined as tuberculosis resistant to isoniazid, rifampin, a quinolone, and a second-line injectable drug (amikacin, kanamycin, and/or capreomycin). Squares indicate unadjusted odds, and circles indicate odds adjusted participant sex, age (in 10-year increments), underweight (body mass index <18.5kg/m2), known contact with MDR-TB (defined as tuberculosis resistant to isoniazid and rifampin), extent of disease on chest radiograph, and empiric prescription of streptomycin or fluoroquinolones along with first-line TB therapy (isoniazid, rifampin, pyrazinamide, and ethambutol) before DST was performed.
When drug resistance is common, DST is slow, and only specialized laboratories perform comprehensive testing, it is reasonable to treat patients conservatively while awaiting final results. Unfortunately, this analysis found that when concern translates to simultaneous prescription of first- and second-line tuberculosis drugs, higher rates of drug resistance were identified. Among 494 cohort participants studied, empiric prescription of fluoroquinolones and streptomycin along with first-line drugs was common, with 26.3% receiving one or both treatments while on first-line treatment. This was more frequent among participants with diabetes and greater lung involvement and occurred for months before DST. We confirmed the association between drug resistance and severe tuberculosis (underweight and cavitary disease). While fluoroquinolone prescription before DST was not associated with fluoroquinolone monoresistance, it was associated with both injectable drug resistance and XDR-TB in both univariable and multivariable analysis. Similarly, the early use of fluoroquinolones and streptomycin together prior to DST increased odds of additional resistance to pyrazinamide, ethambutol, and PAS, underscoring both the progressive development of resistance through inadequate therapy and the importance of DST-directed treatment.
Therapy decisions for tuberculosis include a complex balance of likely efficacy, toxicity, resistance prevention, and prevention of transmission associated with each drug. Years of unfortunate experience have demonstrated how quickly resistance can be amplified through prolonged, ineffective treatment, though recent studies have found primary acquisition of MDR-TB to be more common in some settings than emergence of MDR-TB through inadequate therapy (9, 10). In this study, fluoroquinolone prescription along with first-line treatment before DST was associated with XDR-TB, but not with worse treatment outcomes (OR: 0.96, 95% CI: 0.80-1.17) or mortality (OR: 1.05, 95% CI: 0.93-1.19). Presumably, this reflects changes to treatment after DST, resulting in similarly effective final regimens for participants who received combined first- and second-line therapy before DST. It is possible, however, that some such patients experienced negative outcomes prior to referral, which would not have been reflected in this analysis, and may have transmitted resistant TB to others. In that context, the absence of apparent negative long-term outcomes in this manuscript is not reassuring. Even with the same eventual treatment outcome, quality of life of XDR-TB patients is lower than patients with less-extensive resistance, and that difference persists even after treatment completion, underscoring the importance of reducing transmission of XDR-TB (11).
Our data demonstrate that the practice of prescribing HRZE+FQ, HRZE+S, or HRZE+FQ+S prior to the identification of MDR-TB by DST is common and is independent of risk factors for drug resistance such as prior tuberculosis, a household contact with MDR-TB, cavitary lung disease, or disease severity (Table 1). This is particularly problematic in the setting of high rates of circulating fluoroquinolone resistance, as occurs among MDR-TB isolates in Mumbai. Among people with MDR-TB at this center who complete DST, most MDR-TB strains are resistant to pyrazinamide (82%), ethambutol (86%), and fluoroquinolones (73%) (3). In that context, there is little room for benefit from HRZE+FQ, HRZE+S, or HRZE+S+FQ. For isolates with retained susceptibility to some of these drugs, this strategy is unlikely to provide an adequate number of effective drugs to prevent the rapid development of additional resistance as demonstrated in this analysis.
It is likely that the association of these regimens with newly identified resistance not to fluoroquinolones but to fluoroquinolones and injectable drugs (XDR-TB) reflects significant circulating fluoroquinolone resistance with limited room to demonstrate benefit when this strategy was applied. It is not surprising that resistance to the injectable drugs might quickly follow such a strategy, resulting in XDR-TB and additional resistance to other drugs as outlined in Table 3. Interestingly, duration of therapy prior to DST did not significantly impact resistance profiles despite prolonged exposure prior to DST. One potential explanation is the significant early bactericidal activity of both fluoroquinolones and streptomycin, which reduces the overall bacterial load in a patient infected with a susceptible isolate (12, 13). As a result, drug resistance may have emerged early in treatment, after which only XDR strains remained to be identified. The march of drug resistance for tuberculosis typically starts with isoniazid, followed by rifampin or ethambutol, pyrazinamide, and other second-line drugs (14, 15). While streptomycin shares some resistance-associated mutations in the rrs gene, streptomycin resistance is associated with mutations in either rrs or rpsL (16). It is therefore reasonable to expect that unrecognized drug resistance, including MDR-TB, could accumulate drug resistance after exposure to first-line drugs with either fluoroquinolones or streptomycin. Studies of genotypic drug resistance during MDR-TB treatment will be important to confirm the duration of exposure required to develop resistance to each XDR-TB drug.
Interestingly, this study found a significant association of extent of radiographic lung disease with treatment outcome and non-significant increase in odds of fluoroquinolone among diabetic study participants. Diabetes has been associated with poor drug absorption in several studies, and the presence of extensive and cavitary lung disease has been associated with poor outcomes in several studies (17–20). This suggests a potential benefit to considering diabetes and the extent of lung disease as potential predictors of emerging resistance during treatment, and is an important future area of research.
This study had several limitations. Importantly, most participants were diagnosed and treated for tuberculosis by a variety of providers prior to presentation to Hinduja Hospital and study enrolment. As the treatment described occurred before hospital referral, we can only speculate on the intended plan for testing and regimen duration. Concerns regarding access to DST and long turnaround time for results may have been motivating factors (21). The cost of testing varies across India and can exceed a willingness-to-pay threshold for some patients, who may persuade prescribing physicians to forego testing in favor of empiric treatment considering likelihood of resistance by epidemiologic and clinical factors (22). While this is common, our previous work has underscored the limitations of such an approach (3, 23). This study is also limited by the assumption that resistance developed after treatment was started. Because we included participants who were treated prior to DST, in the absence of molecular epidemiological data, adherence studies, and contact tracing, we cannot confirm whether drug resistance was acquired during treatment or represents primary infection with resistant tuberculosis (10). Due to the observational nature of this study, we cannot conclude that the prescription pattern was the cause of higher resistance rates, but we believe the association identified in this analysis to be a compelling reason to reconsider this practice. In addition, this study site represents a clinic population with low rates of HIV co-infection (<0.5% of participants) and high rates of circulating fluoroquinolone and injectable drug resistance, which may limit generalizability of results to settings with distinct patient populations. Finally, MDR-TB treatment guidelines from both the Indian Government and the WHO have changed substantially in recent years, particularly highlighting the importance of bedaquiline, downgrading the role of injectable drugs, and redefining the definition of XDR-TB to exclude injectable drugs (6, 24, 25). While this reduces the impact of these findings, we believe that the overall lesson that the practice of combining first-line and second-line therapies out of concern for drug resistance should be discouraged, particularly in places like Mumbai with high rates of circulating drug resistance.
In this study, many participants received fluoroquinolones and streptomycin in combination with first-line drugs while awaiting DST. Those prescriptions were associated with increased odds of XDR-TB identified when DST was first completed. To reduce emergence of drug resistance, minimize exposure to toxic therapies, and provide the best possible care, this approach should be discouraged in favor of early DST and DST-guided treatment for MDR-TB.
The datasets presented in this article are not readily available because clinical cohort data were generated as part of an ongoing study. Requests for data should be directed to the corresponding author.
All participants provided written informed consent for this study. Participants <18 years old provided assent and their guardians provided written consent. This study was approved by the institutional review boards at the PD Hinduja National Hospital and Medical Research Centre and the Johns Hopkins University School of Medicine.
ZU contributed to study design, data collection, data analysis, and manuscript preparation. PP contributed to data analysis and manuscript preparation. SS contributed to data collection and manuscript preparation. AG contributed to study design and manuscript preparation. JAT contributed to study design, data analysis, and manuscript preparation. All authors contributed to the article and approved the submitted version.
JAT was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (NIH NIAID awards number K23AI135102 and R21AI122922), the UJMT Fogarty Global Health Fellows Program (NIH Fogarty International Center, NINDS, NIMH, NHBLI and NIEHS award number D43 TW009340), and the Johns Hopkins University School of Medicine Clinician Scientist Career Development Award. AG was supported by NIAID [UM1AI069465], the Ujala Foundation, Gilead Foundation, and Wyncote Foundation. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in the design, analysis, manuscript preparation, or decision to publish this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank the study participants for their contributions to this research.
1. Organization WH. Global Tuberculosis Report 2020. Geneva, Switzerland: World Health Organization (2020).
2. Udwadia ZF, Mehra C. Tuberculosis in India. BMJ (Clinical Res ed) (2015) 350:h1080. doi: 10.1136/bmj.h1080
3. Udwadia ZF, Tornheim JA, Ganatra S, DeLuca A, Rodrigues CS, Gupta A. Few Eligible for the Newly Recommended Short Course MDR-TB Regimen at a Large Mumbai Private Clinic. BMC Infect Dis (2019) 19(1):94. doi: 10.1186/s12879-019-3726-8
4. Mahmoudi A, Iseman MD. Pitfalls in the Care of Patients With Tuberculosis. Common Errors and Their Association With the Acquisition of Drug Resistance. JAMA (1993) 270(1):65–8. doi: 10.1001/jama.1993.03510010071032
5. Sarathy J, Blanc L, Alvarez-Cabrera N, O'Brien P, Dias-Freedman I, Mina M, et al. Fluoroquinolone Efficacy Against Tuberculosis Is Driven by Penetration Into Lesions and Activity Against Resident Bacterial Populations. Antimicrob Agents Chemother (2019) 63(5):e02516–18. doi: 10.1128/AAC.02516-18
6. WHO. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment. Geneva, Switzerland: World Health Organization (2020).
7. Ajbani K, Lin SY, Rodrigues C, Nguyen D, Arroyo F, Kaping J, et al. Evaluation of Pyrosequencing for Detecting Extensively Drug-Resistant Mycobacterium Tuberculosis Among Clinical Isolates From Four High-Burden Countries. Antimicrob Agents Chemother (2015) 59(1):414–20. doi: 10.1128/AAC.03614-14
8. Ralph AP, Ardian M, Wiguna A, Maguire GP, Becker NG, Drogumuller G, et al. A Simple, Valid, Numerical Score for Grading Chest X-Ray Severity in Adult Smear-Positive Pulmonary Tuberculosis. Thorax (2010) 65(10):863–9. doi: 10.1136/thx.2010.136242
9. Seung KJ, Keshavjee S, Rich ML. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harbor Perspect Med (2015) 5(9):a017863. doi: 10.1101/cshperspect.a017863
10. Shah NS, Auld SC, Brust JC, Mathema B, Ismail N, Moodley P, et al. Transmission of Extensively Drug-Resistant Tuberculosis in South Africa. N Engl J Med (2017) 376(3):243–53. doi: 10.1056/NEJMoa1604544
11. Vo NX, Xuan Doan TB, Kha Vo DN, Tran TK, Vo TQ. Assessing Quality of Life for Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis Patients. JPMA J Pakistan Med Assoc (2019) 69(Suppl 2):S137–s57.
12. Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, et al. Early and Extended Early Bactericidal Activity of Levofloxacin, Gatifloxacin and Moxifloxacin in Pulmonary Tuberculosis. Int J Tuberc Lung Dis (2006) 10(6):605–12.
13. Donald PR, Sirgel FA, Venter A, Smit E, Parkin DP, Van de Wal BW, et al. The Early Bactericidal Activity of Streptomycin. Int J Tuberc Lung Dis (2002) 6(8):693–8.
14. Cohen KA, Abeel T, Manson McGuire A, Desjardins CA, Munsamy V, Shea TP, et al. Evolution of Extensively Drug-Resistant Tuberculosis Over Four Decades: Whole Genome Sequencing and Dating Analysis of Mycobacterium Tuberculosis Isolates From KwaZulu-Natal. PLoS Med (2015) 12(9):e1001880. doi: 10.1371/journal.pmed.1001880
15. Manson AL, Cohen KA, Abeel T, Desjardins CA, Armstrong DT, Barry CE 3rd, et al. Genomic Analysis of Globally Diverse Mycobacterium Tuberculosis Strains Provides Insights Into the Emergence and Spread of Multidrug Resistance. Nat Genet (2017) 49(3):395–402. doi: 10.1038/ng.3767
16. Mariam SH, Werngren J, Aronsson J, Hoffner S, Andersson DI. Dynamics of Antibiotic Resistant Mycobacterium Tuberculosis During Long-Term Infection and Antibiotic Treatment. PLoS One (2011) 6(6):e21147. doi: 10.1371/journal.pone.0021147
17. Dooley KE, Tang T, Golub JE, Dorman SE, Cronin W. Impact of Diabetes Mellitus on Treatment Outcomes of Patients With Active Tuberculosis. Am J Trop Med hygiene (2009) 80(4):634–9. doi: 10.4269/ajtmh.2009.80.634
18. Alfarisi O, Mave V, Gaikwad S, Sahasrabudhe T, Ramachandran G, Kumar H, et al. Effect of Diabetes Mellitus on the Pharmacokinetics and Pharmacodynamics of Tuberculosis Treatment. Antimicrob Agents Chemother (2018) 62(11). doi: 10.1128/AAC.01383-18
19. Peetluk LS, Ridolfi FM, Rebeiro PF, Liu D, Rolla VC, Sterling TR. Systematic Review of Prediction Models for Pulmonary Tuberculosis Treatment Outcomes in Adults. BMJ Open (2021) 11(3):e044687. doi: 10.1136/bmjopen-2020-044687
20. Dheda K, Lenders L, Magombedze G, Srivastava S, Raj P, Arning E, et al. Drug-Penetration Gradients Associated With Acquired Drug Resistance in Patients With Tuberculosis. Am J Respir Crit Care Med (2018) 198(9):1208–19. doi: 10.1164/rccm.201711-2333OC
21. Zürcher K, Ballif M, Fenner L, Borrell S, Keller PM, Gnokoro J, et al. Drug Susceptibility Testing and Mortality in Patients Treated for Tuberculosis in High-Burden Countries: A Multicentre Cohort Study. Lancet Infect Dis (2019) 19(3):298–307. doi: 10.1016/S1473-3099(18)30673-X
22. Dowdy DW, van't Hoog A, Shah M, Cobelens F. Cost-Effectiveness of Rapid Susceptibility Testing Against Second-Line Drugs for Tuberculosis. Int J Tuberc Lung Dis (2014) 18(6):647–54. doi: 10.5588/ijtld.13.0776
23. Tornheim JA, Intini E, Gupta A, Udwadia ZF. Clinical Features Associated With Linezolid Resistance Among Multidrug Resistant Tuberculosis Patients at a Tertiary Care Hospital in Mumbai, India. J Clin Tuberc Other Mycobact Dis (2020) 20:100175. doi: 10.1016/j.jctube.2020.100175
24. RNTCP. Guidelines on Programatic Management of Drug-Resistant Tuberculosis in India 2017 Vol. 2017. New Delhi, India: Revised National Tuberculosis Control Programme, Central TB Division, Central TB Division DGoHS, Ministry of Health and Family Welfare (2017).
Keywords: XDR/TB-extensively drug resistance of tuberculosis, drug resistance, fluoroquinolone resistance, streptomycin, emerging resistance, drug susceptibility testing
Citation: Udwadia ZF, Patel PP, Sharma S, Gupta A and Tornheim JA (2022) Empiric Addition of Quinolones to First-Line Tuberculosis Treatment Is Associated With Increased Odds of XDR-TB. Front. Trop. Dis 3:779084. doi: 10.3389/fitd.2022.779084
Received: 17 September 2021; Accepted: 17 January 2022;
Published: 28 February 2022.
Edited by:
Catrin Moore, University of Oxford, United KingdomReviewed by:
Azger Dusthackeer, National Institute of Research in Tuberculosis (ICMR), IndiaCopyright © 2022 Udwadia, Patel, Sharma, Gupta and Tornheim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zarir F. Udwadia, emZ1QGhpbmR1amFob3NwaXRhbC5jb20=; Jeffrey A. Tornheim, dG9ybmhlaW1Aamh1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.