
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 09 December 2022
Sec. Major Tropical Diseases
Volume 3 - 2022 | https://doi.org/10.3389/fitd.2022.1046948
This article is part of the Research Topic Immunology of Tuberculosis View all 6 articles
 Jing-Jing Lin1†
Jing-Jing Lin1† Xiu-Hong Xi2†
Xiu-Hong Xi2† Lu Xia2†
Lu Xia2† Yao-Ju Tan3
Yao-Ju Tan3 Yu Chen4
Yu Chen4 Hong-Qin Di5
Hong-Qin Di5 Zhen-Hua Chen6
Zhen-Hua Chen6 Ting Yu7
Ting Yu7 Jian-Hao Wei2
Jian-Hao Wei2 Peng Fang5
Peng Fang5 Xiao-Ma Lin6
Xiao-Ma Lin6 Bi-Yi Su3
Bi-Yi Su3 Ming-Zhe Yan7
Ming-Zhe Yan7 Yong-Min Yu4
Yong-Min Yu4 Kosuke Okada8
Kosuke Okada8 Norihisa Noguchi9
Norihisa Noguchi9 Toshimitsu Annaka9
Toshimitsu Annaka9 Xu-Hui Liu10*
Xu-Hui Liu10* Shui-Hua Lu11,12*
Shui-Hua Lu11,12*Objectives: To evaluate the diagnostic accuracy of Loop-Mediated Isothermal Amplification Platform (LAMP) in detecting pulmonary tuberculosis (PTB).
Methods: This multicenter prospective study was conducted at six sites in China from June, 2018 to December, 2019. Patients with suspected PTB were consecutively recruited and respiratory samples were collected from all patients. LAMP, Xpert MTB/RIF assay (Xpert), fluorescence smear microscopy, and BACTEC MGIT 960 liquid culture (Mtb culture) were performed for each sample. Diagnostic accuracy indices were calculated against Mtb culture results.
Results: A total of 845 participants were enrolled, but only 799 were included in the analysis. The sensitivities of LAMP, Xpert, and smear microscopy were 78.6% (239/304), 82.2% (250/304), and 63.8% (194/304), respectively, and their specificities were 88.7% (439/495), 86.1% (426/495), and 94.9% (470/495), respectively. The LAMP assay showed substantial agreement with other tests (kappa 0.64–0.79).
Conclusion: The LAMP assay performs as well as Xpert MTB/RIF assay and Mtb culture in tertiary-care hospitals. It can be used as an alternative test for detecting PTB with the advantages of being fast, inexpensive, and easy to operate.
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is a global public health challenge. In, 2018, only 55% of pulmonary tuberculosis (PTB) cases were bacteriologically confirmed (1). In high-income countries with access to the most sensitive diagnostic tests, approximately 80% of PTB cases are bacteriologically confirmed, while the rate of bacteriological confirmation is only 30% in China (1). Rapid molecular tests, such as the Xpert MTB/RIF assay (Xpert; Cepheid, Sunnyvale, CA, USA), have not been generalized to marginalized areas until now, partly because of the relatively high cost and requirement for ongoing specialist technical support; therefore, other methods are used in these areas. For example, fluorescence and acid-fast smear microscopy are still widely used in China, but they are insensitive and do not distinguish between Mtb and non-tuberculous mycobacteria (NTM) (2). BACTEC MGIT 960 liquid culture (Becton Dickinson Biosciences, Sparks, MD, USA) and Lowenstein–Jensen medium culture are the gold standard for Mtb detection. However, even the fastest culture methods are too slow to help with initial TB management, as results are reported after weeks rather than hours or days. Loop-mediated isothermal amplification for TB (TB-LAMP; Eiken Chemical Company, Tokyo, Japan) is a novel nucleic acid amplification assay endorsed by the World Health Organization (WHO) for the detection of PTB. Compared with the Xpert MTB/RIF assay, LAMP is less expensive (50–70% less cost than Xpert in China), easier to operate (result could be read by the naked eye), and comparably fast (3, 4). Some studies have reported the diagnostic accuracy of LAMP in China, but the results have been inconsistent (5–7). Therefore, we conducted a multicenter study with a large sample size to determine the diagnostic accuracy of LAMP in this region.
This multicenter prospective diagnostic accuracy study was conducted in six tertiary-care hospitals (Shanghai Public Health Clinical Center, Guangzhou Chest Hospital, Hunan Chest Hospital, Hebei Chest Hospital, Zhengzhou Sixth People’s Hospital, and Wuhan Jinyintan Hospital) from June, 2018 to December, 2019 (ChiCTR-DDD-17013146). The study protocol was reviewed and approved by the ethics committee of the Shanghai Public Health Clinical Center (2018-S013-02), and the study was performed in accordance with domestic clinical study guidelines. Written informed consent was obtained from the participants (or their parents or guardians for minors). Patients of all ages and both sexes were consecutively enrolled if they met one or more of the following suspected PTB criteria (8): (1) household TB contact in the previous 3 months; (2) fever or cough for more than two weeks; (3) weight loss or failure to gain weight in the previous 3 months; (4) a positive tuberculin skin test or T-SPOT; and (5) chest radiography suggestive of TB (required). In this study, confirmed TB was defined as Mtb culture-positive. Clinically, diagnostic criteria were established according to the diagnostic consensus and domestic guidelines (2, 8, 9). Exclusion criteria included 1) inadequacy or invalid samples for all tests; 2) incomplete clinical data or an indeterminate clinical diagnosis; and 3) exposure to anti-TB drugs for ≥4 weeks (including carbapenems, fluoroquinolones, macrolides, and aminoglycosides).
Sputum or induced sputum was used for diagnostic testing. Samples were processed according to the manufacturer’s instructions (10–12). A 60 µL sample of sputum was used for the LAMP assay(Loopamp PURE DNA Extraction Kit; Eiken Chemical Company, Tokyo, Japan), and the remainder of the sample was divided into three for smear microscopy, Mtb culture (BACTEC MGIT 960 system, BD Biosciences), and Xpert MTB/RIF assay (Xpert; Cepheid, Sunnyvale, CA, USA). Cultures that were positive for acid-fast bacilli were confirmed as Mtb complex (MTBC) organisms using MPT64/MPB64 antigen detection according to the manufacturer’s instructions (Capilia, Hangzhou, China) (2). NTM strains were confirmed by Sanger sequencing. The Limit of Detection (LoD) of Xpert is 131 cfu/mL.The LoD of LAMP assay is 1.28copies/µL when 5µL sample solution is used for reaction. It was performed according to the WHO guidelines (4): (1) A sample of 60 µL of sputum was transferred to a heating tube containing extraction solution; (2) the sample was mixed by inverting it 3–4 times, and the heating tube was placed on the heating block at 90°C for 5 min to inactivate and lyse mycobacteria; (3) take out the heating tube and place it at room temperature for 2 minutes, then invert it 3-5 times; (4) the heating tube was attached to an adsorbent tube, mix the contents thoroughly to ensure no white powder residue; (5) 30 µL of the resulting solution were squeezed from the adsorbent tube to the reaction tube, and invert reaction tube for 2 min to mix the solution and the reagent which is in the lid, then shake the mixture to the bottom; (6) the temperature on the digital display on the incubator was confirmed to be 67°C; (7) the reaction tubes were loaded into the heating block; (8) the sample was amplified for 40 min, and the amplification was stopped automatically after 40 min; (9) a fluorescence detector (Loopamp LF-160; Eiken Chemical Co., Ltd., Tokyo, Japan) was used to check the reaction tubes, and the results were judged with the naked eye, green indicates positive.
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated to evaluate diagnostic accuracy. Categorical variables were compared using Pearson’s chi-square, McNemar’s (paired), or Fisher’s exact test. Statistical p-value of ≤0.05 was considered significant. The 95% confidence intervals (CIs) were estimated for the data with binomial distributions. Kappa values were assessed to determine the agreement between categorical variables (13). Statistical analyses were performed using SPSS software version 22 (IBM Corp, Armonk, NY, USA), Excel for Windows 10 (Microsoft, Redmond, WA, USA), and GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA).
We enrolled 845 patients with suspected PTB. Of these, 10, 15, 6, and 15 cases were excluded due to culture contamination, incomplete data, repetitive testing, and NTM infection, respectively. Thus, 799 cases were analyzed, of which 549 (68.7%) patients were male, and the median age was 53 years (interquartile range; 33, 64). Mtb culture was positive in 304 (38%) samples. Additionally, 332 (41.6%) samples were categorized as Mtb culture-negative clinically diagnosed TB, and 163 (20.4%) as other respiratory diseases. (Figure 1, Table 1).
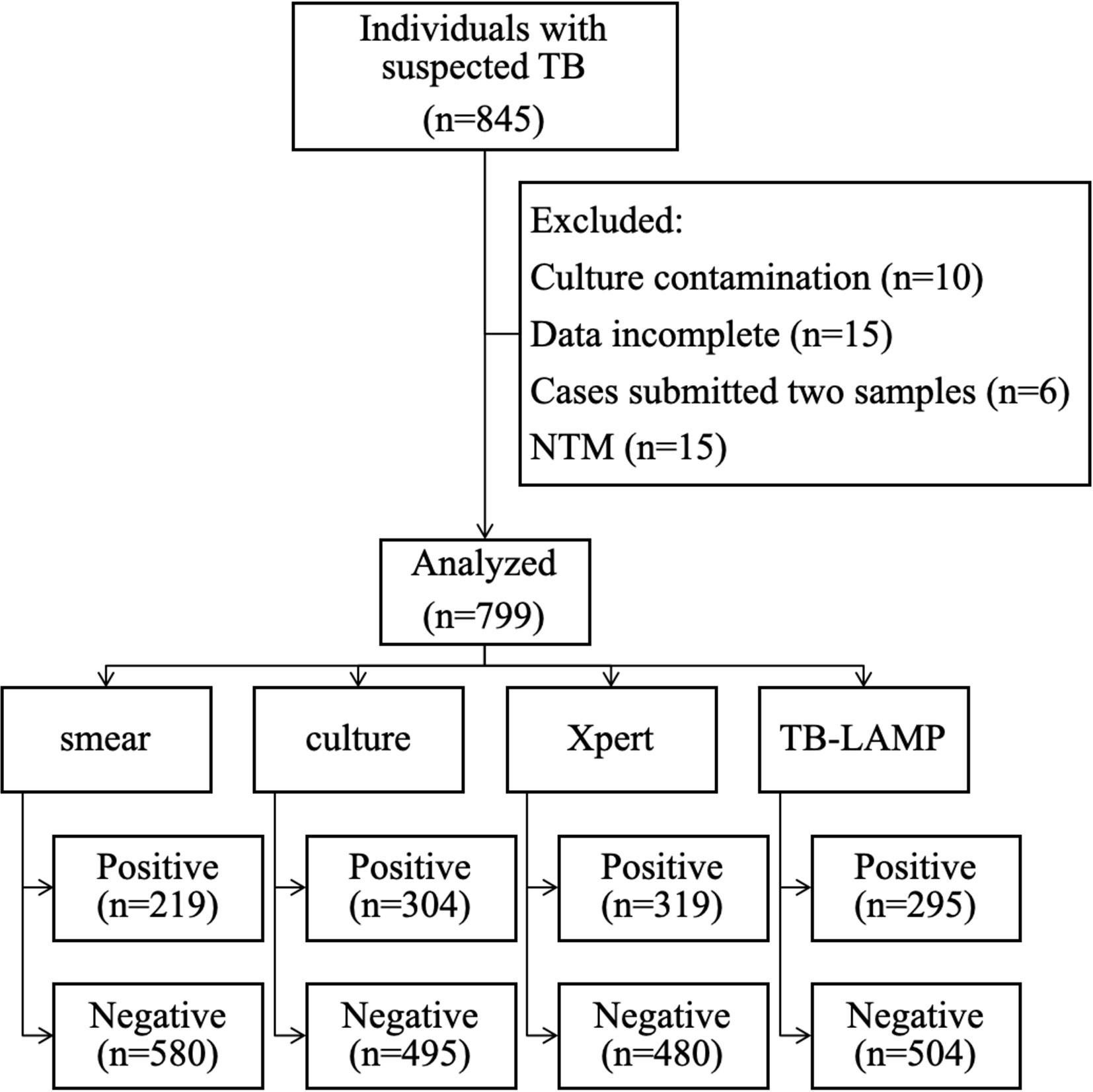
Figure 1 Flow chart of patient enrolment and the results of the diagnostic tests. Abbreviations: NTM, non-tuberculous mycobacteria.
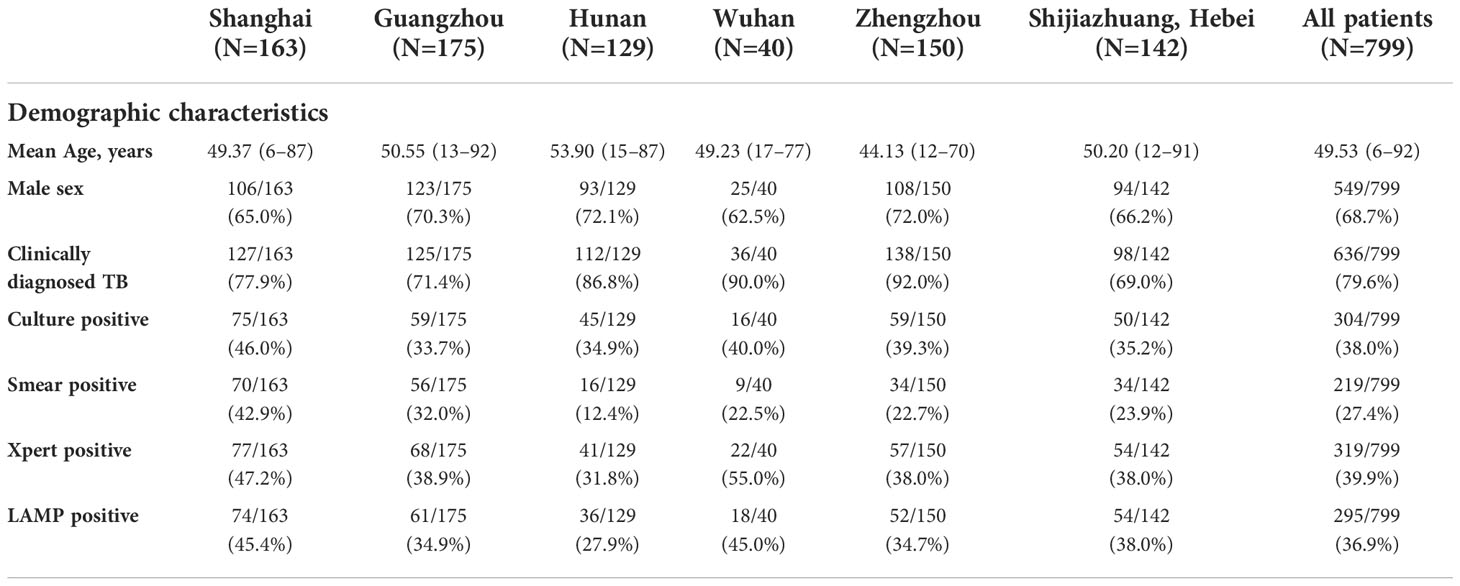
Table 1 Demographic characteristics of the study participants and diagnostic test results according to the recruitment center.
The diagnostic indices of all assays against Mtb cultures are shown in Table 2. LAMP showed an overall sensitivity of 78.6% (95% CI, 73.8–82.9%), similar to that of Xpert (82.2%, p=0.126), and higher than that of smear microscopy (63.8%, p<0.001). In smear-positive and culture-positive cases, the sensitivity of the LAMP assay was 90.7% (176/194), and in smear-negative and culture-positive cases, the sensitivity was 57.3% (63/110). The specificities of LAMP, Xpert, and smear microscopy were 88.7% (95% CI: 85.7–91.3), 86.1% (95% CI: 82.8–88.9), and 94.9% (95% CI: 92.8–96.6), respectively. Furthermore, when stratified by age,the sensitivities of LAMP between adolescent (aged 10–19 years) and adults (aged 20 years or over) were 78.6% (11/14), 78.6% (228/290) (Supplement 1 Table 1).
Compared with clinical diagnosis, the sensitivities of LAMP, Xpert, Mtb culture, and smear microscopy were 46.4%, 50.2%, 47.8%, and 34.4%, respectively, and the specificity was 100% (Table 3).
If the 15 NTM cases had been included in the analysis, smear microscopy and Mtb culture would show lower specificity than nucleic acid assays, with 92.7% (95% CI: 88.2–95.8) and 91.6% (95% CI: 86.8–95.0) specificity when compared with clinical diagnosis, respectively (Supplement 2 Table 2). When against Mtb cultures, the sensitivities of smear microscopy, LAMP and Xpert were 64.9%, 78.4%.75.2% (Supplement 2 Table 2). Sixty-five “false positive” cases (LAMP-positive and Mtb culture-negative) were reviewed, and all cases were categorized as clinically diagnosed TB.
In a single-sample test, the diagnostic consistency between LAMP and other assays was substantially high, with kappa values of 0.789, 0.677, and 0.637 for Xpert, Mtb culture, and smear microscopy, respectively.
TB has been inadequately controlled in China in recent decades. To eliminate the TB epidemic in China, any improvement in TB diagnostics, including cost and turnaround time reduction, is desirable. The use of the gyrB gene as a target for identification of Mtb and the extent to which fluoroquinolone resistance, lineage markers, or other mutations in this locus would impact the diagnostic accuracy of assays, but LAMP, targets the gyrB and IS6110 regions, may reduce the impact.Our study confirmed that the performance of the TB-LAMP assay in PTB diagnosis is non-inferior to the Xpert assay and Mtb culture. Additionally, this assay showed substantial agreement with Xpert, Mtb culture, and smear microscopy, indicating that it can be used as an adjunctive test. The results of this study are comparable with those of another multicenter study conducted in Peru, South Africa, Brazil, and Vietnam (14). In clinical practice, especially in resource-limited settings, LAMP may facilitate the early diagnosis of PTB, as it requires less infrastructure, has a shorter turnover time, and is cheaper (7).
In a systematic review, nine TB-LAMP studies yielded summary estimates of sensitivity of 80.9% and specificity of 96.5% (15). Our study reported a similar sensitivity (78.6%) but a lower specificity (88.7%) against the culture. Ou et al. also conducted a multicenter study in three tertiary hospitals in China and reported a similar sensitivity of 74.88% and specificity of 86.50% (6). We reviewed the database and attributed this lower specificity to four reasons: first, over 60% of the participants were early stage cases, who were asymptomatic or presented mild symptoms with single-lobular infiltration; second, as this study was conducted in tertiary hospitals, a proportion of smear-positive participants (who were also more likely to be culture-positive) were pre-screened and treated before visiting our sites; third, LAMP, nucleic acid amplification testing, could detect uncultured or dead bacteria, but culture could only detect live bacteria. Patients known to have PTB who are mid-treatment may only remain dead, non-viable bacteria in their sputum; fourth, sample contamination, or harsh sample decontamination procedures might be harmful for viability of Mtb but a little impact on existence of DNA.
This TB-LAMP assay, showed high specificity (100%) when compared with clinical diagnosis, and was not affected by NTM infection in this study. This result is similar to those of previous studies in Gambia (16) and India (17).
This study had limitations. First, owing to the lack of clinical data available on subjects, such as HIV status and history of TB, the sensitivity and specificity of LAMP in these populations have not been well-demonstrated. Second, most participants were enrolled in tertiary hospitals and could have been treated for <4 weeks. Thus, the results of this study are not representative of primary care services. Third, infant group (aged under 1 year) was not enrolled, and child group (aged under 10 years) only included 1 patient who was culture-negative, and the number of cases of adolescent group was significantly smaller than that of adult group, so it was hard to conclude that no significant difference in the efficiency of LAMP between different age groups. Further studies about diagnosis accuracy of LAMP need to be done in AIDS patients, infants and adolescents.
LAMP, a cheap and simple assay, holds promise as a rapid, highly sensitive, and specific test for TB case detection in China and other developing countries with a high burden of TB.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The ethics committee of the Shanghai Public Health Clinical Center (2018-S013-02). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
S-HL and X-HL were responsible for the study design and manuscript revision; J-JL, LX, and X-HX participated in conducting the study, collecting data, and writing the manuscript; J-JL, J-HW, YC, Z-HC, H-QD, PF, X-ML, B-YS, Y-JT, M-ZY, TY, and Y-MY enrolled participants in six hospitals; and KO, NN, and TA contributed to the site investigation and provided technical support. All authors contributed to the article and approved the submitted version.
We would like to thank Eiken Chemical Company (Tokyo, Japan) for their technical support. We also thank all the participants and healthcare workers at each site, without whom this study could not be accomplished.
This work received financial and technical support by Shenzhen High-level Hospital Construction Fund (No. G2022061).
Author NN and TA are employed by Eiken Chemical Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2022.1046948/full#supplementary-material
2. Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American thoracic Society/Infectious diseases society of America/Centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis (2017) 64:111–5. doi: 10.1093/cid/ciw778
3. Mitarai S, Okumura M, Toyota E, Yoshiyama T, Aono A, Sejimo A, et al. Evaluation of a simple loop-mediated isothermal amplification test kit for the diagnosis of tuberculosis. Int J Tuberc Lung Dis (2011) 15(9):1211–7, i. doi: 10.5588/ijtld.10.0629
4. World Health Organization. The use of loop-mediated isothermal amplification (LAMP) for the diagnosis of pulmonary tuberculosis- policy guidance. Geneva: World Health Organization (2016).
5. Ou X, Li Q, Xia H, Pang Y, Wang S, Zhao B, et al. Diagnostic accuracy of the PURE-LAMP test for pulmonary tuberculosis at the county-level laboratory in China. PloS One (2014) 9(5):e94544. doi: 10.1371/journal.pone.0094544
6. Ou X, Wang S, Dong H, Pang Y, Li Q, Xia H, et al. Multicenter evaluation of a real-time loop-mediated isothermal amplification (RealAmp) test for rapid diagnosis of mycobacterium tuberculosis. J Microbiol Methods (2016) 129:39–43. doi: 10.1016/j.mimet.2016.07.008
7. Deng S, Sun Y, Xia H, Liu Z, Gao L, Yang J, et al. Accuracy of commercial molecular diagnostics for the detection of pulmonary tuberculosis in china: a systematic review. Sci Rep (2019) 9(1):4553. doi: 10.1038/s41598-019-41074-8
8. National Health and Family Planning Commission of China. Diagnosis for pulmonary tuberculosis (WS 288-2017). Electron J Emerg Infect Dis (2018) 3(1):59–61.
9. Liu XH, Xia L, Song B, Wang H, Qian XQ, Wei JH, et al. Stool-based xpert MTB/RIF ultra assay as a tool for detecting pulmonary tuberculosis in children with abnormal chest imaging: A prospective cohort study. J Infect (2021) 82(1):84–9. doi: 10.1016/j.jinf.2020.10.036
10. World Health Organization. Part II. microscopy. laboratory services in tuberculosis control. Geneva: World Health Organization (1998).
11. Global Laboratory Initiative. Mycobacteriology laboratory manual. Geneva: Global Laboratory Initiative (2014).
12. Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med (2010) 363(11):1005–15. doi: 10.1056/NEJMoa0907847
13. Richard Landis J, Koch GG. The measurement of observer agreement for categorical data. Int Biometric Soc (1977) 33(1):159–74. doi: 10.2307/2529310
14. Pham TH, Peter J, Mello FCQ, Parraga T, Lan NTN, Nabeta P, et al. Performance of the TB-LAMP diagnostic assay in reference laboratories: Results from a multicentre study. Int J Infect Dis (2018) 68:44–9. doi: 10.1016/j.ijid.2018.01.005
15. Nagai K, Horita N, Yamamoto M, Tsukahara T, Nagakura H, Tashiro K, et al. Diagnostic test accuracy of loop-mediated isothermal amplification assay for mycobacterium tuberculosis: systematic review and meta-analysis. Sci Rep (2016) 6:39090. doi: 10.1038/srep39090
16. Bojang AL, Mendy FS, Tientcheu LD, Otu J, Antonio M, Kampmann B, et al. Comparison of LAMP, GeneXpert MTB/RIF and culture for diagnosis of pulmonary tuberculosis in the Gambia. J Infect (2016) 72(3):332–7. doi: 10.1016/j.jinf.2015.11.011
Keywords: tuberculosis, diagnosis, Xpert MTB/RIF assay, smear microscopy, loop-mediated isothermal amplification
Citation: Lin J-J, Xi X-H, Xia L, Tan Y-J, Chen Y, Di H-Q, Chen Z-H, Yu T, Wei J-H, Fang P, Lin X-M, Su B-Y, Yan M-Z, Yu Y-M, Okada K, Noguchi N, Annaka T, Liu X-H and Lu S-H (2022) Diagnostic accuracy of loop-mediated isothermal amplification for pulmonary tuberculosis in China. Front. Trop. Dis 3:1046948. doi: 10.3389/fitd.2022.1046948
Received: 17 September 2022; Accepted: 22 November 2022;
Published: 09 December 2022.
Edited by:
Theolis Barbosa, Oswaldo Cruz Foundation (Fiocruz), BrazilCopyright © 2022 Lin, Xi, Xia, Tan, Chen, Di, Chen, Yu, Wei, Fang, Lin, Su, Yan, Yu, Okada, Noguchi, Annaka, Liu and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shui-Hua Lu, bHVzaHVpaHVhNjZAMTI2LmNvbQ==; Xu-Hui Liu, bGl1eHVodWk2NjZAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.