Abstract
Lymphatic filariasis (LF) is a neglected tropical disease caused by threadlike worms (nematodes) that live in the lymphatic vessels of humans. Although three species of filarial parasites (Wuchereria bancrofti, Brugia malayi and Brugia timori) infect humans; 90% of infection is caused by Wuchereria Bancrofti and humans are its exclusive host. Nigeria is one of over 70 countries endemic for Lymphatic filariasis with an estimated 134 million people at the risk of infection. The Federal Capital Territory (FCT), which is endemic for LF, commenced mass drug administration (MDA) using ivermectin (IVM) and albendazole (ALB) in 2011. While MDA is continuing in two Area Councils, in 2020, we assessed the impact of MDA on LF prevalence in two area councils that had achieved five effective annual rounds of MDA. In 2010, a baseline mapping exercise was conducted in all six area councils of FCT-Abuja. The results revealed that four out of the six area councils were endemic for LF, with prevalence ranging from 1.0%-4.0%. The number of persons treated with ivermectin and albendazole in the four Area Councils was documented during annual MDA and population-based cluster surveys were conducted at least once in each area council during the five years of treatment, to verify the reported geographic and programme MDA coverage. This is the number treated divided by the total population eligible to receive treatment (usually <5years). The survey results confirmed that in two area councils (Abaji and Kuje) the coverage exceeded the target of 65% the while two other Councils did not reach the recommended coverage. A pre-transmission assessment survey (pre-TAS) was conducted in one sentinel site and at least one spot check site in Abaji and Kuje in 2019 and were found to have LF antigenemia (LF Ag) < 2% (range 0.0% to 1.99%). In 2020, transmission assessment surveys (TAS) were conducted in the two area councils that previously passed the Pre-transmission assessment survey. The results showed that the two Evaluation units had achieved the LF Ag threshold required to stop MDA. FCT has made significant progress towards LF elimination with two Area Councils qualifying to stop treatment. However, two other area councils still require a further two years of mass drug administration with effective MDA coverage before these area councils qualify for impact assessment.
1 Introduction
Lymphatic filariasis (LF) is a vector-borne disease caused by one of three filarial parasite species, Wuchereria bancrofti, Brugia malayi and Brugia timori (1). Anopheles is the dominant mosquito species that transmits LF in West Africa, (2). LF causes physical and emotional suffering resulting from the disabling and disfiguring lesions (such as hydrocoele, lymphoedema, lymphangitis and elephantiasis) and economic loss due to diminished productivity and incapacitation. LF mainly affects poor countries and marginalised people (3). Globally, the World Health Organization (WHO) estimates that 120 million people are affected, with an estimated 40 million having clinically significant manifestations. LF is the second most common cause of long-term disability (4, 5).
In 1993 the International Task Force on Disease Eradication identified LF as one of six diseases that could be eliminated globally based on available diagnostic tools and strategies (6). Consequently, in 1997, the World Health Assembly passed resolution WHA 50.29 calling for global elimination of LF as a public health problem. In 2000 the WHO launched the Global Programme to Eliminate LF (GPELF) to provide support to endemic countries and a Global Alliance for the Elimination of LF (GAELF) was established with the two principal objectives of interruption of LF transmission and alleviation/prevention of LF-related disability and suffering (7, 8).
According to WHO recommendations, the main strategy for interruption of transmission is to provide annual mass drug administration (MDA) with albendazole (400 mg) together with diethylcarbamazine (6 mg/kg) or ivermectin (200 μg/kg) (7) to all eligible populations within endemic areas. Annual MDA with a minimum treatment coverage of 65% of the total at-risk population is required for at least five years to achieve the objective of reducing the microfilaraemia prevalence to below 1% (7).
By 2015, 18 of the 73 countries known to be endemic for LF no longer required MDA and were conducting post-MDA surveillance (1). In 2017, Togo was confirmed as the first African country to eliminate LF as a public health problem (9). Globally, an estimated 856.4 million people in 2016 no longer required LF (10).
In Nigeria, the National Lymphatic Filariasis Elimination Programme (NLFEP) was established in 1997 in response to World Health Assembly (WHA) 50.29 resolution of May 1997 and was given a mandate to eliminate LF in Nigeria by 2020. Nigeria has the highest burden of lymphatic filariasis in Africa with an estimated 135 million people at risk of the disease (JRSM, 2019). Between 2008 and 2010, mapping of LF was carried out in the 6 Area Councils (ACs) of the Federal Capital Territory (FCT), using donated ImmunoChromatographic diagnostic test kits. Four of the six ACs (Abaji, Bwari, Gwagwalada and Kuje) were found to be endemic. MDA started in Gwagawalada AC in 2010 using donated medicines (3mg ivermectin and 400mg albendazole) and in 2011 was scaled-up to the remaining 3 endemic ACs. In 2018, a LF pre-transmission assessment survey (Pre-TAS) was conducted in Abaji and Kuje ACs (sentinel and spot check sites) because both ACs had achieved the recommended five effective treatment rounds (at least 65% therapeutic coverage and 100% geographic coverage). Both ACs passed pre-TAS and were therefore qualified for a first Transmission Assessment Survey (TAS 1).
2 Methods
2.1 Study site
Federal Capital Territory (FCT), within which is located Abuja, the capital city of Nigeria, is situated in the central part of Nigeria and falls in the Sudan, and Guinea vegetation. It consists of 6 Area Councils (ACs) with an estimated population of over 2 million (projected from 2006 census using 2.5% growth rate). The population comprises of Gwari, Koro, Ganagana, Gwandara, Afo, and Bassa ethnic groups, who are predominantly dairy farmers. Hausa, Fulani, Igbo, Yoruba ethnic communities also live in the territory. While the city dwellers are mostly civil servants or entrepreneurs, most of the rural dwellers are farmers. Four out of the five PC-NTDs are endemic in FCT - namely onchocerciasis, LF, schistosomiasis and soil-transmitted helminth (STH) infections.
2.2 Disease mapping
Between 2008 and 2010, the Federal Ministry of Health (FMoH) conducted LF mapping in 6 high-risk villages in each of the administrative divisions across FCT to determine LF distribution and endemcity, and to delineate implementation units eligible for MDA. High risk communities were identified using the criteria described in the WHO manual for monitoring and evaluation (M&E) of LF programmes (11). The circulating Wuchereria bancrofti antigen was identified using a rapid-format card test, the immunochromatographic test (ICT) (Alere Inc., Scarborough, USA). Sampling was carried out in the communities by laboratory technicians after they received training on the use of ICT cards and data recording. Besides the antigenaemia data, socio-demographic data (age, sex, community of residence, health area and health district) of each enrollee were also collected. Table 1 below is a summary of the mapping result showing four and two endemic and non-endemic areas respectively.
Table 1
| Area Council | Mapping year | Endemicity status | Prevalence(%) |
|---|---|---|---|
| Abaji | 2010 | endemic | 4.0 |
| Bwari | 2010 | endemic | 2.0 |
| Gwagwalada | 2008 | endemic | 2.0 |
| Kuje | 2008 | endemic | 1.0 |
| Kwali | 2008 | non-endemic | 0.0 |
| Municipal Area Council | 2010 | non-endemic | 0.0 |
LF mapping result showing endemicity in the FCT.
2.3 Mass drug administration
In 2010, integrated MDA for onchocerciasis and LF with ivermectin (3 mg) and albendazole (400mg) commenced in Gwagwalada before it was scaled up to the other ACs in 2011. Since then, all ACs requiring treatment have been reached. While all ACs were endemic for onchocerciasis, only four were co-endemic with LF. On average, annual MDA targeted about 600,000 people in 347 communities across the four endemic area councils ACs using a community directed intervention (CDI) approach. Through this approach, communities were allowed to manage the MDA by volunteers to serve as community drug distributors (CDDs). These distributors, who were required to be literate, were trained by the community health workers on conducting a community census, administering drugs, recording treatment data, and identifying and referring any adverse reactions resulting from the MDA. The CDDs administered between one and four ivermectin tablets (using a dose pole to measure height as a proxy of weight) and one tablet of albendazole to each eligible person. Health workers supervised the CDDs with support from the state and Federal Ministry of Health. MDA was conducted once a year between October and December. Community registers, previously used for onchocerciasis MDA, were modified to include albendazole and provided to all target villages. The registers captured all members of each community, whether eligible for MDA or not. Before each MDA in rural communities (villages), the CDDs conducted a pre-MDA census and updated the community register. Details of the drugs administered to each person were recorded in the registers during the distribution. Prior to MDA, intensive community sensitization was carried out (using town criers, word of mouth and mass media) to raise awareness about the MDA and encourage participation. CDDs either went from house to house or invited community members to come to a fixed point to receive drugs – depending on the choice of each community. The overall target for MDA was to achieve at least 65% programme coverage and 100% geographic coverage each year for 5 years, to ensure the ACs would qualify for a pre-TAS. Based on the programme coverage trend in Table 2 below, only Abaji and Kuje ACs have achieved the recommended coverage, thus qualifying for Pre-TAS.
Table 2
| Area Council | Therapeutic Coverage by Year (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | |
| Abaji | - | 57 | 96 | 91 | 102* | 99 | 79 | 87 | 95 | 91 |
| Bwari | - | 36 | 24 | 29 | 32 | 34 | 38 | 51.1 | 70 | 65 |
| Gwagwalada | 93 | 54 | 30 | 56 | 39 | 48 | 56 | 64.5 | 73 | 76 |
| Kuje | - | 55 | 81 | 76 | 96 | 91 | 90 | 94.2 | 98 | 48 |
LF programme coverage (%).
*Projected population of 2006 census figure was used to calculate the denominator based on an annual growth rate of 2.5%. The over 100% coverage could be due to factors (e g. Immigration or emigration due to flood and security challenges) which makes the denominator lower or higher than the projected population.
2.4 Pre transmission assessment survey
Pre-TAS is the first transmission assessment conducted in an intervention area to determine if transmission has been reduced to a level where it can be remain (or stay) even without control measures. To be eligible for Pre-TAS, in addition to 65% therapeutic coverage and 100% geographic coverage for at least 5 years, the intervention unit must have completed the fifth round of MDA at least 6 months prior to the assessment. The sentinel site (SS) (where mapping was conducted) and spot check site (SCS) (a purposely selected area with high probability of transmission, usually hard-to- reach and not contiguous with the sentinel site) are selected for assessment. The SSs and SCSs must have a population of at least 500 persons aged 5 years and above. Considering these conditions, Abaji and Kuje (Table 2) were the only ACs that met the eligibility for Pre-TAS and were therefore selected for the assessment. Two SSs and SCSs were selected in each AC for the study. Finger-pricked blood samples were collected from 300 persons from both SSs and SCSs by a team of trained laboratory technicians in the two ACs and examined for Wuchereria bancrofti antigen using Filarial Test Kits (FTS, Alere). Persons (>5 years) who spent at least a night in the past year in the sampled sites were eligible to participate in the survey The result of the Pre-TAS is presented in Table 3 below.
Table 3
| State | LGA | Community | Total Valid samples | Total positive | Prevalence (%) | Total number of people that swallowed LF medicine | Remarks |
|---|---|---|---|---|---|---|---|
| FCT | Abaji | Pandagi (SS) | 300 | 0 | 0.0 | 253(84%) | Passed Pre-TAS |
| Yaba (SC) | 300 | 0 | 0.0 | 243(81%) | Passed Pre-TAS | ||
| Kuje | Gaube (SS) | 301 | 0 | 0.0 | 266(88%) | Passed Pre-TAS | |
| Rubochi (SC) | 317 | 1 | 0.3 | 240(76%) | Passed Pre-TAS | ||
| Total | 1,218 | 1 | 0.08 | 1,002(82%) |
Summary of Pre-TAS result.
The threshold for a successful LF Pre-TAS is <2% Ag (Antigenaemia). SS-Sentinel site; SC-spot-check site.
2.5 First transmission assessment survey
The transmission assessment survey determines whether endemic LGAs have reached a critical cut-off point of infection. It is used to determine whether to stop or continue MDA. To be eligible for TAS1, an evaluation unit must have passed Pre TAS (threshold for pass is <2% antigenaemia). Abaji (EU1) and Kuje (EU2) ACs met this criterion and were selected for the assessment. The sampling population for TAS1 is children from 5-7 years old, on the basis that children should not have been exposed to infection if transmission has been interrupted. On the other hand, positive cases from this group could imply recent transmission. The survey was conducted in schools since the EUs have school enrolment of above 75%. The target sample size was 1,532 pupils in each EU taken from 40 selected schools. The location of the schools was determined using the Survey Sample Builder (SSB), a Microsoft Office Excel based-tool developed by the NTDs Support Centre (12). However, the sample size was exceeded with 1, 675 and 1, 644 pupils sampled in Abaji and Kuje respectively. The SSB facilitated the random selection of schools and children from a list of randomized numbers. Consent forms were given to head teachers for endorsement before the exercise. Hard copy forms were used to collect field data which was analyzed with Microsoft Excel. The data points included: sex, age and length of time living in the area. Each child gave their assent and their parents gave consent for each child to participate and was assigned a unique ID code. All school coordinates were taken using an android device. In addition, questionnaires were completed by the head teachers of all the schools about water sources and sanitation facilities.
2.5.1 Data analysis
Hard copy forms were used to collect field data which was entered into Microsoft Excel. All collected field data from the two EUs were verified by going through the field hard copies and using the Health mapper software to confirm the survey sites coordinates. For objectivity, data forms were interchanged among teams. The critical threshold was determined by SSB software and varied by EU.
3 Results
Descriptive statistics using excel were used to analyze and present data in this study. Of the 6 ACs initially surveyed for LF in 2008 and 2010 (Table 1), only Abaji, Bwari, Gwagwalada and Kuje were found to be endemic, with prevalence ranging from 4% (Abaji) to 1% (Kuje). Kwali and Municipal Area council had zero prevalence while Bwari and Gwagwalada had 2% each (Table 1).
For TAS 1, 1, 675 pupils were sampled in 37 schools in Abaji AC while, a total of 1, 644 pupils from 36 schools were sampled in Kuje AC. Table 4 shows a summary of the characteristic of the evaluation units. Only two tested positive for the W.bancrofti antigen in Abaji AC while no positive was recored in Kuje AC. This was far below the threshold of 18 which would have resulted in failure of the TAS 1. Based on WHO recommendation to stop MDA in areas of transmission by Culex, Anopheles or Mansonia in which the prevalence is < 2%, MDA was scaled down. Table 5 is ssummary of the TAS result.
Table 4
| Evaluation Unit | Abaji | Kuje |
|---|---|---|
| Targeted sample size | 1681 | 1719 |
| Collected sample size | 1681 | 1652 |
| Targeted number of schools | 40 | 40 |
| Number of school surveyed | 37 | 36 |
| Boys sampled | 894 | |
| Girls Sampled | 787 | 823 |
Sociodemographic characteristic of the populations surveyed Evaluation unit.
Table 5
| EU | LGAs | Target Sample size | Total registered | Number of Schools targeted | Number of Schools Surveyed | Total Present | Total tested | Total invalid | Total refusal | Total positive | Critical cut-off | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abaji | 1532 | 1,681 | 30 | 37 | 2,421 | 1,675 | 2 | 4 | 2 | 18 | Passed |
| 2 | Kuje | 1532 | 1,652 | 30 | 36 | 1,719 | 1,644 | 0 | 8 | 0 | 18 | Passed |
Sociodemographic characteristic of the populations surveyed Evaluation unit.
4 Discussion
The results from this study show that the FCT is making steady progress to achieve elimination. Two ACs that reported high coverages were assessed and the results have demonstrated that the criteria for stopping MDA have been reached. Consequently, 225,661 persons no longer require MDA in these two ACs shown in Figure 1, even as surveillance is ongoing. This is a major change from the baseline survey where prevalence in Abaji has reduced from 4% to 0.1% and in Kuje from 1% to zero. These results are in line with studies from Kenya, Egypt, Togo and Benin that have shown a similar, significant reduction in LF prevalence and density after five to eight years of LF MDA (13–16).
Figure 1
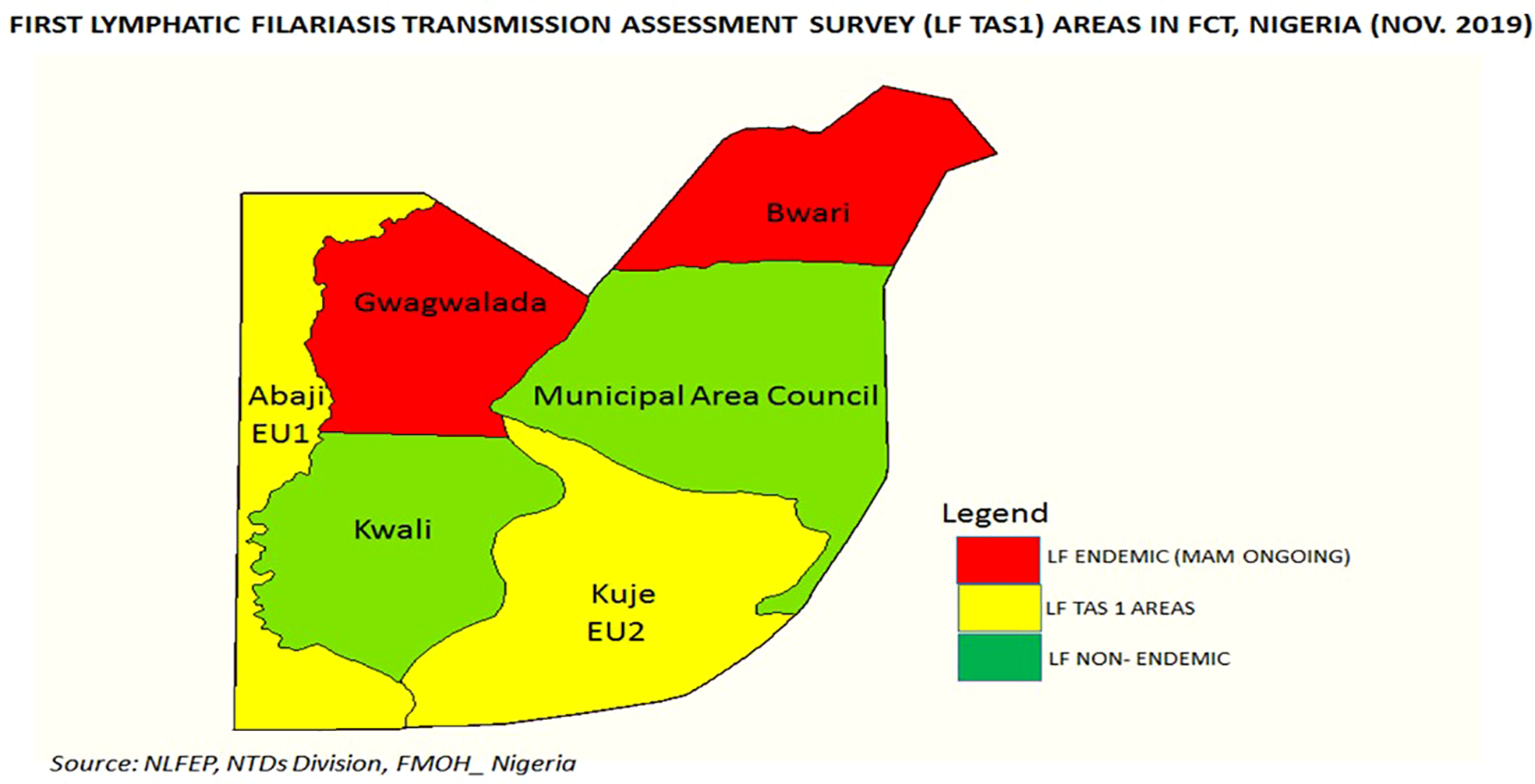
Area councils in the FCT showing the LF endemic areas and survey sites. Source: NLFEP, NTDs Division, Nigeria.
The diagnostics used for TAS1 is the FTS which is the recommended diagnostics by WHO (1) for TAS. Although there has been some concern regarding the sensitivity and specificity of the ICT (used for Pre-TAS) and FTS, the findings of a study (17) comparing FTS and ICT in American Samoa in a post-MDA setting revealed that ICT had lower sensitivity (93.8%) than but the same specificity (100%) as FTS.
4.1 Reflections on LF treatment in urban settings
However, the remaining two ACs in FCT are yet to achieve sufficient rounds of effective coverage to qualify for pre-TAS. Gwagwalada requires two more effective rounds while Bwari requires three. The tasks of achieving effective treatment rounds in these urban ACs are multifaceted and particularly challenging. The concept of community directed treatment with ivermectin (CDTI), where the communities choose and remunerate volunteer CDDs may not work in urban settings where there is limited or no community cohesion, as might be expected in villages. With a general improvement in the standard of living, the clinical signs of LF are less common, and people no longer understand the threat of the disease, as a result of which there less commitment to supporting CDDs. In addition, there are many competing and vertical community health intervention programs that remunerate volunteers and health workers such that volunteers and staff are likely to prioritize the NTD campaigns. MDAs have historically been more suited for rural communities and poses challenges implemented in urban areas with higher population density, greater mobility, and where community boundaries, and therefore target areas, are more difficult to define (18, 19). Re-evaluation of MDA is recommended given the challenge of achieving effective coverage of MDA in such settings.
Most of the focus of the LF elimination programme in FCT to date has been on MDA and there has been little progress in the implementation of the second pillar, ie, morbidity management and disability prevention. It is only in recent years that countries are putting more emphasis on this intervention and scaling up the services for people living with morbidity. A study in Malawi documented the quality of life improved significantly for community members six months after they received hydrocele surgery (20). Another study showed that the lifetime benefits of hydrocelectomy by far exceeded the costs of hydrocele surgeries (21). MMDP activities have generally lagged behind MDA and there is a need to improve the coverage of MMDP services and the number of areas implementing patient-oriented morbidity interventions.
As LF elimination reaches the final mile, MDA strategies must be fine-tuned to ensure that all challenges hindering the attainment of effective treatment coverages are addressed, particularly in urban settings. Secondly, resources must be mobilized through the joint efforts of partners, communities and government to significantly scale up LF MMDP as it is a criterion for validation of elimination of LF as a public health problem.
Acknowledgments
We appreciate the NTD unit of the Federal Ministry of Health for the collaborative work in FCT. Our heartfelt gratitude also goes to the FCT NTD team and Area council coordinators and community leaders within the survey area for their support throughout the survey.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by FCT ethical review committee. Written informed consent to participate in this study was provided by the participants’ legal guardian.
Author contributions
JA-E and JK developed the manuscript; ED contributed to the field work and reviewed the manuscript; GS, NB, CO, RI, BE, SO and BQ reviewed the manuscript. All authors contributed to the manuscript and its revision and approved the final version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
WHO . Strengthening the assessment of lymphatic filariasis transmission and documenting the achievement of elimination in lymphatic filariasis. Geneva, Switzerland: Proceedings of the Meeting of the Neglected Tropical Diseases Strategic and Technical Advisory Group’s Monitoringand Evaluation Subgroup on Disease Specific Indicators (2016).
2
Kelly-Hope LA Molyneux DH Bockarie MJ . Can malaria vector control accelerate the interruption of lymphatic filariasis transmission in africa; capturing a window of opportunity? Parasit Vectors (2013) 6:39. doi: 10.1186/1756-3305-6-39
3
Ottesen EA Duke BO Karam M Behbehani K . Strategies and tools for the control/ elimination of lymphatic filariasis. Bull World Health Organ (1997) 75:491–503.
4
WHO . Managing morbidity and preventive disability in the global programme to eliminate lymphatic filariasis: WHO position statement. Geneva (1995).
5
WHO . Global programme to eliminate lymphatic filariasis: Progress report 2014. Wkly Epidemiol Rec (2015) 90:489–504.
6
Task Force for Global Health . Recommendations of the international task force for disease eradication. MMWR (1993) 42(RR-16):1–38.
7
WHO . Progress report 2000-2009 and strategic plan 2010-2020 of the global programme to eliminate lymphatic filariasis: halfway towards eliminating lymphatic filariasis. Geneva: World Health Organization (2010).
8
Ottesen EA Duke BO Karam M Behbehani K . The global programme to eliminate lymphatic filariasis. Trop Med Int Health (2000) 5(9):591–4. doi: 10.1046/j.1365-3156.2000.00620.x
9
WHO . Togo: first country in sub-Saharan Africa to eliminate lymphatic filariasis (2017). Geneva: World Health Organization. Available at: http://www.who.int/neglected_diseases/news/Togo_saying_goodbye_lymphatic_filariasis/en/ (Accessed 15 March 2021).
10
WHO . Global programme to eliminate lymphatic filariasis: Progress report 2016. Wkly Epidemiol Rec (2017) 92:589–608.
11
WHO . Monitoring and epidemiological assessment of mass drug administration: a manual for national elimination programmes. (World Health Organization (WHO/HTM/NTD/PCT/20114) (2011).
12
The Coalition for Operational Research on Neglected Tropical Diseases (COR-NTD) . Transmission assessment survey sample builder (2009). Available at: https://www.cor-ntd.org/resources/transmission-assessment-survey-sample-builder (Accessed September 18, 2019).
13
Ramzy RM El Setouhy M Helmy H Ahmed ES Abd Elaziz KM Farid HA et al . Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet (2006) 67:992–9. doi: 10.1016/S0140-6736(06)68426-2
14
El-Setouhy M Abd Elaziz KM Helmy H Farid HA Kamal HA Ramzy RM et al . The effect of compliance on the impact of mass drug administration for elimination of lymphatic filariasis in Egypt. Am J Trop Med Hyg (2007) 77:1069–73. doi: 10.4269/ajtmh.2007.77.1069
15
Njenga SM Mwandawiro CS Wamae CN Mukoko DA Omar AA Shimada M et al . Sustained reduction in prevalence of lymphatic filariasis infection in spite of missed rounds of mass drug administration in an area under mosquito nets for malaria control. Parasit Vectors (2011) 4:90. doi: 10.1186/1756-3305-4-90
16
Boko-Collins PM Ogouyemi-Hounto A Adjinacou-Badou EA Gbaguidi-Saizonou L Dossa NI Dare A et al . Assessment of treatment impact on lymphatic filariasis in 13 districts of Benin: progress toward elimination in nine districts despite persistence of transmission in some areas. Parasites Vectors (2019) 12:276. doi: 10.1186/s13071-019-3525-5
17
Sheel M Lau CL Sheridan S Fuimaono S Graves PM . Comparison of immunochromatographic test (ICT) and filariasis test strip (FTS) for detecting lymphatic filariasis antigen in American Samoa. Trop Med Infect Dis (2016) 6(3):132. doi: 10.3390/tropicalmed6030132
18
Gonzales M Baker MC Celestino A Morillo DS Chambliss A Adams S et al . How lymphatic filariasis was eliminated from an urban poor setting in Santo Domingo, Dominican republic. Int Health.11 (2019) 2):108–18. doi: 10.1093/inthealth/ihy059
19
Koudou BG de Souza DK Biritwum NK Bougma R Aboulaye M Elhassan E et al . Elimination of lymphatic filariasis in west African urban areas: is implementation of mass drug administration necessary. Lancet Infect Di (2018) 18(6):e214–20. doi: 10.1016/S1473-3099(18)30069-0
20
Betts H Martindale S Chiphwanya J Mkwanda SZ Matipula D Ndhlovu P et al . Significant improvement in quality of life following surgery for hydrocoele caused by lymphatic filariasis in Malawi: a prospective cohort study. PloS Negl Trop Dis (2020) 14(5):e0008314. doi: 10.1371/journal.pntd.0008314
21
Sawers L Stillwaggon E Chiphwanya J Mkwanda SZ Betts H Martindale S et al . Economic benefits and costs of surgery for filarial hydrocele in Malawi. PloS Negl Trop Dis (2020) 14(3):e0008003. doi: 10.1371/journal.pntd.0008003
Summary
Keywords
lymphatic filariasis, transmission assessment survey, NTDs, LF elimination, Nigeria, lymphatic filariasis (LF), neglected tropical disease (NTD), transmission assessment
Citation
Amanyi-Enegela JA, Kumbur J, Burn N, Sankar G, Davies E, Ishaya R, Ogoshi C, Ekweremadu B, Omoi S and Qureshi B (2022) Assessment of the progress toward elimination of lymphatic filariasis in the Federal Capital Territory- Abuja, Nigeria. Front. Trop. Dis 3:1033802. doi: 10.3389/fitd.2022.1033802
Received
31 August 2022
Accepted
29 September 2022
Published
31 October 2022
Volume
3 - 2022
Edited by
Anuradha R, International Centers for Excellence in Research (ICER), India
Reviewed by
Alfred Kwesi Manyeh, University of Health and Allied Sciences, Ghana; Abdel Jelil Njouendou, University of Buea, Cameroon
Updates
Copyright
© 2022 Amanyi-Enegela, Kumbur, Burn, Sankar, Davies, Ishaya, Ogoshi, Ekweremadu, Omoi and Qureshi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph Kumbur, joseph.kumbur@cbm.org
This article was submitted to Neglected Tropical Diseases, a section of the journal Frontiers in Tropical Diseases
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.