- 1Department of Parasitology, Faculty of Science, Charles University, Prague, Czechia
- 2Department of Zoology, Faculty of Science, Charles University, Prague, Czechia
Sand fly saliva has considerable immunomodulatory effects on Leishmania infections in mammalian hosts. Studies on several Leishmania – sand fly - host combinations have demonstrated that co-inoculation with Leishmania parasites enhances pathogenicity, while pre-exposure of hosts to sand fly bites provides significant protection against infection. However, the third scenario, the effect of sand fly saliva on parasite development in hosts infected before exposure to sand flies, remains an understudied aspect of Leishmania–host–vector interaction. Here we studied the effect of exposure of L. major-infected BALB/c mice to repeated sand fly bites. Mice infected intradermally with sand fly-derived Leishmania were repeatedly bitten by Phlebotomus duboscqi females every two weeks. The lesion development was recorded weekly for ten weeks post-infection and parasite load and distribution in various organs were tested post mortem using qPCR. Repeated sand fly bites significantly enhanced the development of cutaneous lesions; they developed faster and reached larger size than in unexposed mice. Multiple sand fly bites also increased parasites load in inoculated ears. On the other hand, the distribution of parasites in mice body and their infectiousness to vectors did not differ significantly between groups. Our study provides the first evidence that multiple and repeated exposures of infected BALB/c mice to sand fly bites significantly enhance the progress of local skin infection caused by Leishmania major and increase tissue parasite load, but do not affect the visceralization of parasites. This finding appeals to adequate protection of infected humans from sand fly bites, not only to prevent transmission but also to prevent enlarged lesions.
Introduction
Leishmaniases are diseases caused by protozoan Leishmania parasites (Kinetoplastida: Trypanosomatidae) transmitted by insect vectors, phlebotomine sand flies (Diptera: Psychodidae). Leishmaniases are endemic in 98 countries with 350 million people worldwide living at risk and 20 – 40 000 dying annually (1). More than 20 Leishmania species are pathogenic to humans, and each Leishmania species is transmitted with a different spectrum of vector species (2). Among over 800 sand fly species, 98 are proven or suspected vectors (3). The species-specific complex interactions between the host, parasite and sand fly result in the broad spectrum of clinical manifestations typical for human leishmaniases. Cutaneous leishmaniasis is the most prevalent clinical form characterized by localized, diffuse, or disseminated skin lesions, mucocutaneous leishmaniasis with progressive destruction of oronasopharyngeal mucosa and cartilaginous structures is disfiguring and potentially life treating and visceral leishmaniasis is the most severe form, often fatal if left untreated, characterized by fever, loss of weight, splenomegaly, hepatomegaly and/or lymphadenopathy, and anaemia (4, 5).
Sand fly saliva contains a mixture of at least 20 diverse proteins with antihemostatic, anti-inflammatory, and immunomodulatory properties. This cocktail is obligatorily inoculated into the host skin during blood feeding of sand fly female and can help spread Leishmania parasites in naive, previously non-bitten, hosts. This phenomenon is called enhancing effect and can be caused also with some individual salivary proteins or even salivary peptides produced in recombinant form. On the other hand, preimmunization of mice with salivary glands lysates or their pre-exposure to uninfected sand fly bites provided significant protection against infection (reviewed by 6). However, only a single study has been concerned on the effect of saliva in hosts previously infected by Leishmania: on the hamster model, increased transmissibility of L. donovani to vectors after multiple exposures of the host to sand fly bites was reported (7).
In this study, we exposed BALB/c mice infected with sand fly-derived L. major to repeated bites of Phlebotomus duboscqi and provide evidence that vector saliva influences substantially the outcome of L. major infection.
Materials and Methods
Sand flies, Parasites, and Mice
Phlebotomus duboscqi colony (originating from Senegal) was maintained under standard conditions (26°C on 50% sucrose and 12 h light/12 h dark photoperiod) as described previously (8).
Leishmania major (MHOM/IL/81/Friedlin/VI; FVI) was cultured in M199 medium (Sigma) containing 10% heat-inactivated foetal calf serum (Gibson) supplemented with 1% BME vitamins (Basal Medium Eagle, Sigma), 2% sterile urine and 250 µg mL−1 amikacin (Amikin, Bristol-Myers Squibb).
BALB/c mice originated from AnLab. s.r.o. (Harlan Laboratories, USA, Nederland). Animals were maintained in T3 breeding containers (Velaz) equipped with bedding German Horse Span (Pferde) and breeding material (Woodwool), provided with a standard feed mixture ST-1 (Velaz) and water ad libitum, with a 12 h light/12 h dark photoperiod, temperature 22–25°C and humidity 40–60%.
Infection of BALB/c Mice With Sand Fly-Derived Leishmania
Rodents were infected with sand fly-derived Leishmania according to Sadlova et al. (9). Phlebotomus duboscqi females were infected with L. major promastigotes (106 parasites/ml) by feeding through chick-skin membranes and engorged females were maintained under standard conditions till day 8 post blood meal (PBM). Their midguts were checked microscopically for the presence of promastigotes and thoracic midguts with good density of parasites were pooled in sterile saline: 80 infected thoracic midguts were homogenized into 40 µl of sterile saline. Dissected salivary glands (SG) of naïve P. duboscqi were pooled in sterile saline (10 glands per 10 μl of saline) and stored at −20°C. Before mice inoculation, SG was disintegrated by 3 successive immersions into liquid nitrogen and 4 µl added to homogenized guts. Immediately, BALB/c mice anaesthetized with ketamine/xylazine (62.5 mg and 25 mg/kg, respectively) were injected with 5.5 µl of the suspension intradermally into the inner side of the ear pinnae using a syringe. The number of parasites was calculated using the Bürker apparatus and the proportions of metacyclic forms were identified on Giemsa stained smears based on morphological criteria described previously (10). In two repeats of experiments, L. major numbers in the inoculums were 8.5 x 104 per mouse, metacyclics comprised 72% of all forms. Mice were checked weekly for external signs of the diseases till week 10 post-infection (p.i.). At the end of the experiment (10 weeks p.i.), the blood and tissue samples were collected for qPCR analysis. In each of the two independent experiments, 12 mice were infected and 4 mice were used as non-infected controls (NONI group).
Mice Exposure to P. duboscqi Bites
Half of the mice in each experiment were exposed to sand flies on weeks 2, 4, 6, 8 and 10 p.i. (EXPOSED group) while remaining mice were exposed only at the end of experiments on week 10 p. i. (UNEXPOSED group). Anaesthetized mice were inserted into the cotton bag, inoculated ears were removed from the bag and 5 to 7-day-old P. duboscqi females were allowed to feed on both sides of ear pinnae. Engorged sand fly females were separated and maintained under standard conditions. Eight days PBM, females were dissected, their guts were examined under the light microscope and intensity and localization of infections were evaluated as described previously (10, 11).
Mice Sampling and Quantitative PCR
Mice were euthanized by cervical dislocation under anaesthesia 10 weeks p. i. Both ears (inoculated and contralateral), both ear-draining lymph nodes, all four paws, tail, spleen, liver and blood were stored in - 20°C. Extraction of total DNA from mice tissues was performed using a DNA tissue isolation kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer´s instructions. Quantitative (q) PCR for detection and quantification of Leishmania DNA was performed in Bio-Rad iCycler&iQ Real-Time PCR Systems using the SYBR Green detection method (iQ SYBR Green Supermix, Bio-Rad, Hercules, CA).
Statistical Analysis
Statistical analyses of weight gains, lesion development, infectiousness and percentage of infected organs were carried out using the R software (http://cran.r-project.org). The continuous response variable (weight, lesion size) and their relationship with categorical (EXPOSED, UNEXPOSED or NONI group) and continuous (week p.i.) explanatory variables were examined fitting multilevel linear regression models (package “nlme”), taking into account the correlation between repeated measures of the same animal over time. The model used included the interaction term between categorical and continuous explanatory variable. The infectiousness was analysed by proportional test. Percentage of infected organs and parasite load were analysed with GLM with a binomial distribution and negative binomial distribution, respectively. Differences in lesion size in respective weeks were tested by one-tailed T tests. The between-group differences in lesion size were tested with nonparametric Independent Samples Median Test (medians) and Independent Samples Mann-Whitney U Test (distributions) using SPSS software version 23. A P-value of < 0.05 was considered to indicate statistical significance.
Ethical Consideration
Animals were maintained and handled in the animal facility of Charles University in Prague following institutional guidelines and Czech legislation (Act No. 246/1992 and 359/2012 coll. on Protection of Animals against Cruelty in present statutes at large), which complies with all relevant European Union and international guidelines for experimental animals. All experiments were approved by the Committee on the Ethics of Laboratory Experiments of the Charles University in Prague and were performed under permit no. MSMT-10270/2015-5 of the Ministry of the Education, Youth and Sports of the Czech Republic. Investigators were certified for experimentation with animals by the Ministry of Agriculture of the Czech Republic.
Results
Infection Course and Cutaneous Lesions Development
Mice were infected with intradermal inoculation of 8.5 x 104 sand fly–derived parasites, with a prevalence (72%) of metacyclic forms. The course of infections was observed in 24 mice in two independent experiments. In each experiment, infected mice were divided into the EXPOSED group exposed in 2-week intervals to P. duboscqi bites and the UNEXPOSED group, left without exposure. In the first experiment, one mouse in the UNEXPOSED group died before the end of the experiment. In addition, 8 mice (4 in each experiment) were used as the non-infected control (NONI group).
Infected mice did not show significant weight loss, there were no statistical differences in weight gains between uninfected control mice (NONI group) and both infected groups (Supplementary Table S1). In all groups in both experiments, the bodyweight increased significantly with the age of mice (P < 0.0001).
Lesions developed in all infected mice with exception of 2 animals in the UNEXPOSED group in the first experiment. They appeared on week 5 p.i. in the first experiment and on week 2 p.i. in the second experiment. Importantly, lesions appeared one week earlier and they developed to a large size faster in the EXPOSED group compare to the UNEXPOSED group in both experiments (P = 0.048, Figures 1A, B and Supplementary Table S2).
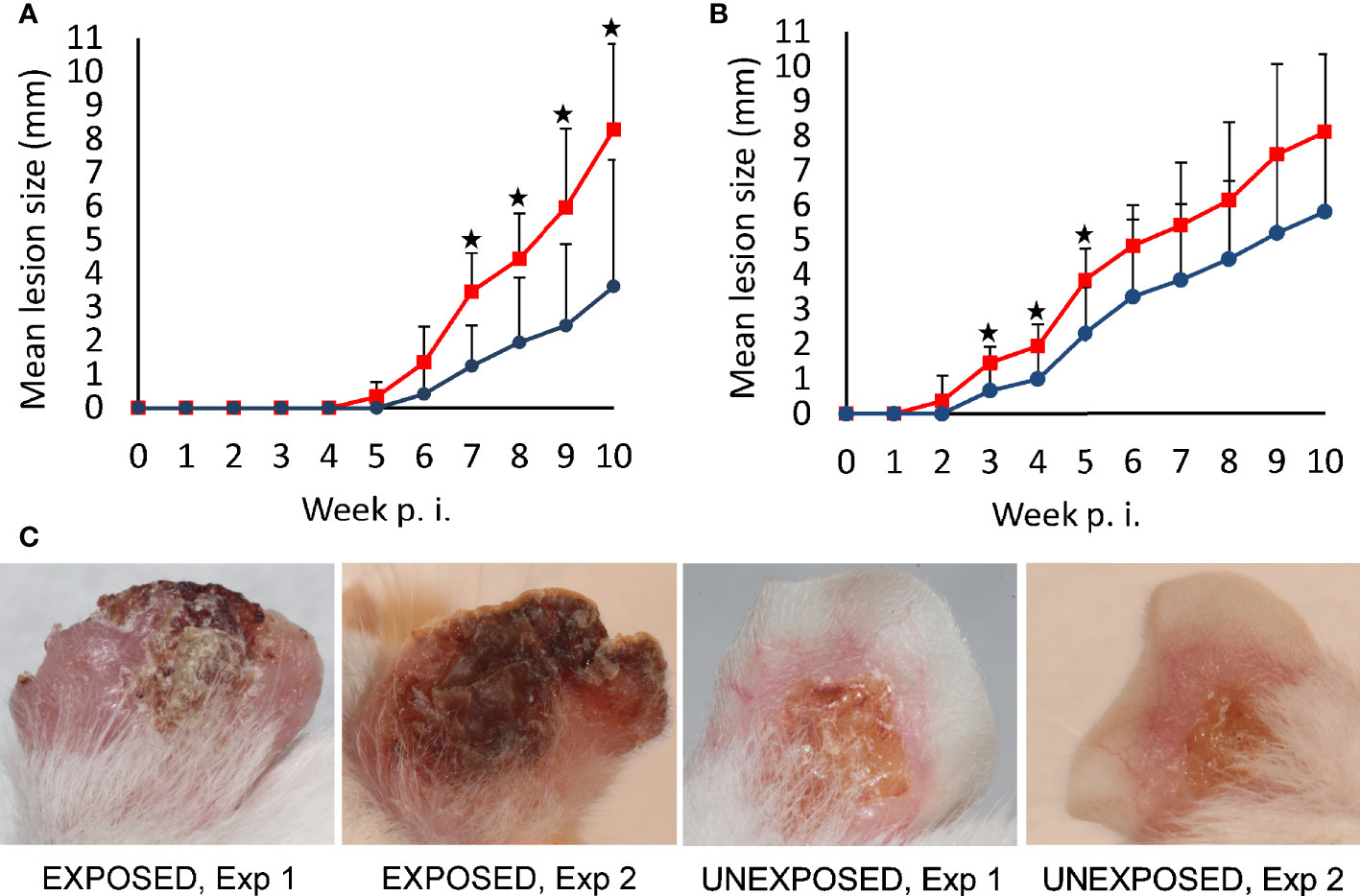
Figure 1 Lesion development in BALB/c mice infected with L. major. Mice infected with intradermal inoculation of sand fly–derived parasites were divided into the EXPOSED group (exposed in 2-week intervals to P. duboscqi bites) and the UNEXPOSED group (left without exposure). The course of infections was observed for 10 weeks in 24 mice in two independent experiments. Between-group differences were tested with multilevel linear regression model on entire data set (P = 0.048). Significant differences between experimental groups in respective weeks, revealed by T tests (one-tailed), are marked by asterisks (A) experiment 1; (B) experiment 2. Red squares, EXPOSED group, blue circles, UNEXPOSED group. (C) typical lesion appearance in BALB/c mice on week 10 p.i., Exp 1, Experiment 1; Exp 2, Experiment 2.
At the end of the experiments on week 10 p.i., lesions reached a significantly larger size in the EXPOSED group compared to the UNEXPOSED group (P = 0.012, Figure 2A). The mean values were 8.3 mm for the EXPOSED group and 4.3 mm for the UNEXPOSED group in the first experiment and 8.1 mm for the EXPOSED group and 5.8 mm for the UNEXPOSED group in the second experiment (Figures 3C, D).
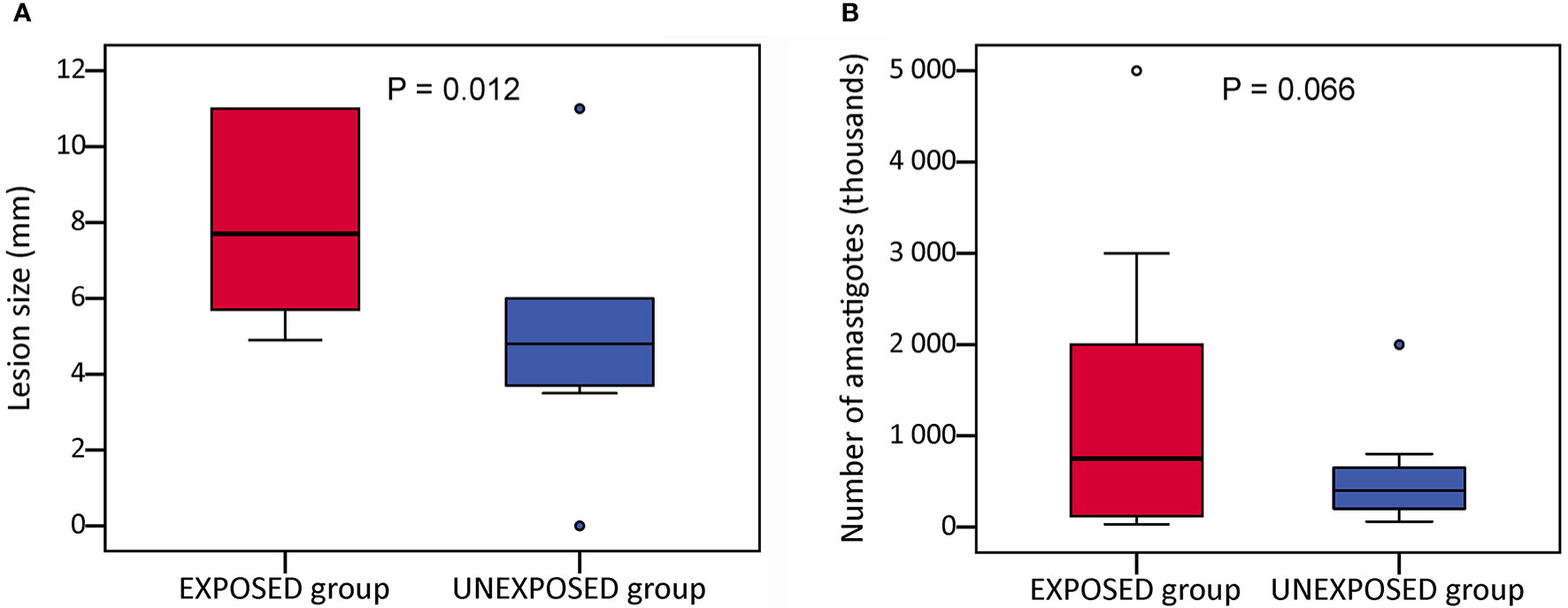
Figure 2 Infection parameters in BALB/c mice on week 10 p.i. Mice infected with intradermal inoculation of sand fly–derived parasites were divided into the EXPOSED group (exposed in 2-week intervals to P. duboscqi bites) and the UNEXPOSED group (left without exposure). The course of infections was observed for 10 weeks in 24 mice in two independent experiments. Parasite load was evaluated post mortem by qPCR. The data presented here is sum from both experiments. (A) the final lesion size; (B) parasite load in infected ears. In the boxplots, the box is bordered by upper and lower quartile (IQR, interquartile range), the horizontal line denotes the median value, whiskers denote 1.5 times IQR and circles denote outliers. Significance of between-group differences in lesion size were tested with nonparametric Independent Samples Median Test, parasite loads were analysed with GLM with a negative binomial distribution.
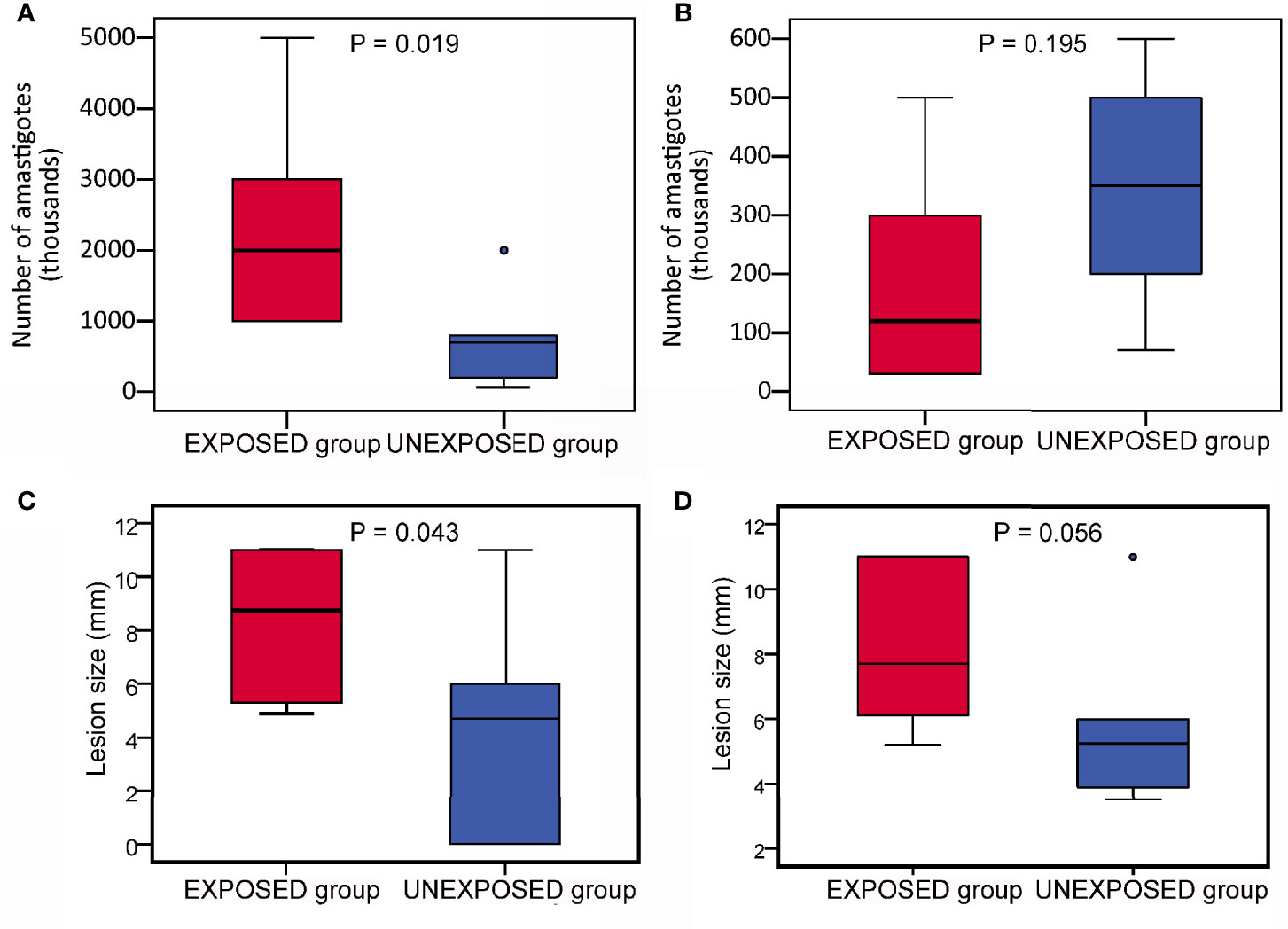
Figure 3 Infection parameters in BALB/c mice on week 10 p.i. in two independent experiments. The parasite load (A, B) and final lesion size (C, D) in infected ears. (A, C) experiment 1; (B, D), experiment 2. In the boxplots, the box is bordered by upper and lower quartile (IQR, interquartile range), the horizontal line denotes the median value, whiskers denote 1.5 times IQR and circles denote outliers. Significance of differences betweed mice exposed to sand flies (EXPOSED group) and control mice (UNEXPOSED group) were analysed with GLM with a negative binomial distribution and one tailed T test.
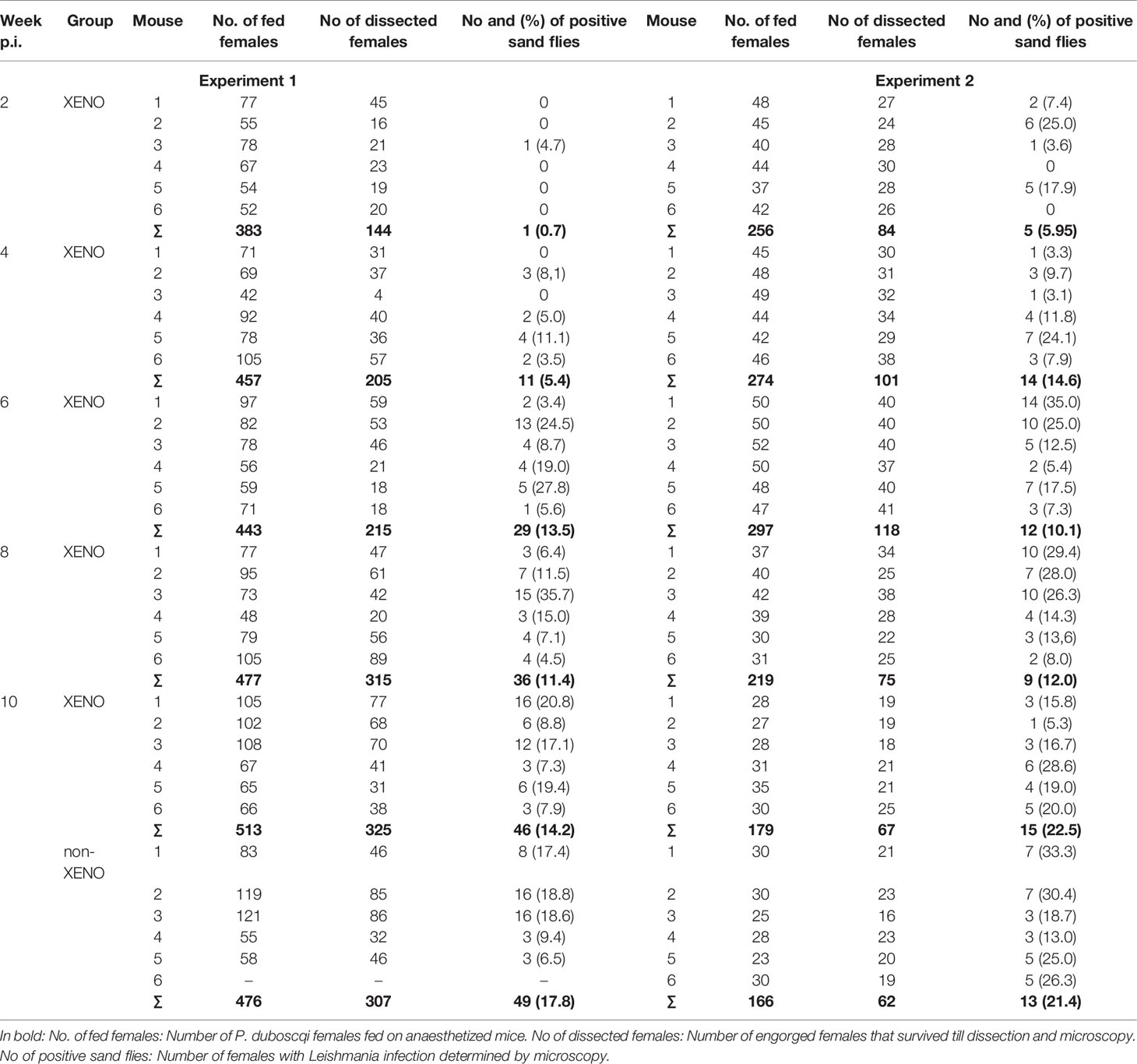
Infectiousness to Phlebotomus duboscqi
Phlebotomus duboscqi is a natural vector of L. major (3) and the colonized flies have been shown to support the development and transmission of the parasite (12). In mice exposed to P. duboscqi females in two-week intervals (EXPOSED group), the relative proportion of mice infectious to sand flies as well as the percentage of infected vectors increased with time till week 6 p.i. in the first experiment and week 4 p.i, in the second experiment. From these time points, all tested mice were infectious, including those two mice without lesions in the UNEXPOSED group (Table 1). On week 10 p.i., the proportion of infected sand flies did not differ significantly between the EXPOSED group and the unexposed UNEXPOSED group, P = 0.259.
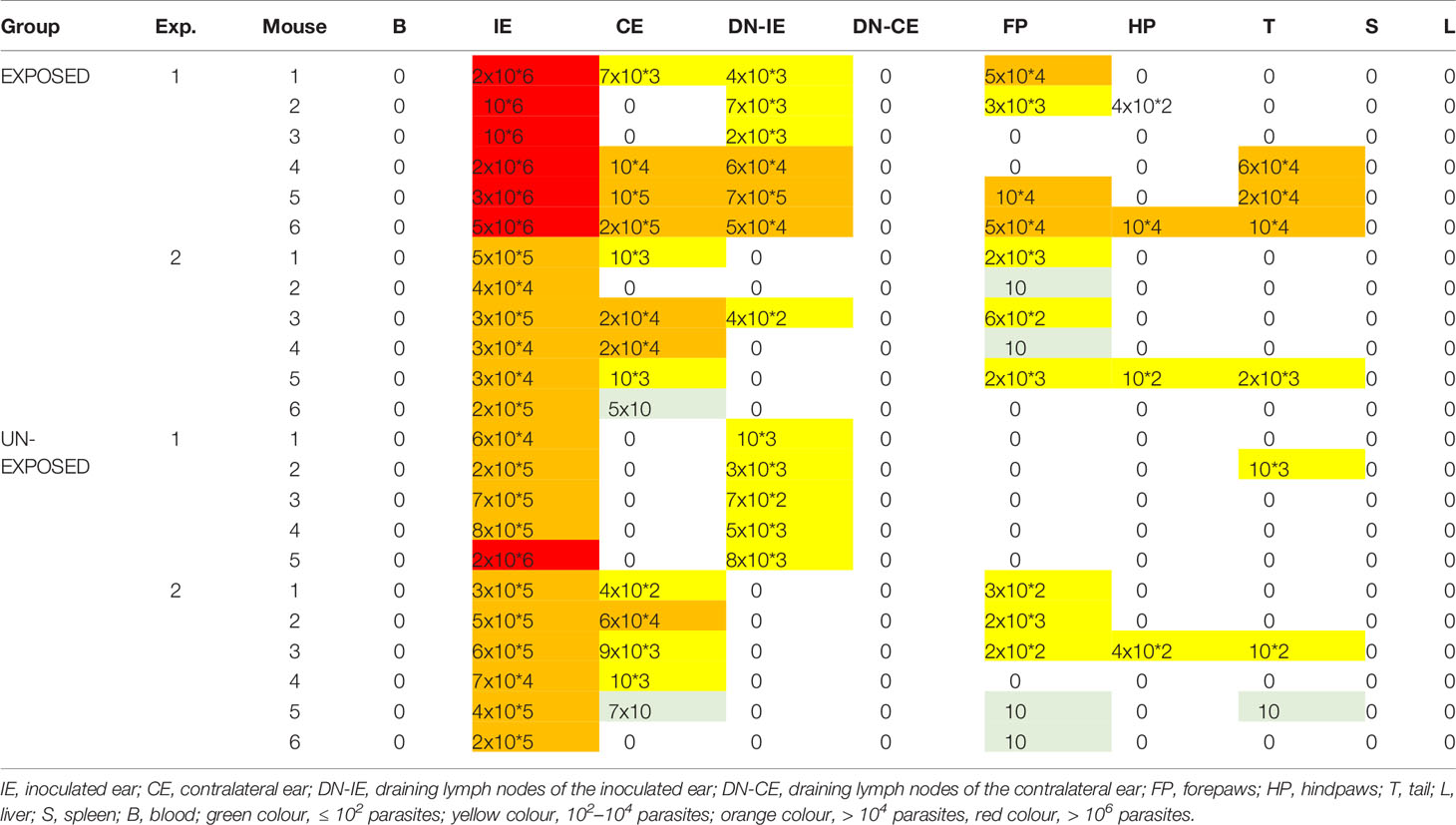
Parasite Load and Anatomical Distribution in BALB/c Mice
Parasites were distributed in the same spectrum of tissues in both groups: inoculated ears were positive in all animals including two mice without apparent lesions, parasites were often found also in contralateral ears and draining lymph nodes of inoculated ears. In some animals of both groups, also paws and tail were infected while blood, draining lymph nodes of contralateral ears, liver and spleen were negative (Table 2). The proportion of infected organs did not differ significantly between the EXPOSED and the UNEXPOSED group (P = 0.130).
Parasite load detected post mortem by qPCR is also summarized in Table 2. The numbers of Leishmania were higher in mice exposed to sand flies according to the higher representation of heavy and moderate parasite load (red and orange coloured) and lower representation of weak infections (green coloured) in respective tissues. The numbers of Leishmania in inoculated ears in the EXPOSED group (mean value 1.3x10*6, median 7.5x10*5) were about twice higher than numbers in the UNEXPOSED group (mean value 5.3x10*5, median 4.0x10*5), and the difference is on the border of statistical significance (P = 0.066, Figure 2B). The two experiments differed in this respect (Figures 3A, B): the EXPOSED group showed significantly higher parasite load than the UNEXPOSED group in the first experiment (P = 0.019) while the differences were not significant in the second experiment (P = 0.195). These results are not surprising considering earlier lesions development in the second experiment: the ear pinnae were more necrotic and a smaller part of the ears remained available for the parasite growth on week 10 p.i. (Figure 1C).
Discussion
This study addresses the substantial question if repeated sand fly bites influence the clinical manifestation of cutaneous leishmaniasis caused by L. major. We used the model of BALB/c mice to compare the disease progress in mice exposed to P. duboscqi females in 2 weeks intervals (EXPOSED group) with mice left without sand fly bites (UNEXPOSED group) and provide the first evidence that repeated sand fly bites significantly enhance the progress of cutaneous lesions and increase tissue parasite load.
The development of Leishmania infection in the host body is a complex process depending on many parasites- and host-associated factors. Different Leishmania species cause different disease forms in the same model animal (reviewed by 13) and even various strains of Leishmania major differ in virulence for the host, as has been demonstrated also for L. major (14, 15). Simultaneously, the genetic background and the immune response of the mammalian host play important role in disease progress (16). BALB/c mouse is the model animal highly susceptible to L. major infection, with the immunity polarized to strong Th2, driven largely by IL-4, that is responsible for suppressing Th1-cell development and inhibiting the secretion of IFN-γ that is required to activate infected macrophages for parasite killing (17–19).
The clinical manifestation of the disease in experimental infections is substantially influenced by methodological parameters like parasite dose and type (procyclic/metacyclic promastigotes or amastigotes) and inoculation route and site (reviewed by 20). In this study, we used the intradermal route of inoculation, which is closest to the natural mode of transmission since parasites are exposed to the localized immune responses in the skin (21). Parasites used for inoculation were predominantly metacyclic infective forms pooled from thoracic midguts of laboratory infected P. duboscqi. The parasite load (8.5 x 104 parasites/mouse) did not exceed the maximal number of L. major parasites transmitted by P. duboscqi females (22). The knowledge and control of parasite load is a big advantage of the used method compare to natural infections by sand fly bite - in the second case, the numbers of transmitted parasites differ in several orders between individual vector females (22–24). To mimic the natural transmission, the homogenate of salivary glands of P. duboscqi females were added to the inoculum. This standardized procedure resulted in the symptomatic course of the disease in BALB/c mice.
It should be noted that in this study, infected mice were exposed to sand flies in two-week intervals while in the real world, sand fly biting is continuous. However, mimicking a real scenario in the laboratory is not possible as the animals cannot be subjected to anaesthesia so often. It is also quite difficult to assess the exact sand fly biting rate on rodent reservoirs of L. major in nature. Turner and Hoogstraal (25) reported the numbers corresponding to 51, 45, 41 and 89 fed Phlebotomus females per two weeks on Tatera, Acomys, Mastomys and Arvicanthis in Sudan. While experimental exposure of rodents used as bait in traps may not exactly reflect the natural situation, influencing their movement, temporal activity pattern, burrowing, aggregation, etc., the natural biting rate may be accurately measured on humans. According to the study on the biting behaviour of P. argentipes in Sri Lanka, the biting rate on humans was 8.4 (range 2-25 bites) per night (26) which would represent 117 (17-210) in two weeks. Although these numbers vary with sand fly species, host, locality, season and many other factors, it is at least a rough estimate of the natural biting rate and in our experiments, bite numbers lied within this range.
It is a well-known phenomenon that salivary molecules are an obligatory part of feeding sand flies on mammalian hosts and they have an immunomodulatory effect on Leishmania infection (27). Like other blood-feeding arthropods, sand flies need to secrete saliva into the wound to facilitate blood-feeding. Sand fly saliva is composed of multiple proteins and peptides with diverse functions that evolved mainly to counteract the host’s hemostatic system. However, salivary proteins are also capable of inducing a strong anti-inflammatory immune response in naïve hosts, which is beneficial for Leishmania parasites (reviewed by 6, 28). Therefore, it is not surprising, that co-injection of Leishmania parasites with salivary gland lysates (SGL) or the recombinant salivary peptides has been shown to enhance Leishmania pathogenicity for mice on many combinations of Leishmania and sand fly species (reviewed by 6). However, to our knowledge, only a single study has been concerned on the additional effect of saliva in hosts previously infected by Leishmania; Valverde et al. (7) described increased transmissibility of L. donovani to vector after multiple exposures of hamsters to sand fly bites.
The current study demonstrated that repeated exposure of the infected host to sand fly bites has an onward “enhancing effect” on the lesion growth to the co-injection of Leishmania with salivary gland lysates. Lesions developed faster and reached a higher size in mice exposed to repeated sand fly bites. Apart from the enhanced lesion development, also tissues parasite load was higher in mice exposed to repeated bites. This differs from the effect of sand fly exposure on L. donovani load in the hamster model; here, repeated sand fly bites enhanced significantly parasite numbers in the peripheral blood but not in the skin (7). This difference may reflect the species-specific organ tropism of different Leishmania - the visceral species L. donovani circulates in the blood, while cutaneous L. major remains localized predominantly in the skin and local lymph draining nodes (29).
In our experiments, the anatomical distribution of parasites was not affected by repeated bites of uninfected sand flies. In both experimental groups, parasites migrated from the site of inoculation (left ear) to its draining lymph nodes and skin on other body parts (contralateral ears, paws and tail) but were never detected in blood or viscera. This corresponds to the previously published pattern of development of L. major in BALB/c mice (30) while visceralisation occurs in BALB/c mice in case of unnatural intravenous or subcutaneous initiation of L. major infection or overdose of the intradermal infection (reviewed by 31). For unknown reasons, migration to ear-draining lymph nodes was reduced in the second experiment in both experimental groups.
Similarly, infectiousness to sand flies did not differ significantly between exposed and unexposed mice in this study. On week 10 p.i., all 24 BALB/c mice infected with L. major were infectious to P. duboscqi females and the mean percentage of infected sand flies varied from 14.2 to 22.5% in respective groups. Similar infectiousness was described for symptomatic dogs infected with L. infantum exposed to Lu. longipalpis (13 - 28%) (32–34), for BALB/c mice infected with L. donovani exposed to P. orientalis (19%) (9) or hamsters infected with L. donovani exposed to L. longipalpis (24%) (7). In the latter study, the infectiousness of hamsters increased significantly post exposures to sand flies while this effect was not observed in our experiments. Here, the differences in infectiousness between exposed and unexposed mice were evaluated at the end of experiments, two weeks post previous exposure of the EXPOSED group while Valverde et al. (7) tested the infectiousness of hamsters just 24h post previous exposure. Therefore, we cannot exclude that if there was a similar short-term effect of saline on infectiousness in order of hours or days, it was not detected due to the experimental design. Similarly, it was not possible to directly compare infectiousness in early weeks p.i. in this study.
Sand fly feeding provides a suitable environment for the survival and multiplication of L. major parasites. Sand fly saliva has chemotactic activities for both neutrophils and macrophages which accelerate the entry of parasites into these cells in the early days (35–39). Besides this effect, sand fly saliva modulates the host immune system in many ways, advantageous for the parasite: inhibits macrophage activation to the production of NO (40), modulates the activity of T cells and macrophages by decreasing the secretion of pro-inflammatory cytokines and enhancing the production of anti-inflammatory cytokines (41), induces apoptosis of neutrophils (42) and inhibits the ability of dendritic cells to present antigens (43). All these effects favor the establishment of Leishmania infection and enhance parasite survival in naive, previously non-bitten hosts. Nevertheless, here we showed a type of onward “enhancing effect” when the disease outcome was significantly exacerbated after further bites of uninfected sand fly females. In our opinion, this effect could be caused by enzyme hyaluronidase present in P. duboscqi saliva (44). Hyaluronidase cleaves hyaluronic acid, a major component of vertebrate extracellular matrix, facilitating the spread of other pharmacologically active compounds of saliva. Hyaluronidase activity was shown to exacerbate skin lesions caused by L. major in concentration-dependent manner but did not affect visceralization (45) and therefore we think that repeated exposure to uninfected bites multiplies the effect of hyaluronidase activity. In addition, several other vector –derived components like proteophosphoglycans, exosomes or sand fly microbiota, co-inoculated with saliva and Leishmania into the host skin, have been shown to modulate host immune response, promote infection and enhance disease pathology (46–48). Presumably, these components inoculated by uninfected sand flies into the skin with Leishmania infection may also contribute significantly to the “enhancing effect”.
In conclusion: our study provides first evidence that sand fly saliva inoculated repeatedly to infected hosts substantially influences the outcome of L. major infection. This finding appeals to adequate protection of infected humans from sand fly bites not only to prevent transmission but also to prevent enlarged lesions
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Committee on the Ethics of Laboratory Experiments of the Charles University in Prague.
Author Contributions
Conceptualization, methodology and supervision: JS. Investigation: BV, TL, and JV. Statistical analysis: DF and TS. Writing-original draft preparation: BV and JS. Writing-review and editing: PV. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Czech Ministry of Education ERD Funds, project CePaViP (CZ.02.1.01/16_019/0000759).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Helena Kulikova, Lenka Krejcirikova and Kristyna Srstkova for their administrative and technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2021.745104/full#supplementary-material
References
1. Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis Worldwide and Global Estimates of its Incidence. PLoS One (2012) 7:e35671. doi: 10.1371/journal.pone.0035671
2. Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, et al. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl Trop Dis (2016) 10:1–40. doi: 10.1371/journal.pntd.0004349
3. Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine Sandflies and the Spreading of Leishmaniases and Other Diseases of Public Health Concern. Med Vet Entomol (2013) 27:123–47. doi: 10.1111/j.1365-2915.2012.01034.x
4. David VD, Craft N. Cutaneous and Mucocutaneous Leishmaniasis. Dermatol Ther (2009) 22:491–502. doi: 10.1111/j.1529-8019.2009.01272.x
5. Ready PD. Epidemiology of Visceral Leishmaniasis. Clin Epidemiol (2014) 6:147–54. doi: 10.2147/CLEP.S44267
6. Lestinova T, Rohousova I, Sima M, de Oliveira CI, Volf P. Insights Into the Sand Fly Saliva: Blood-Feeding and Immune Interactions Between Sand Flies, Hosts, and Leishmania. PLoS Negl Trop Dis (2017) 11:1–26. doi: 10.1371/journal.pntd.0005600
7. Valverde JG, Paun A, Inbar E, Romano A, Lewis M, Ghosh K, et al. Increased Transmissibility of Leishmania Donovani From the Mammalian Host to Vector Sand Flies After Multiple Exposures to Sand Fly Bites. J Infect Dis (2017) 215:1285–93. doi: 10.1093/infdis/jix115
8. Volf P, Volfova V. Establishment and Maintenance of Sand Fly Colonies. J Vectors Ecol (2011) 36:1–9. doi: 10.1111/j.1948-7134.2011.00106.x
9. Sadlova J, Seblova V, Votypka J, Warburg A, Volf P. Xenodiagnosis of Leishmania Donovani in BALB/c Mice Using Phlebotomus Orientalis: A New Laboratory Model. Parasit Vectors (2015) 8:1–8. doi: 10.1186/s13071-015-0765-x
10. Sadlova J, Price HP, Smith BA, Votypka J, Volf P, Smith DF. The Stage-Regulated HASPB and SHERP Proteins Are Essential for Differentiation of the Protozoan Parasite Leishmania Major in Its Sand Fly Vector, Phlebotomus Papatasi. Cell Microbiol (2010) 12:1765–79. doi: 10.1111/j.1462-5822.2010.01507.x
11. Myskova J, Votypka J, Volf P. Leishmania in Sand Flies: Comparison of Quantitative Polymerase Chain Reaction With Other Techniques to Determine the Intensity of Infection. J Med Entomol (2008) 45:133–8. doi: 10.1093/jmedent/45.1.133
12. Ashwin H, Sadlova J, Vojtkova B, Becvar T, Lypaczewski P, Schwartz E, et al. Characterization of a New Leishmania Major Strain for Use in a Controlled Human Infection Model. Nat Commun (2012) 12:1–12. doi: 10.1038/s41467-020-20569-3
13. Loría-Cervera NE, Andrade-Narváez JF. Animal Models for the Study of Leishmaniasis Immunology. Rev Inst Med Trop Sao Paulo (2014) 56:1–11. doi: 10.1590/S0036-46652014000100001
14. Sadlova J, Svobodova M, Volf P. Leishmania Major: Effect of Repeated Passages Through Sandfly Vectors or Murine Hosts. Ann Trop Med Parasitol (1999) 93(6):599–611. doi: 10.1080/00034989958104
15. Elfari M, Schnur LF, Strelkova MV, Eisenberger CL, Jacobson RL, Greenblatt CL, et al. Genetic and Biological Diversity Among Populations of Leishmania Major From Central Asia, the Middle East and Africa. Microbes Infect (2005) 7:93–103. doi: 10.1016/j.micinf.2004.09.010
16. Lipoldová M, Demant P. Genetic Susceptibility to Infectious Disease: Lessons From Mouse Models of Leishmaniasis. Nat Rev Genet (2006) 7:294–305. doi: 10.1038/nrg1832
17. Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, et al. Development of a Natural Model of Cutaneous Leishmaniasis: Powerful Effects of Vector Saliva and Saliva Preexposure on the Long-Term Outcome of Leishmania Major Infection in the Mouse Ear Dermis. J Exp Med (1998) 188:1941–53. doi: 10.1084/jem.188.10.1941
18. Sacks D, Noben-Trauth N. The Immunology of Susceptibility and Resistance to Leishmania Major in Mice. Nat Rev Immunol (2002) 2:845–58. doi: 10.1038/nri933
19. Scott P, Novais FO. Cutaneous Leishmaniasis: Immune Responses in Protection and Pathogenesis. Nat Rev Immunol (2016) 16:581–92. doi: 10.1038/nri.2016.72
20. Loeuillet C, Bañuls A, Hide M. Study of Leishmania Pathogenesis in Mice: Experimental Considerations. Parasit Vectors (2016) 9:144. doi: 10.1186/s13071-016-1413-9
21. Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A Natural Model of Leishmania Major Infection Reveals a Prolonged “Silent” Phase of Parasite Amplification in the Skin Before the Onset of Lesion Formation and Immunity. J Immunol (2000) 165:969–77. doi: 10.4049/jimmunol.165.2.969
22. Kimblin N, Peters N, Debrabant A, Secundino N, Egen J, Lawyer P, et al. Quantification of the Infectious Dose of Leishmania Major Transmitted to the Skin by Single Sand Flies. Proc Natl Acad Sci (2008) 105:10125–30. doi: 10.1073/pnas.0802331105
23. Maia C, Seblova V, Sadlova J, Votypka J, Volf P. Experimental Transmission of Leishmania Infantum by Two Major Vectors: A Comparison Between a Viscerotropic and a Dermotropic Strain. PLoS Negl Trop Dis (2011) 5(6):e1181. doi: 10.1371/journal.pntd.0001181
24. Secundino NFC, De Freitas VC, Monteiro CC, Pires ACAM, David BA, Pimenta PFP. The Transmission of Leishmania Infantum Chagasi by the Bite of the Lutzomyia Longipalpis to Two Different Vertebrates. Parasit Vectors (2012) 5:20. doi: 10.1186/1756-3305-5-20
25. Turner ER, Hoogstraal H. Leishmaniasis in the Sudan Repubic. 23. Sandflies (Phlebotomus) Attracted to Rodent-Baited Traps (Diptera: Psychodidae). J Med Entomol (1965) 2:137–9. doi: 10.1093/jmedent/2.2.137
26. Lane RP, Pile MM, Amerasinghe FP. Anthropophagy and Aggregation Behaviour of the Sandfly Phlebotomus Argentipes in Sri Lanka. Med Vet Entomol (1990) 4:79–88. doi: 10.1111/j.1365-2915.1990.tb00263.x
27. Kamhawi S. The Biological and Immunomodulatory Properties of Sand Fly Saliva and its Role in the Establishment of Leishmania Infections. Microbes Infect (2000) 2:1765–73. doi: 10.1016/S1286-4579(00)01331-9
28. Abdeladhim M, Kamhawi S, Valenzuela JG. What’s Behind a Sand Fly Bite? The Profound Effect of Sand Fly Saliva on Host Hemostasis, Inflammation and Immunity. Infect Genet Evol (2014) 28:691–703. doi: 10.1016/j.meegid.2014.07.028
29. Zhang WW, Mendez S, Ghosh A, Myler P, Ivens A, Clos J, et al. Comparison of the A2 Gene Locus in Leishmania Donovani and Leishmania Major and its Control Over Cutaneous Infection. J Biol Chem (2003) 278:35508–15. doi: 10.1074/jbc.M305030200
30. Vojtkova B, Spitzova T, Votypka J, Lestinova T, Kominkova I, Hajkova M, et al. Central Asian Rodents as Model Animals for Leishmania Major and Leishmania Donovani Research. Microorganisms (2020) 8:1–20. doi: 10.3390/microorganisms8091440
31. McCall LI, Zhang WW, Matlashewski G. Determinants for the Development of Visceral Leishmaniasis Disease. PLoS Pathog (2013) 9(1):e1003053. doi: 10.1371/journal.ppat.1003053
32. Michalsky ÉM, Rocha MF, da Rocha Lima ACVM, França-Silva JC, Pires MQ, Oliveira FS, et al. Infectivity of Seropositive Dogs, Showing Different Clinical Forms of Leishmaniasis, to Lutzomyia Longipalpis Phlebotomine Sand Flies. Vet Parasitol (2007) 147:67–76. doi: 10.1016/j.vetpar.2007.03.004
33. Courtenay O, Quinnell RJJ, Garcez LMM, Shaw JJJ, Dye C. Infectiousness in a Cohort of Brazilian Dogs: Why Culling Fails to Control Visceral Leishmaniasis in Areas of High Transmission. J Infect Dis (2002) 186:1314–20. doi: 10.1086/344312
34. Travi B, Ferro C, Cadena H, Montoya-Lerma J, Adler GH. Canine Visceral Leishmaniasis: Dog Infectivity to Sand Flies From non-Endemic Areas. Res Vet Sci (2002) 72:83–6. doi: 10.1053/rvsc.2001.0527
35. Anjili CO, Mbati PA, Mwangi RW, Githure JI, Olobo JO, Robert LL, et al. The Chemotactic Effect of Phlebotomus Duboscqi (Diptera: Psychodidae) Salivary Gland Lysates to Murine Monocytes. Acta Trop (1995) 60:97–100. doi: 10.1016/0001-706X(95)00112-R
36. de Moura TR, Oliveira F, Rodrigues GC, Carneiro MW, Fukutani KF, Novais FO, et al. Immunity to Lutzomyia Intermedia Saliva Modulates the Inflammatory Environment Induced by Leishmania Braziliensis. PLoS Negl Trop Dis (2010) 4(6):e712. doi: 10.1371/journal.pntd.0000712
37. Prates DB, Barral-Netto M, Barral A, Borges M. New Insights on the Inflammatory Role of Lutzomyia Longipalpis Saliva in Leishmaniasis. J Parasitol Res (2012) 2012. doi: 10.1155/2012/643029
38. Teixeira CR, Teixeira MJ, Gomes RBB, Santos CS, Andrade BB, Raffaele-Netto I, et al. Saliva From Lutzomyia Longipalpis Induces CC Chemokine Ligand 2/Monocyte Chemoattractant Protein-1 Expression and Macrophage Recruitment. J Immunol (2005) 175:8346–53. doi: 10.4049/jimmunol.175.12.8346
39. Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, et al. In Vivo Imaging Reveals an Essential Role for Neutrophils in Leishmaniasis Transmitted by Sand Flies. Science (2008) 321:970–4. doi: 10.1126/science.1159194
40. Hall LR, Titus RG. Vector Saliva Selectively Modulates Macrophage Functions That Inhibit Killing of Leishmania Major and Nitric Oxide Production. J Immunol (1995) 155:3501–6.
41. Mbow ML, Bleyenberg JA, Hall LR, Titus RG. Phlebotomus Papatasi Sand Fly Salivary Gland Lysate Down-Regulates a Th1, But Up-Regulates a Th2, Response in Mice Infected With Leishmania Major. J Immunol (1998) 161:5571–7.
42. Prates DB, Afonso L, Clare J, Miranda C. Lutzomyia Longipalpis Saliva Drives Apoptosis and Enhances Parasite Burden in Neutrophils. J Leukoc Biol (2011) 90:575–82. doi: 10.1189/jlb.0211105
43. Carregaro V, Valenzuela JG, Cunha TM, Waldiceu AV Jr, Grespan R, Matsumura G, et al. Phlebotomine Salivas Inhibit Immune Inflammation-Induced Neutrophil Migration via an Autocrine DC-Derived PGE2/IL-10 Sequential Pathway. J Leukoc Biol (2008) 84:104–14. doi: 10.1189/jlb.1107797
44. Černá P, Mikeš L, Volf P. Salivary Gland Hyaluronidase in Various Species of Phlebotomine Sand Flies (Diptera: Psychodidae ). Insect Biochem Mol Biol (2002) 32:1691–7. doi: 10.1016/S0965-1748(02)00109-1
45. Volfova V, Hostomska J, Cerny M, Votypka J, Volf P. Hyaluronidase of Bloodsucking Insects and its Enhancing Effect on Leishmania Infection in Mice. PLoS Negl Trop Dis (2008) 2(9):e294. doi: 10.1371/journal.pntd.0000294
46. Atayde VD, Aslan H, Townsend S, Hassani K, Kamhawi S, Olivier M. Exosome Secretion by the Parasitic Protozoan Leishmania Within the Sand Fly Midgut. Cell Rep (2015) 13:957–67. doi: 10.1016/j.celrep.2015.09.058
47. Rogers ME. The Role of Leishmania Proteophosphoglycans in Sand Fly Transmission and Infection of the Mammalian Host. Front Microbiol (2012) 3:223. doi: 10.3389/fmicb.2012.00223
Keywords: Leishmania major, Phlebotomus, sand flies, vector saliva, cutaneous lesions, BALB/c mice
Citation: Vojtkova B, Frynta D, Spitzova T, Lestinova T, Votypka J, Volf P and Sadlova J (2021) Repeated Sand Fly Bites of Infected BALB/c Mice Enhance the Development of Leishmania Lesions. Front. Trop. Dis 2:745104. doi: 10.3389/fitd.2021.745104
Received: 21 July 2021; Accepted: 07 October 2021;
Published: 21 October 2021.
Edited by:
Yara M. Traub-Csekö, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
David Sacks, National Institutes of Health (NIH), United StatesClaudia Rios-Velásquez, Instituto Leônidas & Maria Deane (ILMD/Fiocruz Amazônia), Brazil
Patricia Sampaio Tavares Veras, Gonçalo Moniz Institute (IGM), Brazil
Copyright © 2021 Vojtkova, Frynta, Spitzova, Lestinova, Votypka, Volf and Sadlova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jovana Sadlova, c2FkbG92YWpAbmF0dXIuY3VuaS5jeg==
 Barbora Vojtkova
Barbora Vojtkova Daniel Frynta
Daniel Frynta Tatiana Spitzova
Tatiana Spitzova Tereza Lestinova1
Tereza Lestinova1 Petr Volf
Petr Volf Jovana Sadlova
Jovana Sadlova