- Department of Parasitology, Leiden University Medical Center, Leiden, Netherlands
Schistosomiasis is a parasitic disease caused by trematode blood flukes of the genus Schistosoma, affecting over 250 million people mainly in the tropics. Clinically, the disease can present itself with acute symptoms, a stage which is relatively more common in naive travellers originating from non-endemic regions. It can also develop into chronic disease, with the outcome depending on the Schistosoma species involved, the duration and intensity of infection and several host-related factors. A range of diagnostic tests is available to determine Schistosoma infection, including microscopy, antibody detection, antigen detection using the Point-Of-Care Circulating Cathodic Antigen (POC-CCA) test and the Up-Converting Particle Lateral Flow Circulating Anodic Antigen (UCP-LF CAA) test, as well as Nucleic Acid Amplification Tests (NAATs) such as real-time PCR. In this mini review, we discuss these different diagnostic procedures and explore their most appropriate use in context-specific settings. With regard to endemic settings, diagnostic approaches are described based on their suitability for individual diagnosis, monitoring control programs, determining elimination as a public health problem and eventual interruption of transmission. For non-endemic settings, we summarize the most suitable diagnostic approaches for imported cases, either acute or chronic. Additionally, diagnostic options for disease-specific clinical presentations such as genital schistosomiasis and neuro-schistosomiasis are included. Finally, the specific role of diagnostic tests within research settings is described, including a controlled human schistosomiasis infection model and several clinical studies. In conclusion, context-specific settings have different requirements for a diagnostic test, stressing the importance of a well-considered decision of the most suitable diagnostic procedure.
Introduction
Schistosomiasis is a neglected tropical disease (NTD) caused by parasitic blood flukes of the Schistosoma genus, affecting over 250 million people of which the majority reside in sub-Saharan Africa (1, 2). The main species infecting humans are S.mansoni, S.japonicum (both causing intestinal schistosomiasis) and S.haematobium (causing urogenital schistosomiasis) (1, 2). Furthermore, hybrid infections, resulting from interactions between human and animal schistosome species and potentially enhanced by zoonotic transmission, are increasingly being reported and may present a considerable risk of human pathology (3). Schistosomiasis can lead to significant morbidity and even mortality if not treated. The most effective and widely used drug is praziquantel (PZQ), which is safe and efficacious against the adult worm stages of all Schistosoma spp (4). It is commonly used for individual treatment as well as for mass drug administration (MDA), also known as preventive chemotherapy, to at-risk populations for control of schistosomiasis in endemic areas.
To successfully reduce the burden of disease and to eventually move towards elimination of schistosomiasis, the use of sensitive and specific diagnostic tests, to correctly identify those who are infected, is crucial. However, the diagnostic need is strongly related to its context-specific setting, each requiring its own distinctive approach. In this mini review, we will provide a summary of the current diagnostic procedures for human schistosomiasis (part 1) and discuss their appropriate use in context-specific settings (part 2).
1. Diagnostic Procedures
Technically speaking, diagnostic laboratory tests include conventional microscopy, antibody detection methods, antigen detection methods, and Nucleic Acid Amplification Tests (NAATs). Additional clinical diagnostic methods such as clinical markers (e.g. haematuria), physical examinations and imaging techniques, including the recent developments in portable and affordable ultrasound machines, are beyond the scope of this review and have been described in detail elsewhere (2, 5–8).
The current reference standard for diagnosing schistosomiasis is based on the detection of eggs in stool (for intestinal schistosomiasis) or in urine (for urogenital schistosomiasis) by microscopy. For diagnosing individual cases the exact sample preparation can vary, e.g. from a direct stool smear to a glycerine sedimentation technique and information about clinical symptoms and possible exposure history is also often taken into account in the final diagnosis (5). However, for population based surveys or control programs in endemic settings the microscopy-based Kato-Katz (KK) technique and the urine filtration (UF) or urine sedimentation technique to quantitatively assess the intensity of S.mansoni and S.haematobium infections respectively, are most commonly used as a reference standard (9–13). Other available microscopy methods, including techniques such as FECT and FLOTAC, have been described and summarized extensively in Utzinger et al. (5). Even though microscopy techniques are highly specific and can accurately detect high intensity infections, they are not sensitive enough for low intensity infections or post-treatment monitoring (5). New developments in microscopy include optical devices combining digital image recognition with automated data analysis and reporting by using artificial intelligence. Although further technical improvement and validation in the field are needed, these smart and simplified optical diagnostic devices have the potential to identify moderate-to-high intensity infections in stool or urine at low costs (14, 15).
Schistosome-specific antibodies usually develop within a few weeks to months after infection, often before eggs are excreted (2, 5). Various serological methods can be used to detect these antibodies in human plasma or serum (5, 10, 13, 16). Most of these methods are reasonably specific, although a certain level of cross-reactivity with other helminthic diseases is generally accepted (5, 17). Sensitivities of antibody detection methods vary significantly depending on the test format used as well as the infecting species and targeted Schistosoma antigen(s), thereby also affecting the observed timepoint of seroconversion (17, 18). Generally, serology gives neither an indication of the intensity nor the status (past or present) of an infection (19). Still, antibody detection remains useful, especially in the case of travelers who often have not been exposed previously (5, 19). Furthermore, in settings where transmission is assumed to have been interrupted, highly sensitive antibody detection may play an important role to assess small pockets of ongoing risk of infection (20–24).
Living schistosomes release a number of antigens into the hosts’ bloodstream and measurement of such schistosome-specific antigens allows accurate diagnosis of active infections. Most research has focused on Circulating Cathodic Antigen (CCA) and Circulating Anodic Antigen (CAA), two Schistosoma circulating antigens that were already identified in the 1970’s at the Leiden University Medical Center (LUMC), the Netherlands (25, 26). Since then, CCA and CAA have been studied extensively, resulting in over 250 peer-reviewed publications. Both antigens are constantly regurgitated by live Schistosoma worms into the hosts’ circulation, their presence indicating an active infection. Antigen-levels are generally associated with the number of worms present (27). Other unique characteristics of these antigens include the clearance from the blood circulation into the urine with little day-to-day variation (28, 29), and the decrease in levels within days or weeks after PZQ treatment (30–37), making antigen detection highly suitable for individual treatment monitoring.
Detection of CCA can be done via the Point-Of-Care (POC) CCA urine test, a cassette-based lateral flow format which has been evaluated extensively in endemic settings and is commercially available (Rapid Medical Diagnostics, Pretoria, South Africa) for diagnosing intestinal schistosomiasis (12, 38–41). To standardize semi-quantitative visual scoring, a graded and robust scale, called G-scores, became available (42). In combination with a set of reference standards, the G-scores are also useful in dealing with batch-to-batch differences. Furthermore, efforts are ongoing to develop a new test format to overcome observed specificity issues in pregnant women and newborn babies (43).
CAA can be detected in urine and serum via the Up-Converting reporter Particle technology based, Lateral Flow (UCP-LF) CAA test (44). The UCP-LF CAA test has been evaluated in various endemic and non-endemic settings and has demonstrated high specificity and sensitivity for the detection of all human species including hybrids (31, 34, 35, 37, 44–52). Different formats of the UCP-LF CAA test have been developed, including a dry format which allows storage and worldwide shipment of reagents without a cold chain as well as a format applying larger sample volumes thereby increasing sensitivity (44). The most sensitive format requires a more advanced laboratory setting (i.e. sample pre-treatment as well as some centrifugation steps), but is particularly useful for quantifying extremely low worm burdens (32). Although the UCP-LF CAA test is not commercially available yet, efforts are ongoing to develop a more widely available and user-friendly test based on finger prick blood (CAA-RDT) (53).
For the detection and quantification of Schistosoma-specific DNA, several NAATs have been described for different types of clinical samples, including serum, stool and urine (54–61). In particular those that apply urine samples are of interest as this type of sample can be easily and non-invasively acquired (6, 56, 58, 59, 61). Although the described NAATs are mostly in-house assays, some are becoming commercially available (62, 63). The majority of these tests claim a specificity of 100%, while the sensitivity ranges from equal to significantly higher than standard microscopy (5). NAATs have also demonstrated to be very useful for detection and strain typing of hybrid schistosome infections (3). To overcome the need for expensive laboratory equipment and highly trained personnel, more field-friendly alternatives have been developed, such as for example loop-mediated isothermal amplification (LAMP) (64, 65) and recombinase polymerase amplification (RPA) (6, 66), although both need further validation.
Obviously, assay verification and validation are critical steps when implementing diagnostic tests. In particular for antibody detection methods and NAATs, both having a large diversity of assays available, successful participation in an external quality assessment scheme (EQAS) is essential to obtain the highest achievable diagnostic quality (67, 68). Overall, in-house tests require more quality control measurements than commercial tests and they suffer more from variation in test-performance, as well as lack of availability. Still, several non-commercial diagnostic tests for schistosomiasis have proven their diagnostic value in different settings and should therefore not be fully excluded (34, 57, 69). Recently, a list of all currently commercially available diagnostics for schistosomiasis has been made available by the Global Schistosomiasis Alliance Diagnostic Workstream and hopefully this list can be further extended in the near future (63).
2. Diagnostic Approaches for Context-Specific Settings
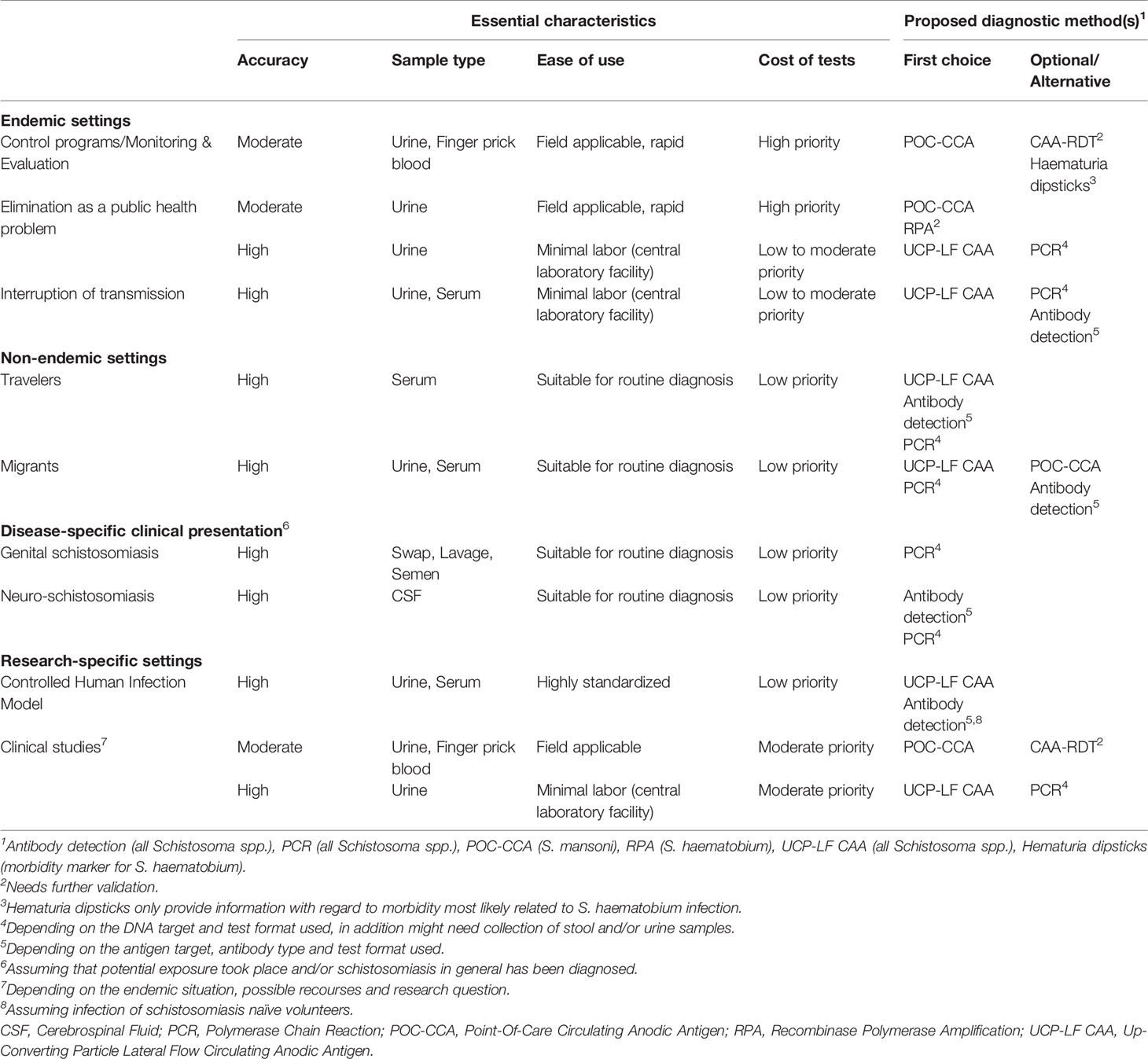
We identified four different settings, namely endemic, non-endemic, disease-specific and research-specific, which can be further subdivided as shown in Table 1. For each situation, diagnostic requirements are discussed with a focus on non-microscopy procedures.
Endemic Settings
In schistosomiasis endemic regions more attention is generally given to diagnosis for public health purposes, than to the identification of infected individuals. The latter often being based on clinical symptoms only, sometimes combined with the detection of eggs in stool or urine. In these settings, the POC-CCA test should be further explored as a user-friendly tool for individual case detection of S.mansoni infections.
Control Programs
Schistosomiasis control in endemic countries relies mainly on transmission intervention measures combined with large-scale administration of PZQ. Programs are based on pilot surveys often performed on a limited number of school-aged children. For monitoring and evaluation of these programs and to determine whether MDA schemes should be adapted or even stopped, more accurate non-microscopy diagnostic procedures are needed (70). The POC-CCA is currently being recommended by the WHO for mapping prevalence of intestinal schistosomiasis as well as for surveillance purposes, as it is a more sensitive and easy-to-use alternative compared to KK (12, 38, 39, 41). As there is no direct rapid diagnostic test for diagnosing S.haematobium infections, an optional method for obtaining an indication of urogenital schistosomiasis prevalence would be hematuria dipsticks, which test for S.haematobium related microhematuria, as hematuria and proteinuria strongly correlate with urogenital schistosomiasis (71). However, even though these methods are not expensive and relatively easy to use, they only provide information on morbidity and do not provide a confirmed diagnosis of infection.
Elimination of Schistosomiasis as a Public Health Problem
In areas where morbidity has been significantly reduced, the next aim is elimination of schistosomiasis as a public health problem. This has been defined by the WHO as <1% of school-aged children with schistosomiasis being categorized as heavily infected (72). For intestinal schistosomiasis, this means >400 eggs per gram of feces and for S.haematobium >50 eggs per 10ml of urine (73), detected by KK or UF, respectively. One notes that these microscopy-based methods are still recommended for determining prevalence and intensity of infection, while their sensitivity is known to be limited (5, 74, 75). As the POC-CCA test has been shown to be a cost-effective alternative for determining S.mansoni prevalence, attempts have been made to estimate equivalent measures of prevalence between POC-CCA and KK (39, 76–79). Likewise, the definition of heavy intensity based on KK or UF should be redefined based on circulating antigen levels. Also, the diagnostic position of the user-friendly RPA assay, as an alternative for the POC-CCA test in case of S.haematobium infections, should be further explored (80).
Interruption of Transmission
Accurate diagnosis of schistosomiasis is also crucial for determining interruption of transmission and eventual elimination, especially in regions where extensive control measures have reduced the prevalence and intensity of infection to very low levels. This is clearly recognized by the WHO and international stakeholders in the NTD Roadmap 2030 where they highlight the need for field-deployable, intelligent diagnostics and sampling strategies to evaluate pre-and post-intervention prevalence, especially for low endemic and near elimination areas (72). Recently, a strategy for the sustained, local interruption of transmission was presented in a viewpoint paper stressing the need for highly sensitive diagnostics (e.g. the UCP-LF CAA test) and intelligent testing procedures such as pooled sampling (81). Applying the UCP-LF CAA test on easily and non-invasively acquired urine samples makes the test an ideal candidate for field use and integration into national control programs. The high accuracy, quantitative outcome and reproducibility of the UCP-LF CAA test makes it amenable to pooled sample testing strategies through which information from whole communities can be obtained presumably in a more cost-effective way (82). Compared to exhaustive individual sampling and testing approaches, appropriate pooling strategies can significantly reduce logistical and laboratory costs of control programs, with minimal loss of sensitivity and specificity (81–83). To what extent NAATs might be suitable for defining interruption of transmission, needs more research (59, 84, 85). Eventually, in settings where transmission appears to have been interrupted, detection of specific antibodies will be useful for assessing exposure in young children (20, 75, 86, 87).
Non-Endemic Settings
In non-endemic settings, diagnosis is usually focused on identifying the infected individual with complete cure as the desired goal. Treatment success can be confirmed by follow-up testing, as there is no risk of re-infection. Distinctive populations can be identified, primarily short-term travelers (including tourists and expatriates) and migrants (including refugees). In general, travelers have not been exposed earlier in life and are therefore considered to be immune-naïve, whereas migrants, when originating from Schistosoma endemic areas, have often been exposed since childhood and are more likely to present with chronic infections. In exceptional cases, migrants originating from non-endemic schistosomiasis areas may have acquired an acute infection when passing through a schistosomiasis endemic area.
Travelers
Only 30-50% of infected travelers present with clinical symptoms (88, 89), but if they develop the so-called Katayama syndrome, it is generally seen several weeks before eggs can be detected. Travelers also often harbor a low worm burden, making appropriate diagnosis even more challenging, even when performed months after exposure (35, 45, 88, 90). Subsequently, schistosome-specific antibody detection plays a central role in the diagnosis of schistosomiasis in previously naïve individuals. Alternatively, detection of Schistosoma DNA in blood has shown to be highly sensitive and specific for early diagnosis of acute schistosomiasis, but also here the test remains positive for many months after treatment (91, 92). Contrary to these findings, there are strong indications that the clearance of S.mansoni DNA in stool or urine occurs within weeks to months following PZQ treatment, but this still needs further validation (93). The diagnostic value of the UCP-LF CAA test in travelers has recently been validated, demonstrating detectable CAA-levels within 4 weeks after exposure, with rapid reduction following appropriate treatment (34, 35).
Migrants
The increasing number of migrants, coming from or passing through Schistosoma endemic regions and arriving in Europe, augments the importance of timely and effective screening for Schistosoma infections (94–96). Detecting Schistosoma-specific antibodies remains the recommended and most used first-line test for screening migrants (97, 98), However, as these methods have their limitations both in sensitivity and specificity in this specific group of suspected infections, the detection of eggs in urine or stool is commonly used to confirm infection, even though its sensitivity is limited (5). A better alternative is the detection of Schistosoma DNA or circulating antigens in clinical samples, as both have demonstrated to be of clinical value when monitoring schistosomiasis in migrants after their arrival in Europe (37). The POC-CCA test can be a useful screening tool for S.mansoni infections when used in a standardized manner (99–101). However, detection of CAA as a routine procedure seems most efficient for migrants originating from different regions, as CAA is found in all Schistosoma species including hybrids and is also most suitable to diagnose low intensity infections (37).
Disease-Specific Clinical Presentation
Genital Schistosomiasis
Genital schistosomiasis, resulting from egg deposition in the genital tissue or fluids, occurs in both males (MGS) and females (FGS) (12, 102). Obviously the first step is to diagnose the presence of schistosomiasis, but there is also a need for diagnostic procedures which can reveal the cause of the organ-specific symptoms. Several studies have demonstrated that the detection of DNA in vaginal swabs, lavage and semen correlates well with the clinical presentation of genital schistosomiasis (103–105). In particular for FGS, this provides a sensitive and more standardized alternative to invasive diagnosis such as colposcopy examination (106, 107).
Neuro-Schistosomiasis
Early diagnosis and treatment of neuro-schistosomiasis, a rare but severe complication of schistosomiasis with spinal-cord involvement, is crucial. The reference standard for confirming neuro-schistosomiasis is the detection of eggs after pathological examination of a tissue biopsy, an invasive and often dangerous procedure. Detection of parasite-specific DNA in cerebrospinal fluid and the demonstration of intra-thecal antibody production seem the most promising diagnostic alternatives (108–110).
Research-Specific Settings
Controlled Human Schistosomiasis Infection Model
To accelerate and assist the development of novel medicines, vaccines and diagnostic tests, an experimental human S.mansoni infection model has been established recently where healthy volunteers were intentionally infected with male-only or female-only parasites. This model provided insight into the development of (acute) schistosomiasis in terms of symptoms, the related immune responses, and the performance of diagnostic tests over time. Following experimental infection, all previously schistosomiasis-naïve volunteers showed detectable antibodies against adult worm gut antigen within 4 to 6 weeks, including those exposed to 10 cercariae only, while the UCP-LF CAA test most accurately reflected worm burdens and appeared highly suitable for monitoring cure (32).
Clinical Studies
Diagnostic tests are also being used and/or evaluated in clinical studies. Currently, the freeBILy project evaluates the POC-CCA and UCP-LF CAA test for the diagnosis of Schistosoma infections in the still often neglected group of pregnant women and their newborn children (111). The project aims to assess the potential of integrating these diagnostics as a schistosomiasis control tool in test-and-treat strategies (111, 112). Another recent clinical trial is the RePST-study in which a panel of adult-worm and egg-related diagnostics were applied to compare standard versus intense treatment in school-aged children with a confirmed S.mansoni infection (113). Cure rates (CRs) based on worm detection (POC-CCA, UCP-LF CAA) were significantly lower than egg-based CRs (KK, PCR) (33, 114). To better understand and optimize treatment strategies, highly sensitive methods such as PCR and UCP-LF CAA should be used in conjunction to provide adequate insight into the host-parasite interaction and metabolic clearance of schistosome circulating antigens.
Other research studies to optimize schistosomiasis control efforts include the SCORE project where the POC-CCA test was evaluated in various endemic settings, contributing to the current WHO recommendation for using the POC-CCA test (38, 39). SCORE also supported further development of the UCP-LF CAA test into more sensitive as well as more user-friendly (dry) formats (44, 47). In addition, preliminary results from a study in Uganda investigating the dynamics of parasite clearance and re-infection based on egg- and antigen-methods, indicate that timing of post-treatment sampling is important, as well as the diagnostic test used to determine Schistosoma CRs and re-infection (115).
Conclusion
The current mini review provides a summary of diagnostic tests with a focus on the requirements for different context-specific settings. Although CAA detection seems the most favorable choice overall, alternative procedures such as antibody detection and NAATs will remain crucial for specific purposes. As ‘no size fits all’, diagnostic tests need to be carefully selected based on the data they provide in order to respond adequately to a specific situation (Figure 1). This is the first and most important step after which further choices will be guided by practicability and economics.
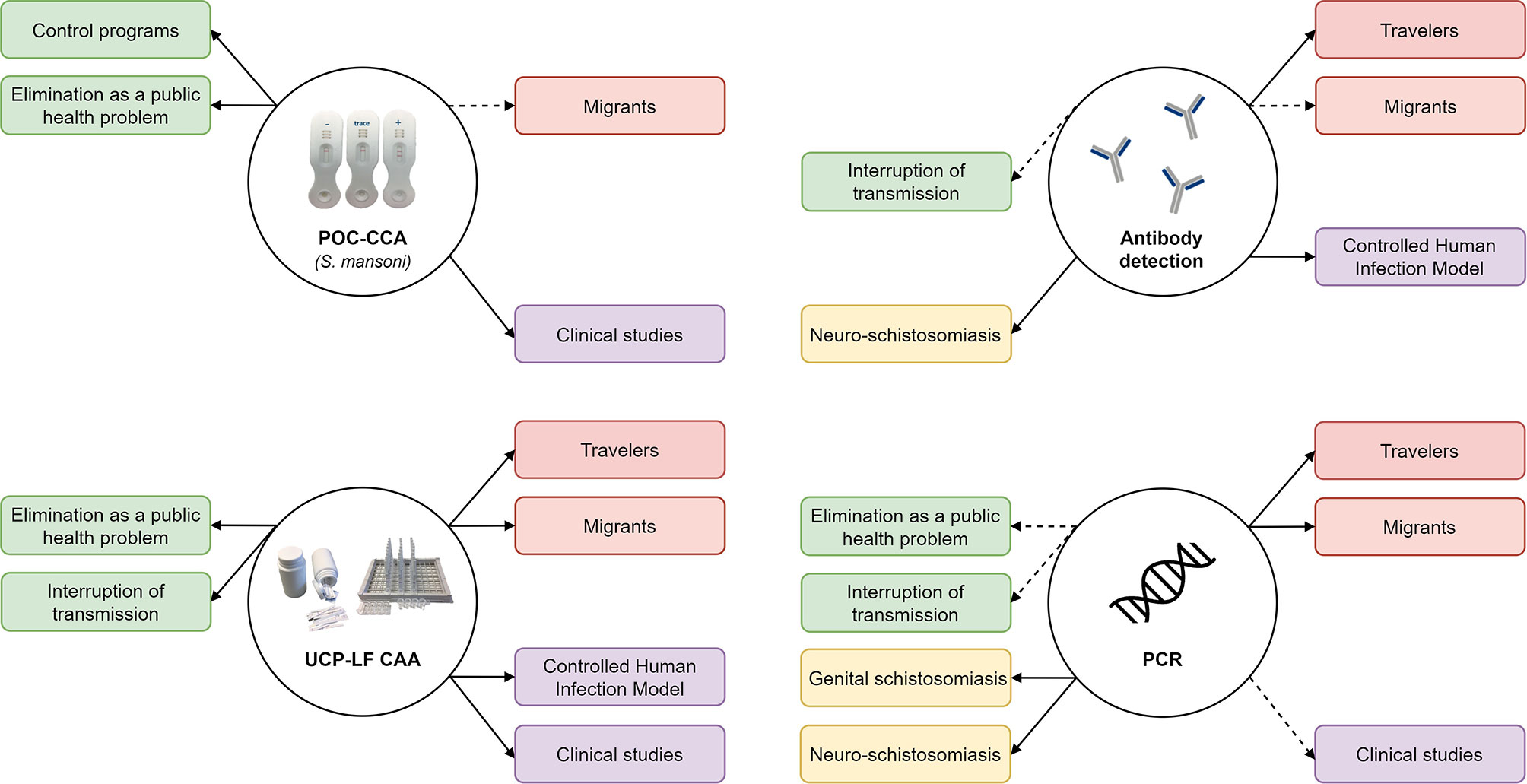
Figure 1 Summary of the applicability of the POC-CCA, UCP-LF CAA, antibody detection and PCR in context-specific settings (solid lines represent first choice diagnostic, dashed lines represent optional or alternative diagnostics). PCR, Polymerase Chain Reaction; POC-CCA, Point-Of-Care Circulation Cathodic Antigen; UCP-LF CAA, Up-Converting Particle Lateral Flow Circulating Anodic Antigen.
Author Contributions
PH wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The Prof. Dr. P.C. Flu foundation is kindly acknowledged for their financial contribution.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with the authors.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank our team-members within the LUMC, in particular Paul Corstjens, Ron Hokke and Meta Roestenberg, as well as our international collaborators, for fruitful discussions over the years leading up to this mini review.
References
1. Colley DG, Bustinduy AL, Secor WE, King CH. Human Schistosomiasis. Lancet (2014) 383(9936):2253–64. doi: 10.1016/S0140-6736(13)61949-2
2. McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers (2018) 4(1):13. doi: 10.1038/s41572-018-0013-8
3. Stothard JR, Kayuni SA, Al-Harbi MH, Musaya J, Webster BL. Future Schistosome Hybridizations: Will All Schistosoma haematobium: Hybrids Please Stand-Up! PLoS Negl Trop Dis (2020) 14(7):e0008201. doi: 10.1371/journal.pntd.0008201
4. Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: Mechanisms of Action, Resistance and New Derivatives for Schistosomiasis. Curr Opin Infect Dis (2008) 21(6):659–67. doi: 10.1097/QCO.0b013e328318978f
5. Utzinger J, Becker SL, van Lieshout L, van Dam GJ, Knopp S. New Diagnostic Tools in Schistosomiasis. Clin Microbiol Infect (2015) 21(6):529–42. doi: 10.1016/j.cmi.2015.03.014
6. Archer J, LaCourse JE, Webster BL, Stothard JR. An Update on non-Invasive Urine Diagnostics for Human-Infecting Parasitic Helminths: What More Could be Done and How? Parasitology (2020) 147(8):873–88. doi: 10.1017/S0031182019001732
7. Kaminstein D, Heller T, Tamarozzi F. Sound Around the World: Ultrasound for Tropical Diseases. Infect Dis Clin North Am (2019) 33(1):169–95. doi: 10.1016/j.idc.2018.10.008
8. Remppis J, Verheyden A, Bustinduy AL, Heller T, García-Tardón N, Manouana GP, et al. Focused Assessment With Sonography for Urinary Schistosomiasis (FASUS)-Pilot Evaluation of a Simple Point-of-Care Ultrasound Protocol and Short Training Program for Detecting Urinary Tract Morbidity in Highly Endemic Settings. Trans R Soc Trop Med Hyg (2020) 114(1):38–48. doi: 10.1093/trstmh/trz101
9. Katz N, Chaves A, Pellegrino J. A Simple Device for Quantitative Stool Thick-Smear Technique in Schistosomiasis Mansoni. Rev Inst Med Trop Sao Paulo (1972) 14:397–400.
10. Weerakoon KG, Gobert GN, Cai P, McManus DP. Advances in the Diagnosis of Human Schistosomiasis. Clin Microbiol Rev (2015) 28(4):939–67. doi: 10.1128/CMR.00137-14
11. Peters PA, Mahmoud AA, Warren KS, Ouma JH, Siongok TK. Field Studies of a Rapid, Accurate Means of Quantifying Schistosoma haematobium Eggs in Urine Samples. Bull World Health Organ (1976) 54(2):159–62.
12. WHO. Schistosomiasis (2020). Available at: https://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis.
13. Bergquist R, van Dam G, Xu J. “Chapter 21”. In: Diagnostic Tests for Schistosomiasis. Schistosoma - Biology, Pathology and Control. Boca Raton: CRC Press (2016).
14. Sukas S, Van Dorst B, Kryj A, Lagatie O, De Malsche W, Stuyver LJ. Development of a Lab-On-a-Disk Platform With Digital Imaging for Identification and Counting of Parasite Eggs in Human and Animal Stool. Micromachines (Basel) (2019) 10(12):852. doi: 10.3390/mi10120852
15. van Grootheest D, Agbana T, Diehl JC, van Diepen A, Bezzubik V, Vdovin G. Large Volume Holographic Imaging for Biological Sample Analysis. J BioMed Opt (2021) 26(1):016502. doi: 10.1117/1.JBO.26.1.016502
16. Hinz R, Schwarz NG, Hahn A, Frickmann H. Serological Approaches for the Diagnosis of Schistosomiasis - A Review. Mol Cell Probes (2017) 31:2–21. doi: 10.1016/j.mcp.2016.12.003
17. van Lieshout L, Roestenberg M. Clinical Consequences of New Diagnostic Tools for Intestinal Parasites. Clin Microbiol Infect (2015) 21(6):520–8. doi: 10.1016/j.cmi.2015.03.015
18. Kinkel HF, Dittrich S, Baumer B, Weitzel T. Evaluation of Eight Serological Tests for Diagnosis of Imported Schistosomiasis. Clin Vaccine Immunol (2012) 19(6):948–53. doi: 10.1128/CVI.05680-11
19. Doenhoff MJ, Chiodini PL, Hamilton JV. Specific and Sensitive Diagnosis of Schistosome Infection: Can it be Done With Antibodies? Trends Parasitolo (2004) 20(1):35–9. doi: 10.1016/j.pt.2003.10.019
20. “Global Health Innovative Technology Fund”. In: Novel Diagnostics for Schistosomiasis Control: Development of Defined Antigens for Detection of Schistosoma Infection-Specific Antibodies in Blood and Urine: Global Health Innovative Technology Fund. Available at: https://www.ghitfund.org/investment/portfoliodetail/detail/123.
21. Crosnier C, Hokke CH, Protasio AV, Brandt C, Rinaldi G, Langenberg MCC, et al. Screening of a Library of Recombinant Schistosoma mansoni Proteins With Sera From Murine and Human Controlled Infections Identifies Early Serological Markers. J Infect Dis (2020). doi: 10.1093/infdis/jiaa329
22. Sotillo J, Pearson MS, Becker L, Mekonnen GG, Amoah AS, van Dam G, et al. In-Depth Proteomic Characterization of Schistosoma haematobium: Towards the Development of New Tools for Elimination. PLoS Negl Trop Dis (2019) 13(5):e0007362. doi: 10.1371/journal.pntd.0007362
23. Yang YYM, Wilson RA, Thomas SRL, Kariuki TM, van Diepen A, Hokke CH. Micro Array-Assisted Analysis of Anti-Schistosome Glycan Antibodies Elicited by Protective Vaccination With Irradiated Cercariae. J Infect Dis (2019) 219(10):1671–80. doi: 10.1093/infdis/jiy714
24. Boissier J, Moné H, Mitta G, Bargues MD, Molyneux D, Mas-Coma S. Schistosomiasis Reaches Europe. Lancet Infect Dis (2015) 15(7):757–8. doi: 10.1016/S1473-3099(15)00084-5
25. Deelder AM, Klappe HT, van den Aardweg GJ, van Meerbeke EH. Schistosoma mansoni: Demonstration of Two Circulating Antigens in Infected Hamsters. Exp Parasitol (1976) 40(2):189–97. doi: 10.1016/0014-4894(76)90081-3
26. Bergquist R. Good Things are Worth Waiting for. Am J Trop Med Hyg (2013) 88(3):409–10. doi: 10.4269/ajtmh.12-0741
27. van Dam GJ, Bogitsh BJ, van Zeyl RJ, Rotmans JP, Deelder AM. Schistosoma mansoni: In Vitro and In Vivo Excretion of CAA and CCA by Developing Schistosomula and Adult Worms. J Parasitol (1996) 82(4):557–64. doi: 10.2307/3283780
28. Polman K, Van Lieshout L, Gryseels B, Deelder AM. Age-Related Worm Load and Worm Fecundity Patterns in Human Populations, as Indicated by Schistosome Circulating Antigens. Mem Inst Oswaldo Cruz (1998) 93(Suppl 1):123–5. doi: 10.1590/S0074-02761998000700017
29. van Lieshout L, de Jonge N, Bassily S, Mansour MM, Deelder AM. Assessment of Cure in Schistosomiasis Patients After Chemotherapy With Praziquantel by Quantitation of Circulating Anodic Antigen (CAA) in Urine. Am J Trop Med Hyg (1991) 44(3):323–8. doi: 10.4269/ajtmh.1991.44.323
30. Kildemoes AO, Vennervald BJ, Tukahebwa EM, Kabatereine NB, Magnussen P, de Dood CJ, et al. Rapid Clearance of Schistosoma mansoni Circulating Cathodic Antigen After Treatment Shown by Urine Strip Tests in a Ugandan Fishing Community - Relevance for Monitoring Treatment Efficacy and Re-Infection. PLoS Negl Trop Dis (2017) 11(11):e0006054. doi: 10.1371/journal.pntd.0006054
31. Sousa MS, van Dam GJ, Pinheiro MCC, de Dood CJ, Peralta JM, Peralta RHS, et al. Performance of an Ultra-Sensitive Assay Targeting the Circulating Anodic Antigen (CAA) for Detection of Schistosoma mansoni Infection in a Low Endemic Area in Brazil. Front Immunol (2019) 10:682. doi: 10.3389/fimmu.2019.00682
32. Langenberg MCC, Hoogerwerf MA, Koopman JPR, Janse JJ, Kos-van Oosterhoud J, Feijt C, et al. A Controlled Human Schistosoma mansoni Infection Model to Advance Novel Drugs, Vaccines and Diagnostics. Nat Med (2020) 26(3):326–32. doi: 10.1038/s41591-020-0759-x
33. Hoekstra PT, Casacuberta-Partal M, van Lieshout L, Corstjens P, Tsonaka R, Assare RK, et al. Efficacy of Single Versus Four Repeated Doses of Praziquantel Against Schistosoma mansoni Infection in School-Aged Children From Cote D’Ivoire Based on Kato-Katz and POC-CCA: An Open-Label, Randomised Controlled Trial (RePST). PLoS Negl Trop Dis (2020) 14(3):e0008189. doi: 10.1371/journal.pntd.0008189
34. Hoekstra PT, van Esbroeck M, de Dood CJ, Corstjens PL, Cnops L, van Zeijl-van der Ham CJ, et al. Early Diagnosis and Follow-Up of Acute Schistosomiasis in a Cluster of Infected Belgian Travellers by Detection of Antibodies and Circulating Anodic Antigen (CAA): A Diagnostic Evaluation Study. Travel Med Infect Dis (2021) 41:102053. doi: 10.1016/j.tmaid.2021.102053
35. van Grootveld R, van Dam GJ, de Dood C, de Vries JJC, Visser LG, Corstjens P, et al. Improved Diagnosis of Active Schistosoma Infection in Travellers and Migrants Using the Ultra-Sensitive in-House Lateral Flow Test for Detection of Circulating Anodic Antigen (CAA) in Serum. Eur J Clin Microbiol Infect Dis (2018) 37(9):1709–16. doi: 10.1007/s10096-018-3303-x
36. van Lieshout L, Polderman AM, Visser LG, Verwey JJ, Deelder AM. Detection of the Circulating Antigens CAA and CCA in a Group of Dutch Travellers With Acute Schistosomiasis. Trop Med Int Health (1997) 2(6):551–7. doi: 10.1046/j.1365-3156.1997.d01-324.x
37. Tamarozzi F, Ursini T, Hoekstra PT, Silva R, Costa C, Gobbi F, et al. Evaluation of Microscopy, Serology, Circulating Anodic Antigen (CAA), and Eosinophil Counts for the Follow-Up of Migrants With Chronic Schistosomiasis: A Prospective Cohort Study. Parasit Vectors (2021) 14(1):149. doi: 10.1186/s13071-021-04655-z
38. WHO. Report of the First Meeting of the WHO Diagnostic Technical Advisory Group for Neglected Tropical Diseases. Geneva: World Health Organization (2019).
39. Bärenbold O, Garba A, Colley DG, Fleming FM, Haggag AA, Ramzy RMR, et al. Translating Preventive Chemotherapy Prevalence Thresholds for Schistosoma mansoni From the Kato-Katz Technique Into the Point-of-Care Circulating Cathodic Antigen Diagnostic Test. PLoS Negl Trop Dis (2018) 12(12):e0006941. doi: 10.1371/journal.pntd.0006941
40. Rapid Medical Diagnostics. In: Rapid Medical Diagnostics. Available at: http://www.rapid-diagnostics.com/.
41. Danso-Appiah A, Minton J, Boamah D, Otchere J, Asmah RH, Rodgers M, et al. Accuracy of Point-of-Care Testing for Circulatory Cathodic Antigen in the Detection of Schistosome Infection: Systematic Review and Meta-Analysis. Bull World Health Organ (2016) 94(7):522–33a. doi: 10.2471/BLT.15.158741
42. Casacuberta-Partal M, Hoekstra PT, Kornelis D, van Lieshout L, van Dam GJ. An Innovative and User-Friendly Scoring System for Standardised Quantitative Interpretation of the Urine-Based Point-of-Care Strip Test (POC-CCA) for the Diagnosis of Intestinal Schistosomiasis: A Proof-of-Concept Study. Acta Trop (2019) 199:105150. doi: 10.1016/j.actatropica.2019.105150
43. Casacuberta-Partal M, Beenakker M, de Dood C, Hoekstra P, Kroon L, Kornelis D, et al. Specificity of the Point-Of-Care Urine Strip Test for Schistosoma Circulating Cathodic Antigen (POC-CCA) Tested in Non-Endemic Pregnant Women and Young Children. Am J Trop Med Hyg (2021) 104(4):1412–1417. doi: 10.4269/ajtmh.20-1168
44. Corstjens P, de Dood CJ, Knopp S, Clements MN, Ortu G, Umulisa I, et al. Circulating Anodic Antigen (CAA): A Highly Sensitive Diagnostic Biomarker to Detect Active Schistosoma Infections-Improvement and Use During SCORE. Am J Trop Med Hyg (2020) 103(1_Suppl):50–7. doi: 10.4269/ajtmh.19-0819
45. Casacuberta-Partal M, Janse JJ, van Schuijlenburg R, de Vries JJC, Erkens MAA, Suijk K, et al. Antigen-Based Diagnosis of Schistosoma Infection in Travellers: A Prospective Study. J Travel Med (2020) 27(4):taaa055. doi: 10.1093/jtm/taaa055
46. Clements MN, Corstjens P, Binder S, Campbell CH Jr., de Dood CJ, Fenwick A, et al. Latent Class Analysis to Evaluate Performance of Point-of-Care CCA for Low-Intensity Schistosoma mansoni Infections in Burundi. Parasit Vectors (2018) 11(1):111. doi: 10.1186/s13071-018-2700-4
47. Corstjens PL, Nyakundi RK, de Dood CJ, Kariuki TM, Ochola EA, Karanja DM, et al. Improved Sensitivity of the Urine CAA Lateral-Flow Assay for Diagnosing Active Schistosoma Infections by Using Larger Sample Volumes. Parasit Vectors (2015) 8:241. doi: 10.1186/s13071-015-0857-7
48. de Dood CJ, Hoekstra PT, Mngara J, Kalluvya SE, van Dam GJ, Downs JA, et al. Refining Diagnosis of Schistosoma haematobium Infections: Antigen and Antibody Detection in Urine. Front Immunol (2018) 9:2635. doi: 10.3389/fimmu.2018.02635
49. Knopp S, Corstjens PL, Koukounari A, Cercamondi CI, Ame SM, Ali SM, et al. Sensitivity and Specificity of a Urine Circulating Anodic Antigen Test for the Diagnosis of Schistosoma haematobium in Low Endemic Settings. PLoS Negl Trop Dis (2015) 9(5):e0003752. doi: 10.1371/journal.pntd.0003752
50. van Dam GJ, Odermatt P, Acosta L, Bergquist R, de Dood CJ, Kornelis D, et al. Evaluation of Banked Urine Samples for the Detection of Circulating Anodic and Cathodic Antigens in Schistosoma mekongi and S. japonicum Infections: A Proof-of-Concept Study. Acta Trop (2015) 141(Pt B):198–203. doi: 10.1016/j.actatropica.2014.09.003
51. van Dam GJ, Xu J, Bergquist R, de Dood CJ, Utzinger J, Qin ZQ, et al. An Ultra-Sensitive Assay Targeting the Circulating Anodic Antigen for the Diagnosis of Schistosoma japonicum in a Low-Endemic Area, People’s Republic of China. Acta Trop (2015) 141(Pt B):190–7. doi: 10.1016/j.actatropica.2014.08.004
52. Vonghachack Y, Sayasone S, Khieu V, Bergquist R, van Dam GJ, Hoekstra PT, et al. Comparison of Novel and Standard Diagnostic Tools for the Detection of Schistosoma mekongi Infection in Lao People’s Democratic Republic and Cambodia. Infect Dis Poverty (2017) 6(1):127. doi: 10.1186/s40249-017-0335-x
53. FIND. Rapid Tests for Schistosomiasis Control & Elimination (2021). Available at: https://www.finddx.org/marginalized-populations/schisto-rdts/.
54. Weerakoon KG, Gordon CA, McManus DP. DNA Diagnostics for Schistosomiasis Control. Trop Med Infect Dis (2018) 3(3):81. doi: 10.3390/tropicalmed3030081
55. Verweij JJ. Application of PCR-Based Methods for Diagnosis of Intestinal Parasitic Infections in the Clinical Laboratory. Parasitology (2014) 141(14):1863–72. doi: 10.1017/S0031182014000419
56. Lodh N, Naples JM, Bosompem KM, Quartey J, Shiff CJ. Detection of Parasite-Specific DNA in Urine Sediment Obtained by Filtration Differentiates Between Single and Mixed Infections of Schistosoma haematobium and S. mansoni From Endemic Areas in Ghana. PLoS One (2014) 9(3):e91144. doi: 10.1371/journal.pone.0091144
57. Meurs L, Brienen E, Mbow M, Ochola EA, Mboup S, Karanja DM, et al. Is PCR the Next Reference Standard for the Diagnosis of Schistosoma in Stool? A Comparison With Microscopy in Senegal and Kenya. PLoS Negl Trop Dis (2015) 9(7):e0003959. doi: 10.1371/journal.pntd.0003959
58. Fernández-Soto P, Gandasegui J, Carranza Rodríguez C, Pérez-Arellano JL, Crego-Vicente B, García-Bernalt Diego J, et al. Detection of Schistosoma mansoni-Derived DNA in Human Urine Samples by Loop-Mediated Isothermal Amplification (LAMP). PLoS One (2019) 14(3):e0214125. doi: 10.1371/journal.pone.0214125
59. Keller D, Rothen J, Dangy J-P, Saner C, Daubenberger C, Allan F, et al. Performance of a Real-Time PCR Approach for Diagnosing Schistosoma haematobium Infections of Different Intensity in Urine Samples From Zanzibar. Infect Dis Poverty (2020) 9(1):128. doi: 10.1186/s40249-020-00726-y
60. Frickmann H, Lunardon LM, Hahn A, Loderstädt U, Lindner AK, Becker SL, et al. Evaluation of a Duplex Real-Time PCR in Human Serum for Simultaneous Detection and Differentiation of Schistosoma mansoni and Schistosoma haematobium Infections - Cross-Sectional Study. Travel Med Infect Dis (2021) 41:102035. doi: 10.1016/j.tmaid.2021.102035
61. Lodh N, Mikita K, Bosompem KM, Anyan WK, Quartey JK, Otchere J, et al. Point of Care Diagnosis of Multiple Schistosome Parasites: Species-Specific DNA Detection in Urine by Loop-Mediated Isothermal Amplification (LAMP). Acta Trop (2017) 173:125–9. doi: 10.1016/j.actatropica.2017.06.015
62. Ajibola O, Gulumbe BH, Eze AA, Obishakin E. Tools for Detection of Schistosomiasis in Resource Limited Settings. Med Sci (2018) 6(2):39. doi: 10.3390/medsci6020039
63. GSA. Communication Piece on Commercially Available Diagnostic Tests (2021). Available at: https://www.eliminateschisto.org/resources/communication-piece-commercially-available-diagnostic-tests.
64. García-Bernalt Diego J, Fernández-Soto P, Febrer-Sendra B, Crego-Vicente B, Muro A. Loop-Mediated Isothermal Amplification in Schistosomiasis. J Clin Med (2021) 8(9):e3126. doi: 10.3390/jcm10030511
65. Avendaño C, Patarroyo MA. Loop-Mediated Isothermal Amplification as Point-Of-Care Diagnosis for Neglected Parasitic Infections. Int J Mol Sci (2020) 21(21):7981. doi: 10.3390/ijms21217981
66. Rosser A, Rollinson D, Forrest M, Webster BL. Isothermal Recombinase Polymerase Amplification (RPA) of Schistosoma haematobium DNA and Oligochromatographic Lateral Flow Detection. Parasit Vectors (2015) 8:446. doi: 10.1186/s13071-015-1055-3
67. Cools P, van Lieshout L, Koelewijn R, Addiss D, Ajjampur SSR, Ayana M, et al. First International External Quality Assessment Scheme of Nucleic Acid Amplification Tests for the Detection of Schistosoma and Soil-Transmitted Helminths, Including Strongyloides: A Pilot Study. PLoS Negl Trop Dis (2020) 14(6):e0008231. doi: 10.1371/journal.pntd.0008231
68. Collier S, Manser M, Chiodini PL. External Quality Assessment Scheme for Parasite Serology; A Review of the Scheme Design and Performance. J Clin Pathol (2010) 63(5):441–4. doi: 10.1136/jcp.2009.071597
69. Aryeetey YA, Essien-Baidoo S, Larbi IA, Ahmed K, Amoah AS, Obeng BB, et al. Molecular Diagnosis of Schistosoma Infections in Urine Samples of School Children in Ghana. Am J Trop Med Hyg (2013) 88(6):1028–31. doi: 10.4269/ajtmh.12-0571
70. Gass K. Time for a Diagnostic Sea-Change: Rethinking Neglected Tropical Disease Diagnostics to Achieve Elimination. PLoS Negl Trop Dis (2020) 14(12):e0008933. doi: 10.1371/journal.pntd.0008933
71. King CH, Bertsch D. Meta-Analysis of Urine Heme Dipstick Diagnosis of Schistosoma haematobium Infection, Including Low-Prevalence and Previously-Treated Populations. PLoS Negl Trop Dis (2013) 7(9):e2431. doi: 10.1371/journal.pntd.0002431
72. WHO. Ending The Neglect to Attain The Sustainable Development Goals -A Road Map for Neglected Tropical Diseases 2021–2030. Geneva, Swtizerland: WHO (2020).
73. WHO. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. Geneva, Switzerland: World Health Organ Tech Rep (2002). Report No.: 912.
74. Knopp S, Ame SM, Hattendorf J, Ali SM, Khamis IS, Bakar F, et al. Urogenital Schistosomiasis Elimination in Zanzibar: Accuracy of Urine Filtration and Haematuria Reagent Strips for Diagnosing Light Intensity Schistosoma haematobium Infections. Parasit Vectors (2018) 11(1):552. doi: 10.1186/s13071-018-3136-6
75. Secor WE, Colley DG. When Should the Emphasis on Schistosomiasis Control Move to Elimination? Trop Med Infect Dis (2018) 3(3):85. doi: 10.3390/tropicalmed3030085
76. Kittur N, Castleman JD, Campbell CH Jr, King CH, Colley DG. Comparison of Schistosoma mansoni Prevalence and Intensity of Infection, as Determined by the Circulating Cathodic Antigen Urine Assay or by the Kato-Katz Fecal Assay: A Systematic Review. Am J Trop Med Hyg (2016) 94(3):605–10. doi: 10.4269/ajtmh.15-0725
77. Prada JM, Touloupou P, Adriko M, Tukahebwa EM, Lamberton PHL, Hollingsworth TD. Understanding the Relationship Between Egg- and Antigen-Based Diagnostics of Schistosoma mansoni Infection Pre- and Post-Treatment in Uganda. Parasit Vectors (2018) 11(1):21. doi: 10.1186/s13071-017-2580-z
78. Colley DG, King CH, Kittur N, Ramzy RMR, Secor WE, Fredericks-James M, et al. Evaluation, Validation, and Recognition of the Point-Of-Care Circulating Cathodic Antigen, Urine-Based Assay for Mapping Schistosoma mansoni Infections. Am J Trop Med Hyg (2020) 103(1_Suppl):42–9. doi: 10.4269/ajtmh.19-0788
79. Clark NJ, Umulisa I, Ruberanziza E, Owada K, Colley DG, Ortu G, et al. Mapping Schistosoma mansoni Endemicity in Rwanda: A Critical Assessment of Geographical Disparities Arising From Circulating Cathodic Antigen Versus Kato-Katz Diagnostics. PLoS Negl Trop Dis (2019) 13(9):e0007723. doi: 10.1371/journal.pntd.0007723
80. Archer J, Barksby R, Pennance T, Rostron P, Bakar F, Knopp S, et al. Analytical and Clinical Assessment of a Portable, Isothermal Recombinase Polymerase Amplification (RPA) Assay for the Molecular Diagnosis of Urogenital Schistosomiasis. Molecules (2020) 25(18):4175. doi: 10.3390/molecules25184175
81. Amoah AS, Hoekstra PT, Casacuberta-Partal M, Coffeng LE, Corstjens PLAM, Greco B, et al. Sensitive Diagnostic Tools and Targeted Drug Administration Strategies are Needed to Eliminate Schistosomiasis. Lancet Infect Dis (2020) 20(7):e165–e72. doi: 10.1016/S1473-3099(20)30254-1
82. Corstjens P, Hoekstra PT, de Dood CJ, van Dam GJ. Utilizing the Ultrasensitive Schistosoma Up-Converting Phosphor Lateral Flow Circulating Anodic Antigen (UCP-LF CAA) Assay for Sample Pooling-Strategies. Infect Dis Poverty (2017) 6(1):155. doi: 10.1186/s40249-017-0368-1
83. Lo NC, Coulibaly JT, Bendavid E, N’Goran EK, Utzinger J, Keiser J, et al. Evaluation of a Urine Pooling Strategy for the Rapid and Cost-Efficient Prevalence Classification of Schistosomiasis. PLoS Negl Trop Dis (2016) 10(8):e0004894. doi: 10.1371/journal.pntd.0004894
84. He P, Gordon CA, Williams GM, Li Y, Wang Y, Hu J, et al. Real-Time PCR Diagnosis of Schistosoma japonicum in Low Transmission Areas of China. Infect Dis Poverty (2018) 7(1):8. doi: 10.1186/s40249-018-0390-y
85. Magalhães FDC, Resende SD, Senra C, Graeff-Teixeira C, Enk MJ, Coelho PMZ, et al. Accuracy of Real-Time Polymerase Chain Reaction to Detect Schistosoma mansoni - Infected Individuals From an Endemic Area With Low Parasite Loads. Parasitology (2020) 147(10):1140–8. doi: 10.1017/S003118202000089X
86. Colley DG, Andros TS, Campbell CH Jr. Schistosomiasis Is More Prevalent Than Previously Thought: What Does it Mean for Public Health Goals, Policies, Strategies, Guidelines and Intervention Programs? Infect Dis Poverty (2017) 6(1):63. doi: 10.1186/s40249-017-0275-5
87. Naus CWA, van Remoortere A, Ouma JH, Kimani G, Dunne DW, Kamerling JP, et al. Specific Antibody Responses to Three Schistosome-Related Carbohydrate Structures in Recently Exposed Immigrants and Established Residents in an Area of Schistosoma mansoni Endemicity. Infect Immun (2003) 71(10):5676–81. doi: 10.1128/IAI.71.10.5676-5681.2003
88. Clerinx J, Van Gompel A. Schistosomiasis in Travellers and Migrants. Travel Med Infect Dis (2011) 9(1):6–24. doi: 10.1016/j.tmaid.2010.11.002
89. Ross AG, Vickers D, Olds GR, Shah SM, McManus DP. Katayama Syndrome. Lancet Infect Dis (2007) 7(3):218–24. doi: 10.1016/S1473-3099(07)70053-1
90. Coltart CE, Chew A, Storrar N, Armstrong M, Suff N, Morris L, et al. Schistosomiasis Presenting in Travellers: A 15 Year Observational Study at the Hospital for Tropical Diseases, London. Trans R Soc Trop Med Hyg (2015) 109(3):214–20. doi: 10.1093/trstmh/tru195
91. Cnops L, Huyse T, Maniewski U, Soentjens P, Bottieau E, Van Esbroeck M, et al. Acute Schistosomiasis With a S. mattheei X S. Haematobium Hybrid Species in a Cluster of 34 Travelers Infected in South Africa. Clin Infect Dis (2020) 72(10):1693–1698. doi: 10.1093/cid/ciaa312
92. Clerinx J, Bottieau E, Wichmann D, Tannich E, Van Esbroeck M. Acute Schistosomiasis in a Cluster of Travelers From Rwanda: Diagnostic Contribution of Schistosome DNA Detection in Serum Compared to Parasitology and Serology. J Travel Med (2011) 18(6):367–72. doi: 10.1111/j.1708-8305.2011.00552.x
93. Vinkeles Melchers NV, van Dam GJ, Shaproski D, Kahama AI, Brienen EA, Vennervald BJ, et al. Diagnostic Performance of Schistosoma Real-Time PCR in Urine Samples From Kenyan Children Infected With Schistosoma haematobium: Day-to-Day Variation and Follow-Up After Praziquantel Treatment. PLoS Negl Trop Dis (2014) 8(4):e2807. doi: 10.1371/journal.pntd.0002807
94. Noori T, Hargreaves S, Greenaway C, van der Werf M, Driedger M, Morton RL, et al. Strengthening Screening for Infectious Diseases and Vaccination Among Migrants in Europe: What Is Needed to Close the Implementation Gaps? Travel Med Infect Dis (2021) 39:101715. doi: 10.1016/j.tmaid.2020.101715
95. Riccardi N, Nosenzo F, Peraldo F, Sarocchi F, Taramasso L, Traverso P, et al. Increasing Prevalence of Genitourinary Schistosomiasis in Europe in the Migrant Era: Neglected No More? PLoS Negl Trop Dis (2017) 11(3):e0005237. doi: 10.1371/journal.pntd.0005237
96. Makhani L, Kopalakrishnan S, Bhasker S, Boggild AK. Five Key Points About Intestinal Schistosomiasis for the Migrant Health Practitioner. Travel Med Infect Dis (2021) 40:101971. doi: 10.1016/j.tmaid.2021.101971
97. European Centre for Disease Prevention and Control. Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants Within the EU/EEA. Stockholm: ECDC (2018).
98. Agbata EN, Morton RL, Bisoffi Z, Bottieau E, Greenaway C, Biggs B-A, et al. Effectiveness of Screening and Treatment Approaches for Schistosomiasis and Strongyloidiasis in Newly-Arrived Migrants From Endemic Countries in the EU/EEA: A Systematic Review. Int J Environ Res Public Health (2019) 16(1):11. doi: 10.3390/ijerph16010011
99. Becker SL, Marti H, Zimmermann S, Vidacek D, Herrmann M, Utzinger J, et al. Application in Europe of a Urine-Based Rapid Diagnostic Test for Confirmation of Schistosoma mansoni Infection in Migrants From Endemic Areas. Euro Surveill (2015) 20(23):21151. doi: 10.2807/1560-7917.ES2015.20.23.21151
100. Chernet A, Kling K, Sydow V, Kuenzli E, Hatz C, Utzinger J, et al. Accuracy of Diagnostic Tests for Schistosoma mansoni Infection in Asymptomatic Eritrean Refugees: Serology and Point-Of-Care Circulating Cathodic Antigen Against Stool Microscopy. Clin Infect Dis (2017) 65(4):568–74. doi: 10.1093/cid/cix366
101. Marchese V, Beltrame A, Angheben A, Monteiro GB, Giorli G, Perandin F, et al. Schistosomiasis in Immigrants, Refugees and Travellers in an Italian Referral Centre for Tropical Diseases. Infect Dis Poverty (2018) 7(1):55. doi: 10.1186/s40249-018-0440-5
102. UNAIDS. Joint United Nations Programme on HIV/AIDS. No More Neglect - Female Genital Schistosomiasis and HIV 2019 [Report No.: UNAIDS/Jc2979] . Available at: https://www.unaids.org/sites/default/files/media_asset/female_genital_schistosomiasis_and_hiv_en.pdf.
103. Kayuni SA, Corstjens P, LaCourse EJ, Bartlett KE, Fawcett J, Shaw A, et al. How can Schistosome Circulating Antigen Assays be Best Applied for Diagnosing Male Genital Schistosomiasis (MGS): An Appraisal Using Exemplar MGS Cases From a Longitudinal Cohort Study Among Fishermen on the South Shoreline of Lake Malawi. Parasitology (2019) 146(14):1785–95. doi: 10.1017/S0031182019000969
104. Pillay P, Downs JA, Changalucha JM, Brienen EAT, Ramarokoto CE, Leutscher PDC, et al. Detection of Schistosoma DNA in Genital Specimens and Urine: A Comparison Between Five Female African Study Populations Originating From S. haematobium and/or S. mansoni Endemic Areas. Acta Trop (2020) 204:105363. doi: 10.1016/j.actatropica.2020.105363
105. Kjetland EF, Hove RJ, Gomo E, Midzi N, Gwanzura L, Mason P, et al. Schistosomiasis PCR in Vaginal Lavage as an Indicator of Genital Schistosoma haematobium Infection in Rural Zimbabwean Women. Am J Trop Med Hyg (2009) 81(6):1050–5. doi: 10.4269/ajtmh.2009.09-0081
106. Sturt AS, Webb EL, Phiri CR, Mweene T, Chola N, van Dam GJ, et al. Genital Self-Sampling Compared With Cervicovaginal Lavage for the Diagnosis of Female Genital Schistosomiasis in Zambian Women: The BILHIV Study. PLoS Negl Trop Dis (2020) 14(7):e0008337. doi: 10.1371/journal.pntd.0008337
107. Pillay P, van Lieshout L, Taylor M, Sebitloane M, Zulu SG, Kleppa E, et al. Cervical Cytology as a Diagnostic Tool for Female Genital Schistosomiasis: Correlation to Cervical Atypia and Schistosoma Polymerase Chain Reaction. Cytojournal (2016) 13:10. doi: 10.4103/1742-6413.180784
108. Harter G, Frickmann H, Zenk S, Wichmann D, Ammann B, Kern P, et al. Diagnosis of Neuroschistosomiasis by Antibody Specificity Index and Semi-Quantitative Real-Time PCR From Cerebrospinal Fluid and Serum. J Med Microbiol (2014) 63(Pt 2):309–12. doi: 10.1099/jmm.0.066142-0
109. de Jongste AH, Tilanus AM, Bax H, Willems MH, van der Feltz M, van Hellemond JJ. New Insights in Diagnosing Schistosoma Myelopathy. J Infect (2010) 60(3):244–7. doi: 10.1016/j.jinf.2009.12.002
110. Bruscky IS, de Melo FL, de Medeiros ZM, Albuquerque FF, Wanderley LB, da Cunha-Correia C. Nested Polymerase Chain Reaction in Cerebrospinal Fluid for Diagnosing Spinal Cord Schistosomiasis: A Promising Method. J Neurol Sci (2016) 366:87–90. doi: 10.1016/j.jns.2016.04.049
111. Hoekstra PT, Schwarz NG, Adegnika AA, Andrianarivelo MR, Corstjens PLAM, Rakotoarivelo RA, et al. Fast and Reliable Easy-to-Use Diagnostics for Eliminating Bilharzia in Young Children and Mothers: An Introduction to the freeBILy Project. Acta Tropica (2020) 211:105631. doi: 10.1016/j.actatropica.2020.105631
112. Honkpehedji YJ, Adegnika AA, Dejon-Agobe JC, Zinsou JF, Mba RB, Gerstenberg J, et al. Prospective, Observational Study to Assess the Performance of CAA Measurement as a Diagnostic Tool for the Detection of Schistosoma haematobium Infections in Pregnant Women and Their Child in Lambaréné, Gabon: Study Protocol of the freeBILy Clinical Trial in Gabon. BMC Infect Dis (2020) 20(1):718. doi: 10.1186/s12879-020-05445-1
113. Hoekstra PT, Casacuberta Partal M, Amoah AS, van Lieshout L, Corstjens P, Tsonaka S, et al. Repeated Doses of Praziquantel in Schistosomiasis Treatment (RePST) - Single Versus Multiple Praziquantel Treatments in School-Aged Children in Côte D’Ivoire: A Study Protocol for an Open-Label, Randomised Controlled Trial. BMC Infect Dis (2018) 18(1):662. doi: 10.1186/s12879-018-3554-2
114. Hoekstra PT. Schistosoma Eggs, Circulating Antigens or DNA? What do These Different Diagnostic Markers Reveal When Monitoring Praziquantel Treatment in a Clinical Trial? (2021). Available at: https://www.youtube.com/watch?v=f1OoEJALth4.
115. Clark J. Reconciling Egg- and Antigen-Based Estimates of Schistosoma mansoni Clearance and Re-Infection: A Modelling Study (2021). Available at: https://www.youtube.com/watch?v=WNFbd9ZViiQ.
Keywords: schistosomiasis, diagnosis, context-specific settings, disease control, imported infection
Citation: Hoekstra PT, van Dam GJ and van Lieshout L (2021) Context-Specific Procedures for the Diagnosis of Human Schistosomiasis – A Mini Review. Front. Trop. Dis 2:722438. doi: 10.3389/fitd.2021.722438
Received: 08 June 2021; Accepted: 22 July 2021;
Published: 05 August 2021.
Edited by:
Francesca Tamarozzi, Sacro Cuore Don Calabria Hospital, ItalyReviewed by:
Cedric Yansouni, McGill University Health Centre, CanadaClive Shiff, Johns Hopkins University, United States
Copyright © 2021 Hoekstra, van Dam and van Lieshout. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pytsje T. Hoekstra, cC50LmhvZWtzdHJhLW1ldml1c0BsdW1jLm5s
 Pytsje T. Hoekstra
Pytsje T. Hoekstra Govert J. van Dam
Govert J. van Dam Lisette van Lieshout
Lisette van Lieshout