
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Trop. Dis. , 20 September 2021
Sec. Vaccines for Tropical Diseases
Volume 2 - 2021 | https://doi.org/10.3389/fitd.2021.709745
This article is part of the Research Topic Vaccines against Enteric Infections View all 4 articles
 Thomas Bentley1,2†
Thomas Bentley1,2† Elizabeth Jones1,2*†
Elizabeth Jones1,2*† Celina Jin1,2
Celina Jin1,2 Maria Moore1,2
Maria Moore1,2 Jonathon Gardner1,2
Jonathon Gardner1,2 Jennifer Hill1,2‡
Jennifer Hill1,2‡ Andrew J. Pollard1,2‡
Andrew J. Pollard1,2‡Background: Salmonella enterica serovar Typhi is estimated to cause 9 to 13 million cases of typhoid fever annually. Typhoid conjugate vaccines represent a promising prophylactic measure to prevent disease, but there are few data assessing persistence of immunity. The effect of a Vi polysaccharide booster vaccine in individuals previously vaccinated with the Vi-tetanus toxoid typhoid conjugate vaccine has not been assessed previously.
Methods: Thirty five healthy adult volunteers received a single dose of the Vi conjugate vaccine (Vi-TT) and 37 received a single dose of Vi polysaccharide vaccine (Vi-PS) prior to oral challenge with live S. Typhi bacteria as part of a randomised controlled, phase 2b study. In addition to data previously published showing persistence of Vi IgG and IgA antibodies for 7 months after Vi vaccination, titres were measured at intervals until 13 months post-vaccination. Ten participants who received Vi-TT (both challenged and unchallenged) were re-vaccinated with Vi-PS at an interval of 19-23 months post-prime. Anti-Vi IgG and IgA titres, and Vi-specific antibody secreting cells and memory B cells were measured at seven days and one month post-boost.
Findings: Vi IgG and IgA antibody titres remained significantly elevated above baseline levels 13 months after priming with Vi-TT, with a 4-fold rise retained in 90% and 88% of recipients (Vi IgG and IgA, respectively). Anti-Vi IgG and IgA antibody titres were found to persist at higher levels in participants who received a single dose of Vi-TT than in those who received Vi-PS. No significant boost in Vi-antibody titre was observed in response to oral challenge with S. Typhi bacteria, one month after vaccination. Following a Vi-PS booster vaccination in those previously vaccinated with Vi-TT, anti-Vi IgG and IgA titres were significantly elevated, with similar titres observed at one month post-boost compared with one month after primary vaccination. The frequency of Vi-specific IgA antibody secreting cells increased significantly 7 days post-boost compared with pre-boost. No memory B cell response was observed following Vi-PS booster vaccination.
Interpretation: Strong persistence of anti-Vi IgG and IgA following Vi-TT vaccination suggests that the conjugate vaccine may offer durable protection, supporting its use in endemic settings.
Despite increasing access to improved water sources globally, typhoid fever continues to be prevalent in low and lower middle income countries (LMIC), with an estimated 9 to 13 million cases and 145,000 to 161,000 deaths annually (1). India and South-East Asia suffer the highest infection rates, and there are frequent epidemics in sub-Saharan Africa, with severity of illness and mortality highest in early childhood (2–4).
Efforts to control and prevent typhoid fever have generally included a combination of public health measures, health education and vaccination programmes. Although antibiotics reduce the fatality rate of patients with an acute infection to around 1%, they have little impact on disease incidence, and improper use of antibiotics can drive the emergence of antibiotic resistant strains (5, 6). Vaccination offers a prophylactic option that may be quicker and more cost effective to implement in the short term than infrastructure improvements. Vaccination against S. Typhi can provide the population with herd immunity, helping to protect those who are vulnerable to disease or cannot safely receive vaccines such as immunocompromised individuals (7, 8). Furthermore, evidence from human typhoid challenge studies suggests that an effective vaccination schedule may reduce onward transmission of S. Typhi by reducing bacterial shedding in stool (9).
Polysaccharide vaccines elicit an antibody response independently of T cells. In the absence of memory B cell formation further doses of vaccine are recommended every 2-3 years (10, 11). Furthermore, polysaccharide vaccines are non-immunogenic in infants; as the ability to respond to polysaccharide antigens develops alongside the maturation of the splenic marginal zone at around 18 to 24 months of age.
Conjugate vaccines utilise a protein carrier to enhance the response to polysaccharide antigen by inducing T cell help and germinal centre formation which results in memory B cell production. Conjugate vaccines have been demonstrated to be efficacious in preventing numerous infections caused by encapsulated pathogens including Haemophilus influenzae type b, pneumococcal infection and meningococcal disease (12–14). Immunogenicity has been demonstrated in infants for whom vaccines were previously unavailable, and higher antibody responses have been elicited in older recipients (15–18). A novel typhoid tetanus toxoid vaccine (Vi-TT) was shown to be efficacious in adults in an established controlled human infection model (CHIM), as well as demonstrating a protective efficacy of 81.6% after one year in children aged 9 months to 16 years in Nepal, in a phase three randomised controlled trial (19, 20). There is mounting evidence that typhoid conjugate vaccines (TCV) are safe, efficacious and highly immunogenic Several TCVs are now prequalified by the WHO and are being rolled out in in endemic countries, however data on persistence of antibodies and duration of protection is unknown (21, 22).
The Vaccines Against Salmonella Typhi (VAST) trial was initiated in 2015 to evaluate the protective efficacies of a typhoid conjugate vaccine (Vi-TT) and the licensed capsular polysaccharide vaccine (Vi-PS) using a CHIM. The 2017 publication of this study reported a protective efficacy for Vi-TT of 55%. Of note, higher Vi IgG titres were elicited in the Vi-TT group at one month after vaccination when compared with Vi-PS vaccinees (19). An extension to the main study, in which participants primed with Vi-TT were later boosted with Vi-PS, was added as part of a project developed to produce high affinity Vi-specific monoclonal antibodies for use as research tools. Here we describe the persistence of Vi antibodies after a single dose of Vi-TT or Vi-PS vaccine, and assess the effect of polysaccharide booster vaccination on antibody titre, antibody secreting cell (ASC) response and memory B cell production in participants primed with Vi conjugate vaccine.
Samples were obtained from volunteers participating in an observer and participant-masked, randomised, controlled, phase 2b clinical trial, evaluating protection from typhoid infection following Vi-TT vaccination using a S. Typhi CHIM (group A) (19). Volunteers in group A were invited to participate in a booster study, along with volunteers from a separate cohort randomised to receive Vi-PS or Vi-TT without subsequent oral challenge as part of a study to develop a Vi IgG serum standard (group B) (23). Five participants were recruited from both group A and group B to form the booster study participants (group C). Details of these groups are presented in Table 1 and Figure 1.
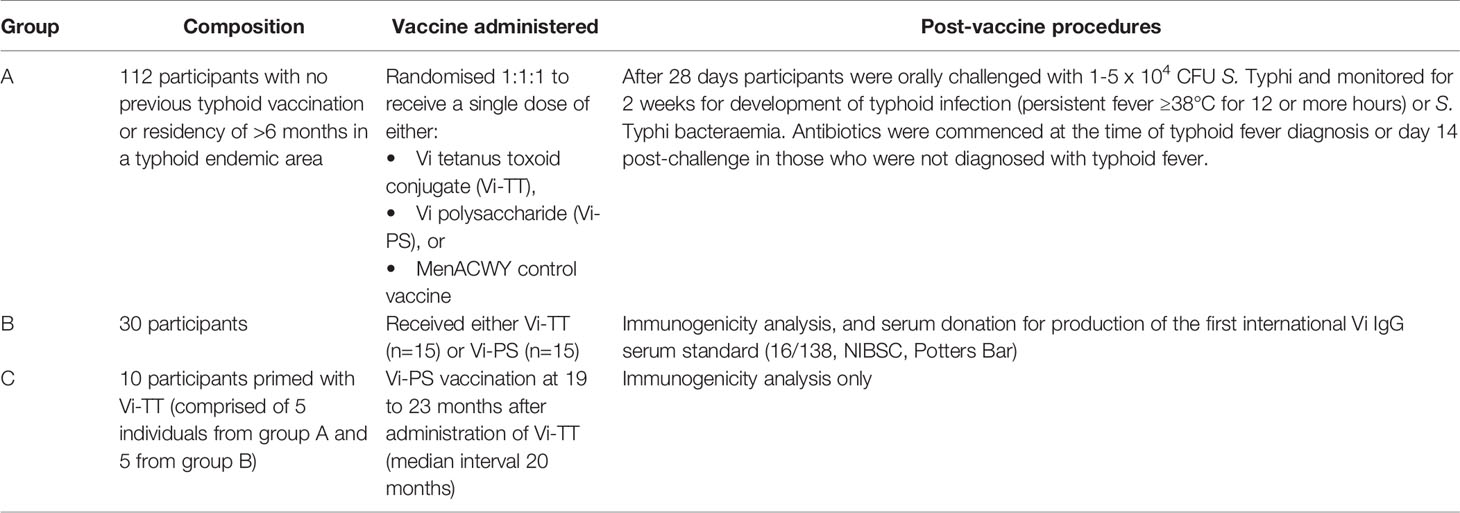
Table 1 Participant groups in the Vaccines Against Salmonella Typhi trial from which samples were taken for analysis in this study.
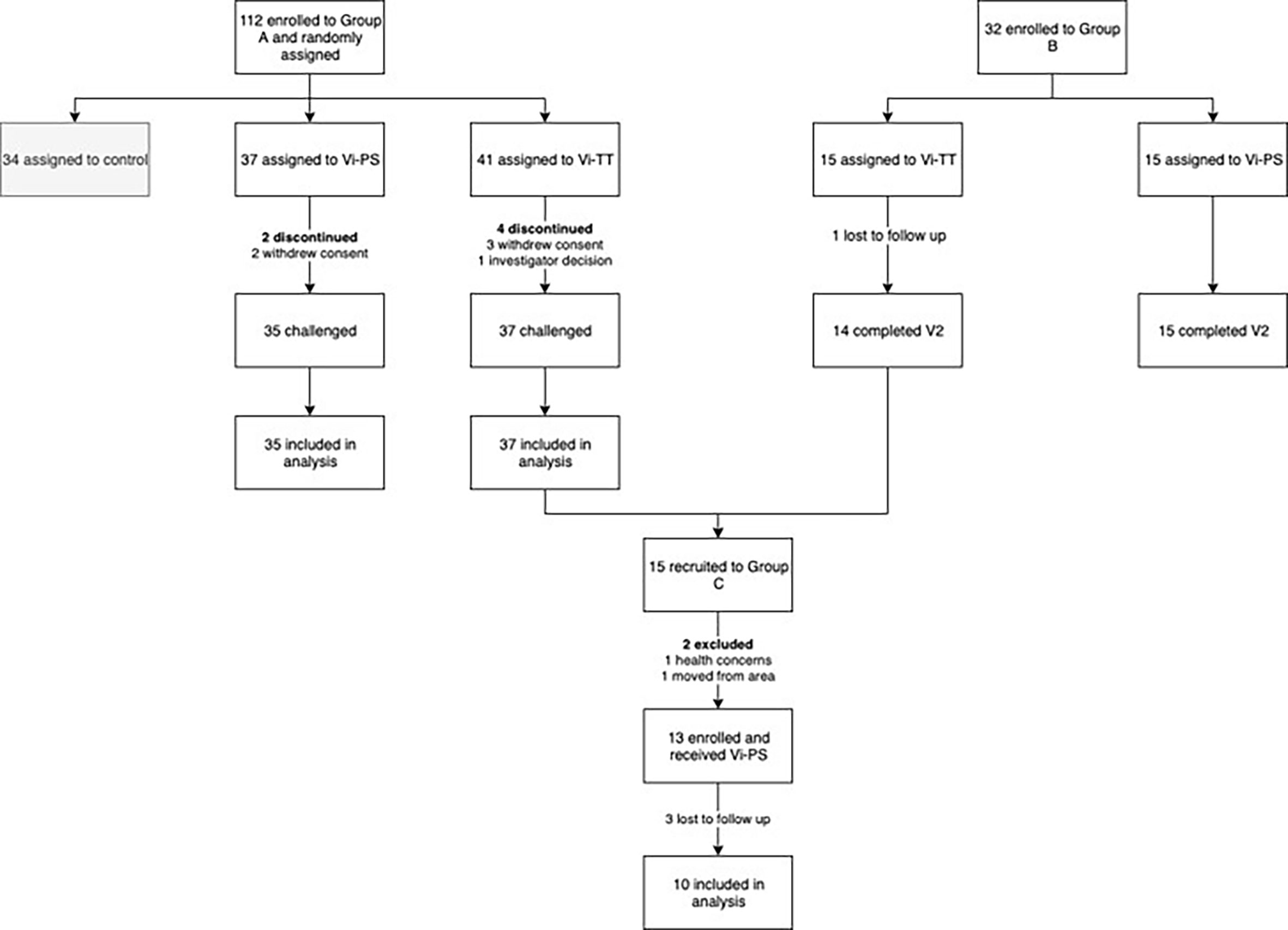
Figure 1 Trial profile. Vi-TT, Vi-tetanus toxoid conjugate vaccine; Vi-PS, Vi-polysaccharide vaccine; V2, study visit 4 - 6 weeks post-vaccination.
Group C participants received a Vi-PS booster vaccination (Typhim Vi™, Sanofi Pasteur).
Serum Vi-specific IgG quantification was performed using a commercial enzyme-linked immunosorbent assay (ELISA) kit (VaccZyme™ Human Anti Salmonella Typhi Vi IgG Enzyme Immunoassay Kit, Binding Site, Birmingham UK), as per the manufacturer’s instructions. Values below the lower limit of assay sensitivity (7.4 EU/ml) were assigned a value of 3.7 EU/ml. For the quantification of serum Vi IgA antibodies the kit was modified to include a sheep anti-human IgA secondary antibody (Binding Site, Birmingham UK) prepared 1:12,000 in 1 x phosphate buffered saline, 10% foetal bovine serum. Values below the lower limit of assay sensitivity (3.125 EU/ml) were assigned a value of 1.56 EU/ml.
Antibodies were quantified in serum samples collected 13 months post primary vaccination for group A only [data for group A PV, 1, 2, 4 and 7 months are published previously (24)], and for all participants recruited into group C prior to boost vaccination (PV Booster), seven days post-boost and 1 month post-boost.
Ex vivo enzyme linked immunosorbent spot (ELISpot) assays were used to detect Vi-specific antibody secreting cells (ASC) for participants in group C prior to booster vaccination (PV Booster), and at 7 and 28 days post-boost. Peripheral Blood Mononuclear Cells (PBMCs) were isolated from fresh whole blood within 6 hours of collection, 2.5 x 105 cells/well were seeded on to ELISpot plates (MultiScreen-HA Filter Plates, Millipore, MAHAS510) pre-coated with Vi antigen (12/244, NIBSC) at 10µg/mL. Cells were incubated overnight (16-24 hours) at 37°C and 5% CO2 before development with alkaline phosphatase conjugated secondary antibodies (goat anti-human IgG, and IgM or IgA, Calbiochem) and a substrate development kit (Bio-rad laboratories ltd). Wells pre-coated with pan goat anti-human immunoglobulin, or PBS were used as positive and negative controls, respectively. The results are expressed as ASCs per million PBMCs, the range of the assay was 3 – 141 Vi specific ASCs per million PBMCs.
ELISpot was used to detect Vi specific memory B cells from cultured stimulated PBMCs for participants in group C at pre vaccination, and at 7 and 28 days post vaccination. Briefly, frozen PBMCs were thawed and stimulated for 5 days with an antigen cocktail containing Staphylococcus aureus Cowans Strain – Pansorbin cells (1:5000, VWR International Ltd), CpG-ODN 2006 (1.7 µg/mL, Source BioScience UK), and Pokeweed Mitogen (83.33ng/mL, Sigma). After 5 days the cells were harvested, washed and counted. The ELISpot assay was then performed as described above. Some wells were also pre-coated with pan goat anti-human immunoglobulin or PBS as positive and negative controls. All samples included in the analysis had positive IgG producing plasmablasts after memory cells culture (10/10).
The data were analysed using GraphPad Prism 8.0.0. Mann-Whitney U tests were used to compare time points between vaccine arms, with a one-way ANOVA Kruskal-Wallis test for multiple comparisons. A Wilcoxon test was used to compare time points within vaccine arms, with a Friedman test adjusting for multiple comparisons. Comparisons between vaccines arms were treated as unpaired, whilst longitudinal comparisons were treated as paired. A p-value of <0.05 was considered to be statistically significant.
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Vi IgG and IgA titres remained significantly elevated above pre-vaccination levels in both group A vaccine arms 13 months after prime, and in the group A Vi-TT recipients 19 – 23 months after prime (median 20 months, n=5) (Vi-PS not measured) (Figure 2). A significant reduction in anti-Vi IgA titre was observed in both vaccine arms, and for anti-Vi IgG in Vi-TT vaccine recipients, between one month post-prime and 13 months post-prime (Figure 2). 90% (26/29) of participants vaccinated with Vi-TT and 85% (23/27) of participants vaccinated with Vi-PS retained an IgG titre at least four times that of the PV titre 13 months after vaccination. 88% (23/26) of participants vaccinated with Vi-TT and 77% (20/26) of participants vaccinated with Vi-PS retained an IgA titre at least four times that of the PV titre 13 months after vaccination. In all five Vi-TT recipients in group A subsequently recruited into group C, anti-Vi IgG and IgA titres measured at 20 months post-prime demonstrated antibody titres four-fold greater than baseline. Participants from group B were not included in the antibody persistence analysis as some volunteers in this group had previously received typhoid vaccines outside of the trial setting.
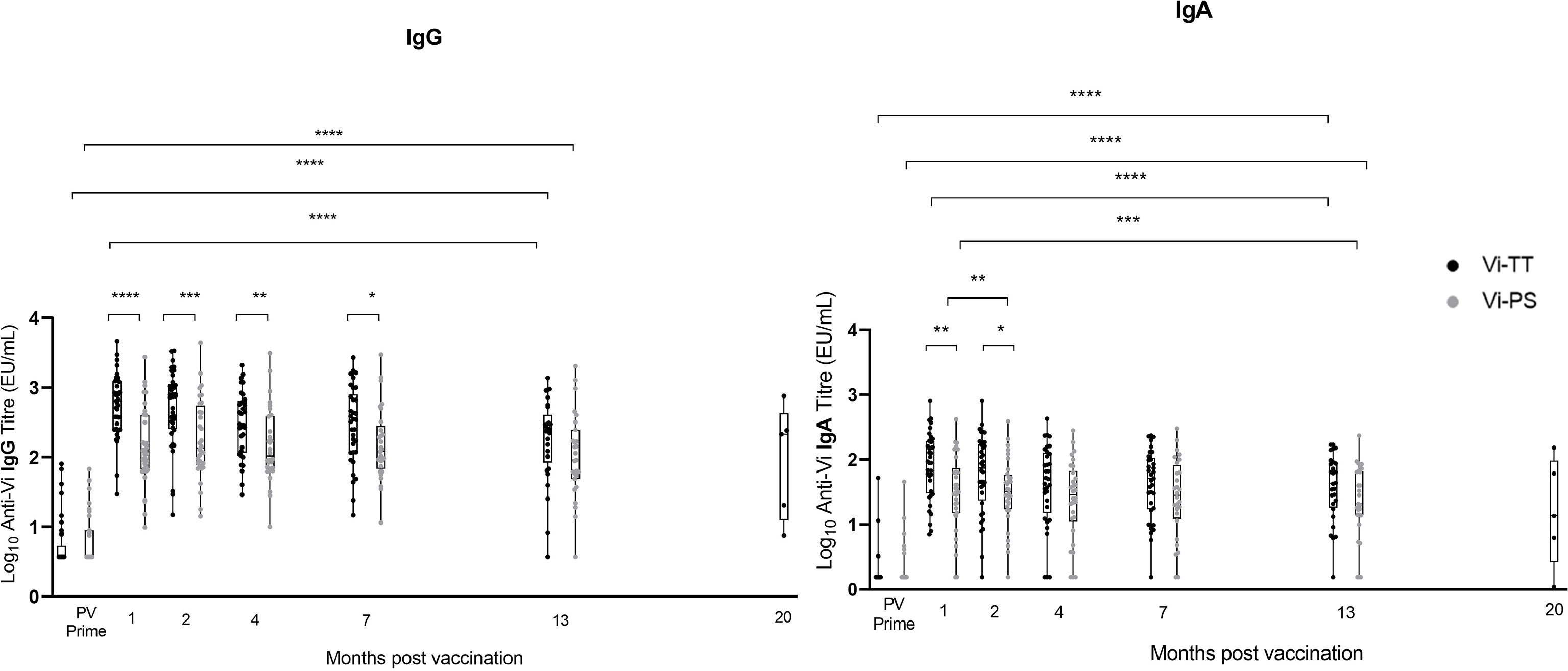
Figure 2 Vi IgG and Vi IgA antibody produced in response to Vi-TT vaccination is significantly elevated over baseline levels beyond 1 year. Vi antibody titres were determined in naïve participants (group A) by ELISA at 13 months post-vaccination (n = 72), and at time points between 19 and 23 months post-vaccination in group C participants (n=5) (shown plotted at the median of 20 months to aid visualisation). To provide context for these data, Vi antibody titres measured prior to vaccination (PV) and at 1, 2, 4, and 7 months post-vaccination are also plotted [published previously (24)]. Comparisons between vaccine arms Vi-PS (grey) and Vi-TT (black), and between time points were performed. Adjusted p-values are indicated. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001. Vertical lines demarcate time points.
Ten participants primed with Vi-TT were boosted with Vi-PS. Demographic information for participants who received a booster vaccination is presented in Supplementary Table 1. Five were from the group A challenge study (three diagnosed with typhoid within 14 days of receiving challenge, and two non-diagnosed), and five from the group B non-challenged cohort. In recipients of a Vi-TT primary vaccine, booster vaccination with Vi-PS induced a significant increase in both anti-Vi IgG and IgA antibody titre at one month (Figure 3).
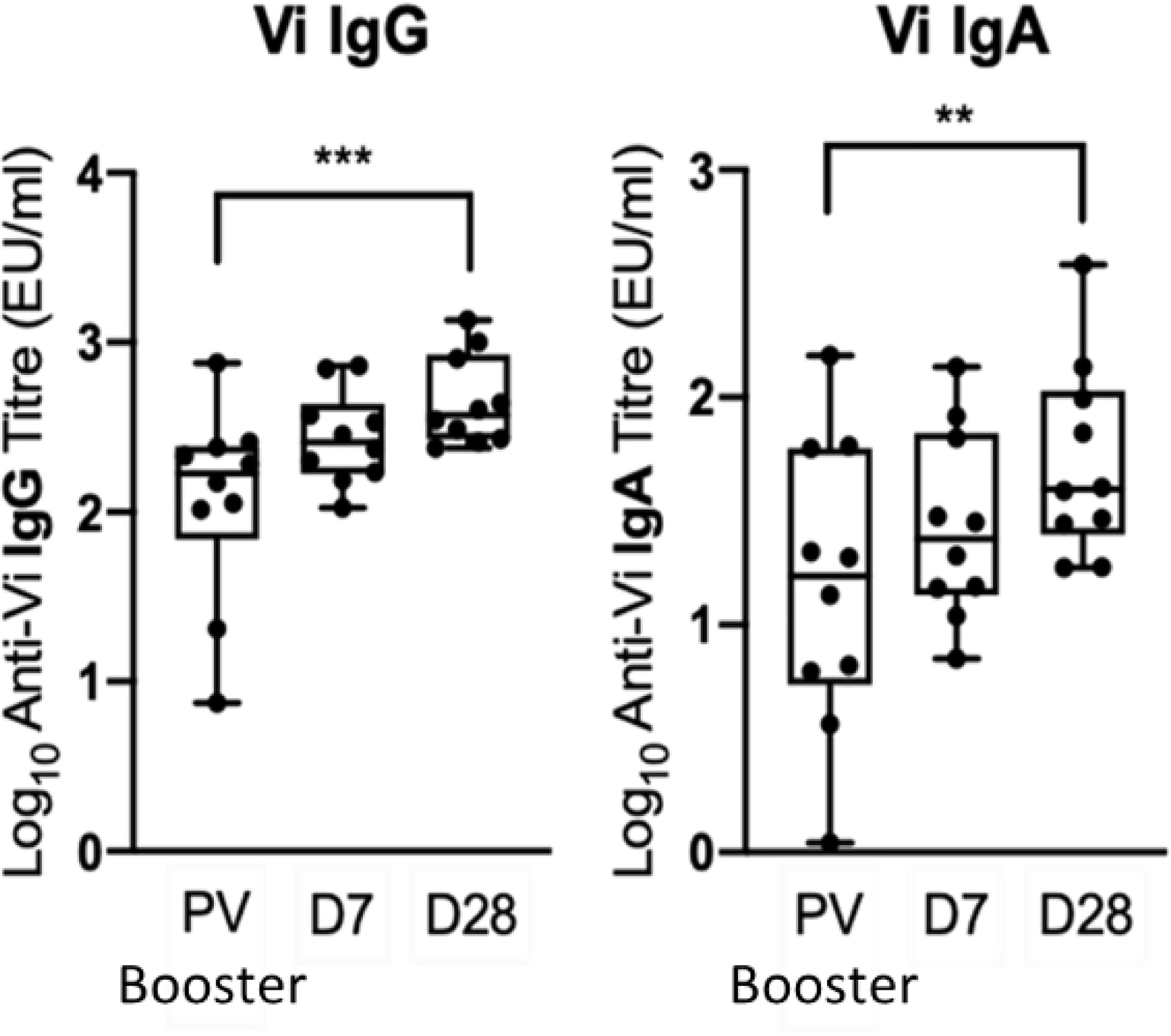
Figure 3 A booster Vi-PS vaccination in participants primed with Vi-TT induced a significant increase in Vi IgG and Vi IgA antibody titre by D28. Vi antibody titres were determined by ELISA prior to Vi-PS vaccination (PV) in individuals primed with Vi-TT, and at 7 and 28-days post-boost, and comparison of titres between time points was performed (n= 10). Adjusted p-values are indicated. **p ≤ 0.01; ***p ≤ 0.001.
Anti-Vi IgG titres were comparable at one month post Vi-TT prime and one month post Vi-PS boost (Figure 4). Anti-Vi IgG titres prior to boost were significantly higher than pre-prime titres, as such the Vi-PS booster vaccination generated a smaller fold rise above pre-boost titres after one month as compared with primary vaccine response. Correlation of pre-boost anti-Vi IgG and fold change in anti-Vi IgG (pre-boost to day 28 post-boost) demonstrated a strong negative relationship (Spearman’s correlation coefficient = -0.90, p = 0.0008) (Figure 5). A negative relationship between titre prior to boost and the fold change in titre generated by boost was also observed for anti-Vi IgA (Spearman’s correlation coefficient = -0.61, p = 0.064).
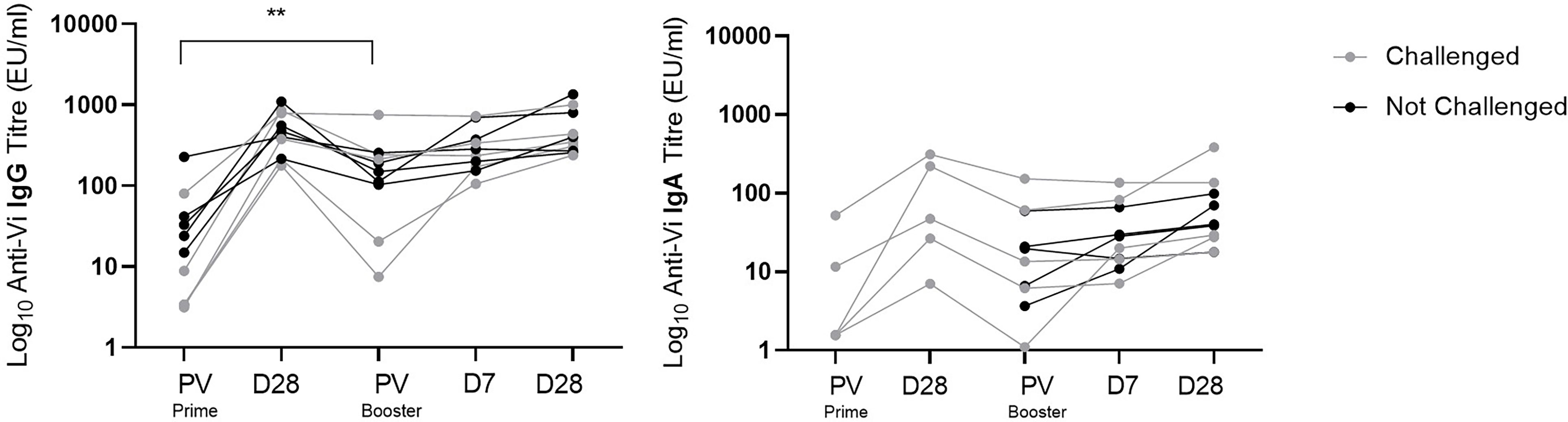
Figure 4 Vi specific IgG and IgA antibody response to Vi-TT conjugate prime and Vi-PS booster vaccination. Vi antibody titres were measured with ELISA, and comparison of titres between time points was performed. Interval between D28 post-prime and PV Booster ranged from 19 to 23 months. Adjusted p-values are indicated. **p ≤ 0.01. PV, pre-vaccination.
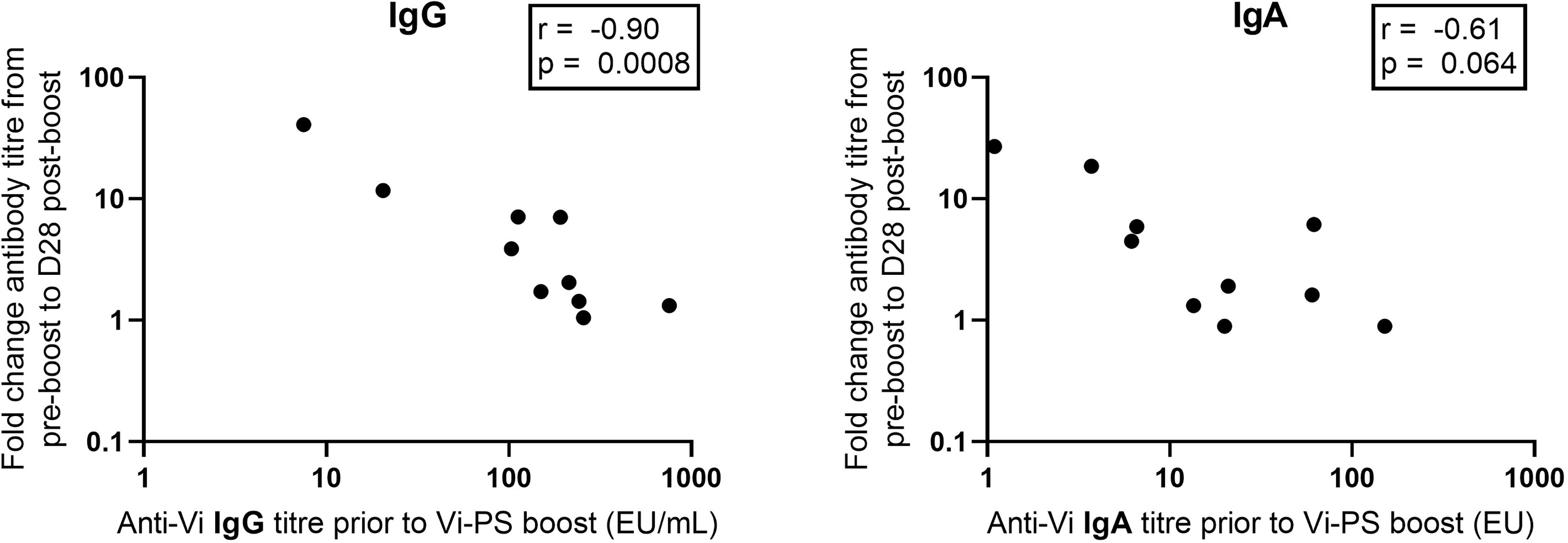
Figure 5 Vi specific IgG and IgA pre-boost antibody titre versus fold change between pre-boost and D28 post-boost anti-Vi IgG and IgA antibody titre. Vi antibody titres were assayed with ELISA.
To assess the effect of exposure to S. Typhi on anti-Vi IgG titres in participants immunised with Vi-TT, the decay in antibody at one and 20 months post primary Vi-TT vaccination was compared between individuals who underwent S. Typhi challenge (n=5), and those who were not exposed (n=5). A Mann-Whitney test comparing fold change between these two groups showed no significant difference (p=0.42), suggesting that a single oral exposure to S. Typhi has no effect on the persistence of serum anti-Vi IgG.
Vi-PS booster vaccination induced a significant increase in the frequency of Vi IgA ASCs between pre-boost (median = 3.0 ASCs/106 PBMCs) and 7 days post-boost (median = 7.7 ASCs/106 PBMCs), while no significant change was observed for ASCs producing anti-Vi IgG or IgM (Figure 6). In contrast, the initial Vi-TT vaccination induced significant increases in both Vi specific IgG and IgM ASCs (25). No data were collected on the anti-Vi IgA ASC response to primary conjugate vaccination.

Figure 6 Vi-PS booster vaccination induces an anti-Vi IgA ASC response but no change in anti-Vi IgG or IgM ASCs. Peripheral ASC numbers were determined by ex vivo ELISpot, and comparison of titres between time points was performed *p ≤ 0.05; **p ≤ 0.01. PV, pre-vaccination. Prime data collected from group A participants who were also enrolled in group C, n = 5. Boost data collected from all group C participants, n = 10.
No significant change in the frequencies of Vi-specific IgG or IgA memory B cells were observed seven and 28 days after Vi-PS booster vaccination (Figure 7).
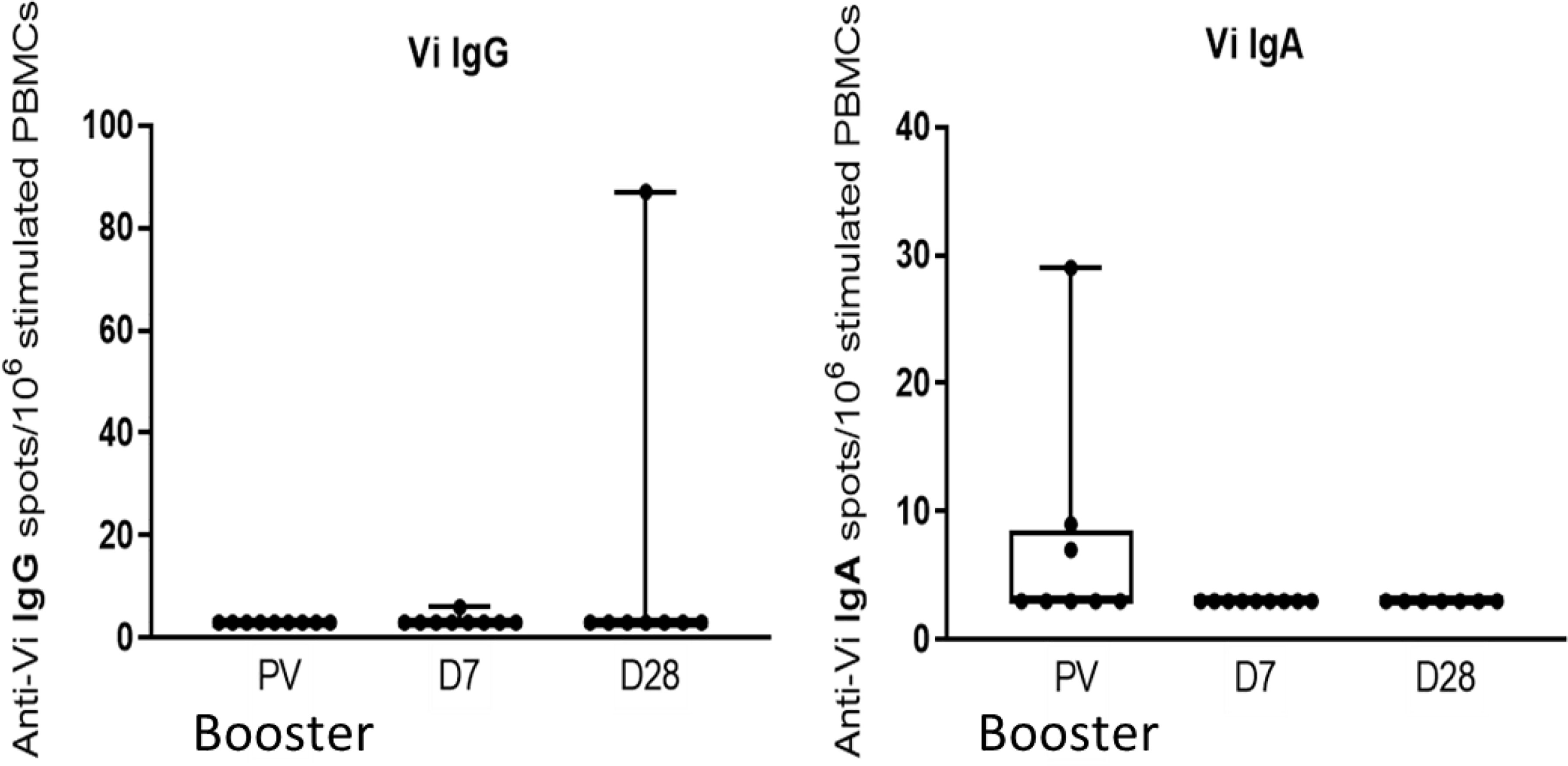
Figure 7 Vi-PS booster vaccination does not induce a significant increase in circulating IgG or IgA Vi-specific memory cells. Activated memory cells were assayed by memory ELISpot, and comparison between vaccine arms was performed. Adjusted p-values are indicated. PV, pre-vaccination.
In this study we describe the persistence of the Vi antibody response to plain Vi polysaccharide and Vi tetanus toxoid conjugate vaccines, showing a similar kinetic profile with a significant decline in both Vi IgG and IgA titres between one and 13 months after Vi-TT vaccination. In addition, we show that individuals primed with Vi-TT and boosted with Vi-PS at 19-23 months, have an increase in Vi antibody titre, reaching similar levels as those measured one month post-prime. The response to the Vi-PS boost is characterised by a significant increase in circulating Vi-specific IgA ASCs, but no rise in IgM or IgG ASCs nor Vi-specific memory B cells.
Significant waning of both anti-Vi IgG and IgA from measured peak levels was observed sooner in the Vi-TT group than amongst those primed with Vi-PS, perhaps as a consequence of the high titres induced with Vi-TT vaccination. While Vi-TT generated higher anti-Vi IgG and IgA responses one month after vaccination, at 13 months post-vaccination, anti-Vi IgG levels were comparable between Vi-TT and Vi-PS recipients while anti-Vi IgA titres were comparable at four months for both vaccine groups and remaining so at 13 months. Thirteen months after primary vaccination, anti-Vi IgG and IgA titres remained elevated above baseline (both Vi-TT and Vi-PS), and with the elevation above baseline retained at 20 months in the small cohort of Vi-TT recipients (n=5) sampled at this late time point. These data suggest that Vi-TT induces Vi antibody responses that persistent for up to at least 20 months, comparable to the Vi-PS vaccine. Although persistence of higher antibody levels does not necessarily translate to direct protection from disease, recent analysis of Vi-specific serological correlates of protection against typhoid underline the importance of anti-Vi IgG antibody in preventing disease after exposure as well as highlighting a lesser described significance of anti-Vi IgA (24, 26).
The rise in antibody titre one month post Vi-TT prime was similar to that seen in response to the Vi-rEPA and Vi-CRM conjugate vaccines (15, 16, 27, 28). At 19 – 23 months (median interval of 20 months) following Vi-TT vaccination the GMT of anti-Vi IgG was 65.7 EU/ml (n= 5), similar to the GMT of 82.2 EU/ml reported by Mohan et al. using the same assay at 720 days (23.7 months) post-Vi-TT vaccination in an Indian population (17). Multiple episodes of natural exposure or subclinical infection in the Indian population both prior to and following vaccination might explain the slightly higher titre observed compared with our cohort of UK adults. We have seen no significant boosting of titres after single exposure to S. Typhi in the context of our challenge model, the higher level of persisting antibodies in endemic populations might be due to various factors such as exposure to other strains with higher Vi expression, multiple exposures, or other differences in the host or environment.
Demonstrating medium-term maintenance of immunological features which correlate with protection against typhoid, our data contribute to the mounting evidence supporting the use of Vi conjugate vaccines. Subsequently, this Vi-TT vaccine has been administered to over 10,000 children aged between 9 months and 16 years in Nepal, a typhoid endemic country, where it has demonstrated an efficacy of 81.6% after 1 year (20). Pakistan was the first country to introduce Vi-TT into its routine immunization programme and here more than 10 million doses have been administered to combat the outbreak of extensively drug resistant (XDR) typhoid in the Sindh province (21). While Vi antibody titre data can be used to guide expectations of duration of protection afforded by vaccination, there is currently no definitive protective threshold. It is important to look at other immune parameters such as antibody effector functions and cellular responses to understand the mechanism and duration of protection afforded by Vi vaccination, in the most comprehensive analysis of features which protect following Vi-TT to date, measures of IgA quantity and binding were found to be the most strongly associated with protection (24).
Conjugate vaccines hold promise for improving protection in view of their increased immunogenicity in infants and because they are T-dependent antigens. Furthermore, greater initial B-cell responses have been shown to be associated with greater antibody persistence at one year of age suggesting that the magnitude of initial germinal centre reaction is an important long-term determinant of protection (29).
Of note, the majority of participants in this study were exposed to S. Typhi one month after vaccination (‘challenge’). Comparison of longitudinal antibody profiles from these individuals with a small set of vaccinated but unchallenged participants suggests exposure to S. Typhi in this context does not significantly boost Vi antibody titres. The effect of repeated exposures, more similar to real-life settings where Vi seropositivity is related to levels of typhoid transmission is unknown (30).
The Vi-PS booster in previously Vi-TT-vaccinated volunteers induced a significant increase in antibody titre within 1 month of vaccination (both IgG and IgA). Analysis of changes at the level of the individual indicate the magnitude of the increase in antibody levels was inversely associated with circulating antibody level at the time of the booster. This relationship might reflect a negative feedback mechanism, potentially in place to prevent aberrant responses. This is consistent with clinical trials of a diphtheria-conjugated Vi typhoid vaccine, which showed that an additional conjugate vaccine booster 6 weeks after an initial vaccination did not increase antibody titres above those observed post-prime, and another in which revaccination again had no incremental effect on Vi antibody titre in relation to post-prime (27, 31). In contrast a study in which two to five year olds received Vi-rEPA noted a ten-fold rise in Vi IgG in response to revaccination four weeks after the primary dose (15). The differences noted in the response to a second dose of a conjugate vaccine may relate to differences in the products, including the amount of unconjugated polysaccharide in the vaccine and the carrier protein. Repeated doses of Vi-PS vaccines; may induce hyporesponsiveness, but several studies demonstrate that a second dose of Vi-PS given at appropriate intervals gives rise to antibody levels at least as high as those following the prime, and the WHO recommend revaccination every 3 years (17, 32, 33). Here we show that boosting with a Vi-polysaccharide after a Vi-conjugate prime can successfully return Vi IgG levels to those present after the primary vaccine, but no evidence for an enhanced response to the second dose. These findings may imply that natural exposure in endemic settings could also boost immunity after administration of conjugate vaccines and potentially sustain protection in populations. Boosting of antibody levels may not be enough to directly confer protection, since antibody dependent effector functions have been shown to more closely correlate with protection rather than titre alone, as demonstrated by Jin et al (24). Assessment of antibody effector functions after boosting was beyond the scope of this project.
Previous studies have shown that polysaccharide-conjugate vaccines induce long lived antigen specific memory B cells (34). We have previously shown that Vi-TT vaccination induces Vi specific memory B cells which are significantly elevated above baseline 28 days after vaccination, while Vi-PS showed no significant change in memory B cell formation (25). Our data add to this and suggest that Vi specific memory B cells are not detectable in peripheral blood 19-23 months after Vi-TT vaccination. Increases in Vi antibodies following booster vaccination with Vi-PS are not accompanied by an expansion in Vi specific memory B cells detectable in peripheral blood, while boosting with Vi-TT was not explored within this study. Administration of a pneumococcal polysaccharide vaccine boost following a conjugate vaccine prime was observed to give rise to a decrease in capsular polysaccharide-specific memory B cells, in keeping with the hypothesis that polysaccharide antigens drive pre-existing switched memory B cells into terminal differentiation and are unable to replenish the pool of memory B cells (35). While one possibility for the failure to detect memory B cells in response to the Vi-PS boost is that the circulating Vi specific memory B cells are low frequency or that the activated memory B cells may home to bone marrow and are therefore they are not detected in the peripheral blood. It is also possible that the Vi-PS boost may negatively impact Vi-specific memory B cells, and hence potentially antibody responses to subsequent Vi exposure. We were unable to compare boosting with Vi-PS with Vi-TT as this was not studied - the project was specifically designed to generate Vi specific ASC for the production of monoclonal antibodies after a Vi-PS boost. Boosting with Vi-TT would also induce expansion of tetanus toxoid ASCs making it less suitable for Vi specific ASC isolation. However, exploring boosting with Vi-TT to restore Vi antibody levels and memory B cells should be considered; studies of memory B cell responses to pneumococcal conjugate vaccines set a strong precedent for this (36, 37).
Limitations of our work include the relatively short period of study; later time points are much needed to inform on longer term persistence of antibodies; and the small number of individuals who received a Vi-PS booster vaccination, the original aim of revaccination being to stimulate responses for a study to generate Vi monoclonal antibodies. Data from phase III trials of Vi-TT over a longer period are expected imminently and will address the first of these shortcomings.
In conclusion, we present data demonstrating that typhoid conjugate vaccination gives rise to initially higher Vi antibody titres compared with Vi-PS vaccination, with antibodies persisting at similar levels to those in response to Vi-PS at the end of the first year of vaccination. This study provides further evidence to support the use of conjugate vaccines in endemic areas, to both reduce the burden of typhoid fever and to combat growing antibiotic resistance. Future studies should explore the merits of different strategies to boost Vi responses following Vi conjugate vaccination.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by The South Central Oxford A Ethics Committee (14/SC/1427). The patients/participants provided their written informed consent to participate in this study.
AP, JH, CJ, and EJ conceived and designed the work. CJ, JH, EJ, MM, JG, and JP carried out the clinical trial. EJ, TB, and CJ acquired data for the work. EJ, TB, and JH analysed the work. TB, EJ, JH, CJ, and AP interpreted data for the work. TB, EJ, JH, CJ, and AP wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by The Bill & Melinda Gates Foundation (OPP1084259) and the European Commission FP7 grant “Advanced Immunization Technologies” (ADITEC).
The views expressed in this article do not necessarily represent the views of the UK Department of Health and Social Care, Joint Committee on Vaccination and Immunisation, or World Health Organization.
AP is Chair of the UK Department of Health and Social Care’s Joint Committee on Vaccination and Immunisation; and is a member of the World Health Organization’s Strategic Advisory Group of Experts. The University of Oxford has entered into a partnership with AstraZeneca on coronavirus vaccine development.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fitd.2021.709745/full#supplementary-material
1. Srinivasan M, Sindhu KN, John J, Kang G. Opportunities for Typhoid Vaccination in India. Indian Pediatr (2019) 56:453–8. doi: 10.1007/s13312-019-1566-7
2. Buckle GC, Walker CLF, Black RE. Typhoid Fever and Paratyphoid Fever: Systematic Review to Estimate Global Morbidity and Mortality for 2010. J Glob Health (2012) 2:1–9. doi: 10.7189/jogh.01.010401
3. Kim JH, Mogasale V, Im J, Ramani E, Marks F. Updated Estimates of Typhoid Fever Burden in Sub-Saharan Africa. Lancet Global Health (2017) 5:e969. doi: 10.1016/S2214-109X(17)30328-5
4. Vollaard AM, Ali S, Van Asten HAGH, Widjaja S, Visser LG, Surjadi C, et al. Risk Factors for Typhoid and Paratyphoid Fever in Jakarta, Indonesia. J Am Med Assoc (2004) 291:2607–15. doi: 10.1001/jama.291.21.2607
5. Crump JA, Luby SP, Mintz ED. The Global Burden of Typhoid Fever. Bull World Health Organ. Bull World Health Organ (2004) 82:346–53. doi: 10.1590/S0042-96862004000500008
6. Kariuki S, Gordon MA, Feasey N, Parry CM. Antimicrobial Resistance and Management of Invasive Salmonella Disease. Vaccine (2015) 33:C21–9. doi: 10.1016/j.vaccine.2015.03.102
7. Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, et al. Population-Based Incidence of Typhoid Fever in an Urban Informal Settlement and a Rural Area in Kenya: Implications for Typhoid Vaccine Use in Africa. PloS One (2012) 7:e29119. doi: 10.1371/journal.pone.0029119
8. Bodhidatta L, Taylor DN, Thisyakom U, Echeverria P. Control of Typhoid Fever in Bangkok, Thailand, by Annual Immunization of Schoolchildren With Parenteral Typhoid Vaccine. Clin Infect Dis (1987) 9:841–5. doi: 10.1093/clinids/9.4.841
9. Gibani MM, Voysey M, Jin C, Jones C, Thomaides-Brears H, Jones E, et al. The Impact of Vaccination and Prior Exposure on Stool Shedding of Salmonella Typhi and Salmonella Paratyphi in 6 Controlled Human Infection Studies. Clin Infect Dis (2019) 68:1265–73. doi: 10.1093/cid/ciy670
10. Lindberg AA. Glycoprotein Conjugate Vaccines. Vaccine (1999) 17:S28–36. doi: 10.1016/S0264-410X(99)00232-7
11. Froeschle JE, Decker MD. Duration of Vi Antibodies in Participants Vaccinated With Typhim Vi (Typhoid Vi Polysaccharide Vaccine) in an Area Not Endemic for Typhoid Fever. Vaccine (2010) 28:1451–3. doi: 10.1016/j.vaccine.2009.11.051
12. Morris SK, Moss WJ, Halsey N. Haemophilus Influenzae Type B Conjugate Vaccine Use and Effectiveness. Lancet Infect Dis (2008) 8:435–43. doi: 10.1016/S1473-3099(08)70152-X
13. Aliberti S, Mantero M, Mirsaeidi M, Blasi F. The Role of Vaccination in Preventing Pneumococcal Disease in Adults. Clin Microbiol Infect (2014) 20:52–8. doi: 10.1111/1469-0691.12518
14. Papaevangelou V, Spyridis N. MenACWY-TT Vaccine for Active Immunization Against Invasive Meningococcal Disease. Expert Rev Vaccines (2012) 11:523–37. doi: 10.1586/erv.12.32
15. Lin FYC, Ho VA, Khiem HB, Trach DD, Bay P, Kossaczka TC, et al. The Efficacy of a Salmonella Typhi Vi Conjugate Vaccine in Two-To-Five-Year-Old Children. N Engl J Med (2001) 344:1263–9. doi: 10.1056/NEJM200104263441701
16. Thiem VD, Lin FYC, Canh DG, Son NH, Anh DD, Mao ND, et al. The Vi Conjugate Typhoid Vaccine is Safe, Elicits Protective Levels of IgG Anti-Vi, and is Compatible With Routine Infant Vaccines. Clin Vaccine Immunol (2011) 18:730–5. doi: 10.1128/CVI.00532-10
17. Mohan VK, Varanasi V, Singh A, Pasetti MF, Levine MM, Venkatesan R, et al. Safety and Immunogenicity of a Vi Polysaccharide-Tetanus Toxoid Conjugate Vaccine (Typbar-TCV) in Healthy Infants, Children, and Adults in Typhoid Endemic Areas: A Multicenter, 2-Cohort, Open-Label, Double-Blind, Randomized Controlled Phase 3 Study. Clin Infect Dis (2015) 61:393–402. doi: 10.1093/cid/civ295
18. Capeding MR, Teshome S, Saluja T, Syed KA, Kim DR, Park JY, et al. Safety and Immunogenicity of a Vi-DT Typhoid Conjugate Vaccine: Phase I Trial in Healthy Filipino Adults and Children. Vaccine (2018) 36:3794–801. doi: 10.1016/j.vaccine.2018.05.038
19. Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, et al. Efficacy and Immunogenicity of a Vi-Tetanus Toxoid Conjugate Vaccine in the Prevention of Typhoid Fever Using a Controlled Human Infection Model of Salmonella Typhi: A Randomised Controlled, Phase 2b Trial. Lancet (2017) 390:2472–80. doi: 10.1016/S0140-6736(17)32149-9
20. Shakya M, Colin-Jones R, Theiss-Nyland K, Voysey M, Pant D, Smith N, et al. Phase 3 Efficacy Analysis of a Typhoid Conjugate Vaccine Trial in Nepal. N Engl J Med (2019) 381:2209–18. doi: 10.1056/NEJMoa1905047
21. World Health Organisation. Pakistan Introduces Typhoid Conjugate Vaccine (TCV) Into Routine Immunization Schedule in World First (2019). Available at: http://www.emro.who.int/pak/pakistan-news/pakistan-introduces-typhoid-conjugate-vaccine-tcv-into-routine-immunization-schedule-in-world-first.html#:~:text=Pakistan-,Pakistan%20introduces%20typhoid%20conjugate%20vaccine%20(TCV)%20into%20routine%20immunizat.
22. Andrews JR, Baker S, Marks F, Alsan M, Garrett D, Gellin BG, et al. Typhoid Conjugate Vaccines: A New Tool in the Fight Against Antimicrobial Resistance. Lancet Infect Dis (2019) 19:e26–30. doi: 10.1016/S1473-3099(18)30350-5
23. Rijpkema S, Hockley J, Logan A, Rigsby P, Atkinson E, Jin C, et al. Establishment of the First International Standard for Human Anti-Typhoid Capsular Vi Polysaccharide IgG. Biologicals (2018) 56:29–38. doi: 10.1016/j.biologicals.2018.09.001
24. Jin C, Hill J, Gunn BM, Yu WH, Dahora LC, Jones E, et al. Vi-Specific Serological Correlates of Protection for Typhoid Fever. J Exp Med (2021) 218. doi: 10.1084/jem.20201116
25. Cross DL, Verheul MK, Leipold MD, Obermoser G, Jin C, Jones E, et al. Vi-Vaccinations Induce Heterogeneous Plasma Cell Responses That Associate With Protection From Typhoid Fever. Front Immunol (2020) 11. doi: 10.3389/fimmu.2020.574057
26. Dahora LC, Jin C, Spreng RL, Feely F, Mathura R, Seaton KE, et al. IgA and IgG1 Specific to Vi Polysaccharide of Salmonella Typhi Correlate With Protection Status in a Typhoid Fever Controlled Human Infection Model. Front Immunol (2019) 10. doi: 10.3389/fimmu.2019.02582
27. Szu SC, Lin KFY, Hunt S, Chu C, Thinh ND. Phase I Clinical Trial of O-Acetylated Pectin Conjugate, a Plant Polysaccharide Based Typhoid Vaccine. Vaccine (2014) 32:2618–22. doi: 10.1016/j.vaccine.2014.03.023
28. Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, et al. Immunogenicity and Safety of the Vi-CRM197 Conjugate Vaccine Against Typhoid Fever in Adults, Children, and Infants in South and Southeast Asia: Results From Two Randomised, Observer-Blind, Age De-Escalation, Phase 2 Trials. Lancet Infect Dis (2014) 14:119–29. doi: 10.1016/S1473-3099(13)70241-X
29. Rohner GB, Snape MD, Kelly DF, John T, Morant A, Yu L-M, et al. The Magnitude of the Antibody and Memory B Cell Responses During Priming With a Protein-Polysaccharide Conjugate Vaccine in Human Infants Is Associated With the Persistence of Antibody and the Intensity of Booster Response. J Immunol (2008) 180:2165–73. doi: 10.4049/jimmunol.180.4.2165
30. Watson CH, Baker S, Lau CL, Rawalai K, Taufa M, Coriakula J, et al. A Cross-Sectional Seroepidemiological Survey of Typhoid Fever in Fiji. PloS Negl Trop Dis (2017) 11. doi: 10.1371/journal.pntd.0005786
31. Medise BE, Soedjatmiko S, Rengganis I, Gunardi H, Sekartini R, Koesno S, et al. Six-Month Follow Up of a Randomized Clinical Trial-Phase I Study in Indonesian Adults and Children: Safety and Immunogenicity of Salmonella Typhi Polysaccharide-Diphtheria Toxoid (VI-DT) Conjugate Vaccine. PloS One (2019) 14. doi: 10.1371/journal.pone.0211784
32. Pakkanen SH, Kantele JM, Rombo L, Kantele A. Specific and Cross-Reactive Plasmablast Response in Humans After Primary and Secondary Immunization With Vi Capsular Polysaccharide Typhoid Vaccine. Scand J Immunol (2017) 86:207–15. doi: 10.1111/sji.12583
33. Roggelin L, Vinnemeier CD, Fischer-Herr J, Johnson-Weaver BT, Rolling T, Burchard GD, et al. Serological Response Following Re-Vaccination With Salmonella Typhi Vi-Capsular Polysaccharide Vaccines in Healthy Adult Travellers. Vaccine (2015) 33:4141–5. doi: 10.1016/j.vaccine.2015.05.080
34. Kelly DF, Snape MD, Cutterbuck EA, Green S, Snowden C, Diggle L, et al. CRM197-Conjugated Serogroup C Meningococcal Capsular Polysaccharide, But Not the Native Polysaccharide, Induces Persistent Antigen-Specific Memory B Cells. Blood (2006) 108:2642–7. doi: 10.1182/blood-2006-01-009282
35. Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EAL, Diggle L, et al. Pneumococcal Conjugate and Plain Polysaccharide Vaccines Have Divergent Effects on Antigen-Specific B Cells. J Infect Dis (2012) 205:1408–16. doi: 10.1093/infdis/jis212
36. Clutterbuck EA, Salt P, Oh S, Marchant A, Beverley P, Pollard AJ. The Kinetics and Phenotype of the Human B-Cell Response Following Immunization With a Heptavalent Pneumococcal-CRM197 Conjugate Vaccine. Immunology (2006) 119:328–37. doi: 10.1111/j.1365-2567.2006.02436.x
Keywords: typhoid fever, enteric fever, Salmonella typhi (S. typhi), vaccination, humoral immunity, Vi vaccine
Citation: Bentley T, Jones E, Jin C, Moore M, Gardner J, Hill J and Pollard AJ (2021) Persistence of Antibody After a Vi-Tetanus Toxoid Conjugate Vaccine and Effect of Boosting With a Plain Polysaccharide Vaccine on Vi Antibody and Antigen-Specific B Cells. Front. Trop. Dis 2:709745. doi: 10.3389/fitd.2021.709745
Received: 14 May 2021; Accepted: 30 August 2021;
Published: 20 September 2021.
Edited by:
Jean-Louis Excler, International Vaccine Institute, South KoreaReviewed by:
Yun-Chi Chen, Morgan State University, United StatesCopyright © 2021 Bentley, Jones, Jin, Moore, Gardner, Hill and Pollard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth Jones, ZWxpemFiZXRoLmpvbmVzQHBhZWRpYXRyaWNzLm94LmFjLnVr
†These authors have contributed equally to this work
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.