
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Trop. Dis. , 05 August 2021
Sec. Emerging Tropical Diseases
Volume 2 - 2021 | https://doi.org/10.3389/fitd.2021.707865
This article is part of the Research Topic Highlights in Emerging Tropical Diseases 2021/22 View all 5 articles
While molecular assays, such as reverse-transcription polymerase chain reaction (RT-PCR), have been widely used throughout the coronavirus disease 2019 (COVID-19) pandemic, the technique is costly and resource intensive. As a means to reduce costs and increase diagnostic efficiency, pooled testing using RT-PCR has been implemented. However, pooling samples for antigen testing has not been evaluated. Here, we propose a proof-of-concept pooling strategy for antigen testing that would significantly expand SARS-CoV-2 surveillance, especially for low-to-middle income countries, schools, and workplaces. Our laboratory-based testing demonstrates that combining of up to 20 nasal swab specimens per pool can expand surveillance with antigen tests, even if a pool contains only one positive sample.
With ongoing worldwide transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and emergent variants, laboratory testing has played an important role in detecting the virus in symptomatic and asymptomatic individuals (1, 2). Molecular assays, such as reverse-transcription polymerase chain reaction (RT-PCR), are accurate but costly and resource and supply chain intensive, challenging large scale surveillance efforts, especially for low-to-middle income countries (3). The financial and logistics barriers associated with RT-PCR restricts the ability to rapidly identify and quarantine infected individuals and hinders our ability to estimate community prevalence and epidemic trajectory (4). These limitations underscore the need for novel and dynamic approaches to individual diagnostics, community surveillance, and epidemic control.
Pooling subsamples and processing them in groups by RT-PCR has been proposed as a testing strategy to reduce costs and increase efficiency (5). If a pool tests negative by RT-PCR, then all of the constituent samples are assumed negative. If a pool tests positive by RT-PCR, then the constituent samples are putatively positive and must be re-tested individually or in smaller pools. A recent study demonstrated that a range of positive pools could be identified, even if the Ct value of a single sample was up to 34 (6). Simple RT-PCR pooling designs can also be used to assess prevalence without individual specimen identification, by using quantitative viral load measurements in each positive pool, where the viral load measurement from a pool is proportional to the sum of the diluted viral loads from each positive sample in the pool (7).
Public health surveillance tools, such as rapid antigen tests, have also been advocated for since early in the pandemic to help control SARS-CoV-2 spread (4, 8–12). Rapid antigen tests are optimized to detect the presence of SARS-CoV-2 proteins in individuals during the acute, infectious phase of COVID-19, and can be self-administered or performed at the point-of-care, leading to faster sample to result time and more frequent testing (4). However, pooled testing using rapid antigen tests, has not been evaluated. In this study, we examined a proof-of-concept pooling method for public health surveillance of COVID-19 using a rapid antigen test.
The study included a clinical dilution panel provided by the non-profit PATH (www.path.org). The panel was prepared from human nasal swab eluate discards from COVID-19 patients, collected within seven days post-symptoms onset. A single swab eluate positive for SARS-CoV-2 by RT-PCR was diluted into a single nasal eluate negative for SARS-CoV-2 by RT-PCR. Dilutions were blinded, coded, and then aliquoted frozen at -80°C.
The primary studies under which the samples were collected received ethical clearance from PATH Institutional Review Board (IRB) (approval number 00004244). All excess samples and corresponding data were banked and de-identified prior to analysis. This study received an exemption determination from the PATH IRB.
Aliquots from the clinical dilution panel were thawed and 200 μl was used for extraction with the QIAamp Viral RNA Mini Kit (Qiagen, Germany). Nucleic acids were eluted in 50 μl and 10 μl were used for qRT-PCR using the CDC’s 2019-nCoV Real-Time RT-PCR N1 assay on the QuantStudio 5 Real-Time PCR System (Applied Biosystems, Foster City, CA). Viral loads were determined by measuring the concentration of the RNA using a qualified standard to determine genomic RNA levels, as previously described (13). Briefly, each reaction contained extracted RNA, 1X TaqPath 1-Step RT-qPCR Master Mix, CG, the CDC N1 forward and reverse primers, and probe. Viral copy numbers were quantified using N1 quantitative PCR standards in 16-fold dilutions to generate a standard curve. Quantification of the Importin-8 housekeeping gene RNA level was performed to determine the quality of nasal swab eluate discard sample.
Based on a number of published studies for sample pooling using RT-PCR, we opted for nasal swab eluate pools consisting of a total of 20 combined samples (14–16). 50 μl from a single RT-PCR confirmed positive nasal swab specimen dilution was mixed with 50 μl from nineteen RT-PCR confirmed negative nasal swab specimens. The total volume of each pool was 1 mL. For the non-pooled testing, the RT-PCR confirmed nasal swab specimen dilutions were not mixed with any negative nasal swab specimens, and were tested directly on the rapid antigen test. For pooled and single sample testing, 100 μl of the sample was applied to the COVID-19 rapid antigen test and allowed to react for 15 minutes before test result image capture.
The rapid antigen test (E25Bio, Inc., Cambridge, MA) contains a monoclonal antibody and a nanoparticle conjugate that detects the nucleocapsid protein of SARS-CoV-2. Interaction of the immobilized Test and Control line antibodies with antigen and nanoparticle conjugate produces visible bands, indicating whether a test is positive or negative. The lot used in this study was validated using recombinant SARS-CoV-2 nucleocapsid protein (SinoBiological, China) from 0.1 ng/ml to 500 ng/ml in a total volume of Solution Buffer (E25Bio, Inc., Cambridge, MA) of 100 μl (data not shown).
Rapid antigen test result images were captured via the iPhone E25Bio Passport appliation (currently only available via TestFlight) and analyzed using image processing software, Image J (NIH), to machine-read and quantify test results. The average pixel intensity was quantified at the Test line, Control line, and background areas. The background-adjusted Test line signal was then normalized to the background-subtracted Control line and expressed as an arbitrary unit (A.U.).
To analyze the effect of sample pooling on the analytical sensitivity of a rapid antigen test, we compared antigen detectability of single RT-PCR confirmed nasal swab specimens with varying Ct values versus when pooled into 19 RT-PCR confirmed negative nasal swab specimens. Antigen detectability was determined by applying to a rapid antigen test 100 μl of a single RT-PCR positive nasal swab specimen or 100 μl of a pooled sample. Each pooled sample consisted of 50 μl of a RT-PCR positive nasal swab dilution specimen and a 950 μl negative nasal swab mixture (50 μl from 19 RT-PCR confirmed negative nasal swab specimens).
Our results show that the rapid antigen test detected positive pools between Ct values 18.3 (VL: 7.6E7) and 30.1 (VL: 4.8E4), compared to 18.3 and 31.9 (VL: 3.5E4) for single nasal swab specimens (Figure 1). The sensitivity, specificity, positive predictive value (PPV) and negative predicative value (NPV) for the pooled testing strategy was 86.67% (13/15), 100% (20/20), 100%, and 90.91%, respectively. The sensitivity, specificity, positive predictive value (PPV) and negative predicative value (NPV) for the single sample testing strategy was 99.33% (14/15), 100% (20/20), 100%, and 95.24%, respectively. These data suggest that combining of up to 20 samples per pool can expand surveillance with rapid antigen tests, even if a pool contains only one positive sample. Only in the case of a positive pool test result is additional rapid antigen testing and/or RT-PCR of individual samples or smaller pools required.
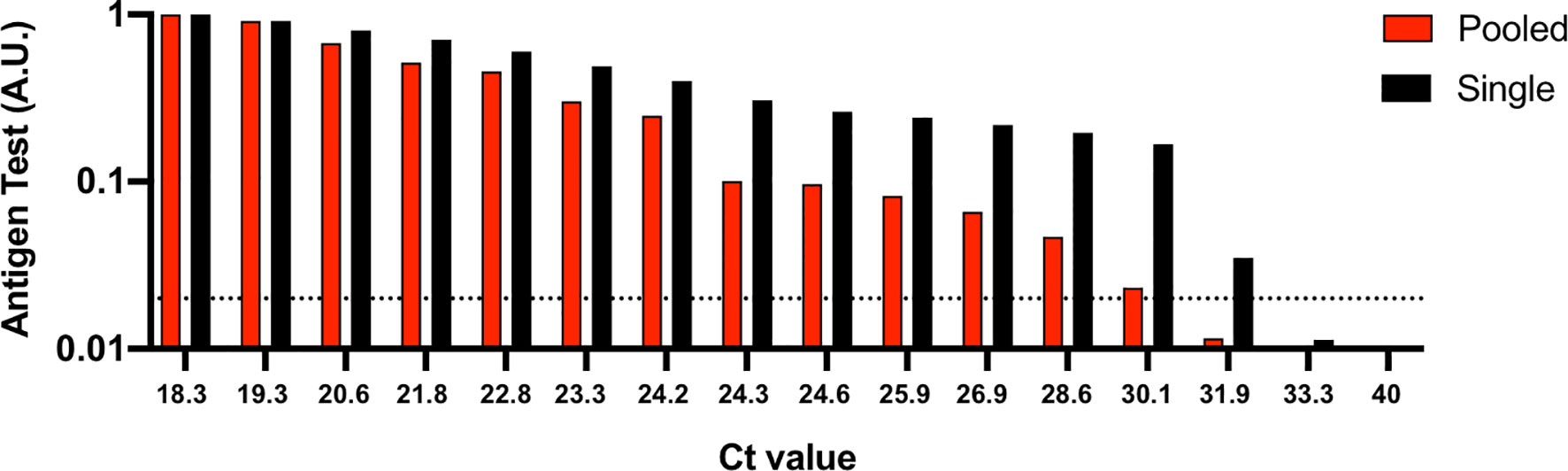
Figure 1 Analytical sensitivity of a rapid antigen test using single nasal swab specimens versus when pooled. 50 μl of the positive nasal swab specimens with Ct values ranging between 18.3 and 40 were spiked into 50 μl of RT-PCR confirmed negative nasal swab specimens from 19 individuals. 100 μl of the 1 ml pooled samples or 100 μl of the single positive nasal swab specimens were applied to the rapid antigen test and allowed to react for 15 minutes before test results were image captured. To determine relative nucleocapsid antigen levels detected by the antigen test, images were analyzed using image processing software and reported as an arbitrary unit (A.U.). Ct, cycle threshold. Horizontal dashed line, limit of detection for the rapid antigen test.
We propose a proof-of-concept pooling strategy for antigen testing that is easy to implement and can further expand SARS-CoV-2 surveillance. In general, for single sample testing, a nasal swab is collected from an individual, resuspended into 0.5-1 mL solution (e.g., viral transport media, saline solution, antigen test kit buffer solution, etc.), and 100 μl of the nasal swab specimen is then applied to a rapid antigen test. However, performing individual antigen testing on a single nasal swab specimen can present further cost and logistical limitations when the frequency of antigen testing is increased, especially for wide-scale surveillance. For pooled sample testing, nasal swabs are collected from up to 20 individuals, each of the nasal swabs are then resuspended into 0.3-0.5 mL solution, and 50 μl from each nasal swab specimen is pooled into a mixture before applying 100 μl to a rapid antigen test (Figure 2). If a pool tests negative by an antigen test, then all of the constituent nasal swab specimens are assumed negative. If a pool tests positive by the antigen test, then the constituent nasal swab specimens are putatively positive and should be re-tested in mini pools. If a mini pool remains positive, then antigen testing on individual nasal swab specimens can be performed.
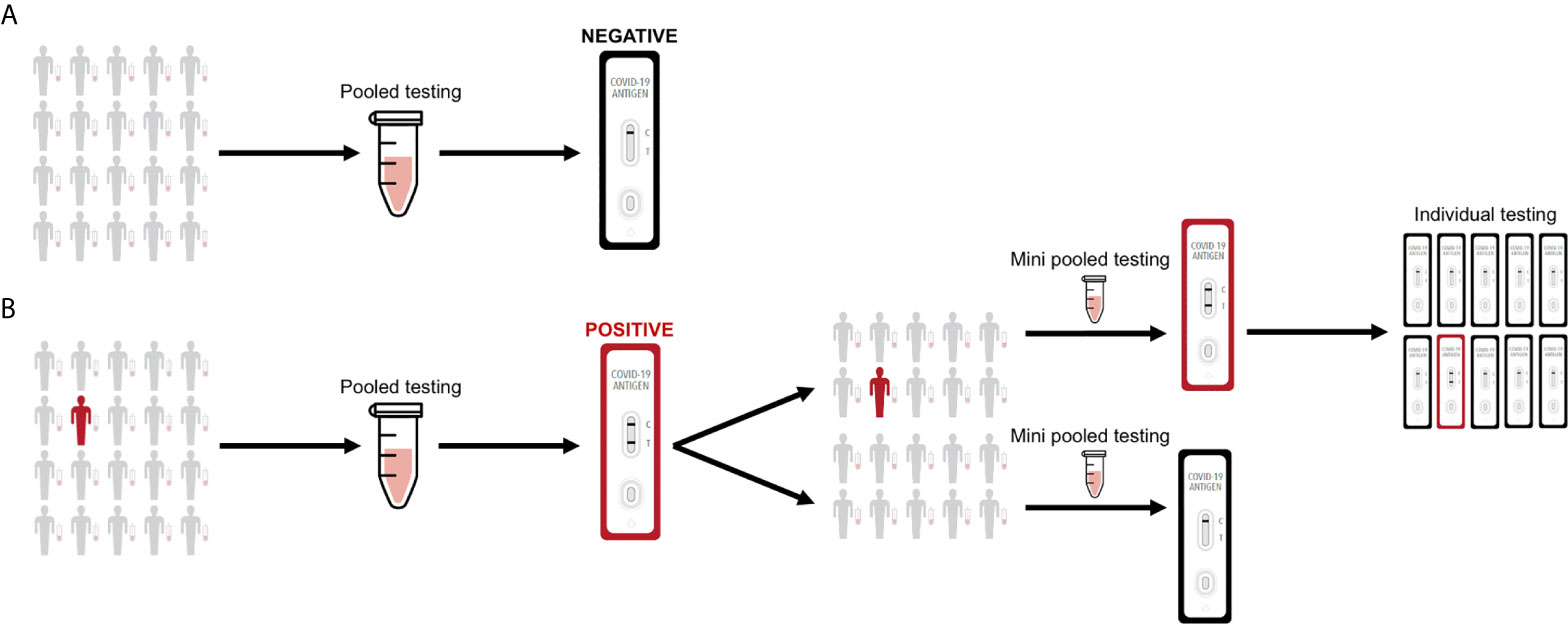
Figure 2 Sample pooling strategy for antigen testing. (A) Nasal swab specimens are collected, where 50 μl from each individual is pooled into a single mixture and 100 μl is then applied to the rapid test. If a pool tests negative by the antigen test, then all of the constituent nasal swab specimens are assumed negative. (B) If a pool tests positive by the antigen test, then the constituent nasal swab specimens are putatively positive and should be re-tested in mini pools. If a mini pool remains positive, then antigen testing on individual nasal swab specimens can be performed.
Pooled testing using rapid antigen tests is a cost-effective and public health conscious approach to expand SARS-CoV-2 surveillance, thus further enabling societal reopening, especially in instances where capacity and resources are constrained. Our results show that a rapid antigen test can detect SARS-CoV-2 positive nasal swab specimens up to Ct value 30.1, even when the positive specimen is combined with 19 negative nasal swab specimens. A recently published study demonstrated that in a 9-fold pooled testing strategy using RT-PCR, a single positive sample was detected with a Ct value as high as 34 (16). Our results are striking given SARS-CoV-2 antigen detectability in a 20-fold pooled sample, suggesting pooled antigen testing can be implemented to identify individuals during the infectious phase of COVID-19. Studies have shown that individuals are most infectious around the time of symptoms onset, where viral load in the upper respiratory tract are highest and Ct values are generally below 30 (17–19).
While our experiments suggest that a pooling strategy for antigen testing may be beneficial for SARS-CoV-2 surveillance and identification of individuals during the infectious phase of COVID-19, there are limitations. Specimens collected and tested beyond the acute infection phase (7 days post-infection) will likely escape detection by a rapid antigen test, regardless if the specimen is pooled or non-pooled. Enhancing efficiency at the expense of sensitivity must be appropriately considered depending on the purpose of testing and resources available. However, modeling and clinical studies have shown that increasing the frequency of antigen testing to a minimum of two times per week can increase the performance metrics of an antigen test (4, 12). Furthermore, pooling adds complexity because samples must be archived, or individuals would need to undergo additional swabbing for potential re-testing. Finally, other factors, including COVID-19 prevalence and incidence rates, virological (e.g., infecting SARS-CoV-2 or variant, viral load, defective virus, etc.) and host considerations, and the antigen test used (e.g., analytical sensitivity, etc.) will likely impact the sensitivity and efficacy of a pooling strategy.
We have shown that a simple design for antigen testing using a sample pooling strategy is straightforward and can enhance SARS-CoV-2 surveillance. Future studies will be important to determine whether pooling strategies hold for COVID-19 antibody detection in sera using rapid tests or for antigen detection from other microorganisms. While there are other costs and logistical limitations associated with pooling that we do not consider here, our study highlights the potential use of antigen tests as a public health tool to help combat the COVID-19 pandemic during specific epidemiological scenarios.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by PATH Institutional Review Board Approval Number 00004244. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: BH. Formal analysis: BH, NS, and AH. Investigation: NS and AH. Methodology: BH, NS, and AH. Project administration: BH. Resources: BH. Supervision: BH. Validation: BH, NS, and AH. Visualization: BH, NS, and AH. Writing—original draft: BH. Writing—review and editing: BH, NS, and AH. All authors contributed to the article and approved the submitted version.
The authors declare that this study received funding from E25Bio. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
NS, AH, and BH are employed by E25Bio, Inc., a biotechnology company that develops diagnostic assays for fever-causing infectious diseases.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Babiker A, Myers CW, Hill CE, Guarner J. SARS-Cov-2 Testing. Am J Clin Pathol (2020) 153(6):706–8. doi: 10.1093/ajcp/aqaa052
2. Krause PR, Fleming TR, Longini IM, Peto R, Briand S, Heymann DL, et al. SARS-Cov-2 Variants and Vaccines. N Engl J Med (2021) 385(2):179–86. doi: 10.1056/NEJMsr2105280
3. Du Z, Pandey A, Bai Y, Fitzpatrick MC, Chinazzi M, Pastore YPA, et al. Comparative Cost-Effectiveness of SARS-Cov-2 Testing Strategies in the USA: A Modelling Study. Lancet Public Health (2021) 6(3):e184–91. doi: 10.1016/S2468-2667(21)00002-5
4. Larremore DB, Wilder B, Lester E, Shehata S, Burke JM, Hay JA, et al. Test Sensitivity Is Secondary to Frequency and Turnaround Time for COVID-19 Screening. Sci Adv (2020) 7(1):eabd5393. doi: 10.1126/sciadv.abd5393
5. Tromberg BJ, Schwetz TA, Perez-Stable EJ, Hodes RJ, Woychik RP, Bright RA, et al. Rapid Scaling Up of Covid-19 Diagnostic Testing in the United States - The NIH Radx Initiative. N Engl J Med (2020) 383(11):1071–7. doi: 10.1056/NEJMsr2022263
6. Lohse S, Pfuhl T, Berko-Gottel B, Rissland J, Geissler T, Gartner B, et al. Pooling of Samples for Testing for SARS-Cov-2 in Asymptomatic People. Lancet Infect Dis (2020) 20(11):1231–2. doi: 10.1016/S1473-3099(20)30362-5
7. Cleary B, Hay JA, Blumenstiel B, Harden M, Cipicchio M, Bezney J, et al. Efficient Prevalence Estimation and Infected Sample Identification With Group Testing for SARS-CoV-2. medRxiv (2020).
8. Lambert-Niclot S, Cuffel A, Le Pape S, Vauloup-Fellous C, Morand-Joubert L, Roque-Afonso AM, et al. Evaluation of a Rapid Diagnostic Assay for Detection of SARS-Cov-2 Antigen in Nasopharyngeal Swabs. J Clin Microbiol (2020) 58(8). doi: 10.1128/JCM.00977-20
9. Mak GC, Cheng PK, Lau SS, Wong KK, Lau CS, Lam ET, et al. Evaluation of Rapid Antigen Test for Detection of SARS-Cov-2 Virus. J Clin Virol (2020) 129:104500. doi: 10.1016/j.jcv.2020.104500
10. Mertens P, De Vos N, Martiny D, Jassoy C, Mirazimi A, Cuypers L, et al. Development and Potential Usefulness of the COVID-19 Ag Respi-Strip Diagnostic Assay in a Pandemic Context. Front Med (Lausanne) (2020) 7:225. doi: 10.3389/fmed.2020.00225
11. Porte L, Legarraga P, Vollrath V, Aguilera X, Munita JM, Araos R, et al. Evaluation of a Novel Antigen-Based Rapid Detection Test for the Diagnosis of SARS-Cov-2 in Respiratory Samples. Int J Infect Dis (2020) 99:328–33. doi: 10.1016/j.ijid.2020.05.098
12. Nash B, Badea A, Reddy A, Bosch M, Salcedo N, Gomez AR, et al. Validating and Modeling the Impact of High-Frequency Rapid Antigen Screening on COVID-19 Spread and Outcomes. medRxiv (2021). doi: 10.1101/2020.09.01.20184713
13. Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, et al. SARS-Cov-2 Viral Load Is Associated With Increased Disease Severity and Mortality. Nat Commun (2020) 11(1):5493. doi: 10.1038/s41467-020-19057-5
14. Griesemer SB, Van Slyke G, St George K. Assessment of Sample Pooling for Clinical SARS-Cov-2 Testing. J Clin Microbiol (2021) 59(4). doi: 10.1128/JCM.01261-20
15. Mahmoud SA, Ibrahim E, Thakre B, Teddy JG, Raheja P, Ganesan S, et al. Evaluation of Pooling of Samples for Testing SARS-Cov- 2 for Mass Screening of COVID-19. BMC Infect Dis (2021) 21(1):360. doi: 10.1186/s12879-021-06061-3
16. Sawicki R, Korona-Glowniak I, Boguszewska A, Stec A, Polz-Dacewicz M. Sample Pooling as a Strategy for Community Monitoring for SARS-Cov-2. Sci Rep (2021) 11(1):3122. doi: 10.1038/s41598-021-82765-5
17. He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19. Nat Med (2020) 26(5):672–5. doi: 10.1038/s41591-020-0869-5
18. Singanayagam A, Patel M, Charlett A, Lopez Bernal J, Saliba V, Ellis J, et al. Duration of Infectiousness and Correlation With RT-PCR Cycle Threshold Values in Cases of COVID-19, England, January to May 2020. Euro Surveill (2020) 25(32). doi: 10.2807/1560-7917.ES.2020.25.32.2001483
Keywords: SARS-CoV-2, COVID-19, rapid antigen test, pooled testing methodology, surveillance
Citation: Salcedo N, Harmon A and Herrera BB (2021) Pooling of Samples for SARS-CoV-2 Detection Using a Rapid Antigen Test. Front. Trop. Dis 2:707865. doi: 10.3389/fitd.2021.707865
Received: 10 May 2021; Accepted: 22 July 2021;
Published: 05 August 2021.
Edited by:
Jorge Enrique Gómez Marín, University of Quindío, ColombiaReviewed by:
Diana Carolina Hernández Castro, Rosario University, ColombiaCopyright © 2021 Salcedo, Harmon and Herrera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bobby Brooke Herrera, YmJoZXJyZXJhQGUyNWJpby5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.