
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Transplant. , 17 March 2025
Sec. Abdominal Transplantation
Volume 4 - 2025 | https://doi.org/10.3389/frtra.2025.1572928
 Tara B. Gavcovich1,2*†,‡
Tara B. Gavcovich1,2*†,‡ Vaka K. Sigurjonsdottir1,2*†,‡
Vaka K. Sigurjonsdottir1,2*†,‡ Marissa J. DeFreitas1,2,‡
Marissa J. DeFreitas1,2,‡ Claudia Serrano1,2
Claudia Serrano1,2 Esther Rivas1,2
Esther Rivas1,2 Migdalia Jorge1,2
Migdalia Jorge1,2 Wacharee Seeherunvong1,2,‡
Wacharee Seeherunvong1,2,‡ Chryso Katsoufis1,2,‡
Chryso Katsoufis1,2,‡ Wendy Glaberson1,2,‡
Wendy Glaberson1,2,‡ Melisa Oliva3,‡
Melisa Oliva3,‡ Adela D. Mattiazzi4,‡
Adela D. Mattiazzi4,‡ Carolyn Abitbol1,2,‡
Carolyn Abitbol1,2,‡ Jayanthi Chandar1,2,‡
Jayanthi Chandar1,2,‡
Background: Long-term survival of kidney allografts is limited by multiple factors, including nonadherence. High intrapatient variability in tacrolimus levels (≥30%) is associated with de novo donor-specific antibody (dnDSA) formation, increased risk of rejection and graft loss.
Methods: We prospectively analyzed the association between tacrolimus intrapatient variability and nonadherence in pediatric kidney transplant recipients. We derived a composite adherence score from 0 to 3 points based on (1) Basel Assessment of Adherence to Immunosuppressive Medical Scale©; (2) healthcare team score; and (3) intentionally missed laboratory or clinic visits. A score of 1 or more was considered nonadherent. Tacrolimus 12 h trough levels, patient characteristics and clinical outcomes were collected. Tacrolimus IPV was calculated as the coefficient of variation.
Results: The nonadherent group had a significantly higher median tacrolimus intrapatient variability (31%) as compared to the adherent cohort (20%) (p < 0.001.) Tac IPV demonstrated strong predictive performance for adherence (AUC 0.772), with a particularly high sensitivity of 90% at thresholds up to 20%, offering a practical and actionable framework for assessing adherence-related risks in clinical practice.
Conclusions: Tacrolimus intrapatient variability may be a useful biomarker to identify nonadherence and high-risk patients, allowing for early interventions to prevent adverse graft outcomes.
Allograft rejection due to insufficient immunosuppression is a major contributor to early graft loss (1–3). Not taking medications consistently or inconsistent follow-up with the medical care team is a known risk factor for poor graft outcomes, with nonadherence being the most common in adolescent patients (1, 4, 5). Post-transplant maintenance immunosuppression typically includes tacrolimus, a calcineurin inhibitor, with a narrow therapeutic index requiring frequent drug level monitoring to balance effective drug concentrations while minimizing toxicity (6–8). High tacrolimus intrapatient variability (Tac IPV) has been studied for over two decades and measured with two different methods, the coefficient of variation (CV) and Medication Level Variability Index (MLVI). Tac IPV has been increasingly recognized as a biomarker for graft rejection and loss (7, 9–12) and a marker of nonadherence (13). The Medication Adherence in children who had a Liver Transplant (MALT) prospective multi-site study evaluated whether MLVI predicts late acute rejection. A total of 379 participants were followed prospectively and results showed that a higher prerejection MLVI predicted adverse graft outcomes (14).
The International Consensus on Managing Modifiable Risk in Transplantation recommends monitoring nonadherence as a fifth vital sign (15). As expected, nonadherence has also been associated with worse graft outcomes, leading to increased incidence of rejection, de novo donor specific antibody (dnDSA) formation, decreased renal function and ultimately graft loss (1, 16, 17). While multiple factors contribute to raising the Tac IPV, including tube feeding, feeding intolerance, infection, drug or food interactions and dose adjustments (6, 8, 12, 18–21), medication nonadherence is thought to be the strongest contributor (22). Several studies have shown that tacrolimus variability responds to behavioral interventions, strongly supporting that is affected by behavior (23–26). This relationship, however, has not been described well in adults or children, especially since nonadherence is difficult to measure consistently in the clinical setting (27, 28). The prevalence of nonadherence varies across studies, and depends on heterogenous measurement tools, which adds to the inconsistent analyses (29). Measures of nonadherence include direct (observation, drug assays) and indirect (self-report, collateral report, prescription refills, electronic monitoring) parameters, and there is no single ideal method given the limitations of each (27, 29–31).
Since adherence measures can be quite unreliable and burdensome to patients and clinicians, tacrolimus variability has been suggested as a potential objective biomarker for nonadherence, specifically in pediatric liver transplant patients (14). We hypothesized that nonadherence was a strong contributor to high Tac IPV. The aim of this study was to investigate the relationship between Tac IPV and adherence in a cohort of children and young adult kidney transplant recipients.
This is a prospective, single center, cross-sectional study. This research was approved by the institutional review board of the University of Miami Miller School of Medicine (IRB #20220914). All participants provided informed consent. All pediatric recipients of isolated kidney transplants who presented to Miami Transplant Institute (MTI) for a post-transplant visit from September 2022 to December 2022 were considered eligible for inclusion in the study. Patients < 10 months post-transplant, not on tacrolimus immunosuppression, or with fewer than three tacrolimus levels in the study period were excluded. The standard induction protocol included thymoglobulin on post-transplant day 0 (one dose total of thymoglobulin), basiliximab on post-transplant day 0 and post-transplant day 3 or 4 (two doses total of basiliximab), along with a steroid taper. Maintenance immunosuppression included tacrolimus and mycophenolate mofetil, with or without prednisone, based on immunologic risk. Sirolimus was selectively added for some recipients to diminish target tacrolimus levels and to limit nephrotoxicity. The goal tacrolimus (or combined tacrolimus and sirolimus) level was 6–8 in the first three months post-transplant and 5–7 thereafter. Adherence data was collected on the day of enrollment during the clinic visit. Baseline and follow-up laboratory data was collected prospectively through November 2023. Historical data on episodes of rejection and dnDSA formation was also collected. All participants were followed for at least 6 months after enrollment. Demographic and clinical characteristics such as sex, age at transplantation, duration after transplant, underlying renal disease, donor source, pre-transplant panel reactive antibody (PRA), human leukocyte antigen (HLA) matching, and insurance type were obtained from the electronic medical record (EMR).
In addition to the validated Basel Assessment of Adherence to Immunosuppressive Medical Scale©(BAASIS©), we developed a composite adherence score (CAS) which included the BAASIS© to enhance our assessment of adherence and address limitations of self-report, which are known to include biases such as recall inaccuracies and social desirability bias, leading to underreporting of nonadherence. We used a CAS ranging from 0 to 3 total points based on three parameters: (1) Basel Assessment of Adherence to Immunosuppressive Medical Scale©(BAASIS©); (2) healthcare team score; and (3) intentionally missed laboratory or clinic visits. Each measure was awarded 1 point if considered nonadherent. The final CAS score was 0 to 3. A perfect adherence score corresponded to a total score of 0, and nonadherence was defined as a score of 1–3.
The BAASIS© is a written questionnaire that is widely used in research and clinical practice, and has been validated in kidney transplant recipients to assess adherence to immunosuppressive medications (32–34). It consists of five questions on timing and taking of immunosuppressive medications, including missed doses, drug holidays, time deviation, and dose changes or discontinuation of the medications without physician consultation. The questionnaire was filled out independently by the patient (if ≥15 years old) or caregiver (if younger) at the time of enrollment, based on a 4-week recall. Nonadherence was defined as “yes” to any of the questions.
The transplant clinical team (three physicians, two nurse coordinators, and one nurse practitioner closely involved in the follow-up care of the kidney transplant recipients) scored recipients’ adherence on 4-point scale (poor, suboptimal, fair, good), as described by Schafer et al. (27). A patient received a score of 4 if all clinicians estimated his/her adherence as good, a score of 2 or 3 if any of the providers estimated his/her adherence as less than good (fair or suboptimal), but not poor, and a score of 1 if any clinician estimated his/her adherence as poor, independently of the estimations given by the other clinicians. A perfect adherence score corresponded to a total score of 4, and nonadherence was defined as a score of 1–3.
Transplant nurse coordinators track missed clinic and laboratory visits as a standard. Nonadherence was defined as report of more than one intentionally missed clinic and/or laboratory visit. An intentionally missed visit was defined as patient and/or caregiver not providing an explanation for missing the visit, not trying to reschedule the visit, and/or not calling the healthcare team prior to missing the visit.
We collected all available 12 h trough tacrolimus levels in the six to twelve-month period following enrollment. Levels drawn while hospitalized, during sickness, or non-trough levels were excluded. Tac IPV was calculated using the CV according to the equation CV = σ/μ × 100%, where σ is the standard deviation of the tacrolimus levels and μ is the mean tacrolimus level.
Baseline characteristics and demographics were summarized descriptively using median (interquartile ranges) and counts (percentages) where appropriate. Receiver operating characteristic (ROC) curves were generated to evaluate diagnostic performance, and the area under the curve (AUC) was calculated. A p value < 0.05 was considered statistically significant. Analyses were performed using GraphPad Prism 10.0 software.
From September 2022 through December 2022, 75 patients were enrolled and followed through November 2023, with a total of 725 tacrolimus levels (median, 9 levels per patient; interquartile range 6,13) analyzed. Twelve patients did not meet inclusion criteria. No eligible patient refused to participate. The median follow-up time was 12 months (IQR 10,12). Median age at transplant was 14 years (IQR 7.5,16.5). The median post-transplant time at enrollment was 3.1 years (IQR 1.5,15.2). Demographic characteristics, stratified by adherence as defined by CAS, are summarized in Table 1. In addition to tacrolimus, 28% of participants were also on sirolimus (19 patients) or abatacept (2 patients). All participants continued tacrolimus throughout the study period.
The median Tac IPV among all participants was 24% (IQR 17,33). Among participants who were not on sirolimus or abatacept, the median tacrolimus level was 5.5 ng/ml (IQR 4.5,6.7). Among patients on concomitant sirolimus or abatacept, and therefore with lower tacrolimus goals, the median tacrolimus level was 3.2 ng/ml (IQR 2.5,4.3).
Using the BAASIS© alone, the nonadherence rate was 29%; the nonadherent group had a median Tac IPV of 32%, vs. 22% among the adherent cohort (p < 0.001, Figure 1a).
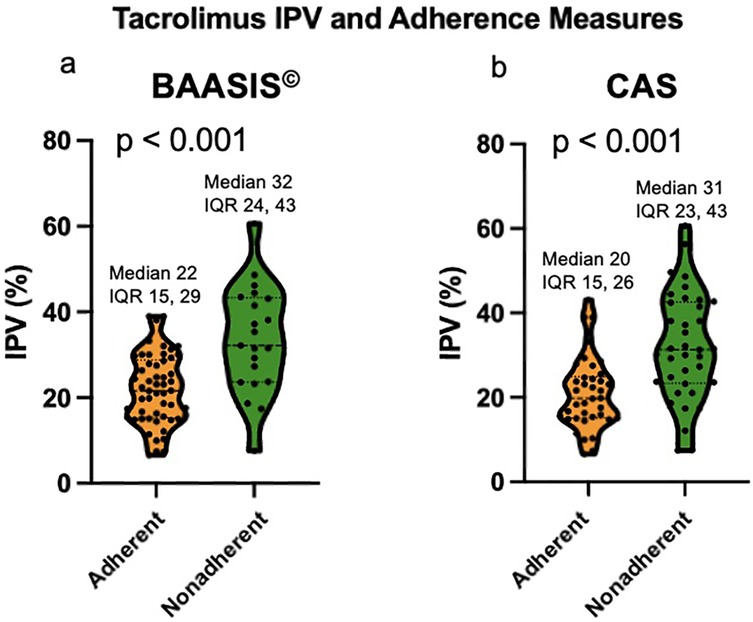
Figure 1. (a) Violin plot of Tac IPV comparing adherent and nonadherent patients based on BAASIS©. (b) Violin plot of Tac IPV comparing adherent and nonadherent patients based on CAS. IPV: intrapatient variability; BAASIS©: Basel Assessment of Adherence to Immunosuppressive Medical Scale; CAS: composite adherence score.
Using the CAS, the nonadherence rate was 49%; the nonadherent group had a significantly higher median Tac IPV of 31%, as compared to the adherent cohort with a median Tac IPV of 20% (p < 0.001, Figure 1b). Table 1 compares the clinical and demographic characteristics between patients classified as adherent vs. nonadherent. The performance of the Tac IPV in predicting nonadherence in pediatric kidney transplant recipients was assessed using an ROC curve. The AUC was 0.77 (95% CI, 0.66 to 0.88), indicating a moderately strong ability to distinguish between adherent and nonadherent patients. To stratify patients into risk categories, we analyzed Tac IPV thresholds along the ROC curve. The optimal cut-off point was a Tac IPV of 17%, where sensitivity reached 92%, suggesting that IPV values below this threshold were highly associated with adherence (Figure 2). Generally, patients with a Tac IPV 20% or less could be categorized as low risk, with high sensitivity at 90% and specificity at 60% (Figure 2). ROC curve based on BAASIS© alone had an AUC of 0.74 (95% CI, 0.61 to 0.86).
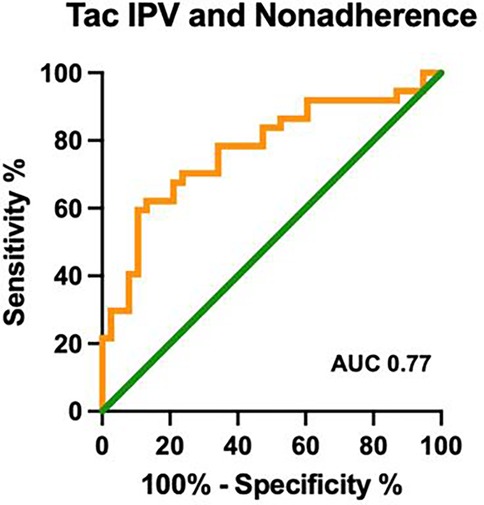
Figure 2. ROC curve of Tac IPV in identifying nonadherence. ROC: receiver operating characteristic; Tac IPV: tacrolimus intrapatient variability; AUC: area under the curve; CAS: composite adherence score.
This prospective study highlights the importance of tacrolimus intrapatient variability as a biomarker for identifying nonadherence in pediatric kidney transplant recipients. Our findings demonstrate a significant association between high Tac IPV and nonadherence, supporting its potential as a reliable, objective tool for detecting patients at increased risk of adverse outcomes. These results are consistent with prior studies across both pediatric and adult transplant populations, which have demonstrated that increased Tac IPV is associated with higher rates of rejection and graft loss (1, 4, 5, 35, 36).
In our study, median Tac IPV in the entire cohort was 24%, lower than studies that did not censor out data (12), and higher than other studies assessing highly adherent patients (12, 26, 27, 37). Median Tac IPV was lower in adherent patients, 20% vs. 31% in nonadherent. In the MALT study, MLVI correlates strongly with adherence measures, including electronic monitoring and multidisciplinary panel evaluations, reinforcing its role as a behavioral metric rather than a reflection of pharmacologic variability (14). The MALT study further demonstrated that the MLVI is not influenced by metabolic or absorption anomalies, common drug-drug interactions, or prescription practices.
Additionally, the MLVI is best utilized as a threshold construct rather than a continuous measure, consistent with our findings from the ROC analysis. Tac IPV exhibited strong predictive performance for adherence, with an AUC of 0.77, supporting its utility as a robust marker in clinical practice. Notably, Tac IPV was particularly sensitive at thresholds up to 20% for identifying adherent patients, providing a practical and clinically actionable framework for assessing adherence-related risks.
The use of a threshold-based approach offers significant advantages in risk stratification and intervention planning. Specifically, Tac IPV exceeding 30% should prompt heightened clinical suspicion for nonadherence, warranting further evaluation and direct discussions to uncover and address potential barriers to adherence. This practical application allows for early identification of at-risk patients and facilitates targeted interventions that may improve both adherence and clinical outcomes. These findings reinforce the utility of variability indices such as MLVI and Tac IPV as objective tools to improve risk stratification and optimize clinical care.
Leino et al. evaluated baseline patterns of Tac IPV in an adherent cohort of adult kidney and liver transplant recipients. The study population demonstrated 99.9% adherence, as measured by patient daily diary, pill counts and the electronic medication event monitoring system (MEMS); the median weekly Tac IPV was calculated at 15.2% (37). This finding indirectly suggests that tacrolimus levels are not variable in adherent patients. In a post-hoc analysis of a dataset from a randomized controlled trial, Ko et al. looked at the relationship between adherence, as measured by self-report and MEMS, and Tac IPV in adult kidney transplant recipients. The median Tac IPV was not significantly different between adherent and nonadherent groups, 16 vs. 16.5% (26). This was concordant with a paper by Gokoel et al. that also showed a lower mean Tac IPV of 17.9% and no relationship between adherence and Tac IPV among stable adult kidney transplant recipients (25). The baseline Tac IPV in our cohort was higher than in these recent adult studies, suggesting an underlying difference (25, 26, 37). The baseline Tac IPV in a recent pediatric study from Piburn, et al. was 30%, higher than in our study, which is likely explained by their retrospective design as well as inclusion of all uncensored trough levels (12).
A highly variable drug level has been defined in many studies as ≥30%, but in the two studies by Ko et al. and Gokoel et al., median Tac IPV was low, probably because the degree of nonadherence was not sufficient in these cohorts to test the hypothesis (25, 26). Given the difficulty to engage nonadherent patients in research, trials are often biased towards a sample of adherent patients as supported by a recent systematic analysis (28). In the study by Ko et al., the cohort consisted of motivated patients that participated in a randomized controlled trial, with a mean age at transplant of 43 years (26). In our cohort, almost half of the patients were nonadherent at a median age of 17 years. It is well known that recipients aged 14 to 16 years have the greatest risk of kidney graft failure (1, 5, 16, 38). As described by Piburn et al., the baseline trend of Tac IPV started to increase in adolescence and young adulthood, which could indicate an increased incidence of nonadherence by this age group (12). In our cohort, nonadherent patients had a higher Tac IPV and were more likely to have a history of biopsy-proven rejection and formation of dnDSAs, suggesting that nonadherent behavior probably preceded our assessment. We identified an association between adherence and Tac IPV, where the nonadherent group demonstrated a high-risk Tac IPV of ≥30%, which had not been previously done prospectively.
One of the largest strengths of this study was the prospective study design, and the real time collection of data, allowing us to limit confounders such as improper timed levels, levels drawn during hospitalization or comorbid illness, or a change in therapeutic goal during acute infection or graft rejection. Limitations include the lack of objective measures of adherence. This shows the difficulty of truly assessing adherence in the clinical setting and in research. While BAASIS© is a validated tool for assessing adherence, it is limited by self-report bias, which can result in underestimation of nonadherence. Self-reported adherence, such as BAASIS©, has known biases including social desirability bias and recall inaccuracies, leading to underreporting of nonadherence. Additionally, nonadherent patients are less likely to participate in studies, introducing selection bias or the streetlight effect, where adherence is assessed primarily in the most accessible patients. To enhance the accuracy of adherence assessment and mitigate the limitations of self-report, we developed the CAS, incorporating more objective markers such as intentionally missed clinic/laboratory visits and healthcare team assessments, providing a more robust and clinically relevant evaluation of adherence.
The composite score has not yet been validated, which limits our study; however, the BAASIS© is a validated measure used among kidney transplant recipients to assess adherence to immunosuppressive medications and remains a component of our CAS (32–34, 39). Further, BAASIS© alone demonstrated sufficient statistical power in detecting the correlation between adherence and Tac IPV, as evidenced by the strong relationship observed in our analysis. However, the CAS provided greater sensitivity in identifying Tac IPV, our surrogate marker of nonadherence, reinforcing the importance of multi-method adherence assessment. The study was not blinded; therefore, the providers scoring for nonadherence were also caring for the patients, allowing them to make informed assessments of adherence, but also allowing for a possibility of bias. Our study was further limited by being from a single center and having a relatively short follow-up time.
To conclude, this study demonstrates the important role of Tac IPV as a biomarker for identifying nonadherence and predicting adverse graft outcomes in pediatric kidney transplant recipients. Tac IPV was significantly higher in nonadherent patients and the low risk threshold for Tac IPV was identified to be less than 20%. Adolescents, a population particularly vulnerable to lapses in adherence, present unique challenges that require targeted strategies to ensure consistent immunosuppression. Tac IPV offers an objective and noninvasive metric to identify at-risk patients early, enabling timely interventions to mitigate the risk of acute rejection and graft loss. Furthermore, the potential modifiability of Tac IPV through adherence-promoting interventions highlights its clinical relevance not only as a diagnostic tool but also as a target for improving long-term outcomes.
The long-term care of pediatric kidney transplant recipients necessitates a multidisciplinary approach, with adherence monitoring at its core. Tac IPV serves as a valuable adjunct in this paradigm, bridging the gap between subjective assessments and actionable insights. Future multicenter, prospective studies are essential to validate Tac IPV as a reliable biomarker and to explore its role in guiding personalized interventions. By integrating Tac IPV monitoring into routine clinical practice, clinicians can better address the complexities of nonadherence, ultimately improving outcomes for pediatric and adolescent kidney transplant recipients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by University of Miami Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
TG: Writing – original draft, Writing – review & editing. VS: Writing – original draft, Writing – review & editing. MD: Writing – review & editing. CS: Writing – review & editing. ER: Writing – review & editing. MJ: Writing – review & editing. WS: Writing – review & editing. CK: Writing – review & editing. WG: Writing – review & editing. MO: Writing – review & editing. AM: Writing – review & editing. CA: Writing – review & editing, Supervision. JC: Writing – review & editing, Supervision.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frtra.2025.1572928/full#supplementary-material
95% CI, 95% confidence interval; ABMR, antibody-mediated rejection; AUC, area under the curve; BAASIS©, Basel Assessment of Adherence to Immunosuppressive Medical Scale; CAS, composite adherence score; CI, confidence interval; CV, coefficient of variation; dnDSA, de novo donor-specific antibody; eGFR, estimated glomerular filtration rate); EMR, electronic medical record; ESKD, end-stage kidney disease; HLA, human leukocyte antigen; IQR, interquartile range; MTI, Miami Transplant Institute; PRA, panel reactive antibody; RCT, randomized controlled trial; ROC, receiver operating characteristic; Tac IPV, tacrolimus intrapatient variability; TCMR, T-cell mediated rejection.
1. Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant. (2009) 14(5):603–13. doi: 10.1111/j.1399-3046.2010.01299.x
2. Hsu DT. Biological and psychological differences in the child and adolescent transplant recipient. Pediatr Transplant. (2005) 9(3):416–21. doi: 10.1111/j.1399-3046.2005.00352.x
3. Dharnidharka VR, Fiorina P, Harmon WE. Kidney transplantation in children. N Engl J Med. (2014) 371(6):549–58. doi: 10.1056/NEJMra1314376
4. Zelikovsky N, Schast AP, Palmer J, Meyers KEC. Perceived barriers to adherence among adolescent renal transplant candidates. Pediatr Transplant. (2008) 12(3):300–8. doi: 10.1111/j.1399-3046.2007.00886.x
5. Denhaerynck K, Steiger J, Bock A, Schäfer-Keller P, Köfer S, Thannberger N, et al. Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant. (2007) 7(1):108–16. doi: 10.1111/J.1600-6143.2006.01611.X
6. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. (2004) 43(10):623–53. doi: 10.2165/00003088-200443100-00001
7. Shuker N, Van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev. (2015) 29(2):78–84. doi: 10.1016/J.TRRE.2015.01.002
8. Gonzales HM, McGillicuddy JW, Rohan V, Chandler JL, Nadig SN, Dubay DA, et al. A comprehensive review of the impact of tacrolimus intrapatient variability on clinical outcomes in kidney transplantation. Am J Transplant. (2020) 20(8):1969–83. doi: 10.1111/ajt.16002
9. Larkins N, Matsell DG. Tacrolimus therapeutic drug monitoring and pediatric renal transplant graft outcomes. Pediatr Transplant. (2014) 18(8):803–9. doi: 10.1111/petr.12369
10. Whalen HR, Glen JA, Harkins V, Stevens KK, Jardine AG, Geddes CC, et al. High intrapatient tacrolimus variability is associated with worse outcomes in renal transplantation using a low-dose tacrolimus immunosuppressive regime. Transplantation. (2017) 101(2):430–6. doi: 10.1097/TP.0000000000001129
11. Vanhove T, Vermeulen T, Annaert P, Lerut E, Kuypers DRJ. High intrapatient variability of tacrolimus concentrations predicts accelerated progression of chronic histologic lesions in renal recipients. Am J Transplant. (2016) 16(10):2954–63. doi: 10.1111/ajt.13803
12. Piburn KH, Sigurjonsdottir VK, Indridason OS, Maestretti L, Patton MV, McGrath A, et al. Patterns in tacrolimus variability and association with de novo donor-specific antibody formation in pediatric kidney transplant recipients. Clin J Am Soc Nephrol. (2022) 17(8):1194–203. doi: 10.2215/CJN.16421221
13. Duncan-Park S, Dunphy C, Becker J, D'Urso C, Annunziato R, Blatter J, et al. Remote intervention engagement and outcomes in the clinical trials in organ transplantation in children consortium multisite trial. Am J Transplant. (2021) 21(9):3112–22. doi: 10.1111/ajt.16567
14. Shemesh E, Bucuvalas JC, Anand R, Mazariegos GV, Alonso EM, Venick RS, et al. The medication level variability index (MLVI) predicts poor liver transplant outcomes: a prospective multi-site study. Am J Transplant. (2017) 17(10):2668–78. doi: 10.1111/ajt.14276
15. Neuberger JM, Bechstein WO, Kuypers DRJ, Burra P, Citterio F, De Geest S, et al. Practical recommendations for long-term management of modifiable risks in kidney and liver transplant recipients. Transplantation. (2017) 101(4S):S1–S56. doi: 10.1097/TP.0000000000001651
16. Holmberg C. Nonadherence after pediatric renal transplantation. Curr Opin Pediatr. (2019) 31(2):219–25. doi: 10.1097/MOP.0000000000000734
17. Hsiau M, Fernandez HE, Gjertson D, Ettenger RB, Tsai EW. Monitoring nonadherence and acute rejection with variation in blood immunosuppressant levels in pediatric renal transplantation. Transplantation. (2011) 92(8):918–22. doi: 10.1097/TP.0b013e31822dc34f
18. Taber DJ, Hirsch J, Keys A, Su Z, McGillicuddy JW. Etiologies and outcomes associated with tacrolimus levels out of a typical range that lead to high variability in kidney transplant recipients. Ther Drug Monit. (2021) 43(3):401–7. doi: 10.1097/FTD.0000000000000863
19. Gold A, Tönshoff B, Döhler B, Süsal C. Association of graft survival with tacrolimus exposure and late intra-patient tacrolimus variability in pediatric and young adult renal transplant recipients—an international CTS registry analysis. Transpl Int. (2020) 33(12):1681–92. doi: 10.1111/tri.13726
20. Süsal C, Döhler B. Late intra-patient tacrolimus trough level variability as a major problem in kidney transplantation: a collaborative transplant study report. Am J Transplant. (2019) 19(10):2805–13. doi: 10.1111/ajt.15346
21. Shah PB, Ennis JL, Cunningham PN, Josephson MA, McGill RL. The epidemiologic burden of tacrolimus variability among kidney transplant recipients in the United States. Am J Nephrol. (2019) 50(5):370–4. doi: 10.1159/000503167
22. Kuypers DRJ. Intrapatient variability of tacrolimus exposure in solid organ transplantation: a novel marker for clinical outcome. Clin Pharmacol Ther. (2020) 107(2):347–58. doi: 10.1002/cpt.1618
23. Herblum J, Dacouris N, Huang M, Koestler J, Catano D, Petersen KH. Retrospective analysis of tacrolimus intrapatient variability as a measure of medication adherence. Can J Kidney Health Dis. (2021) 8:205435812110217. doi: 10.1177/20543581211021742
24. McGillicuddy JW, Chandler JL, Sox LR, Taber DJ. Exploratory analysis of the impact of an mHealth medication adherence intervention on tacrolimus trough concentration variability: post hoc results of a randomized controlled trial. Ann Pharmacother. (2020) 54(12):1185–93. doi: 10.1177/1060028020931806
25. Gokoel SRM, Zwart TC, Moes DJAR, van der Boog PJM, de Fijter JW. No apparent influence of nonadherence on tacrolimus intrapatient variability in stable kidney transplant recipients. Ther Drug Monit. (2020) 42(5):702–9. doi: 10.1097/FTD.0000000000000772
26. Ko H, Kim HK, Chung C, Han A, Min SK, Ha J, et al. Association between medication adherence and intrapatient variability in tacrolimus concentration among stable kidney transplant recipients. Sci Rep. (2021) 11(1):5397. doi: 10.1038/s41598-021-84868-5
27. Schäfer-Keller P, Steiger J, Bock A, Denhaerynck K, De Geest S. Diagnostic accuracy of measurement methods to assess non-adherence to immunosuppressive drugs in kidney transplant recipients. Am J Transplant. (2008) 8(3):616–26. doi: 10.1111/j.1600-6143.2007.02127.x
28. Duncan S, Annunziato RA, Dunphy C, LaPointe Rudow D, Shneider BL, Shemesh E. A systematic review of immunosuppressant adherence interventions in transplant recipients: decoding the streetlight effect. Pediatr Transplant. (2018) 22(1):0.1111/petr.13086. doi: 10.1111/petr.13086
29. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. (2005) 353(5):487–97. doi: 10.1056/NEJMra050100
30. Butler JA, Peveler RC, Roderick P, Horne R, Mason JC. Measuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoring. Transplantation. (2004) 77(5):786–9. doi: 10.1097/01.TP.0000110412.20050.36
31. De Geest S, Vanhaecke J. Methodological issues in transplant compliance research. Transplant Proc. (1999) 31(4):81S–3. doi: 10.1016/S0041-1345(99)00137-2
32. Denhaerynck K, Dobbels F, Košťálová B, De Geest S. Psychometric properties of the BAASIS: a meta-analysis of individual participant data. Transplantation. (2023) 107(8):1795–809. doi: 10.1097/TP.0000000000004574
33. Dobbels F, Berben L, De Geest S, Drent G, Lennerling A, Whittaker C, et al. The psychometric properties and practicability of self-report instruments to identify medication nonadherence in adult transplant patients: a systematic review. Transplantation. (2010) 90(2):205–19. doi: 10.1097/TP.0b013e3181e346cd
34. de Marsicano Ede O, Fernandes Nda S, Colugnati F, Grincenkov FR, Fernandes NM, De Geest S, et al. Transcultural adaptation and initial validation of Brazilian-Portuguese version of the Basel assessment of adherence to immunosuppressive medications scale (BAASIS) in kidney transplants. BMC Nephrol. (2013) 14(1):108. doi: 10.1186/1471-2369-14-108
35. O’Regan JA, Canney M, Connaughton DM, O'Kelly P, Williams Y, Collier G, et al. Tacrolimus trough-level variability predicts long-term allograft survival following kidney transplantation. J Nephrol. (2016) 29(2):269–76. doi: 10.1007/s40620-015-0230-0
36. Prytula AA, Bouts AH, Mathot RAA, van Gelder T, Croes LK, Hop W, et al. Intra-patient variability in tacrolimus trough concentrations and renal function decline in pediatric renal transplant recipients. Pediatr Transplant. (2012) 16(6):613–8. doi: 10.1111/j.1399-3046.2012.01727.x
37. Leino AD, King EC, Jiang W, Vinks AA, Klawitter J, Christians U, et al. Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: establishing baseline values. Am J Transplant. (2019) 19(5):1410–20. doi: 10.1111/ajt.15199
38. Van Arendonk KJ, James NT, Boyarsky BJ, Garonzik-Wang JM, Orandi BJ, Magee JC, et al. Age at graft loss after pediatric kidney transplantation. Clin J Am Soc Nephrol. (2013) 8(6):1019–26. doi: 10.2215/CJN.10311012
Keywords: kidney transplant, pediatric, adherence, tacrolimus variability, tacrolimus, pediatric kidney transplant recipients, medication nonadherence
Citation: Gavcovich TB, Sigurjonsdottir VK, DeFreitas MJ, Serrano C, Rivas E, Jorge M, Seeherunvong W, Katsoufis C, Glaberson W, Oliva M, Mattiazzi AD, Abitbol C and Chandar J (2025) Intrapatient tacrolimus variability is associated with medical nonadherence among pediatric kidney transplant recipients. Front. Transplant. 4:1572928. doi: 10.3389/frtra.2025.1572928
Received: 7 February 2025; Accepted: 3 March 2025;
Published: 17 March 2025.
Edited by:
Alan Langnas, University of Nebraska Medical Center, United StatesReviewed by:
Osama Ashry Gheith, Mansoura University, EgyptCopyright: © 2025 Gavcovich, Sigurjonsdottir, DeFreitas, Serrano, Rivas, Jorge, Seeherunvong, Katsoufis, Glaberson, Oliva, Mattiazzi, Abitbol and Chandar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tara B. Gavcovich, dGJnMjFAbWlhbWkuZWR1; Vaka K. Sigurjonsdottir, dnhzNjExQG1lZC5taWFtaS5lZHU=
†These authors have contributed equally to this work
‡ORCID:
Tara B. Gavcovich
orcid.org/0009-0004-3991-1618
Vaka K. Sigurjonsdottir
orcid.org/0000-0003-2776-4601
Marissa J. DeFreitas
orcid.org/0000-0001-9331-1905
Wacharee Seeherunvong
orcid.org/0000-0003-0485-5762
Chryso Katsoufis
orcid.org/0000-0002-8872-1165
Wendy Glaberson
orcid.org/0000-0003-0865-3279
Melisa Oliva
orcid.org/0000-0001-8851-3967
Adela D. Mattiazzi
orcid.org/0000-0002-3388-5648
Carolyn Abitbol
orcid.org/0000-0002-0984-3771
Jayanthi Chandar
orcid.org/0000-0002-2859-4184
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.