
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Transplant., 03 April 2025
Sec. Abdominal Transplantation
Volume 4 - 2025 | https://doi.org/10.3389/frtra.2025.1564460
 Luccas Marcolin Miranda1*†
Luccas Marcolin Miranda1*† Pedro Emanuel Carneiro De Lima2
Pedro Emanuel Carneiro De Lima2 Nathalia De Carvalho Dias Miranda3
Nathalia De Carvalho Dias Miranda3 Giovanna Zaniolo Margraf1
Giovanna Zaniolo Margraf1 Juliano Riella4
Juliano Riella4
Introduction: The shortage of organs remains one of the most challenging global problems nowadays. Donor's therapeutic hypothermia was suggested to decrease kidney delayed graft function (DGF) when compared to normothermia in previous trials, but the role of such intervention is still controversial. To assess this, we performed a systematic review and meta-analysis of randomized clinical trials (RCTs) investigating the benefits of donor hypothermia in DGF rate and Graft Failure.
Methods: MEDLINE, Embase, and Cochrane databases were systematically searched for studies of deceased organ donors who underwent hypothermia or normothermia prior to kidney transplantation. Statistical analysis was performed using R Studio version 3.6. Heterogeneity was assessed using I2 statistics and a Baujat Plot.
Results: Four different RCTs were analyzed, including more than 3,000 recipients. Donor hypothermia was associated with a lower, but not statistically significant, rate of DGF (RR 0.87; 95% CI 0.71–1.08; P = .21) and graft failure (RR 0.70; 95% CI 0.45–1.10; P = .12). When analyzing only expanded criteria donors, a significantly lower rate of DGF was observed in the hypothermia-treated group (RR 0.65; 95% CI 0.47–0.89; P = .008). Sensitivity analysis identified one study as an outlier, probably due to protocol deviation. When excluded from the analysis, a significant reduction in DGF rate was observed among the hypothermia-treated group (RR 0.80; 95% CI 0.67–0.94; P = .007).
Conclusion: Our meta-analysis could not find a statistical difference between donor therapeutic hypothermia and normothermia in preventing DGF or Graft Failure. However, these results may be influenced by outliers and the limitations of the included studies. Further research is needed to clarify the role of donor hypothermia in kidney transplantation. If proven beneficial, it could be a promising alternative to sites where preservation techniques are limited, such as low-income countries.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024581665, PROSPERO (CRD42024581665).
The shortage of organ donors remains one of the most challenging global problems nowadays (1). Even though the raw number of transplants has doubled within the last three decades, the number of patients on the waiting list has increased six-fold (1). This demand has also been reflected in the donor profile. In fact, expanded criteria donors (ECDs) were introduced in an attempt to reduce graft shortages (2).
Within kidney transplantation, this change can be seen by the increase in the use of HIV-positive and HCV-positive donors, and by the rising number of donors with an elevated Kidney Donor Profile Index (KDPI) (1, 3). Additionally, delayed graft function (DGF), defined as the need for dialysis within the first week after transplant, has also followed this trend, increasing over the past decade (4). As a result, there has been growing scientific interest in transplant and organ preservation research (1).
In this context and guided by an apparent protective role of hypothermia on the renal function of patients with cardiac arrest, Niemann et al. conducted in 2015 a randomized clinical trial demonstrating the benefit of donor therapeutic hypothermia in preventing DGF (5, 6). Although additional trials were conducted, the role of donor hypothermia is still controversial within the current literature (2, 7–10).
Given this ongoing controversy, a systematic review and meta-analysis of the available randomized clinical trials was performed to assess whether therapeutic hypothermia could indeed decrease kidney DGF and graft failure rates.
This systematic review and meta-analysis was performed following the Cochrane Collaboration Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Eligible studies included the following criteria: (1) Were Randomized Clinical Trials (RCTs); (2) Whose population was brain-dead deceased kidney donors; and (3) Compared donor's therapeutic hypothermia with normothermia. Furthermore, the studies were only included if they reported at least one of the outcomes of interest from this research: (1) Delayed graft function (DGF); (2) Graft failure or graft survival at 1 year; (3) Recipient mortality; or (4) Donor adverse events (hypotension, cardiac arrest, cardiac arrhythmias and/or systemic hypertension).
Additionally, studies were excluded from this research if: (1) Donors who underwent normothermia and therapeutic hypothermia were not randomized; or (2) The full paper was not available in English.
A systematic search was performed in PubMed (MEDLINE), EMBASE, and The Cochrane Central Register of Controlled Trials. The search block was built with a varied combination of the terms “kidney transplantation”, “hypothermia”, “deceased organ donor”, and their synonyms with Boolean operators. The Cochrane highly sensitive search strategies for the identification of randomized clinical trials were also included (6.4 Cochrane Handbook for Systematic Reviews of Interventions) (11). The protocol for this systematic review and meta-analysis was registered on PROSPERO (CRD42024581665).
Two authors (LMM and NDM) conducted an independent and blinded search. Abstracts were selected for full-text reading based on the inclusion criteria. The papers selected for full-text review were evaluated independently by each author, with results cross-checked. Any disagreements were addressed by the senior reviewer (JR). Moreover, the reference lists of all included studies were examined for any additional relevant titles.
Two researchers (LMM and PEL) independently extracted the data of interest from the included studies, which was then reviewed by the senior author (JR). The following data from individual studies were extracted: (1) study characteristics: study site, period, design, number of donors and recipients, follow-up time, population, and definition of hypothermia and normothermia; (2) donor characteristics: age, sex, height, weight, body mass index (BMI), proportion of standard and extended criteria donors (SCD and ECD respectively), prior treatment with hypothermia, creatine and estimated glomerular filtration rate at enrollment and before surgery, and kidney donor profile index (KDPI); (3) recipient characteristics: age, sex, height, weight, body mass index (BMI), donor-recipient weight ratio, positive hepatitis C virus (HCV), HLA mismatches, duration of RRT therapy before transplant, presence of previous renal transplant, and cold ischemia time; and (4) outcomes: delayed graft function (DGF), graft failure or survival, recipient mortality and donor adverse events.
The primary outcomes of this research were delayed graft function (DGF), defined as the need for dialysis within 1 week (7 days) of transplantation, and graft failure after 12 months, determined by allograft failure or dependency of renal replacement therapy (RRT). Donor's adverse events and recipients' mortality were addressed as secondary outcomes.
The risk of bias assessment was conducted using Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB-2), following the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions (11). Two authors (LMM and NDM) independently performed the evaluation, and disagreements were resolved through consensus. A study was categorized as having a high risk of bias if one or more domains assessed by the RoB-2 tool were rated as having a high or unclear risk.
Categorical results were expressed using Risk Ratios (RR) with 95% confidence intervals (95% CI). The Mantel-Haenszel test was used with a random effects model, and heterogeneity was assessed using Higgins and Thompson's I² statistic. An I² > 50% was considered significant for heterogeneity. Furthermore, the contribution of each trial to the overall heterogeneity was accessed through a Baujat Plot. Sensitivity analyses were performed for both primary outcomes by excluding each study from the outcome evaluation with the leave-one-out method. All statistical analyses were performed using R Studio version 3.6 software.
Finally, the subgroups addressed within this research were: (1) by country; (2) only ECD donors; and (3) by the adjunctive use of machine perfusion.
The systematic search yielded 164 results. Following the removal of duplicates and the exclusion of studies that did not meet the eligibility criteria based on abstract screening, 19 studies were selected for full-text review. Of these, 4 studies, encompassing data presented in 6 articles, fulfilled the inclusion criteria and were incorporated into the analysis (Figure 1 and Table 1).
Donors in all studies underwent therapeutic hypothermia (34°C–35°C) in the intervention group, while donors in the control group were maintained at normothermia (36.5°C–37.5°C). Of the four studies included in this research, three were conducted in the USA and one in France. The study population contained both SCD and ECD for all studies except for the HYPOREME trial whose population embraced only ECD donors (2). The percentage of females varied between 36% and 51% among kidney donors and 35%–42% among recipients. Mean age varied between 33.92 and 71.8 years between donors and 47.32–65.9 years among recipients. Only two papers included donors whose kidneys underwent adjunctive use of machine perfusion (2, 10). A summary of the studies and the characteristics of donors and recipients is available in Table 1 (Refer to Supplementary Tables S1, S2 for additional information).
Only one study was classified as having a high risk of bias (Supplementary Table S3) due to identified biases within the protocol deviation domain (10). Given the high level of heterogeneity observed, a random-effects model was applied to all statistical analyses.
DGF was reported in all four trials included. Donor hypothermia led to a numerically lower, but not statistically significant, rate of DGF (RR 0.87; 95% CI 0.71–1.08; P = .21) (Figure 2).
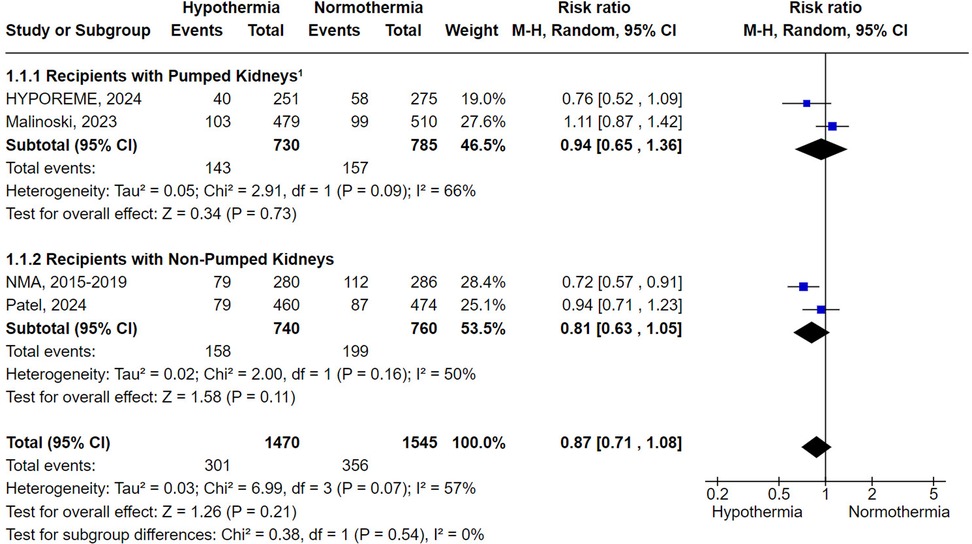
Figure 2. Delayed graft function with donor's therapeutic hypothermia vs. normothermia stratified by subgroup analysis involving the adjunctive use of machine perfusion. 1Around 10% of the kidneys in the HYPOREME trial were not submitted to machine perfusion, being a significant proportion due to severe atherosclerotic disease or unfavorable anatomy. Since these factors are already associated with poorer DGF outcomes and the subdivision of the trial's population would distort sample size calculation, the HYPOREME trial was analyzed in an intention-to-treat manner concerning the use of machine perfusion.
Subgroup analyses based on the adjunctive use of machine perfusion reached similar results for non-pumped and pumped kidneys (RR 0.81; 95% CI 0.63–1.05; P = .11 and RR 0.94; 95% CI 0.65–1.36; P = .73, respectively) (Figure 2). Similarly, the subgroup analysis limited to studies conducted in the USA did not achieve statistical significance (RR 0.90; 95% CI 0.70–1.17; P = .45) (Supplementary Figure S1). In contrast, the subgroup analysis focusing exclusively on ECDs demonstrated that hypothermia was statistically superior to normothermia in preventing DGF (RR 0.65; 95% CI 0.47–0.89; P = .008) (Figure 3).
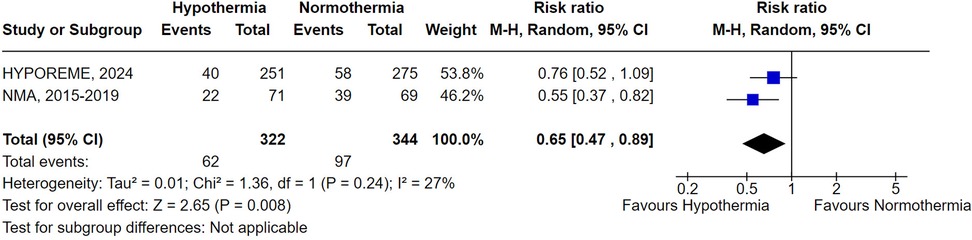
Figure 3. Delayed graft function with donor's therapeutic hypothermia vs. normothermia including only extended criteria donors.
Graft failure or necessity of RRT in 12 months was reported in 3 trials. Similar to DGF, therapeutic hypothermia was associated with a lower, but not statistically significant, incidence of graft failure or RRT dependency after 12 months (RR 0.70; 95% CI 0.45–1.10; P = .12) (Figure 4).
Due to limited data, only the subgroup analysis involving studies conducted in the USA was performed. Consistent with the overall analysis, hypothermia led to a non-statistically significant reduction in graft failure and RRT necessity (RR 0.68; 95% CI 0.42–1.10; P = .12) (Supplementary Figure S2).
Mortality outcomes were reported in two trials, while adverse events were documented in three of the four included studies (Supplementary Figures S3, S4). Neither the analyses of mortality nor adverse events reached statistical significance. Hypothermia was associated with a non-statistically significant decrease in recipient mortality (RR 0.91; 95% CI 0.58–1.43; P = .69) and an increase in donor adverse events (RR 1.28; 95% CI 0.45–3.62; P = .64).
When Malinoski et al. (2023) study (10), classified with a high risk of bias in the RoB-2 tool, was excluded from the analysis, donor hypothermia was associated with a statistically significant lower rate of DGF when compared to normothermia (RR 0.80; 95% CI 0.67–0.94; P = .007) (Figure 5). Additionally, the exclusion of this study also led to a non-significant heterogeneity (I2 = 4%). This observation is consistent with the Baujat Plot analysis which demonstrated that the study not only contributed disproportionately to the overall heterogeneity, occupying the highest value in the x-axis, but was also the study that most contributed to the overall pooled result (Supplementary Figure S5).
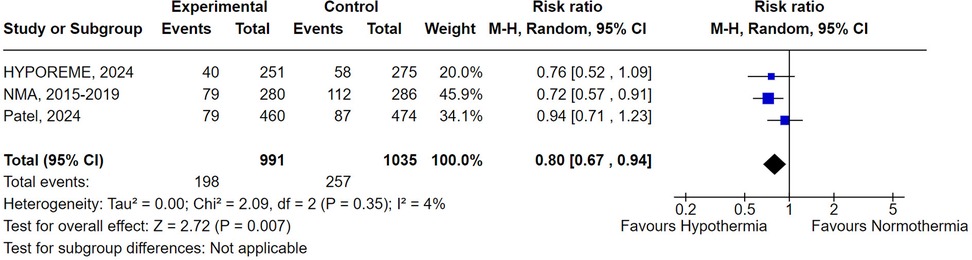
Figure 5. Delayed graft function with donor's therapeutic hypothermia vs. normothermia excluding Malinoski et al. (2023) (10).
The remaining sensitivity analyses for DGF and graft failure reached similar results to the overall pooled analyses, demonstrating a numerically, but not statistically significant, benefit of hypothermia in decreasing the rate of both DGF and graft failure (Supplementary Figures S6, S7).
The use of hypothermia as a therapeutic approach is not new. The induction of hypothermia dates back to the 1940s, when patients were routinely treated for cardiac arrest and traumatic brain injury with deep hypothermia (<30°C) (12). As expected, significant problems arose and the interest in hypothermia fizzled out until the 1980s (12). The positive results observed at that time, coupled with the understanding that mild hypothermia (31°C–35°C) could improve neurological outcomes without having many side effects, rekindled the interest of researchers in the topic (12).
Today, known benefits of hypothermia include the interruption of the apoptotic pathway, suppression of ischemic and ischemic-reperfusion immune responses, and reduction of free-radical production, events that are often present in DGF (12, 13). In fact, the pathophysiology of DGF, which resembles that of acute tubular necrosis, occurs due to a variety of processes that could be mitigated by hypothermia, including immunological reactions, endothelial damage, and ischemic injury (12, 13). However, despite these known benefits, the effect of donor hypothermia on renal function is still unclear (5).
Previous cohorts nested in randomized clinical trials have observed an association between spontaneous donor hypothermia and lower creatinine levels prior to organ procurement (7, 14). Furthermore, a study involving patients with cardiac arrest has suggested a potential protective effect of mild hypothermia on renal function (6). Still, randomized clinical trials were not conclusive in addressing this benefit, and the role of donor hypothermia in kidney transplantation remains uncertain.
To our knowledge, this is the first meta-analysis to address this topic. The analysis, including over 3,000 patients, revealed a numerical reduction in DGF and graft failure rates, though these findings were not statistically significant. However, after excluding Malinoski et al. (10), identified as an outlier in the sensitivity analysis, the decrease in DGF rates associated with donor hypothermia reached statistical significance and heterogeneity was significantly reduced.
A possible explanation for such a phenomenon would be the high risk of bias of the included outlier. Malinoski's work originally aimed to compare isolated hypothermia with hypothermic machine perfusion (HMP) or combination therapy. However, 27% (269/989) of the intended patients did not use hypothermic machine perfusion. To avoid selection bias, an intention-to-treat analysis was conducted (15). However, the study does not provide information on the distribution of these 269 patients across the study groups, which justifies its classification under the high-risk of bias category in the protocol deviation domain of the RoB-2 tool (16). Since our meta-analysis intended to compare normothermia and hypothermia in donors, the analyses included the study groups utilizing combination therapy and isolated machine perfusion. Therefore, unequal distribution of patients who did not undergo machine perfusion could introduce bias into our analysis, given the well-established benefit of HMP in preventing DGF (16). This imbalance may potentially explain why Malinoski's study was the only one to favor normothermia over hypothermia in preventing DGF. Furthermore, supporting our hypothesis, is a recent Cochrane review examining the role of normothermic and hypothermic machine perfusion in kidney transplantation (16). The review has also highlighted the potential bias of Malinoski's work, classifying it under the high-risk of bias category as well (16).
The use of machine perfusion (MP) dates back to 1968 when Belzer et al. successfully preserved a human kidney using HMP (17). However, the machine's considerable size posed significant challenges for transportation (16). Nowadays, MP technologies have evolved and differ substantially, including continuous modalities where perfusion is maintained during transport, or end-ischemic when the therapy is initiated at the implanting center after a period of cold storage during transport (16). Furthermore, MP techniques also vary in terms of oxygen provision (oxygenated vs. non-oxygenated) and flow patterns (pulsatile vs. non-pulsatile) (16).
Currently, guidelines recommend the use of HMP which is considered to be the standard of care for deceased kidney donors (18). Known benefits of HMP include lower rates and reduced duration of DGF, as well as improved overall graft survival when compared to static cold storage (16, 19). Moreover, these benefits are supported by robust up-to-date evidence, originating from well conducted RCTs and a large meta-analysis (16).
To address the possible influence of machine perfusion techniques in the context of hypothermia, we conducted subgroup analyses evaluating DGF in both pumped and non-pumped kidneys (Figure 5). Despite a clear graph skew demonstrating lower DGF rates in the hypothermia-treated group when considering only kidneys that have not undergone HMP, there was no statistical difference between the intervention and control. The same was true considering the analysis of the pumped kidneys, although hypothermia appeared to have a lesser impact on this population compared to the non-pumped organs. However, caution should be taken when interpreting such results given the possible lack of sufficient data, as only two trials were included within each analysis.
It is well established that extended criteria donors (ECDs) are often associated with inferior outcomes as the chronic conditions commonly present in these donors increase the risk of DGF (5). This elevated risk is driven by various physiological factors including a pro-inflammatory environment, oxidative stress, and ischemic injury which can be mitigated by the application of hypothermia (5, 12, 13, 20). Consequently, ECDs may, in theory, derive greater benefit from this therapy (5, 12, 13, 20). Our subgroup analysis supports this hypothesis; however, caution is warranted, as only two trials reported DGF events within this specific subgroup.
Due to the global organ shortage, great effort is being made to develop strategies to optimize organ usage without negatively affecting outcomes. The DONORS trial, protocolized in 2019, is a Brazilian multi-site cluster randomized controlled trial that proposes to assess the benefit of an evidence-based bedside checklist with goals and recommendations for the management of brain-dead organ donors (21). The trial suggests that clinical management strategies focused on hemodynamic stabilization, optimal ventilatory support, and temperature control may enhance organ quality and lead to improved clinical outcomes for transplant recipients (21).
In this context, if proven beneficial, donor therapeutic hypothermia could be a promising strategy as it is an easily feasible, low-cost intervention (2, 5, 7, 20, 22). Furthermore, even if its benefits are less pronounced or outweighed by those of machine perfusion, incorporating donor hypothermia into temperature control management strategies could be valuable as perfusion devices are not widely available in all transplant centers. In fact, machine perfusion is used in only 32%–38% of all kidneys considered for transplantation in the United States, and is rarely available in developing countries (10). Moreover, donor hypothermia may also lead to additional benefits beyond the potential reduction in DGF rate. Hypothermia was associated with statistically significant lower serum creatinine concentration and a higher estimated glomerular filtration rate (eGFR) at all timepoints evaluated in the HYPOREME trial, for example (2). Although not meta-analyzed, as this was reported in a single trial, these results are of clinical significance as several studies have already linked impaired kidney graft function at 1 year with increased risk of graft failure and cardiovascular death (23–26).
It is important to note, however, that Malinoski's trial, despite being limited by biases, has demonstrated that undergoing HMP when appropriate leads to lower rates of DGF when compared to undergoing hypothermia alone (10). Therefore, the value of therapeutic hypothermia in kidney transplantation is probably as an adjunctive therapy to machine perfusion, which should only be considered as an alternative to perfusion devices when these are not available. This approach is of particular interest in low-income countries, where access to perfusion devices may be limited.
Another important factor to consider is that donor therapeutic hypothermia is not without potential adverse effects. It can increase donor-related complications including cardiac arrest and other cardiovascular events, which are associated with organ loss (7, 21). Our findings did show a numerical increase in these events, but the difference was not statistically significant. Likewise, none of the trials that have evaluated the number of individual or total organs transplanted from each donor have reported significant differences between study arms, being the overall failure of non-kidney organ transplants also similar across the study groups (5, 10, 20). In addition, donor hypothermia does not seem to be associated with decreased urine output, hemodynamic instability, or elevated lactate blood concentration which could potentially negatively affect organ utilization and graft outcomes (2, 10).
Regarding rejection rates, these were reported in a single trial and, as a result, could not be meta-analyzed as initially planned (2). Nevertheless, the results from that trial showed no statistical differences between the intervention and control (2). Similarly, our meta-analysis has also not found a significant difference in recipient mortality between donor hypothermia and normothermia (Supplementary Figure S3), suggesting that donor therapeutic hypothermia is probably a safe intervention and that its benefits may outweigh its risks.
Despite the substantial interest in organ preservation research, very few studies explore interventions in donors (1, 5). These may reflect logistical and ethical aspects that surround donor-intervention research and the subsequent use of such organs (27, 28). A waitlist candidate offered a compatible organ that has been exposed to an intervention, for instance, might decline it specifically as a result of that exposition, while candidates who accept such organs will be directly exposed to the associated risks, thus making it difficult to carry out research exploring donor's interventions (27, 28). Without large high-quality trials, in turn, it is difficult to address the clinical benefits and risks of these potential interventions.
Moreover, although legally, research conducted on deceased donors does not require consent or ethics review committee approval, the intervention is rarely without any risk, raising a clear ethical issue that surrounds the implementation of interventions, such as hypothermia, in this type of donor (27, 28). Therefore, although donor therapeutic hypothermia is an easily feasible low-cost intervention in which benefits may outweigh the risks, logistical and ethical concerns may pose important barriers to its widespread clinical adoption.
Our meta-analysis has several limitations. Firstly, when analyzing the references of the full-text screened articles, the work of Kepu et al. was identified (29). This work randomly assigned 38 donors to therapeutic hypothermia or normothermia and included DGF as an observed outcome (29). The results section in the abstract mentioned a significantly lower DGF rate within the hypothermia-treated group (6%) when compared to normothermia (24%) (29). However, the article was not included in the present meta-analysis, as only the abstract was available in English. Therefore, we recommend expanding further research in order to assess non-English studies.
Moreover, although included studies defined hypothermia and normothermia groups equally, cooling protocols differed among the studies, and even within the same trial. Different cooling methods vary in terms of efficiency and safety, with intravascular cooling, gel pads, and water-circulating blankets being more effective compared to conventional cooling and air-circulating blankets, for example (30). This variability could introduce a bias that may influence the results of this analysis.
Thirdly, the role of hypothermia in the context of new technologies including normothermic machine perfusion (NMP) and normothermic regional perfusion (NRP) should also be investigated. In fact, portable NMP technologies have now entered phase 1 trials (16). Despite the non-significant benefit of NMP when compared to static cold storage observed in a recent RCT (31), a recent systematic review outlined the feasibility and possible benefit of both NMP and NRP within donors after circulatory death (DCD), which were also not included in our analysis (32). Moreover, hypothermic machine perfusion techniques also vary significantly, potentially impacting graft outcomes (16). Therefore, to effectively evaluate outcomes such as graft failure, ensuring equivalence between the machine perfusion techniques used is recommended.
Furthermore, although several sub-analyses were performed to address subgroups of interest, it was impossible to perform an analysis of DGF including only SCDs. Only one trial has reported DGF separately for standard criteria donors and the study has terminated early, making it difficult to draw conclusions on the impact of hypothermia among this population (5). Similarly, other subpopulations of interests such as HIV-positive donors and recipients transplanted after the HIV Organ Policy Equity (HOPE) Act and HCV-positive patients were also not addressed due to the lack of sufficient data.
Finally, only four randomized trials were included in this analysis, highlighting the need for further studies to more accurately address the role of donor's hypothermia in kidney transplantation.
An important limitation of the present meta-analysis is the lack of standardization in the design and reporting of included trials. Because of this, the authors have prepared a list of recommendations in an attempt to reduce the biases and assure comparability of future trials. These recommendations are summarized in Table 2.
Firstly, it is important to assure comparability of the cooling methods used across study groups as this may have a direct impact in graft outcomes. Cooling techniques that can effectively lead to rapid hypothermia induction, for example, can reduce the risks of short-term side effects such as shivering and metabolic disorders (33). In this context, intravascular cooling seems to be the most efficient cooling method for both inducing and maintaining therapeutic hypothermia (30, 33, 34). These benefits, however, must be weighed against the potential risks and barriers of this cooling method, including the necessity of an invasive procedure and its procedural risks (33). Other reliable alternatives are gel pads and water-circulating blankets, non-invasive techniques that show an overall similar efficiency to intravascular cooling (30, 33, 34). Conversely, conventional cooling and air-circulating blankets seem to be inferior to intravascular cooling, gel pads, or water-circulating blankets, and should be avoided when more reliable methods are available (30, 33, 34). The choice of a particular cooling method should be made by the clinician considering efficiency, safety and availability. If great variability is present, we advise conducting a sub-analysis stratifying outcome results per cooling method used.
Furthermore, the core temperature measurement method utilized should also be similar across study groups. The gold-standard site for measuring the core temperature is the pulmonary artery, but this method requires an invasive and complex insertion procedure (33, 35, 36). Alternatively, measuring the core temperature in the bladder, esophagus or rectum is less invasive and still provides reliable temperature measurements (33, 35, 36). In choosing the measurement site, it is important to consider factors such as accuracy, team expertise, and the limitations inherent to each technique.
Thirdly, considering the benefits of perfusion devices, we suggest stratifying outcome results by the use of machine perfusion. Additionally, we also recommend describing the type of perfusion devices used in respect to flow pattern (pulsatile vs. non-pulsatile) and oxygenation (oxygenated vs. non-oxygenated). Currently, there is limited data and no consensus regarding the optimal flow pattern or if oxygenated machine perfusion is superior to non-oxygenated devices, but this may be of importance when interpreting results in a near future (37–40). Moreover, it is also important that studies describe if the perfusion was maintained during transport or initiated at the transplanting center after a period of cold storage. This is often not reported, and not only might be of clinical interest, but give important insights on studies heterogeneity (16).
Lastly, the inclusion of additional outcomes, aside from donor adverse events, graft failure, recipient mortality, and DGF is also strongly recommended. Analyzing other potential benefits of hypothermia such as higher eGFR, and additional risks such as rejection rates, would provide important clinical evidence to better understand the advantages and limitations of donor hypothermia in kidney transplantation. Similarly, stratifying outcome results per donor type (ECD vs. SCD) is also recommended given the potential different impacts of therapeutic hypothermia within these two populations.
Our meta-analysis did not find a statistical difference between normothermia and therapeutic hypothermia in preventing DGF or graft failure. However, these results may be influenced by outliers and the limitations of the included studies. Further research is needed to better understand the role of therapeutic hypothermia in DGF rates, preferentially controlling for the cooling methods used, and for the adjunctive usage of machine perfusion techniques. Further analysis including non-English studies is also recommended. If proven beneficial, it could serve as a promising alternative to sites with limited access to other preservation techniques.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
LMM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. PEL: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. NDM: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. GM: Writing – review & editing. JR: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frtra.2025.1564460/full#supplementary-material
BMI, body-mass index; DGF, delayed graft function; ECD, extended criteria donor; eGFR, estimated glomerular filtrarion rate; HCV, hepatitis C virus; HIV, human immunodefficiency virus; HLA, human leukocyte antigen; HMP, hypothermic machine perfusion; HOPE, HIV organ policy equity (HOPE); JR, Juliano Riella, MD; KDPI, kidney profile index; LMM, Luccas M. Miranda; MP, machine perfusion; NDM, Nathalia Dias Miranda; PEL, Pedro Emanuel Lima; PRISMA, preferred reporting items for systematic reviews and meta-analysis; RCTs, randomized clinical trials; RoB-2, version 2 of the cochrane risk-of-bias tool for randomized trials; RR, risk ratio; RRT, renal replacement therapy; SCD, standard criteria donor; USA, United States of America.
1. Kupiec-Weglinski JW. Grand challenges in organ transplantation. Front Transplant (2022) 1:897679. doi: 10.3389/frtra.2022.897679
2. Canet E, Brule N, Pere M, Feuillet F, Blancho G, Martin-Lefevre L, et al. Hypothermia for expanded criteria organ donors in kidney transplantation in France (HYPOREME): a multicentre, randomised controlled trial. Lancet Respir Med. (2024) 12:693–702. doi: 10.1016/S2213-2600(24)00117-6
3. Israni AK, Zaun DA, Gauntt K, Schaffhausen CR, Lozano C, McKinney WT, et al. OPTN/SRTR 2022 annual data report: deceased organ donation. Am J Transplant (2024) 24:S457–88. doi: 10.1016/j.ajt.2024.01.018
4. Lentine KL, Smith JM, Miller JM, Bradbrook K, Larkin L, Weiss S, et al. OPTN/SRTR 2021 annual data report: kidney organ procurement and transplantation network, United Network for Organ Sharing (2023).
5. Niemann CU, Feiner J, Swain S, Bunting S, Friedman M, Crutchfield M, et al. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med. (2015) 373:405–14. doi: 10.1056/NEJMoa1501969
6. Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention*. Crit Care Med. (2008) 36:1780–6. doi: 10.1097/CCM.0b013e31817437ca
7. Westphal GA, Robinson CC, Cavalcanti AB, Gonçalves ARR, Guterres CM, Teixeira C, et al. Brazilian guidelines for the management of brain-dead potential organ donors. The task force of the AMIB, ABTO, BRICNet, and the general coordination of the national transplant system. Ann Intensive Care. (2020) 10:169. doi: 10.1186/s13613-020-00787-0
8. Anwar ASMT, Lee J. Medical management of brain-dead organ donors. Acute Crit Care. (2019) 34:14–29. doi: 10.4266/acc.2019.00430
9. Patel MS, Salcedo-Betancourt JD, Saunders C, Broglio K, Malinoski D, Niemann CU. Therapeutic hypothermia in low-risk nonpumped brain-dead kidney donors: a randomized clinical trial. JAMA Netw Open. (2024) 7:e2353785. doi: 10.1001/jamanetworkopen.2023.53785
10. Malinoski D, Saunders C, Swain S, Groat T, Wood PR, Reese J, et al. Hypothermia or machine perfusion in kidney donors. N Engl J Med. (2023) 388:418–26. doi: 10.1056/NEJMoa2118265
11. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions, version 6.5. The Cochrane Collaboration (2024). Available at: www.training.cochrane.org/handbook
12. Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. (2009) 37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241
13. Halloran PF, Hunsicker LG. Delayed graft function: state of the art, November 10–11, 2000. Summit meeting, Scottsdale, Arizona, USA. Am J Transplant. (2001) 1:115–20. doi: 10.1034/j.1600-6143.2001.10204.x
14. Schnuelle P, Mundt HM, Drüschler F, Schmitt WH, Yard BA, Krämer BK, et al. Impact of spontaneous donor hypothermia on graft outcomes after kidney transplantation. Am J Transplant. (2018) 18:704–14. doi: 10.1111/ajt.14541
15. Schnuelle P, Krämer BK. Donor conditioning and organ pre-treatment prior to kidney transplantation: reappraisal of the available clinical evidence. J Clin Med. (2024) 13:4073. doi: 10.3390/jcm13144073
16. Tingle SJ, Thompson ER, Figueiredo RS, Moir JA, Goodfellow M, Talbot D, et al. Normothermic and hypothermic machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst Rev. (2024) 7:CD011671. doi: 10.1002/14651858.CD011671.pub3
17. Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N Engl J Med. (1968) 278:608–10. doi: 10.1056/NEJM196803142781108
18. Breda A, Budde K, Figueiredo A, Lledó García E, Olsburgh J, Regele H, et al. EAU guidelines on renal transplantation (2024). Available online at: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-Guidelines-on-Renal-Transplantation-2024.pdf (Accessed February 18, 2025).
19. Moers C, Smits JM, Maathuis M-HJ, Treckmann J, van Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. (2009) 360:7–19. doi: 10.1056/NEJMoa0802289
20. Malinoski D, Patel MS, Axelrod DA, Broglio K, Lewis RJ, Groat T, et al. Therapeutic hypothermia in organ donors: follow-up and safety analysis. Transplantation. (2019) 103:e365–8. doi: 10.1097/TP.0000000000002890
21. Westphal GA, Robinson CC, Biasi A, Machado FR, Rosa RG, Teixeira C, et al. DONORS (donation network to optimise organ recovery study): study protocol to evaluate the implementation of an evidence-based checklist for brain-dead potential organ donor management in intensive care units, a cluster randomised trial. BMJ Open. (2019) 9:e028570. doi: 10.1136/bmjopen-2018-028570
22. Axelrod DA, Malinoski D, Patel MS, Broglio K, Lewis R, Groat T, et al. Modeling the economic benefit of targeted mild hypothermia in deceased donor kidney transplantation. Clin Transplant (2019) 33:e13626. doi: 10.1111/ctr.13626
23. Salvadori M, Rosati A, Bock A, Chapman J, Dussol B, Fritsche L, et al. Estimated one-year glomerular filtration rate is the best predictor of long-term graft function following renal transplant. Transplantation. (2006) 81:202–6. doi: 10.1097/01.tp.0000188135.04259.2e
24. Kasiske BL, Israni AK, Snyder JJ, Skeans MA, Patient Outcomes in Renal Transplantation (PORT) Investigators. The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis. (2011) 57:466–75. doi: 10.1053/j.ajkd.2010.10.054
25. Meier-Kriesche HU, Baliga R, Kaplan B. Decreased renal function is a strong risk factor for cardiovascular death after renal transplantation. Transplantation. (2003) 75:1291–5. doi: 10.1097/01.TP.0000061602.03327.E2
26. Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. (2002) 62:311–8. doi: 10.1046/j.1523-1755.2002.00424.x
27. Abt PL, Marsh CL, Dunn TB, Hewitt WR, Rodrigue JR, Ham JM, et al. Challenges to research and innovation to optimize deceased donor organ quality and quantity. Am J Transplant. (2013) 13(6):1400–4. doi: 10.1111/ajt.12243
28. Mone T, Heldens J, Niemann CU. Deceased organ donor research: the last research frontier? Liver Transpl. (2013) 19(2):118–21. doi: 10.1002/lt.23579
29. Liu K, Zhang G, Li Z, Ruan D, Gao L, Wang H, Zheng W, et al. Effect of hypothermia status in donors on renal graft function after renal transplantation from donation after citizen’s death. Organ Transplant. (2017) 8:376–80. doi: 10.3969/j.issn.1674-7445.2017.05.008
30. Hoedemaekers CW, Ezzahti M, Gerritsen A, van der Hoeven JG. Comparison of cooling methods to induce and maintain normo- and hypothermia in intensive care unit patients: a prospective intervention study. Crit Care. (2007) 11:R91. doi: 10.1186/cc6104
31. Hosgood SA, Callaghan CJ, Wilson CH, Smith L, Mullings J, Mehew J, et al. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: a randomized controlled trial. Nat Med. (2023) 29:1511–9. doi: 10.1038/s41591-023-02376-7
32. Klein Nulend R, Hameed A, Singla A, Yuen L, Lee T, Yoon P, et al. Normothermic machine perfusion and normothermic regional perfusion of DCD kidneys before transplantation: a systematic review. Transplantation. (2025) 109:362–75. doi: 10.1097/TP.0000000000005132
33. Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. (2009) 37:1101–20. doi: 10.1097/CCM.0b013e3181962ad5
34. Sonder P, Janssens GN, Beishuizen A, Henry CL, Rittenberger JC, Callaway CW, et al. Efficacy of different cooling technologies for therapeutic temperature management: a prospective intervention study. Resuscitation. (2018) 124:14–20. doi: 10.1016/j.resuscitation.2017.12.026
35. Lefrant JY, Muller L, de La Coussaye JE, Benbabaali M, Lebris C, Zeitoun N, et al. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. (2003) 29:414–8. doi: 10.1007/s00134-002-1619-5
36. Shin J, Kim J, Song K, Kwak Y. Core temperature measurement in therapeutic hypothermia according to different phases: comparison of bladder, rectal, and tympanic versus pulmonary artery methods. Resuscitation. (2013) 84:810–7. doi: 10.1016/j.resuscitation.2012.12.023
37. Lo Faro ML, Akhtar MZ, Boffa C, Ploeg R. Should pulsatile preservation be the gold standard in kidney transplantation? Curr Transplant Rep. (2015) 2:105–12. doi: 10.1007/s40472-015-0063-8
38. Sevinc M, Stamp S, Ling J, Carter N, Talbot D, Sheerin NS. Comparison of the outcome of kidney transplant after pulsatile or continuous ex vivo hypothermic machine perfusion of kidneys donated after cardiac death: analysis of kidney pairs. Transplant Proc. (2019) 51:1785–90. doi: 10.1016/j.transproceed.2019.03.025
39. Pravisani R, Baccarani U, Molinari E, Cherchi V, Bacchetti S, Terrosu G, et al. PO2 21% oxygenated hypothermic machine perfusion in kidney transplantation: any clinical benefit? Int J Artif Organs. (2022) 45:666–71. doi: 10.1177/03913988221107946
Keywords: delayed graft function, graft failure, graft survival, hypothermia, kidney transplantation
Citation: Marcolin Miranda L, De Lima PEC, Dias Miranda NDC, Margraf GZ and Riella J (2025) Donor's therapeutic hypothermia vs. normothermia in kidney transplantation: a meta-analysis of randomized controlled trials. Front. Transplant. 4:1564460. doi: 10.3389/frtra.2025.1564460
Received: 21 January 2025; Accepted: 11 March 2025;
Published: 3 April 2025.
Edited by:
Alan Langnas, University of Nebraska Medical Center, United StatesReviewed by:
David Peter Al-Adra, University of Wisconsin-Madison, United StatesCopyright: © 2025 Marcolin Miranda, De Lima, Dias Miranda, Margraf and Riella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luccas Marcolin Miranda, bHVjYy5tYXJjb2xpbm1pcmFuZGFAZ21haWwuY29t
†ORCID:
Luccas Marcolin Miranda
orcid.org/0009-0005-8885-0409
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.