
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Transplant., 08 January 2025
Sec. Abdominal Transplantation
Volume 3 - 2024 | https://doi.org/10.3389/frtra.2024.1483943
Introduction: The clinical characteristics of de novo inflammatory bowel disease (dnIBD) diagnosed after solid organ transplant (SOT) are not well-described, particularly since the advent of biologic therapy for treatment of IBD.
Methods: We conducted a single-center, retrospective review of SOT recipients between 2010 and 2022 at the University of Minnesota Medical Center who were diagnosed with IBD after transplant.
Results: Of 89 patients at our center with IBD and a history of SOT, five (5.6%) patients were diagnosed with IBD post-transplant (three liver, one kidney, and one simultaneous liver and kidney): three patients were female and four were Caucasian. Mean age at transplant and IBD diagnosis were 46.7 and 49.4 years respectively. Indication for transplant were alcohol-related cirrhosis (n = 2), idiopathic fulminant hepatic failure (n = 1), metabolic dysfunction-associated steatotic liver disease (n = 1), and IgA nephropathy (n = 1). Four patients were diagnosed with ulcerative colitis (UC) and one with Crohn's disease (CD). Three patients (all with UC) required escalation to a biologic therapy. Four patients were in clinical remission from IBD at last follow-up, one patient required IBD surgery, while there was no rejection and no deaths following IBD diagnosis.
Conclusion: dnIBD post-SOT is uncommon, while newer IBD therapies may be safe and effective. Further study is required to better understand the natural history and IBD outcomes of this population relative to non-SOT patients.
Inflammatory bowel disease (IBD) is a chronic, inflammatory condition of the gastrointestinal tract (GI), which includes ulcerative colitis (UC) and Crohn's disease (CD) (1). IBD's pathophysiology remains poorly understood but it is likely due to complex interactions between environmental factors, genetics, immune responses, and the microbiota (2). Solid organ transplantation (SOT) in patients with IBD is uncommon but those with primary sclerosing cholangitis (PSC) have slow progression to end-stage-liver-disease, with a median duration of time to liver transplantation (LT) of 15–20 years post diagnosis (3, 4).
Lifelong immunosuppression is required to prevent organ rejection after SOT; therefore, the development of de-novo (new onset) IBD (dnIBD) post SOT seems counterintuitive with immunosuppression use. Standard immunosuppression post SOT includes calcineurin inhibitors, which have a variable relationship with IBD. Tacrolimus, a calcineurin inhibitor, is associated with worsening IBD disease activity in pre-existing IBD (5, 6). Conversely, tacrolimus has been used as treatment for CD. Additionally, cyclosporine, is used as salvage therapy in cases of severe IBD refractory to intravenous steroids in hospitalized patients (7). However, dnIBD post SOT has been described, albeit with most cases following LT (8, 9). A recent case series from Japan described six cases of dnIBD in a cohort of patients who underwent living donor kidney transplantation (10).
In 1998, the FDA approved infliximab, a monoclonal antibody targeting tumor necrosis factor-alpha (TNF-α), for treatment of CD. The introduction of biologic therapy has transformed clinical outcomes for patients with IBD with higher rates of remission and a reduction in surgical interventions, while also reducing hospitalization rates and enhancing patients' quality of life (11, 12). Biologic therapies are integral to the treatment of moderate to severe IBD, with current guidelines recommending their use as first-line treatment particularly in the era of top-down approach to therapy for IBD (12). A recent meta-analysis reported that biologic and small molecule therapies appear to be well-tolerated in SOT patients with IBD, although data on the use of biologic therapy in patients with dnIBD after SOT are limited (13).
At this time, there is no clear consensus on management or surveillance of dnIBD after SOT, particularly surrounding the use of biologic therapy. In fact, diagnosis of dnIBD in SOT patients is challenging given the broad differential diagnosis, specifically infection and medication side effects. The aim of this study was to characterize the clinical presentation, management and clinical outcomes of individuals developing IBD after SOT at our institution over a period when the use of biologic therapy for non-SOT IBD was well-established.
We conducted a retrospective review of the electronic medical records of patients who underwent SOT at University of Minnesota Medical Center from 1/1/2010 through 12/31/2022. Patients were categorized with dnIBD if they developed IBD during any period after SOT, without a pre-SOT IBD diagnosis. Individuals with clinical signs or endoscopic evidence of IBD prior to SOT were excluded; four out of five patients included in the study had a normal colonoscopy prior to transplant. Records were reviewed for demographic information, SOT indication and outcomes, and IBD diagnosis and outcomes. Clinical documentation was reviewed for presenting symptoms and severity at time of IBD diagnosis, as described by the treating physicians' overall impression. Objective disease activity scores obtained from clinical documentation and endoscopy reports were reviewed when available. Response to therapy was determined by the treating provider's documented impression. Individuals with SOT and other forms of colitis who did not meet criteria for IBD were excluded from the study. Infection was ruled out in all cases at the time of diagnosis with negative clostridium difficile polymerase chain reaction (PCR) and enteric pathogen panel (when available), or negative stool bacterial culture, and ova and parasites testing. This study was conducted with the approval of the University of Minnesota Institutional Review Board (STUDY00017400).
Eighty-nine patients with a history of SOT and a diagnosis of IBD were initially identified during the study period: five patients were diagnosed with IBD after SOT and included in the study. Three (60%) patients were women. The mean age at transplant was 46.7 years (range, 24–66 years), mean age at IBD diagnosis was 49.4 years (range, 27–68 years), and the mean time from SOT to diagnosis of IBD was 3.1 years (range, 3–3.75 years). Four (80%) patients were non-Hispanic white, and one patient was Black/African American (Table 1). Four (80%) patients have no family history of IBD, with one patient having a family history of CD.
Three (60%) patients received LT, one received kidney transplant (KT), and one received simultaneous liver-kidney (SLK) transplant. Indications for SOT included IgA nephropathy, alcohol-related liver disease, and metabolic dysfunction-associated steatotic liver disease (MASLD). No patient had a history of transplant rejection prior to diagnosis of IBD. Four (80%) patients were taking tacrolimus-based immunosuppression regimens at the time of diagnosis of IBD, one patient (20%) was taking mycophenolate mofetil (MMF) in addition to tacrolimus, and another patient (20%) was taking mycophenolic acid (MPA) and low dose prednisone (5 mg per day) (Table 1).
Four (80%) patients presented with hematochezia, while the other patient presented with diarrhea. Four (80%) patients were diagnosed with UC and one with CD. Two (40%) patients presented with severe disease based on the treating providers' impressions of endoscopic Mayo score of 3.7 Three (75%) patients with UC presented with ulcerative pancolitis. The patient with CD presented with diarrhea and abdominal pain and was diagnosed with ileal CD complicated by a stricture (Table 1). Endoscopic and histopathologic features were consistent with IBD in all patients (Figures 1A, B, D).
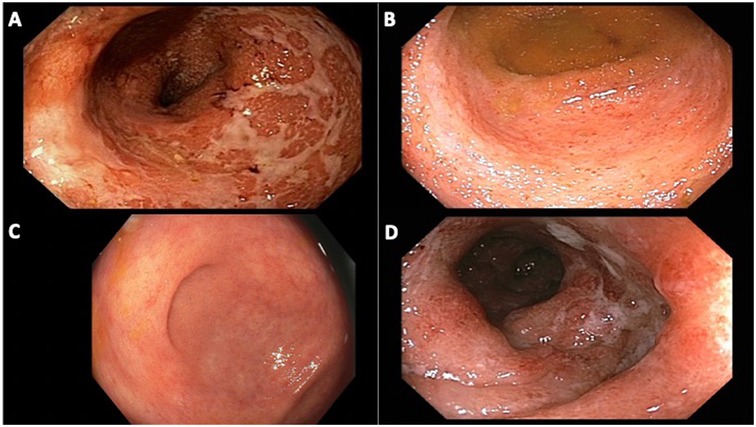
Figure 1. Endoscopic images of patients from our cohort with ulcerative colitis. (A) patient 1, rectum with diffuse erythema and congestion, ulcerated mucosa, at time of diagnosis of ulcerative proctocolitis; (B) patient 2, cecum with congestion, erosions and friability, at time of diagnosis of ulcerative pan-colitis; (C) patient 3, cecum with minimal patchy erythema and granularity, on oral mesalamine for 18 months after diagnosis of ulcerative pancolitis; (D) patient 4, sigmoid colon with diffuse edema, patchy erythema and ulcerations, at time of diagnosis of ulcerative pan-colitis.
Two patients were on MMF and MPA at the time of diagnosis, which are known to induce IBD-like inflammation. Histologic features of MMF/MPA-induced colitis, such as apoptotic bodies and eosinophilic infiltrates, were not seen (Figure 2). Both cases underwent expert pathology review and were deemed consistent with IBD rather than drug-induced colitis. In both cases, discontinuation of MMF/MPA was considered if no improvement was observed following IBD treatment.

Figure 2. Pathology images of patients from our cohort with ulcerative colitis. H&E staining showing marked lamina propria lymphoplasmacytic inflammation, irregular crypt, and neutrophilic cryptitis and crypt abscess. (A) 50X. (B) 100X. (C) 200X. (D) 200X.
Within the first 6-months of diagnosis, three individuals required escalation to an advanced therapy with vedolizumab. Two patients, both with severe UC, were treated with corticosteroids at the time of diagnosis. By six months, both transitioned to vedolizumab: one experienced clinical remission, while the other did not and was subsequently transitioned to ustekinumab.
Two patients with mild UC were initially treated with 5-aminosalicylic acid (ASA). One experienced clinical remission on mesalamine monotherapy while the other had ongoing disease activity and was transitioned to vedolizumab. This patient experienced clinical remission on vedolizumab.
The patient with CD continued treatment with azathioprine, which was already being used as immunosuppression for his LT. No dose adjustments were made to azathioprine. This patient was documented as being in clinical remission at 6-months post-diagnosis.
The patient with CD underwent right hemicolectomy at ∼12 months after IBD diagnosis. Ileal biopsies taken at a surveillance colonoscopy showed evidence of a villous adenoma and a possible adenocarcinoma. Histopathology of the resection specimen showed two synchronous ileal adenocarcinomas- one adenocarcinoma was poorly differentiated and the other was moderately differentiated. Azathioprine was continued at the same dose post-ileal resection and the patient was in full remission at last follow-up.
At the time of last follow-up, three of the patients with UC were in clinical remission: one on mesalamine monotherapy and two on vedolizumab (Figure 1C). Two UC patients in clinical remission were also in endoscopic and histologic remission. The patient with UC on vedolizumab and mesalamine had mild inflammation on endoscopy. The final patient, who had been transitioned to ustekinumab within the first 6 months of IBD diagnosis, had poorly controlled disease at the time of last follow-up and was initiating ozanimod (Table 1).
One patient with UC, who was maintained on mesalamine, developed recurrent clostridium difficile infection since IBD diagnosis and received fecal microbiota transplant. The patient with CD developed two ileal adenocarcinomas (see above), but no dysplasia was noted in the patients with UC. No patients developed acute organ rejection, however, one patient developed graft failure due to chronic antibody-mediated rejection and recurrent IgA nephropathy. Transplant immunosuppression medications were not changed over the course of IBD therapy. There were no patients with documented extraintestinal disease after IBD diagnosis and no patients had died by the time of last follow-up.
In this report, we describe our experience with dnIBD following SOT, which accounted for 5.6% of our center's IBD-SOT population. Clinical presentations of dnIBD were similar to those in non-SOT IBD, with MMF/MPA-associated colitis considered in two patients. Most SOT patients with dnIBD at our center required an advanced therapy, which favored biologic agents with preferable safety profiles. Importantly, no major treatment complications were noted in our patients.
The pathophysiology of IBD remains incompletely understood, but likely involves a variety of factors including genetics, environmental, immune responses, and microbes (2, 14). Genome studies have identified more than 200 genetic mutations that have been linked to IBD (15). T-cells of the adaptive immune response have been implicated in the development of IBD, as these responses are heightened in UC and CD (15). Almost paradoxically, immunosuppression in SOT patients is primarily directed at regulation of the alloimmune T-cell response to an allograft (15). Of note, most of the patients in our cohort did not have immune-related indications for transplantation, which can be associated with development of IBD.
Other factors such as medication use and the microbiome, are also important in the development of de novo IBD. The use of medications such as statins and non-steroidal anti-inflammatory drugs (NSAIDs), have been associated with an increased risk of developing IBD (16). Transplantation results in changes to a patient's microbiome, while the use of immunosuppression itself leads to immune responses against the gut microbiome that are dysregulated, which increases the risk of IBD development (17). In our cohort, one patient had a history of IgA nephropathy, which is associated with IBD, although the exact relationship (and pathophysiology) remains poorly understood (18–20). Finally, smoking has been implicated with the development of IBD, particularly CD- the only patient in our cohort with CD was a former smoker (21).
MMF-induced colitis is an important consideration in SOT patients presenting with diarrhea and/or hematochezia. Two patients in our cohort were taking either MMF or MPA at the time of diagnosis of IBD. MMF toxicity can affect the entire GI tract, while MMF-induced colitis has been reported in up to 9% of SOT recipients on MMF who undergo colonoscopy (22, 23). Histological findings of MMF-induced colitis may be clinically and endoscopically indistinguishable from IBD, although histology shows a predominance of eosinophils in the mucosa with a lack of apoptotic micro-abscesses and endocrine cell aggregates in the lamina propria (5, 22). Discontinuation of MMF may cause a quick resolution of symptoms, but symptoms can persist for months in specific cases, raising the concern for IBD (5, 23). In both of our cases who were taking MMF/MPA, drug-induced colitis was considered, but a satisfactory diagnosis of IBD was made after pathology review and close observation over time. This highlights the importance of multidisciplinary teams including a transplant hepatologist, gastrointestinal pathologist and IBD specialist working together to balance the safety of changing SOT medications while treating intestinal inflammation (23).
Our study provides necessary granular detail on the use of biologic therapy in SOT patients with dnIBD. In particular, this level of detail can provide important context to clinicians needing to make decisions on the use advanced therapies in patients with more severe disease. There is currently limited data on the use and efficacy of advanced therapies in SOT patients with dnIBD (24, 25). A recent meta-analysis provided minimal safety information on SOT patients with dnIBD who received biologic therapies (13). In general, the use of biologic therapy for all indications in SOT recipients is safe and effective, albeit with a concern for the development of severe infections (24). Biologic and small molecule therapies in SOT patients with pre-existing IBD are largely well-tolerated: a meta-analysis showed that infectious complications were similar to rates seen in SOT patients without IBD, as were rates of colectomy and discontinuation of biologic therapy (13).
In our cohort, vedolizumab was the most used biologic agent. Vedolizumab is a gut-selective, anti-integrin blocker that binds to leucocyte integrin α4β7 that is considered to have a lesser effect on the immune system when compared to other monoclonal antibodies, making it a preferable medication for SOT recipients (26). A recent systematic review and meta-analysis showed that patients with UC on vedolizumab had a lower risk of serious infections when compared to infliximab, although another study reported that infliximab may be more effective at induction of remission in these patients (27, 28). However, a systematic review and meta-analysis evaluating outcomes of biologic and small molecule therapies, specifically in SOT patients with major emphasis of a diagnosis of IBD pre-transplant, suggested that rates of severe infections were higher in patients taking vedolizumab, albeit the studies used were not adjusted for disease severity and other confounders (13). Only one patient on systemic immunosuppression for IBD in our cohort developed a serious or severe infection. Our experience suggests that providers may prioritize (perceived) safety when selecting an advanced therapy. Furthermore, transplant immunosuppression regimens were stable in our cohort: we hypothesize that treating gastroenterologists may have deferred to transplant immunosuppression as “primary immunosuppression” and subsequently chosen milder IBD treatments. Qualitative studies examining therapeutic relationships between transplant and gastroenterology providers are needed to better understand decision-making regarding immunosuppression in this patient population.
Our cohort adds to the literature by describing outcomes of IBD development post SOT. It is possible that patients who develop IBD while taking SOT immunosuppression may have a more aggressive underlying disease phenotype, however clinical outcomes in our cohort were favorable as four of five patients achieved clinical remission at the time of last follow-up. A multicenter retrospective study of SOT in an pre-existing IBD population described a clinical remission rate of 61% in patients following transplantation (29). One patient in our cohort did require surgery for a malignant ileal stricture. In non-SOT patients with CD, rates of surgery are decreasing, possibly related to advances in medical therapy (30, 31). In two studies of patients with pre-existing IBD who underwent SOT, rates of surgery were low both before and after transplantation (29, 32).
Of note, no patients in our cohort developed acute rejection following diagnosis of dnIBD over the follow-up period. As mentioned previously, transplant immunosuppression medication regimens were stable over the follow-up period. Treatments for IBD primarily target T-cell activity in the immune system, while activation of the T-cell response is one of the main cascades responsible for the development of acute rejection (33). It is possible that the presence of IBD may in fact be a protective factor against rejection in SOT patients: acute rejection was also rare in a cohort of patients with pre-existing IBD who underwent non-LT SOT (32).
Our study has clear limitations related to both the retrospective nature of the data collection and the small sample size. The retrospective study design leads to variable time follow-up and limits the ability to address confounding factors: specifically, we cannot be 100% sure that there was no IBD preceding SOT. However, medical records were extensively manually reviewed and there was, at a minimum, no clinical IBD activity. As a retrospective analysis, there is potentially important data on risk factors, e.g., HLA typing, that is not available for our review. Additionally, the small sample size limits the ability to infer accurate trends among this population but does allow for data collection in more granular detail. Larger sample sizes are clearly needed to accurately estimate the true rate of clinical outcomes in this unique patient population. Additionally, as a single center study, the results may not be generalizable, but we anticipate that most transplant centers reflect a population similar to ours. These are unavoidable limitations of this analysis. However, our study provides information to guide future studies, which are required to better understand the natural history and outcomes of dnIBD after SOT.
In summary, we report our experience of five patients who developed IBD following SOT. dnIBD post SOT is uncommon but should be considered in SOT patients presenting with typical IBD symptoms. The key highlight of our small study is that most individuals required specific treatment of IBD in the post-transplant setting, with some requiring an advanced therapy to achieve clinical remission. And, when choosing IBD therapy, there appears to be a provider bias towards medications with a favorable safety profile. Multicenter studies are required better understand clinical outcomes, impact of transplant type, and long-term impact of biologic therapies in patients with dnIBD after SOT.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by University of Minnesota Institutional Review Board (STUDY00017400). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
WJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing. BV: Conceptualization, Formal Analysis, Resources, Writing – review & editing. NL: Conceptualization, Formal Analysis, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ASA, 5-aminosalicylic acid; CD, Crohn's disease; dnIBD, de novo inflammatory bowel disease; IBD, inflammatory bowel disease; KT, kidney transplantation; LT, liver transplantation; MASLD, metabolic dysfunction-associated steatotic liver disease; MMF, mycophenolate mofetil; MPA, mycophenolic acid; PSC, primary sclerosing cholangitis; SLK, simultaneous liver-kidney transplant; SOT, solid organ transplant; UC, ulcerative colitis.
1. Martinez Montiel MDP, Casis Herce B. Inflammatory bowel disease and solid organ transplantation. Rev Esp Enferm Dig. (2020) 112: 60–65. doi: 10.17235/reed.2020.7361/2020
2. Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. (2019) 12(2):113–22. doi: 10.25122/jml-2018-0075
3. Boonstra K, Weersma RK, van Erpecum KJ, Rauws EA, Marcel Spanier BW, Poen AC, et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology. (2013) 58(6):2045–55. doi: 10.1002/hep.26565
4. Filipec Kanizaj T, Mijic M. Inflammatory bowel disease in liver transplanted patients. World J Gastroenterol. (2017) 23(18):3214. doi: 10.3748/wjg.v23.i18.3214
5. Indriolo A. Clinical management of inflammatory bowel disease in the organ recipient. World J Gastroenterol. (2014) 20(13):3525. doi: 10.3748/wjg.v20.i13.3525
6. Verdonk RC, Dijkstra G, Haagsma EB, Shostrom VK, Van den Berg AP, Kleibeuker JH, et al. Inflammatory bowel disease after liver transplantation: risk factors for recurrence and de novo disease. Am J Transplant. (2006) 6(6):1422–9. doi: 10.1111/j.1600-6143.2006.01333.x
7. Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. (2020) 158(5):1450–61. doi: 10.1053/j.gastro.2020.01.006
8. Ghouri YA, Tahan V, Shen B. Secondary causes of inflammatory bowel diseases. World J Gastroenterol. (2020) 26(28):3998–4017. doi: 10.3748/wjg.v26.i28.3998
9. Hampton DD, Poleski MH, Onken JE. Inflammatory bowel disease following solid organ transplantation. Clin Immunol. (2008) 128(3):287–93. doi: 10.1016/j.clim.2008.06.011
10. Ogata M, Kato M, Miyauchi T, Murata-Hasegawa M, Sakurai Y, Shinoda K, et al. de novo inflammatory bowel disease in kidney transplant recipients: a single-center case series study. Inflamm Intest Dis. (2024) 9(1):96–102. doi: 10.1159/000538334
11. Berg DR, Colombel JF, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis. (2019) 25(12):1896–905. doi: 10.1093/ibd/izz059
12. Aberra FN, Lichtenstein GR. Infliximab in ulcerative colitis. Gastroenterol Clin North Am. (2006) 35(4):821–36. doi: 10.1016/j.gtc.2006.09.002
13. Taneja V, Anand RS, El-Dallal M, Dong J, Desai N, Taneja I, et al. Safety of biologic and small molecule therapy for inflammatory bowel disease among solid organ transplant recipients: systematic review and meta-analysis. Inflamm Bowel Dis. (2024) 30(4):585–93. doi: 10.1093/ibd/izad108
14. Zhang Y-Z. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. (2014) 20(1):91. doi: 10.3748/wjg.v20.i1.91
15. Flynn S, Eisenstein S. Inflammatory bowel disease presentation and diagnosis. Surg Clin North Am. (2019) 99(6):1051–62. doi: 10.1016/j.suc.2019.08.001
16. Kumbala D, Zhang R. Essential concept of transplant immunology for clinical practice. World J Transplant. (2013) 3(4):113. doi: 10.5500/wjt.v3.i4.113
17. Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. (2019) 94(1):155–65. doi: 10.1016/j.mayocp.2018.09.013
18. Rehnberg J, Symreng A, Ludvigsson JF, Emilsson L. Inflammatory bowel disease is more common in patients with IgA nephropathy and predicts progression of ESKD: a Swedish population-based cohort study. J Am Soc Nephrol. (2021) 32(2):411–23. doi: 10.1681/ASN.2020060848
19. Nakayama T, Kaneko H, Okada A, Suzuki Y, Fujiu K, Takeda N, et al. Association of inflammatory bowel disease with incident IgA nephropathy. Clin J Am Soc Nephrol. (2024) 19(6):704–11. doi: 10.2215/CJN.0000000000000457
20. Ravipati P, Reule S, Bren A, Bu L, Vaughn BP, Nachman PH. Kidney biopsy findings and clinical outcomes of US veterans with inflammatory bowel disease. Glomerular Dis. (2023) 3(1):233–40. doi: 10.1159/000534062
21. Gibson CM, Childs-Kean LM, Naziruddin Z, Howell CK. The alteration of the gut microbiome by immunosuppressive agents used in solid organ transplantation. Transpl Infect Dis. (2021) 23(1):e13397. doi: 10.1111/tid.13397
22. Sharma A, Giorgakis E. Gut microbiome dysbiosis in the setting of solid organ transplantation: what we have gleaned from human and animal studies. World J Transplant. (2022) 12(7):157–62. doi: 10.5500/wjt.v12.i7.157
23. Farooqi R, Kamal A, Burke C. Mycophenolate-induced colitis: a case report with focused review of literature. Cureus. (2020) 12(1):e6774. doi: 10.7759/cureus.6774
24. Solitano V, Facciorusso A, Jess T, Ma C, Hassan C, Repici A, et al. Comparative risk of serious infections with biologic agents and oral small molecules in inflammatory bowel diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2023) 21(4):907–921.e2. doi: 10.1016/j.cgh.2022.07.032
25. Peyrin-Biroulet L, Arkkila P, Armuzzi A, Danese S, Guardiola J, Jahnsen J, et al. Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: a systematic review and meta-analysis. BMC Gastroenterol. (2022) 22(1):291. doi: 10.1186/s12876-022-02347-1
26. Meyer F, Weil-Verhoeven D, Prati C, Wendling D, Verhoeven F. Safety of biologic treatments in solid organ transplant recipients: a systematic review. Semin Arthritis Rheum. (2021) 51(6):1263–73. doi: 10.1016/j.semarthrit.2021.08.013
27. Sands BE, Peyrin-Biroulet L, Loftus EV Jr, Danese S, Colombel JF, Toruner M, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. (2019) 381(13):1215–26. doi: 10.1056/NEJMoa1905725
28. Wyant T, Leach T, Sankoh S, Wang Y, Paolino J, Pasetti MF, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. (2015) 64(1):77–83. doi: 10.1136/gutjnl-2014-307127
29. Schnitzler F, Friedrich M, Stallhofer J, Schonermarck U, Fischereder M, Habicht A, et al. Solid organ transplantation in patients with inflammatory bowel diseases (IBD): analysis of transplantation outcome and IBD activity in a large single center cohort. PLoS One. (2015) 10(8):e0135807. doi: 10.1371/journal.pone.0135807
30. Zhulina Y, Udumyan R, Tysk C, Montgomery S, Halfvarson J. The changing face of Crohn’s disease: a population-based study of the natural history of Crohn’s disease in Örebro, Sweden 1963–2005. Scand J Gastroenterol. (2016) 51(3):304–13. doi: 10.3109/00365521.2015.1093167
31. Ramadas AV, Gunesh S, Thomas GAO, Williams GT, Hawthorne AB. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut. (2010) 59(9):1200–6. doi: 10.1136/gut.2009.202101
32. Ribaldone DG, Vieujean S, Julsgaard M, Armandi A, Zingone F, Savarino E, et al. Non-hepatic solid organ transplant in patients with inflammatory bowel disease: an ECCO CONFER multicentre case series. J Crohn’s Colitis. (2023) 17(7):1097–102. doi: 10.1093/ecco-jcc/jjad030
Keywords: solid organ transplant, Crohn's disease, ulcerative colitis, immunosuppression, inflammatory bowel disease
Citation: Johnson WM Jr, Vaughn BP and Lim N (2025) Diagnosis and management of de novo inflammatory bowel disease after solid organ transplantation in the era of biologic therapy: a case series. Front. Transplant. 3:1483943. doi: 10.3389/frtra.2024.1483943
Received: 20 August 2024; Accepted: 23 December 2024;
Published: 8 January 2025.
Edited by:
Alan Langnas, University of Nebraska Medical Center, United StatesReviewed by:
David Peter Al-Adra, University of Wisconsin-Madison, United StatesCopyright: © 2025 Johnson, Vaughn and Lim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicholas Lim, bmxpbUB1bW4uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.