
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Transplant. , 27 February 2024
Sec. Abdominal Transplantation
Volume 3 - 2024 | https://doi.org/10.3389/frtra.2024.1352220
This article is part of the Research Topic Editors' Showcase 2023: Abdominal Transplantation View all 4 articles
 Paolo De Simone1,2*
Paolo De Simone1,2* Giacomo Germani3
Giacomo Germani3 Quirino Lai4
Quirino Lai4 Juri Ducci1
Juri Ducci1 Francesco Paolo Russo5
Francesco Paolo Russo5 Stefano Gitto6
Stefano Gitto6 Patrizia Burra3,5
Patrizia Burra3,5
Despite global expansion, social disparities impact all phases of liver transplantation, from patient referral to post-transplant care. In pediatric populations, socioeconomic deprivation is associated with delayed referral, higher waitlist mortality, and reduced access to living donor transplantation. Children from socially deprived communities are twice as much less adherent to immunosuppression and have up to a 32% increased incidence of graft failure. Similarly, adult patients from deprived areas and racial minorities have a higher risk of not initiating the transplant evaluation, lower rates of waitlisting, and a 6% higher risk of not being transplanted. Social deprivation is racially segregated, and Black recipients have an increased risk of post-transplant mortality by up to 21%. The mechanisms linking social deprivation to inferior outcomes are not entirely elucidated, and powered studies are still lacking. We offer a review of the most recent evidence linking social deprivation and post-liver transplant outcomes in pediatric and adult populations, as well as a literature-derived theoretical background model for future research on this topic.
The success of liver transplantation (LT) depends on multiple factors, including medical, surgical, biological, psychological, and social determinants (1). However, the impact of social determinants of healthcare on LT has not been fully explored, though their role in other medical and surgical fields has been largely investigated (2). Understanding the contribution of socioeconomic deprivation (SED) to health outcomes has become an increasingly popular topic of interest (3). Many social and environmental factors affect individual health, including economic stress, limited access to healthcare facilities, poor air quality, high-density housing, inadequate infrastructure maintenance, and lack of safe outdoor spaces (3–6). After controlling for individual medical variables, SED independently predicts specific poor health outcomes. This indicates that social and environmental barriers beyond the control of individual patients play a major role in driving health outcomes (7, 8).
SED is a crucial factor to consider for the best possible outcome of pediatric and adult LT (9). If we can understand the extent to which deprivation characteristics are associated with post-transplant outcomes, we can identify actionable objectives for health improvement (9). This will also help develop fair and equitable metrics for benchmarking and reimbursement and improve the quality of transplant care.
In this review, we use concepts from ethics and public health and examine literature studies to describe the impact of SED on LT outcomes. In addition, we provide a theoretical foundation to direct future studies on this subject.
SED, or poverty in a broader sense, is the lack of social and economic resources necessary for a good quality of life (10, 11). SED is a complex measure that considers various factors such as individual, family, community, geographical, and national variables, and it depends heavily on the country, ethnicity, and culture (as illustrated in Table 1). SED components are constantly changing, and items are being added according to location (i.e., Europe vs. the US vs. Asia), communities (i.e., neighborhood), culture, study designs, and objectives (12, 13).
There are four essential components in SED (11): (1) Socioeconomic status—which includes individual and household income, employment status, and type of occupation; (2) Housing—which refers to living in rental or owned properties and the number of people living together; (3) Family disposition—whether an individual lives alone or with a partner, and the presence of children; and (4) Type of neighborhood—which refers to the availability of community and social resources (i.e., transportation, social services, health facilities, public schools, recreation areas, and activities, etc…). Some authors list additional social factors affecting transplant health outcomes, including racism, discrimination, violence, limited medical knowledge (i.e., medical illiteracy), unstable housing, transportation, childcare, and food insecurity (9).
Although ethnicity does not make up the components of SED, there exists a complex relationship between SED and race because racially segregated communities frequently face discrimination, marginalization, and limited access to healthcare (14). As with non-liver transplantation (15–17), differences in patient and graft survival rates among LT recipients have been well documented in the literature (18, 19). Recently, the US Congress requested that the National Institutes of Health sponsor a study on equity in the US organ transplant system by the National Academies of Sciences, Engineering, and Medicine (20). This was due to significant performance variation, with inequality in race, ethnicity, location, and socioeconomic status (20). Reports on survival after LT have shown differences across race and ethnicity in the US (21). Race has two related meanings in the causal pathways leading to transplant outcomes. Firstly, it is a social classification with observable biological consequences (i.e., graft rejection) that do not always accurately correspond to genetic variation/predisposition (19). Secondly, it is an indicator of potential exposure to systematic racism, implicit bias, and limited access to healthcare (22). A significant body of literature posits SED to explain outcome differences across racial groups (20). However, the role of socio-economic deprivation (SED) in perpetuating racial disparities in access to and outcomes of organ transplantation remains poorly understood (19).
Research on SED has been conducted in recent years in the US, with investigations in pediatric (23–29) and adult LT populations (30–36). Limited evidence is available from Europe (35), and no information can be derived from Asian countries. In pediatric populations, the impact of SED has been investigated regarding waitlist registrations (25), post-transplant outcomes (23, 26–28), and immunosuppression adherence (29). Additionally, some authors have investigated the effect of environmental risk factors (i.e., air pollution) (24). For adult LT recipients, the focus has been on referral patterns (30, 34), waitlist registrations (31), mode of liver transplant evaluation (33), post-transplant survival (36), quality of life (32) and the risk of post-transplant hepatocellular carcinoma (HCC) recurrence (35).
Table 2 summarizes the findings of the current review.
Based on literature data, SED accounts for limited or delayed access to pre-transplant care, and the impact of neighborhood deprivation is particularly significant for racial/ethnic minority children (25). Black and Hispanic children have higher lab PELD/MELD scores than White children, indicating possible delays in referral, listing, or transplantation (9, 25, 28). In a recent, extensive registry analysis of 7,716 patients, Black and Hispanic children had increased unadjusted hazard of waitlist mortality than White children [subhazard ratio (sHR) = 1.44; 95% CI = 1.18–1.75 for Black patients and sHR = 1.48; 95% CI = 1.25–1.76 for Hispanic children, respectively] (25). However, after adjusting for neighborhood deprivation, insurance, and Model for End-Stage Liver Disease (MELD)/Pediatric End-Stage Liver Disease (PELD), Black and Hispanic children did not have increased hazard of waitlist mortality (sHR, 1.12; 95% CI, 0.91, 1.39 and sHR, 1.21; 95% CI, 1.00, 1.47, respectively) (25). Similarly, Black and Hispanic children had decreased likelihood of LDLT (sHR, 0.58; 95% CI, 0.45, 0.75 and sHR, 0.61; 95% CI, 0.49, 0.75, respectively), but adjustment attenuated the effect of Black and Hispanic race/ethnicity on likelihood of LDLT (sHR, 0.79; 95% CI, 0.60, 1.02 and sHR, 0.89; 95% CI, 0.70, 1.11, respectively) (25). These results indicate that incorporating SED and disease severity into the model reduces the differences observed between children of different ethnicities (25). However, a more nuanced understanding of how neighborhood adversity impacts clinical results is still lacking (25).
Non-standard exception requests may also explain waitlist disparities (9). In 2019, 75% of pediatric transplant recipients had an exception score at the time of transplant (37). Still, children of non-White race, including Black, Hispanic, Asian, American Indian/Alaska Native, Native Hawaiian/Other Pacific Islander, and multiracial children, had 13% lower rates of exception score requests submitted by the transplant team (38). Children with exception approval have a decreased risk of graft loss, while those with exception denial have a higher risk of post-transplant death (39).
Another potential contributor to waitlist disparities may be differential access to living donor liver transplantation (LDLT). For Black children, Mogul et al. found a reduced incidence of LDLT vs. White children utilizing the Scientific Registry of Transplant Recipients (SRTR) (40). This was confirmed by Wadhwani et al., who reported that Black and Hispanic children were about half as likely to undergo LDLT compared to White children (sHR = 0.58; 95% CI = 0.45–0.75 for Black children and sHR = 0.61; 95% CI = 0.49–0.75 for Hispanic recipients, respectively) (28). This disparity in LDLT rates for children may lead to longer wait times, higher waitlist morbidity and mortality, and inferior post-transplant graft and patient survival (9).
Neighborhood SED has also been associated with adverse outcomes before transplantation. In unadjusted analyses, each 0.1 increase in the deprivation index was associated with a 9% increased sub-hazard of waitlist mortality (28). The distance to care also impacts waitlist mortality. Children over 200 miles from their transplant center have a 75% higher risk of waitlist death, possibly reflecting a delay in referral or listing for transplantation (41). Notably, in their recent paper, Henson et al. highlighted several potential barriers to evaluating and selecting for LT, including poverty, educational attainment, access to healthy food, and access to technology (34).
Social adversity has a negative impact on the outcomes of LT. Using SRTR data, Wadhwani et al. found that neighborhood SED was associated with an increased risk of graft failure and death over a 10-year timespan after transplant (28). In multivariable analysis adjusted for race, each 0.1 increase in the deprivation index was associated with a 11.5% (95% CI: 1.6%–23.9%) increased hazard of graft failure and a 9.6% (95% CI: −0.04% to 20.7%) increased hazard of death. Notably, when the proportion of patients from SED neighborhoods increases for a given transplant center, patients have a 32% increased hazard of graft failure (26). However, the impact of neighborhood disadvantage can be mitigated by medical practices. High-performing pediatric liver transplant centers achieved good long-term outcomes despite caring for socioeconomically deprived children, demonstrating that there may be transplant center practices that mitigate the risks of SED (26). Addressing treatment non-adherence may be one such strategies, since pediatric recipients living in the most deprived neighborhood deprivation index quartile were twice as likely to be non-adherent to immunosuppressive medication (29). The insurance status has an additional impact on post-transplant outcome, and children less than 18 years old with Medicaid insurance have a relative risk of 1.42 of post-transplant mortality (42).
Living in neighborhoods with high air pollution can increase the risk of graft failure and death post-transplant in children, even after accounting for sociodemographic factors (24). Recently, Yalung et al. investigated the role of environmental factors and found that air pollution was linked to a 54% higher risk of graft failure or death (HR: 1.54; 95% CI: 1.29, 1.83; P < 0.001) after adjusting for race, insurance status, rurality, and neighborhood socioeconomic status (24).
The socioeconomic status of individuals and communities significantly impacts the outcomes of the pre-transplant evaluations (30). For every 0.1 increase in the overall Social Vulnerability Index (SVI), there was an 8% reduction in the rate of waitlisting, as per HR 0.92 (95% CI 0.87–0.96, p < 0.001) (30). The domains significantly contributing to this correlation were socioeconomic status, household characteristics, housing type, transportation, and racial and ethnic minority status (30). Patients from vulnerable communities had a 6% lower transplant rate (HR 0.94, 95% CI 0.91–0.98, P = 0.007), and socioeconomic and household characteristics in the SVI domain significantly contributed to this association (30). At the individual level, lack of government insurance and unemployment were associated with lower rates of waitlisting and transplantation, but there was no association with mortality before or while on the waitlist (30).
According to a recent analysis carried out at a single center, liver disease patients who live in socially deprived communities have a higher risk of not being listed compared to patients with higher socioeconomic status (33). The analysis found that patients from socially deprived neighborhoods are also at a greater risk of not initiating the evaluation post-referral and dying without initiating the evaluation (33). The results showed that the adjusted relative risk (aRR) for not being listed was 1.14 (95% CI = 1.05–1.22, P < 0.001). The aRR for not initiating the evaluation post-referral was 1.20 (95% CI = 1.01–1.42, P = 0.03). Lastly, the aRR for dying without initiating evaluation was 1.55 (95% CI = 1.09–2.2, P = 0.01) (33). The study found that White patients with low SED have similar rates of being listed compared to White patients with high SED. However, patients from social minority groups who live in neighborhoods with low SED are 31% more likely not to be listed for a transplant compared to patients from the same minority group living in neighborhoods with high SED. The results were statistically significant (aRR = 1.31; 95% CI = 1.12–1.5; P < 0.001) (33).
The impact of social adversity appears to be greater for certain indications to LT. In a recent analysis of the UNOS registry 2008–2019, Cullaro et al. have shown that patients from the most deprived areas are the least likely to be listed for alcohol-related liver disease (OR = 0.97, 95% CI = 0.95–0.98) and have an increased rate of waitlist mortality (OR = 1.1; 95% CI = 1.06–1.14) (31).
Compared to pediatric patients, there is limited information on how social adversity impacts adult LT recipients. Initial surveys (1987–2001) on the influence of neighborhood income, education, and insurance showed that education had a marginal influence on outcomes, and patients with Medicare and Medicaid had lower survival than those with private insurance (36). More recently, in a proportional hazards model analysis, LT recipients with the lowest socioeconomic status have an increased risk of death within 2 years after transplantation (HR = 1.17; 95% CI = 1.02–1.35) (43). After adjusting for differences in recipient characteristics, donor organ quality, transplant center volume/quality, geographic region, and DSA, being in the lowest SED quartile remained an independent predictor for patient but not graft survival (43). Adjusting for individual hospitals had minimal impact on patient survival hazard ratio, indicating that differences in SED groups did not result from hospital care (43).
Health-related quality of life (HRQoL) seems poorer in recipients from disadvantaged areas. In a recent publication, Sgrò et al. investigated HRQoL in 331 patients and found that greater SED was associated with lower post-transplantation HRQoL scores, with a difference of 9.7 points (95% CI: 4.6–14.9, P < 0.001) between the most and least deprived quintiles (32). Recipients living in areas of least deprivation were less likely to suffer from anxiety (OR = 0.05; 95% CI: 0.00–0.28; P = 0.003) or depression (OR = 0.13, 95% CI = 0.02–0.56; P = 0.009) (32).
While socially disadvantaged patients with HCC show inferior survivals (44), a group of French investigators failed to show any impact of SED on the post-transplant outcome of patients with HCC (35). In their registry analysis of 3,865 recipients, the European deprivation index (EDI) did not impact overall survival after LT for HCC, while the number of tumor nodules and time on the waiting list were the independent prognostic factors predicting survival (35).
The literature has extensively analyzed the impact of race and ethnicity, particularly in the US context. Black patients have worse outcomes than White patients, including lower graft function (45), inferior graft survival (46), and worse overall survival (47), revealing the role of racial disparities. This disparity has remained consistent over time (i.e., before and after the MELD era) (47) and persists after controlling for patient-level factors, such as socioeconomic status (43) and clinical covariates (45). Recent reports confirm that Black patients have a 21% higher mortality risk than White patients, but no effect modification by transplant center volume was found (48).
Although evidence shows socioeconomic determinants impact LT outcomes, their mechanisms in clinical practice remain elusive. However, a clear understanding by clinicians is crucial for equitable patient outcomes in liver disease and transplantation. To ensure equitable care and outcomes in LT, having a common language across the transplant community is crucial. Understanding the social determinants of health and engaging in integrated person-centered liver patient care across classical medical specialty boundaries is essential, which can help identify pre-transplant patients and recipients requiring more intensive resources (49). This will help equalize outcomes and ensure everyone receives the care they need. Still, reappraisal of disease-related medical challenges must be conducted to increase awareness among liver transplant physicians and reduce the social stigmatization associated with liver disease (49, 50).
Our literature search identified three areas in which SED may affect the results of LT. These areas ought to be considered as potential topics for future research and interventions (Figure 1).
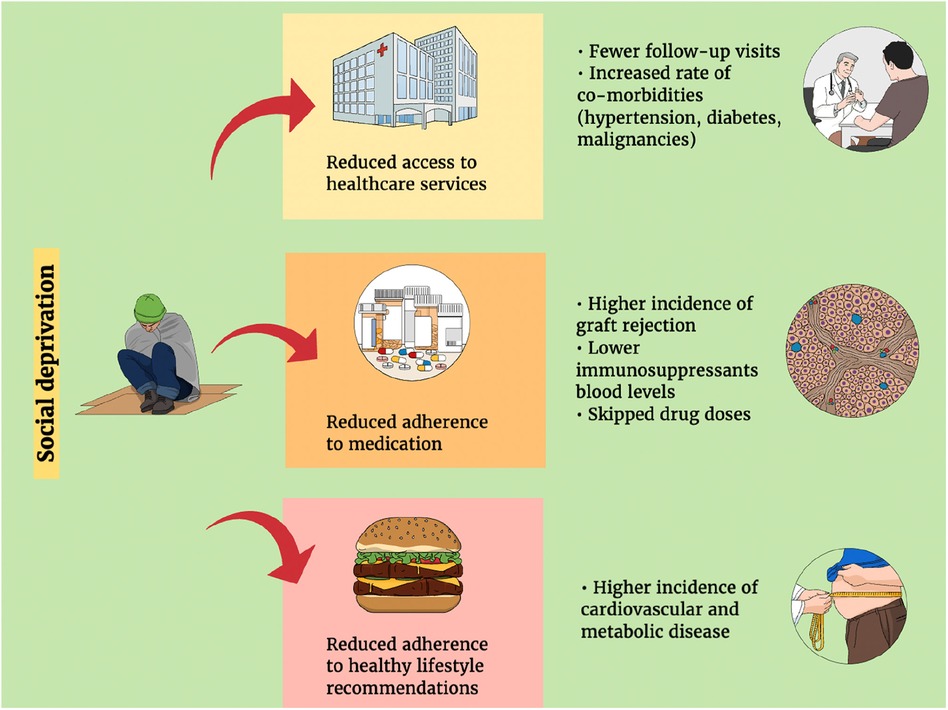
Figure 1. The implications of socio (economic) deprivation on the outcomes of liver transplantation.
In transplant (9, 16, 25, 30, 31, 33, 34, 36) and non-transplant populations (51, 52–54), SED is associated with reduced access to healthcare services and facilities. Liver disease patients from deprived areas and minorities are waitlisted in greater severity per PELD/MELD scores (9, 25, 30, 39, 40) and less frequently referred to living donor LT (25). Social barriers, such as stigma (i.e., negative or discriminatory attitudes of others), have a significant impact on liver diseases and patients' referral patterns, leading to discrimination, reduction in health-care-seeking behavior, and reduced allocation of resources, which all result in poor clinical outcomes (49). Due to delayed referral to appropriate care, waitlist mortality is higher in socially deprived populations (25, 31, 33, 39, 40). It has also been noted that a greater distance from the transplant center where a patient was referred to may worsen their disease severity (41).
Some indications of liver transplantation still bear the consequence of public stigma and SED. This is particularly true for alcohol use disorders, acute alcohol-related hepatitis, alcohol-related chronic liver disease, and metabolic dysfunction-associated steatotic liver disease (MASLD) (49, 50, 55, 56). Globally, there has been a significant increase in alcohol use disorder among women, ethnic and racial minorities, and individuals living in poverty, who also experience poor access to alcohol treatment (56). This has resulted in a rise in alcohol-related liver diseases (56). Rising rates of MASLD and associated fibrosis have been observed in Hispanics, women aged >50, and individuals experiencing food insecurity (56). Limited access to viral hepatitis screening and treatment for racial and ethnic minorities, as well as uninsured or underinsured individuals, leads to higher mortality rates and later diagnoses of HCC (56). However, favorable results can be obtained after LT through an accurate selection of transplant candidates, multidisciplinary integration of pre-transplant care with family support, and close post-transplant integrated care (57). People with alcohol-related disorders are subject to public stigma, self-stigma (negative attitudes, including shame, about their condition), and structural stigma (policies that intentionally or unintentionally limit opportunities for people with the disease) (58). Patients with alcohol-related liver disease often describe healthcare settings as stigmatizing, and removing blaming for alcohol use is central to facilitating access to healthcare and transplantation (49, 50, 58). Furthermore, a significant proportion of individuals with alcohol-related disorders are from historically underrepresented racial/ethnic, sex/gender, and sociocultural groups and those vulnerable in their social determinants of health, creating additional barriers to treatment (50).
In the post-transplant period, deprivation and racial disparities are associated with reduced graft and patient survival, especially for pediatric populations (25, 26, 29). Increased non-adherence to immunosuppression (29, 59) and the impact of environmental disadvantages (24) could be contributing factors, but additional mechanisms should be considered. Quality and quantity of care within the household (60), availability of caregivers and resources (60), attending follow-up visits (61), and involvement of other health care practitioners (61), such as recipient coordinators, pharmacists, dermatologists, and addiction specialists (61), may improve outcomes. Peer and social pressure on pediatric/adolescent recipients (62) and cognitive determinants of health status and adherence (63) are interesting areas that require further investigation. Higher SED may disincentive adherence to pre- and post-transplant lifestyle recommendations, leading to worse outcomes due to the role of non-surgical and non-medication risk factors on liver disease and transplant outcomes (64). In the population of patients with alcohol use disorders, the available literature has documented a higher relapse rate post-transplantation for patients with lower social support and socioeconomic status through a complex interplay of social, economic, psychological, and behavioral mechanisms (65). Post-transplant recurrence of MASLD has been associated with metabolic derangements such as insulin-dependent diabetes, hyperlipoproteinemia, and graft steatosis within 2 years after transplantation (66). The role of social deprivation in this setting still needs clarification.
Extensive literature evidence in transplantation shows that higher SED (29, 58, 67, 68), being divorced, having a history of substance or alcohol use, having mental health needs, missing clinic appointments, and not maintaining medication logs are associated with a higher probability of immunosuppressive medication non-adherence (69).
Health literacy refers to an individual's ability to access, understand, and use health-related information and services to make informed decisions about their health. This has recently been recognized as a critical factor in treatment compliance and overall health-related quality of life (70). Inadequate adherence to treatment due to difficulties in understanding medical information related to medication and lifestyle recommendations following organ transplantation or the inability to find appropriate information can increase the risk of re-hospitalization and transplant organ failure (71). Health literacy follows a socioeconomic gradient (72), and it might be speculated that transplant individuals from socially deprived areas are more exposed to illiteracy and consequent increased treatment non-adherence. To date, this hypothesis must be investigated in LT recipients.
Decreased adherence after kidney transplantation is associated with black race (73), but also with transplant center and dosing frequency (73). Investigating non-adherence to immunosuppression after transplantation is challenging, requiring direct measurements (such as electronic monitoring), blood drug exposure level testing, and collateral reports (such as patients' self-reports and/or caregivers' opinions) (59). Longitudinal observations should be conducted and correlated with the socioeconomic determinants of interest in each transplant population. In the case of a liver transplant, detecting the impact of non-adherence might be more challenging due to the immunologic privilege of the liver graft and the lower probability of acute cellular rejection compared to other solid organ transplant categories (74).
Reduced adherence to lifestyle recommendations can be linked to social deprivation, a third area that needs to be explored. Along with constant medical care, the active participation of patients in their healthcare plan and adherence to lifestyle recommendations are crucial for improving the outcomes of LT (75). To reduce the rate of chronic attrition after a transplant, it is important to address pre-existing health conditions and complications that may arise from immunosuppressive drugs (76). This involves managing conditions like diabetes, hypertension, and obesity and making healthy lifestyle choices, such as quitting smoking, engaging in regular physical activity, and following a healthy diet. It is also crucial to seek prompt medical attention from the transplant center. From previous research in non-transplant populations, children living in socially deprived areas have a twofold higher risk of obesity than children from families with higher socioeconomic status (77). Likewise, children from families with medium and low education have twice the risk for obesity compared to children with high parental education (77). In the UK, areas with high rates of obesity are often concentrated around economically depressed urban areas in the north of England, leading to health inequalities across the country (78). Therefore, we may speculate that SED act along the same trajectories in transplant populations by contributing to an increase in the negative impact of co-morbidities and immunosuppression and non-immunosuppressive medication complications.
Addressing the negative consequences of social adversity on the outcomes of LT requires comprehensive strategies that are multi-level and multi-dimensional. These strategies should be based on the chronic care model (CCM) and should cover the entire continuum of LT care, starting from the pre-transplant phase and extending to the long-term follow-up.
All levels, including patients, caregivers, stakeholders, clinics, academia, communities, and institutions, should be involved, and interventions should address the biological, psychological, and social determinants of transplant outcomes. To a greater extent, the US National Academies of Sciences, Engineering, and Medicine have recently laid out the details of these initiatives, addressing all strata of patients (79).
(1) Increase awareness. The first strategy is to raise healthcare professionals' awareness of SED's impact on LT outcomes. This requires promoting research, data measurement, and exchange among clinicians/researchers. Standardizing research language and measures across institutions and countries is crucial due to SED components' varied definitions and implications. Research on the interaction between the environment (i.e., air/water pollution, food insecurity) behaviors (i.e., diet, exercise) and biology (i.e., immune response, post-transplant cancer risk) should be promoted by academia, scientific societies, and research institutions.
(2) Adjust care delivery to patients' needs. The second initiative aims to provide sustainable, patient-centered care that minimizes routine disruption to patients and their families (i.e., care adjustment). Socioeconomic profiling should be integrated into pre- and post-transplant care via tailored questionnaires or census block deprivation indexes. Adjusting care to patients' needs also requires removing obstacles hindering communication/interaction between patients and transplant centers. For instance, telehealth appointments can be used instead of in-person visits, tailored to the patient's health status and distance from the transplant center. Transplant centers should also establish collaborative networks with local referring institutions to increase adherence to follow-up visits for patients from socially disadvantaged areas. They should also improve data exchange and promote crosstalk among multiple institutions. Engaging family caregivers from the beginning of the transplant process is crucial to ensuring patient participation in pre- and post-transplant care.
(3) Advocacy. The transplant community should advocate for eliminating inequities to access to pre and post-transplant care by introducing norms/regulations, social (i.e., housing and transport vouchers), and financial (i.e., reimbursements) incentives for patients from socially disadvantaged areas and seeking transplant care. We also recommend that deprivation indexes (both individual and at the census block levels) be introduced in the case-mix evaluation to better understand the connection between socioeconomic disadvantage (SED) and transplant outcomes. Public, personal, and structural stigma should also be removed to enhance access to pre- and post-transplant care for patients with alcohol-related liver disease and disorders and for those with social disadvantages. Public discourse on the role of social determinants in transplant care, both nationally and internationally, should be favored among clinicians, patients, caregivers, and stakeholders.
Personalizing LT recipient care based on medical, surgical, immunologic, psychologic, and socioeconomic factors improves outcomes. There is growing evidence, derived mainly from studies in the US, that socioeconomic deprivation plays a crucial role in delaying prompt patient referral, hindering access to care, and discouraging participation in follow-up care. Public, personal, and structural stigma should also be removed to enhance access to pre- and post-transplant care. The socioeconomic profile of liver disease patients seeking transplant care should be integrated into the pre-transplant evaluation process and their post-transplant care plan.
PD: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. GG: Conceptualization, Data curation, Validation, Writing – review & editing. QL: Conceptualization, Data curation, Validation, Writing – review & editing. JD: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. FR: Supervision, Validation, Visualization, Writing – review & editing. SG: Conceptualization, Investigation, Validation, Writing – review & editing. PB: Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
PDS serves on the advisory boards of Novartis, Astellas, and Chiesi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zarrinpar A, Busuttil R. Liver transplantation: past, present, and future. Nat Rev Gastroenterol Hepatol. (2013) 10:434–40. doi: 10.1038/nrgastro.2013.88
2. Yao L, Robert SA. The contributions of race, individual socioeconomic status, and neighborhood socioeconomic context on the self-rated health trajectories and mortality of older adults. Res Aging. (2008) 30(2):251–73. doi: 10.1177/0164027507311155
3. Gaskin DJ, Roberts ET, Chan KS, McCleary R, Buttorff C, Delarmente BA. No man is an island: the impact of neighborhood disadvantage on mortality. Int J Environ Res Public Health. (2019) 16(7):1265. doi: 10.3390/ijerph16071265
4. Kirby JB, Kaneda T. Neighborhood socioeconomic disadvantage and access to health care. J Health Soc Behav. (2005) 46(1):15–31. doi: 10.1177/002214650504600103
5. Ross CE, Mirowsky J. Neighborhood disadvantage, disorder, and health. J Health Soc Behav. (2001) 42(3):258–76. doi: 10.2307/3090214
6. Wing JJ, Sánchez BN, Adar SD, Meurer WJ, Morgenstern LB, Smith MA, et al. Synergism of short-term air pollution exposures and neighborhood disadvantage on initial stroke severity. Stroke. (2017) 48(11):3126–9. doi: 10.1161/STROKEAHA.117.018816
7. Lusk JB, Hoffman MN, Clark AG, Bae J, Corsino L, Hammill BG. Neighborhood socioeconomic deprivation and 30-day mortality and readmission for patients admitted for diabetes management. Diabetes Care. (2022) 45(11):e169–170. doi: 10.2337/dc22-0913
8. Lusk JB, Hoffman MN, Clark AG, Bae J, Luedke MW, Hammill BG. Association between neighborhood socioeconomic status and 30-day mortality and readmission for patients with common neurologic conditions. Neurology. (2023) 100(17):e1776–86. doi: 10.1212/WNL.0000000000207094
9. Ebel NH, Lai JC, Bucuvalas JC, Wadhwani SI. A review of racial, socioeconomic, and geographic disparities in pediatric liver transplantation. Liver Transpl. (2022) 28(9):1520–8. doi: 10.1002/lt.26437
10. Townsend P. Poverty in the United Kingdom: A survey of Household Resources and Standards of Living. Berkeley and Los Angeles: University of California Press (1979).
11. Fabrizi E, Mussida C, Parisi ML. Comparing material and social deprivation indicators: identification of deprived populations. Soc Indic Res. (2023) 165:999–1020. doi: 10.1007/s11205-022-03058-6
12. Alkire S, Foster J. Counting and multidimensional poverty measurement. J Pub Econ. (2011) 95(7-8):476–87. doi: 10.1016/j.jpubeco.2010.11.006
13. Fusco A, Guio AC, Marlier E. Building a material deprivation index in a multinational context: lessons from the EU experience: lessons from the EU experience. In: Berenger V, Bresson F, editors. Poverty and Social Exclusion Around the Mediterranean Sea. Economic Studies in Inequality, Social Exclusion, and Well-Being, Vol. 9. Boston, MA: Springer. (2013). p. 43–71. doi: 10.1007/978-1-4614-5263-8_2
14. Williams DR, Rucker TD. Understanding and addressing racial disparities in health care. Health Care Financ Rev. (2000) 21(4):75–90.11481746
15. Luan FL, Kommareddi M, Cibrik DM, Samaniego M, Ojo AO. Influence of recipient race on the outcome of simultaneous pancreas and kidney transplantation. Am J Transplant. (2010) 10:2074–81. doi: 10.1111/j.1600-6143.2010.03211.x
16. Fan PY, Ashby VB, Fuller DS, Boulware LE, Kao A, Norman SP, et al. Access and outcomes among minority transplant patients, 1999-2008, with a focus on determinants of kidney graft survival. Am J Transplant. (2010) 10(4 pt 2):1090–107. doi: 10.1111/j.1600-6143.2009.03009.x
17. Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Conte JV, et al. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. Ann Thorac Surg. (2010) 89:1956–63. doi: 10.1016/j.athoracsur.2010.02.093
18. Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. (2002) 359:287–93. doi: 10.1016/S0140-6736(02)07494-9
19. Kemmer N. Ethnic disparities in liver transplantation. Gastroenterol Hepatol (N Y). (2011) 7(5):302–7.21857831
20. Kizer KW, English RA, Hackmann M. Committee on a fairer and more equitable, cost-effective, and transparent system of donor organ procurement, allocation, and distribution. In: National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Board on Health Sciences Policy, editors. Realizing the Promise of Equity in the Organ Transplantation System. Washington DC: The National Academies Press (2022). doi: 10.17226/26364.
21. OPTN/SRTR. 2016 Annual data report: preface. Am J Transplant. (2018) 18(suppl 1):1–9. doi: 10.1111/ajt.14559
22. Fuentes A, Ackermann RR, Athreya S, Bolnick D, Lasisi T, Lee SH, et al. AAPA statement on race and racism. Am J Phys Anthropol. (2019) 169(3):400–2. doi: 10.1002/ajpa.23882
23. Shifman HP, Huang CY, Beck AF, Bucuvalas J, Perito ER, Hsu EK, et al. Association of state medicaid expansion policies with pediatric liver transplant outcomes. Am J Transplant. (2023) 24:239–49. doi: 10.1016/j.ajt.2023.09.017
24. Yalung JE, Shifman HP, Rasnick Manning E, Beck A, Bucuvalas J, Lai JC, et al. Ambient air pollution is associated with graft failure/death in pediatric liver transplant recipients. Am J Transplant. (2023) S1600-6135(23):00817-1. doi: 10.1016/j.ajt.2023.10.015
25. Wadhwani SI, Ge J, Gottlieb L, Lyles C, Beck AF, Bucuvalas J, et al. Racial/ethnic disparities in wait-list outcomes are only partly explained by socioeconomic deprivation among children awaiting liver transplantation. Hepatology. (2022) 75(1):115–24. doi: 10.1002/hep.32106
26. Wadhwani SI, Huang CY, Gottlieb L, Beck AF, Bucuvalas J, Kotagal U, et al. Center variation in long-term outcomes for socioeconomically deprived children. Am J Transplant. (2021) 21(9):3123–32. doi: 10.1111/ajt.16529
27. Wadhwani SI, Gottlieb L, Bucuvalas JC, Lyles C, Lai JC. Addressing social adversity to improve outcomes for children after liver transplant. Hepatology. (2021) 74(5):2824–30. doi: 10.1002/hep.32073
28. Wadhwani SI, Beck AF, Bucuvalas J, Gottlieb L, Kotagal U, Lai JC. Neighborhood socioeconomic deprivation is associated with worse patient and graft survival following pediatric liver transplantation. Am J Transplant. (2020) 20(6):1597–605. doi: 10.1111/ajt.15786
29. Wadhwani SI, Bucuvalas JC, Brokamp C, Anand R, Gupta A, Taylor S, et al. Association between neighborhood-level socioeconomic deprivation and the medication level variability for children following liver transplantation. Transplantation. (2020) 104(11):2346–53. doi: 10.1097/TP.0000000000003157
30. Yilma M, Cogan R, Shui AM, Neuhaus JM, Light C, Braun H, et al. Community-level social vulnerability and individual socioeconomic status on liver transplant referral outcome. Hepatol Commun. (2023) 7(7):e00196. doi: 10.1097/HC9.0000000000000196
31. Cullaro G, Ge J, Lee BP, Lai JC, Wadhwani SI. Association between neighborhood-based material deprivation and liver transplant waitlist registrants demographics and mortality. Clin Transplant. (2023) 38:e15189. doi: 10.1111/ctr.15189
32. Sgrò A, Cambridge WA, McLean KA, Drake TM, Camilleri-Brennan J, Knight SR, et al. Is socioeconomic deprivation associated with worse quality of life, anxiety and depression in liver transplant recipients? A cross-sectional study in a national transplantation programme. BMJ Open. (2023) 13(8):e070422. doi: 10.1136/bmjopen-2022-070422
33. Strauss AT, Moughames E, Jackson JW, Malinsky D, Segev DL, Hamilton JP, et al. Critical interactions between race and the highly granular area deprivation index in liver transplant evaluation. Clin Transplant. (2023) 37(5):e14938. doi: 10.1111/ctr.14938
34. Henson JB, Chan NW, Wilder JM, Muir AJ, McElroy LM. Characterization of social determinants of health of a liver transplant referral population. Liver Transpl. (2023) 29(11):1161–71. doi: 10.1097/LVT.0000000000000127
35. Menahem B, Dejardin O, Alves A, Launay L, Lubrano J, Duvoux C, et al. Socioeconomic deprivation does not impact liver transplantation outcome for HCC: a survival analysis from a national database. Transplantation. (2021) 105(5):1061–8. doi: 10.1097/TP.0000000000003340
36. Yoo HY, Thuluvath PJ. Outcome of liver transplantation in adult recipients: influence of neighborhood income, education, and insurance. Liver Transpl. (2004) 10:235–43. doi: 10.1002/lt.20069
37. Kwong AJ, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, et al. OPTN/SRTR 2019 annual data report: liver. Am J Transplant. (2021) 21:208–315. doi: 10.1111/ajt.16494
38. Hsu EK, Shaffer M, Bradford M, Mayer-Hamblett N, Horslen S. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. Am J Transpl. (2015) 15:436–44. doi: 10.1111/ajt.13089
39. Braun HJ, Perito ER, Dodge JL, Rhee S, Roberts JP. Nonstandard exception requests impact outcomes for pediatric liver transplant candidates. Am J Transpl. (2016) 16:3181–91. doi: 10.1111/ajt.13879
40. Mogul DB, Luo X, Chow EK, Massie AB, Purnell TS, Schwarz KB, et al. Impact of race and ethnicity on outcomes for children waitlisted for pediatric liver transplantation. J Pediatr Gastroenterol Nutr. (2018) 66:436–41. doi: 10.1097/MPG.0000000000001793
41. Adler JT, Bababekov YJ, Markmann JF, Chang DC, Yeh H. Distance is associated with mortality on the waitlist in pediatric liver transplantation. Pediatr Transpl. (2017) 21. doi: 10.1111/petr.12842
42. Dick AAS, Winstanley E, Ohara M, Blondet NM, Healey PJ, Perkins JD, et al. Do funding sources influence long-term patient survival in pediatric liver transplantation? Pediatr Transplant. (2021) 25:e13887. doi: 10.1111/petr.13887
43. Quillin RC III, Wilson GC, Wima K, Hohmann SF, Sutton JM, Shaw JJ, et al. Neighborhood level effects of socioeconomic status on liver transplant selection and recipient survival. Clin Gastroenterol Hepatol. (2014) 12:1934–41. doi: 10.1016/j.cgh.2014.05.020
44. Ruffolo LI, Zambrano D, Dale BS, Nimmagadda SV, Hack M, Gaba H, et al. Inferior survival is associated with socioeconomic deprivation in hepatocellular carcinoma. J Surg Res. (2022) 279:228–39. doi: 10.1016/j.jss.2022.05.035
45. Quillin RC III, Wilson GC, Wima K, Hanseman DJ, Sutton JM, Shaw JJ, et al. Independent effect of black recipient race on short term outcomes after liver transplantation. Surgery. (2015) 157:774–84. doi: 10.1016/j.surg.2014.10.018
46. Hong JC, Kosari K, Benjamin E, Duffy JP, Ghobrial RM, Farmer DG, et al. Does race influence outcomes after primary liver transplantation? A 23-year experience with 2,700 patients. J Am Coll Surg. (2008) 206:1009–16. doi: 10.1016/j.jamcollsurg.2007.12.019
47. Ananthakrishnan AN, Saeian K. Racial differences in liver transplantation outcomes in the MELD era. Am J Gastroenterol. (2008) 103:901–10. doi: 10.1111/j.1572-0241.2008.01809.x
48. Ross-Driscoll K, Kramer M, Lynch R, Plantiga L, Wedd J, Patzer R. Variation in racial disparities in liver transplant outcomes across transplant centers in the United States. Liver Transpl. (2021) 27(4):558–67. doi: 10.1002/lt.25918
49. Karlsen TH, Sheron N, Zelber-Sagi S, Carrieri P, Dusheiko G, Bugianesi E, et al. The EASL-lancet liver commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet. (2022) 399(10319):61–116. doi: 10.1016/S0140-6736(21)01701-3
50. Sedarous M, Flemming JA. Culture, stigma, and inequities creating barriers in alcohol use disorder management in alcohol-associated liver disease. Clin Liver Dis (Hoboken). (2023) 21(5):130–3. doi: 10.1097/CLD.0000000000000026
51. Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. (2013) 48(2 Pt 1):539–59. doi: 10.1111/j.1475-6773.2012.01449.x
52. Walker AF, Hu H, Cuttriss N, Anez-Zabala C, Yabut K, Haller MJ, et al. The neighborhood deprivation Index and provider geocoding identify critical catchment areas for diabetes outreach. J Clin Endocrinol Metab. (2020) 105(9):3069–75. doi: 10.1210/clinem/dgaa462
53. Ulhaq A, McMahon AD, Buchanan S, Goold S, Conway DI. Socioeconomic deprivation and NHS orthodontic treatment delivery in Scotland. Br Dent J. (2012) 213(4):E5. doi: 10.1038/sj.bdj.2012.724
54. Gilliland JA, Shah TI, Clark A, Sibbald S, Seabrook JA. A geospatial approach to understanding inequalities in accessibility to primary care among vulnerable populations. PLoS One. (2019) 14(1):e0210113. doi: 10.1371/journal.pone.0210113
55. Germani G, D'Arcangelo F, Grasso M, Burra P. Advances and controversies in acute alcohol-related hepatitis: from medical therapy to liver transplantation. Life (Basel). (2023) 13(9):1802. doi: 10.3390/life13091802
56. Kardashian A, Serper M, Terrault N, Nephew LD. Health disparities in chronic liver disease. Hepatology. (2023) 77(4):1382–403. doi: 10.1002/hep.32743
57. Germani G, Mathurin P, Lucey MR, Trotter J. Early liver transplantation for severe acute alcohol-related hepatitis after more than a decade of experience. J Hepatol. (2023) 78(6):1130–6. doi: 10.1016/j.jhep.2023.03.007
58. Schomerus G, Leonhard A, Manthey J, Morris J, Neufeld M, Kilian C, et al. The stigma of alcohol-related liver disease and its impact on healthcare. J Hepatol. (2022) 77(2):516–24. doi: 10.1016/j.jhep.2022.04.026
59. Burra P, Germani G, Gnoato F, Lazzaro S, Russo FP, Cillo U, et al. Adherence in liver transplant recipients. Liver Transpl. (2011) 17:760–70. doi: 10.1002/lt.22294
60. López-Navas A, Ríos A, Riquelme A, Martínez-Alarcón L, Pons JA, Miras M, et al. Psychological care: social and family support for patients awaiting a liver transplant. Transplant Proc. (2011) 43(3):701–4. doi: 10.1016/j.transproceed.2011.01.095
61. Neuberger J. Follow-up of liver transplant recipients. Best Pract res Clin Gastroenterol. (2020) 46:101682. doi: 10.1016/j.bpg.2020.101682
62. Burra P. The adolescent and liver transplantation. J Hepatol. (2012) 56(3):714–22. doi: 10.1016/j.jhep.2011.07.032
63. Wilkinson A, Whitehead L. Evolution of the concept of self-care and implications for nurses: a literature review. Int J Nurs Stud. (2009) 46:1143–7. doi: 10.1016/j.ijnurstu.2008.12.011
64. Nobili V, Carter-Kent C, Feldstein AE. The role of lifestyle changes in the management of chronic liver disease. BMC Med. (2011) 9:70. doi: 10.1186/1741-7015-9-70
65. Chuncharunee L, Yamashiki N, Thakkinstian A, Sobhonslidsuk A. Alcohol relapse and its predictors after liver transplantation for alcoholic liver disease: a systematic review and meta-analysis. BMC Gastroenterol. (2019) 19(1):150. doi: 10.1186/s12876-019-1050-9
66. Villeret F, Dharancy S, Erard D, Abergel A, Barbier L, Besch C, et al. Inevitability of disease recurrence after liver transplantation for NAFLD cirrhosis. JHep Rep. (2023) 5(3):100668. doi: 10.1016/j.jhepr.2022.100668
67. Mols RE, Bakos I, Løgstrup BB, Horváth-Puhó E, Gustafsson F, Eiskjær H. Adherence to pharmacotherapies after heart transplantation in relation to multimorbidity and socioeconomic position: a nationwide register-based study. Transpl Int. (2023) 36:11676. Available online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11676 DOI=10.3389/ti.2023.11676
68. Lamba S, Nagurka R, Desai KK, Chun SJ, Holland B, Koneru B. Self-reported non-adherence to immune-suppressant therapy in liver transplant recipients: demographic, interpersonal, and intrapersonal factors. Clin Transplant. (2012) 26(2):328–35. doi: 10.1111/j.1399-0012.2011.01489.x
69. Weng FL, Israni AK, Joffe MM, Hoy T, Gaughan CA, Newman M, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol. (2005) 16(6):1839–48. doi: 10.1681/ASN.2004121059
70. Bae SH, Lee JJ, Son SY, Kim HY, Ju MK. A cross-sectional analysis of health literacy and compliance to treatment in organ transplant recipients. J Clin Med. (2023) 12(3):977. doi: 10.3390/jcm12030977
71. Pullen LC. A path toward improving health literacy and transplant outcomes. Am J Transplant. (2019) 19:1871–2. doi: 10.1111/ajt.15475
72. Sorensen K, Pelikan JM, Rothlin F, Ganahl K, Slonska Z, Doyle G, et al. Health literacy in Europe: comparative results of the European health literacy survey (HLS-EU). Eur J Public Health. (2015) 25:1053–8. doi: 10.1093/eurpub/ckv043
73. Gandolfini I, Palmisano A, Fiaccadori E, Cravedi P, Maggiore U. Detecting, preventing and treating non-adherence to immunosuppression after kidney transplantation. Clin Kidney J. (2022) 15(7):1253–74. doi: 10.1093/ckj/sfac017
74. Mastoridis S, Martinez-Llordella M, Sanchez-Fueyo A. Immunotolerance in liver transplantation. Semin Liver Dis. (2017) 37(2):95–108. doi: 10.1055/s-0037-1602762
75. Fuochi E, Anastasio L, Lynch EN, Campani C, Dragoni G, Milani S, et al. Main factors influencing long-term outcomes of liver transplantation in 2022. World J Hepatol. (2023) 15(3):321–53. doi: 10.4254/wjh.v15.i3.321
76. De Simone P, Carrai P, Coletti L, Ghinolfi D, Petruccelli S, Filipponi F. Modification of immunosuppressive therapy as risk factor for complications after liver transplantation. Best Pract Res Clin Gastroenterol. (2017) 31(2):199–209. doi: 10.1016/j.bpg.2017.03.001
77. Nguyen TH, Götz S, Kreffter K, Lisak-Wahl S, Dragano N, Weyers S. Neighbourhood deprivation and obesity among 5656 pre-school children-findings from mandatory school enrollment examinations. Eur J Pediatr. (2021) 180(6):1947–54. doi: 10.1007/s00431-021-03988-2
78. Tackling obesity: the role of the NHS in a whole-system approach. The King’s Fund. Retrieved November 20, 2023. Available online at: https://www.kingsfund.org.uk/publications/tackling-obesity-nhs (Accessed February 10, 2024).
79. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Integrating Social Needs Care into the Delivery of Health Care to Improve the Nation’s Health. Integrating Social Care into the Delivery of Health Care: Moving Upstream to Improve the Nation’s Health. Washington (DC): National Academies Press (US) (2019).
Keywords: liver transplantation, social deprivation, disparities, equity, outcomes
Citation: De Simone P, Germani G, Lai Q, Ducci J, Russo FP, Gitto S and Burra P (2024) The impact of socioeconomic deprivation on liver transplantation. Front. Transplant. 3:1352220. doi: 10.3389/frtra.2024.1352220
Received: 7 December 2023; Accepted: 13 February 2024;
Published: 27 February 2024.
Edited by:
Stuart Knechtle, Duke University, United StatesReviewed by:
Denise Lo, Emory University, United States© 2024 De Simone, Germani, Lai, Ducci, Russo, Gitto and Burra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo De Simone cGFvbG8uZGVzaW1vbmVAdW5pcGkuaXQ=
Abbreviations ACR, acute cellular rejection; aRR, adjusted relative risk; CCM, chronic care model; CI, confidence interval; DSA, donor service area; EDI, European deprivation index; HCC, hepatocellular carcinoma; HR, hazard ratio; HR-QoL, health-related quality of life; LDLT, living donor liver transplantation; LT, liver transplantation; KT, kidney transplantation; MELD, model for end-stage liver disease; OR, odds ratio; PELD, pediatric MELD; SED, socioeconomic deprivation; sHR, subpopulation HR; SRTR, scientific registry of transplant recipients; SVI, social vulnerability index; UNOS, united network for organ sharing.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.