- 1Department of Medicine, Rutgers Robert Wood Johnson Medical School, New Brunswick, NJ, United States
- 2Department of Biostatistics and Epidemiology, Rutgers School of Public Health, New Brunswick, NJ, United States
- 3Division of Medical Oncology, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, United States
Background: Immune checkpoint inhibitors (ICI) are standard of care therapy for patients with cutaneous malignancies, the most frequently diagnosed cancers in solid organ transplant (SOT) recipients. The activity and rate of allograft rejection in SOT recipients with advanced skin cancers treated with ICI is understudied.
Methods: We conducted a retrospective analysis of SOT recipients with advanced melanoma, cutaneous squamous cell carcinoma (cSCC), and merkel cell carcinoma (MCC) who were treated with ICI. Unpublished cases from our institution and published cases from the literature were aggregated. Demographics, type of immunosuppressive therapy, type of ICI(s) administered, prior systemic therapies, tumor response to ICI, and evidence of organ rejection and/or failure were recorded. Objective response rates (ORR) and rates of graft rejection and failure are reported.
Results: Ninety patients were identified; four patients from our institution and 86 unique patients from a literature review. ORR to first-line ICI for the entire cohort was 41.1% (37/90). ORR by tumor type was 31% (18/58), 64.3% (18/28), and 25.0% (1/4) for melanoma, cSCC, and MCC, respectively. The rate of graft rejection was 37.8% (34/90) with 61.8% (21/34) of these cases progressing to graft failure. Number of immunosuppressive agents (0, 1, 2, or 3) was inversely associated with rate of graft failure.
Conclusions: In this retrospective analysis, ICIs demonstrate clinical activity in SOT recipients with cutaneous malignancies; however, the rate of graft rejection is high. Treatment plans should be individualized through thorough interdisciplinary discussion. Immunosuppressive modifications may be considered prior to starting treatment, but when feasible, enrollment on clinical trials is preferred.
Introduction
Cancer is one of the leading causes of death in patients who are solid organ transplant (SOT) recipients. In 2022, over 42,000 solid organ transplants were performed in the United States and the total number of transplants recorded surpassed 1 million (1). Over 100,000 patients are now on the transplant waiting list each year (2). SOT recipients have a 2- to 4- fold increased risk of developing cancer compared to the general population (3, 4). This risk is partially attributed to lifelong immunosuppressive drugs that are required to prevent graft rejection as well as infection with oncogenic viruses (5, 6). Skin cancers are the most frequent malignancy among SOT recipients, accounting for almost 40% of post-transplant malignancies (7, 8). When compared to the general population and depending partially on geography, SOT recipients have a 65- to 250- fold increased risk of developing cutaneous squamous cell carcinoma (cSCC), a 2- to 8- fold increased risk of developing melanoma and a threefold increased risk of developing Merkel cell carcinoma (MCC) (6, 9). Identifiable risk factors for development of post-transplant skin cancers may include a history of pre-transplant skin cancer, male sex, white race, ultraviolet radiation exposure, type and dosing of immunosuppression, age greater than 50 at time of transplant, and recipients of thoracic transplants (8, 10).
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy, particularly in the management of advanced cutaneous malignancies. Monoclonal antibodies against cytotoxic T-lymphocyte antigen 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand-1 (PD-L1) are approved by the Food and Drug Administration (FDA) for treatment of cSCC, melanoma, and/or MCC (11). ICIs function by blocking the negative regulation of effector T-lymphocytes, allowing for potentiation of anti-tumor immune responses (12). ICIs are standard of care for the management of advanced skin cancers, however the same co-inhibitory signals that ICIs suppress are implicated in maintaining organ tolerance, which has been demonstrated in several pre-clinical studies (13–19). As a result, ICIs have historically been avoided in patients with a history of SOT due to concerns of causing severe, life-threatening graft rejection (20). Furthermore, the impact of immunosuppressive therapies on the efficacy of concurrent ICI therapy is understudied (21). SOT recipients are typically excluded from clinical trials using ICIs, resulting in limited knowledge of their safety and efficacy in this patient population.
While there have been multiple case reports and several retrospective reviews investigating the use of ICIs in SOT recipients (22–69), results are variable regarding antitumor efficacy and rates of graft rejection (70). No reports to our knowledge have solely focused on the experience of SOT patients with advanced skin cancers treated with ICI. The aim of this study is to document the clinical outcomes of SOT patients with advanced skin cancer treated with ICI at our institution and to aggregate it with the available retrospective literature on this population, which we comprehensively compile here. We also aim to identify clinical factors that may be associated with increased risk of allograft rejection.
Materials and methods
Single institution retrospective case series
We searched the Rutgers Cancer Institute of New Jersey (CINJ) electronic medical record (EMR) for SOT recipients who developed an advanced cutaneous malignancy and were treated with ICI by the CINJ Cutaneous Oncology group between January 2019 and May 2022. This retrospective study was approved by the Rutgers Institutional Review Board (#2022000836). Information collected on each patient included: demographics including age and sex, type of SOT, type of immunosuppressive therapy for SOT, type of cutaneous malignancy, type of ICI(s) administered, prior systemic therapies, interval time from transplant to ICI administration, tumor response to ICI, and evidence of organ rejection and/or failure.
Literature review
A systematic search of PubMed and Google Scholar was manually conducted to identify relevant publications up until April 2022, without any date restrictions. Search terms included “checkpoint inhibitor”, “transplant”, “PD-1 inhibitor”, “CTLA-4 inhibitor”, “nivolumab”, “pembrolizumab”, “ipilimumab”, and “cemiplimab”. All types of publications were reviewed, including case reports and case series if they were written in English and reported on the use of ICI in SOT recipients who developed advanced skin cancer. To be included in this study, the articles were required to provide patient-specific information regarding age, sex, tumor type, type of immunosuppression, allograft status after ICI treatment, and the cancer response to ICI treatment. Article titles and abstracts were evaluated for relevance. Eligible articles were reviewed in their entirety to ensure that all inclusion criteria were met. The same data collected from patients in the CINJ EMR were also extracted from the existing publications.
Graft response to ICI treatment was considered to either be “acute rejection,” “graft failure”, or “no rejection”. For the patients who experienced acute rejection, if the allograft was not rescued and no longer functioned according to the author of the paper (i.e., the patient subsequently became reliant on dialysis or experienced liver failure), then the patient was deemed to have graft failure.
Cancer response to first-line ICI was separated into 4 categories: progressive disease (PD), stable disease (SD), partial response (PR), and complete response (CR). Objective response rates (ORR) were calculated as the proportion of patients who had a CR or PR. While the majority of papers explicitly stated the tumor response, there were four patients in which tumor response was determined by the authors based on the paper's descriptions of the patient and one patient in which the tumor response was non-evaluable due to death from graft rejection. Cancer response was determined based on response achieved with first-line ICI.
Data from the published cases were compiled with our previously unpublished single institution retrospective case series of SOT skin cancer patients treated with ICI. Data were stratified based on tumor type, number of immunosuppressive drugs, and tumor response to ICI. Descriptive statistics were used to report tumor response to ICI and rates of graft rejection or failure. Fisher's exact test was used to compare ORR between tumor types, transplant types, rejection rates between number of immunosuppressants and type of ICI, and rejection rates depending on if there was a change in immunosuppression time of ICI initiation.
Results
Baseline patient characteristics
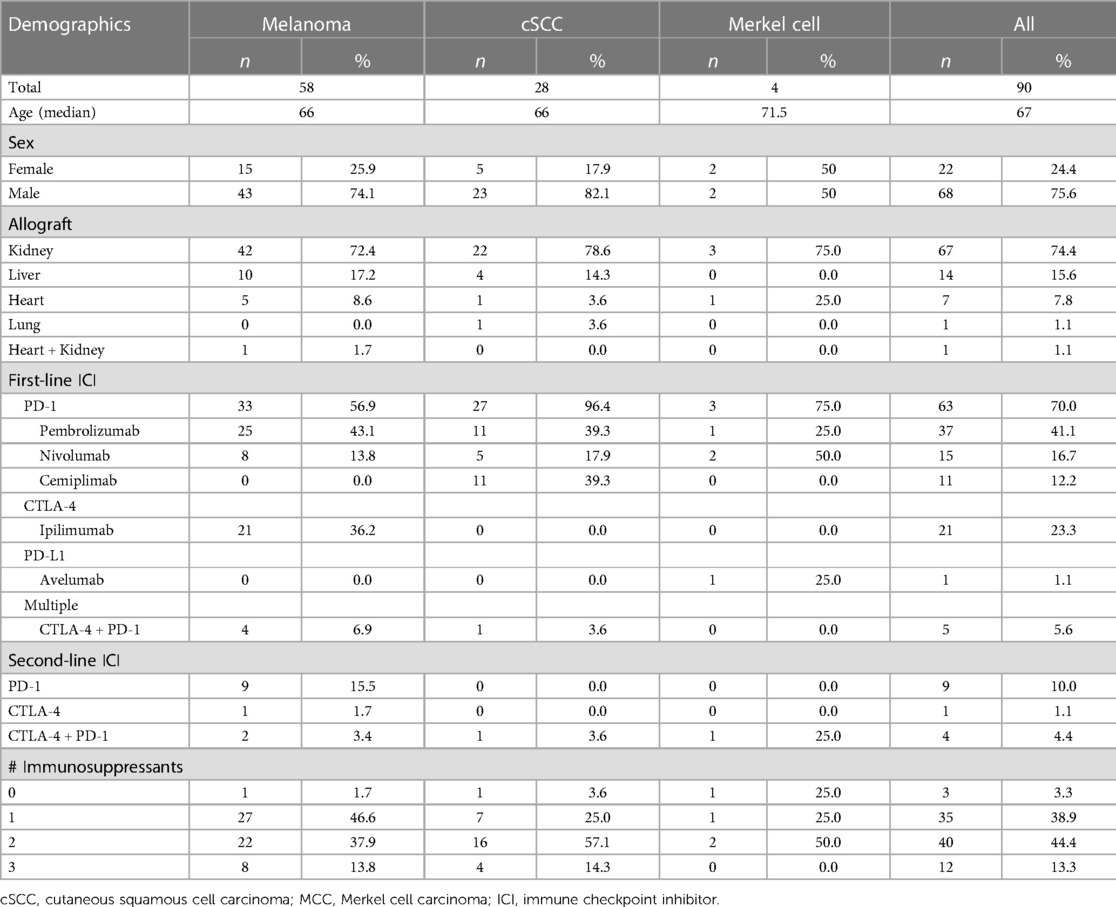
Four patients who met inclusion criteria were identified in the CINJ EMR. The literature search yielded 973 articles. Forty-five of the publications described 86 unique patients who met all inclusion criteria. Thus, 90 total SOT patients treated with ICI for advanced skin cancer were included in our final analysis (Supplementary Table S1). Patient characteristics are shown in Table 1. Median age at time of skin cancer treatment with an ICI was 67 years and there were more men (n = 68, 75.6%) than women (n = 22, 24.4%). Of these 90 cases, most had melanoma (n = 58, 64.4%) followed by cSCC (n = 28, 31.1%) and MCC (n = 4, 4.44%). The most common SOT was kidney (n = 67, 74.4%), followed by liver (n = 14, 15.6%), heart (n = 7, 7.8%), and lung (n = 1, 1.1%). One patient had a heart and kidney transplant (1.1%).
Type of ICI for entire cohort
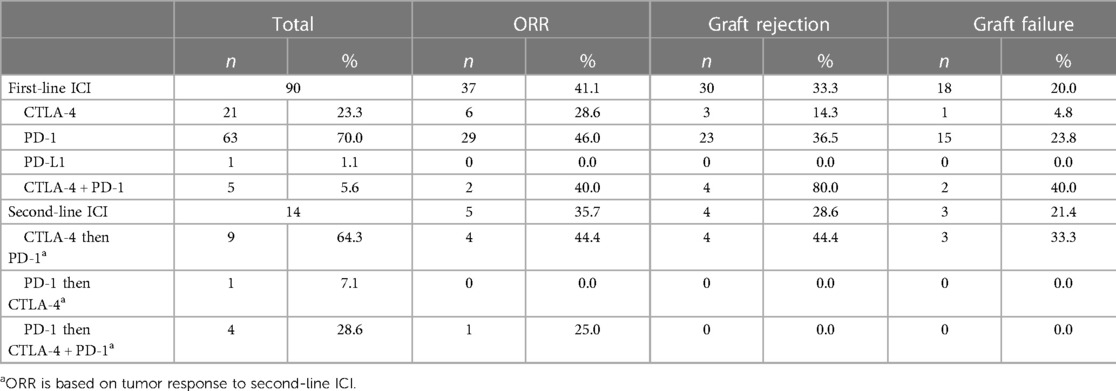
Of the 90 patients, 33 (36.7%) received prior non-ICI systemic therapy, 37 (41.1%) were treatment-naive, and the remaining 20 cases did not specify. The first-line immunotherapy most commonly consisted of anti-PD-1 monotherapy (n = 63, 70.0%) including pembrolizumab (n = 37, 41.1%), nivolumab (n = 15, 16.7%), and cemiplimab (n = 11, 12.2%). One patient received avelumab (n = 1, 1.1%). Twenty-one patients initially received single agent ipilimumab (23.3%). Five patients were initially treated with a combination of ipilimumab and a PD-1 inhibitor. Additionally, 14 patients (15.5%) were treated with second-line immunotherapy with an alternative ICI regimen due to disease progression on the initial ICI regimen. Median time from transplantation to initiation of ICI was 9 years (ranging from 0.2 to 32 years) in the 70 cases that reported it.
Response to ICI for entire cohort
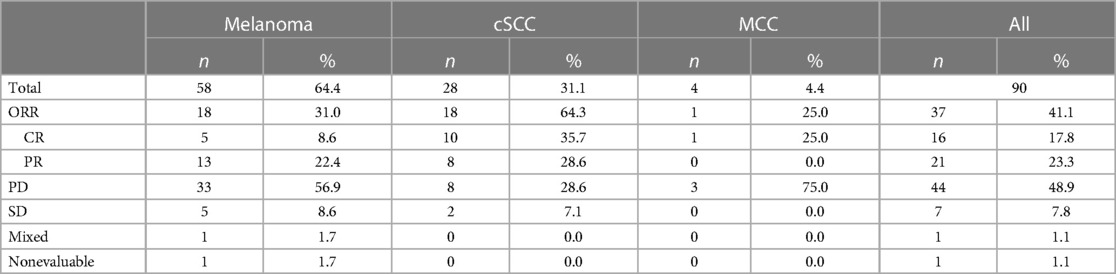
Objective responses to any ICI regimen for the entire cohort were: 17.8% CR (n = 16), 23.3% PR (n = 21), 7.8% SD (n = 7), and 48.9% PD (n = 44) for an objective response rate (ORR) (CR + PR) of 41.1%. One patient had a mixed response and one patient was considered unevaluable due to the patient expiring from graft failure before disease response could be evaluated. The majority of patients were treated with PD-1 inhibitors as first line ICI as opposed to ipilimumab monotherapy or ipilimumab plus nivolumab, with ORRs of 46%, 28.6%, and 40.0%, respectively. There were no significant differences in ORR between patients treated first-line with PD-1inhibitor vs. ipilimumab, PD-1 inhibitor monotherapy vs. ipilimumab plus nivolumab and ipilimumab monotherapy vs. ipilimumab plus a PD-1 inhibitor (p = 0.20, p = 1 and p = 0.63, respectively). Of note, there were 9 patients who had disease progression on ipilimumab and were started on a second-line PD-1 inhibitor, with an ORR of 44.4% (4/9) on the second-line agent. Of the patients who received a first-line PD-1 inhibitor, 4 were treated with second-line combination ipilimumab and nivolumab (ORR 25%, 1/4) while 1 was treated with second-line ipilimumab (ORR 0%, 0/1).
By tumor type, ORRs were 64.3% (18/28) for cSCC, 31% (18/58) for melanoma, and 25.0% (1/4) for MCC (Table 2). By type of SOT, ORRs were 57.1% for liver, 41.8% for kidney, and 0% for heart. The difference in ORR between patients with kidney and liver transplants was not significant (p = 0.38). However, as there was no tumor response in patients with heart transplants, the ORR was significantly higher when compared to patients with kidney (p = 0.04) and liver (p = 0.02) transplants.
SOT recipients with melanoma
The most common type of SOT in patients with melanoma was kidney (n = 42, 72.4%), followed by liver (n = 10, 17.2%), heart (n = 5, 8.6%), then heart and kidney (n = 1, 1.7%). The first line ICI regimen for melanoma patients was either ipilimumab (n = 21, 36.2%), a PD-1 inhibitor (n = 33, 56.9%), or a combination of ipilimumab and anti-PD-1 (n = 4, 6.9%). There were 9 patients who had disease progression on ipilimumab and were then treated with a second-line PD-1 inhibitor. Tumor responses were CR (n = 5, 8.6%), PR (n = 13, 22.4%), SD (n = 5, 8.6%), PD (n = 33, 56.9%), or mixed response (n = 1, 1.7%) for an ORR of 31%. There were 24 cases (41.4%) of acute graft rejection and 14 of those cases (58.3%) resulted in graft failure while the other 10 allografts were rescued with temporary discontinuation of ICI and immunosuppression.
SOT recipients with cSCC
Twenty-eight patients (31.1%) had cSCC and the vast majority had a renal transplant (n = 22, 78.6%) followed by liver (n = 4, 14.3%), heart (n = 1, 3.6%), and lung (n = 1, 3.6%) transplants. All patients received frontline PD-1 inhibition except for one patient who received ipilimumab and nivolumab which was administered after disease progression on carboplatin and taxol, and then cetuximab. Tumor responses were CR (n = 10, 35.7%), PR (n = 8, 28.6%), SD (n = 2, 7.1%), or PD (n = 8, 28.6%) for an ORR of 64.3%. There were 9 cases (32.1%) of acute graft rejection and 6 of those cases (66.7%) resulted in graft failure.
SOT recipients with MCC
Three patients with MCC were treated with a PD-1 inhibitor and one received a PD-L1 inhibitor. Tumor responses were CR (n = 1, 25%) or PD (n = 3, 75%) with an ORR of 25%. There was 1 case of acute kidney rejection that eventually progressed to graft failure.
Impact of ICI on graft in the entire cohort
There were 34 cases (37.8%) of acute graft rejection of which 21 resulted in graft failure (61.8%). In the overall cohort, 21/90 (23.3%) patients had graft failure. On average, graft rejection occurred 49 days after ICI initiation (ranging from 4 to 240 days), with a median of 28.5 days. Patients treated with a combination of CTLA-4 and PD-1 inhibitors (4/5) had the highest rate of graft rejection at 80%, followed by graft rejection rates of 36.5% (23/63) for first-line PD-1 inhibitors, and 14.3% (3/21) for first-line ipilimumab (Table 3). In addition, of the 9 patients who were treated with ipilimumab then a PD-1 inhibitor for second-line treatment, 4 of them experienced graft rejection after the first dose of the PD-1 inhibitor while 1 experienced graft rejection after the 4th cycle of ipilimumab prior to starting the PD-1 inhibitor. There was 1 patient who was treated with a PD-1 inhibitor followed by second line ipilimumab and 3 patients who were treated with a PD-1 inhibitor followed by second line ipilimumab and nivolumab. None of those 4 patients experienced graft rejection. Graft rejection rates were higher with combination ipilimumab plus nivolumab compared to first-line ipilimumab monotherapy (p = 0.01). There was no difference in rates of rejection for patients treated with anti-PD-1 vs. ipilimumab (p = 0.06) or ipilimumab plus nivolumab vs. anti-PD-1 monotherapy (p = 0.08). The one patient treated with a PD-L1 inhibitor did not have a graft rejection. Rates of acute graft rejection and failure were 41.8% (n = 28) and 26.9% (n = 18) for kidney transplants, 28.6% (n = 4) and 21.4% (n = 3) for liver transplant, and 14.3% (n = 1) and 0% for heart transplant, respectively. There were no significant differences between graft rejection rates by transplant type (kidney vs. liver p = 0.55, kidney vs. heart p = 0.23, and liver vs. heart p = 0.62). Mean time from transplant to ICI initiation for patients with no acute graft rejection vs. acute rejection was 9.3 years and 11.7 years respectively.
Number and type of immunosuppressive agents for SOT
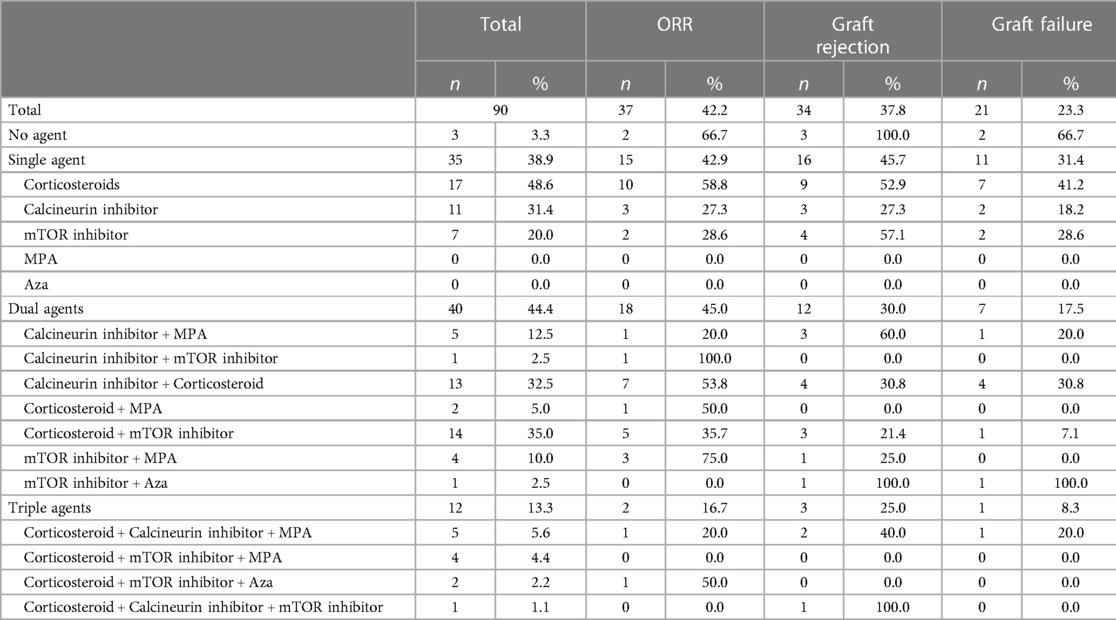
At the time of ICI initiation, patients were maintained on either 0 (n = 3), 1 (n = 35), 2 (n = 40), or 3 (n = 12) of the following immunosuppressive agents for their SOT: corticosteroids (prednisone), calcineurin inhibitors (tacrolimus, cyclosporine), mTOR inhibitors (sirolimus, everolimus), mycophenolic acid, and azathioprine.
The three patients who were not on any immunosuppressive agents at the time of ICI initiation all experienced graft rejection and two of those patients then experienced graft failure. Patients treated with 1, 2, and 3 immunosuppressive agent(s) had a graft rejection rate of 45.7% (16/35), 30% (12/40), and 25% (3/12), respectively and a graft failure rate of 31.4% (11/35), 17.5% (7/40), and 8.3% (1/12), respectively (Table 4). Patients on 2 or 3 immunosuppressive agents had lower rates of graft rejection compared to patients on 0 immunosuppressants (p = 0.04 and 0.04, respectively). There were no statistically significant differences in graft rejection rates when comparing patients treated with 1 vs. 2 (p = 0.23), 1 vs. 3 (p = 0.31), or 2 vs. 3 (p = 1.0) immunosuppressive agents, nor any differences in graft failure rates (p = 0.18, 0.15, and 0.66 respectively).
In patients treated with only a single immunosuppressive agent, those on prednisone monotherapy had the best response to ICI (ORR 58.8%), but also had a higher graft rejection rate of 52.9% and a graft failure rate of 41.2%. When comparing monotherapies, calcineurin inhibitors had the lowest rate of graft rejection (27.3%) and graft failure (18.2%). In patients receiving two or more immunosuppressive agents, those on an mTOR inhibitor and mycophenolic acid had the highest ORR at 75% (3/4), and the lowest rate of graft failure at 0%. Patients treated with both prednisone and an mTOR inhibitor also had lower rates of graft rejection (21.4%, 3/14) and failure (7.1%, 1/14) (Table 4).
In this series, it is difficult to evaluate whether a modification to the immunosuppressive regimen affected risk of graft rejection. Thirty-nine patients underwent a change in their immunosuppressive regimen prior to starting ICI therapy and 19 (48.7%) developed graft rejection. However, out of the 29 patients who had no change in their immunosuppressive regimen, 7 (24.1%) developed graft rejection in comparison. There was a statistically significant difference in rate of graft rejection between these two groups (p = 0.047).
Patients with melanoma were numerically more likely to have acute graft rejection than those with cSCC, while patients with cSCC were more likely to have their acute graft rejection progress to graft failure but the differences were not significant (p = 0.48 and p = 1). Liver transplant recipients tend to have lower rates of graft rejection and clinically, and it is possible that the selection of the type of and number of immunosuppressive agents may differ by type of transplant (71). In this series, patients with liver transplants received single agent immunosuppression (64.3%) at a numerically higher rate than patients with kidney transplants (31.3%), who were more commonly on 2 immunosuppressive agents at ICI initiation (50.7%). By type of SOT, rates of rejection were 41.8% (28/67), 28.6% (4/14), and 14.3% (1/7) for kidney, liver, and heart respectively and proceeded to graft failure in 64.3% (18/28), 75% (3/4), and 0% (0/1), respectively. Despite the difference in number of immunosuppressive agents, there were no significant differences in rate of graft rejection or graft failure when comparing liver to kidney transplants (p = 0.55 and p = 1, respectively).
The most common cause of death was disease progression, not graft failure, as has been commented on in another study (72). Of the 49 patients who died, 41 patients died due to progression of their underlying disease whereas 6/49 died from graft failure alone or a combination of graft failure and progression of disease. Of note, since the majority of patients received kidney transplants, if the allograft failed, patients underwent dialysis, which bias these results, and long term follow-up is not available. The two patients who declined dialysis passed away.
Discussion
Use of ICI in SOT with cancer is a clinical challenge due to the risks of life-threatening graft rejection. This retrospective review represents one of the largest collections of SOT patients with advanced skin cancer treated with an ICI, mostly anti-PD-1 monotherapy. It also adds an additional 4 cases to the literature from our single institution experience. Tumor stabilization or regression was noted in 48.9% of cases and the ORR was 41.1% for the entire cohort. Responses to anti-PD-1 in SOT patients with cSCC (ORR 60.7%) were higher than that reported in clinical trials of non-SOT patients (ORR 35%–50%) and higher than in other case series of SOT patients with cSCC (73–75). Patient or tumor-based characteristics of individual cSCC patients included here that may have accounted for this discrepancy are not available. SOT patients with melanoma had similar response rates to anti-PD-1 compared with responses in non-SOT patients treated with nivolumab or pembrolizumab (76, 77). There were too few cases of SOT patients with MCC to draw conclusions regarding response rates. The durability of responses and long-term overall survival is unable to be assessed due to the retrospective nature of the study and because the data was available only through published case series, with the exception of 4 patients from our institution. However, this study confirms the efficacy of ICI for advanced skin cancers in the SOT population, which has been highlighted in several other series (48, 70).
Despite the relatively preserved activity of ICI in skin cancer patients with SOT, graft rejection occurred in 37.8% of patients and more than half of those (61.8%) progressed to graft failure and either became reliant on hemodialysis, in the setting of kidney transplant failure, or passed away (48). There were no differences in rejection rates by type of transplant, but 4 out of 5 (80%) patients who were treated with a combination of ipilimumab and nivolumab experienced acute rejection and 2 out of 5 (40%) had graft failure. However, there were three SOT patients who were first treated with a PD-1 inhibitor and then followed by a combination of ipilimumab and nivolumab who did not develop graft rejection. The sample size in this study is too small to draw conclusions regarding impact of dual checkpoint inhibition on graft function (59). Only one patient was treated with a PD-L1 inhibitor in our cohort which limits our assessment of whether rates of graft rejection differ between PD-1 and PD-L1 inhibitors. One retrospective study included 6 kidney transplant patients, who did not fit our inclusion criteria, treated with anti-PD-L1 and report a graft rejection rate of 0% (75). Graft rejection in our series occurred in a numerically higher proportion of patients who received anti-PD-1 monotherapy compared to ipilimumab monotherapy, but the difference was not statistically significant and there have been conflicting reports in other retrospective series (56, 75, 78). Prior series have similarly and consistently shown that graft rejection for SOT patients occurs in about 40% of cases, however rates of graft failure are more variable (48, 56, 70, 72, 79). Despite high rates of graft rejection, cause of death was most commonly from disease progression. This may be impacted by the ability of patients with renal transplant rejection to be rescued with dialysis (70, 72, 80). Finally, there was no significant difference in time from transplant and initiation of ICI in patients who had graft rejection and those who did not. While some studies show higher rejection rates if ICI is administered earlier after a transplant, other studies have not verified that correlation (21, 75, 81).
The optimal approach to immunosuppressive regimens in SOT receiving ICI is unknown but being studied in several clinical trials. Although our study found that rates of graft rejection were higher in patients who had modifications to their immunosuppressive regimen prior to starting ICI therapy compared to those who did not have a change in their immunosuppressive regimen, not all cases reported on whether changes were made. Additionally, there are multiple confounding factors that need to be accounted for to better evaluate this question. Interestingly, in a phase I clinical trial studying pembrolizumab in kidney transplant recipients, it was also noted that maintaining baseline immunosuppression before ICI may reduce the risk of graft rejection (82). However, this requires further study. Tailoring the immunosuppression for SOT patients receiving ICI should be carefully discussed between the oncology and transplant teams to modify risks and clinical trial participation, where able, is encouraged. In our series, patients receiving no immunosuppression had the best tumor responses whereas patients on three immunosuppressive agents were poorly responsive to ICI, consistent with other studies (83, 84). Rates of graft failure were clearly inversely correlated with number of immunosuppressive agents, ranging from only 8.3% with 3 agents to 31.4% with one agent.
Type and/or combination of immunosuppressive agent(s) likely also matters. In patients treated with a single immunosuppressive agent, calcineurin inhibitors were associated with the lowest proportion of graft rejection (27.3%) and graft failure (18.2%), as previously reported, however tumor responses were also lower (ORR 27.3%) (70, 84). A clinical trial (NCT03816332) studied the efficacy of an ipilimumab, nivolumab, prednisone and tacrolimus regimen in patients with kidney transplants and advanced skin cancers, although in the small sample size, tacrolimus and prednisone did not prevent rejection in 2/8 patients (85). In our series, while tumor responses were highest in patients receiving prednisone only (ORR 58.8%), rates of graft rejection (52.9%) and failure (41.2%) were much higher. In patients receiving dual agent immunosuppression, the regimen that suggested the best balanced tumor response with lower risk of graft rejection or failure was prednisone plus an mTOR inhibitor. This finding is supported by results of CONTRAC-1 (NCT04339062), a clinical trial in which SOT patients with cSCC were treated with cemiplimab. The immunosuppressive regimen was standardized and involved administration of mTOR inhibition with a prednisone taper surrounding each cycle of cemiplimab (40 mg on days 1–3, 20 mg on days 4–6, and 10 mg on days 7–20). Preliminary results from the study showed ORR 50% in 8 evaluable patients and no kidney allograft rejection or loss (86).
The potential benefit of immunosuppressive regimens that contain mTOR inhibitors has been explored in another multi-center retrospective cohort study in which mTOR inhibitors were associated with lower rates of kidney graft rejection for SOT recipients treated with ICIs (75). In addition, mTOR inhibitors may have potential anti-tumor properties for post-transplant patients with cSCC, which may partially explain the improved ORR for patients with cSCC compared to melanoma in our study (87). Two case reports have further delved into the role of mTOR inhibitors in uncoupling the efficacy and toxicity of PD-1 inhibition, but the results appear inconclusive due to the limited sample size. Although one study notes that mTOR inhibitors may abate pembrolizumab associated T-cell activation, another notes that alloreactive T-cells may still be present despite persistent mTOR immunosuppression and may be induced by PD-1 inhibitors (49, 88). Future studies should focus on strategies to optimize immunosuppression for the allograft so that T-cell responses are directed towards tumor rather than both tumor and allograft.
This study has multiple limitations. This was a retrospective review of published cases from the literature in addition to cases reported from our own institution. The effect of publication bias here cannot be understated and may underestimate the incidence of graft rejection on ICI initiation. Cases in the literature that documented graft rejection but did not report on ICI responses were excluded. Differences in survival cannot be determined as the follow-up for each study was variable or not reported. Although we adhered to standard inclusion criteria for selection of published articles studied here, the actual treatment approaches and diagnosis and management of graft rejection or failure were individualized. Due to the overall small sample size, although Fisher's exact test was used, statistical analysis was largely descriptive and multivariable analysis was not performed. Prospective controlled studies are needed to confirm any trends observed here.
Overall, the use of ICI in SOT patients with skin cancer requires thorough multidisciplinary discussion, consideration of other available treatment options, if effective, and possible modification of the patient's immunosuppressive regimen to decrease risk of graft rejection and/or failure, however this requires further study. Risks and benefits including the possibility of fatal graft failure must be discussed at length with each patient. ICI remains the standard of care for advanced skin cancers and has the highest potential for long-term durable responses. In many cases, risks of administration of ICI in SOT patients may be necessary when balancing the risks of death from progressive skin cancer. Clinical trials are ongoing to better understand how to optimize immunomodulation in this population. The number of immunosuppressants and the biology of each type of organ transplant could affect rates of rejection and further research must be done to understand the impact of ICIs in an organ-specific manner as well. Strategies to prevent and manage graft rejection should also be studied, for example, with use of IL-6 antibodies or other agents. Donor-derived cell-free DNA (dd-cfDNA) may be a useful biomarker in cancer patients treated with ICI to detect acute rejection (50, 64). Although this was a population typically excluded from clinical trials, as the numbers of SOT patients increase, more research is needed to address this unique population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Rutgers Institutional Review Board (#2022000836). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. HL: Writing–review & editing, Formal Analysis. LP: Writing – review & editing. RS: Writing–review & editing. RG: Writing–review & editing. SW: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing–review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
SW has consulted for Lyell Immunopharma and Incyte. RG is an employee of Merck.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frtra.2023.1284740/full#supplementary-material
References
1. 2022 organ transplants again set annual records. United Network for Organ Sharing (2023). Available at: https://unos.org/news/2022-organ-transplants-again-set-annual-records/
2. Neergaard L. US counts millionth organ transplant while pushing for more. New York: Associated Press News. (2022). Available at: https://apnews.com/article/science-health-organ-transplants-government-and-politics-308bfae0c70c3377d595b9a0a3a5a381
3. Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Saf. (2000) 23(2):101–13. doi: 10.2165/00002018-200023020-00002
4. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. (2007) 370(9581):59–67. doi: 10.1016/S0140-6736(07)61050-2
5. Engels EA, Pfeiffer RM, Fraumeni JF Jr., Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. (2011) 306(17):1891–901. doi: 10.1001/jama.2011.1592
6. Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. (2005) 80(2 Suppl):S254–64. doi: 10.1097/01.tp.0000186382.81130.ba
7. O’Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part I. Epidemiology of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. (2011) 65(2):253–61. doi: 10.1016/j.jaad.2010.11.062
8. Greenberg JN, Zwald FO. Management of skin cancer in solid-organ transplant recipients: a multidisciplinary approach. Dermatol Clin. (2011) 29(2):231–41, ix. doi: 10.1016/j.det.2011.02.004
9. Euvrard S, Kanitakis J, Claudy A. Skin cancers after organ transplantation. N Engl J Med. (2003) 348(17):1681–91. doi: 10.1056/NEJMra022137
10. Garrett GL, Blanc PD, Boscardin J, Lloyd AA, Ahmed RL, Anthony T, et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the United States. JAMA Dermatol. (2017) 153(3):296–303. doi: 10.1001/jamadermatol.2016.4920
11. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers (Basel). (2020) 12(3):738. doi: 10.3390/cancers12030738
12. Hwu P. Treating cancer by targeting the immune system. N Engl J Med. (2010) 363(8):779–81. doi: 10.1056/NEJMe1006416
13. Li W, Zheng XX, Kuhr CS, Perkins JD. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am J Transplant. (2005) 5(5):978–86. doi: 10.1111/j.1600-6143.2005.00823.x
14. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. (2022) 40(2):127–37. doi: 10.1200/JCO.21.02229
15. Grob JJ, Gonzalez R, Basset-Seguin N, Vornicova O, Schachter J, Joshi A, et al. Pembrolizumab monotherapy for recurrent or metastatic cutaneous squamous cell carcinoma: a single-arm phase II trial (KEYNOTE-629). J Clin Oncol. (2020) 38(25):2916–25. doi: 10.1200/JCO.19.03054
16. Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. (2016) 374(26):2542–52. doi: 10.1056/NEJMoa1603702
17. Murakami N, Riella LV. Co-inhibitory pathways and their importance in immune regulation. Transplantation. (2014) 98(1):3–14. doi: 10.1097/TP.0000000000000169
18. Riella LV, Watanabe T, Sage PT, Yang J, Yeung M, Azzi J, et al. Essential role of PDL1 expression on nonhematopoietic donor cells in acquired tolerance to vascularized cardiac allografts. Am J Transplant. (2011) 11(4):832–40. doi: 10.1111/j.1600-6143.2011.03451.x
19. Yang J, Popoola J, Khandwala S, Vadivel N, Vanguri V, Yuan X, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. (2008) 117(5):660–9. doi: 10.1161/CIRCULATIONAHA.107.741025
20. Smedman TM, Line PD, Guren TK, Dueland S. Graft rejection after immune checkpoint inhibitor therapy in solid organ transplant recipients. Acta Oncol. (2018) 57(10):1414–8. doi: 10.1080/0284186X.2018.1479069
21. Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. (2017) 123(11):1904–11. doi: 10.1002/cncr.30642
22. Lipson EJ, Bodell MA, Kraus ES, Sharfman WH. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol. (2014) 32(19):e69–71. doi: 10.1200/JCO.2013.49.2314
23. Morales RE, Shoushtari AN, Walsh MM, Grewal P, Lipson EJ, Carvajal RD. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer. (2015) 3:22. doi: 10.1186/s40425-015-0066-0
24. Qin R, Salama AK. Report of ipilimumab in a heart transplant patient with metastatic melanoma on tacrolimus. Melanoma Manag. (2015) 2(4):311–4. doi: 10.2217/mmt.15.27
25. Ranganath HA, Panella TJ. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother. (2015) 38(5):211. doi: 10.1097/CJI.0000000000000077
26. Alhamad T, Venkatachalam K, Linette GP, Brennan DC. Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant. (2016) 16(4):1332–3. doi: 10.1111/ajt.13711
27. Gastman BR, Ernstoff MS. Tolerability of immune checkpoint inhibition cancer therapy in a cardiac transplant patient. Ann Oncol. (2016) 27(12):2304–5. doi: 10.1093/annonc/mdw293
28. Herz S, Hofer T, Papapanagiotou M, Leyh JC, Meyenburg S, Schadendorf D, et al. Checkpoint inhibitors in chronic kidney failure and an organ transplant recipient. Eur J Cancer. (2016) 67:66–72. doi: 10.1016/j.ejca.2016.07.026
29. Jose A, Yiannoullou P, Bhutani S, Denley H, Morton M, Picton M, et al. Renal allograft failure after ipilimumab therapy for metastatic melanoma: a case report and review of the literature. Transplant Proc. (2016) 48(9):3137–41. doi: 10.1016/j.transproceed.2016.07.019
30. Lipson EJ, Bagnasco SM, Moore J Jr., Jang S, Patel MJ, Zachary AA, et al. Tumor regression and allograft rejection after administration of anti-PD-1. N Engl J Med. (2016) 374(9):896–8. doi: 10.1056/NEJMc1509268
31. Ong M, Ibrahim AM, Bourassa-Blanchette S, Canil C, Fairhead T, Knoll G. Antitumor activity of nivolumab on hemodialysis after renal allograft rejection. J Immunother Cancer. (2016) 4:64. doi: 10.1186/s40425-016-0171-8
32. Spain L, Higgins R, Gopalakrishnan K, Turajlic S, Gore M, Larkin J. Acute renal allograft rejection after immune checkpoint inhibitor therapy for metastatic melanoma. Ann Oncol. (2016) 27(6):1135–7. doi: 10.1093/annonc/mdw130
33. Deltombe C, Garandeau C, Renaudin K, Hourmant M. Severe allograft rejection and autoimmune hemolytic Anemia after anti-PD1 therapy in a kidney transplanted patient. Transplantation. (2017) 101(9):e291. doi: 10.1097/TP.0000000000001861
34. Dueland S, Guren TK, Boberg KM, Reims HM, Grzyb K, Aamdal S, et al. Acute liver graft rejection after ipilimumab therapy. Ann Oncol. (2017) 28(10):2619–20. doi: 10.1093/annonc/mdx281
35. Kittai AS, Oldham H, Cetnar J, Taylor M. Immune checkpoint inhibitors in organ transplant patients. J Immunother. (2017) 40(7):277–81. doi: 10.1097/CJI.0000000000000180
36. Kwatra V, Karanth NV, Priyadarshana K, Charakidis M. Pembrolizumab for metastatic melanoma in a renal allograft recipient with subsequent graft rejection and treatment response failure: a case report. J Med Case Rep. (2017) 11(1):73. doi: 10.1186/s13256-017-1229-z
37. Miller DM, Trowbridge RM, Desai A, Drews RE. Kaposi’s varicelliform eruption in a patient with metastatic melanoma and primary cutaneous anaplastic large cell lymphoma treated with talimogene laherparepvec and nivolumab. J Immunother Cancer. (2018) 6(1):122. doi: 10.1186/s40425-018-0437-4
38. Owonikoko TK, Kumar M, Yang S, Kamphorst AO, Pillai RN, Akondy R, et al. Cardiac allograft rejection as a complication of PD-1 checkpoint blockade for cancer immunotherapy: a case report. Cancer Immunol Immunother. (2017) 66(1):45–50. doi: 10.1007/s00262-016-1918-2
39. Schvartsman G, Perez K, Sood G, Katkhuda R, Tawbi H. Immune checkpoint inhibitor therapy in a liver transplant recipient with melanoma. Ann Intern Med. (2017) 167(5):361–2. doi: 10.7326/L17-0187
40. Winkler JK, Gutzmer R, Bender C, Lang N, Zeier M, Enk AH, et al. Safe administration of an anti-PD-1 antibody to kidney-transplant patients: 2 clinical cases and review of the literature. J Immunother. (2017) 40(9):341–4. doi: 10.1097/CJI.0000000000000188
41. DeLeon TT, Salomao MA, Aqel BA, Sonbol MB, Yokoda RT, Ali AH, et al. Pilot evaluation of PD-1 inhibition in metastatic cancer patients with a history of liver transplantation: the mayo clinic experience. J Gastrointest Oncol. (2018) 9(6):1054–62. doi: 10.21037/jgo.2018.07.05
42. Grant MJ, DeVito N, Salama AKS. Checkpoint inhibitor use in two heart transplant patients with metastatic melanoma and review of high-risk populations. Melanoma Manag. (2018) 5(4):MMT10. doi: 10.2217/mmt-2018-0004
43. Kuo JC, Lilly LB, Hogg D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: a case report and literature review. Melanoma Res. (2018) 28(1):61–4. doi: 10.1097/CMR.0000000000000410
44. Lesouhaitier M, Dudreuilh C, Tamain M, Kanaan N, Bailly E, Legoupil D, et al. Checkpoint blockade after kidney transplantation. Eur J Cancer. (2018) 96:111–4. doi: 10.1016/j.ejca.2018.03.019
45. Sadaat M, Jang S. Complete tumor response to pembrolizumab and allograft preservation in renal allograft recipient on immunosuppressive therapy. J Oncol Pract. (2018) 14(3):198–9. doi: 10.1200/JOP.2017.027326
46. Tio M, Rai R, Ezeoke OM, McQuade JL, Zimmer L, Khoo C, et al. Anti-PD-1/PD-L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur J Cancer. (2018) 104:137–44. doi: 10.1016/j.ejca.2018.09.017
47. Zehou O, Leibler C, Arnault JP, Sayegh J, Montaudie H, Remy P, et al. Ipilimumab for the treatment of advanced melanoma in six kidney transplant patients. Am J Transplant. (2018) 18(12):3065–71. doi: 10.1111/ajt.15071
48. Abdel-Wahab N, Safa H, Abudayyeh A, Johnson DH, Trinh VA, Zobniw CM, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. (2019) 7(1):106. doi: 10.1186/s40425-019-0585-1
49. Esfahani K, Al-Aubodah TA, Thebault P, Lapointe R, Hudson M, Johnson NA, et al. Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun. (2019) 10(1):4712. doi: 10.1038/s41467-019-12628-1
50. Hurkmans DP, Verhoeven J, de Leur K, Boer K, Joosse A, Baan CC, et al. Donor-derived cell-free DNA detects kidney transplant rejection during nivolumab treatment. J Immunother Cancer. (2019) 7(1):182. doi: 10.1186/s40425-019-0653-6
51. Lee BT, Horwich BH, Chopra S, Ahearn A, Han HH. Checkpoint inhibitor-induced rejection of a liver allograft: a combination of acute T cell-mediated and antibody-mediated rejection. Liver Transpl. (2019) 25(12):1845–8. doi: 10.1002/lt.25622
52. Singh P, Visger Von J, Prosek J, Rovin B, Pesavento TE, Olencki T, et al. Preserved renal allograft function and successful treatment of metastatic merkel cell cancer post nivolumab therapy. Transplantation. (2019) 103(2):e52–3. doi: 10.1097/TP.0000000000002502
53. Bystrup Boyles T, Schødt M, Hendel HW, Krarup-Hansen A, Junker N. Pembrolizumab as first line treatment of merkel cell carcinoma patients–a case series of patients with various co-morbidities. Acta Oncol (Madr). (2020) 59(7):793–6. doi: 10.1080/0284186X.2020.1747637
54. Danesh MJ, Mulvaney PM, Murakami N, Riella LV, Silk AW, Hanna GJ, et al. Impact of corticosteroids on allograft protection in renal transplant patients receiving anti-PD-1 immunotherapy. Cancer Immunol Immunother. (2020) 69(9):1937–41. doi: 10.1007/s00262-020-02644-2
55. Hanna DL, Law SJ, Merrick SA, Heptinstall L, Bass P, Dupont P, et al. The successful use of pembrolizumab in a renal transplant recipient with metastatic melanoma. Melanoma Res. (2020) 30(3):321–4. doi: 10.1097/CMR.0000000000000651
56. Kumar V, Shinagare AB, Rennke HG, Ghai S, Lorch JH, Ott PA, et al. The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist. (2020) 25(6):505–14. doi: 10.1634/theoncologist.2019-0659
57. Owoyemi I, Vaughan LE, Costello CM, Thongprayoon C, Markovic SN, Herrmann J, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: a single-center analysis. Cancer. (2020) 126(21):4780–7. doi: 10.1002/cncr.33134
58. Soellradl I, Kehrer H, Cejka D. Use of ipilimumab and pembrolizumab in metastatic melanoma in a combined heart and kidney transplant recipient: a case report. Transplant Proc. (2020) 52(2):657–9. doi: 10.1016/j.transproceed.2019.09.014
59. Trager MH, Coley SM, Dube G, Khan S, Ingham M, Samie FH, et al. Combination checkpoint blockade for metastatic cutaneous malignancies in kidney transplant recipients. J Immunother Cancer. (2020) 8(1):e000908. doi: 10.1136/jitc-2020-000908
60. Venkatachalam K, Malone AF, Heady B, Santos RD, Alhamad T. Poor outcomes with the use of checkpoint inhibitors in kidney transplant recipients. Transplantation. (2020) 104(5):1041–7. doi: 10.1097/TP.0000000000002914
61. Brumfiel CM, Patel MH, Aqel B, Lehrer M, Patel SH, Seetharam M. Immune checkpoint inhibitor therapy in a liver transplant recipient with autoimmune disease and metastatic cutaneous squamous cell carcinoma. JAAD Case Rep. (2021) 14:78–81. doi: 10.1016/j.jdcr.2021.05.012
62. Gambichler T, Hessam S, Luttringhaus T, Boms S. Prompt response of advanced cutaneous squamous cell carcinoma to cemiplimab in a kidney transplant patient receiving treatment with everolimus. Clin Exp Dermatol. (2021) 47(3):608–9. doi: 10.1111/ced.15018
63. Goodman AE, Karapetyan L, Pugliano-Mauro M, Levenson JE, Luke JJ. Case report: single dose anti-PD1 in a patient with metastatic melanoma and cardiac allograft. Front Immunol. (2021) 12:660795. doi: 10.3389/fimmu.2021.660795
64. Lakhani L, Alasfar S, Bhalla A, Aala A, Rosenberg A, Ostrander D, et al. Utility of serial donor-derived cell-free DNA measurements for detecting allograft rejection in a kidney transplant recipient after PD-1 checkpoint inhibitor administration. Transplant Direct. (2021) 7(2):e656. doi: 10.1097/TXD.0000000000001113
65. Paoluzzi L, Ow TJ. Safe administration of cemiplimab to a kidney transplant patient with locally advanced squamous cell carcinoma of the scalp. Curr Oncol. (2021) 28(1):574–80. doi: 10.3390/curroncol28010057
66. Tan B, Baxter M, Casasola R. Acute renal transplant rejection following nivolumab therapy for metastatic melanoma. BMJ Case Rep. (2021) 14(2):e238037. doi: 10.1136/bcr-2020-238037
67. Tsung I, Worden FP, Fontana RJ. A pilot study of checkpoint inhibitors in solid organ transplant recipients with metastatic cutaneous squamous cell carcinoma. Oncologist. (2021) 26(2):133–8. doi: 10.1002/onco.13539
68. Bastos S, Masmoudi W, Pinard C, Duval-Modeste AB, Joly P, Hebert V. Efficacy of nivolumab in the treatment of metastatic cutaneous squamous cell carcinoma in a kidney-transplant patient with a history of allograft rejection. Ann Dermatol Venereol. (2022) 149(3):198–9. doi: 10.1016/j.annder.2022.01.003
69. O'Connell KA, Schmults CD. Treatment of metastatic cutaneous squamous cell carcinoma in a solid organ transplant recipient with programmed death-1 checkpoint inhibitor therapy. J Eur Acad Dermatol Venereol. (2022) 36(Suppl 1):45–8. doi: 10.1111/jdv.17407
70. Portuguese AJ, Tykodi SS, Blosser CD, Gooley TA, Thompson JA, Hall ET. Immune checkpoint inhibitor use in solid organ transplant recipients: a systematic review. J Natl Compr Canc Netw. (2022) 20(4):406–416.e11. doi: 10.6004/jnccn.2022.7009
71. Abrol N, Jadlowiec CC, Taner T. Revisiting the liver’s role in transplant alloimmunity. World J Gastroenterol. (2019) 25(25):3123–35. doi: 10.3748/wjg.v25.i25.3123
72. Fisher J, Zeitouni N, Fan W, Samie FH. Immune checkpoint inhibitor therapy in solid organ transplant recipients: a patient-centered systematic review. J Am Acad Dermatol. (2020) 82(6):1490–500. doi: 10.1016/j.jaad.2019.07.005
73. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. (2018) 379(4):341–51. doi: 10.1056/NEJMoa1805131
74. Hughes BGM, Munoz-Couselo E, Mortier L, Bratland Å, Gutzmer R, Roshdy O, et al. Pembrolizumab for locally advanced and recurrent/metastatic cutaneous squamous cell carcinoma (KEYNOTE-629 study): an open-label, nonrandomized, multicenter, phase II trial. Ann Oncol. (2021) 32(10):1276–85. doi: 10.1016/j.annonc.2021.07.008
75. Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, et al. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. (2021) 100(1):196–205. doi: 10.1016/j.kint.2020.12.015
76. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
77. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. (2015) 372(26):2521–32. doi: 10.1056/NEJMoa1503093
78. Aguirre LE, Guzman ME, Lopes G, Hurley J. Immune checkpoint inhibitors and the risk of allograft rejection: a comprehensive analysis on an emerging issue. Oncologist. (2019) 24(3):394–401. doi: 10.1634/theoncologist.2018-0195
79. Rossi E, Schinzari G, Maiorano BA, Esposito I, Acampora A, Romagnoli J, et al. Immune-checkpoint inhibitors in renal transplanted patients affected by melanoma: a systematic review. Immunotherapy. (2022) 14(1):65–75. doi: 10.2217/imt-2021-0195
80. Ferrándiz-Pulido C, Leiter U, Harwood C, Proby CM, Guthoff M, Scheel CH, et al. Immune checkpoint inhibitors in solid organ transplant recipients with advanced skin cancers-emerging strategies for clinical management. Transplantation. (2023) 107(7):1452–62. doi: 10.1097/tp.0000000000004459
81. Au KP, Chok KSH. Immunotherapy after liver transplantation: where are we now? World J Gastrointest Surg. (2021) 13(10):1267–78. doi: 10.4240/wjgs.v13.i10.1267
82. Carroll RP, Boyer M, Gebski V, Hockley B, Johnston JK, Kireta S, et al. Immune checkpoint inhibitors in kidney transplant recipients: a multicentre, single-arm, phase 1 study. Lancet Oncol. (2022) 23(8):1078–86. doi: 10.1016/S1470-2045(22)00368-0
83. d’Izarny-Gargas T, Durrbach A, Zaidan M. Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: a systematic review. Am J Transplant. (2020) 20(9):2457–65. doi: 10.1111/ajt.15811
84. Alzahrani N, Al Jurdi A, Riella LV. Immune checkpoint inhibitors in kidney transplantation. Curr Opin Organ Transplant. (2023) 28(1):46–54. doi: 10.1097/MOT.0000000000001036
85. Schenk KM, Stein JE, Chandra S, Davar D, Eroglu Z, Khushalani NI, et al. Nivolumab (NIVO) + tacrolimus (TACRO) + prednisone (PRED) +/− ipilimumab (IPI) for kidney transplant recipients (KTR) with advanced cutaneous cancers. J Clin Oncol. (2022) 40(16_suppl):9507. doi: 10.1200/JCO.2022.40.16_suppl.9507
86. Hanna GJ, Dharaneeswaran HJ, Giobbie-Hurder A, Harran JJ, Liao Z, Pai L, et al. Cemiplimab for kidney organ transplant recipients with advanced cutaneous squamous cell carcinoma: CONTRAC-1. J Clin Oncol. (2023) 41(16_suppl):9519. doi: 10.1200/JCO.2023.41.16_suppl.9519
87. Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. (2012) 367(4):329–39. doi: 10.1056/NEJMoa1204166
Keywords: immune checkpoint inhibitor, solid organ transplant, melanoma, cutaneous squamous cell carcinoma, merkel cell carcinoma
Citation: Ji S, Liu H, Pachella L, Stephenson RD, Groisberg R and Weiss SA (2023) Use of immune checkpoint inhibitors in solid organ transplant recipients with advanced cutaneous malignancies. Front. Transplant. 2:1284740. doi: 10.3389/frtra.2023.1284740
Received: 30 August 2023; Accepted: 13 October 2023;
Published: 30 October 2023.
Edited by:
Gaurav Gupta, Virginia Commonwealth University, United StatesReviewed by:
Naoka Murakami, Harvard Medical School, United StatesWilliam Kitchens, Emory University, United States
© 2023 Ji, Liu, Pachella, Stephenson, Groisberg and Weiss. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah A. Weiss c2F3ZWlzc0BjaW5qLnJ1dGdlcnMuZWR1
 Stephanie Ji
Stephanie Ji Hao Liu2
Hao Liu2 Laura Pachella
Laura Pachella Ryan D. Stephenson
Ryan D. Stephenson Roman Groisberg
Roman Groisberg Sarah A. Weiss
Sarah A. Weiss