- 1Division of Transplantation, Department of Surgery, University of Wisconsin, Madison, WI, United States
- 2Baylor Annette C. and Harold C. Simmons Transplant Institute, Baylor University Medical Center, Dallas, TX, United States
Organ transplantation is characterized by a sequence of steps that involve operative trauma, organ preservation, and ischemia-reperfusion injury in the transplant recipient. During this process, the release of damage-associated molecular patterns (DAMPs) promotes the activation of innate immune cells via engagement of the toll-like receptor (TLR) system, the complement system, and coagulation cascade. Different classes of effector responses are then carried out by specialized populations of macrophages, dendritic cells, and T and B lymphocytes; these play a central role in the orchestration and regulation of the inflammatory response and modulation of the ensuing adaptive immune response to transplant allografts. Organ function and rejection of human allografts have traditionally been studied through the lens of adaptive immunity; however, an increasing body of work has provided a more comprehensive picture of the pivotal role of innate regulation of adaptive immune responses in transplant and the potential therapeutic implications. Herein we review literature that examines the repercussions of inflammatory injury to transplantable organs. We highlight novel concepts in the pathophysiology and mechanisms involved in innate control of adaptive immunity and rejection. Furthermore, we discuss existing evidence on novel therapies aimed at innate immunomodulation and how this could be harnessed in the transplant setting.
Introduction
The immune system consists, broadly, of two intricately interconnected arms: the innate and the adaptive. The innate arm is responsible for a rapid and broad response to invading pathogens, as well as to diverse physical and metabolic insults (1). This response involves the activation of a complex network of cellular and soluble mediators of inflammation that play a crucial role in maintaining immune surveillance at critical tissue sites. However, the failure to resolve the response can lead to persistent inflammation, tissue damage, and fibrosis (2, 3). The innate immune system uses pattern recognition receptors (PRRs) to detect conserved pathogenic motifs, such as lipid and carbohydrate moieties. PRRs are expressed on various immune and non-immune cells and can sense pathogen-associated molecular patterns and danger signals. Toll-like receptors (TLRs) are the most extensively studied PRRs, found on the cell surface and endosomes of cells, and they recognize bacterial lipopolysaccharides and viral nucleic acids. Intracellular signaling pathways are initiated upon PRR activation, resulting in the mobilization of inflammatory and immune responses (4, 5). The innate immune system's hard-wired responsiveness provides a critical first line of defense against invading pathogens and tissue damage, ensuring a rapid and effective response to potential threats.
In contrast, the adaptive arm is triggered by the presence of specific antigens, and it involves a highly targeted and specific response. This response is characterized by clonal expansion and affinity maturation of T and B cells, resulting in the generation of high-affinity receptors for antigenic peptides (6). The innate arm plays a vital role in initiating and enhancing the adaptive response by providing signals that trigger the recruitment and activation of cells of the adaptive immune system. The coordinated and integrated interplay between the innate and adaptive arms is essential for the acquisition of effective and long-lasting immunity against invading pathogens (7). The innate arm provides a rapid and non-specific response, while the adaptive arm provides a highly specific and targeted response. Therefore, the ability of the immune system to generate a successful defense against invading pathogens depends on the functional interdependence between these two arms of the immune system (8).
The innate pathway has been extensively studied in the context of transplantation. Understanding the role of these innate pathways in graft dysfunction and rejection is of paramount importance. By exploring the intricate interplay between the innate immune system and the transplantation process, we can identify novel therapeutic targets for promoting graft acceptance.
This review aims to provide a comprehensive analysis of the complement and TLR systems in the context of transplantation. We will examine the mechanisms underlying their activation, their contribution to graft dysfunction and rejection, and the potential for targeted manipulation of specific components to steer the immune response towards graft protection and acceptance. In doing so, we hope to deepen our understanding of the complex immune interactions that underlie innate immune responses in transplantation and to provide valuable insights into the development of new immunomodulatory strategies for improving transplant outcomes.
Components of the innate immune system and their role in transplant
Complement
The complement system is a vital component of the innate immune system. It is triggered by pattern recognition molecules (PRMs) that recognize specific patterns on the surface of invasive pathogens or damaged cells. The classical and lectin complement activation pathways converge on C3, the pivotal component of complement, while the alternative pathway generates a C3 convertase from C3b and factor B (fB). The resulting cleavage of C3 generates complement effectors, including the membrane attack complex (MAC), C3a, and C5a, which recruit the adaptive immune system and mediate inflammation (Figure 1) (9, 10).
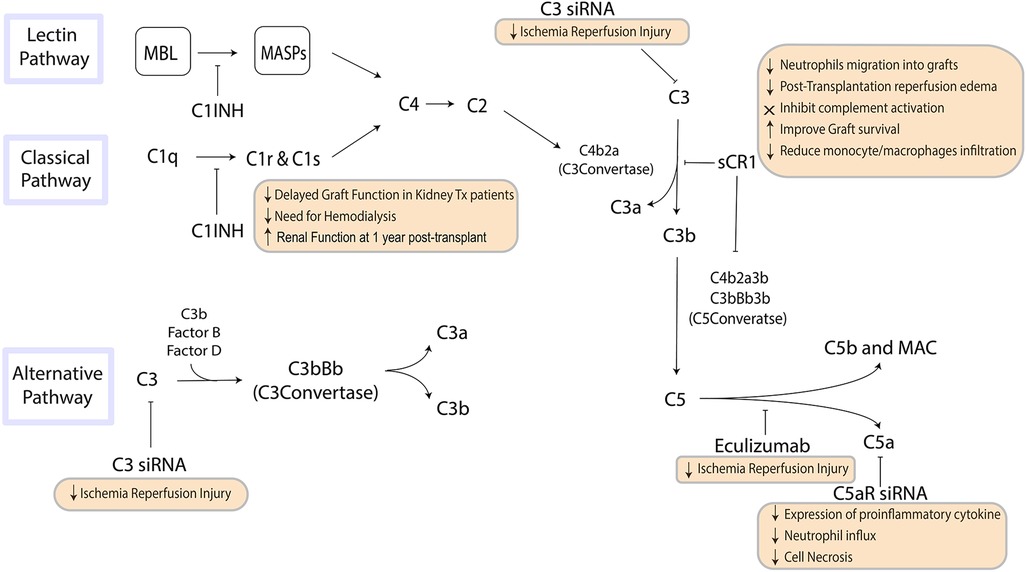
Figure 1. The pathways of the complement system are a crucial component of innate immunity that influence inflammation and the recruitment of the adaptive immune system. Steps in this pathway can be targeted to suppress inflammation and influence immune response to organ transplantation. MBL, mannose-binding lectin; MASP, mannan-binding lectin-associated serine protease; MAC, membrane attack complex.
Allograft rejection is a complex process that involves both innate and adaptive immune responses, and complement activation has been widely implicated in the initiation and progression of allograft injury during ischemia reperfusion injury (IRI) and in the pathophysiology of rejection (10–12). Complement activation during IRI is triggered by locally released CL-11, which activates C3a/C3aR interaction leading to glomerular and tubular injury and stimulates secondary epithelial cell chemokine production that contributes to local inflammation (13, 14).
The role of complement activation in acute rejection is more complex. The inflammatory response triggered by complement activation in a transplanted organ is mediated via generation of biologically active complement fragments. These fragments mediate chemotaxis and activate neutrophils and macrophages, resulting in a cascade of biological effects that depend on the site and trigger of complement activation (15). Once the MAC is formed, cells are induced to produce IL-1α and IL-8, further increasing local tissue inflammation (16).
Complement activation is detrimental in itself, as it damages the transplanted organ, but it also leads to recruitment of the adaptive immune system by enhancing T-cell activity. C5a has been shown to be a strong chemoattractant, and C5aR deficiency has demonstrated a protective effect in mouse models of IRI (17). Understanding the varying roles of complement within the transplant process is critical to predicting transplant outcomes and developing strategies to minimize the risk of allograft rejection.
Toll-like receptors
IRI is a multifaceted process that entails the activation of diverse pathways such as toll-like receptor (TLR) signaling, extracellular signaling molecules, alterations in gene expression, production of reactive oxygen species (ROS), regulation of cell death, activation of innate and adaptive immune factors, and the triggering of hypoxia-inducible factors (18). In general, this process constitutes a classic positive feedback loop in which damaged cells at the transplantation site release redox-sensitive damage-associated molecular patterns (DAMPs) that promote local accumulation of recipient-derived monocytes and polymorphonuclear leukocytes (PMNs), such as neutrophils (19). These cells sustain immune cascades and amplify the destruction of foreign tissue. This self-amplified cytotoxic cascade is initiated by the release of DNA/RNA complexes and/or acetylation of the non-histone chromatin-associated protein such as high mobility group box 1 (HMGB1), a representative danger signal. Additionally, degradation of the extracellular matrix can release molecules such as heparan sulfate, hyaluronan, fibrinogen, fibronectin A domain, or tenascin C, which can further amplify the response to tissue damage (20).
Acute kidney injury (AKI) is a complex disorder characterized by a rapid decline in renal function resulting from various etiologies. Neutrophils and macrophages are among the earliest effector cells involved in AKI, where they are found to persist for several days. The contribution of these cells to the pathophysiology of IRI results from their adherence to endothelium, release of ROS and proteases, and their ability to synthesize and secrete cytokines/chemokines that recruit other effector cells to the organ, thereby amplifying local inflammation (21–23).
Zhang et al. investigated the inactivation of neutrophils using mAbs against high mobility group box 1 (HMGB1) in a mouse model of AKI. They found that the treatment alleviated IRI-induced renal dysfunction by suppressing the activation of the HMGB1-Toll-like receptor 4 (TLR4)-interleukin (IL)-23-IL-17A signaling axis. This resulted in the reduction of neutrophil-mediated inflammation, chemokine expression, oxidative stress, and apoptosis (24). Neutrophil trafficking is also dependent on the crosstalk between donor non-classical and classical monocytes in a lung transplant IRI model (25).
Depleting neutrophils entirely may not be clinically feasible, given their critical role in pathogen clearance and immune surveillance. Instead, selectively blocking the recruitment of circulating leukocytes to the inflammatory site may be a better approach. In a recent study, researchers used mAb treatment to block the vascular surface marker CD321, a marker for the transmigration of circulating leukocytes into the inflamed tissue. This blockade attenuated damage responses by hepatic IRI, evaluated by serum liver enzymes, inflammatory cytokines, and hepatocyte cell death (26).
The IL-6 pathway
As a critical regulator of inflammation, IL-6 plays a pivotal role in the pathophysiology of transplant rejection (27). It is noteworthy that upregulation of IL-6 during brain death promotes a proinflammatory state even before organ procurement (28, 29), and during cold preservation, there is a detectable increase in graft IL-6 levels (30). This increase in IL-6 then upregulates adhesion molecules, inflammatory cytokines such as IL-17 and IFN-γ, and molecules that regulate migration in endothelial cells (31). Importantly, a growing body of evidence suggests that IL-6 blockade can mitigate the pro-inflammatory environment associated with brain death and IRI (32). Evidence from experimental and human studies links IL-6 to allograft injury, with blockade of the IL-6/IL-6R signaling pathway shown to mitigate these effects (33, 34). In mouse skin transplantation models, IL-6 promotes T cell alloreactivity and impairs Treg function (35). In addition, inhibition of IL-6 signaling reduces the production of donor-specific antibodies and modulates immune regulatory and effector cells (36, 37). Studies in murine models demonstrate that IL-6 plays a critical role in the pathogenesis of acute and chronic allograft rejection, and blocking IL-6 reduces/prevents rejection and fibrosis (38–41). In human transplant recipients, higher levels of IL-6 are consistently associated with worse outcomes, including a higher risk for acute rejection (42–44).
Coagulation cascade
The coagulation system, through the extrinsic and intrinsic pathways, activates factor X. Initiated by vascular injury, the extrinsic pathway catalyzes thrombin production, vital for stimulating platelets and vascular cells. The intrinsic pathway is autoactivated when circulating factor XII contacts negatively charged surfaces, under the regulation of antithrombin, tissue factor pathway inhibitor, and activated protein C. Importantly, coagulation interacts closely with the innate immune system and the complement cascade, influencing thrombogenesis and fibrinolysis (45).
Thrombin and tissue factor interact with protease-activated receptors on immune cells, releasing inflammatory mediators crucial for transplantation and IRI. In delayed graft function (DGF), ischemia/reperfusion-triggered coagulation activation, fibrin deposition, and robust expression of pro-inflammatory agents primes adaptive alloimmune responses and leads to allograft dysfunction (46, 47).
Mechanistically, this is explained by infiltrating dendritic cells expressing protease-activated receptor 1 (PAR-1), which influences cytokine gene expression and enhances T helper-1 bias in DGF (48). Interstitial coagulation activation, indicative of renal graft rejection, involves fibrin stimulation of fibrosis-contributing cells, and post-transplantation complications like microvascular thrombi, endothelial dysfunction, and fibrin deposition can lead to graft dysfunction, particularly in highly sensitized recipients (49, 50).
The fibrinolytic system, responsible for removing fibrin, prevents vessel occlusion and is significant in transplantation (51). The cause of organ donors' death can affect fibrinolysis activation, with injured donors exhibiting enhanced fibrinolytic activity (52). DGF correlates with increased urokinase (uPA) and urokinase-type plasminogen activator receptor (uPAR) expression, which impacts graft function and renal filtration. Chronic renal graft failure is characterized by fibrin deposition due to a disrupted plasminogen/plasmin system inhibiting fibrinolysis (53, 54).
Therapeutic strategies
Many current therapies in transplantation target the adaptive immune system in order to dampen the immune response to the new organ. The adaptive immune system and innate immune system are intimately linked, and the various components of the innate immune system may serve as novel therapeutic targets (Figure 2).
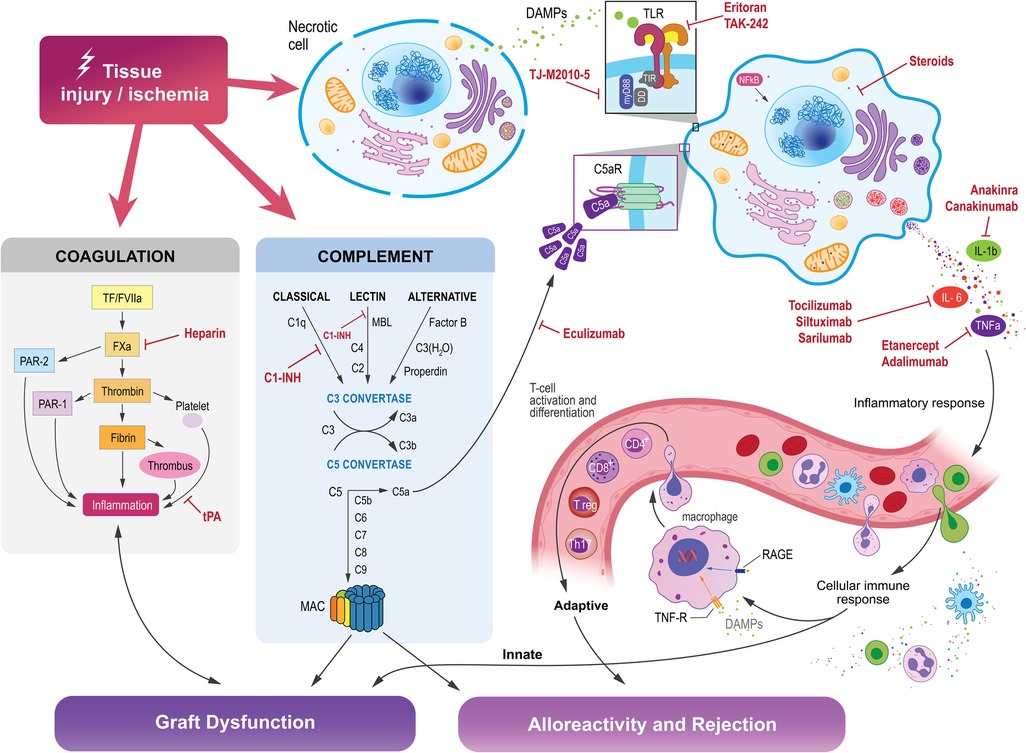
Figure 2. Tissue injury and ischemia activates the innate immune system, which is comprised of intricately interconnected pathways that influence the activation of the adaptive immune system, which can ultimately lead to graft dysfunction, alloreactivity, and rejection in organ transplantation. Molecules within each of these pathways may be targeted to suppress those reactions and promote transplant organ function and acceptance by the host immune system.
Complement inhibition
There is increasing experimental and clinical evidence indicating that complement blockade can be an effective therapeutic target for improving outcomes in organ transplantation (55). A range of therapeutic agents, including monoclonal antibodies, small molecules, and small interfering RNA (siRNA) agents, have been developed to inhibit complement cascade activation. For example, the administration of a soluble complement receptor-1 antagonist (sCR1) can inactivate the C3 and C5 convertases, resulting in reduced neutrophil migration into grafts and reduced post-transplantation reperfusion edema in animal models of lung allotransplantation (56). In clinical trials, sCR1 has been shown to effectively inhibit complement activation, and treating recipient mice with a C5aR antagonist before transplantation has been shown to improve graft survival and reduce monocyte/macrophage infiltration (57, 58).
Eculizumab, a humanized monoclonal antibody that binds to C5 and inhibits its cleavage to C5a, has been approved by the FDA to block complement cascade activation and constitutes another approach to attenuating IRI in organ transplantation (59, 60). Clinical case reports have shown promising results for eculizumab in patients with atypical hemolytic uremic syndrome undergoing kidney transplantation (61). Another potential strategy to inhibit complement cascade activation is to silence C3 using siRNA. In a mouse model of kidney IRI, the systemic administration of C3-specific siRNA reduced renal injury and mouse mortality by diminishing renal C3 synthesis, as C3 is the central component of complement cascade activation where all three pathways converge (62, 63). Silencing the C5a receptor (C5aR) gene using siRNA before the induction of ischemia resulted in reduced expression of the proinflammatory cytokine TNF-α and chemokines MIP-2 and KC, resulting in reductions in neutrophil influx and cell necrosis in the kidneys (64).
In a preclinical context, C1 inhibitors (C1 INH), which are approved for hereditary angioedema treatment, can prevent the activation of both the classical and lectin pathways (65). Research using a brain-dead rat model has shown that C1 INH can mitigate complement activation resulting from brain death (66). Our preliminary studies using a mouse model of renal IRI showed that pre-treating animals with C1 INH led to enhanced renal function, improved survival rates, and decreased C5a release, C3b deposition, as well as neutrophil/macrophage infiltration in the grafts. Furthermore, we observed a significant reduction in tissue fibrosis and TGF-β1 levels 30 and 90 days post-ischemia (67). Utilizing a nonhuman primate kidney transplant model, we demonstrated that managing brain-dead donors with recombinant human C1 INH and heparin substantially diminished complement pathway activation, attenuated systemic inflammatory response, and crucially, averted DGF in all instances compared to controls (68). A subsequent study suggested that C1 INH, when administered systemically to either the brain-dead donor or recipient only, could decrease complement deposition on biopsy and prevent DGF (69). Lastly, in a pig kidney auto-transplant model, C1 INH treatment expedited the recovery of glomerular function and significantly lessened long-term graft fibrosis (70, 71).
Clinical trials pertaining to kidney transplantation have thus far explored the use of C1 and C5 inhibitors as well as a C3 inhibitor. The primary objective of these trials was to avert early graft failure due to DGF, and to prevent both early and late rejection episodes, particularly antibody-mediated rejection (ABMR). Currently, only one phase I/II trial (NCT02134314) comparing the use of the C1-esterase with a placebo has been completed. Although there were no significant differences between groups in the incidence of DGF, the duration of dialysis was notably shortened in the C1-esterase inhibitor group (72). Furthermore, in a follow-up study conducted 3.5 years post-transplantation, a significantly improved estimated glomerular filtration rate (eGFR) was found in the C1-esterase inhibitor group (73).
Recent alternative anti-complement therapies have shown promise by directly administering complement inhibitors to donor kidneys ex vivo before transplantation. This method focuses on local kidney complement inhibition, minimizing the impact on the recipient's systemic complement pool. Mirococept, derived from human CR1, was tested in the EMPIRIKAL trial to evaluate its effect on DGF duration. The initial trial demonstrated the feasibility and safety of ex vivo Mirococept delivery to kidney allografts and highlighted the need for dose calibration (74). Subsequent pig kidney studies showed strong localization to the treated kidney's tubular epithelium and capillaries, with minimal recipient release. The EMPIRIKAL trial is now revising its human study protocol based on the optimal higher dose achieved in pigs (EMPIRIKAL-2).
TLR inhibition
Inhibitors and antagonists that target TLR signaling or downstream components have the potential to reduce IRI and improve allograft survival, and several studies on animal models suggest that blocking antibodies against TLR pathway molecules can be an effective therapeutic strategy. For example, the administration of an anti-HMGB1 neutralizing antibody reduced kidney IRI in mice, as shown by lower levels of inflammation and apoptosis in tubular epithelial cells, and reduced infiltration of neutrophils and macrophages (75). In addition, in a model of cardiac transplantation, neutralizing HMGB1 resulted in a reduction of inflammatory CD11b + Ly6C high myeloid cells both at the level of the allograft and the spleen (76).
There have been numerous attempts to block the TLR pathway as a strategy to attenuate ischemia-reperfusion injury (IRI) and enhance allograft survival. One such attempt involves the use of eritoran, a TLR4 antagonist that has been tested in transplantation (77). Eritoran is a synthetic structural analog of the lipid A portion of lipopolysaccharide (LPS) that binds to the MD2-TLR4 receptor and terminates MD2/TLR4-mediated signaling. This action blocks LPS from binding at the cell surface and subsequently inhibits the pro-inflammatory signaling cascade that follows (78). In a rat transplantation model, eritoran treatment induced less monocyte infiltration; lower levels of TNF-α, IL-1β, IL-6, and MCP1; and prolonged survival. There is also evidence to suggest that eritoran may block the HMGB1-TLR4 interaction, which could explain its ability to attenuate IRI. In a mouse model of liver IRI, eritoran was shown to inhibit the TLR4-dependent release of HMGB1, indicating a potential mechanism by which eritoran exerts its protective effects (79).
Another TLR-4 inhibitor, TAK-242, is a small compound that mitigates the release of inflammatory cytokines triggered by pathogens, primarily by impeding TLR-4-mediated signaling pathways. Furthermore, it exhibits suppressive effects on the synthesis of nitric oxide (NO) or tumor necrosis factor (TNF)-alpha, specifically when induced by the TLR4-specific ligand LPS (80). A study investigated the effects of TAK-242 on liver transplant viability in a model of swine Maastricht-category-III cardiac death. TAK-242 was administered prior to induction of cardiac death with mRNA and protein levels of TLR4 signaling pathway evaluated at different time points after induction of cardiac death. The results showed that the mRNA and protein levels of important immune and inflammatory response molecules such as TLR4, NF-ϰB, MCP-1, TNF-α, IL-6, ICAM-1, and MPO, increased significantly after cardiac death. However, infusion of TAK-242 one hour before induction blocked the increase of many of these molecules; though notably, the increase of TLR4 levels was not affected by the TAK-242 infusion (81).
Other potential targets in the innate immune system
IL-6 inhibitors
Tocilizumab, a potent antagonist of the interleukin-6 receptor, has shown remarkable efficacy in reducing HLA antibodies in highly sensitized patients awaiting kidney transplantation and those with antibody-mediated rejection. In 2015, Vo et al. conducted a phase I/II open-label trial (NCT01594424) with ten unresponsive end-stage renal disease patients. The six-month treatment regimen with monthly tocilizumab and IVIg administration resulted in a reduction of DSA levels, enabling five patients to undergo kidney transplantation. Biopsies revealed no evidence of antibody-mediated rejection (AMR) six months post-transplant, but two patients who stopped tocilizumab experienced mild AMR a year later. The study suggested that targeting the IL-6 pathway could offer an alternative for desensitization (82).
A subsequent study by this group evaluated the use of tocilizumab as a rescue therapy for DSA-positive chronic antibody-mediated rejection (cAMR) and transplant glomerulopathy in patients who had previously failed standard treatments. Monthly administration of tocilizumab yielded an 80% graft survival rate at six years, decreased DSA levels, and stabilized renal function without significant adverse events. Four patients did experience graft loss after discontinuing tocilizumab, suggesting rebound IL-6 pathway signaling may contribute to generation of an alloimmune response (83).
Furthermore, tocilizumab has been studied as a first-line therapy for cAMR in kidney transplantation, resulting in GFR stabilization and a significant decrease in DSA with improved pathology observed at six months. IL-6 blockade may also be a potential treatment option for regulating T-cell alloimmune response in kidney transplantation. A recent randomized-controlled trial found that kidney transplant recipients who received tocilizumab had increased T-reg frequency and a blunted T-effector cytokine response (84). A multicenter phase II clinical trial investigating the efficacy of tocilizumab in cardiac transplantation is currently under way to evaluate the impact of tocilizumab on acute and chronic cardiac allograft rejection (NCT03644667).
TNF and IL-1 receptor antagonists
The use of anti-inflammatory agents to counteract the harmful effects of proinflammatory cytokines has been extensively investigated, primarily in islet transplantation. Anti-cytokine strategies, such as TNF-alpha inhibition and IL-1 beta antagonism, have demonstrated promising results in improving islet cell function and promoting insulin-independence following transplantation.
TNF-a inhibitors have shown reduced inflammatory markers in animal models and long-lasting efficacy in treating autoimmune disorders (85, 86). The pharmacological characteristics of TNF inhibitors can vary considerably, with Etanercept (ETA) binding and neutralizing lymphotoxin-α3 and lymphotoxin-α2β1, while Infliximab (INF) does not possess these properties (87, 88). A recent qualitative analysis of anti-inflammatory agents in clinical islet transplantation summarized findings from multiple studies evaluating Etanercept monotherapy in allo-islet cell transplantation protocols (89). Although the studies differed in insulin independence (II) definitions and assessment time points, the overall results suggest that Etanercept may contribute to long-term maintenance of II. Despite some inconsistencies in outcomes, an Etanercept-based protocol could be associated with lower islet equivalent (IEQ) requirements (90, 91).
A single prospective, randomized study examined Infliximab as an adjunct to the Edmonton protocol, but no significant clinical benefits or substantial differences were observed in oral glucose tolerance test (OGTT) or intravenous glucose tolerance test (IVGTT) outcomes (92).
IL-1β, a pro-inflammatory cytokine, has been linked to β-cell destruction and diabetes development (93). Interleukin-1 receptor antagonists (IL-1RA) have been demonstrated to effectively counteract the effects of proinflammatory cytokines, including nitric oxide production, necrosis, apoptosis, glucose-stimulated insulin secretion, and mitochondrial dysfunction (94). Several studies have shown that interleukin-1 receptor antagonist (IL-1RA) can improve pancreatic islet transplantation outcomes in mice, primates, and humans (95, 96). In two human trials, anakinra combined with other immunosuppressive agents during the first 1–2 weeks post-transplantation resulted in better engraftment without increased infection risk (97, 98).
Limited data exist on IL-1 beta-directed therapies in transplantation outside the islet field. In renal transplantation, the safety and efficacy of combining IL-1 beta-directed therapy with standard immunosuppressive treatment were evaluated in three patients, with no significant complications except for minor infections (99). Case reports and series indicate that anakinra can be safely used alongside immunosuppressive agents in renal transplant recipients with IL-1 driven diseases, although one patient experienced neutropenia and decreased allograft function, with an unclear link to IL-1 blockade (100).
Coagulation cascade inhibition
The coagulation system, which regulates bleeding and clotting, also plays an important role in innate immunity; this, and its interaction with the fibrinolytic system influences IRI. Targeting coagulation factors is complicated by their presence within fibrin clots, often protecting them from inhibitors (45). Therapies such as rapamycin can mitigate fibrinolysis disruption by reducing plasminogen activator inhibitor type 1 (PAI-1) expression, slowing the progression of interstitial fibrosis (101). Furthermore, in porcine models of renal autotransplant, the use of Xa and dual Xa/IIa inhibitors has been shown to be beneficial to graft outcome (102, 103). In addition to inhibiting the complement system, C1 INH has also been shown to inhibit enzymes in the coagulation and fibrinolytic systems, including factor XII and plasmin, respectively (104). As discussed above, in pre-clinical models and ongoing clinical studies, C1 INH has demonstrated a beneficial effect on graft function.
Steroids
Glucocorticoids (GCs) play a crucial role in immunosuppression following renal transplantation by mitigating inflammatory responses and leukocyte infiltration. They also facilitate the resolution of inflammation through the suppression of vascular permeability, leukocyte distribution/trafficking, and modulation of cellular death/survival and differentiation processes (105, 106). While GCs were initially believed to exert their anti-inflammatory effects through the inhibition of pro-inflammatory cytokine-encoding gene regulators, recent studies have uncovered alternative mechanisms, including negative interference with inflammatory mediator synthesis, suppression of immune cell activation, and cooperation between the glucocorticoid receptor and transcription factors to induce anti-inflammatory genes (107).
GCs are known to induce neutrophilic leukocytosis by promoting neutrophil maturation and mobilization, an effect that can be blocked by L-selectin adhesion protein inhibition. They also hinder the entire neutrophil activation process, including respiratory burst-related enzyme expression, chemotaxis, phagocytosis, and cytokine secretion. In neutrophils, GCs suppress transcription factors related to pro- and anti-inflammatory genes, leading to upregulated expression of interleukin and pro-inflammatory leukotriene receptors and reduced apoptosis sensitivity, thus extending neutrophil lifespan. Furthermore, endogenous glucocorticoids under stress conditions affect natural killer (NK) cells by reducing their cytolytic activity and inducing pro-inflammatory cytokine synthesis through an epigenetic mechanism, enhancing the expression of IL-6 and INF-γ and promoting histone acetylation in their enhancer regions.
GCs also exhibit direct effects on innate immune cells, as demonstrated by in vitro studies on methylprednisolone-treated monocytes, which show increased anti-inflammatory cytokine expression and reduced antigen-presenting capabilities (108, 109). In vivo data from renal transplant recipients treated with methylprednisolone reveal alterations in monocyte populations and TLR4 downregulation on GC-treated monocytes, a critical component in monocyte activation during sepsis and the immune response to transplanted organs (110, 111). Steroids also impact dendritic cell differentiation and maturation, with dexamethasone-exposed monocyte-derived dendritic cells displaying decreased expression of specific markers, impaired antigen-presenting cell function, and reduced cytokine secretion (112).
The anti-inflammatory effect of steroids in transplantation has been widely investigated in the deceased donor setting and initial studies have not consistently show benefits of donor methylprednisolone treatment in transplantation outcome (113). In addition, a large-scale, randomized, double-blind trials in kidney transplantation found that while methylprednisolone pretreatment improved inflammatory and apoptotic gene expression profiles, it did not impact delayed graft function or serum creatinine decline (114, 115). Therefore, high-dose methylprednisolone before organ recovery is not recommended for kidney transplantation.
Although the trial's results were inconclusive, preliminary evidence suggests potential benefits of donor steroid treatment in liver transplantation, prompting continued research efforts into the routine use of steroids in combined hormonal resuscitation protocols (116).
Myeloid differentiation factor 88 (MyD88)
Myeloid differentiation factor 88 (MyD88) is a protein that plays a role in signal transduction through TLRs, prompting the production of pro-inflammatory cytokines that promote leukocyte recruitment. Specifically, MyD88 links the IL-1 receptor or other TLR family members to IL-1R-associated kinase (IRAK) kinases that leads to downstream events including NFkB activation and MAP kinase activation (117). Thus, it has a far-reaching effect on the inflammatory response of the immune system.
TJ-M2010-5 is a MyD88 inhibitor that targets and interacts with the MyD88 TIR domain, interfering with dimerization. This molecule has been shown in murine models to play a role in slowing liver fibrosis, and decreasing myocardial ischemic reperfusion injury (118), cerebral reperfusion injury (119), and hepatic reperfusion injury (120). Alleviation of hepatic reperfusion injury with this molecule has been particularly successful when coupled with hepatic hypothermic oxygenated perfusion (121). While these studies have been performed using animal models, they demonstrate some significant promise as a viable therapeutic option for the prevention or reduction of ischemia reperfusion injury in human transplantation.
Resveratrol
Resveratrol is a naturally-occurring phenol produced by plants that has been shown to play a role in inflammatory disease prevention and progression. Resveratrol is thought to be produced in response to oxidative stress, and several studies have shown it is able to regulate hepatic metabolism of lipids hence improving metabolic lipid homeostasis and slowing the progression of fatty liver disease (122). Resveratrol also functions as an anti-oxidant, and its main effect is linked to the reduction of reactive nitrogen species, direct elimination of free radicals, improvement in antioxidant enzymatic activity and promotion of synthesis of antioxidant.
Resveratrol interacts with several different immunomodulatory targets including Sirtuins. Sirtuins are NAD+-dependent histone deacetylases, and they are thought play a significant role in immunomodulatory responses and are of significant interest as targets to treat autoimmune disease (123). They are currently studied for their role in promoting organ transplant tolerance, in particular SIRT1 inhibition, which may affect Th17/Treg cell balance (124, 125).
Overall Resveratrol has the potential to play a crucial role in prevention and treatment of liver disease such as anti-oxidant, steatosis and immune-modulator. Additional studies are ongoing with the goal of highlighting efficacy and safety.
Vitamin D
Vitamin D plays an important role in the regulation of the immune system, and its deficiency has been shown to play a role in immune dysregulation, particularly in the context of autoimmunity (126). Renal failure can lead to disruption of vitamin D metabolism. In fact, studies have shown that vitamin D deficiency may be a risk factor for rejection or decreased graft function (127–129). As such, studies have demonstrated that vitamin D supplementation may serve as a means to promote immunity and maintain graft function (130).
Conclusion
In order for the immune system to function appropriately, there must be a complex synergy between the innate and adaptive arms. The innate immune system is essential for the highly specialized adaptive immune system to function. Furthermore, modulation of this complex system is essential for successful organ transplantation, particularly to prevent ischemia reperfusion injury and rejection, and to promote graft function. There is an abundance of research that highlights components of the innate immune system, their role in transplantation, and how those components may be harnessed for therapeutic benefit. Ongoing studies are identifying novel therapeutic targets within the innate immune system that have the potential to greatly impact management of transplant patients.
Author contributions
CP: Conceptualization, Resources, Visualization, Writing – original draft, Writing – review & editing. RT: Conceptualization, Resources, Visualization, Writing – original draft, Writing – review & editing. JD: Conceptualization, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We extend our gratitude to Dr. David Foley and the Division of Transplantation at the University of Wisconsin for their support. We would also like to thank Sally Griffith-Oh for her generous contributions to the generation of the included figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med. (2000) 343(1):37–49. doi: 10.1056/NEJM200007063430107
2. Medzhitov R. Origin and physiological roles of inflammation. Nature. (2008) 454(7203):428–35. doi: 10.1038/nature07201
3. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. (2012) 18(7):1028–40. doi: 10.1038/nm.2807
4. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. (2001) 1(2):135–45. doi: 10.1038/35100529
5. Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. (2005) 17(1):1–14. doi: 10.1093/intimm/dxh186
6. Bonilla FA, Oettgen HC. Adaptive immunity. J Allergy Clin Immunol. (2010) 125(2 Suppl 2):S33–40. doi: 10.1016/j.jaci.2009.09.017
7. Jain A, Pasare C. Innate control of adaptive immunity: beyond the three-signal paradigm. J Immunol. (2017) 198(10):3791–800. doi: 10.4049/jimmunol.1602000
8. Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. (2015) 16(4):343–53. doi: 10.1038/ni.3123
9. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. (2010) 11(9):785–97. doi: 10.1038/ni.1923
10. Asgari E, Zhou W, Sacks S. Complement in organ transplantation. Curr Opin Organ Transplant. (2010) 15(4):486–91. doi: 10.1097/MOT.0b013e32833b9cb7
11. Sacks S, Lee Q, Wong W, Zhou W. The role of complement in regulating the alloresponse. Curr Opin Organ Transplant. (2009) 14(1):10–5. doi: 10.1097/MOT.0b013e32831ec551
12. Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat Rev Immunol. (2012) 12(6):431–42. doi: 10.1038/nri3225
13. de Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc. (2007) 39(2):481–4. doi: 10.1016/j.transproceed.2006.12.012
14. Hepburn NJ, Ruseva MM, Harris CL, Morgan BP. Complement, roles in renal disease and modulation for therapy. Clin Nephrol. (2008) 70(5):357–76. doi: 10.5414/CNP70357
15. McCullough JW, Renner B, Thurman JM. The role of the complement system in acute kidney injury. Semin Nephrol. (2013) 33(6):543–56. doi: 10.1016/j.semnephrol.2013.08.005
16. Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. (2013) 190(8):3831–8. doi: 10.4049/jimmunol.1203487
17. Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, et al. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol. (2012) 23(9):1474–85. doi: 10.1681/ASN.2011111072
18. Arumugam T, Okun E, Tang S, Thundyil J, Taylor S, Woodruff T. Toll-like receptors in ischemia-reperfusion injury. Shock. (2009) 32(1):4–16. doi: 10.1097/SHK.0b013e318193e333
19. Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. (2010) 464(7285):104–7. doi: 10.1038/nature08780
20. Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. (2010) 28:367–88. doi: 10.1146/annurev.immunol.021908.132603
21. Anders HJ, Schaefer L. Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol. (2014) 25(7):1387–400. doi: 10.1681/ASN.2014010117
22. Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. (2004) 66(2):480–5. doi: 10.1111/j.1523-1755.2004.761_2.x
23. Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, De Sarro A, et al. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther. (2005) 312(3):1170–8. doi: 10.1124/jpet.104.078659
24. Zhang J, Li Q, Zou YR, Wu SK, Lu XH, Li GS, et al. HMGB1-TLR4-IL-23-IL-17A axis accelerates renal ischemia-reperfusion injury via the recruitment and migration of neutrophils. Int Immunopharmacol. (2021) 94:107433. doi: 10.1016/j.intimp.2021.107433
25. Kurihara C, Lecuona E, Wu Q, Yang W, Núñez-Santana FL, Akbarpour M, et al. Crosstalk between nonclassical monocytes and alveolar macrophages mediates transplant ischemia-reperfusion injury through classical monocyte recruitment. JCI Insight. (2021) 6(6). doi: 10.1172/jci.insight.147282
26. Yin E, Fukuhara T, Takeda K, Kojima Y, Fukuhara K, Ikejima K, et al. Anti-CD321 antibody immunotherapy protects liver against ischemia and reperfusion-induced injury. Sci Rep. (2021) 11(1):6312. doi: 10.1038/s41598-021-85001-2
27. Miller CL, Madsen JC. IL-6 directed therapy in transplantation. Curr Transplant Rep. (2021) 8(3):191–204. doi: 10.1007/s40472-021-00331-4
28. Watts RP, Thom O, Fraser JF. Inflammatory signalling associated with brain dead organ donation: from brain injury to brain stem death and posttransplant ischaemia reperfusion injury. J Transplant. (2013) 2013:521369. doi: 10.1155/2013/521369
29. Zitur LJ, Chlebeck PJ, Odorico SK, Danobeitia JS, Zens TJ, Van Kooten C, et al. Brain death enhances activation of the innate immune system and leads to reduced renal metabolic gene expression. Transplantation. (2019) 103(9):1821–33. doi: 10.1097/TP.0000000000002744
30. Jurewicz M, Takakura A, Augello A, Movahedi Naini S, Ichimura T, Zandi-Nejad K, et al. Ischemic injury enhances dendritic cell immunogenicity via TLR4 and NF-kappa B activation. J Immunol. (2010) 184(6):2939–48. doi: 10.4049/jimmunol.0901889
31. Romano M, Sironi M, Toniatti C, Polentarutti N, Fruscella P, Ghezzi P, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. (1997) 6(3):315–25. doi: 10.1016/S1074-7613(00)80334-9
32. Uehara M, Solhjou Z, Banouni N, Kasinath V, Xiaqun Y, Dai L, et al. Ischemia augments alloimmune injury through IL-6-driven CD4. Sci Rep. (2018) 8(1):2461. doi: 10.1038/s41598-018-20858-4
33. Raasveld MH, Weening JJ, Kerst JM, Surachno S, ten Berge RJ. Local production of interleukin-6 during acute rejection in human renal allografts. Nephrol Dial Transplant. (1993) 8(1):75–8. doi: 10.1093/oxfordjournals.ndt.a092278
34. Waiser J, Budde K, Katalinic A, Kuerzdörfer M, Riess R, Neumayer HH. Interleukin-6 expression after renal transplantation. Nephrol Dial Transplant. (1997) 12(4):753–9. doi: 10.1093/ndt/12.4.753
35. Shen H, Goldstein DR. IL-6 and TNF-alpha synergistically inhibit allograft acceptance. J Am Soc Nephrol. (2009) 20(5):1032–40. doi: 10.1681/ASN.2008070778
36. Kim I, Wu G, Chai NN, Klein AS, Jordan S. Anti-interleukin 6 receptor antibodies attenuate antibody recall responses in a mouse model of allosensitization. Transplantation. (2014) 98(12):1262–70. doi: 10.1097/TP.0000000000000437
37. Wu G, Chai N, Kim I, Klein AS, Jordan SC. Monoclonal anti-interleukin-6 receptor antibody attenuates donor-specific antibody responses in a mouse model of allosensitization. Transpl Immunol. (2013) 28(2-3):138–43. doi: 10.1016/j.trim.2013.03.003
38. Fogal B, Yi T, Wang C, Rao DA, Lebastchi A, Kulkarni S, et al. Neutralizing IL-6 reduces human arterial allograft rejection by allowing emergence of CD161+ CD4+ regulatory T cells. J Immunol. (2011) 187(12):6268–80. doi: 10.4049/jimmunol.1003774
39. Liang Y, Christopher K, Finn PW, Colson YL, Perkins DL. Graft produced interleukin-6 functions as a danger signal and promotes rejection after transplantation. Transplantation. (2007) 84(6):771–7. doi: 10.1097/01.tp.0000281384.24333.0b
40. Booth AJ, Grabauskiene S, Wood SC, Lu G, Burrell BE, Bishop DK. IL-6 promotes cardiac graft rejection mediated by CD4+ cells. J Immunol. (2011) 187(11):5764–71. doi: 10.4049/jimmunol.1100766
41. Zhao X, Boenisch O, Yeung M, Mfarrej B, Yang S, Turka LA, et al. Critical role of proinflammatory cytokine IL-6 in allograft rejection and tolerance. Am J Transplant. (2012) 12(1):90–101. doi: 10.1111/j.1600-6143.2011.03770.x
42. Krams SM, Falco DA, Villanueva JC, Rabkin J, Tomlanovich SJ, Vincenti F, et al. Cytokine and T cell receptor gene expression at the site of allograft rejection. Transplantation. (1992) 53(1):151–6. doi: 10.1097/00007890-199201000-00031
43. Gorczynski RM, Adams RB, Levy GA, Chung SW. Correlation of peripheral blood lymphocyte and intragraft cytokine mRNA expression with rejection in orthotopic liver transplantation. Surgery. (1996) 120(3):496–502. doi: 10.1016/S0039-6060(96)80069-9
44. Kita Y, Iwaki Y, Demetris AJ, Starzl TE. Evaluation of sequential serum interleukin-6 levels in liver allograft recipients. Transplantation. (1994) 57(7):1037–41. doi: 10.1097/00007890-199404150-00009
45. Stallone G, Pontrelli P, Rascio F, Castellano G, Gesualdo L, Grandaliano G. Coagulation and fibrinolysis in kidney graft rejection. Front Immunol. (2020) 11:1807. doi: 10.3389/fimmu.2020.01807
46. Pawlicki J, Cierpka L, Król R, Ziaja J. Analysis of coagulation parameters in the early period after kidney transplantation. Transplant Proc. (2007) 39(9):2754–5. doi: 10.1016/j.transproceed.2007.08.050
47. Thiagarajan RR, Winn RK, Harlan JM. The role of leukocyte and endothelial adhesion molecules in ischemia-reperfusion injury. Thromb Haemost. (1997) 78(1):310–4. doi: 10.1055/s-0038-1657545
48. Pontrelli P, Cariello M, Rascio F, Gigante M, Verrienti R, Tataranni T, et al. Thrombin may modulate dendritic cell activation in kidney transplant recipients with delayed graft function. Nephrol Dial Transplant. (2015) 30(9):1480–7. doi: 10.1093/ndt/gfv129
49. Grandaliano G, Di Paolo S, Monno R, Stallone G, Ranieri E, Pontrelli P, et al. Protease-activated receptor 1 and plasminogen activator inhibitor 1 expression in chronic allograft nephropathy: the role of coagulation and fibrinolysis in renal graft fibrosis. Transplantation. (2001) 72(8):1437–43. doi: 10.1097/00007890-200110270-00018
50. Manook M, Kwun J, Sacks S, Dorling A, Mamode N, Knechtle S. Innate networking: thrombotic microangiopathy, the activation of coagulation and complement in the sensitized kidney transplant recipient. Transplant Rev (Orlando). (2018) 32(3):119–26. doi: 10.1016/j.trre.2018.01.001
51. Bronisz M, Rość D, Bronisz A, Manitius J, Nartowicz E. The role of intrinsic fibrinolytic system activation in pathogenesis of hemostasis disturbances in hemodialyzed patients with chronic renal failure. Ren Fail. (2004) 26(3):223–9. doi: 10.1081/JDI-120039519
52. Ziętek Z, Iwan-Ziętek I, Sulikowski T, Sieńko J, Zukowski M, Kaczmarczyk M, et al. The effect of cause of cadaveric kidney donors death on fibrinolysis and blood coagulation processes. Transplant Proc. (2011) 43(8):2866–70. doi: 10.1016/j.transproceed.2011.08.011
53. Roelofs JJ, Rowshani AT, van den Berg JG, Claessen N, Aten J, ten Berge IJ, et al. Expression of urokinase plasminogen activator and its receptor during acute renal allograft rejection. Kidney Int. (2003) 64(5):1845–53. doi: 10.1046/j.1523-1755.2003.00261.x
54. Staniszewska M, Dziedziejko V, Kwiatkowska E, Tkacz M, Puchałowicz K, Safranow K, et al. Plasma concentration of urokinase plasminogen activator receptor is a marker of kidney allograft function. Ir J Med Sci. (2018) 187(4):1083–7. doi: 10.1007/s11845-018-1767-4
55. Grafals M, Thurman JM. The role of complement in organ transplantation. Front Immunol. (2019) 10:2380. doi: 10.3389/fimmu.2019.02380
56. Schmid RA, Zollinger A, Singer T, Hillinger S, Leon-Wyss JR, Schöb OM, et al. Effect of soluble complement receptor type 1 on reperfusion edema and neutrophil migration after lung allotransplantation in swine. J Thorac Cardiovasc Surg. (1998) 116(1):90–7. doi: 10.1016/S0022-5223(98)70246-6
57. Keshavjee S, Davis RD, Zamora MR, de Perrot M, Patterson GA. A randomized, placebo-controlled trial of complement inhibition in ischemia-reperfusion injury after lung transplantation in human beings. J Thorac Cardiovasc Surg. (2005) 129(2):423–8. doi: 10.1016/j.jtcvs.2004.06.048
58. Gueler F, Rong S, Gwinner W, Mengel M, Bröcker V, Schön S, et al. Complement 5a receptor inhibition improves renal allograft survival. J Am Soc Nephrol. (2008) 19(12):2302–12. doi: 10.1681/ASN.2007111267
59. Kaabak M, Babenko N, Shapiro R, Zokoyev A, Dymova O, Kim E. A prospective randomized, controlled trial of eculizumab to prevent ischemia-reperfusion injury in pediatric kidney transplantation. Pediatr Transplant. (2018) 22(2). doi: 10.1111/petr.13129
60. Grenda R, Durlik M. Eculizumab in renal transplantation: a 2017 update. Ann Transplant. (2017) 22:550–4. doi: 10.12659/AOT.905917
61. Favi E, Molinari P, Alfieri C, Castellano G, Ferraresso M, Cresseri D. Case report: eculizumab plus obinutuzumab induction in a deceased donor kidney transplant recipient with DEAP-HUS. Front Immunol. (2022) 13:1073808. doi: 10.3389/fimmu.2022.1073808
62. Zheng X, Feng B, Chen G, Zhang X, Li M, Sun H, et al. Preventing renal ischemia-reperfusion injury using small interfering RNA by targeting complement 3 gene. Am J Transplant. (2006) 6(9):2099–108. doi: 10.1111/j.1600-6143.2006.01427.x
63. Zheng X, Zhang X, Sun H, Feng B, Li M, Chen G, et al. Protection of renal ischemia injury using combination gene silencing of complement 3 and caspase 3 genes. Transplantation. (2006) 82(12):1781–6. doi: 10.1097/01.tp.0000250769.86623.a3
64. Zheng X, Zhang X, Feng B, Sun H, Suzuki M, Ichim T, et al. Gene silencing of complement C5a receptor using siRNA for preventing ischemia/reperfusion injury. Am J Pathol. (2008) 173(4):973–80. doi: 10.2353/ajpath.2008.080103
65. Davis AE. Biological effects of C1 inhibitor. Drug News Perspect. (2004) 17(7):439–46. doi: 10.1358/dnp.2004.17.7.863703
66. Poppelaars F, Jager NM, Kotimaa J, Leuvenink HGD, Daha MR, van Kooten C, et al. C1-Inhibitor treatment decreases renal injury in an established brain-dead rat model. Transplantation. (2018) 102(1):79–87. doi: 10.1097/TP.0000000000001895
67. Danobeitia JS, Ziemelis M, Ma X, Zitur LJ, Zens T, Chlebeck PJ, et al. Complement inhibition attenuates acute kidney injury after ischemia-reperfusion and limits progression to renal fibrosis in mice. PLoS One. (2017) 12(8):e0183701. doi: 10.1371/journal.pone.0183701
68. Danobeitia JS, Zens TJ, Chlebeck PJ, Zitur LJ, Reyes JA, Eerhart MJ, et al. Targeted donor complement blockade after brain death prevents delayed graft function in a nonhuman primate model of kidney transplantation. Am J Transplant. (2020) 20(6):1513–26. doi: 10.1111/ajt.15777
69. Eerhart MJ, Reyes JA, Blanton CL, Danobeitia JS, Chlebeck PJ, Zitur LJ, et al. Complement blockade in recipients prevents delayed graft function and delays antibody-mediated rejection in a nonhuman primate model of kidney transplantation. Transplantation. (2022) 106(1):60–71. doi: 10.1097/TP.0000000000003754
70. Castellano G, Melchiorre R, Loverre A, Ditonno P, Montinaro V, Rossini M, et al. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am J Pathol. (2010) 176(4):1648–59. doi: 10.2353/ajpath.2010.090276
71. Castellano G, Intini A, Stasi A, Divella C, Gigante M, Pontrelli P, et al. Complement modulation of anti-aging factor klotho in ischemia/reperfusion injury and delayed graft function. Am J Transplant. (2016) 16(1):325–33. doi: 10.1111/ajt.13415
72. Jordan SC, Choi J, Aubert O, Haas M, Loupy A, Huang E, et al. A phase I/II, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients. Am J Transplant. (2018) 18(12):2955–64. doi: 10.1111/ajt.14767
73. Huang E, Vo A, Choi J, Ammerman N, Lim K, Sethi S, et al. Three-year outcomes of a randomized, double-blind, placebo-controlled study assessing safety and efficacy of C1 esterase inhibitor for prevention of delayed graft function in deceased donor kidney transplant recipients. Clin J Am Soc Nephrol. (2020) 15(1):109–16. doi: 10.2215/CJN.04840419
74. Kassimatis T, Qasem A, Douiri A, Ryan EG, Rebollo-Mesa I, Nichols LL, et al. A double-blind randomised controlled investigation into the efficacy of mirococept (APT070) for preventing ischaemia reperfusion injury in the kidney allograft (EMPIRIKAL): study protocol for a randomised controlled trial. Trials. (2017) 18(1):255. doi: 10.1186/s13063-017-1972-x
75. Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, et al. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. (2010) 21(11):1878–90. doi: 10.1681/ASN.2009101048
76. Zou H, Yang Y, Gao M, Zhang B, Ming B, Sun Y, et al. HMGB1 Is involved in chronic rejection of cardiac allograft via promoting inflammatory-like mDCs. Am J Transplant. (2014) 14(8):1765–77. doi: 10.1111/ajt.12781
77. Liu M, Gu M, Xu D, Lv Q, Zhang W, Wu Y. Protective effects of toll-like receptor 4 inhibitor eritoran on renal ischemia-reperfusion injury. Transplant Proc. (2010) 42(5):1539–44. doi: 10.1016/j.transproceed.2010.03.133
78. Chen F, Zou L, Williams B, Chao W. Targeting toll-like receptors in sepsis: from bench to clinical trials. Antioxid Redox Signal. (2021) 35(15):1324–39. doi: 10.1089/ars.2021.0005
79. Mcdonald KA, Huang H, Tohme S, Loughran P, Ferrero K, Billiar T, et al. Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling. Mol Med. (2015) 20(1):639–48. doi: 10.2119/molmed.2014.00076
80. Samarpita S, Kim JY, Rasool MK, Kim KS. Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug. Arthritis Res Ther. (2020) 22(1):16. doi: 10.1186/s13075-020-2097-2
81. Shao Z, Jiao B, Liu T, Cheng Y, Liu H, Liu Y. TAK-242 treatment ameliorates liver ischemia/reperfusion injury by inhibiting TLR4 signaling pathway in a swine model of Maastricht-category-III cardiac death. Biomed Pharmacother. (2016) 84:495–501. doi: 10.1016/j.biopha.2016.09.036
82. Vo AA, Choi J, Kim I, Louie S, Cisneros K, Kahwaji J, et al. A phase I/II trial of the interleukin-6 receptor-specific humanized monoclonal (tocilizumab)+intravenous immunoglobulin in difficult to desensitize patients. Transplantation. (2015) 99(11):2356–63. doi: 10.1097/TP.0000000000000741
83. Choi J, Aubert O, Vo A, Loupy A, Haas M, Puliyanda D, et al. Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant. (2017) 17(9):2381–9. doi: 10.1111/ajt.14228
84. Chandran S, Leung J, Hu C, Laszik ZG, Tang Q, Vincenti FG. Interleukin-6 blockade with tocilizumab increases tregs and reduces T effector cytokines in renal graft inflammation: a randomized controlled trial. Am J Transplant. (2021) 21(7):2543–54. doi: 10.1111/ajt.16459
85. Westwell-Roper C, Dai DL, Soukhatcheva G, Potter KJ, van Rooijen N, Ehses JA, et al. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. (2011) 187(5):2755–65. doi: 10.4049/jimmunol.1002854
86. Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. (1990) 71(1):152–6. doi: 10.1210/jcem-71-1-152
87. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. (2008) 117(2):244–79. doi: 10.1016/j.pharmthera.2007.10.001
88. Kaymakcalan Z, Sakorafas P, Bose S, Scesney S, Xiong L, Hanzatian DK, et al. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin Immunol. (2009) 131(2):308–16. doi: 10.1016/j.clim.2009.01.002
89. Szempruch KR, Banerjee O, McCall RC, Desai CS. Use of anti-inflammatory agents in clinical islet cell transplants: a qualitative systematic analysis. Islets. (2019) 11(3):65–75. doi: 10.1080/19382014.2019.1601543
90. Faradji RN, Tharavanij T, Messinger S, Froud T, Pileggi A, Monroy K, et al. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. (2008) 86(12):1658–65. doi: 10.1097/TP.0b013e31818fe448
91. Gangemi A, Salehi P, Hatipoglu B, Martellotto J, Barbaro B, Kuechle JB, et al. Islet transplantation for brittle type 1 diabetes: the UIC protocol. Am J Transplant. (2008) 8(6):1250–61. doi: 10.1111/j.1600-6143.2008.02234.x
92. Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, et al. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. (2005) 5(8):2037–46. doi: 10.1111/j.1600-6143.2005.00957.x
93. Yan LL, Ye LP, Chen YH, He SQ, Zhang CY, Mao XL, et al. The influence of microenvironment on survival of intraportal transplanted islets. Front Immunol. (2022) 13:849580. doi: 10.3389/fimmu.2022.849580
94. Schwarznau A, Hanson MS, Sperger JM, Schram BR, Danobeitia JS, Greenwood KK, et al. IL-1beta receptor blockade protects islets against pro-inflammatory cytokine induced necrosis and apoptosis. J Cell Physiol. (2009) 220(2):341–7. doi: 10.1002/jcp.21770
95. Sahraoui A, Kloster-Jensen K, Ueland T, Korsgren O, Foss A, Scholz H. Anakinra and tocilizumab enhance survival and function of human islets during culture: implications for clinical islet transplantation. Cell Transplant. (2014) 23(10):1199–211. doi: 10.3727/096368913X667529
96. Danobeitia JS, Hanson MS, Chlebeck P, Park E, Sperger JM, Schwarznau A, et al. Donor pretreatment with IL-1 receptor antagonist attenuates inflammation and improves functional potency in islets from brain-dead nonhuman primates. Cell Transplant. (2015) 24(9):1863–77. doi: 10.3727/096368914X681045
97. Maffi P, Berney T, Nano R, Niclauss N, Bosco D, Melzi R, et al. Calcineurin inhibitor-free immunosuppressive regimen in type 1 diabetes patients receiving islet transplantation: single-group phase 1/2 trial. Transplantation. (2014) 98(12):1301–9. doi: 10.1097/TP.0000000000000396
98. Takita M, Matsumoto S, Shimoda M, Chujo D, Itoh T, Sorelle JA, et al. Safety and tolerability of the T-cell depletion protocol coupled with anakinra and etanercept for clinical islet cell transplantation. Clin Transplant. (2012) 26(5):E471–84. doi: 10.1111/ctr.12011
99. Mulders-Manders CM, Baas MC, Molenaar FM, Simon A. Peri- and postoperative treatment with the interleukin-1 receptor antagonist anakinra is safe in patients undergoing renal transplantation: case series and review of the literature. Front Pharmacol. (2017) 8:342. doi: 10.3389/fphar.2017.00342
100. Direz G, Noël N, Guyot C, Toupance O, Salmon JH, Eschard JP. Efficacy but side effects of anakinra therapy for chronic refractory gout in a renal transplant recipient with preterminal chronic renal failure. Joint Bone Spine. (2012) 79(6):631. doi: 10.1016/j.jbspin.2012.04.009
101. Pontrelli P, Rossini M, Infante B, Stallone G, Schena A, Loverre A, et al. Rapamycin inhibits PAI-1 expression and reduces interstitial fibrosis and glomerulosclerosis in chronic allograft nephropathy. Transplantation. (2008) 85(1):125–34. doi: 10.1097/01.tp.0000296831.91303.9a
102. Tillet S, Giraud S, Delpech PO, Thuillier R, Ameteau V, Goujon JM, et al. Kidney graft outcome using an anti-Xa therapeutic strategy in an experimental model of severe ischaemia-reperfusion injury. Br J Surg. (2015) 102(1):132–42; discussion 42. doi: 10.1002/bjs.9662
103. Tillet S, Giraud S, Kerforne T, Saint-Yves T, Joffrion S, Goujon JM, et al. Inhibition of coagulation proteases Xa and IIa decreases ischemia-reperfusion injuries in a preclinical renal transplantation model. Transl Res. (2016) 178:95–106.e1. doi: 10.1016/j.trsl.2016.07.014
104. Berger M, Baldwin WM, Jordan SC. Potential roles for C1 inhibitor in transplantation. Transplantation. (2016) 100(7):1415–24. doi: 10.1097/TP.0000000000000995
105. Steiner RW, Awdishu L. Steroids in kidney transplant patients. Semin Immunopathol. (2011) 33(2):157–67. doi: 10.1007/s00281-011-0259-7
106. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. (2011) 335(1):2–13. doi: 10.1016/j.mce.2010.04.005
107. Xavier AM, Anunciato AK, Rosenstock TR, Glezer I. Gene expression control by glucocorticoid receptors during innate immune responses. Front Endocrinol (Lausanne). (2016) 7:31. doi: 10.3389/fendo.2016.00031
108. Lee SW, Tsou AP, Chan H, Thomas J, Petrie K, Eugui EM, et al. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. (1988) 85(4):1204–8. doi: 10.1073/pnas.85.4.1204
109. Debets JM, Ruers TJ, van der Linden MP, van der Linden CJ, Buurman WA. Inhibitory effect of corticosteroids on the secretion of tumour necrosis factor (TNF) by monocytes is dependent on the stimulus inducing TNF synthesis. Clin Exp Immunol. (1989) 78(2):224–9.12412753
110. Rogacev KS, Zawada AM, Hundsdorfer J, Achenbach M, Held G, Fliser D, et al. Immunosuppression and monocyte subsets. Nephrol Dial Transplant. (2015) 30(1):143–53. doi: 10.1093/ndt/gfu315
111. Kaczorowski DJ, Nakao A, Mollen KP, Vallabhaneni R, Sugimoto R, Kohmoto J, et al. Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation. (2007) 84(10):1279–87. doi: 10.1097/01.tp.0000287597.87571.17
112. Rozkova D, Horvath R, Bartunkova J, Spisek R. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of toll-like receptors. Clin Immunol. (2006) 120(3):260–71. doi: 10.1016/j.clim.2006.04.567
113. D'Aragon F, Belley-Cote E, Agarwal A, Frenette AJ, Lamontagne F, Guyatt G, et al. Effect of corticosteroid administration on neurologically deceased organ donors and transplant recipients: a systematic review and meta-analysis. BMJ Open. (2017) 7(6):e014436. doi: 10.1136/bmjopen-2016-014436
114. Kainz A, Wilflingseder J, Mitterbauer C, Haller M, Burghuber C, Perco P, et al. Steroid pretreatment of organ donors to prevent postischemic renal allograft failure: a randomized, controlled trial. Ann Intern Med. (2010) 153(4):222–30. doi: 10.7326/0003-4819-153-4-201008170-00003
115. Reindl-Schwaighofer R, Kainz A, Jelencsics K, Heinzel A, Berlakovich G, Remport Á, et al. Steroid pretreatment of organ donors does not impact on early rejection and long-term kidney allograft survival: results from a multicenter randomized, controlled trial. Am J Transplant. (2019) 19(6):1770–6. doi: 10.1111/ajt.15252
116. Kotsch K, Ulrich F, Reutzel-Selke A, Pascher A, Faber W, Warnick P, et al. Methylprednisolone therapy in deceased donors reduces inflammation in the donor liver and improves outcome after liver transplantation: a prospective randomized controlled trial. Ann Surg. (2008) 248(6):1042–50. doi: 10.1097/SLA.0b013e318190e70c
117. Deguine J, Barton GM. Myd88: a central player in innate immune signaling. F1000Prime Rep. (2014) 6:97. doi: 10.12703/P6-97
118. Miao Y, Ding Z, Zou Z, Yang Y, Yang M, Zhang X, et al. Inhibition of MyD88 by a novel inhibitor reverses two-thirds of the infarct area in myocardial ischemia and reperfusion injury. Am J Transl Res. (2020) 12(9):5151–69.33042411
119. Li Z, Zhao M, Zhang X, Lu Y, Yang Y, Xie Y, et al. TJ-M2010-5, a novel CNS drug candidate, attenuates acute cerebral ischemia-reperfusion injury through the MyD88/NF-κB and ERK pathway. Front Pharmacol. (2022) 13:1080438. doi: 10.3389/fphar.2022.1080438
120. Xie Y, Du D, Zhang L, Yang Y, Zou Z, Li Z, et al. TJ-M2010-5, a self-developed MyD88 inhibitor, attenuates liver fibrosis by inhibiting the NF-κB pathway. Chem Biol Interact. (2022) 354:109839. doi: 10.1016/j.cbi.2022.109839
121. Zhou W, Peng S, Du P, Zhou P, Xue C, Ye Q. Hypothermic oxygenated perfusion combined with TJ-M2010-5 alleviates hepatic ischemia-reperfusion injury in donation after circulatory death. Int Immunopharmacol. (2022) 105:108541. doi: 10.1016/j.intimp.2022.108541
122. Izzo C, Annunziata M, Melara G, Sciorio R, Dallio M, Masarone M, et al. The role of resveratrol in liver disease: a comprehensive review from in vitro to clinical trials. Nutrients. (2021) 13(3). doi: 10.3390/nu13030933
123. Hamaidi I, Kim S. Sirtuins are crucial regulators of T cell metabolism and functions. Exp Mol Med. (2022) 54(3):207–15. doi: 10.1038/s12276-022-00739-7
124. Beier UH, Wang L, Bhatti TR, Liu Y, Han R, Ge G, et al. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol. (2011) 31(5):1022–9. doi: 10.1128/MCB.01206-10
125. Malaguarnera L. Influence of resveratrol on the immune response. Nutrients. (2019) 11(5). doi: 10.3390/nu11050946
126. Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. (2013) 5(7):2502–21. doi: 10.3390/nu5072502
127. Buyukdemirci S, Oguz EG, Cimen SG, Sahin H, Cimen S, Ayli MD. Vitamin D deficiency may predispose patients to increased risk of kidney transplant rejection. World J Transplant. (2022) 12(9):299–309. doi: 10.5500/wjt.v12.i9.299
128. Obi Y, Hamano T, Ichimaru N, Tomida K, Matsui I, Fujii N, et al. Vitamin D deficiency predicts decline in kidney allograft function: a prospective cohort study. J Clin Endocrinol Metab. (2014) 99(2):527–35. doi: 10.1210/jc.2013-2421
129. Bienaimé F, Girard D, Anglicheau D, Canaud G, Souberbielle JC, Kreis H, et al. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol. (2013) 24(5):831–41. doi: 10.1681/ASN.2012060614
Keywords: innate immune system, transplantation, complement, toll-like receptor, ischemia-reperfusion injury, therapeutics
Citation: Praska CE, Tamburrini R and Danobeitia JS (2023) Innate immune modulation in transplantation: mechanisms, challenges, and opportunities. Front. Transplant. 2:1277669. doi: 10.3389/frtra.2023.1277669
Received: 15 August 2023; Accepted: 23 November 2023;
Published: 8 December 2023.
Edited by:
Stefan Reuter, University Hospital Münster, GermanyReviewed by:
Mingqing Song, Duke University, United StatesSteven Wisel, Cedars Sinai Medical Center, United States
© 2023 Praska, Tamburrini and Danobeitia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Sebastian Danobeitia anVhbi5kYW5vYmVpdGlhQGJzd2hlYWx0aC5vcmc=
 Corinne E. Praska
Corinne E. Praska Riccardo Tamburrini
Riccardo Tamburrini Juan Sebastian Danobeitia1,2*
Juan Sebastian Danobeitia1,2*