
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Transplant. , 19 September 2023
Sec. Cell and Stem Cell Transplantation
Volume 2 - 2023 | https://doi.org/10.3389/frtra.2023.1238494
This article is part of the Research Topic Rising Stars: Cell and Stem Cell Transplantation 2022 View all 5 articles
 Vera Radici1*
Vera Radici1* Cinzia Giagulli2
Cinzia Giagulli2 Eugenia Accorsi Buttini1
Eugenia Accorsi Buttini1 Mirko Farina1
Mirko Farina1 Nicola Polverelli1
Nicola Polverelli1 Duilio Brugnoni2
Duilio Brugnoni2 Marco Chiarini3
Marco Chiarini3 Anna Galvagni3
Anna Galvagni3 Camillo Almici4
Camillo Almici4 Emilio Ferrari4
Emilio Ferrari4 Andrea Bianchetti4
Andrea Bianchetti4 Stefania Masneri4
Stefania Masneri4 Alessandro Leoni1,5
Alessandro Leoni1,5 Federica Re1,5
Federica Re1,5 Simona Bernardi1,5
Simona Bernardi1,5 Michele Malagola1
Michele Malagola1 Alessandro Re6
Alessandro Re6 Arnaldo Caruso2
Arnaldo Caruso2 Domenico Russo1
Domenico Russo1
Background: The COVID-19 pandemic has had a significant impact on the management and care of onco-hematological patients, particularly those with lymphoproliferative disorders who are at higher risk for COVID-19 associated bacterial and fungal superinfections.
Case presentation: We present the successful treatment of a 44-year-old male patient with refractory mantle cell lymphoma treated with chimeric antigen receptor T (CAR-T) cell therapy, despite concurrent COVID-19 infection. The patient developed grade II cytokine release syndrome, requiring admission to the intensive care unit. The CAR-T cells expanded effectively, and the patient achieved complete metabolic remission. During the treatment course, the patient experienced complications including COVID-19-associated pulmonary aspergillosis and a co-infection with Stenotrophomonas maltophilia and the SARS-CoV-2 omicron variant. Prompt antifungal and antibacterial therapy, along with appropriate COVID-19 treatment, led to the resolution of these infections. Dexamethasone was also administered to reduce inflammation and aid hematologic recovery. Despite the presence of multiple infections, the patient achieved complete remission of lymphoma, highlighting the effectiveness of CAR-T cell therapy in this high-risk patient.
Conclusion: Despite the challenges posed by concurrent infections, the decision to proceed with CAR-T cell therapy in this patient proved to be successful, resulting in complete remission of lymphoma. Early initiation of supportive therapies and the use of dexamethasone contributed to the resolution of complications. This case underscores the importance of individualized decision-making and the potential benefits of CAR-T cell therapy in similar high-risk patients.
The pandemic sustained by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2/COVID-19) had a dramatic impact on the management and care of onco-hematological patients (1). Due to the disease and therapy-related immunosuppression, patients with lymphoproliferative disorders are particularly exposed to increased morbidity and mortality for COVID-19 associated bacterial and fungal superinfections (2).
We report a case of a refractory mantle cell lymphoma (MCL) successfully treated with chimeric antigen receptor T (CAR-T) cell therapy while bacterial, fungal, and COVID-19 infections were occurring.
A 44-year-old male patient was diagnosed in June 2021 with MCL, pleomorphic variant, stage IV B, high-risk mantle cell lymphoma international prognostic index (MIPI), without significant co-morbidities. As a first-line treatment, the patient received six cycles of alternating R-CHOP (rituximab, cyclophosphamide (CTX), doxorubicin, vincristine, and prednisone)/R-DHAP (rituximab, dexamethasone, high-dose cytarabine, and cisplatin). According to the Lugano criteria (3), he achieved only a partial response (PR) given the persistence of an abdominal mass (19 × 11 mm), documented by computed tomography (CT) scan.
In December 2021, ibrutinib was started, but the patient was urgently readmitted for massive hemoperitoneum 1 month later. Once the acute complication was solved, the patient started the first course of R-BAC (rituximab, bendamustine, cytarabine) resulting in a stable PR after the completion of three courses, in February 2022. One month later, a paucisymptomatic SARS-CoV-2 infection was documented, resulting in nasal swab negativization after Nirmatrelvir/Ritonavir treatment. Previously the patient had received three doses of the Pfizer vaccine. Genotyping was performed utilizing real-time PCR (RT-PCR) multiplex technique, unveiling the B.1.1.529 variant (referred to as omicron). Subsequently, sequencing of the S, N, and M genes was conducted using the Sanger method, disclosing the presence of the B.1.1.529 BA.5 lineage variant (see the Material and Methods section).
In May 2022, a further increase of mesenteric adenopathies indicated a novel disease progression. Vincristine (VCR, 2 mg) and corticosteroids (PDN, 1 mg/kg) were promptly administered with containment intent. In the meantime, the SARS-CoV-2 PCR test on nasal swab samples was alternating as positive and negative, while the patient always remained asymptomatic.
Considering the rapid progression of the disease and the COVID-19 asymptomatic infection, the patient was included in a CAR-T cell program. As bridging chemotherapy, the patient received high-dose cytarabine (ARA-C) followed by a short course of corticosteroids and CTX. In addition, a dose of Evusheld (tixagevimab 300 mg + cilgavimab 300 mg) was administered for an increased risk of progression to the severe form of COVID-19 and the susceptibility of this drug to the variant involved. Despite a further nasal swab positivity for COVID-19, the patient remained asymptomatic. The patient’s individual HEMATOTOX score before lymphodepleting chemotherapy was high, due to C-reactive protein >3 mg/dl and ferritin >2,000 ng/ml (4).
Due to his peculiar clinical condition, both lymphodepletion therapy (including fludarabine and cyclophosphamide) and Brexu-cel were administered in a negative-pressure room at the Department of Infectious Diseases on 12 July 2022. Although the nasal swab was positive for COVID-19 by RT-PCR, the patient continued to be persistently asymptomatic.
On day +6 from CAR-T infusion, the patient developed a grade I cytokine release syndrome (CRS), characterized by a fever of 38.7 °C in the absence of other symptoms. For the persistence of symptoms, three doses of Tocilizumab were administered every 8 h, and empirical antibiotic therapy with piperacillin-tazobactam was started. On day +9, due to a worsening of CRS (grade II) characterized by fever, hypotension, and desaturation, the patient was admitted to the intensive care unit (ICU) and treated with dexamethasone and low-flow oxygen therapy with an improvement of symptoms. On day +10, the circulating CAR-T cells were first checked, reaching a peak of 88% of total lymphocytes on day +14 (good expander) and then decreasing to 8% at day +60 (Figure 1). On day +21, a CT scan documented the radiological signs of possible fungal pneumonia. Stenotrophomonas maltophilia and Aspergillus niger were isolated on sputum. Moxifloxacin and liposomal amphotericin B were started and, because of the persistence of neutropenia (grade IV), the G-CSF dose was doubled.
On day +28, a CT scan (Figure 2) showed a worsening of pulmonary infection and the patient required high-flow oxygen therapy. On day +30 a bronchoalveolar lavage (BAL) provided the presence of S. maltophilia, multisensitive Klebsiella pneumoniae, and, unexpectedly, of the SARS-CoV-2 omicron variant. Accordingly, SARS-CoV-2 pneumonia was treated with Remdesivir (200 mg IV on day 1, then 100 mg IV on days 2–5) and liposomal B amphotericine was replaced with isavuconazole, since the Aspergillus positivity (BAL galattomannan positivity was 1.23) was emerging under the previous antifungal therapy. Therefore, clinical findings were compatible with a diagnosis of proven COVID-19 associated pulmonary aspergillosis (CAPA) (5). Quickly, the patient became afebrile and progressively reduced his oxygen requirement. A short course of dexamethasone (10 mg four times a day) was given as an effort to lower the signs of inflammation (ferritin >5,000 μg/L, IL-6 > 3,000 ng/L). Then, a rapid decrease in inflammatory status, a reduced oxygen need, and an increased neutrophil count were observed. Figure 3 shows the cycle-threshold (Ct) evolution for the different genes (gene E, N RdRp, and S) over the time span between lymphodepletion and after the CAR-T cells. Here, it is possible to compare the different Cts for the different genes detected by the kit (E, N RdRp, and S genes). All samples analyzed have Ct < 35, so there are no weakly positive samples, as 35 is the cutoff used to distinguish samples from positive to weakly positive.
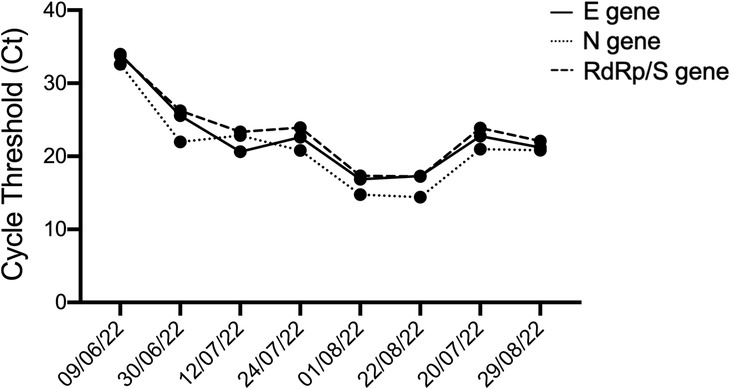
Figure 3. Cycle threshold (Ct) values for the E, N, and RdRP/S genes at different time intervals, before and after CAR-T therapy, to determine dynamics of the SARS-CoV-2 viral load. Each dot represents a different time point.
The early Immune Effector Cell-Associated Hematotoxicity (ICAHT) score (6) was grade IV and the late score grade III. For persistent cytopenia G-CSF refractory 4 weeks after CAR-T infusion, an incremental work-up was performed to define the differential diagnoses. The latter included a bone marrow aspiration and biopsy. Flow cytometric immunophenotyping excluded the presence of lymphoma cells, and showed an increased presence of reactive lymphocytes, probably secondary to virosis. Between day +30 and day +90, the patient remained severely lymphopenic (<200 cells/µl), with circulating CAR-T cells progressively decreasing to less than 3% from day +90 onward.
A positron emission tomography (PET) scan performed at day +42 showed complete metabolic remission (CR). Thus the patient was finally discharged in CR and proper condition on day +45 after CAR-T cell infusion. At day +120, he is well, still in complete remission, and COVID free (Figure 4).
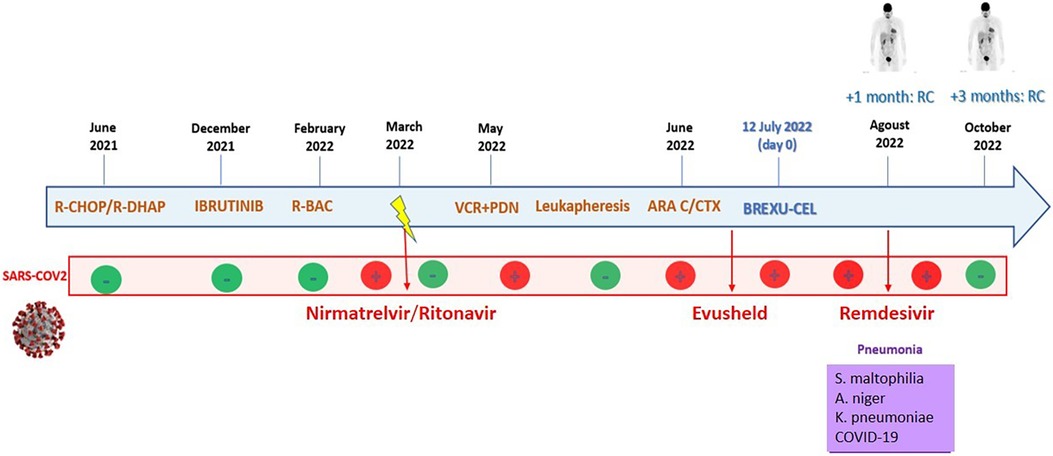
Figure 4. The patient's timeline. Inside the blue arrow, the main lines of therapy with the corresponding dates are reported. The second red line shows samples of SARS-CoV-2 PCR nasal swabs. Then, in the violet box, the infectious complications during CAR-T cell hospitalization are reported.
Nasopharyngeal specimens were collected at the Brescia Civic Hospital, (Brescia, Lombardy, Italy), using FLOQSwabs in the universal transport medium (UTM) (COPAN, Brescia, Italy). Viral RNA was extracted from 300 µl of UTM with Nimbus automatic system (Arrow Diagnostics, Genoa, Italy), according to the manufacturer's instructions. Amplification was performed on BioRad CFX PCR machine (Bio-Rad Laboratories S.r.l., Milan, Italy) using the Allplex™ SARS-CoV-2 Assay (Seegene Inc., Seoul, Korea), a multiplex real-time RT-PCR assay that enables simultaneous amplification and detection of four target genes of SARS-CoV-2 (E, RdRP, S, and N genes) with internal control (IC) to monitor the whole process of nucleic acid extraction and amplification. Ct values were automatically calculated using the Seegene Viewer analysis software (Seegene Inc., Seoul, Korea). A plot of cycle threshold (Ct) values was performed using Prism 8 software (GraphPad, San Diego, CA, USA). SARS-CoV-2 variant typing was performed on extracted RNA using the Allplex™ SARS-CoV-2 Variants II Assay, which detects the presence of the K417N mutation in the S gene.
Total RNA was extracted from 200 µl of nasopharyngeal swabs using a QIAamp DSP Virus Kit® (Qiagen, Hilden, Germany) according to the manufacturer's instructions. RNA was eluted in 30 µl AVE and stored at −80 °C until use. SARS-CoV-2 RNA was reverse-transcribed and PCR amplified using a SuperScript™ IV One-Step RT-PCR System with Platinum™ Taq DNA Polymerase (Thermo Fisher Scientific, Carlsbad, CA, USA) in a 50 µl reaction. PCR primers used in the reaction were: SARS2-S-F3, 5′-TAT CTT GGC AAA CCA CGC GAA CAA and SARS2-S-R3, 5′-ACC CTT GGA GAG TGC TAG TTG CCA TCT C. Then, PCR products were checked on a 1% agarose gel, purified through QIAquick PCR Purification Kit® (Qiagen, Hilden, Germany), and quantified using the Qubit DNA HS Assay Kit (Thermo Fisher Scientific). The purified PCR products were sequenced with the BigDye Terminator v3.1 cycle sequencing kit on SeqStudio Genetic Analyzer (Thermo Fisher Scientific). The derived sequences were analyzed with Geneious software (v. 11.1.5) (Biomatters Ltd., Auckland, New Zealand), using the sequence NC_045512.2 as a SARS-CoV-2 reference.
The infection of immunocompromised individuals with SARS-CoV-2 presents distinct challenges, marked by prolonged viral shedding and compromised inflammatory responses. This phenomenon has been observed in our patient who, due to their immunocompromised state as a result of ongoing chemotherapy, exhibited protracted viral shedding without experiencing symptoms or virus-related complications (7–11). This unique case prompted a complex decision-making process involving the CAR-T Team physicians and the patient.
Previous studies have highlighted the poor outcomes of COVID-19 in immunocompromised patients, particularly in the context of CAR-T recipients, where mortality rates can escalate up to 40% (12). This raised concerns about initiating CAR-T therapy in a SARS-CoV-2 positive recipient due to the potential for disease progression, pneumonia, and the development of superinfections, such as CAPA (5).
A notable aspect of this case report is its novelty, demonstrating the feasibility of CAR-T therapy in a patient with a concurrent SARS-CoV-2 infection. While the decision was intricate, the patient's positivity for the virus did not preclude the pursuit of CAR-T therapy, especially considering the absence of pulmonary involvement.
The comprehensive management employed for this patient's case played a crucial role in the positive outcome. Swift initiation of tocilizumab, combined with aggressive antibacterial, antifungal, and anti-COVID treatments alongside high-flow oxygen support, effectively resolved CRS and CAPA. In addition, the use of dexamethasone proved instrumental in controlling inflammation and promoting hematologic recovery, mitigating the risks associated with pancytopenia.
Looking ahead, the management of SARS-CoV-2 positive recipients who are candidates for allogeneic hematopoietic stem cell transplantation or CAR-T therapy necessitates careful consideration of evolving treatment strategies (13–16). Despite the complexities, the successful outcome for our patient underscores the potential for a multidisciplinary approach and underscores the importance of individualized patient care in such intricate cases.
In conclusion, this case report offers valuable insights into the feasibility of CAR-T therapy for SARS-CoV-2 positive immunocompromised patients. It underscores the significance of early intervention, comprehensive management, and collaborative decision-making in achieving positive outcomes for patients who face dual challenges of immunosuppression and COVID-19 infection. Furthermore, it points toward the potential of emerging treatment strategies in guiding the management of SARS-CoV-2 positive recipients in the realm of cellular therapy.
In summary, the decision to administer CAR-T cells to this high-risk patient proved to be a winning strategy. In our case, despite the presence of multiple infections, a complete remission of the lymphoma was obtained, secondary to a good expansion of CAR-T cells, which is consistent with the conclusions of several studies, showing a strong correlation between CAR-T cell expansion and disease outcome (17–19). Indeed, the patient at day +120 is well, in complete remission, and COVID free (Figure 4).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
VR, EAB, MC and AG collected the data. VR, EAB, MF, MC, and DR wrote the manuscript. CG and CA dealt with the detection of SARs-CoV-2 and variant typing, Viral RNA Extraction, and Sanger sequencing. DB, MC, and AG performed flow cytometry analysis and the corresponding graph. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19) In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2022). Available at: https://www.ncbi.nlm.nih.gov/books/NBK554776/.
2. Firas EC, Jeffery JA, Roy FC. How I treat and prevent COVID-19 in patients with hematologic malignancies and recipients of cellular therapies. Blood. (2022) 140(7):673–84. doi: 10.1182/blood.2022016089
3. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
4. Rejeski K, Perez A, Subklewe M, Penack O, Bücklein V, Jentzsch L, Mougiakakos D, et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J Immunother Cancer. (2022) 10(5):e004475. doi: 10.1136/jitc-2021-004475
5. Koehler P, Bassetti M. Cornely OA, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. (2021) 21(6):e149–62. doi: 10.1016/S1473-3099(20)30847-1
6. Rejeski K, Subklewe M, Yakoub-Agha I, Bachy E, Balduzzi A, Barba P, et al. Immune effector cell-associated hematotoxicity (ICAHT): EHA/EBMT consensus grading and best practice recommendations. Blood. (2023) 142(10):865–77. doi: 10.1182/blood.2023020578
7. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. (2020) 39(5):405–7. doi: 10.1016/j.healun.2020.03.012
8. Cesaro S, Ljungman P, Mikulska M, Hirsch HH, von Lilienfeld-Toal M, Cordonnier C, et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia. (2022) 36:1467–80. doi: 10.1038/s41375-022-01578-1
9. Garcia-Vidal C, Puerta-Alcalde P, Mateu A, Cuesta-Chasco G, Meira F, Lopera C, et al. Prolonged viral replication in patients with hematologic malignancies hospitalized with COVID-19. Haematologica. (2022) 107(7):1731–5. doi: 10.3324/haematol.2021.280407
10. Arcani R, Colle J, Cauchois R, Koubi M, Jarrot PA, Jean R, et al. Clinical characteristics and outcomes of patients with haematologic malignancies and COVID-19 suggest that prolonged SARS-CoV-2 carriage is an important issue. Ann Hematol. (2021) 100(11):2799–803. doi: 10.1007/s00277-021-04656-z
11. Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. (2020) 383(26):2586–8. doi: 10.1056/NEJMc2031670
12. Spanjaart AM, Ljungman P, Mielke S, Tridello G, Ortiz-Maldonado V, Urbano-Ispizua A, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) infectious diseases working party and the European Hematology Association (EHA) lymphoma group. Leukemia. (2021) 35(12):3585–8. doi: 10.1038/s41375-021-01466-0
13. Russo D, Polverelli N, Malagola M, Farina M, Leoni A, Bernardi S, et al. Changes in stem cell transplant activity and procedures during SARS-CoV2 pandemic in Italy: an Italian Bone Marrow Transplant Group (GITMO) nationwide analysis (TransCOVID-19 survey). Bone Marrow Transplant. (2021) 56(9):2272–5. doi: 10.1038/s41409-021-01287-w
14. Malagola M, Polverelli N, Gandolfi L, Zollner T, Bernardi S, Zanaglio C, et al. Severe acute respiratory syndrome coronavirus-2 pandemia: facts and perspectives in a bone marrow transplant unit. Front Oncol. (2020) 10:1626. doi: 10.3389/fonc.2020.01626
15. Mikulska M, Sepulcri C, Bassetti M, Magne F, Balletto E, Baldi F, et al. Triple combination therapy with 2 antivirals and monoclonal antibodies for persistent or relapsed severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients. Clin Infect Dis. (2023) 77(2):280–6. doi: 10.1093/cid/ciad181
16. Van Doesum JA, Salmanton-García J, Pagano L, Di Blasi R, Falces-Romero I, Cabirta A, et al. Impact of SARS-CoV-2 vaccination and monoclonal antibodies on outcome post-CD19-directed CAR T-cell therapy: an EPICOVIDEHA survey. Blood Adv. (2023) 7(11):2645–55. doi: 10.1182/bloodadvances.2022009578
17. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, Timmerman JM, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. (2020) 382:1331–42. doi: 10.1056/NEJMoa1914347
18. Porter DL, Hwang W-T, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. (2015) 7(303):303ra139. doi: 10.1126/scitranslmed.aac5415
Keywords: COVID-19, infection, CAR-T cell, lymphoma, mantle cell lymphoma (MCL)
Citation: Radici V, Giagulli C, Accorsi Buttini E, Farina M, Polverelli N, Brugnoni D, Chiarini M, Galvagni A, Almici C, Ferrari E, Bianchetti A, Masneri S, Leoni A, Re F, Bernardi S, Malagola M, Re A, Caruso A and Russo D (2023) Successful CAR-T cell therapy in a refractory MCL patient with bacterial, fungal and COVID-19 infection: a case report. Front. Transplant. 2:1238494. doi: 10.3389/frtra.2023.1238494
Received: 11 June 2023; Accepted: 28 August 2023;
Published: 19 September 2023.
Edited by:
Roberto Passera, University of Turin, ItalyReviewed by:
Elisa Roldan Galvan, Sheffield Teaching Hospitals NHS Foundation Trust, United Kingdom© 2023 Radici, Giagulli, Accorsi Buttini, Farina, Polverelli, Brugnoni, Chiarini, Galvagni, Almici, Ferrari, Bianchetti, Masneri, Leoni, Re, Bernardi, Malagola, Re, Caruso and Russo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vera Radici dmVyYS5yYWRpY2lAaG90bWFpbC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.