- 1Berlin Center for Advanced Therapies (BeCAT), Charité—Universitätsmedizin Berlin, Berlin, Germany
- 2Center for Translational Medicine, Universitätsklinikum der Ruhr-Universität Bochum, Medizinische Klinik I, Herne, Germany
- 3MicroDiscovery GmbH, Berlin, Germany
- 4Universitätsklinikum Carl Gustav Carus, Medizinische Klinik III—Bereich Nephrologie, Dresden, Germany
- 5Chirurgische Klinik, Universitätsklinikum Knappschaftskrankenhaus Bochum, Bochum, Germany
- 6Institute of Medical Immunology, Charité—Universitätsmedizin Berlin, Berlin, Germany
Human herpesvirus 6 (HHV-6) is a common opportunistic pathogen in kidney transplant recipients. Two distinct species of HHV-6, HHV-6A and HHV-6B, have been identified, of which the latter seems to be dominant. However, it is unclear whether they increase the likelihood of other viral reactivations. We characterized a multi-centre cohort of 93 patients along nine study visits for viral load. We tested for the following viruses: HHV-6A and HHV-6B, the herpesviruses cytomegalovirus (CMV) and Epstein-Barr virus (EBV) and the polyomavirus BK (BKV). We detected HHV-6A viral load in 48 (51.6%) patients, while the incidence of HHV-6B was much lower, being detected in 6 (6.5%) patients. The incidence of HHV-6A was higher than of BKV, CMV and EBV. HHV-6A also demonstrated higher viral loads than the rest of viruses. There was a non-significant trend of association between HHV-6A and HHV-6B as co-infection, whereas no increased incidence of other viruses among patients with HHV-6A reactivation was observed. There was no negative effect of high HHV-6A (>10,000 copies/ml) load on markers of renal graft and hepatic function or blood count twelve months post-transplant. In contrast to previously published data, our results show a clear dominance of HHV-6A in peripheral blood when compared to HHV-6B, with higher incidence and viral load levels. Despite the high HHV-6A loads observed, we did not identify any negative effects on posttransplant outcome.
Introduction
Human herpesvirus 6 (HHV-6) infects over 90% of the healthy population within the first three years of life and is a common opportunistic pathogen in kidney transplant recipients (1). Two distinct species of HHV-6, HHV-6A and HHV-6B, have been identified (1, 2), although the genome of these two viruses have a 90% similarity (3). HHV-6B has been observed to be dominant in peripheral blood and to reactivate often after solid organ transplantation, while HHV6-A seems to be dominant in the central nervous system (1, 2). HHV-6 primary infection occurs in the early childhood and is usually asymptomatic or mild, with rare systemic complications; most symptomatic infections seem to be caused by HHV-6B (4). An even higher incidence of HHV-6 seropositivity (96.4%) has been observed among adult recipients of solid organ transplants (5). As a consequence of immunosuppression, renal transplant recipients (RTR) experience reactivation in 23%–55% of the cases (1). While most reactivations are asymptomatic, they may be associated with graft dysfunction and hepatic dysfunction (1). However, the frequency of these complications and the role of the HHV-6 subtypes, as well as their association with other viral reactivations, have been insufficiently studied. Similarly, to HHV6, herpesviruses cytomegalovirus (CMV) and Epstein-Barr virus (EBV) and the polyomavirus BK (BKV) infection also occurs during childhood, with an approximate prevalence of 80%, 60%, and 90%, respectively (2–4). Reactivations of BKV, CMV and EBV can result in the appearance with clinically relevant symptoms. Especially individuals with an immune system compromised by dialysis or immunosuppressive drugs, i.e., after a solid organ transplantation, bear a high reactivation risk with serve health consequences (5).
Here, we characterized a multi-centre cohort of 93 patients along nine study visits for viral load in peripheral blood (Supplementary Table S1). We tested for the following viruses: HHV-6A and HHV-6B, CMV, EBV and BKV. Patients were tested pre-transplant and one week, two weeks, one month, two months, three months, six months, nine months and twelve months post-transplant. A total of 696 samples were analysed. For more details on the employed methods, see the Supplementary Methods.
We detected HHV-6A viral load in 48 (51.6%) patients, while the incidence of HHV-6B was much lower, being detected in 6 (6.5%) patients. The incidence of HHV-6A was higher than of BKV (29.2%), CMV (27.7%) and EBV (7.7%) (Figure 1A). HHV-6A also demonstrated higher viral peak loads [13,600 (1,484–2,378,404) copies/ml] than all other analysed viruses (Figure 1B). Most reactivations occurred for both HHV-6 species pre-transplant or within the first two weeks post-transplant (HHV-6A: 58.3%; HHV-6B: 71.4%). Although not significant there was a clear trend in the association between HHV-6A and HHV-6B [OR: 5.04 (0.53–247.07); P = 0.20, Figure 1C], the lack of significance was probably due to the very low incidence of HHV-6B reactivation. The OR of BKV and CMV were similar (1.56 [0.46–5.59] for BKV and 1.38 [0.4–4.97] for CMV). In contrast, the incidence between HHV-6A and EBV showed a reversed associated trend, but also not significant [OR 0.52 (0.04–4.84) Figure 1C]. Lastly, we examined the effects of high viral load of the more common HHV-6A on transplant outcome. Here, we did not find any negative effect of high viral loads (>10,000 copies/ml; N = 25) load on markers of renal graft and hepatic function or blood count twelve months post-transplant, when comparing them with patients with no detectable HHV-6A viral load (Supplementary Figure S1).
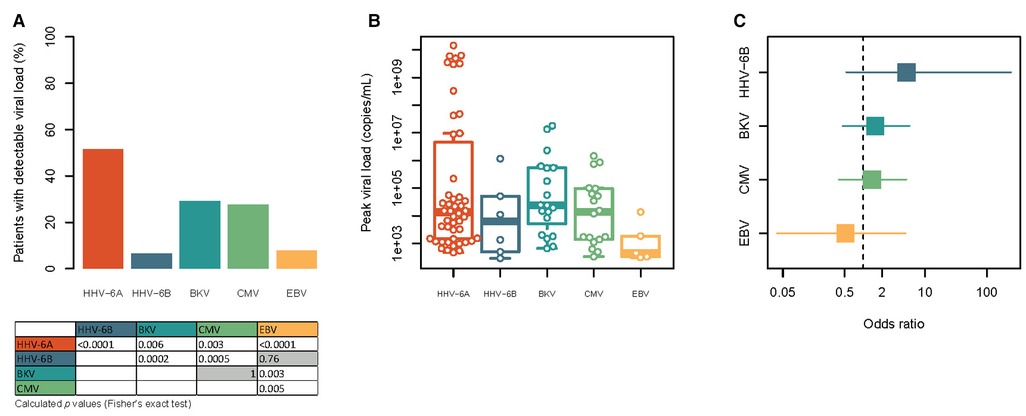
Figure 1. Comparison of the frequency, viral load and mutual association of HHV-6A, HHV-6B, BKV, CMV and EBV in the study cohort. (A) Frequency of patients with detectable viremia for HHV-6A, HHV-6B, BKV, CMV and EBV at least one study visit. Statistical significances were tested with Fisher's exact test and are shown in the table below the graph. Non-significant results are shown with gray background. For reasons of clarity only non-redundant comparisons are shown. (B) Height of the peak viral load observed for each patient with detectable viraemia for each of the viruses. Note the logarithmic scale. All variables tested by Kruskal-Wallis test with Dunn's post-hoc test showed no significances. (C) Forest plot of the association of HHV-6A with the other five viruses. The square points indicate the odds ratio, while the line indicates the 95% confidence interval. The vertical dashed line represents an odds ratio of 1. Note the logarithmic scale.
One limitation of our study is that monitoring the HHV-6 reactivation in the healthy population and among patients with end stage renal disease could not be performed. Thus, it is difficult to judge whether the observed reactivation of HHV-6 in RTR is more prevalent compared to other individuals, especially since we observed a frequent HHV-6 reaction before kidney transplantation. Another aspect of our study is that we did not evaluate specifically chromosomally integrated HHV-6 (ciHHV-6). However, the reported incidence for the healthy population is 0.2% to 2.9% while∼2.0% for renal transplant patients (6, 7), why, this would affect in theory a negligible 3.7% of our HHV-6 positive patient collective. Retrospectively, by calculating the ratio between peak viral load (for HHV-6A) and white blood cell (WBC) counts for every patient we identified just one potentially positive iciHHV-6A positive patient (ratio 1.01) which was not excluded from that study (Supplementary Figure S3). The other patients showed ratios beyond >2.0 or <0.3.
In summary, our results from a multi-centre cohort show a clear dominance of HHV-6A in peripheral blood when compared to HHV-6B and other transplant-associated viruses, with both higher incidence and viral load levels. This cohort are in strong contrast to previously published data (1, 2). We did not identify any significant associations of HHV-6 reactivation with transplant outcome or transplant-associated viral infections such as BKV, CMV and EBV. Moreover, despite the high HHV-6A loads observed we did not identify any negative effects on the graft, nor on hepatic and bone marrow function. Furthermore, it cannot be excluded that other infections also play a role (8). Therefore, the diagnostic utility of HHV-6-PCR should be analysed in larger prospective studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of the Charité-Universitätsmedizin Berlin. The patients/participants provided their written informed consent to participate in this study.
Author contributions
NB, CB, CT, US, MA, PR, CH, PZ, RV, TW, and MO: contributed to the study design, sample collection, and/or sample management. PW and SK: carried out experiments. AB-N, TR, KR and NB: performed data interpretation. AB, TR and NB: drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
CB was employed by MicroDiscovery GmbH.
The authors PR and NB declared that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frtra.2023.1188535/full#supplementary-material
References
1. Lautenschlager I, Razonable RR. Human herpesvirus-6 infections in kidney, liver, lung, and heart transplantation: review. Transpl Int. (2012) 25:493–502. doi: 10.1111/j.1432-2277.2012.01443.x
2. Ablashi D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, DiLuca D, et al. Classification of HHV-6A and HHV-6B as distinct viruses. Arch Virol. (2014) 159:863–70. doi: 10.1007/s00705-013-1902-5
3. Flamand L, Komaroff AL, Arbuckle JH, Medveczky PG, Ablashi DV. Review, part 1: human herpesvirus-6—basic biology, diagnostic testing, and antiviral efficac. Antivir Ther. (2010) 82:1560–8. doi: 10.1002/jmv.21839
4. Dewhurst S, McIntyre K, Schnabel K, Hall CB. Human herpesvirus 6 (HHV-6) variant B accounts for the majority of symptomatic primary HHV-6 infections in a population of U.S. Infants. J Clin Microbiol. (1993) 31:416–8. doi: 10.1128/jcm.31.2.416-418.1993
5. Cervera C, Marcos MA, Linares L, Roig E, Benito N, Pumarola T, et al. A prospective survey of human herpesvirus-6 primary infection in solid organ transplant recipients. Transplantation. (2006) 82:979–82. doi: 10.1097/01.tp.0000229938.12722.ee
6. Aimola G, Beythien G, Aswad A, Kaufer BB. Current understanding of human herpesvirus 6 (HHV-6) chromosomal integration. Antiviral Res. (2020) 176:104720. doi: 10.1016/j.antiviral.2020.104720
7. Pellet PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, Descamps V, et al. Chromosomally integrated human herpesvirus 6: questions and answers. Rev Med Virol. (2011) 22:144–55. doi: 10.1002/rmv.715
Keywords: renal tranplantation, BK viraemia, CMV (citomegalovirus), epstein—barr virus, HHV-6A/B, transplant outcome
Citation: Blazquez-Navarro A, Roch T, Wehler P, Kaliszczyk S, Bauer C, Thieme C, Rosiewicz KS, Stervbo U, Anft M, Reinke P, Hugo C, Zgoura P, Viebahn R, Westhoff T, Or-Guil M and Babel N (2023) High incidence and viral load of HHV-6A in a multi-centre kidney transplant cohort. Front. Transplant. 2:1188535. doi: 10.3389/frtra.2023.1188535
Received: 17 March 2023; Accepted: 8 June 2023;
Published: 26 June 2023.
Edited by:
Friedrich Thaiss, University of Hamburg, GermanyReviewed by:
Rasmus Gustafsson, Karolinska Institutet (KI), SwedenLawrence J. Stern, University of Massachusetts Medical School, United States
© 2023 Blazquez-Navarro, Roch, Wehler, Kaliszczyk, Bauer, Thieme, Rosiewicz, Stervbo, Anft, Reinke, Hugo, Zgoura, Viebahn, Westhoff, Or-Guil and Babel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nina Babel bmluYS5iYWJlbEBjaGFyaXRlLmRl
 Arturo Blazquez-Navarro
Arturo Blazquez-Navarro Toralf Roch
Toralf Roch Patrizia Wehler1,2
Patrizia Wehler1,2 Sviatlana Kaliszczyk
Sviatlana Kaliszczyk Constantin Thieme
Constantin Thieme Kamil S. Rosiewicz
Kamil S. Rosiewicz Ulrik Stervbo
Ulrik Stervbo Petra Reinke
Petra Reinke Michal Or-Guil
Michal Or-Guil Nina Babel
Nina Babel