
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Toxicol., 26 February 2025
Sec. Developmental and Reproductive Toxicology
Volume 7 - 2025 | https://doi.org/10.3389/ftox.2025.1477822
Background: The specific and non-specific toxicities of cryoprotective agents (CPAs) for semen or spermatozoa cryopreservation/vitrification (SC/SV) remain challenges to the success of assisted reproductive technologies.
Objective: We searched for and integrated the physicochemical and toxicological characteristics of small-molecule CPAs as well as curated the information of all extenders reported for carnivores to provide a foundation for new research avenues and computational cryobiology.
Methods: The PubMed database was systematically searched for CPAs reported in SC/SV of carnivores from 1964 to 2024. The physicochemical features, ADMET parameters, toxicity classes, optimized structures, biological activities, thermodynamic equilibrium constants, and kinetic parameters were curated and assessed computationally.
Results: Sixty-two relevant papers pertaining to CPAs used in SC/SV were found, and 11 CPAs were selected. Among the properties of CPAs, the molecular weight range (59–758 g/mol), melting point (−60°C to 236°C), XlogP3 (−4.5 to 12.9), topological polar surface area (TPSA; 20–160 Å2), Caco2 permeability (−0.62 to 1.55 log(Papp) in 10–6 cm/s), volume of distribution (−1.04 to 0.19 log L/kg), unbound fraction of a CPA in plasma (0.198–0.895), and Tetrahymena pyriformis toxicity (log µg/L; −2.230 to 0.285) are reported here. Glutathione, dimethyl formamide, methyl formamide, and dimethyl sulfoxide were used as the P-glycoprotein substrates. Ethylene glycol, dimethyl sulfoxide, dimethyl formamide, methyl formamide, glycerol, and soybean lecithin showed Caco2 permeabilities in this order, whereas fructose, glutathione, glutamine, glucose, and citric acid were not Caco2-permeable. The CPAs were distributed in various compartments and could alter the physiological properties of both seminal plasma and spermatozoa. Low volume distributions of all CPAs except glucose indicate high water solubility or high protein binding because higher amounts of the CPAs remain in the seminal plasma.
Conclusion: ADMET information of the CPAs and extenders in the bipartite compartments of seminal plasma and intracellular spaces of spermatozoa are very important for systematic definition and integration because the nature of the extenders and seminal plasma could alter the physiology of cryopreserved spermatozoa.
• Specific and non-specific toxicities of cryoprotective agents (CPAs) must be considered in assisted reproductive technologies
• Non-specific toxicity is caused by ice crystallization and subsequent thermal cytoinjuries
• Specific toxicity depends on the innate toxic properties of the CPAs and their concentrations
• Small-molecule CPAs are a large category of chemicals used in semen cryopreservation/vitrification (SC/SV)
• The bipartite compartment of seminal plasma and intracellular space of spermatozoa alters the thermodynamic and toxicological features of small-molecule CPAs
• Glycerol and ethylene glycol have been used to prepare semen extenders in carnivorous SC/SV for over 60 years
Semen cryopreservation (SC) and semen vitrification (SV) are two techniques used for long-term biobanking of the male gametes of mammals and other species. These cryobiotechnological methods have been used for more than half a century without considerable progress compared to the methods introduced in the original works (Foote, 1964; Foote and Leonard, 1964), and the original formulations of the semen extenders have often been modified without any systematic testing. Although some process improvements have been attempted on the initial SC recipes, the post-thaw fitness (viability and motility) of spermatozoa do not differ significantly over those of canonical efforts. Therefore, it is important to develop novel interdisciplinary and multidisciplinary methodologies to conserve the viability of spermatozoa during SC/SV or long-term biobanking. Spermatozoa viability is mainly influenced by the semen plasma (SP), extenders, and thermodynamic behaviors of the cryoprotective agents (CPAs; Figure 1). For example, Hyakutake et al. (2015) showed that the motility of bovine spermatozoa differs in the presence of Newtonian and non-Newtonian fluids or extenders; in this context, if the spermatozoa pellets have been separated from SP and rediluted at predetermined concentrations, the exact quality of the extender will strictly impact the fitness of the spermatozoa more than whole semen dilution. Since semen is a non-Newtonian fluid, the mixing (dilution) of whole semen and extenders will produce new properties that the naïve spermatozoa do not have in their prior-experience motion repertoire to swim in this new-content pond. In this regard, Mane et al. (2023) used microchannel methods and showed that the highest motility of spermatozoa is observed in methylcellulose solution that acts as a pseudoplastic non-Newtonian fluid. In summary, understanding the biophysical properties (i.e., microfluidic features) of extenders and their perturbations in the presence of CPAs is critical steps in designing smart extenders or CPAs.
Spermatozoa naturally pass through various survival barriers (intratesticular, urethral, airy, vaginal, uterine, and tubal) with diverse physicochemical and thermodynamic characteristics that may be harsh on their innate motility and viability. Therefore, more biomathematical models and simulations are required to explain the swimming patterns of spermatozoa or spermatozoon communities in various milieus. The outcomes of these investigations could offer new avenues for designing artificial SP as well as a new generation of extenders for precision medicine and personalized treatments of infertility associated with the transport and motility of spermatozoa. Hence, complete understanding of these spermicidal barriers would be the second step in designing smart extenders or CPAs. Finally, the third step in designing smart extenders or CPAs involves dissecting the chemical and physical interactions of all components of semen, spermatozoa, and exogenous additives, and this requires deep understanding of the interactions between the exogenous and endogenous components of semen extenders.
In addition to the aforementioned steps highlighting the smart design of semen extenders, a major bottleneck that must be addressed involves thermal shocks that can lead to cryoinjury (cold-induced injury) and/or pyroinjury (heat-induced injury), which are collectively known as thermoinjuries. In this continuum, the most common thermoinjuries that occur during SC and SV can be summarized as decreasing post-thaw fitness of the spermatozoa, including decreased or altered motility as well as decreased viability. In this context, any alterations such as derangements in plasma membrane integrity (PMI), acrosomal membrane integrity (AcI), mitochondrial membrane potential (MMP) integrity, and DNA integrity as well as morphological abnormalities, percentage remnant cytoplasmic droplets, vulnerability to oxidative or reductive stresses, apoptosis, spermatozoa dyskinesia, zona pellucida disbinding assay, and altered -omes (transcriptome, genome, proteome, metabolome, and physiome) can fulminate to increase male-factor subfertility. To overcome these thermoinjuries, SC and SV optimizations have been pursued, and the application of CPAs is the top priority among all optimization programs to specify the inborn (specific) and acquired (non-specific) toxicities.
Despite the aforementioned requirements to optimize the SC and SV, the study of cryobiotechnological approaches is inadequate. The biophysical and biochemical aspects of semen as a non-Newtonian fluid and its behaviors during freezing as well as the optimum roles of CPAs employed in semen extenders to avoid cellular cryoinjuries during SC and SV (AbdelHafez et al., 2009) must be studied further. In this context, cryoinjury of spermatozoa is a critical and cardinal issue during SC that encompasses derangements of the PMI, AcI, DNA integrity, etc., which decrease the post-thaw fitness of spermatozoa. Luvoni (2006) and Bencharif and Dordas-Perpinya (2020) focused on the non-specific toxicities of CPAs, but evaluation of the specific toxicities of CPAs is an incentive for improving the SC/SV. In such cases, the spermatozoa experience two temperature shocks, namely, cold shock during freezing and heat shock during thawing, both of which can result in ice crystallization or cellular dehydration. To overcome these thermal shocks, we could add the CPAs during freezing and remove them during thawing to decrease the toxicity to the spermatozoa. In summary, despite the introduction of several commercial extenders and CPAs in the market, the specific and non-specific toxicities of CPAs have not been studied in detail.
To the best of our knowledge, there is no available database pertaining to the physicochemical properties of currently available CPAs used for SC/SV of animals that can enlighten researchers on the physicochemical and biological spaces of CPAs and their boundaries (cut-offs) or provide current integrated information on designing/discovering new CPAs. Therefore, the present work focuses on the computational physicochemistry, thermodynamics, and toxicology of small-molecule CPAs employed for the SC/SV of carnivores to aid research on the advantages of computational cryobiotechnology.
We comprehensively and historically perused publications regarding carnivorous SC/SV on PubMed dating from 1964 to 10 January 2024 as well as searched for papers using the search string “[(spermatozoa) AND (cryoprotectant)] AND (carnivores)” that adhered to the PRISMA guidelines (Liberati et al., 2009), as demonstrated in Figure 2. These studies were carefully screened, and eligible works were selected based on the following inclusion criteria: (1) studies published in English, (2) studies aimed at reporting carnivorous SC/SV, and (3) studies reporting CPAs and extenders. We excluded papers focused on other animals or the cryopreservation of different organs as well as review articles; however, the references of such works were scrutinized to find relevant works.
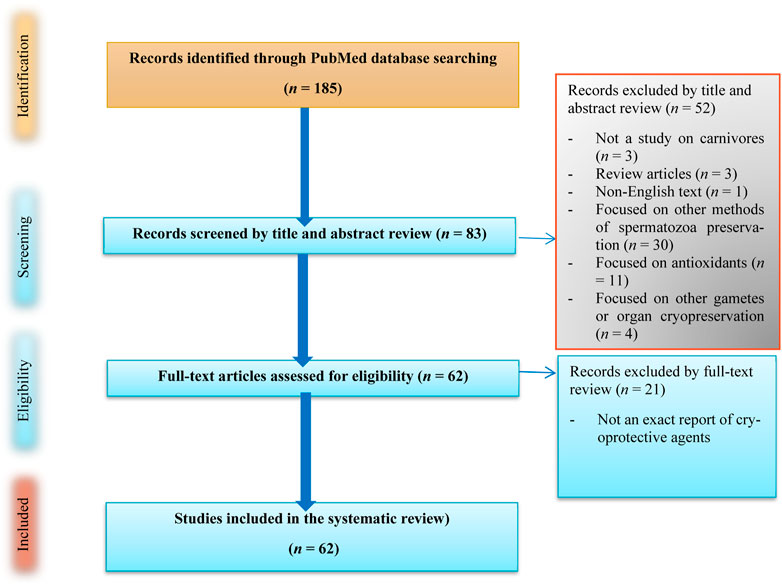
Figure 2. Flow diagram showing the literature search on cryoprotective agents used in carnivorous semen cryopreservation/vitrification.
The physicochemical attributes of CPAs used in carnivorous SC were curated from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Then, the chemical properties, molecular structures, canonical simplified molecular input line entry system (SMILS), XlogP3, logS, cellular locations, molecular weights in g/mol, melting points in °C, topological polar surface areas (TPSAs; Å2), and toxicity classes of common small-molecule CPAs were computed.
A knowledge-based approach based on an additive atom/group model starting from the known logP value of a similar reference compound (Cheng et al., 2007) was employed to compute XlogP3. Here, the logP value is a constant defined as log10 (partition coefficient) with the partition coefficient P = [organic]/[aqueous], where [ ] indicate the concentrations of the solutes in the organic and aqueous partitions. A negative value of logP indicates that the compound has a higher affinity for the aqueous phase (more hydrophilic); when logP = 0, the compound is equally partitioned between the lipid and aqueous phases; a positive value of logP denotes a higher concentration of the lipid phase (i.e., the compound is more lipophilic). For instance, logP = 1 indicates a 10:1 partitioning of the organic:aqueous phases. In contrast to XlogP3, logS is related to the water solubility (mol/L) of a chemical and is expressed as a common solubility unit in log10 value. The molecular polar surface area (PSA) refers to the surface area corresponding to the polar atoms and is a descriptor of the passive molecular transport through the membranes for predicting the transport properties of chemicals (Ertl et al., 2000).
SwissADME (http://www.swissadme.ch/) (Daina et al., 2017) was used to compute the biological activities. The bioavailability radar offers a speedy assessment of the medication resemblance of a particle (Daina et al., 2017). Six physicochemical properties (lipophilicity, size, polarity, solubility, flexibility, and saturation) were computed for each of the CPAs, and these descriptors were established with physicochemical ranges along each axis. The pink areas outline the best possible regions for each of the ranges. Briefly, the fitness parameters include lipophilicity (LIPO) as logP (XLOGP3) values ranging from −0.7 to 5.0; SIZE as molecular weights in the range of 150–500 g/mol; polarity (POLAR) as TPSA values ranging from 20 to 130 Å2; insolubility (INSOLU) as solubility with logS (ESOL) values ranging from −6 to 0; insaturation (INSATU) as saturation fractions of carbons with sp3 hybridization (fraction Csp3) ranging from 0.25 to 1; and flexibility (FLEX) as the number of rotatable bonds ranging from 0 to 9 (Daina et al., 2017).
The boiled-egg construction depicts a snapshot of the human intestinal absorption (HIA) and blood–brain barrier (BBB) permeability of a substance (Daina and Zoete, 2016), where the blue dots represent the P-glycoprotein (PGP) substrates (PGP+) and red dots indicate the PGP non-substrates (PGP−). The outer gray area represents compounds with lower gastrointestinal absorption and limited BBB penetration; the white area represents the physicochemical space of the molecule with the highest probability of passive HIA; the yellow area represents the physicochemical space of the molecule with the highest likelihood of BBB penetration.
For the most part, a chemical reaction has some basic equilibrium constant parameters; however, these are not effortlessly obtained from experiments. Herein, we show that the computed thermodynamic properties of CPAs used for carnivorous SC are dependent on Chemeo, which includes high-quality compound properties (www.chemeo.com), and the Joback method that produces an anticipated value of 813.3 K. The actuation entropy (S°), enactment enthalpy (ΔfH°), and Gibbs free energy obtained from the thermodynamic properties contrast between the explicit reactant and change states compared to individual response stages subbed into the progress state theory (Benkert et al., 2007), as shown in Equations 1, 2 to gage the rate steady (K) and preexponential factor (A). The thermodynamic equilibrium constant of each response was obtained from the ratio of the forward to retrogressive rate steady values for the resulting correlation.
where kB is the Boltzmann constant, h is the Planck constant, and n is the molecularity.
We reported the initial values of all chemical properties of CPAs, from which their proton affinities were computed. The basicity was determined from the presence or absence of the reaction shown in Equation 3 and its inverse reaction, where A is an amino acid, B is a reference base, and AH+ and BH+ are the protonated versions of the amino acids and bases, respectively (Gorman et al., 1992; PAaJd, 2006).
In addition, the optimized structures of the chosen CPA mixtures were predicted using density functional theory (DFT), GaussView (Frisch HBS et al., 2009), and Gaussian 09 program (Frisch et al., 2009) with 6-311++G (d, p) premise sets. The potential limitations of the chosen tools were addressed in the study, and the parameters chosen were essential for the transparency and robustness of the study based on careful consideration of their strengths. For example, SwissADME is known for its ability to predict the absorption, distribution, metabolism, and excretion (ADME) properties, which are crucial for assessing the suitability of a cryoprotectant, and PubChem is a widely used and authoritative resource for chemical information.
The features of computational toxicology including absorption, distribution, metabolism, excretion, and toxicity (ADMET) were predicted using a web ADMET server (http://biosig.unimelb.edu.au/pkcsm/prediction) (Pires et al., 2015). This allowed determination and expression of the ADMET boundaries through investigation of human influences on medication (pharmacokinetic properties), neutral nature, and the unwavering quality of the restorative media on small-molecule CPAs. Using the SciDaviz package (Benkert et al., 2007), the correlation distribution properties were plotted in graphs in the vertical direction and structured based on expansion with different CPAs.
A set of endpoints including water solubility (log mol/L), colorectal adenocarcinoma cells (Caco2) permeability (log(Papp) in 10–6 cm/s), HIA (% absorbed), skin permeability (logKp), PGP substrate, PGP I inhibitor, and PGP II inhibitor was computed to predict the various absorption components of the ADMET assay (https://biosig.lab.uq.edu.au/pkcsm/theory). For instance, using the in vitro absorption data of 674 compounds, pkCSM predicts the apparent cellular permeability coefficient of the compound of interest. For the pkCSM predictive model, high Caco2 permeability translates to values greater than 0.9.
Another set of endpoints including steady-state volume distribution (VDss; human; log L/kg), unbound fraction (human; Fu), BBB permeability (log BB), central nervous system (CNS) permeability (logPs), and total clearance (log mL/min/kg) was computed to predict the various distribution and excretion components of the ADMET assay (https://biosig.lab.uq.edu.au/pkcsm/theory). A set of substrates and inhibitors of the cytochrome (CYP) class including the CYP2D6 and CYP3A4 substrates as well as inhibitors of CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4 were computationally assessed to predict the metabolism part of the ADMET assay (https://biosig.lab.uq.edu.au/pkcsm/theory). An array of toxicological endpoints, including AMES toxicity maximum tolerated dose (MTD; human; log mg/kg/day), Ether-à-go-go-related gene (hERG) I inhibitor, hERG II inhibitor, rat oral acute toxicity (LD50 mol/kg), rat oral chronic toxicity (LOAEL; (log mg/kg-bodyweight/day), hepatotoxicity, skin sensitization, Tetrahymena pyriformis toxicity (log µg/L), minnow toxicity (log mM), and toxicity class, were then computed to predict the various toxicity components of the ADMET assay (https://biosig.lab.uq.edu.au/pkcsm/theory).
Furthermore, a set of toxicological endpoints was employed to show the ecofriendliness of the CPA of interest. For instance, the AMES test is a globally accepted bacterial assay for evaluating the potential genotoxicity, mutagenicity, and even carcinogenicity of chemicals (Zeiger, 2019). The relevancy of the AMES test for SC/SV requires further investigation; however, CPA remnants can alter the reproductive microbiota of packs or pets that have received AI, especially if repeated services have been attempted. This relevancy can be examined by measuring the ability of the CPA to induce reverse mutations at selected loci in several bacterial strains. The cardiotoxic hERG binding of a CPA may seem irrelevant to its evaluation. The human hERG codes for the alpha subunit of the potassium ion channel known as Kv11.1, which is dubbed so because of its role in coordinating the electrical activity of the heart. The flexible nature of hERG to bind to various ligands was used in its selection as a target in the ADMET assay of all compounds to avoid cardiotoxicity (Garrido et al., 2020). Skin sensitization is a predictor of potential adverse effects such as dermatitis for chemicals applied to the skin. The MTD is commonly estimated as the highest dose that can be administered for a specific duration that will not compromise the survival of the animal through causes other than carcinogenicity (Shayne, 2024). Itis noted that MTD is sometimes also referred to as the minimum toxic dose. The acute median lethal dose (LD50) is the dose of a chemical that will kill 50% of the test organisms within 24 h of exposure. Acute toxicity studies are usually conducted for various routes of administration in rodents to provide the baseline values for novel chemicals of interest (Gadaleta et al., 2019). The computation model for LD50 was built on over 10,000 compounds tested in rats and predicted in units of mol/L. In addition, chronic studies such as rat oral chronic toxicity aim to identify the minimum dose of a substance that results in the least observable adverse effect level (LOAEL) and maximum dose for the no observable adverse effect level (NOAEL). This predictor was built using the LOAEL library comprising 567 compounds.
Tetrahymena pyriformis toxicity (log µg/L) is a globally accepted screening test for the detection of environmental toxicants (Maurya and Pandey, 2020). Here, the laboratory-adopted protozoan Tetrahymena pyriformis is the most commonly used ciliated model for primary toxicological research, and the dose that inhibits 50% of its growth is the toxic endpoint, which has been identified as values > −0.5 log µg/L. At a higher organism level, minnow toxicity is used to predict the lethal concentration (LC50) of any chemical that would kill 50% of flathead minnows; here, LC50 values below 0.5 mM (log LC50 <-0.3) indicate high acute toxicity. Finally, based on the hepatic injuries induced by 531 compounds, pkCSM computationally predicts the hepatotoxicity of the compound of interest.
The Toxtree v.3.1.0-1851-1525442531402 software platform (http://toxtree.sourceforge.net/) was employed to predict the toxicity class based on the Cramer rule (Cramer et al., 1976; Munro et al., 1996; Patlewicz et al., 2008). Here, the toxicological hazard of oral administration is estimated from the molecular structure, and three classes are defined as follows. Low oral toxicity or Class I substances have simple chemical structures and efficient modes of metabolism; here, the no observable effect level (NOEL) at the fifth percentile is 3.0 mg/kg-bodyweight/day and human exposure threshold is 1.8 mg/person/day. The NOEL is the highest dose or exposure level of a chemical that produces no noticeable (observable) toxic effects. Intermediate oral toxicity or Class II substances possess structures that are less innocuous than Class I substances but do not contain structural features suggestive of toxicity; here, the NOEL at the fifth percentile is 0.91 mg/kg-bodyweight/day and human exposure threshold is 0.54 mg/person/day. High oral toxicity or Class III substances have chemical structures that permit no strong initial presumption of safety or may even show significant toxicity or have reactive functional groups; here, the NOEL at the fifth percentile is 0.15 mg/kg-bodyweight/day and human exposure threshold is 0.09 mg/person/day. The hazardous substances data bank (HSDB) at https://www.nlm.nih.gov/toxnet/index.html is a reliable index for deciphering the biosafety of extenders and CPAs used in the SC/SV of carnivores. The toxin and toxin target database (T3DB; http://www.t3db.ca/toxins/) was employed to compute the other toxicological features.
The data were described in terms of mean ± standard error of the mean (SEM) for all features of the selected CPAs in this study. Furthermore, all descriptive statistics of the numeric features were analyzed using IBM SPSS Statistics ver. 20 (https://www.ibm.com/spss).
First, 185 articles were screened, and no duplicate works were found. Subsequently, the publications were vetted by title revision, and three review articles were excluded. Thus, 182 articles were selected based on the titles, including those concerned with in vivo experiments, research linked to female animals, and toxic substances, along with publications involving species such as humans, mice, and bulls (Figure 2). Among the remaining articles, 83 full-text articles were assessed and discussed by the coauthors to find the best and most effective CPA candidates for dogs (Table 1). Finally, 62 full-text appropriate papers on SC/SV as well as their physicochemical and some toxicological properties of small molecules were discussed (Table 1).
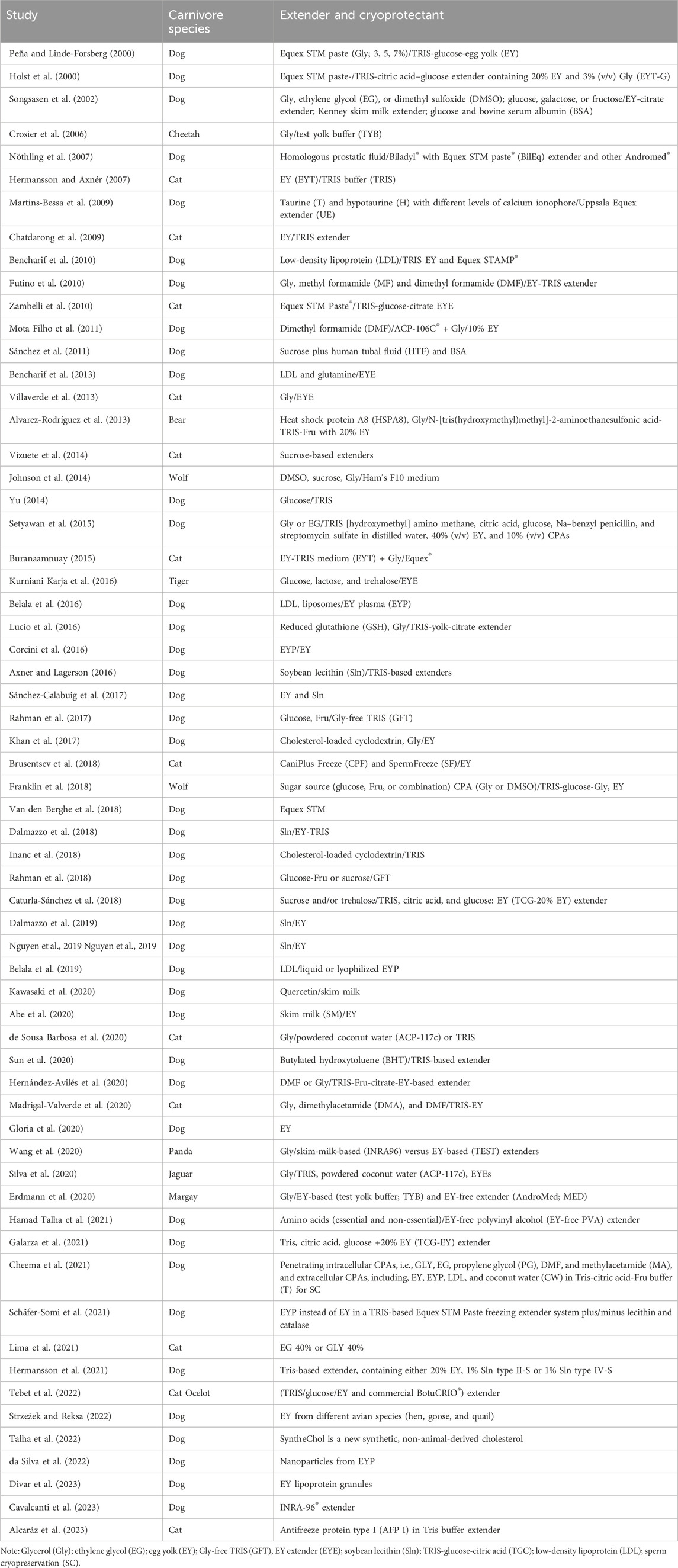
Table 1. Categorization of studies dedicated to the addition of cryoprotectants to the spermatozoa or semen cryopreservation/vitrification of carnivores.
In summary, Table 1 shows an array of extenders employed in the SC/SV of carnivores. Although various extenders have been commercially distributed as chemically defined media with unknown or lesser-known chemical compositions (e.g., INRA-96 extender®), many of the commercial blends contain materials ranging from amino acids to biological fluids such as skim milk. SC is an essential biotechnology in canine reproduction, and several studies have attempted to introduce novel CPAs and/or cryopreservation methods to achieve better results, which have been systematically reviewed herein (Table 1). Readers are referred to the references mentioned in Table 1 for detailed comparisons of the SC and SV methods along with the CPAs and their major roles in decreasing cryoinjuries.
The cellular locations of small-molecule CPAs are predicted and presented in Table 2. Briefly, none of the CPAs were localized in the cell membrane, while ethylene glycol (EG), citric acid (CA), glutathione (GSH), dimethyl formamide (DMF), and dimethyl sulfoxide (DMSO) were localized in the cytoplasm. Moreover, EG, glycerol (Gly), fructose (Fru), glucose (Glc), CA, glutamine (Gln), GSH, DMF, methyl formamide (MF), and DMSO were localized in the extracellular compartments. Some CPAs were also localized in the mitochondria (e.g., Gly, CA, Gln, and GSH), lysosomes, Golgi apparatus (e.g., Glc), and endoplasmic reticulum (e.g., GSH and Glc). All physiochemical properties of the CPAs may influence their transfer through membranes. The physicochemical characteristics of some selected small-molecule CPAs used in carnivorous SC/SV were curated from databases to obtain cues regarding their behaviors and toxicities.
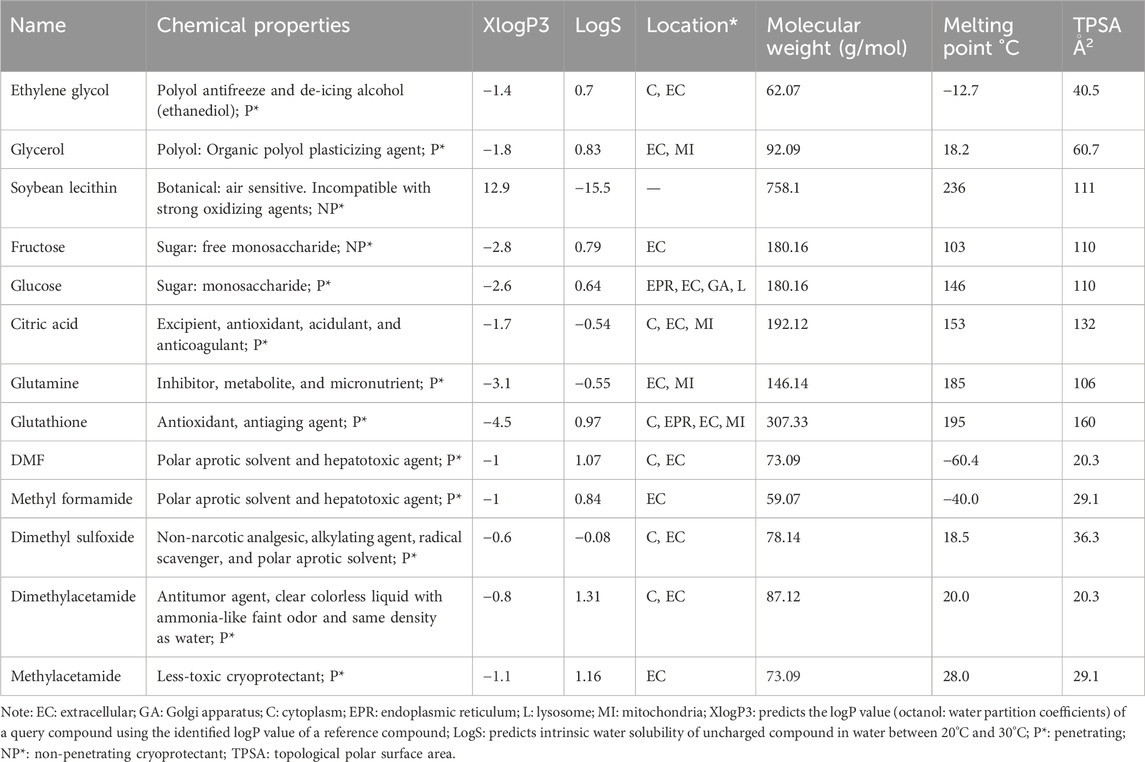
Table 2. Physicochemical characteristics of selected small-molecule cryoprotectants used in carnivorous semen cryopreservation/vitrification based on the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) and T3DB (http://www.t3db.ca/toxins/).
The TPSAs of both EG and Gly were less than 100 Å2 (Table 2); therefore, they can cross the cell membrane more easily than CPAs that have TPSAs larger than 100 Å2. The TPSAs of the CPAs used for carnivores ranged from 20.3 (DMF) to 160 Å2 (GSH), and we cannot categorize the CPAs based on their TPSAs because they do not represent a rule of thumb (Table 2). The hydroxyl groups of EG or Gly make them polar substances that penetrate the cell membrane because of their low molecular weights. Two novel acetamide derivatives (methylacetamide and dimethylacetamide) are also used as CPAs for carnivorous SC/SV (Table 2).
The physicochemical attributes of CPAs used for carnivorous SC/SV are presented in Table 2, and the descriptive statistics of their quantitative features are shown in Supplementary Table S1. Based on the XlogP descriptor, all CPAs except soybean lecithin (Sln) show negative values, meaning that they are hydrophilic; however, they are considered permeating CPAs. The degree of hydrophilicity ranges from −4.5 for GSH to −0.6 for DMSO (Table 2). Among the CPAs, Sln showed an XlogP value equal to 12.9, which reflects the supralipophilic nature of this non-permeating CPA.
Based on the PubChem information, the CPAs were distributed in various cellular locations. All CPAs are distributable in the extracellular compartment as their logS values support this finding (Table 2). The organelle distribution of CPAs can alter all functional aspects of spermatozoa during the thawing and/or freezing phases of SC/SV (Table 2). The logS values of small-molecule CPAs ranged from −15.5 for Sln as the most hydrophobic to 1.33 for DMA as the most hydrophilic agent (Table 2). The molecular weights of the CPAs vary from 59.07 for MF to 758.1 for Sln, while the melting points range from −60.4°C for DMF to 236°C for Sln (Table 2).
All penetrating CPAs show optimal ranges for the physicochemical properties, except GSH, which deviates in terms of flexibility and polarity (Figure 3). Sln is a non-penetrating CPA that deviates in terms of size, insolubility, lipophilicity, and flexibility (Figure 3). None of the CPAs except DMSO exhibit predicted high BBB penetration, as demonstrated by their location inside the yellow ellipse (yolk) outlining BBB absorption (Figure 3). Glc is located in the outer gray area, indicating that it is a compound with lower HIA and limited BBB penetration. Many CPAs are located at the boundary between the gray and white sections. Gln is situated in the white area and represents a molecule with the highest probability of passive HIA. GSH is out of range of the boiled-egg scheme. None of the CPAs are PGP+, and they cannot interfere with the transfer of other drugs.
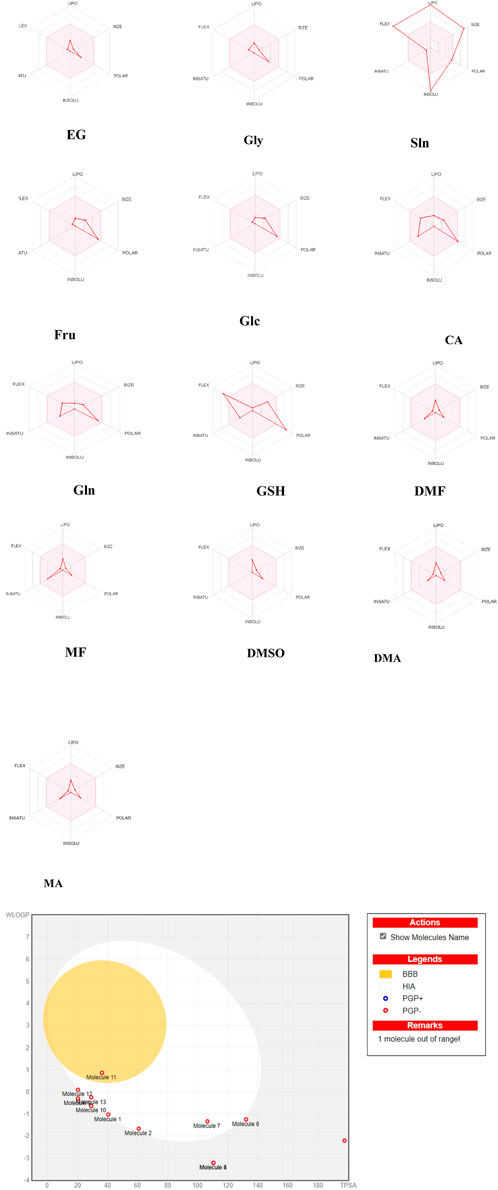
Figure 3. Bioavailability radar of the cryoprotectants used in carnivorous semen cryopreservation based on SwissADME (http://www.swissadme.ch/). The pink areas represent the optimal ranges for the physicochemical properties (size: molecular weight between 150 and 500 g/mol, lipophilicity: XlogP3 between −0.7 and +5.0, polarity: topological polar surface area (TPSA) between 20 and 130 Å2, saturation: fraction of carbons in the sp3 hybridization not less than 0.25, solubility: logS not higher than 6, and flexibility: no more than nine rotatable bonds). Human intestinal absorption (HIA), brain–blood barrier (BBB) penetration, P-glycoprotein (PGP) as an efflux transporter that can pump drugs out of cells, lipophilicity (WlogP), and polarity (TPSA) were calculated. The white region is the physicochemical space of molecules with the highest probability of being absorbed by the gastrointestinal tract that is extrapolated for dissolving semen, and the yellow region (yolk) is the physicochemical space of molecules with the highest probabilities of permeating the brain that is extrapolated for cytoplasmic penetration of spermatozoa. The yolk and white areas are compatible properties for computing the polarity and lipophilicity of small molecules. Note: ethylene glycol (EG; molecule 1); glycerol (Gly; molecule 2); soybean lecithin (Sln; molecule 3 is out of the range); fructose (Fru; molecule 4); glucose (Glc; molecule 5 is located in the same place as molecule 4); citric acid (CA; molecule 6); glutamine (Gln; molecule 7); glutathione (GSH; molecule 8 is shown without name on the right side); dimethyl formamide (DMF; molecule 9 located under molecule 12); methyl formamide (MF; molecule 10); dimethyl sulfoxide (DMSO; molecule 11); dimethylacetamide (DMA; molecule 12); methylacetamide (MA; molecule 13). GSH was out of range in the boiled-egg construction.
Based on the computational thermodynamic properties, Fru, Glc, and CA do not show proton affinities, whereas EG and Gly are protonated. Figure 4 predicts the optimized geometries of common small-molecule CPAs used for carnivorous SC/SV. At 813° K, the enthalpy of Gly is 3.5% higher than those of DMF and DMSO, whereas that of EG is 1.46% lower in the gaseous phase. Although most exothermic (ΔfH° < 0) cases are spontaneous, certain endothermic (ΔfH° > 0) cases can be included in this as well. Entropy is a thermodynamic parameter that describes the number of possible arrangements for a system in a particular state. The molecular structures demonstrate the increases in randomness of the particles upon melting of the solid, particularly as the liquid vaporizes (entropy S°gas >> S°liquid > S°solid). The entropy of EG S°gas (311.8 J/mol k) is higher than that of S°liquid (166.9 J/mol k). The Gibbs free energy (ΔfG° = ΔfH° - T S°) is calculated by combining the effects of enthalpy (ΔfH°) and entropy (S°) on a process. The ΔfG° (813 K) values for all CPAs are spontaneously formed at this temperature because the Gibbs free energies of the CPAs are less than zero. The Gibbs free energy of CA (−931.5 kJ/mol) is higher than those of all other compounds.
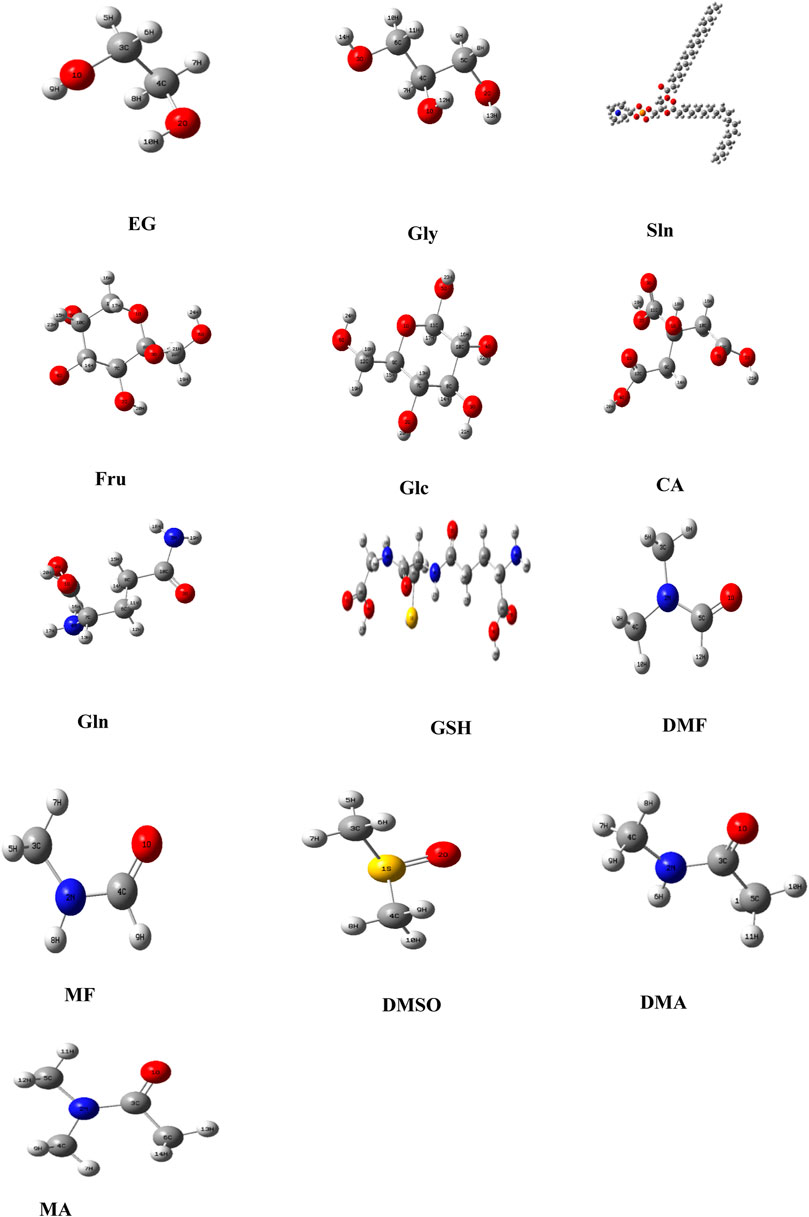
Figure 4. Optimized geometries of the selected cryoprotectants used in carnivorous semen cryopreservation/vitrification based on DFT/B3LYP-6311++. Note: Ethylene glycol (EG); glycerol (Gly); soybean lecithin (Sln); fructose (Fru); glucose (Glc); citric acid (CA); glutamine (Gln); glutathione (GSH); dimethyl formamide (DMF); methyl formamide (MF); dimethyl sulfoxide (DMSO); dimethylacetamide (DMA); methylacetamide (MA).
Table 3 shows that EG has the lowest proton affinity (PAff) among the CPAs. The PAff of Gln (937.8 kJ/mol) is greater than that of DMSO (832.1 kJ/mol); however, when the alkyl chains are lengthened, the PAff cross (at the butyl acetate and methyl pentanoate pair) becomes slightly smaller for acetates with long chains. Because the protons are accompanied by the absorption of HO⁻ groups, the Fru, Glc, and CA systems have low affinities, with the molecules collecting 20–25 times more on the inside than outside.
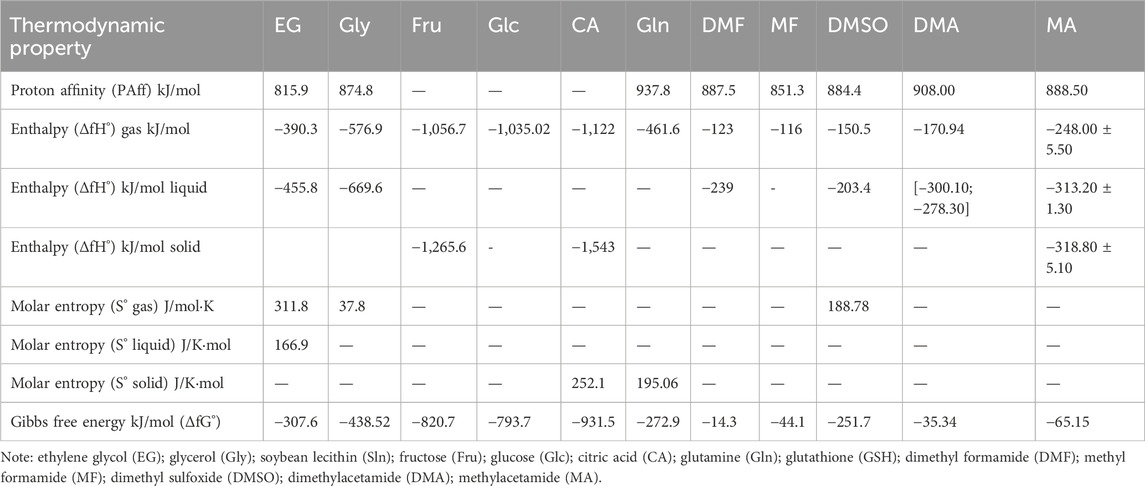
Table 3. Thermodynamic properties of cryoprotectants used in carnivorous semen cryopreservation based on Chemeo, high-quality chemical properties (www.chemeo.com), and Joback method predicted at 813.3 °K.
Based on the data shown in Table 4, all CPAs reported for carnivorous SC/SV are healthy for the brain except GSH, DMF, MF, and DMSO, which show tendencies to be PGP (transmembrane efflux pump) substrates. None of the CPAs were PGP I and PGP II inhibitors. None of the selected CPAs could penetrate skin, and their logKp values were negative, indicating that they cannot cross skin tissue upon accidental skin exposure. Because the logKp values of all CPAs do not exceed −2.5, they do not have excellent skin permeability. The intestinal absorption percentages of CA and GSH are 0, whereas those of DMSO and Sln are 100% through the intestinal tract (Table 4). In particular, EG, DMSO, DMF, MF, Gly, and Sln show Caco2 permeabilities in this order, whereas Fru, GSH, Gln, Glc, and CA are not Caco2-permeable (Table 4). EG, DMSO, DMF, MF, Fru, and Gly are water-soluble compounds, whereas Sln, Glc, CA, Gln, and GSH show poor water solubilities (Table 4).
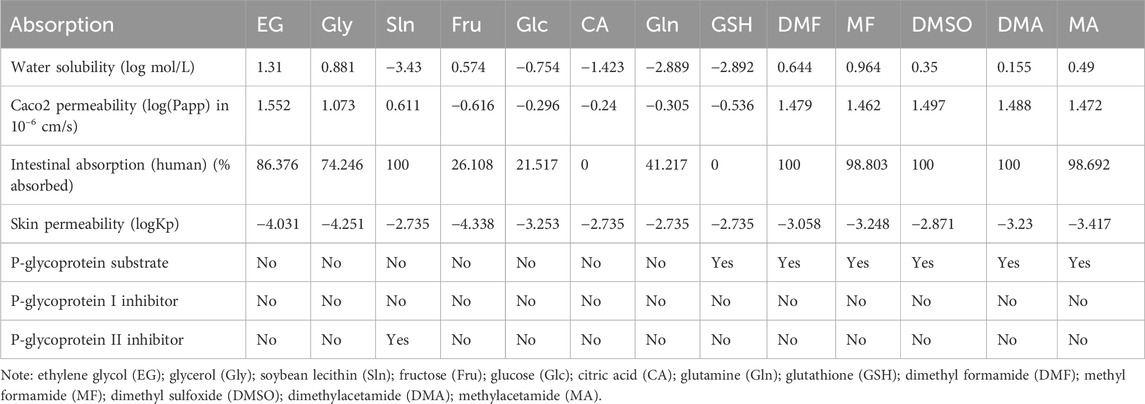
Table 4. Pharmacokinetic properties of transmembrane transport and penetration of selected small-molecule cryoprotectants used in carnivorous semen cryopreservation/vitrification.
It is noted that the distributions of the CPAs in various compartments may alter the rheological properties of both SP and spermatozoa during SC/SV. The low VDss values of all CPAs except Glc indicate high water solubility or high plasma protein binding because the CPAs remain predominantly in the plasma, whereas high VDss values indicate significant concentration in the tissues, for example, due to tissue binding or high lipid solubility (Table 5). The relevancy of the computational data for BBB permeability (logBB), CNS permeability (logPs), and total clearance (log mL/min/kg) of the CPAs are obscured at this step; however, similar definitions like permeability of the cell membrane of the spermatozoa may be pursued computationally in the future (Table 5).
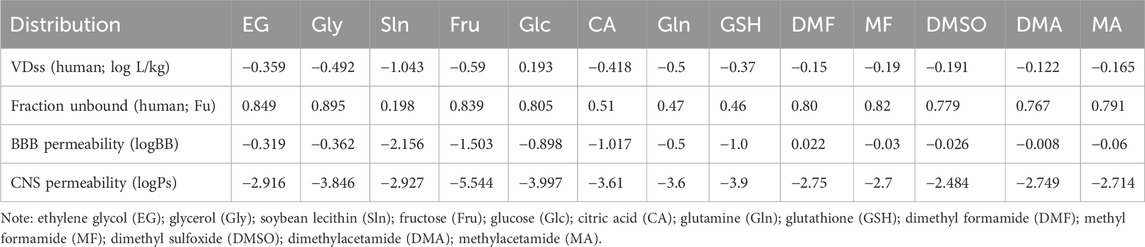
Table 5. Distribution of selected small-molecule cryoprotectants used in carnivorous semen cryopreservation/vitrification.
All penetrating CPAs are not substrates or inhibitors of the main CYPs, whereas Sln is a CYP3A4 substrate (Table 6). None of the CPAs are renal OCT2 substrates, and the highest total clearance was found for Sln (Table 6). Computationally, the small-molecule CPAs reported for SC/SV of carnivores can be categorized into three toxicity classes. Here, EG, Gly, Glc, and CA are low-toxicity (Class I); Gln is an intermediate-toxicity (Class II); and Sln, Fru, GSH, DMF, MF, and DMSO are high-toxicity (Class III) compounds (Table 7).
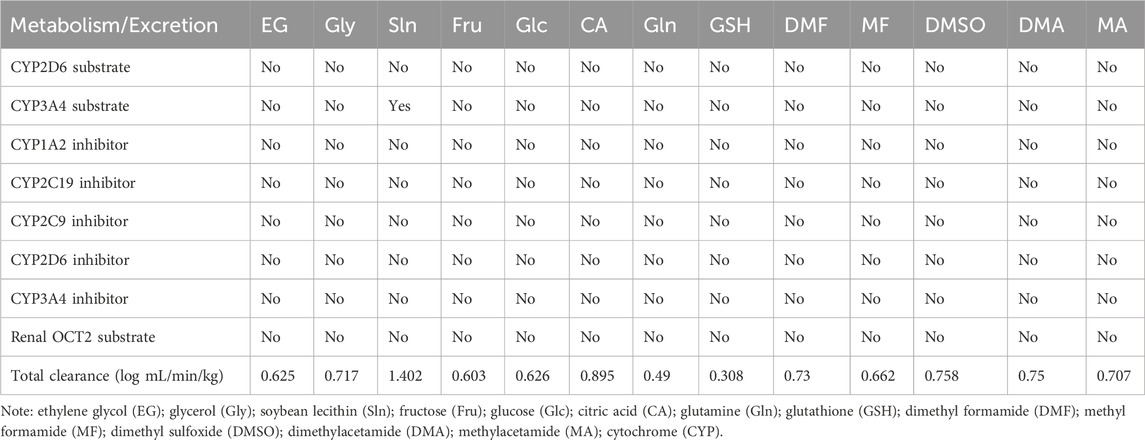
Table 6. Metabolism and excretion of selected small-molecule cryoprotectants used in carnivorous semen cryopreservation/vitrification.
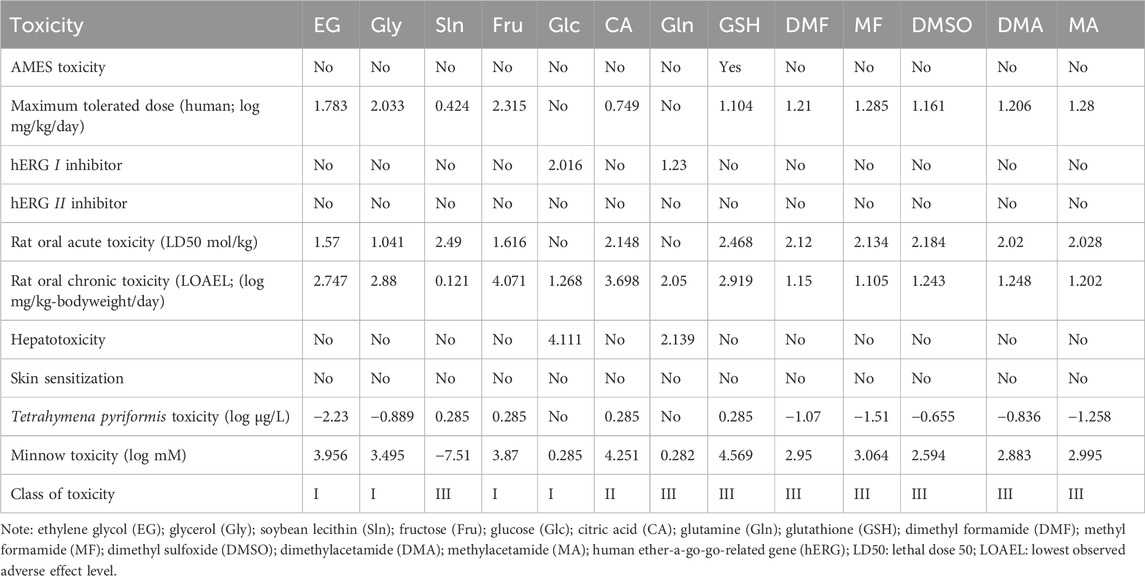
Table 7. Toxicities of selected small-molecule cryoprotectants used in carnivorous semen cryopreservation.
The toxicity profiles of the CPAs are presented in Table 7; however, these data are not useful for daily clinical SC/SV and artificial insemination (AI). Among the CPAs discussed here, only GSH is positive in the AMES test (Table 7). Glc and Gln show hERG I inhibitory effects, whereas the remaining CPAs show no hERG I or hERG II inhibitory effects (Table 7). The rat oral acute toxicity (LD50 mol/kg) doses of EG and Gly are the lowest among the CPAs (Table 7), whereas the NOAEL of rat oral chronic toxicity of Sln is lowest among CPAs (Table 7). Glc and Gln show hepatotoxicity among the CPAs, whereas none of the CPAs show skin sensitization (Table 7). Sln, Fru, EG, CA, GSH, DMF, and MF show T. pyriformis toxicity values greater than −5 log µg/L and are therefore considered toxic (Table 7). Thus, Gly and DMSO are not toxic to T. pyriformis in the computational sense. Among all the CPAs, Sln is the only compound found to be toxic to minnows (Table 7).
Different extenders are used for carnivorous SC/SV, and various formulas are available commercially (e.g., Equex STM paste, TRIS-sugar extenders, skim milk extenders, and protein-fortified extenders); however, 42 out of 62 studies reported the egg yolk (EY)-based extenders considered in the present study. To date, EY and its byproducts, such as EY plasma (EYP), TRIS-EY, and EYs buffered with salts (e.g., citrate) have been used as dominant extenders for the SC/SV of carnivores (Figure 5). Lipid derivatives of EY, including low-density lipoprotein (LDL) and lecithin, have also been used. EY was either used as a single material for the SC/SV of carnivores or fortified with buffers, bases, salts, or sugars. To the best of our knowledge, there is no integrated study available pertaining to the rheological properties of EY or EY-based extenders and their cryoprotective potentials. EY is a viscous fluid and must be diluted before use as an extender or basic material for preparing new marketable extenders. As shown schematically in Figure 5, EY or EY-based extenders can also be mixed with other materials or extenders, including Gly, ACP-160 C®, sugars like Fru and Glc, bases like TRIS and citrate, and botanicals like soybean-based biomaterials, in various proportions. However, researchers have focused on the biological properties of these formulations, and there is no reliable information about their biophysical properties, such as pH, viscosity, molecular weight, and biosafety.
More investigations are needed to assess the rheological, physicochemical, and toxicological properties of extenders used for the SC/SV of pet animals. For instance, polyvinyl alcohol (PVA) has been reportedly used as a non-EY-based extender (Hamad Talha et al., 2021), and its pH value for a 4% aqueous solution ranges from 5 to 8 along with strong hydrophilic behaviors (PubChem NTP; Gudeman and Peppas, 1995). Surprisingly, PVA shows contraceptive effects when used in products designed for intravaginal administration in human women (Sanders and Matthews, 1990); therefore, its HSDB information and complete separation from semen before AI should be studied carefully. Thus, the eutectic effects of extenders and CPAs must be investigated computationally and experimentally.
Several aspects of the toxicities of CPAs were explored in a seminal paper (Best, 2015). To inhibit ice formation during cryopreservation at cryogenic temperatures, the authors focused on penetrating CPAs; this work highlights that increased CPA concentration may lead to toxicity and suggests strategies to overcome this problem by optimizing the cooling and warming rates or optimizing the time of addition of the individual CPAs during cooling. One of the most striking features reported in Best (2015) was the classification of the toxicities into specific and non-specific categories; accordingly, toxicity can be specific to a particular CPA (specific toxicity) or a consequence of being a CPA (non-specific toxicity). More specifically, CPAs are believed to prevent ice formation by interfering with the hydrogen bonding between water molecules, and this effect has been noted to cause non-specific toxicity. Herein, we discuss both specific and non-specific toxicities of CPAs to offer researchers a knowledge-based computational roadmap for designing smart extenders or CPAs. Therefore, we focus on the toxicities of common small-molecule CPAs used in carnivorous SC/SV.
It is recommended to remove CPAs during thawing and before AI; however, the success rate of this operation cannot be determined precisely. Clinicians often do not pursue this step and employ CPA-contaminated semen in AI that can cause cellular toxicities in both spermatozoa and female reproductive systems, thereby resulting in lower fertility rates or teratogenic sequelae. The latter should be discussed separately and is not a goal in our hybrid approach integrating a systematic review with computational efforts. Therefore, it is essential to compute the toxicities of CPAs or novel extenders before their use in all assisted reproductive technologies (ARTs) such as SC/SV (Dohle, 2010). To address these concerns, the physicochemical, thermodynamic, and toxicological properties of small-molecule CPAs that have contributed to the rapid evaluation, validation, and preclinical assessments of CPAs are exemplified and discussed in this work.
Generally, CPAs can be categorized into intracellular (endocellular and permeating) and extracellular (exocellular and non-permeating) types depending on their ability to penetrate the plasma membranes of spermatozoa. Both EG and Gly are permeating CPAs that can reduce the concentrations of electrolytes and prevent cell shrinkage in a hypertonic solution (Zhmakin, 2009). If intracellular CPAs cannot efflux the cell quickly enough during thawing, free water may rush into the spermatozoa and the resulting cell swelling may cause cytolysis (Zhmakin, 2009). In this context, molecular PSA, i.e., the surface area of the polar atoms, is a descriptor that correlates passive molecular transport through the membrane for predicting the transport property of a chemical (Ertl et al., 2000). The TPSA of both EG and Gly is less than 100 Å2; therefore, they can cross the cell membrane more easily than CPAs with TPSAs greater than 100 Å2. Interestingly, the wide range of TPSAs of the CPAs used for carnivores has led us to conclude that CPAs cannot be categorized based on their TPSA datasets because they do not represent a rule of thumb. The hydroxyl groups of EG or Gly make them polar substances that allow penetration of the cell membrane because of their low molecular weights. The molecular mass of a penetrating CPA is typically less than 100 Da (Wowk, 2007); however, we found that other physicochemical properties could facilitate CPA penetration of the spermatozoa. The melting point of a semen sample decreases after appropriate mixing with a CPA; however, the impact of the melting point of a CPA on spermatozoa must be discussed. The freezing point depression of a semen sample is dependent on the concentrations of the added solutes; however, its dependency on the innate melting or freezing points of the added CPAs has not been discussed experimentally. Thus, EG, MF, and DMF should be under the ice point (0°C) among the selected CPAs. The prediction of the cryogenic potential of a CPA is based on its innate or acquired physicochemical properties, which require more thermodynamic investigations.
Based on the results of the computational thermodynamic properties, Fru, Glc, and CA do not show proton affinities, whereas EG and Gly are protonated. As a result, the low-affinity systems of Fru, Glc, and CA are not more diffusional but rather dependent on metabolic energy and hence transported actively. When a system in a state of dynamic equilibrium is acted upon by stress (e.g., a change in concentration, pressure, or temperature), the equilibrium will change the temperature to minimize the effects of the stress, according to Le Châtelier’s principle (Quílez-Pardo and Solaz-Portolés, 1995). Therefore, thermodynamic studies of CPAs highlight the importance of using temperature-dependent thermal characteristics to forecast the thermal history accurately.
A small molecule (or metabolite) is a low-molecular-weight organic compound that is typically involved in a biological process as a substrate or product. Metabolomics frequently focuses on small molecules with masses in the range of 50–1,500 Da (https://www.ebi.ac.uk/). The molecular weights of all selected small-molecule CPAs in this work were less than 1,500 Da; therefore, as endogenous (e.g., Gly) or natural or synthetic exogenous (e.g., Sln and DMF) metabolites, these can be analyzed with software and metabolomics servers for further feature findings (data not presented here).
Among the CPAs that have been reported for carnivorous SC/SV, GSH, DMF, MF, and DMSO are PGP (transmembrane efflux pump) substrates and cannot penetrate the biological membranes (e.g., spermatozoa plasma membrane) (Mealey and Fidel, 2015) of cryopreserved semen in situations where repeated services are required. All reported CPAs were not PGP I and PGP II inhibitors in this review; therefore, they cannot interfere with the absorption of other medications by inhibiting the transmembrane efflux pump. None of the selected CPAs can penetrate skin, and if they remain in the thawed semen, their absorption through the skin tissues of the reproductive tracts of carnivores would not be problematic. To the best of our knowledge, there are no comparable and relevant data regarding the importance of logKp for CPAs. Clinically, accidental rupture of the rectal tissue during AI may be relevant to this finding, which could lead to intestinal absorption or possible specific toxicity. To investigate this, the Caco2 cell line is widely used as an in vitro model for predicting human drug absorption. The translation of the findings from computational and experimental data to spermatozoa permeation in the case of extracellular or intracellular CPAs requires further investigation. EG, DMSO, DMF, MF, DMA, MA, Fru, and Gly were investigated as water-soluble compounds, whereas Sln, Glc, CA, Gln, and GSH were investigated as poorly water-soluble compounds. Therefore, a drug-discovery-type approach must be used in the discovery and design of less-toxic CPAs (Murray and Gibson, 2022).
Extrapolation of the VDss and Fu results to the bipartite compartment of SP and spermatozoa may be erroneous at this point; however, this comparison may provide some cues regarding the distribution of CPAs between the SP and spermatozoa. The chemical composition of SP varies among carnivores and may influence SC/SV with/without CPA addition. Primarily, SP is a heterogeneous medium containing an array of biochemical components, including ions, energy substrates, organic compounds, nitrogenous components, and reducing substances (Juyena and Stelletta, 2012; Aisen et al., 2021). We cannot rule out the interactions between the CPAs and components (mainly proteins) of the SP and spermatozoa. Therefore, the distribution of CPAs between the SP and spermatozoa cytoplasm as well as the ratios of unbounded to bounded fractions of the CPAs are unexplained aspects of SC/SV that should be modeled, computed, and validated (Figure 6). In summary, it appears that some CPAs are distributed in various compartments and could alter the rheological properties of both SP and spermatozoa during SC/SV. The low VDss values of all CPAs except Glc indicate high water solubility or high plasma protein binding because the CPAs remain predominantly in the plasma; conversely, a high VDss value implies significant concentration in the tissues, for example, due to tissue binding or high lipid solubility. The relevancy of the data obtained computationally for BBB permeability (logBB), CNS permeability (logPs), and total clearance (log mL/min/kg) for the CPAs are obscured at this step; however, similar definitions such as the blood–testis barrier, spermatozoa cell membrane permeability, and post-SC/SV alterations may be explored computationally in the future (Antonouli et al., 2024).
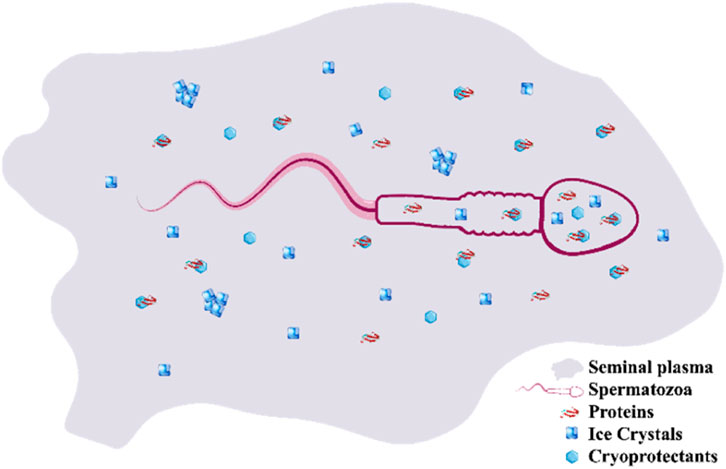
Figure 6. Bipartite compartment of spermatozoa and seminal plasma contains both penetrating and non-penetrating cryoprotectants that are bound or unbound to proteins of the seminal plasma or membrane of the spermatozoa.
The cellular locations of small-molecule CPAs are predicted in this study. Accordingly, none of the CPAs are localized in the cell membrane, while EG, CA, GSH, DMF, DMA, MA, and DMSO are localized in the cytoplasm. Moreover, EG, Gly, Fru, Glc, CA, Gln, GSH, DMF, MF, and DMSO are localized in the extracellular compartment. Some CPAs are also localized in the mitochondria (e.g., Gly, CA, Gln, and GSH), lysosomes, Golgi apparatus (e.g., Glc), and endoplasmic reticulum (e.g., GSH and Glc). Therefore, the bioaccumulation of a CPA in any cellular compartment would determine its specific toxicity.
In a pioneering work, it was reported that GSH could lead to mutagenicity even at the normal levels found in mammalian tissues (Glatt et al., 1983). Glc and Gln showed hERG I inhibitory effects, whereas the remaining CPAs showed no hERG I or hERG II inhibitory effects. hERG is also expressed in the heart tissues of dogs and is known as a canonical target for screening the pro-arrhythmogenic and non-arrhythmogenic activities of HERG-blocking agents (Schneider et al., 2005). The relevancy of hERG may seem vague for the evaluation of CPA toxicity; however, when tracing the toxicodynamics of CPAs in spermatozoa, an array of ion channels such as the Na/K-ATPase (NKA) and sperm-specific cation channel (Catsper channel) (Sultana Syeda et al., 2020; Sun et al., 2017) would be candidates because their blockage interferes with the normal (electro)physiology of spermatozoa during their voyage from the testes to the fertilization sites in the uterine tubes.
Newtonian and non-Newtonian fluids or extenders may alter the viability of spermatozoa in different ways because they offer spermatozoa a new milieu for swimming. The extender compositions and CPAs employed in SC/SV are the main determinants of a successful ART program and can avoid iatrogenic male-factor failure in this regard. The systematic review presented herein shows that the addition of glycerol (glycerinating) and EY (luteinizing) are the two common methods of preparing semen extenders in over 60 years of research on carnivorous SC/SV. Thermoinjuries including cryoinjury and pyroinjury are the major technological and methodological barriers in the smart design of semen extenders. To overcome these thermoinjuries, the specific and non-specific toxicities of CPAs should be determined and optimized as the core concepts of cell cryopreservation. In this context, non-specific toxicity is caused by ice crystallization, while specific toxicity is dependent on the innate toxic properties of the CPAs of interest and are related to their concentrations. Herein, we attempted to include both types of toxicities of CPAs to build an applied-knowledge-based computational package for designing smart extenders or CPAs.
In the present study, we introduce several biophysical, thermodynamic, and biological features that can shed light on semen cryobiotechnology; however, the impetus to curate and integrate a reference database is heavily sensed here. When antibiotics are present in the mixture of semen and extenders, the biophysical properties of SC/SV of semen and spermatozoa may be altered in addition to the antimicrobial effects. In some cases, clinicians may have to repeat AI owing to the low infertility rates of packs or pets or the occurrences of enzootic diseases; therefore, evaluation of CPA toxicity should be considered among other factors.
IK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing–original draft. LM: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing–original draft. AS: Data curation, Formal analysis, Investigation, Methodology, Software, Writing–original draft. ZH: Data curation, Formal analysis, Investigation, Methodology, Software, Writing–original draft. HS: Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing–review and editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftox.2025.1477822/full#supplementary-material
AbdelHafez, F., Bedaiwy, M., El-Nashar, S. A., Sabanegh, E., and Desai, N. (2009). Techniques for cryopreservation of individual or small numbers of human spermatozoa: a systematic review. Hum. Reprod. update 15, 153–164. doi:10.1093/humupd/dmn061
Abe, Y., Asano, T., Wakasa, I., Kume, A., Yokozawa, S., Umemiya-Shirafuji, R., et al. (2020). Cryopreservation of canine spermatozoa using a skim milk-based extender and a short equilibration time. Reproduction Domest. Animals 55, 1548–1553. doi:10.1111/rda.13806
Aisen, E. G., Huanca López, W., Pérez Durand, M. G., Torres Mamani, E., Villanueva Mori, J. C., Ousset, M. J., et al. (2021). Spermatozoa obtained from alpaca vas deferens. Effects of seminal plasma added at post-thawing. Front. Veterinary Sci. 8, 611301. doi:10.3389/fvets.2021.611301
Alcaráz, L. P., Pereira, P. V. S., Oliveira, T. A., Correia, L. F. L., Vasconcelos, E. M., Brandão, F. Z., et al. (2023). Effect of the addition of antifreeze protein type I on the quality of post-thawed domestic cat epididymal sperm. Zygote Camb. Engl. 31 (3), 240–245. doi:10.1017/S0967199422000521
Alvarez-Rodríguez, M., Alvarez, M., Borragan, S., Martinez-Pastor, F., Holt, W. V., Fazeli, A., et al. (2013). The addition of heat shock protein HSPA8 to cryoprotective media improves the survival of brown bear (Ursus arctos) spermatozoa during chilling and after cryopreservation. Theriogenology 79, 541–550. doi:10.1016/j.theriogenology.2012.11.006
Antonouli, S., Di Nisio, V., Messini, C., Samara, M., Salumets, A., Daponte, A., et al. (2024). Sperm plasma membrane ion transporters and male fertility potential: a perspective under the prism of cryopreservation. Cryobiology 114, 104845. doi:10.1016/j.cryobiol.2023.104845
Axner, E., and Lagerson, E. (2016). Cryopreservation of dog semen in a tris extender with 1% or 2% soya bean lecithin as a replacement of egg yolk. Reproduction Domest. Animals 51, 262–268. doi:10.1111/rda.12675
Belala, R., Briand-Amirat, L., Martinot, A., Thorin, C., Michaud, S., Desherces, S., et al. (2019). A comparison of liquid and lyophilized egg yolk plasma to low density lipoproteins for freezing of canine spermatozoa. Reproduction Domest. Animals 54, 1131–1138. doi:10.1111/rda.13476
Belala, R., Briand-Amirat, L., Vinciguerra, L., Tainturier, D., Kaidi, R., Thorin, C., et al. (2016). Effect of equilibration time on the motility and functional integrity of canine spermatozoa frozen in three different extenders. Res. Veterinary Sci. 106, 66–73. doi:10.1016/j.rvsc.2016.03.010
Bencharif, D., Amirat-Briand, L., Garand, A., Anton, M., Schmitt, E., Desherces, S., et al. (2010). Freezing canine sperm: comparison of semen extenders containing Equex and LDL (Low Density Lipoproteins). Animal reproduction Sci. 119, 305–313. doi:10.1016/j.anireprosci.2010.01.009
Bencharif, D., Amirat-Briand, L., Le Guillou, J., Garand, A., Anton, M., Schmitt, E., et al. (2013). Canine-chilled sperm: study of a semen extender made with low-density lipoproteins from hen egg yolk supplemented with glutamine. Reproduction Domest. Animals 48, 258–266. doi:10.1111/j.1439-0531.2012.02142.x
Bencharif, D., and Dordas-Perpinya, M. (2020). Canine semen cryoconservation: emerging data over the last 20 years. Reproduction Domest. Animals 55, 61–65. doi:10.1111/rda.13629
Best, B. P. (2015). Cryoprotectant toxicity: facts, issues, and questions. Rejuvenation Res. 18, 422–436. doi:10.1089/rej.2014.1656
Brusentsev, E., Kizilova, E., Mokrousova, V., Kozhevnikova, V., Rozhkova, I., and Amstislavsky, S. (2018). Characteristics and fertility of domestic cat epididymal spermatozoa cryopreserved with two different freezing media. Theriogenology 110, 148–152. doi:10.1016/j.theriogenology.2017.12.038
Buranaamnuay, K. (2015). Determination of appropriate cryopreservation protocols for epididymal cat spermatozoa. Reproduction Domest. Animals 50, 378–385. doi:10.1111/rda.12496
Caturla-Sánchez, E., Sánchez-Calabuig, M., Pérez-Gutiérrez, J., Cerdeira, J., Castaño, C., and Santiago-Moreno, J. (2018). Vitrification of dog spermatozoa: effects of two cryoprotectants (sucrose or trehalose) and two warming procedures. Cryobiology 80, 126–129. doi:10.1016/j.cryobiol.2017.11.001
Cavalcanti, T. P., Pereira, A. G., Bezerra, L. G. P., Moreira, S. S. J., da Silva, A. M., Matos, Y. G., et al. (2023). Short-term preservation of canine sperm-binding ability and other metrics using the INRA-96 in comparison to Tris-egg yolk extender. Reproduction Domest. animals = Zuchthygiene 58 (9), 1320–1329. doi:10.1111/rda.14448
Chatdarong, K., Thuwanut, P., Suksamai, P., Patanatiradaj, S., and Sangwornrachasup, A. (2009). Survival of frozen-thawed cat spermatozoa pre-cooled in the epididymides. Reproduction Domest. Animals 44, 377–380. doi:10.1111/j.1439-0531.2009.01412.x
Cheema, R. S., Kaur, S., Mavi, G. K., Singh, A. K., Honparkhe, M., and Gandotra, V. K. (2021). In vitro evaluation of Labrador dog spermatozoa cryopreserved in Tris-citric acid-fructose buffer supplemented with different combinations of extracellular and intracellular cryoprotectants. Anim. Biotechnol. 32, 352–365. doi:10.1080/10495398.2019.1698434
Cheng, T., Zhao, Y., Li, X., Lin, F., Xu, Y., Zhang, X., et al. (2007). Computation of octanol− water partition coefficients by guiding an additive model with knowledge. J. Chem. Inf. Model. 47, 2140–2148. doi:10.1021/ci700257y
Corcini, C., Goularte, K., Bongalhardo, D., Lucia, Jr T., Jardim, R., and Varela Junior, A. (2016). Effect of egg yolk plasma on dog sperm cryopreservation. Andrologia 48, 114–115. doi:10.1111/and.12411
Cramer, G., Ford, R., and Hall, R. (1976). Estimation of toxic hazard—a decision tree approach. Food Cosmet. Toxicol. 16, 255–276. doi:10.1016/s0015-6264(76)80522-6
Crosier, A. E., Pukazhenthi, B. S., Henghali, J. N., Howard, J., Dickman, A. J., Marker, L., et al. (2006). Cryopreservation of spermatozoa from wild-born Namibian cheetahs (Acinonyx jubatus) and influence of glycerol on cryosurvival. Cryobiology 52, 169–181. doi:10.1016/j.cryobiol.2005.10.011
Daina, A., Michielin, O., and Zoete, V. (2017). SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 7, 42717. doi:10.1038/srep42717
Daina, A., and Zoete, V. (2016). A boiled- egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 11 (11), 1117–1121. doi:10.1002/cmdc.201600182
Dalmazzo, A., de Souza Ramos Angrimani, D., Losano, J. D. A., Rocha, C. C., Sobrinho, C. A. B., Chinait Gurgel, J. R., et al. (2019). Insights into soy lecithin and egg yolk-based extenders for chilling canine spermatozoa. Zygote 27, 17–24. doi:10.1017/S0967199418000576
Dalmazzo, A., Losano, J. D. A., Rocha, C. C., Tsunoda, R. H., Angrimani, D. d. S. R., Mendes, C. M., et al. (2018). Effects of soy lecithin extender on dog sperm cryopreservation. Anim. Biotechnol. 29, 174–182. doi:10.1080/10495398.2017.1334662
da Silva, E. A., Corcini, C. D., de Assis Araújo Camelo Junior, F., Martins, D., Meneghello Gheller, S. M., Hädrich, G., et al. (2022). Probe ultrasonification of egg yolk plasma forms low-density lipoprotein nanoparticles that efficiently protect canine semen during cryofreezing. J. Biol. Chem. 298, 101975. doi:10.1016/j.jbc.2022.101975
de Sousa Barbosa, B., Izzo, R. G., Silva, H. V. R., Nunes, T. G. P., Brito, B. F., Silva, T. F. P. d., et al. (2020). Recovery and cryopreservation of epididymal sperm from domestic cat using powdered coconut water (ACP-117c) and TRIS extenders. Cryobiology 92, 103–108. doi:10.1016/j.cryobiol.2019.11.042
Divar, M. R., Mogheiseh, A., Mohammadi, F., and Mavalizadeh, L. (2023). Effects of extender filtration and egg yolk concentration on canine semen cryopreservation. Reproduction Domest. Animals 58, 272–287. doi:10.1111/rda.14284
Dohle, G. R. (2010). Male infertility in cancer patients: review of the literature. Int. J. urology 17, 327–331. doi:10.1111/j.1442-2042.2010.02484.x
Erdmann, R. H., Blank, M. H., Ribeiro, R. N., José de Oliveira, M., Cubas, Z. S., Pradiee, J., et al. (2020). Cryopreservation of margay (Leopardus wiedii) spermatozoa: effects of different extenders and frozen protocols. Theriogenology 143, 27–34. doi:10.1016/j.theriogenology.2019.11.032
Ertl, P., Rohde, B., and Selzer, P. (2000). Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 43, 3714–3717. doi:10.1021/jm000942e
Foote, R., and Leonard, E. (1964). The influence of pH, osmotic pressure, glycine, and glycerol on the survival of dog sperm in buffered-yolk extenders. Cornell Veterinarian 54, 78–89.
Franklin, A. D., Waddell, W. T., and Goodrowe, K. L. (2018). Red wolf (Canis rufus) sperm quality and quantity is affected by semen collection method, extender components, and post-thaw holding temperature. Theriogenology 116, 41–48. doi:10.1016/j.theriogenology.2018.05.007
Frisch Hbs, G. W. T. M. J., Scuseria, G. E., Robb, M. A., Cheeseman, J. R., Montgomery, J. A., Vreven, T., et al. (2009). Gaussian09, revision A. Wallingford CT: Inc.
Futino, D., Mendes, M., Matos, W., Mondadori, R., and Lucci, C. (2010). Glycerol, methyl-formamide and dimethyl-formamide in canine semen cryopreservation. Reproduction Domest. Animals 45, 214–220. doi:10.1111/j.1439-0531.2008.01208.x
Gadaleta, D., Vuković, K., Toma, C., Lavado, G. J., Karmaus, A. L., Mansouri, K., et al. (2019). SAR and QSAR modeling of a large collection of LD50 rat acute oral toxicity data. J. Cheminform. 11, 58. doi:10.1186/s13321-019-0383-2
Galarza, D. A., Landi, G., Mejía, E., Samaniego, J. X., Méndez, S., Soria, M. E., et al. (2021). Cryopreservation of dog epididymal spermatozoa by conventional freezing or ultra-rapid freezing with nonpermeable cryoprotectant. Cryobiology 103, 15–21. doi:10.1016/j.cryobiol.2021.10.002
Garrido, A., Lepailleur, A., Mignani, S. M., Dallemagne, P., and Rochais, C. (2020). hERG toxicity assessment: useful guidelines for drug design. Eur. J. Med. Chem. 195, 112290. doi:10.1016/j.ejmech.2020.112290
Glatt, H., Protić-Sabljić, M., and Oesch, F. (1983). Mutagenicity of glutathione and cysteine in the Ames test. Science 220, 961–963. doi:10.1126/science.6342137
Gloria, A., Zambelli, D., Carluccio, A., Cunto, M., Ponzio, P., and Contri, A. (2020). Is the protective effect of egg yolk against osmotic and cryogenic damage on dog spermatozoa dose-dependent? Animal reproduction Sci. 213, 106259. doi:10.1016/j.anireprosci.2019.106259
Gorman, G. S., Speir, J. P., Turner, C. A., and Amster, I. J. (1992). Proton affinities of the 20 common. alpha.-amino acids. J. Am. Chem. Soc. 114, 3986–3988. doi:10.1021/ja00036a062
Gudeman, L. F., and Peppas, N. A. (1995). Preparation and characterization of pH-sensitive, interpenetrating networks of poly (vinyl alcohol) and poly (acrylic acid). J. Appl. Polym. Sci. 55, 919–928. doi:10.1002/app.1995.070550610
Hamad Talha, N. A., Jeon, Y., and Yu, I.-J. (2021). Cryopreservation of dog spermatozoa using essential and non-essential amino acids solutions in an egg yolk-free polyvinyl alcohol extender. CryoLetters 42, 44–52.
Hermansson, U., and Axnér, E. (2007). Epididymal and ejaculated cat spermatozoa are resistant to cold shock but egg yolk promotes sperm longevity during cold storage at 4 degrees C. Theriogenology 67, 1239–1248. doi:10.1016/j.theriogenology.2007.01.008
Hermansson, U., Johannisson, A., and Axnér, E. (2021). Cryopreservation of dog semen in a Tris extender with two different 1% soybean preparations compared with a Tris egg yolk extender. Veterinary Med. Sci. 7, 812–819. doi:10.1002/vms3.445
Hernández-Avilés, C., Ruíz-Cristancho, A., Vergara-Galván, M., Zambrano-Varón, J., and Jiménez-Escobar, C. (2020). The Effect of NN-dimethylformamide on the membrane characteristics of canine spermatozoa after cryopreservation, and its relationship with post-thaw motility. Top. companion animal Med. 38, 100372. doi:10.1016/j.tcam.2019.100372
Holst, B. S., Larsson, B., Linde-Forsberg, C., and Rodriguez-Martinez, H. (2000). Evaluation of chilled and frozen-thawed canine spermatozoa using a zona pellucida binding assay. J. reproduction Fertil. 119, 201–206. doi:10.1530/jrf.0.1190201
Hyakutake, T., Suzuki, H., and Yamamoto, S. (2015). Effect of non-Newtonian fluid properties on bovine sperm motility. J. biomechanics 48, 2941–2947. doi:10.1016/j.jbiomech.2015.08.005
Inanc, M. E., Tekin, K., Olgac, K. T., Yilmaz, B., Cil, B., Tasdemir, U., et al. (2018). Effect of cholesterol loaded cyclodextrin on semen cryopreservation of Aksaray Malakli shepherd dogs of different ages. Animal reproduction Sci. 193, 191–200. doi:10.1016/j.anireprosci.2018.04.068
Johnson, A. E., Freeman, E. W., Wildt, D. E., and Songsasen, N. (2014). Spermatozoa from the maned wolf (Chrysocyon brachyurus) display typical canid hyper-sensitivity to osmotic and freezing-induced injury, but respond favorably to dimethyl sulfoxide. Cryobiology 68, 361–370. doi:10.1016/j.cryobiol.2014.04.004
Juyena, N. S., and Stelletta, C. (2012). Seminal plasma: an essential attribute to spermatozoa. J. Androl. 33, 536–551. doi:10.2164/jandrol.110.012583
Kawasaki, Y., Sakurai, D., Yoshihara, T., Tsuchida, M., Harakawa, S., and Suzuki, H. (2020). Effect of quercetin on the motility of cryopreserved canine spermatozoa. Cryobiology 96, 50–54. doi:10.1016/j.cryobiol.2020.08.006
Khan, J., Tahir, M., Khalid, A., Sattar, A., and Ahmad, N. (2017). Effect of cholesterol-loaded cyclodextrins on cryosurvival of dog spermatozoa. Reproduction Domest. Animals 52, 265–268. doi:10.1111/rda.12893
Kurniani Karja, N. W., Fahrudin, M., Setiadi, M. A., Tumbelaka, L. I., Sudarwati, R., Hastuti, Y. T., et al. (2016). Characteristics and fertility of Sumatran tiger spermatozoa cryopreserved with different sugars. Cryoletters 37, 264–271.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6 (7), e1000100. doi:10.1371/journal.pmed.1000100
Lima, S. L., Soares, A. R., Stalker, L., Santos, R. R., and Domingues, S. F. (2021). Epididymal tail solid-surface vitrification as an effective method for domestic cat sperm cryobanking. Zygote 29, 452–458. doi:10.1017/S096719942100006X
Lucio, C. F., Silva, L. C. G., Regazzi, F. M., Angrimani, D. S. R., Nichi, M., Assumpção, M. E. O., et al. (2016). Effect of reduced glutathione (GSH) in canine sperm cryopreservation: in vitro and in vivo evaluation. Cryobiology 72, 135–140. doi:10.1016/j.cryobiol.2016.02.001
Luvoni, G. C. (2006). Gamete cryopreservation in the domestic cat. Theriogenology 66, 101–111. doi:10.1016/j.theriogenology.2006.03.012
Madrigal-Valverde, M., Bittencourt, R. F., de Lisboa Ribeiro Filho, A., Araujo, G. R., Lents, M. P., Santos, E. S., et al. (2020). Can amides be alternative cryoprotectors for the preservation of feline semen? Cryobiology 97, 138–143. doi:10.1016/j.cryobiol.2020.09.004
Mane, N. S., Shah, K., Mane, S., Banerjee, A., and Tripathi, S. (2023). “Effect of Newtonian and shear thinning medium on human sperm motion within a microchannel,” in Fluid mechanics and fluid power (vol. 2). FMFP 2021. Lecture notes in mechanical engineering. Editors S. Bhattacharyya, and A. C. Benim (Singapore: Springer). doi:10.1007/978-981-19-6970-6_71
Martins-Bessa, A., Rocha, A., and Mayenco-Aguirre, A. (2009). Effects of taurine and hypotaurine supplementation and ionophore concentrations on post-thaw acrosome reaction of dog spermatozoa. Theriogenology 71, 248–253. doi:10.1016/j.theriogenology.2008.07.006
Maurya, R., and Pandey, A. K. (2020). Importance of protozoa Tetrahymena in toxicological studies: a review. Sci. Total Environ. 741, 140058. doi:10.1016/j.scitotenv.2020.140058
Mealey, K. L., and Fidel, J. (2015). P-glycoprotein mediated drug interactions in animals and humans with cancer. J. Vet. Intern. Med. 29 (1), 1–6. doi:10.1111/jvim.12525Q13
Mota Filho, A., Teles, C. H. A., Jucá, R. P., Cardoso, J. F. S., Uchoa, D. C., Campello, C. C., et al. (2011). Dimethylformamide as a cryoprotectant for canine semen diluted and frozen in ACP-106C. Theriogenology 76, 1367–1372. doi:10.1016/j.theriogenology.2011.05.010
Munro, I. C., Ford, R. A., Kennepohl, E., and Sprenger, J. (1996). Correlation of structural class with no-observed-effect levels: a proposal for establishing a threshold of concern. Food Chem. Toxicol. 34, 829–867. doi:10.1016/s0278-6915(96)00049-x
Murray, K. A., and Gibson, M. I. (2022). Chemical approaches to cryopreservation. Nat. Rev. Chem. 6 (8), 579–593. doi:10.1038/s41570-022-00407-4
Nguyen, V. V., Ponchunchoovong, S., Kupittayanant, S., and Kupittayanant, P. (2019). Effects of egg yolk and soybean lecithin on sperm quality determined by computer-assisted sperm analysis and confocal laser scanning microscope in chilled canine sperm. Veterinary Med. Sci. 5, 345–360. doi:10.1002/vms3.158
Nöthling, J., Dolieslager, S., Fillekes, R., and Colenbrander, B. (2007). Thawing dog spermatozoa in just-boiled water: submersion time and effect on sperm quality compared to thawing in water at 70 degrees C. Theriogenology 68, 530–537. doi:10.1016/j.theriogenology.2007.04.047
Patlewicz, G., Jeliazkova, N., Safford, R., Worth, A., and Aleksiev, B. (2008). An evaluation of the implementation of the Cramer classification scheme in the Toxtree software. SAR QSAR Environ. Res. 19, 495–524. doi:10.1080/10629360802083871
Peña, A., and Linde-Forsberg, C. (2000). Effects of spermatozoal concentration and post-thaw dilution rate on survival after thawing of dog spermatozoa. Theriogenology 54, 703–718. doi:10.1016/s0093-691x(00)00384-8
Pires, D. E., Blundell, T. L., and Ascher, D. B. (2015). pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 58, 4066–4072. doi:10.1021/acs.jmedchem.5b00104
Quílez-Pardo, J., and Solaz-Portolés, J. J. (1995). Students' and teachers' misapplication of Le Chatelier's principle: implications for the teaching of chemical equilibrium. J. Res. Sci. Teach. 32, 939–957. doi:10.1002/tea.3660320906
Rahman, M. A., Park, S.-H., and Yu, I.-J. (2017). Effect of monosaccharides in glycerol-free tris extender on reactive oxygen species and apoptosis in dog sperm cryopreservation. CryoLetters 38, 51–57.
Rahman, M. A., Park, S.-H., and Yu, I.-J. (2018). Dog sperm cryopreservation in glucose-fructose or sucrose supplemented glycerol-free tris: effect of post-thaw incubation time on gene expression related to apoptosis and motility. Cryoletters 39, 45–52.
Sánchez, R., Risopatrón, J., Schulz, M., Villegas, J., Isachenko, V., Kreinberg, R., et al. (2011). Canine sperm vitrification with sucrose: effect on sperm function. Andrologia 43, 233–241. doi:10.1111/j.1439-0272.2010.01054.x
Sánchez-Calabuig, M. J., Maillo, V., Beltrán-Breña, P., de la Fuente Martínez, J., Galera-Carrillo, S., Pérez-Gutiérrez, J. F., et al. (2017). Cryopreservation of canine sperm using egg yolk and soy bean based extenders. Reprod. Biol. 17, 233–238. doi:10.1016/j.repbio.2017.05.007
Sanders, J., and Matthews, H. (1990). Vaginal absorption of polyvinyl alcohol in Fischer 344 rats. Hum. and Exp. Toxicol. 9, 71–77. doi:10.1177/096032719000900202
Schäfer-Somi, S., Binder, C., Burak, J., Papadopoulos, N., Ilas, J., Boersma, A., et al. (2021). Using egg yolk in a TRIS-Equex STM paste extender for freezing of dog semen is superior to egg yolk plasma, also after addition of lecithin and catalase. Cryobiology 100, 63–71. doi:10.1016/j.cryobiol.2021.03.009
Schneider, J., Hauser, R., Andreas, J. O., Linz, K., and Jahnel, U. (2005). Differential effects of human ether-a-go-go-related gene (HERG) blocking agents on QT duration variability in conscious dogs. Eur. J. Pharmacol. 512 (1), 53–60. doi:10.1016/j.ejphar.2005.01.042
Setyawan, E. M. N., Kim, M. J., Oh, H. J., Kim, G. A., Jo, Y. K., Lee, S. H., et al. (2015). Maintaining canine sperm function and osmolyte content with multistep freezing protocol and different cryoprotective agents. Cryobiology 71, 344–349. doi:10.1016/j.cryobiol.2015.08.010
Shayne, C. (2024). “Gad. Maximum tolerated dose,” in Philip wexler, encyclopedia of toxicology. Fourth Edition. Academic Press, 43–44. doi:10.1016/B978-0-12-824315-2.00532-7
Silva, H. V. R., Nunes, T. G. P., Brito, B. F., Campos, L. B., Silva, A. M. d., Silva, A. R., et al. (2020). Influence of different extenders on morphological and functional parameters of frozen-thawed spermatozoa of jaguar (Panthera onca). Cryobiology 92, 53–61. doi:10.1016/j.cryobiol.2019.10.195
Songsasen, N., Yu, I., Murton, S., Paccamonti, D. L., Eilts, B. E., Godke, R. A., et al. (2002). Osmotic sensitivity of canine spermatozoa. Cryobiology 44, 79–90. doi:10.1016/S0011-2240(02)00009-3
Strzeżek, R., and Reksa, A. (2022). Effect of different egg yolk sources on dog semen quality following cryopreservation. Pol. J. Veterinary Sci. 25, 187–189. doi:10.24425/pjvs.2022.140856
Sultana Syeda, S., Sánchez, G., McDermott, J. P., Hong, K.Ho, Blanco, G., and Georg, G. I. (2020). The Na+ and K+ transport system of sperm (ATP1A4) is essential for male fertility and an attractive target for male contraception. Biol. Reproduction 103 (2), 343–356. doi:10.1093/biolre/ioaa093
Sun, L., Wu, C., Xu, J., Zhang, S., Dai, J., and Zhang, D. (2020). Addition of butylated hydroxytoluene (BHT) in tris-based extender improves post-thaw quality and motion dynamics of dog spermatozoa. Cryobiology 97, 71–75. doi:10.1016/j.cryobiol.2020.10.006
Sun, Xh., Zhu, Yy., Wang, L., Liu, H. L., Ling, Y., Li, Z. L., et al. (2017). The Catsper channel and its roles in male fertility: a systematic review. Reprod. Biol. Endocrinol. 15, 65. doi:10.1186/s12958-017-0281-2
Talha, N. A. H., Jeon, Y., and Yu, I. J. (2022). Effect of synthetic cholesterol (Synthechol®) supplementation in an egg yolk-free extender on dog sperm cryopreservation. Cryoletters 43, 99–109. doi:10.54680/fr22210110212
Tebet, J. M., Ferreira de Souza, F., Mello Martins, M. I., Chirinéa, V. H., Candido de Carvalho, J., Papa, F. O., et al. (2022). Assessment of thawed sperm quality from feline species: ocelot (Leopardus pardalis) and oncilla (Leopardus gutullus). Theriogenology 177, 56–62. doi:10.1016/j.theriogenology.2021.10.004
Van den Berghe, F., Paris, M. C. J., Briggs, M. B., Farstad, W. K., and Paris, DBBP (2018). A two-step dilution tris-egg yolk extender containing Equex STM significantly improves sperm cryopreservation in the African wild dog (Lycaon pictus). Cryobiology 80, 18–25. doi:10.1016/j.cryobiol.2017.12.095
Villaverde, AISB, Fioratti, E. G., Penitenti, M., Ikoma, M. R. V., Tsunemi, M. H., Papa, F. O., et al. (2013). Cryoprotective effect of different glycerol concentrations on domestic cat spermatozoa. Theriogenology 80, 730–737. doi:10.1016/j.theriogenology.2013.06.010
Vizuete, G., Jiménez, E., Agüera, E., and Pérez-Marín, C. (2014). Impact of ultra-rapid freezing on the motility, morphology, viability and acrosome integrity of epididymal cat sperm diluted in sucrose-based extenders. Reproduction Domest. animals 49, e5–e8. doi:10.1111/rda.12253
Wang, D.-H., Liu, Y. L., Cai, Z. G., An, J. H., Lan, J. C., Chen, J. S., et al. (2020). Effects of extender type on the quality of post-thaw giant panda (Ailuropoda melanoleuca) semen. Cryobiology 94, 95–99. doi:10.1016/j.cryobiol.2020.04.003
Yu, I.-J. (2014). Canine sperm cryopreservation using glucose in glycerol-free Tris. CryoLetters 35, 101–107.
Zambelli, D., Raccagni, R., Cunto, M., Andreani, G., and Isani, G. (2010). Sperm evaluation and biochemical characterization of cat seminal plasma collected by electroejaculation and urethral catheterization. Theriogenology 74, 1396–1402. doi:10.1016/j.theriogenology.2010.06.011
Zeiger, E. (2019). The test that changed the world: the Ames test and the regulation of chemicals. Mutat. Research/Genetic Toxicol. Environ. Mutagen. 841, 43–48. doi:10.1016/j.mrgentox.2019.05.007
Keywords: spermatozoa, cryoprotectant, cryopreservation, seminal plasma, carnivores, thermodynamics, toxicology
Citation: Karimi I, Mohammad LJ, Suvitha A, Haidari Z and Schiöth HB (2025) Comprehensive overview of the toxicities of small-molecule cryoprotectants for carnivorous spermatozoa: foundation for computational cryobiotechnology. Front. Toxicol. 7:1477822. doi: 10.3389/ftox.2025.1477822
Received: 09 August 2024; Accepted: 20 January 2025;
Published: 26 February 2025.
Edited by:
Rosaria Meccariello, University of Naples Parthenope, ItalyReviewed by:
Bupesh Giridharan, Nagaland University, IndiaCopyright © 2025 Karimi, Mohammad, Suvitha, Haidari and Schiöth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helgi B. Schiöth, aGVsZ2kuc2NoaW90aEB1dS5zZQ==; Isaac Karimi, aXNhYWNfa2FyaW1pMjAwMEB5YWhvby5jb20=, a2FyaW1paXNhYWNAcmF6aS5hYy5pcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.