1 Introduction
Ah receptor (AHR) is a ligand-dependent transcription factor and environmental sensor (Poland et al., 1976; Gu et al., 2000). The receptor has been discovered in studies of toxicity of the persistent AHR ligand TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) leading to deregulation of the receptor. Recently, a number of physiological receptor functions have been identified. One of its multiple physiological functions is involved in intestinal barrier integrity and host-microbiome interaction (Stockinger et al., 2021; Bock, 2020). The microbiome of human colon includes anaerobic archaea and bacteria generating vitamin B12 (Mahammadzadeh et al., 2022; Sokolovskaya et al., 2020). Surprisingly, vitamin B12 has been recently identified as natural AHR antagonist (Kim et al., 2020). This important finding appears to be unrecognized by many scientists in the AHR field. A previous minireview (Bock, 2023) and the present opinion on links between Ah receptor, vitamin B12 and itaconate are intended to stimulate the interest in the unsolved finding that vitamin B12 is a direct antagonist of the AHR.
2 Links between Ah receptor, vitamin B12 and itaconate
2.1 Vitamin B12 and folic acid as natural AHR antagonists
Both vitamin B12 and folic acid have been identified as natural antagonists of the ligand-modulated AHR (Kim et al., 2020). Indead, the two vitamins directly bind to the receptor in the cytosol, blocking AHR’s nuclear translocation and transcriptional signaling. In this way, they possibly compete with many environmental toxic ligands such as TCDD, dietary phytochemicals and microbial products including indoles and pigmented virulence factors (Moura-Alves et al., 2014). Vitamin B12 and folic acid are known to be cofactors of the enzyme methionine synthase in the one-carbon cycle facilitating the de novo synthesis of nucleotides and methylation of DNA and protein (Ducker and Rabinowitz, 2017). Here, the focus is on B12-dependent methylmalonyl-CoA mutase (MUT) that is involved in lipid metabolism, particularly in converting propionyl-CoA to the TCA cycle metabolite succinyl-CoA. However, the second vitamin B12-dependent enzyme, methionine synthase, should not be neglected in studies of possible links between AHR and vitamin B12, as discussed in section 3.
2.2 Decreased vitamin B12 due to generation of toxic itaconyl-CoA in mitochondria
It has been demonstrated that macrophages activated by microbial pathogen-associated molecular patterns and cytokines upregulate expression of the enzyme cis-aconitate decarboxylase (CAD), also termed ACOD1 and Irg1, generating the antimicrobial and antiinflammatory itaconate (Michelucci et al., 2013; Lang and Siddique, 2024). CAD is induced by the key antioxidant regulator Nrf2 (Mills et al., 2018) and by AHR since Nrf2 has been demonstrated to operate in mutual crosstalk with the AHR (Miao et al., 2005; Shin et al., 2007). Increased itaconate levels in mitochondria lead to toxic itaconyl-CoA that has been demonstrated to form a stable biradical when bound to the enzyme MUT leading to decreased vitamin B12 (Shen et al., 2017; Ruetz et al., 2019).
The toxic reaction of itaconyl-CoA with MUT is supported by studies of TCDD-treated mice. These studies demonstrated that B12-dependent MUT activity is inhibited in mitochondria, leading to decreased vitamin B12 serum levels thereby redirecting propionyl-CoA metabolism to an alternative ß-oxidation-like pathway resulting in acrylyl-CoA conjugate buildup leading to non-alcoholic fatty liver (NAFLD) (Orlowska et al., 2022).
2.3 Decreased vitamin B12 leading to death of macrophage-ingested bacteria
Importantly, decreased B12 levels in macrophages also inhibit survival of macrophage-ingested bacteria (Figure 1) (Ryan et al., 2020). This inhibition is necessary since bacteria such as M. tuberculosis managed to degrade itaconate (Sasikaran et al., 2014). Macrophages may temporarily tolerate a localized decrease of B12.
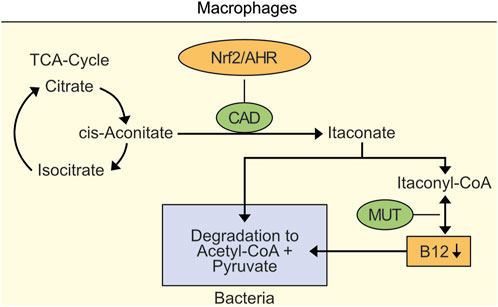
Figure 1. Localized decrease of vitamin B12 in macrophages as an example for inhibition of survival of macrophage-ingested bacteria. Nrf2- and AHR-induced cis-aconitate decarboxylase (CAD) leads to accumulation of itaconate and toxic itaconyl-CoA. Toxic Itaconyl-CoA has been demonstrated to decrease B12 levels by inhibiting methylmalonyl-CoA mutase (MUT). Importantly, decreased B12 levels inhibit survival of macrophase-ingested bacteria.
3 Links between AHR and vitamin B12 signalling in intestinal tissue repair
As mentioned in the introduction, AHR is involved in intestinal barrier integrity and host-microbiome interaction (Stockinger et al., 2021). In particular, AHR signalling is required for the resolution of injury-induced colonic stem cells (Sha et al., 2022). In this context it is interesting that vitamin B12 is a limiting factor for induced cellular plasticity and tissue repair. In the dextran sulfate sodium model of acute ulcerative colitis serum vitamin B12 was observed to be depleted and vitamin B12 supplementation enhanced specific markers of epigenetic histone methylation (Kovatcheva et al., 2023). Hence, there are links between AHR and vitamin B12 signaling in the intestine.
4 Discussion
It is understood that both AHR and vitamin B12 are involved in complex, tissue-dependent signalling networks. Tissue-dependent links between AHR and the two vitamin B12-dependet enzymes, methylmalonyl-CoA mutase (MUT) and methionine synthase have been discussed here. Notably, persistent AHR activation has to be avoided since it may lead to toxicity and cancer. In studies using a constitutively-active AHR mutant it has been demonstrated that the gastric mucosa is particularly affected (Dantsuka et al., 2019). Interestingly, the gastric pump inhibitor omeprazole has been identified as AHR agonist (Diaz et al., 1990). The discussed opinion deals with tissue-dependent links between AHR, vitamin B12 and itaconate, for example, with findings how the decrease of vitamin B12 leads to death of macrophage-ingested bacteria. It is hoped the discussion may stimulate the interest of AHR scientists in attempts to substantiate and understand the surprising finding that vitamin B12 is a direct antagonist of the AHR (Kim et al., 2020).
Author contributions
KB: Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Valuable help of Thomas Staiger in preparing figure 1 is greatly appreciated.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bock, K. W. (2020). Aryl hydrocarbon receptor (AHR) functions: balancing opposing processes including inflammatory reactions. Biochem. Pharmacol. 178, 114093. doi:10.1016/j.bcp.2020.114093
Bock, K. W. (2023). Aryl hydrocarbon receptor (AHR): towards understanding intestinal microbial ligands including vitamin B12 and folic acid as natural antagonists. Biochem. Pharmacol. 214, 115658. doi:10.1016/j.bcp.2023.115658
Dantsuka, A., Ichii, O., Hanberg, A., Elewa, Y. H. A., Otsuka-Kanazawa, S., Nakamura, T., et al. (2019). Histopathological features of the proper gastric glands in FVB/N-background mice carrying constitutively-active aryl-hydrocarbon receptor. BMC Gastroenterol. 19, 102. doi:10.1186/s12876-019-1009-x
Diaz, D., Fabre, I., Daujat, M., Saint Aubert, B., Bories, P., Michel, H., et al. (1990). Omeprazole is an aryl hydrocarbon-like inducer of human hepatic cytochrome P450. Gastroenterology 99, 737–747. doi:10.1016/0016-5085(90)90963-2
Ducker, G. S., and Rabinowitz, J. D. (2017). One-carbon metabolism in health and disease. Cell Metab. 25, 27–42. doi:10.1016/j.cmet.2016.08.009
Gu, Y. Z., Hogenesch, J. B., and Bradfield, C. A. (2000). The PAS superfamily: sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 40, 519–561. doi:10.1146/annurev.pharmtox.40.1.519
Kim, D. J., Venkataraman, A., Jain, P. C., Wiesler, E. P., DeBlasio, M., Klein, J., et al. (2020). Vitamin B12 and folic acid alleviate symptoms of nutritional deficiency by antagonizing aryl hydrocarbon receptor. PNAS 117, 15837–15845. doi:10.1073/pnas.2006949117
Kovatcheva, M., Melendez, E., Chondronasiou, D., Pietrocola, F., Bernad, R., Caballe, A., et al. (2023). Vitamin B12 is a limiting factor for induced cellular plasticity and tissue repair. Nat. Metab. 5, 1911–1930. doi:10.1038/s42255-023-00916-6
Lang, R., and Siddique, MNAA (2024). Control of immune cell signaling by the immuno-metabolite itaconate. Front. Immunol. 15, 1352165. doi:10.3389/fimmu.2024.1352165
Mahammadzadeh, R., Mahnert, A., Duller, S., and Moissl-Eichinger, C. (2022). Archaeal key-residents within the human microbiome: characteristics, interactions and involvement in health and disease. Curr. Opin. Microbiol. 67, 102146. doi:10.1016/j.mib.2022.102146
Miao, W., Hu, L., Scrivens, J., and Batist, G. (2005). Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 280, 20340–20348. doi:10.1074/jbc.M412081200
Michelucci, A., Cordes, T., Ghelfi, J., Pailot, A., Reiling, O., Goldmann, O., et al. (2013). Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. PNAS 110, 7820–7825. doi:10.1073/pnas.1218599110
Mills, E. L., Ryan, D. G., Prag, H. A., Dikovskaya, D., Menon, D., Zaslona, Z., et al. (2018). Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 556, 113–117. doi:10.1038/nature25986
Moura-Alves, P., Fae, K., Houthuys, E., Dorhoi, A., Kreuchwig, A., Furkert, J., et al. (2014). AhR sensing of bacterial pigments regulates antibacterial defence. Nature 512, 387–392. doi:10.1038/nature13684
Orlowska, K., Fling, R. R., Nault, R., Sink, W. J., Schilmiller, A. L., and Zacharewski, T. (2022). Dioxin-elicited decrease in cobalamin redirects propionyl-CoA metabolism to the ß-oxidation-like pathway resulting in acrylyl-CoA conjugate buildup. J. Biol. Chem. 298, 192301. doi:10.1016/j.jbc.2022.102301
Poland, A., Glover, E., and Kende, A. S. (1976). Stereospecific high affinity binding of 2,3,7.8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 251, 4936–4946. doi:10.1016/s0021-9258(17)33205-2
Ruetz, M., Campanello, G. C., Purchal, M., Shen, H., McDevitt, L., Gouda, H., et al. (2019). Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science 366, 589–593. doi:10.1126/science.aay0934
Ryan, D. G., Frezza, C., and O'Neill, L. A. J. (2020). TCA cycle signalling and the evolution of eukaryotes. Curr. Opin. Biotechnol. 68, 72–88. doi:10.1016/j.copbio.2020.09.014
Sasikaran, J., Ziemski, M., Zadora, P. K., Fleig, A., and Berg, I. A. (2014). Bacterial itaconate degradation promotes pathogenicity. Nat. Chem. Biol. 10, 371–377. doi:10.1038/nchembio.1482
Sha, K., Maradana, M. R., Delas, M. J., Medidji, A., Graelmann, F., Llorian, M., et al. (2022). Cell-intrinsic aryl hydrocarbon receptor signalling is required for the resolution of injury-induced colonic stem cells. Nat. Commun. 13, 1827. doi:10.1038/s41467-022-29098-7
Shen, H., Campanello, G. C., Flicker, D., Grabarek, Z., Hu, J., Luo, C., et al. (2017). The human knockout gene CLYBL connects itaconate to vitamin B12. Cell 171, 771–782.e11. doi:10.1016/j.cell.2017.09.051
Shin, S., Wakabayashi, N., Misra, V., Biswal, S., Lee, G. H., Agoston, E. S., et al. (2007). Nrf2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol. Cell Biol. 27, 7188–7197. doi:10.1128/MCB.00915-07
Sokolovskaya, O. M., Shelton, A. N., and Taga, M. E. (2020). Sharing vitamins: cobamids unveil microbial interactions. Science, 369. doi:10.1126/science.aba0165
Keywords: Ah receptor, vitamin B12, itaconate, macrophages, bacteria
Citation: Bock KW (2024) Ah receptor, vitamin B12 and itaconate: how localized decrease of vitamin B12 prevents survival of macrophage-ingested bacteria. Front. Toxicol. 6:1491184. doi: 10.3389/ftox.2024.1491184
Received: 04 September 2024; Accepted: 28 November 2024;
Published: 11 December 2024.
Edited by:
Hardeep Singh Tuli, Maharishi Markandeshwar University, IndiaReviewed by:
Sandipan Mukherjee, University of Washington, United StatesCopyright © 2024 Bock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karl Walter Bock, Ym9ja0B1bmktdHVlYmluZ2VuLmRl
 Karl Walter Bock
Karl Walter Bock