- 1INAIL, National Institute for Insurance against Accidents at Work, Department of Occupational and Environmental Medicine, Epidemiology and Hygiene, Rome, Italy
- 2Department of Chemistry, University of Rome Sapienza, Rome, Italy
- 3Department of Neuroscience, Reproductive and Odontostomatological Sciences-Audiology Section Naples, University of Naples Federico II, Naples, Italy
- 4Catholic University of the Sacred Hearth, Faculty of Medicine and Surgery, Rome, Italy
Introduction: In the shipbuilding industry, workers are exposed to a variety of dangerous chemicals, styrene being one of them. The International Agency for Research on Cancer classified styrene as a chemical belonging to group 2A, which means it is probably carcinogenic to humans. This study aimed at evaluating the oxidative stress effects due to occupational exposure to styrene and other chemicals.
Materials and methods: Styrene urinary metabolites, such as mandelic acid and phenylglyoxylic acid, and the urinary biomarkers of oxidative stress, i.e., oxidation products of DNA and RNA and of proteins, were measured in a group of 17 workers and compared to the concentrations found in a group of 17 healthy volunteers who had not been exposed to chemicals.
Results and discussion: Statistically significant differences were found for 8-oxo-7,8-dihydroguanine (8-oxoGua) and 8-oxo-7,8-dihydro-2′-deoxiguanosine (8-oxodGuo) concentrations that are higher in workers than in the control group. The workers performing the tasks of painting are the most exposed to styrene and show higher concentrations of 8-oxo-7,8-dihydroguanosine (8-oxoGuo). Workers performing the tasks of wood refining and welding are less exposed to styrene but have higher concentrations of 8-oxoGua and 8-oxodGuo.
Conclusion: The exposure scenario in shipbuilding is a complex one, in which different xenobiotics are simultaneously present. The oxidative stress effect biomarkers, obtained from the oxidation product of RNA and DNA, are promising, sensitive, but not specific.
1 Introduction
The shipbuilding industry is a dynamic and competitive sector and is one of the oldest production industries, important from both an economic and social perspective. The production processes of shipyards are divided into two categories: the new shipbuilding and ship repair industry, both representing workplaces at a high risk of occupational diseases. The processes involve surface preparation, welding, painting, fiberglass manufacturing, molding, and solvent cleaning. All these involve the exposure of workers to several chemicals, such as degreasers, solvents, abrasives, and styrene vapors. The main health risk is associated to exposure to styrene vapors, solvents, and wood dust. Finally, exposure to fumes resulting from metal combustion during welding and cutting processes is also possible (Ünsalan and Celebi, 2010; Makarova and Tkalich, 2021).
Styrene is an aromatic hydrocarbon widely used in the production of plastics and used in the fiberglass-reinforced plastic industry, owing to the great reactivity of the vinyl double bond which makes it easily polymerizable and co-polymerizable, even at room temperature, but more rapidly at high temperatures (Rueff et al., 2009). The highest exposure to styrene was measured in occupational settings, particularly in the fabrication of reinforced plastic products (Paci et al., 2013; Kim, 2015; Banton et al., 2019). Styrene exposure occurs mainly through the inhalation of its vapors during lay-up or spray-up operations and lamination and curing steps (Lees et al., 2003; Rueff et al., 2009) and, to a minor extent, via skin contact (Costa et al., 2012). Exposure to styrene causes adverse effects in the peripheral and central nervous system, irritation of the skin and respiratory system, and mild liver damage; it is immediately absorbed through the skin and via the lungs, distributed in adipose tissues, and metabolized in the body. The International Agency for Research on Cancer (IARC) classified styrene as a chemical that is probably carcinogenic to humans, group 2A (IARC, 2019).
In humans, styrene is oxidized by cytochrome P-450-(CYP) into 7,8-styrene oxide (SO) which is considered to be directly responsible for the genotoxic effect. Then, SO is hydrolyzed by microsomal epoxide hydrolase (mEH) in phenyl ethylene glycol which is further metabolized to mandelic acid (MA) and phenylglyoxylic acid (PGA) (Carbonari et al., 2015).
MA and PGA represent 85% and 10% of the total amount of absorbed styrene excreted in urine, respectively (ACGIH, 2001). The dose biomarkers suggested by the American Conference of Governmental Industrial Hygienists (ACGIH) for occupational exposure are MA + PGA, with a BEI of 400 mg/g of creatinine or 0.2 mg/L of styrene in venous blood, both measured at the end of the work shift, corresponding to a threshold limit value–time-weighted average (TLV–TWA; concentration limit for a normal 8-h workday and a 40-h workweek to which nearly all workers may be repeatedly exposed, day after day, without adverse effects) of 85 mg/m3 which is equivalent to 20 ppm (ACGIH, 2020).
As previously stated, in addition to styrene, shipbuilding industry workers are also exposed to other dangerous volatile organic compounds (VOCs), some of which, according to the IARC, have both neurotoxic and carcinogenic properties. Benzene is classified by the IARC in group 1 (IARC, 2018), xylene and toluene are classified in group 3, and ethylbenzene is classified in group 2B (IARC, 2000; Cheng et al., 2019). To protect the health of workers, the airborne concentration levels of VOCs must comply with the occupational exposure limits (OELs) and the appropriate personal protective equipment must be worn if needed (Sisto et al., 2020).
In the shipbuilding industry, workers are also exposed to wood dust as carpentry activities are carried out. Wood dust comprises cellulose and other substances that depend on wood species. The size of the particles and their quantities depend on the operations during wood processing (Vallières et al., 2015).
Wood dust is classified by the IARC as carcinogenic, group 1, to humans for cancers of the nasal cavity, paranasal sinus, and nasopharynx, with an association between this type of cancer and exposure to hardwood dust (IARC, 2012). Exposure to hardwood dust may cause respiratory symptoms and diseases, the most serious health effect being the risk of nasal and sinonasal cancer. According to the Directive (EU) 2017/2398 (Directive EU, 2017/2398, 2017), the limit values for occupational exposure to hardwood dusts is 3 mg/m3 until 17 January 2023, and then, it will be reduced to 2 mg/m3. If hardwood dusts are mixed with other wood dusts, the limit value applies to all wood dusts present in that mixture.
Oxidative stress involves oxidative damage to nucleic acids, lipids, and proteins. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are the cause for the progression of several diseases, such as cardiovascular and neurodegenerative diseases, age-related diseases, alterations in the reproductive system, and cancer (Jacob et al., 2013; Pigini et al., 2022). In humans, the urinary biomarkers of oxidation of nucleic acids and proteins can be measured as the effect biomarkers of chemical exposures (Cavallo et al., 2021; Ghelli et al., 2021). They are formed as a result of the reaction of the hydroxyl radical •OH generated in cells as a consequence of the exposure to pollutants, dangerous substances, and lifestyles (smoking habits, nutrition, and alcohol) (Prasad et al., 2017). The hydroxyl radical • OH is characterized by a very high reactivity and easily reacts with the guanine (DNA base most susceptible to oxidation because of its low redox potential) bound to deoxyribose in DNA and ribose in RNA, releasing 8-oxo-7,8-dihydroguanine (8-oxoGua), 8-oxo-7,8-dihydro-2′-deoxiguanosine (8-oxodGuo), and 8-oxo-7,8-dihydroguanosine (8-oxoGuo) in urine (Cadet et al., 2012; Carrieri et al., 2019). In human urine, 8-oxoGua and 8-oxodGuo are derived from DNA repair mechanisms and also by the turnover of DNA damaged by oxidation (Chao et al., 2021; Tanaka and Chock, 2021) The concentrations of these indicators, measured in the urine, represent the share of oxidative damage suffered by the nucleic acids and the nucleotide pool; they also represent the share of oxidative damage the organism was able to repair spontaneously or following the activation of specific existing repair mechanisms.
Both O2•− and NO• react to form peroxynitrite ONOO−, a compound that reacts with tyrosine and an amino acid found in most proteins, respectively, giving 3-nitrotyrosine (3-NO2Tyr), which can be used as a marker of “nitro-oxidative” damage to proteins (Campolo et al., 2020).
In the present study, the occupational exposure to styrene of a group of shipbuilding industry workers was assessed by measuring, before and after the work shift, the urinary concentration of MA and PGA. The objective of this study was to assess the effects of the occupational exposure not only to styrene but also to the mixture of all the other chemicals used in the workplace, such as solvents and wood dusts. The oxidative stress was also evaluated by measuring urinary biomarkers. The results found in the exposed workers were compared to those of a group of healthy volunteers not occupationally exposed to chemicals.
2 Materials and methods
2.1 Studied population
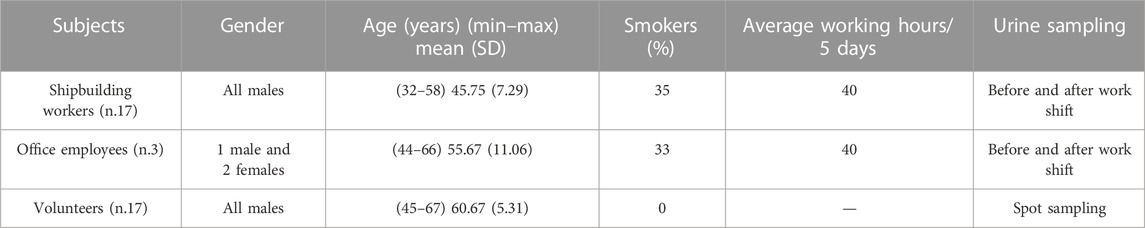
The studied group included 17 male workers of a shipbuilding industry, who finished and assembled fiberglass-reinforced plastic parts of the boats. The population we used as control comprises 17 healthy volunteers without any exposure to chemicals. Only subjects living in very unpolluted areas were included in the control group. The volunteers were recruited among colleagues, their relatives, and students. The shipbuilding industry is located in a seaside town in central Italy. The produced boats are small- and medium-sized yachts that are assembled and refined. The present study was approved by the Ethical Committee of the Fondazione Policlinico Universitario Agostino Gemelli, Università Cattolica del Sacro Cuore, prot ID 5117 (nonprofit study), date 03/08/2022. All subjects provided their written informed consent to participate in the study and filled a questionnaire for the collection of the following information: gender, age, occupation, smoking status, and general health status. Workers were equipped with the most appropriate personal protective devices. The assessment of the exposure to chemical agents was carried out by the employer according to the Italian legislation, and results were in agreement with the occupational exposure limits.
This study can be defined as a non-interventionist/observational study, on the basis of the European Directive 2001/20/EC (2001); all experiments were conducted according to the Declaration of Helsinki, following the International Code of Ethics for Occupational Health Professionals published by the International Committee of Occupational Health (ICOH) (ICOH, 2014). Urine sampling was conducted during the medical surveillance of workers organized by the employer. The information gathered was used as aggregate data referring to the whole group of workers, with no risk of individual identification.
Table 1 shows the main subjects’ characteristics. Among workers exposed to styrene and other chemicals, different tasks were individuated, carpenter (seven workers), mechanical worker and welder (two workers), painter (three workers), and worker without any specific task (five workers). Furthermore, office employees (three workers) are exposed to chemicals as their workplace is very close to the industrial area.
2.2 Urine collection
All urine samples were collected on the same day in sterile polypropylene containers and divided into three aliquots, which were immediately transported to our laboratory and stored frozen at −25°C until analysis. In the workers’ urines, biomarkers were measured before and after the work shift, while for the control group, a spot urine sample was used. One aliquot of each urine sample was used to determine the urinary metabolites MA and PGA, one for the analysis of the oxidative stress biomarkers, and the third one for the determination of the creatinine concentration. The final concentration of all analytes was expressed as the ratio of the creatinine concentration in order to normalize their values with respect to the variability of urine dilution. This strategy was suggested by other authors (Heavner et al., 2006; Cone et al., 2009; Ozdemir et al., 2021). Urinary creatinine was determined by the Jaffè method using the alkaline picrate test with UV/VIS detection at 490 nm (Kroll et al., 1986). The samples having a creatinine concentration higher than 3 g/L or lower than 0.3 g/L have to be discarded, according to the recommendations of the ACGIH (ACGIH, 2016). The concentrations of creatinine lower than 0.3 g/L or higher than the upper limit of 3 g/L were not found in this study.
2.3 Chemicals and supplies
The analytical reference standards of DL mandelic acid (MA) and phenylglyoxylic acid (PGA) were purchased from Fluka (Sigma-Aldrich, Germany), and 8-oxoGua, 8-oxodGuo, and 8-oxoGuo were purchased from Spectra 2000 SRL (Rome, Italy). The deuterium-labeled internal standards ± mandelic-d5 acid (MA-d5) 99.4% and sodium phenyl-d5-glyoxylate 99.8% (PGA-d5) and the isotope-labeled internal standards (13C15N2), 8-oxodGuo, and (13C15N2) 8-oxoGuo were obtained from C/D/N Isotopes Inc. (Pointe-Claire, QC, Canada). The (13C15N2) 8-oxoGua (98%) was obtained from Cambridge Isotope Laboratories Inc. (Tewksbury, MA, United States). The 3-NO2Tyr was purchased from the Cayman Chemical Company (Ann Arbor, MI, United States) and 3-NO2Tyrd3 from Toronto Research Chemicals (Toronto, ON, Canada).
Glacial acetic acid 30% NH3, formic acid 50%, dimethyl sulfoxide, sodium hydroxide solution (50%–52% in water), and CHROMASOLV® gradient grade 99.9% methanol and acetonitrile for HPLC/MS 99.9% carbon disulfide low benzene content were obtained from Sigma-Aldrich (Saint Louis, MO, United States). Purified water obtained from a Milli-Q Plus system (Millipore, Milford, MA, United States) was used for preparing the mobile phase and for diluting the samples.
Anotop 10LC syringe filter devices (0.2 m pore size and 10 mm diameter) were purchased from Whatman Inc. (Maidstone, United Kingdom). The Kinetex Polar C18 column 100 A (100 × 4.6 mm, 2.6 μm) and Kinetex Polar C18 column 100 A (150 × 4.6 mm and 2.6 m), supplied by Phenomenex (Torrance, CA, United States), were used throughout the study.
All urine samples were analyzed by liquid chromatography/tandem mass spectrometry (HPLC/MS-MS) using an API 4000 triple-quadrupole mass spectrometry detector equipped with a TurboIonSpray (TIS) probe (AB Sciex, Framingham, MA, United States) coupled to a Series 200 LC quaternary pump (PerkinElmer, Norwalk, CT, United States). Detection was carried out in the multiple reaction monitoring (MRM) mode, and parameters were optimized for the analytes by the automated infusion quantitative optimization procedure and then refined by flow injection analysis (FIA) using the pure standards. The 1.5 version of Analyst® software (AB Sciex, Framingham, MA, United States) was used for instrument control and data analysis.
2.4 Determination of styrene metabolites (MA and PGA)
The MA and PGA quantitative analyses were carried out according to a validated analytical method (Paci et al., 2013). In brief, 1 mL urine samples were diluted with 1 mL of 2% acetic acid, added with 100 µL of internal standard (MA-d5 and PGA-d5) solution (10 mg/L) and filtered on a 0.2-μm syringe filter; 20 μL was injected into the HPLC-MS/MS system for quantitative analysis. Detection was carried out in the negative ion, MRM mode. The elution was carried out using a gradient of acetonitrile (phase A) and formic acid 0.2% (phase B), at a flow rate of 500 μL/min. Total run time was 10 min. The retention time of MA and its internal standard (MA-d5) was approximately 4.5 min, while for PGA and its internal standard (PGA-d5), the retention time was 4.0 min. The following m/z ion combinations (precursor→product) used both for identification and quantification were monitored, and the transitions were as follows: m/z −150.9→-107.3 for MA and -155.9→-112.0 for its internal standard MA-d5; −148.8→-105.1 for PGA and −153.8→-126.0 for its internal standard PGA-d5. The limit of detection (LOD) was 0.02 mg/L for MA and 0.015 mg/L for PGA, and the limit of quantification (LOQ) was 0.075 and 0.040 mg/L for MA and PGA, respectively. Accuracy was always higher than 82% and variability lower than 11% for both analytes. (Paci et al., 2013).
2.5 Biomarker oxidative stress (8-oxoGua, 8-oxodGuo, 8-oxoGuo, and 3-NO2Tyr)
The quantitative analysis of 8-oxoGua, 8-oxoGuo, 8-oxodGuo, and 3-NO2Tyr was determined according to the method proposed by Andreoli et al. (2010) with modifications in the sample thawing, dilution solvents, chromatographic column, and mobile phases. The samples were completely thawed in lukewarm water at approximately 37°C (Shih, Y-M., 2019), vortexed, and centrifuged at 10,000 x g for 5 min; the urine supernatant was added with internal standard and injected into the HPLC-MS/MS system. Detection was carried out in the positive ion, MRM mode. The elution was carried out using a gradient of methanol 9:1 v/v (phase A) and acetic acid 0.5% v/v (phase B) in water at a flow rate of 500 μL/min. Total run time was 18 min. The precursor→product ionic transitions monitored were 168.0→140.0 and 171.0 →143.0 for 8-oxoGua and its internal standard ((13C15N2) 8-oxoGua), 284.3→ 168.0 and 287.13 →171.1 for 8-oxodGuo and its internal standard ((13C15N2) 8-oxodGuo), 300.24→168.2 and 302.24→171.0 for 8-oxoGuo and its internal standard ((13C15N2) 8-oxoGuo), and 226.99 → 181.0 and 229.99 → 184.0 for 3-NO2Tyr and its internal standard (3-NO2Tyr d3). The concentrations of biomarkers and analytes were measured by using urine calibration curves. Eight different internal standards were used, spanning the range of interest. The concentrations were determined by measuring the ratio of the area of the analyte or biomarker to the area of the respective internal standard.
2.6 Statistical analysis
Statistical analyses were performed using an additional program of Microsoft Office Excel, Analysis Tool Pak (Microsoft Corporation, Redmond, WA, United States), after checking the log-normality of the distribution of data. Students’ t-test was used to analyze the difference between log-transformed values of the urinary concentrations, measured before and after the work shift in workers (paired t-test) and between exposed workers and non-exposed subjects (unpaired t-test). The linear bivariate correlation between variables was also determined on the log-transformed values. In all tests, p-value lower than 0.05 was considered as statistically significant.
Multivariate analyses were carried out with statistical software R (R version 4.2.1 (2022-06-23)). The sparse PLS-DA (sPLS-DA) technique was applied with the aim of determining the combination of variables, maximally discriminating between the exposed from the control group and discriminating different working tasks. sPLS-DA permits to project the data in a subspace containing only the variables that explain the major fraction of variances. If the maximum number of variables is set, n, starting from an initial space dimension N, the principal components (PCs) will be created as a linear combination of the n variables maximally contributing in explaining the variance. For sPLS-DA, a package of R in the library mixOmics was used. A factor variable “task” was introduced and was characterized by four levels: “c” for carpenters, “m” individuating welders and mechanical workers, “p” for painters, and “w” for a generic worker. The groups “c” and “m” were subsequently gathered in a unique group named group 1, and the painter and generic worker, groups “p” and “w,” were grouped in a unique group named group 2.
sPLS-DA was applied to the exposed workers belonging to the two groups previously described in order to individuate the variables and to better discriminate different tasks. All the oxidative stress variables, 8-oxoGua, 8-oxoGuo 8-oxodGuo, and 3-NO2Tyr, and the dose biomarkers, i.e., the styrene metabolites, MA and PGA, were used in this analysis. Another analysis was performed only on the oxidative stress variables in order to study what combination better discriminates exposed from non-exposed subjects.
3 Results and discussion
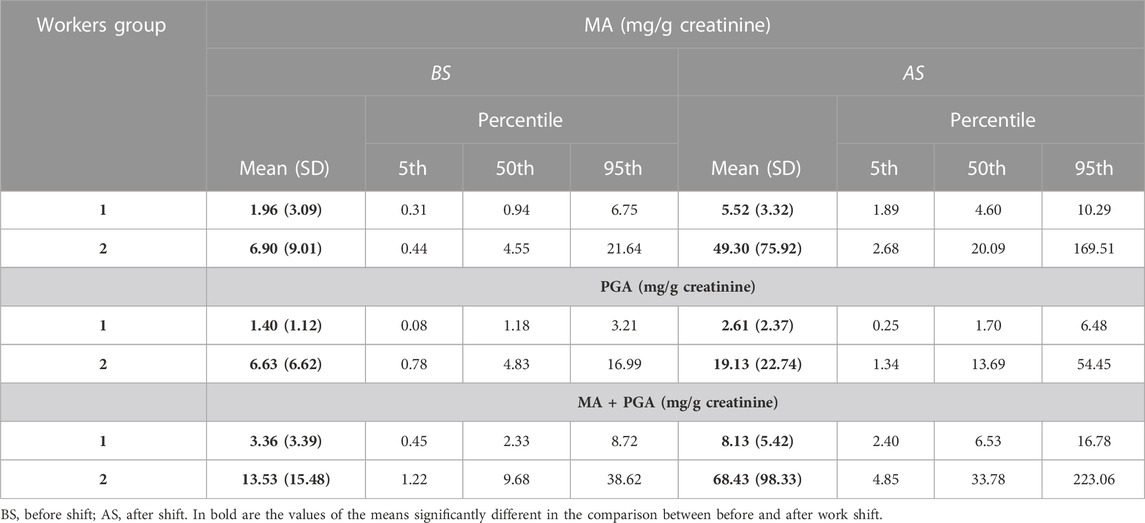
In this study, the analyte concentration levels show the descriptive statistics expressed in terms of mean with the standard deviation, 5th, 50th or median, and 95th percentile, as reported in Table 2 for the urinary metabolites MA and PGA expressed in mg/g creatinine.
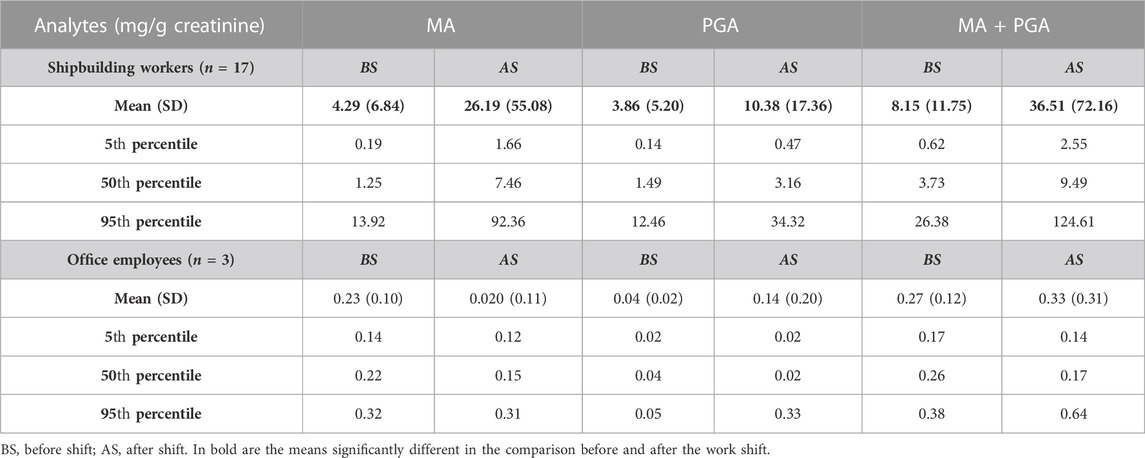
TABLE 2. Concentrations of the urinary metabolites MA and PGA in the exposed subjects: descriptive statistics.
The mean values of MA and PGA measured after the work shift and their sum are higher than those measured before (p = 0.00007; p = 0.025; and p = 0.0003, respectively) for the shipbuilding workers. However, the sum of the two metabolites at the end of the shift is much lower than the ACGIH BEI of 400 mg/g creatinine (ACGIH, 2020). The urinary metabolites of office employees are very low, as expected, as they should not be exposed to styrene, and there is no statistically significant difference between before and after the work shift. The high metabolite levels at the beginning of the work shift is an indication of a possible slowdown of styrene metabolism caused by the co-exposure to other organic solvents and, in particular, acetone, which is used as a cleaning agent for the tools, as observed in a previous study (Bonanni et al., 2015).
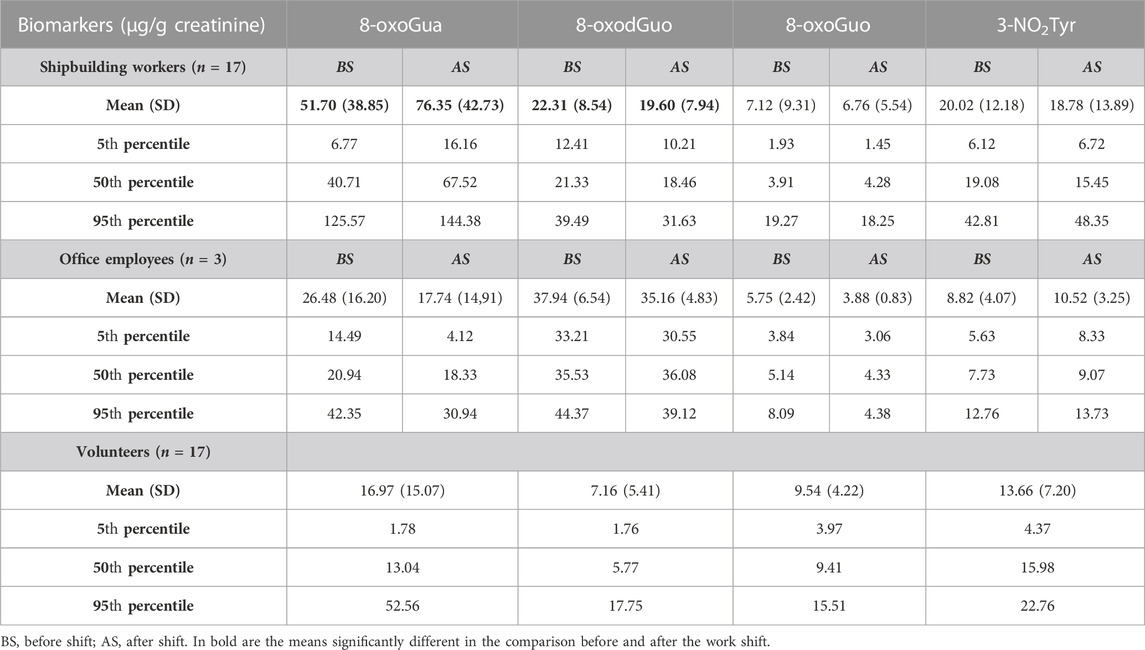
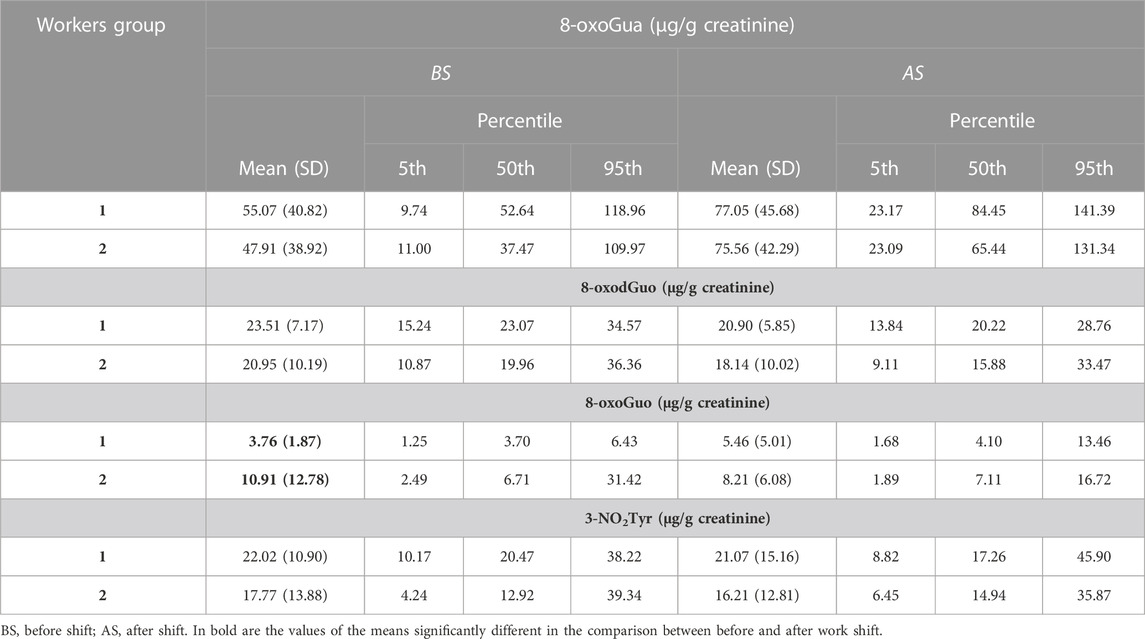
Table 3 shows the descriptive statistics for the four oxidative stress biomarkers measured in the same urine samples, expressed in µg/g creatinine. The values are compared to those of 17 healthy volunteers, all males, involved in this study as a control group.
In terms of all the biomarkers considered in this study, both styrene metabolites and oxidative stress biomarkers, no statistically significant differences were found between smokers and non-smokers in the exposed group, both at the beginning and end of the work shift. We interpreted this finding to be related to the fact that the exposure to toxic chemicals, coming from the working environment, largely exceeds the exposure that could be related to the smoking habit.
For what concerns the biomarkers of oxidative stress, the statistical comparison performed by the paired t-test shows that the mean concentration values of 8-oxoGua in the shipbuilding workers are higher after the work shift than before. A statistically significant difference was also found for 8-oxoGua and 8-oxodGuo values that are higher in the shipbuilding workers than in the control group, both when measured before and after the work shift. The values of 8-oxoGua and 3-NO2Tyr of the shipbuilding workers are higher than those of the office employees.
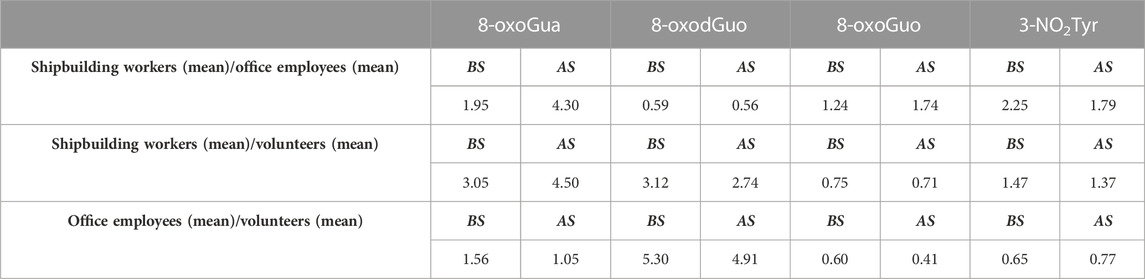
With the aim of facilitating reading of the results, we reported in Table 4 the ratio between the average concentrations of the oxidative stress biomarkers in exposed workers and in the group of healthy volunteers.
The log-correlations (Pearson’s) among the exposure biomarkers (the styrene metabolites) and the effect biomarkers (the oxidative stress biomarkers) were also studied, and the results are reported in Table 5. For the shipbuilding workers, the correlation was studied separately before and after the work shift, while for the office employees, the values of MA, PGA, and their sum measured before and after the work shift were combined as there was no statistically significant difference between the two series.
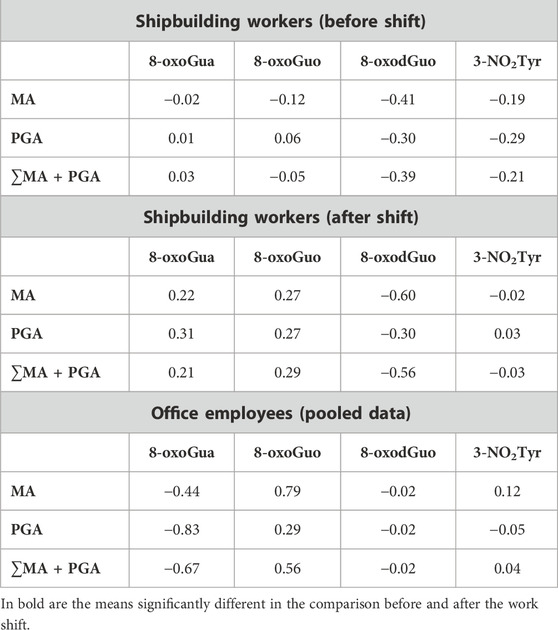
TABLE 5. Pearson’s correlation of exposure and effect biomarkers (calculated on log-transformed values).
The results show that the urinary 8-oxoGuo is positively correlated in shipbuilding workers with MA, PGA, and ∑MA + PGA. This result confirms the results observed in a previous study (Manini et al., 2009), showing high levels of urinary 8-oxoGuo and correlating with internal exposure metabolites (∑MA + PGA), in styrene-exposed workers. Another study on the levels of urinary biomarkers of oxidatively generated damage to DNA and RNA in different groups of workers compared to the general population concluded that the urinary 8-oxoGuo, which is related to RNA oxidation, seems to be the most suitable biomarker to detect short-term, reversible effects of exposure to dangerous chemicals (Tranfo et al., 2019) and metals (Buonaurio et al., 2021. Furthermore, in agreement with other recent works (Broedbaek et al., 2011; Kjær et al., 2017; Gan et al., 2018), urinary 8-oxoGuo may be associated with an increase in mortality, considering it as a potential biomarker to identify individuals at high risk of developing age-related diseases.
The correlation coefficients between styrene metabolites and urinary 8-oxoGuo are not very high, and they do not reach statistical significance. On the other hand, we should consider that the workers of the shipbuilding industry are exposed not only to styrene but also to other hazardous chemicals, such as organic solvents contained in paints and wood dust that could contribute to oxidative stress. In office employees, MA and the sum MA + PGA are also well correlated to the urinary 8-oxoGuo, if we include the concentrations measured before the work shift, but this is not true for PGA. Therefore, the oxidative stress cannot be derived from styrene exposure only but from different sources. In fact, mandelic acid can be found in some foods (mainly almonds), but it is also used in cosmetics and as an antibiotic. The very low number of office employees does not permit drawing conclusions regarding the association between MA and PGA and the urinary concentration of 8-oxoGuo in this group.
3.1 sPLS-DA discrimination by task
The distributions of the dose and oxidative stress biomarkers have been studied separately for the different working tasks: carpenters (n = 7), mechanical workers and welders (n = 2), painters (n = 3), and workers without a specific task (n = 5). Figures 1A–F show the boxplots for these four groups for each biomarker.
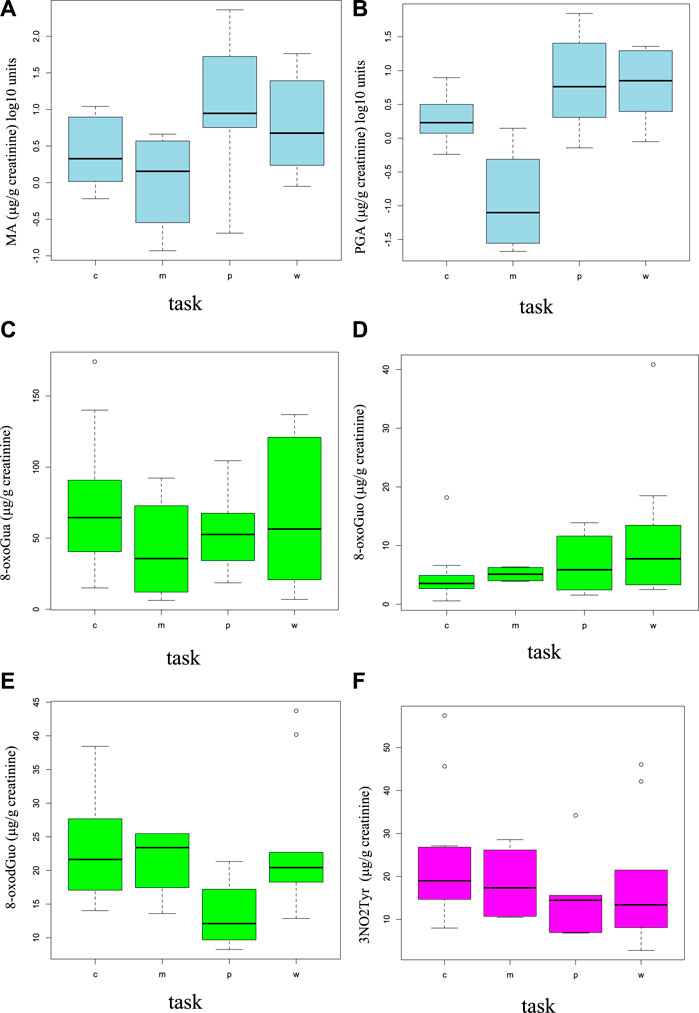
FIGURE 1. Boxplots showing the distributions (median in the center and the first and fourth quartiles as rectangle delimiters) of the styrene dose exposure biomarkers (MA and PGA (A, B)) and the oxidative stress biomarkers [8-oxoGua (C), 8-oxoGuo (D), 8-oxodGuo (E), and 3-NO2Tyr (F)] in different working tasks (“c” = carpenter, “m” = welder and mechanical worker, “p” = painter, and “w” = generic worker).
The painters are the workers having the maximum average levels of MA and PGA urinary concentrations. The maximum average concentrations of 8-oxoGua and 8-oxodGuo were found in carpenters. Due to the poor number of workers in different task groups, the workers were grouped in only two groups, based on their styrene exposure. Group 1, including carpenters, welders, and mechanical workers (n = 9), has the lowest styrene exposure, while group 2, including painters and generic workers engaged in the assembly of parts of the boats (n = 8), has a higher styrene exposure.
Tables 6, 7 show the urinary concentrations of the dose and oxidative stress biomarkers for the two groups.
The mean values for MA, PGA, and their sum are statistically higher after the work shift in both groups and in group 2 that comprises painters and generic workers with respect to group 1 (t-test, p < 0.05, data in bold).
Regarding the oxidative stress biomarkers, only 8-oxoGuo is higher in group 2 than in group 1, but only before the work shift (data in bold). This can be interpreted as a long half-life of the considered biomarkers that is still excreted in the morning after the exposure to chemicals. The number of subjects is small, so conclusions cannot be drawn regarding this finding.
Figure 2, A and B, shows the results of the sPLS-DA analysis discriminating group 1 and group 2 (all data for both BS and AS), respectively.
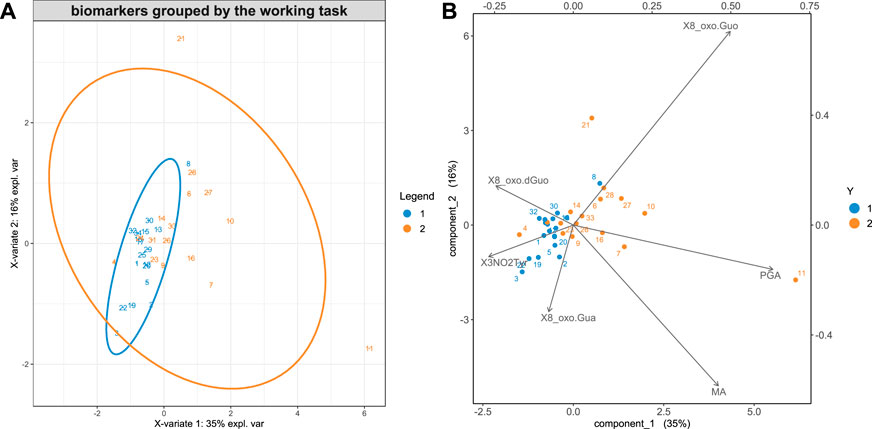
FIGURE 2. (A) The cases are plotted in the principal component 2d plane individuated by sPLS-DA to find the maximum discrimination between group 1 and 2. (B) The variables are also represented in the principal component plane.
Figure 2 shows that the workers of group 2 have a larger exposure to styrene, as they excrete a higher concentration of styrene metabolites, MA and PGA, in their urine. In group 2, the 8-oxoGuo is higher than in group 1, which is characterized by higher concentrations of 8-oxoGua and 8-oxodGuo. A previous study suggested that 8-oxoGuo could be the most sensitive biomarker to the short-term, reversible effects of exposure to chemical agents even under conditions that could be considered safe, as the styrene exposure below the occupational exposure limits (Tranfo et al., 2019). 8-OxodGuo, derived from damage caused to the DNA, both if repaired and/or not repaired, is typically higher when the exposure to carcinogenic agents occurs, which, in the case of group 1, formed by carpenters and welders, could be identified with wood dust and welding fumes, respectively.
3.2 sPLS-DA discrimination between workers and the control group
The results of the exposed workers (n = 34, before and after the work shift) were compared to those of the 17 volunteers shown in Table 1. This choice was supported by the fact that the three employees shared a common environment with the workers and, consequently, were also exposed to different airborne pollutants, and therefore, they cannot be considered non-exposed subjects. In addition, both in the group of exposed workers and in that of office employees, a certain percentage (approximately 30%) of smokers were present contributing to the oxidative stress. The non-exposed, healthy volunteers are also non-smokers, making the discrimination between the exposed and non-exposed more effective.
The distributions of 8-oxoGua and 8-oxodGuo in the groups of exposed and control subjects are shown in the boxplots of Figure 3, A and B, as, of the four biomarkers related to the oxidative stress, only these two were found differently concentrated at a statistically significant level between the two groups (p = 5.57*10^-7 and p = 5.77*10^-9 for 8-oxoGua and 8-oxodGuo, respectively).
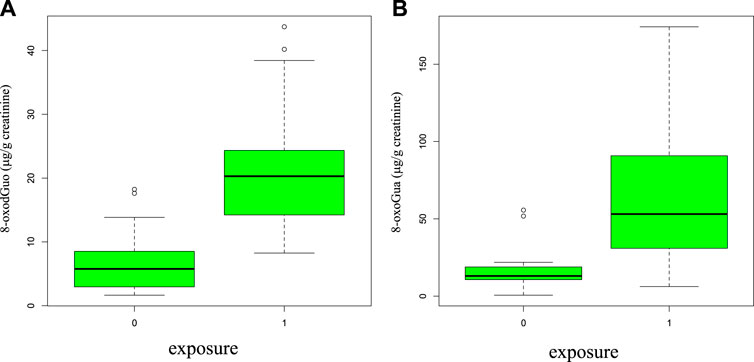
FIGURE 3. Boxplot representing the statistical distributions (median in the center and the first and fourth quartiles as rectangle delimiters) of 8-oxodGuo (A) and 8-oxoGua (B) in the group of exposed (group = 1, shipbuilding workers, all tasks) and control subjects (group = 0, 17 healthy volunteers).
Including all the four oxidative stress biomarkers into the sPLS-DA analysis, a good discrimination of the two aforementioned groups, exposed and controls, is obtained as shown in Figure 4.
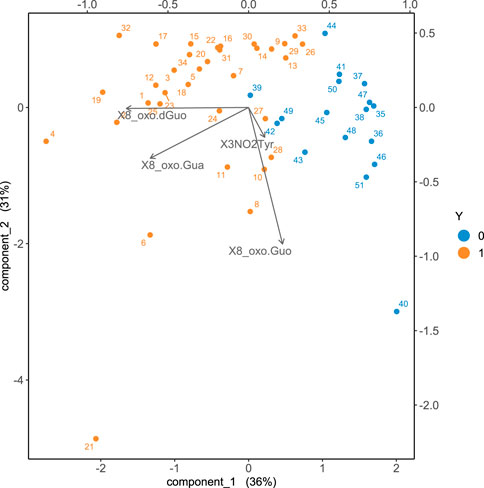
FIGURE 4. Biplot obtained with sPLS-DA discriminating the exposed from the control group. The cases of the non-exposed subjects are represented in blue, whilst the exposed workers are represented in orange. The arrows represent the original variables projected in the 2d plane principal component space.
As it can be appreciated by visually inspecting the plot in Figure 4, the exposed subjects are characterized by increased values of 8-oxoGua and 8-oxodGuo biomarkers whilst the concentrations of 8-oxoGuo are larger in the controls. The statistical power of the discrimination between exposed and controls is quantified by means of the ROC curve, as shown in Figure 5.
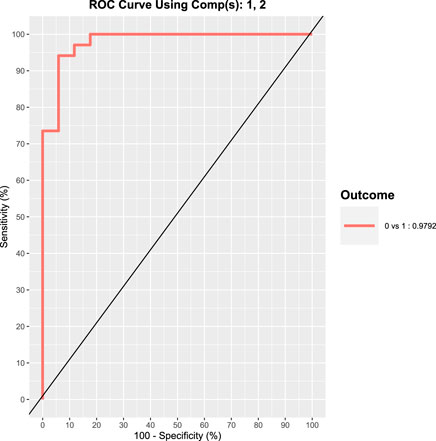
FIGURE 5. ROC curve representing the statistical power of the discrimination between exposed (17 shipbuilding workers) and control subjects (17 healthy volunteers) based on the oxidative stress biomarker excretion.
This curve shows a very high sensitivity and specificity in discriminating controls from exposed subjects, measured by the area under curve that is approximately 98%. Further studies will be devoted to the development of a test based on 8-oxoGua and 8-oxodGuo in order to discriminate, at high levels of sensitivity and specificity, subjects exposed to carcinogenic agents from subjects who are not exposed to carcinogenic agents.
3.3 Limitations of the study
This study is strongly limited by the small number of subjects. In particular, it is difficult to compare between the different working tasks with such a small sample size. The limitation of this study is strictly related to the production structure as Italy is dominated by small and medium enterprises characterized by handcrafted features. This work reflects this structure. The small number of subjects was counteracted by a very precise evaluation of dose metabolites in combination with oxidative stress biomarkers. In any case, it must be stressed that what was found is based on a small sample and must be confirmed on a larger one.
4 Conclusion
This study aimed to measure the levels of oxidative stress biomarkers in workers exposed to styrene in a shipbuilding industry in which workers finish and assemble fiberglass plastic parts of the boats. In order to assess styrene exposure, the concentrations of urinary styrene metabolites, MA and PGA, were measured both at the beginning and end of the work shift.
When the oxidative stress biomarkers were compared in exposed workers and in volunteers without any professional exposure to chemicals, a significant discrimination was obtained. The area under the ROC curve, discriminating exposed from control subjects, was approximately 98%. The discrimination was found mainly due to the concentrations of 8-oxoGua and 8-oxodGuo that are biomarkers known to be related to nucleic acid damage.
The stratification of results according to different working tasks showed larger exposure to styrene in painters and generic workers, who show significantly higher concentrations of 8-oxoGuo and relatively lower concentrations of 8-oxoGua and 8-oxodGuo, with respect to the group of carpenters and welders.
The oxidative stress biomarker profile in the shipbuilding industry is quite complex due to the complexity of the working tasks, involving different exposure factors, that could contribute to the increase in the oxidative stress biomarker excretion in the urine. In fact, the workers are exposed not only to styrene but also to paints, wood dust, coming from the refining of the parts of the boat, and welding fumes. Even if the oxidative stress biomarkers are sensitive to the occupational exposure to chemicals and could be used as an early warning for their health effects, a thorough analysis of the exposure sources is necessary to determining the level of oxidative stress induced by the exposure to different xenobiotics.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Università Cattolica del sacro Cuore, Policlinico Universitario Agostino Gemelli. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DP: conceptualization, data curation, investigation, methodology, and writing–original draft. EP: data curation, investigation, methodology, and writing–review and editing. RG: data curation, investigation, and writing–review and editing. GT: conceptualization, supervision, and writing–review and editing. MS: writing–review and editing. AF: investigation, resources, supervision, and writing–review and editing. LT: data curation, investigation, and writing–review and editing. RS: conceptualization, formal analysis, funding acquisition, methodology, resources, and writing–review and editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article, Grant INAIL BRIC Id08 PAR 2022-2024.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that they were editorial board members of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
American Conference of the Governmental Industrial Hygienists (ACGIH) (2001). Documentation of the biological exposure indices. 7th edition. Ohio, United States: ACGIH.
American Conference of the Governmental Industrial Hygienists (ACGIH) (2016). Documentation of the of the threshold limit values and biological exposure indices. Cincinnati, OH, USA: ACGIH.
American Conference of the Governmental Industrial Hygienists (ACGIH) (2020). TLVs and BEIs: based on the documentation of the threshold limit values for chemical substances and physical agents and biological exposure indices Cincinnati. USA: OH.
Andreoli, R., Manini, P., De Palma, G., Alinovi, R., Goldoni, M., Niessen, W. M. A., et al. (2010). Quantitative determination of urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine, 8-oxo-7,8-dihydroguanine, 8-oxo-7,8-dihydroguanosine, and their non-oxidized forms: daily concentration profile in healthy volunteers. Biomarkers 15, 221–231. doi:10.3109/13547500903434501
Banton, M., Bus, J., Collins, J., Delzell, E., Gelbke, H. P., Kester, J., et al. (2019). Evaluation of potential health effects associated with occupational and environmental exposure to styrene - an update. J. Toxicol. Environ. Health Part B 22, 1–130. doi:10.1080/10937404.2019.1633718
Bonanni, R. C., Gatto, M. P., Paci, E., Gordiani, A., Gherardi, M., and Tranfo, G. (2015). Biomonitoring for exposure assessment to styrene in the fibreglass reinforced plastic industry: determinants and interferents. Ann. Occup. Hyg. 59 (8), 1000–1011. doi:10.1093/annhyg/mev047
Broedbaek, K., Siersma, V., Henriksen, T., Weimann, A., Petersen, M., Andersen, J. T., et al. (2011). Urinary markers of nucleic acid oxidation and long-term mortality of newly diagnosed type 2 diabetic patients. Diabetes Care 34 (12), 2594–2596. doi:10.2337/dc11-1620
Buonaurio, F., Astolfi, M. L., Pigini, D., Tranfo, G., Canepari, S., Pietroiusti, A., et al. (2021). Oxidative stress biomarkers in urine of metal carpentry workers can Be diagnostic for occupational exposure to low level of welding fumes from associated metals. Cancers 13, 3167. doi:10.3390/cancers13133167
Cadet, J., Loft, S., Olinski, R., Evans, M. D., Bialkowski, K., Richard, J., et al. (2012). Biologically relevant oxidants and terminology, classification and nomenclature of oxidatively generated damage to nucleobases and 2-deoxyribose in nucleic acids. Free Radic. Res. 46 (4), 367–381. doi:10.3109/10715762.2012.659248
Campolo, N., Issoglio, F. M., Estrin, D. A., Bartesaghi, S., and Rad, R. (2020). 3-Nitrotyrosine and related derivatives in proteins: precursors, radical intermediates and impact in function. Essays Biochem. 64 (1), 111–133. doi:10.1042/EBC20190052
Carbonari, D., Mansi, A., Proietto, A. R., Paci, E., Bonanni, R. C., Gherardi, M., et al. (2015). Influence of genetic polymorphisms of styrene-metabolizing enzymes on the levels of urinary biomarkers of styrene exposure. Toxicol. Lett. 233 (2), 156–162. doi:10.1016/j.toxlet.2015.01.002
Carrieri, M., Pigini, D., Martinelli, A., Paci, E., Maratini, F., Salamon, F., et al. (2019). Effect of benzene exposure on the urinary biomarkers of nucleic acid oxidation in two cohorts of gasoline pump attendants. Int. J. Environ. Res. Public Health 16, 129. doi:10.3390/ijerph16010129
Cavallo, D., Ursini, C. L., Fresegna, A. M., Ciervo, A., Maiello, R., Buresti, G., et al. (2021). Occupational exposure in industrial painters: sensitive and non invasive biomarkers to evaluate early cytotoxicity, genotoxicity and oxidative stress. Int. J. Environ. Res. Public Health 18, 4645. doi:10.3390/ijerph18094645
Chao, M. R., Evans, M. D., Hu, C. W., Ji, Y., Møller, P., Rossner, P., et al. (2021). Biomarkers of nucleic acid oxidation-A summary state-of-the-art. Redox Biol. 42, 101872. doi:10.1016/j.redox.2021.101872
Cheng, S., Zhang, J., Wang, Y., Zhang, D., Teng, G., Chang-Chien, G. P., et al. (2019). Global research trends in health effects of volatile organic compounds during the last 16 years: a bibliometric analysis. Aerosol Air Q. Res. 19, 1834–1843. doi:10.4209/aaqr.2019.06.0327
Cone, E. J., Caplan, Y. H., Moser, F., Robert, T., Shelby, M. K., and Black, D. L. (2009). Normalization of urinary drug concentrations with specific gravity and creatinine. J. Anal. Toxicol. 33, 1–7. doi:10.1093/jat/33.1.1
Costa, C., Costa, S., Silva, S., Coelho, P., Botelho, M., Gaspar, J., et al. (2012). DNA damage and susceptibility assessment in industrial workers exposed to styrene. J. Toxicol. Environ. Health, Part A 75, 735–746. doi:10.1080/15287394.2012.688488
Directive 2001/20/EC (2001). Directive 2001/20/EC of the European parliament and of the council of 4 april. Available at: https://ec.europa.eu/health/sites/default/files/files/eudralex/vol1/dir200120/dir200120en.pdf (accessed on June 24, 2021).
Directive (EU) 2017/2398 (2017). Directive (EU) 2017/2398 of the European Parliament and of the Council of 12 December amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work. Available at: https://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=CELEX%3A32017L2398.
Gan, W., Liu, X. L., Yu, T., Zou, Y. G., Li, T. T., Wang, S., et al. (2018). Urinary 8-oxo-7, 8-dihydroguanosine as a potential biomarker of aging. Front. Aging Neurosci. 10, 34. doi:10.3389/fnagi.2018.00034
Ghelli, F., Bellisario, V., Squillacioti, G., Grignani, E., Garzaro, G., Buglisi, M., et al. (2021). Oxidative stress induction in woodworkers occupationally exposed to wood dust and formaldehyde. J. Occup. Med. Toxicol. 16, 4. doi:10.1186/s12995-021-00293-4
Heavner, D. L., Morgan, W. T., Sears, S. B., Richardson, J. D., Byrd, G. D., and Ogden, M. W. (2006). Effect of creatinine and specific gravity normalization techniques on xenobiotic biomarkers in smokers' spot and 24h urines. J. Pharm. Biomed. Anal. 40, 928–942. doi:10.1016/j.jpba.2005.08.008
IARC (2018). Monographs on the evaluation of carcinogenic risks to humans. Benzene. Lyon, Fr. 120, 1–309.
IARC Monographs (2012). Arsenic, metals, fibres, and dusts, volume 100 C Vol.62. France: A review of human carcinogens -Lyon.
IARC Monographs on the Evaluation of Carcinogenic Risks to Humans (2000). Some industrial chemicals. Lyon, Fr. 77, 1–573.
IARC Monograph Working Group (2019). “Styrene, styrene-7, 8-oxide, and quinoline,” in IARC working group on the evaluation of carcinogenic risks to humans (Lyon, France: International Agency for Research on Cancer), 1–355.
International Commission on Occupational Health (ICOH) (2014). International Code of Ethics for occupational health professionals. Available at: http://www.icohweb.org/site/multimedia/code_of_ethics/code-of-ethics-en.pdf (accessed on June 24, 2021).
Jacob, K. D., Noren Hooten, N., Trzeciak, A. R., and Evans, M. K. (2013). Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev. 134, 139–157. doi:10.1016/j.mad.2013.02.008
Kim, K. W. (2015). Effects of styrene-metabolizing enzyme polymorphisms and lifestyle behaviors on blood styrene and urinary metabolite levels in workers chronically exposed to styrene. Toxicol. Res. 31 (4), 355–361. doi:10.5487/TR.2015.31.4.355
Kjær, L. K., Cejvanovic, V., Henriksen, T., Petersen, K. M., Hansen, T., Pedersen, O., et al. (2017). Cardiovascular and all-cause mortality risk associated with urinary excretion of 8-oxoGuo, a biomarker for RNA oxidation, in patients with type 2 diabetes: a prospective cohort study. Diabetes Care Dec 40 (12), 1771–1778. doi:10.2337/dc17-1150
Kroll, M. H., Chesler, R., Hagengruber, C., Blank, D. W., Kestner, J., and Rawe, M. (1986). Automated determination of urinary creatinine without sample dilution: theory and practice. Clin. Chem. 32, 446–452. doi:10.1093/clinchem/32.3.446
Lees, P. S. J., Stefaniak, A., Emmett, E. A., and Dalton, P. (2003). Exposure assessment for study of olfactory function in workers exposed to styrene in the reinforced-plastics industry. Am. J. industrial Med. 44, 12–23. doi:10.1002/ajim.10236
Makarova, V., and Tkalich, V. (2021). Evaluation of the efficiency of dust and gas collecting units of woodworking shops in shipbuilding industry. Earth science" IOP Conf. Ser. Earth Environ. Sci. 666, 042049. doi:10.1088/1755-1315/666/4/042049
Manini, P., De Palma, G., Andreoli, R., Marczynski, B., Hanova, M., Mozzoni, P., et al. (2009). Biomarkers of nucleic acid oxidation, polymorphism in, and expression of, hOGG1 gene in styrene-exposed workers. Toxicol. Lett. 190, 41–47. doi:10.1016/j.toxlet.2009.06.862
Ozdemir, S., Sears, C. G., Harrington, J. M., Poulsen, A. H., Buckley, J., Howe, C. J., et al. (2021). Relationship between urine creatinine and urine osmolality in spot samples among men and women in the Danish diet cancer and health cohort. Toxics 9, 282. doi:10.3390/toxics9110282
Paci, E., Pigini, D., Caporossi, L., De Rosa, M., Santoro, A., Sisto, R., et al. (2013). Matrix effect in the quantitative determination of mandelic and phenylglyoxylic acid in urine samples by HPLC-MS/MS with isotopic dilution. Curr. Anal. Chem. 9, 439–446. doi:10.2174/1573411011309030013
Pigini, D., Caporossi, L., Paci, E., Capanna, S., Viganò, P., Alteri, A., et al. (2022). Phthalate exposure and biomarkers of oxidation of nucleic acids: results on couples attending a fertility center. Toxics 10, 61. doi:10.3390/toxics10020061
Prasad, S., Gupta, S. C., and Tyagi, A. K. (2017). Reactive oxygen species (ROS) and cancer: role of antioxidative Nutraceuticals. Cancer Lett. 387, 95–105. doi:10.1016/j.canlet.2016.03.042
Rueff, J., Teixeira, J. P., Santos, L. S., and Gaspar, J. F. (2009). Genetic effects and biotoxicity monitoring of occupational styrene exposure. Clin. Chim. Acta 399, 8–23. doi:10.1016/j.cca.2008.09.012
Shih, Y.-M., Cooke, M. S., Pan, C. H., Chao, M. R., and Hu, C. W. (2019). Clinical relevance of guanine-derived urinary biomarkers of oxidative stress, determined by LC-MS/MS. Redox Biol. 20, 556–565. doi:10.1016/j.redox.2018.11.016
Sisto, R., Capone, P., Cerini, L., Paci, E., Pigini, D., Gherardi, M., et al. (2020). Occupational exposure to volatile organic compounds affects microRNA profiling: towards the identification of novel biomarkers. Toxicol. Rep. 7, 700–710. doi:10.1016/j.toxrep.2020.05.006
Sisto, R., Cavallo, D., Ursini, C. L., Fresegna, A. M., Ciervo, A., Maiello, R., et al. (2020). Direct and oxidative DNA damage in a group of painters exposed to VOCs: dose – response relationship. Front. Public Health 8, 445. doi:10.3389/fpubh.2020.00445
Tanaka, M., and Chock, P. B. (2021). Oxidative modifications of RNA and its potential roles in biosystem. Front. Mol. Biosci. 8, 685331. doi:10.3389/fmolb.2021.685331
Tranfo, G., Paci, E., Carrieri, M., Marchetti, E., Sisto, R., Gherardi, M., et al. (2019). Levels of urinary biomarkers of oxidatively generated damage to DNA and RNA in different groups of workers compared to general population. Int. J. Environ. Res. Public Health. 16, 2995. doi:10.3390/ijerph16162995
Ünsalan, D., and Celebi, U. (2010). “The risk of occupational safety and health in shipbuilding industry in Turkey,” in Proceedings of the 3rd International Conference on Maritime and Naval Science and Engineering, Anchorage, Alaska, 20-23 September 2010.
Keywords: occupational exposure, mandelic acid, phenylglyoxylic acid, oxidative stress biomarkers, biological monitoring
Citation: Pigini D, Paci E, Guglielmetti R, Tranfo G, Spagnoli M, Fetoni A, Tricarico L and Sisto R (2023) Oxidative stress in occupational exposure to styrene vapors and dangerous chemicals in the shipbuilding industry. Front. Toxicol. 5:1319896. doi: 10.3389/ftox.2023.1319896
Received: 11 October 2023; Accepted: 07 November 2023;
Published: 22 November 2023.
Edited by:
Camilo Dias Seabra Pereira, Federal University of São Paulo, BrazilReviewed by:
Andy Povey, The University of Manchester, United KingdomMarcus S. Cooke, University of South Florida, United States
Copyright © 2023 Pigini, Paci, Guglielmetti, Tranfo, Spagnoli, Fetoni, Tricarico and Sisto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renata Sisto, ci5zaXN0b0BpbmFpbC5pdA==
 Daniela Pigini
Daniela Pigini Enrico Paci1
Enrico Paci1 Giovanna Tranfo
Giovanna Tranfo Mariangela Spagnoli
Mariangela Spagnoli Annarita Fetoni
Annarita Fetoni Renata Sisto
Renata Sisto