
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Toxicol. , 27 November 2023
Sec. Environmental Toxicology
Volume 5 - 2023 | https://doi.org/10.3389/ftox.2023.1280230
 Devin I. Alewel1†
Devin I. Alewel1† Thomas W. Jackson1†
Thomas W. Jackson1† Katherine M. Rentschler1
Katherine M. Rentschler1 Mette C. Schladweiler2
Mette C. Schladweiler2 Anna Astriab-Fisher2
Anna Astriab-Fisher2 Stephen H. Gavett2
Stephen H. Gavett2 Paul A. Evansky2
Paul A. Evansky2 Urmila P. Kodavanti2*†
Urmila P. Kodavanti2*†Introduction: Acrolein is a significant component of anthropogenic and wildfire emissions, as well as cigarette smoke. Although acrolein primarily deposits in the upper respiratory tract upon inhalation, patterns of site-specific injury in nasal versus pulmonary tissues are not well characterized. This assessment is critical in the design of in vitro and in vivo studies performed for assessing health risk of irritant air pollutants.
Methods: In this study, male and female Wistar-Kyoto rats were exposed nose-only to air or acrolein. Rats in the acrolein exposure group were exposed to incremental concentrations of acrolein (0, 0.1, 0.316, 1 ppm) for the first 30 min, followed by a 3.5 h exposure at 3.16 ppm. In the first cohort of male and female rats, nasal and bronchoalveolar lavage fluids were analyzed for markers of inflammation, and in a second cohort of males, nasal airway and left lung tissues were used for mRNA sequencing.
Results: Protein leakage in nasal airways of acrolein-exposed rats was similar in both sexes; however, inflammatory cells and cytokine increases were more pronounced in males when compared to females. No consistent changes were noted in bronchoalveolar lavage fluid of males or females except for increases in total cells and IL-6. Acrolein-exposed male rats had 452 differentially expressed genes (DEGs) in nasal tissue versus only 95 in the lung. Pathway analysis of DEGs in the nose indicated acute phase response signaling, Nrf2-mediated oxidative stress, unfolded protein response, and other inflammatory pathways, whereas in the lung, xenobiotic metabolism pathways were changed. Genes associated with glucocorticoid and GPCR signaling were also changed in the nose but not in the lung.
Discussion: These data provide insights into inhaled acrolein-mediated sex-specific injury/inflammation in the nasal and pulmonary airways. The transcriptional response in the nose reflects acrolein-induced acute oxidative and cytokine signaling changes, which might have implications for upper airway inflammatory disease susceptibility.
Acrolein, an electrophilic α,β-unsaturated aldehyde, is a volatile respiratory irritant formed during biomass burning, ambient photochemical transformation of anthropogenic pollutants, and tobacco combustion or pyrolysis (ATSDR, 2007; Stevens and Maier, 2008). The U.S. Environmental Protection Agency ranks acrolein as a prominent nationwide risk to health among hazardous air pollutants, and an estimated 97% of the non-carcinogenic respiratory hazard associated with mainstream cigarette smoke exposure is due to acrolein inhalation (U.S. EPA, 2006; Fowles and Dybing, 2003; Alwis et al., 2015). Acrolein exposure is implicated in the pathogenesis of multiple respiratory diseases, such as chronic obstructive pulmonary disease and lung cancer, and induces injury via sustained oxidative and inflammatory signaling, as well as tissue remodeling through extracellular matrix destruction and necrosis (Yeager et al., 2016).
In nose-breathing rodents, chemical reactivity with airway lining affects site-specific deposition and injury (Cichocki et al., 2014). Water-soluble vapors such as acrolein are scrubbed within the nasal turbinates, resulting in the stimulation of nasal mucosal trigeminal nerve endings (Alarie, 1973; Harkema, 1990). Chemo-sensory responses are largely mediated through nociceptive unmyelinated C-fibers, which are the most numerous neural projections in the airway (Mazzone and Undem, 2016; Gambeta et al., 2020). Acrolein activates Transient Receptor Potential Vanilloid and Ankyrin receptors (TRPV1 and TRPA1) at nerve endings, stimulating afferent signaling to the trigeminal ganglion and then the brain (Bautista et al., 2006; Achanta and Jordt, 2017). Some volatile gaseous pollutants with low solubility, such as ozone, deposit into the distal lung space, triggering vagal sensory neurons (Mazzone and Undem, 2016) to send nerve signals that traverse through the jugular and nodose ganglia and into the brain stem and stress-regulating regions such as the hypothalamus (Mazzone and Undem, 2016). Upper or lower airway chemical deposition following acrolein or ozone exposure triggers neurally mediated sympathetic-adrenal-medullary (SAM) and hypothalamic-pituitary-adrenal (HPA) neuroendocrine changes associated with hyperglycemia, circulating pituitary hormone alterations, and adrenal-hormone release (Miller et al., 2015; 2016; Snow et al., 2017; Henriquez et al., 2019; Alewel et al., 2023b).
Acute acrolein inhalation induces immediate bronchoconstriction (reduced ventilatory capacity), cardiovascular dysfunction, and release of adrenal catecholamines and glucocorticoids, which is believed to be modulated through a combined effort of sympathetic and parasympathetic innervation (Hazari et al., 2014; Snow et al., 2017). Following inhalation, acrolein rapidly reacts with cellular macromolecules to induce a myriad of airway epithelial effects, including depletion of airway antioxidant stores [e.g., glutathione (GSH)], production of reactive oxygen species, and induction of inflammatory cascades such as NFκB (Moghe et al., 2015). Acrolein also targets organelle-specific functions within the endoplasmic reticulum (ER) and mitochondria, resulting in protein unfolding and mitophagy (Moghe et al., 2015), which are etiological processes of chronic respiratory diseases associated with repeated acrolein exposure (Cloonan and Choi, 2016). Although the molecular effects of acrolein are fairly well characterized, a comparative assessment of transcriptional changes in nasal versus pulmonary airways in vivo has not been conducted, and little is known about the relative contribution of nasal versus pulmonary airways in health effects of upper airway irritants (Harkema et al., 1999; Bromberg, 2016). Such an assessment of upper versus lower airways would be critical in validating in vitro toxicity results derived from exposures of non-nasal respiratory cells (Cheah et al., 2013; Yeager et al., 2016; Xiong et al., 2018). Furthermore, given the neural and systemic endocrine effects of acrolein (Morris et al., 1999; Achanta and Jordt, 2017; Alewel et al., 2023b), a global transcriptional assessment of nasal and lung tissue could provide mechanistic insights into the potential contribution of circulating adrenal-derived stress hormones and local tissue changes in mediating nasal versus pulmonary response to acrolein.
Computational inhalation dosimetry approaches have been developed in rodents for inhalants such as acrolein to predict airway injury outcomes in humans (Cichocki et al., 2014). Owing to the primary deposition of acrolein in the nasal airways, greater injury responses in this region are expected; however, it is also likely that differential cellular composition and context with the surrounding milieu could influence the type of changes that occur in nasal versus lung tissue. Among the histologically unique zones of the rat nasal mucosa, the respiratory epithelium (used here for transcriptomics) comprises nearly 45% of the nasal surface area and experiences approximately 80% of airflow (Harkema, 1990; Chamanza and Wright, 2015). Further, this zone possesses cuboidal cells, which can metabolically interact with acrolein through GSH conjugation and other acrolein metabolizing enzymes, and brush cells, which play a role in nasal irritant detection (Saunders et al., 2013; Chamanza and Wright, 2015). Based on site-specific respiratory distribution of acrolein upon inhalation, we hypothesized that an assessment of global transcriptomic alterations in nasal versus pulmonary tissues would provide mechanistic insights regarding the pathways/gene signatures that are differentially impacted. More specifically, gene signatures elicited by acrolein may indicate patterns consistent with responses to adrenal-derived stress hormones involved in acrolein pathophysiology (Snow et al., 2017; Alewel et al., 2023b). In this study, we exposed male and female Wistar-Kyoto (WKY) rats nose-only to air or acrolein for an acute 4 h exposure duration and examined lavage markers of injury and inflammation. Due to exacerbated responses in males when compared to females in terms of nasal effects and propensity for neuroendocrine activation (Alewel et al., 2023b), we further performed nasal and pulmonary mRNA sequencing (RNAseq) in male rats to assess acrolein-induced changes in mRNA expression.
Male and female WKY rats for both cohorts were purchased from Charles River Laboratories (Raleigh, NC). Animals were housed in the U.S. EPA Office of Research and Development Research Triangle Park animal facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care at atmospheric housing conditions of 21°C ± 1°C, 50%–65% relative humidity under a 12 h light/dark cycle. Rats were pair-housed in polycarbonate cages containing hardwood chip bedding and Enviro-dri wrinkle paper for enrichment, and provided with Purina (5,001) rat chow (Brentwood, MO) and water ad libitum, except during exposure. Exposure occurred at 12–13 weeks of age, and males weighed ∼250–300 g and females weighed ∼150–200 g. Sexes were housed and exposed separately. All experimental procedures received prior approval from the U.S. EPA Health Institutional Animal Care and Use Committee.
Data presented here for nasal and bronchoalveolar lavage injury markers were collected from our recent acrolein exposure study, cohort 1 (Alewel et al., 2023b). A new cohort of male rats (cohort 2), used for collecting tissues for transcriptomic analysis, underwent exact same exposure conditions. In brief, rats for each cohort were exposed to air or acrolein (n = 8/sex/group) (exposures for each sex conducted separately). Over 2 consecutive days prior to exposure, rats were acclimated to nose-only inhalation tubes (Lab Products, Seaford, DE). On day 1, rats underwent two 2 h acclimations 4 h apart, and on day 2, rats underwent a single 4 h acclimation. On the day of exposure, veterinary-grade artificial tears (Akorn Animal Health, Inc., Lake Forest, IL) were applied to the eyes and rats were exposed via nose-only inhalation to air (0 ppm) or acrolein for ∼4 h. Previously, to assess in real-time acrolein concentration-related effects on respiratory parameters during the 4 h exposure period, head-out plethysmography (HOP) was performed using a graded half-log exposure concentration paradigm, where for the first 30 min rats were exposed to 0, 0.10, 0.316, and 1 ppm acrolein (7.5 min/concentration) followed by 3.5 h of exposure at 3.16 ppm in the same animals. A separate air control group was also included that was exposed in parallel to filtered air for 4 h. Plethysmography, serum/plasma, and some nasal lavage fluid (NALF) and bronchoalveolar lavage fluid (BALF) data are reported in detail in our prior publication (Alewel et al., 2023b). To be consistent with that exposure paradigm, in this study for collecting nasal and lung tissue, the same protocol was used. Necropsies for sample collection in each cohort occurred immediately after exposure. Although the highest concentration of acrolein used here (3.16 ppm) is higher than ambient traffic-related acrolein pollution levels (De Woskin et al., 2003), woodsmoke and mainstream cigarette smoke contain up to 50 ppm and 90 ppm acrolein, respectively (Einhorn, 1975; Hristova et al., 2012).
Acrolein was obtained as a gas stored under inert nitrogen for transport (Airgas, Morrisville, NC). For exposures, mass flow controllers modulated flow of acrolein and dilution air (purified air) to generate desired concentrations using a modified in-house gas blending system (MKS, Andover, MA). Acrolein was directed to flow through a 52-outlet port nose-only inhalation tower with air flow of 0.35 L/min per inhalation port. Targeted acrolein concentrations delivered to nose-only ports in exposure tower were continuously measured during exposure using an Agilent 6890 gas chromatography analyzer with flame ionization detection and 624 capillary columns (Supelco, Bellefonte, PA), and flows were adjusted as necessary to maintain the targeted acrolein concentrations (no deviations in target concentration were noted). Temperature (21.8°C ± 0.3°C) and relative humidity (38.9% ± 2.7%) in the room where the nose-only chambers were located were monitored once per hour during exposure.
Immediately after exposure, rats were transported for necropsy to be weighed and euthanized via an intraperitoneal injection of sodium pentobarbital (>200 mg/kg) (Fatal-plus; Covetrus, Portland, ME) in a staggered manner between air- and acrolein-exposed rats (all necropsies completed within 1-2 h after end of exposure). For the animals in the first cohort, BALF was collected. The left lung lobe was tied off, and calcium- and magnesium-free phosphate buffered saline (PBS) warmed to 37°C was instilled into the lung through the trachea. BALF instillation volume was based upon a total lung capacity being 28 mL/kg body weight and right lung weight as approximately 60% of total lung weight. NALF was collected from the same cohort of animals through a retrograde instillation of 2 mL warm PBS through the pharyngeal opening of the nasal cavity, allowing lavage fluid to freely drip through nares. Another 1 mL of air was instilled in the same manner to collect any residual NALF. Aliquots of whole NALF and BALF were used for total cell quantification, using a Z1 Coulter Counter (Coulter, Inc., Miami, FL). Albumin levels in NALF and BALF were assessed using kits from Sekisui Diagnostics (Burlington, MA), with the protocol for this assay adapted for use on a Konelab Arena 30 clinical analyzer (Thermo Chemical Lab Systems, Espoo, Finland). From the second cohort of animals, un-lavaged left lung was removed and snap frozen in liquid nitrogen. From the same cohort, the head was removed and split down the sagittal crest to reveal the nasal cavities. Lateral and septal wall nasal respiratory epithelium was scraped from the nasal cavity, collected on parafilm, and frozen in liquid nitrogen. Olfactory epithelial tissues, which are visually distinct, were avoided while collecting septal and lateral wall respiratory epithelial tissues. In rodents, the olfactory mucosa is anatomically blocked from main inspired airflow (Harkema, 1990; Chamanza and Wright, 2015). Therefore, to not introduce variability in mRNA assessment that may arise from differential expression profiles between structurally and functionally unique nasal mucosal zones, we focused our assessment on nasal respiratory epithelium. All samples were stored at −80°C for later RNA analysis.
NALF and BALF cytokines interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), C-X-C motif chemokine ligand 1 (CXCL1), interleukin-1β (IL-1β), interferon-γ (IFN-γ), and interleukin-13 (IL-13) were analyzed using V-PLEX pro-inflammatory panel 2 rat kits (Mesoscale Discovery Inc., Rockville, MD). The manufacturer’s protocol was followed for assessment in multiplex format. Electrochemiluminescence signals were measured using MESO QuickPlex SQ 120 platform (Mesoscale Discovery Inc., Rockville, MD). Protein signals were measured using a 4-parameter logistic model to determine sample concentration using MSD Discovery Workbench analysis software (Mesoscale Discovery Inc., Rockville, MD).
Nasal epithelial tissue (n = 5-6/group) and uniform portions of the left lung (n = 6–8/group) from male rats were used for RNA extraction and processed with RNeasy mini kits (Qiagen, Valencia, CA) as directed by the manufacturer. Following extraction, RNA quantity and purity (260/230 and 260/280 ratios) were determined using a Nanodrop 1000 (ThermoFisher Scientific Inc., Waltham, MA). RNA integrity was assessed by the RNA 6000 LabChip® kit and a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) using a RIN cutoff of 7. mRNA samples were randomized and processed on an Apollo324 automated system (Takara Bio Inc., Kusatsu, Japan) for library prep with PrepX mRNA 48 protocol v19, using the PrepXTM RNA-seq for Illumina Library Kit (Takara), SuperScript III reverse transcriptase (ThermoFisher), and AMPure XP Beads (Agilent). PCR amplification with 48 index primers was run for 16 cycles and resulting PCR product quality was analyzed again via Qubit (ThermoFisher) and Bioanalyzer (Agilent). An RNAseq library was prepared with Wafergen’s PrepX mRNA 48 protocol and dsDNA products were prepared from cDNA. Each library was sequenced according to Illumina NextSeq 500, with a final concentration of 2.2 pM + 2% PhiX and run for 75 cycles (Illumina Inc., San Diego, CA).
The data for NALF and BALF albumin, total cells, and cytokine proteins were analyzed using GraphPad Prism software v9. Due to low basal levels of proteins in NALF, some samples for albumin content in air-exposed groups were below the detection limit (BDL). For these samples, BDL values were imputed with the limit of detection (LOD) using LOD/√2. Likewise, in some cytokines, particularly among air-exposed controls in nasal lavage, few samples that were BDL were imputed in the same manner using LOD/√2 for each analyte for statistical comparison. First, outliers were identified and discarded using a robust regression outlier test (ROUT) with a false discovery threshold of 0.01 (Q = 1%). To meet the assumptions of normality and homoscedasticity within an analysis of variance (ANOVA), data underwent Shapiro-Wilks normality (α = 0.05) and Levene’s equality of variance tests. Data that did not meet ANOVA assumptions of equal variance and/or normality were Log transformed [Y = Log (Y)] prior to analysis. NALF and BALF markers were analyzed separately using a two-way ANOVA (sex, exposure). A Tukey’s multiple comparisons post-hoc was used and significance was determined at p
Sequenced mRNA reads were mapped to the rat genome (rn7) using ensemble release 105 in the Partek Flow suite, R version 4.1.3. In total, 24,091 genes were mapped. Genes that did not have at least 1 read in 10 samples were removed, leaving 20,580 genes. An average of 15.5 million reads were mapped per sample with a standard deviation of 5.5 million. One nasal sample was removed because of low total reads (∼300,000). Principal components analysis was performed to identify any samples that did not cluster and should be removed; none were removed in this step. The remaining samples had 15.8 million reads per sample with a standard deviation of 5.3 million, with an average read per gene of 766. Differential gene expression was assessed in DESeq2 package in R (v1.34.0) (Love et al., 2014) and normalized reads were assessed for acrolein effects. Differential expression analyses were performed within tissue to assess the effects of acrolein. Gene expression was considered different when the fold change (FC) was greater than 1.2 (upregulation) or less than 0.833 (downregulation) and the Benjamini–Hochberg false discovery rate
For each heatmap, normalized read counts for each gene were averaged and calculated as the standard deviation from the mean, reflecting row z-score of each gene as up- (yellow) or downregulated (blue). Genes were clustered according to average-linkage Euclidean distancing using the online tool http://heatmapper.ca (last accessed 22 June 2023). We utilized Ingenuity Pathway Analysis (IPA) software (QIAGEN, Redwood City, CA; www.qiagen.com/ingenuity; last accessed 22 June 2023), which compares our mRNA expression dataset to previously reported databases and reports overlaps in expression changes for interpretation of major pathway and gene changes. Top canonical pathways were selected based upon significance between overlap of molecules in our dataset and molecular changes consistent with a particular network (adj. p-value <0.1). To infer involvement of upstream regulators, we determined an |activation z-score|
To assess overall upper and lower airway injury and inflammation patterns in response to acrolein inhalation, we measured albumin and total cells in NALF and BALF (Figure 1). A single exposure to acrolein resulted in higher NALF albumin levels in male and female rats when compared to respective air groups, but did not change BALF albumin levels (Figure 1A). Interestingly, although both sexes demonstrated nasal protein leakage, only acrolein-exposed males had higher NALF total cell counts, which we previously reported as neutrophilic and eosinophilic inflammation (Alewel et al., 2023b) (Figure 1B). Further, acrolein exposure significantly elevated BALF total cell counts in both sexes (Figure 1B).
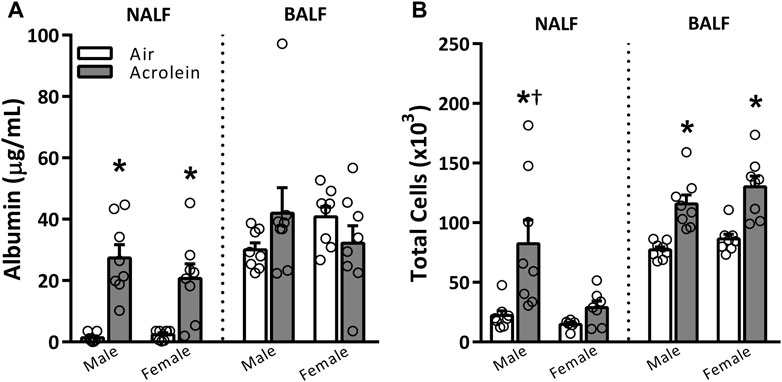
FIGURE 1. Acute acrolein inhalation induces both vascular leakage and inflammation in nasal airways but only inflammation in the pulmonary airways (males > females). Markers of injury and inflammation, albumin (A) and total cell counts (B), respectively, were measured in the nasal lavage fluid (NALF) and bronchoalveolar lavage fluid (BALF) in samples collected immediately following air or acrolein inhalation. Data are mean ± SEM. * indicates a significant acrolein effect; † indicates a significant sex-effect (n = 6–8/group; p < .05).
NALF and BALF samples were further assessed to examine the potential involvement of various pro-inflammatory cytokines (Figure 2). The only cytokine impacted in both sexes was IL-6, which, compared to air group, was higher in NALF of acrolein-exposed males and females and BALF of acrolein-exposed males, and nearly significant in female BALF (p = 0.053) (Figure 2A). Other cytokine alterations followed a sex-specific response only in NALF, suggesting an effect at the site of nasal acrolein deposition. TNF-α, IL-1β, and IFN-γ levels were higher in NALF of acrolein-exposed males, whereas no other changes in these cytokines were noted in NALF of females or BALF of either sex (Figures 2B, D, E). CXCL1 and IL-13 NALF and BALF levels were not impacted by acrolein exposure in either sex, except for a decrease in BALF CXCL1 in acrolein-exposed females (Figures 2C, F). Few cytokines showed differences among air control groups, where NALF IFN-γ levels and BALF CXCL1 levels were higher in female air controls compared to males (Figures 2C, E).
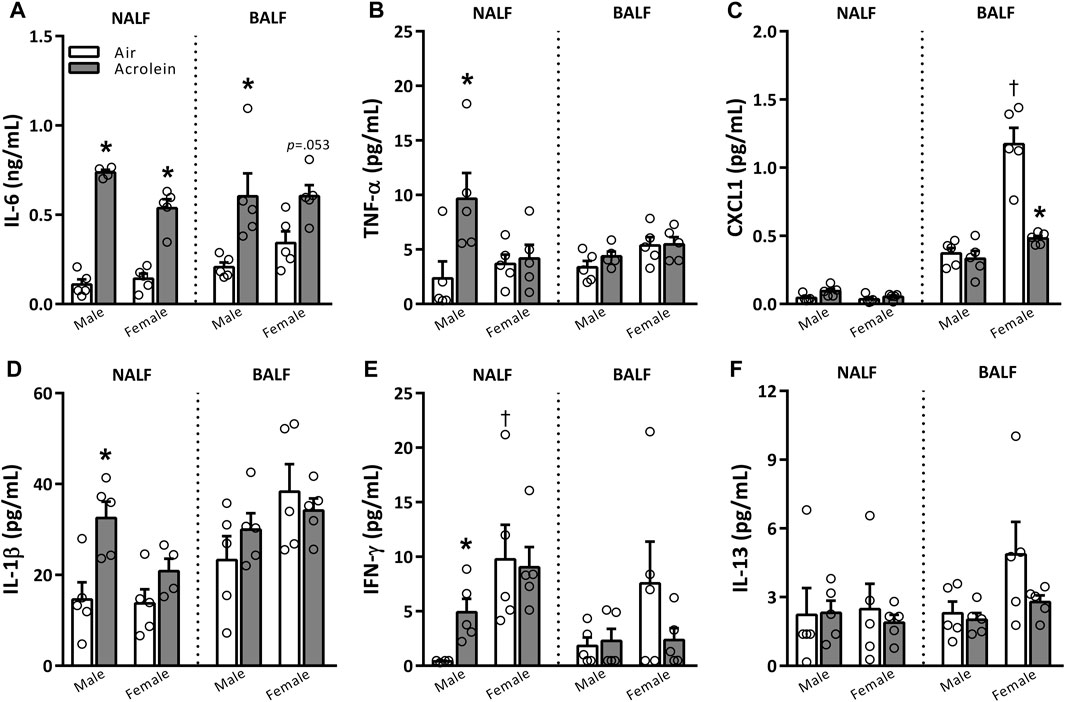
FIGURE 2. Acrolein alters respiratory pro-inflammatory cytokine levels in a sex- and site-specific manner. A panel of pro-inflammatory cytokine proteins were assessed in nasal lavage fluid (NALF) and bronchoalveolar lavage fluid (BALF), including interleukin-6 (IL-6, (A)), tumor necrosis factor-α (TNF-α, (B)), CXC motif ligand 1 (CXCL1, (C)), interleukin-1β (IL-1β, (D)), interferon-γ (IFN-γ, (E)), and interleukin-13 (IL-13, (F)). Data are mean ± SEM. * indicates a significant acrolein effect; † indicates a significant sex-effect (n = 4-5/group; p < .05).
To assess nasal versus lung transcriptional responses to acrolein, we performed global mRNA analysis in each tissue. Only males were used due to their greater susceptibility to acrolein-induced airway injury and inflammation when compared to females. Figure 3 shows volcano plots of RNAseq data obtained from nasal and lung tissues. Acrolein inhalation changed expression of 452 genes in the nasal epithelium (310 upregulated and 142 downregulated) (Figure 3A). Comparatively, acrolein exposure resulted in nearly 5-fold fewer DEGs in lung, changing expression of 95 genes (80 upregulated and 15 downregulated) (Figure 3B). To determine the overall effects on biological processes within nasal and lung tissue, IPA core analysis was performed. Figures 3C, D show the graphical summary of the interactive influence on key signaling regulators up- or downregulated after acrolein exposure in the nasal and lung tissue, respectively. This core analysis illustrates relations among predicted biological pathways, transcription factors, and important signaling modulators being impacted by acrolein exposure in the nasal versus lung tissue. Overall, tissue-specific differences can be seen in the number and function of biological processes involved in transcriptional alterations (Figure 3). This IPA core analysis demonstrates that cell signaling pathways and transcriptional activations involving innate inflammatory processes mediated by oxidative stress are induced in the nasal tissues with inhibition of IL-10 signaling. The increases in mRNA transcriptions for IL-6, IFN-γ, IL-1β, and TNF genes (Figure 3C) coincide with increases in these cytokine proteins in the NALF. The absence of these pathways activated in the lung is consistent with the lack of increases in these cytokines in the BALF. Rather, the pathways changed in the lung tissue show that cell growth and proliferation processes might be activated (Figure 3D).
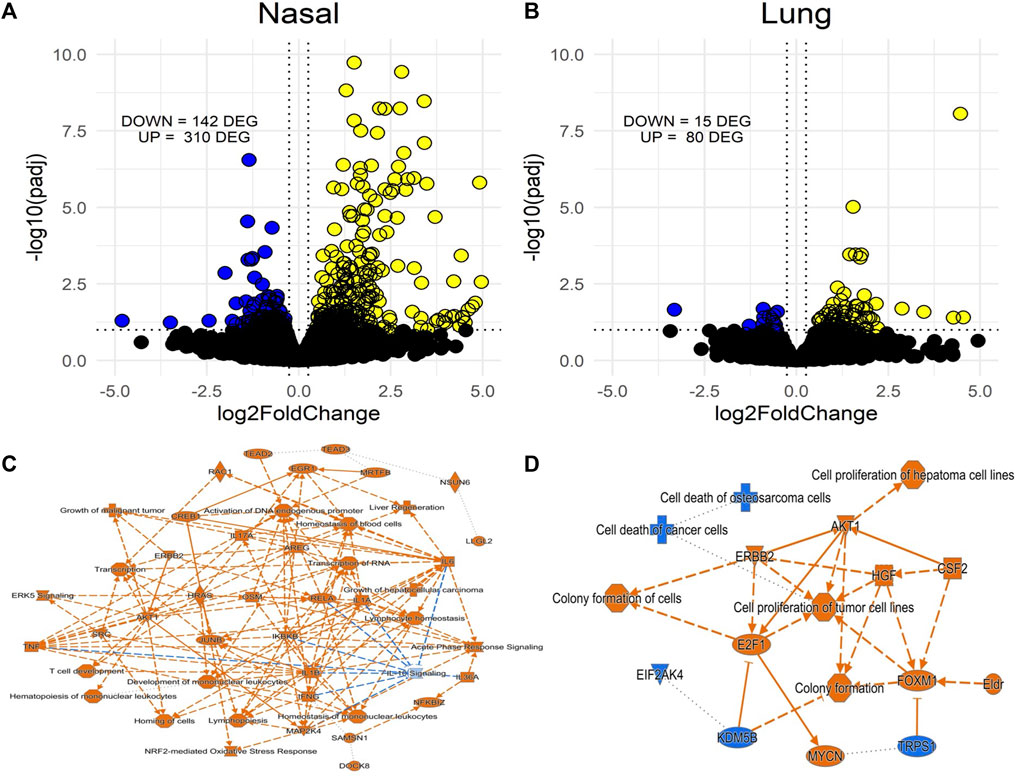
FIGURE 3. Volcano plots of differentially expressed genes (DEGs) from RNAseq in nasal (A) and lung tissue (B) of acrolein-exposed male rats and accompanying Ingenuity Pathway Analysis (IPA) activity plots showing primary signaling networks being overrepresented or underrepresented in nasal epithelial (C) and lung tissue (D). More transcriptomic alterations were observed in nasal tissue, 310 upregulated and 142 downregulated DEGs, compared to lung tissue, which had 80 upregulated and 15 downregulated DEGs. Activity plots show overall inferred relationships between molecules and functions based on DEGs. Significance threshold was set at an absolute fold change in expression >20% (Log2FC) and adj. p-value <.1 (-Log10padj), with cutoffs shown by dotted lines on volcano plots (blue are downregulated and yellow are upregulated DEG). For activity plots, color indicates a positive (orange) or negative (blue) relationship, and lines indicate a direct (solid) or indirect (dashed) relationship.
To highlight the similarities and differences of acrolein effects on cellular processes between the nasal and pulmonary tissues, the top 20 up- and 20 downregulated genes in nasal tissues were compared with same genes in the lung and vice versa (Figure 4). The heat map in Figure 4A displays the top 20 DEGs up- and downregulated in the nose (ranked based on fold change), with the listing of changes in same genes in the lung. In the nose, the most upregulated gene was Hmox1 (Log2FC = 4.63), a gene regulating iron homeostasis and oxidative imbalance (Chen et al., 2020), and the most downregulated gene was Gmnc (Log2FC = −1.40), a gene involved in the differentiation of ciliated epithelial cells (Zhou et al., 2015) (Figure 4A). Other top upregulated nasal genes included multiple FOS family genes (Fos, Fosl1, and Fosb) involved in acrolein toxicity (Yeager et al., 2016), and those downregulated included genes regulating ion channel functions and cellular transport (Figure 4A). It is noteworthy most of these same genes did not respond to acrolein exposure in lung tissue. While acrolein exposure caused only a fifth of the number of genes to be differentially expressed in the lung when compared to nasal airways, Hmox1 was commonly upregulated in both tissues (Figure 4B). The heat map in Figure 4B displays the top DEGs in the lung from acrolein exposure, with the same genes selected and matched for nasal airways for comparison. In the lung, the most upregulated DEG was Slc16a5 (Log2FC = 1.98) involved in transport of monocarboxylate metabolites such as pyruvate and lactate (Ren et al., 2022), and the most downregulated gene was Card9 (Log2FC = −0.95), an upstream regulator involved in activation of NFκB and apoptosis signaling (Bertin et al., 2000). This pattern of change depicts that in the lung, acrolein transcriptional response likely did not involve the activation of NFκB signaling despite observed increases in BALF cells. Other genes downregulated in the lung included Hpse2, Ahr, and Irs1, all involved in cellular metabolism. Overall, few overlapping genes were differentially expressed in both tissues following acrolein exposure. We found 7 overlapping genes, Hmox1, Cebpd, Akr1b10, Slc7a11, Slc16a5, Hap1, and Tsku, differentially expressed in both nasal and lung tissue (Supplementary Table S2).
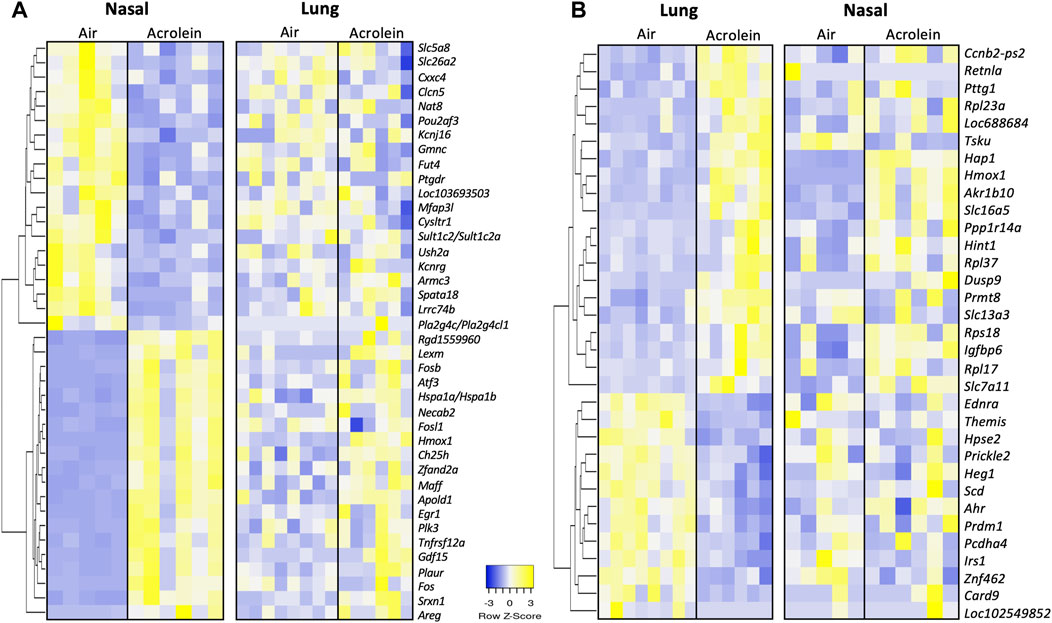
FIGURE 4. Heatmaps of top 20 up- and 20 downregulated differentially expressed genes (DEGs) in either nasal tissue with matching lung genes (A) or lung with matching nasal tissue genes (B). Genes in either nasal tissue or lung were selected based upon highest or lowest fold change in expression, where row z-score are shown in heatmap hierarchically clustered by average-linkage Euclidean distance (nasal, n = 5-6; lung, n = 6–8). The same genes were selected and match ordered for tissue comparison of top DEGs. Upregulated genes are shown in yellow and downregulated genes are shown in blue. Supplementary Table S1 provides further gene information.
To obtain insight into processes affected by acrolein exposure, we performed canonical pathway analysis of mRNA expression data. Table 1 shows the top 15 most significantly altered pathways (adj. p-value <0.1) in each tissue following acrolein exposure. Notable pathways enriched by acrolein exposure in nasal tissue included acute phase response (APR) signaling, Nrf2-mediated oxidative stress, IL-6 signaling, unfolded protein response, ERK5 signaling, and HMGB1 signaling. Pathways downregulated by acrolein exposure in nasal RNAseq data were IL-10 and ATM signaling (Table 1). Unlike widespread transcriptional changes indicative of oxidative stress response and activation of inflammatory processes in the nasal tissue, the enriched canonical pathways in the lung indicated changes in xenobiotic metabolism.
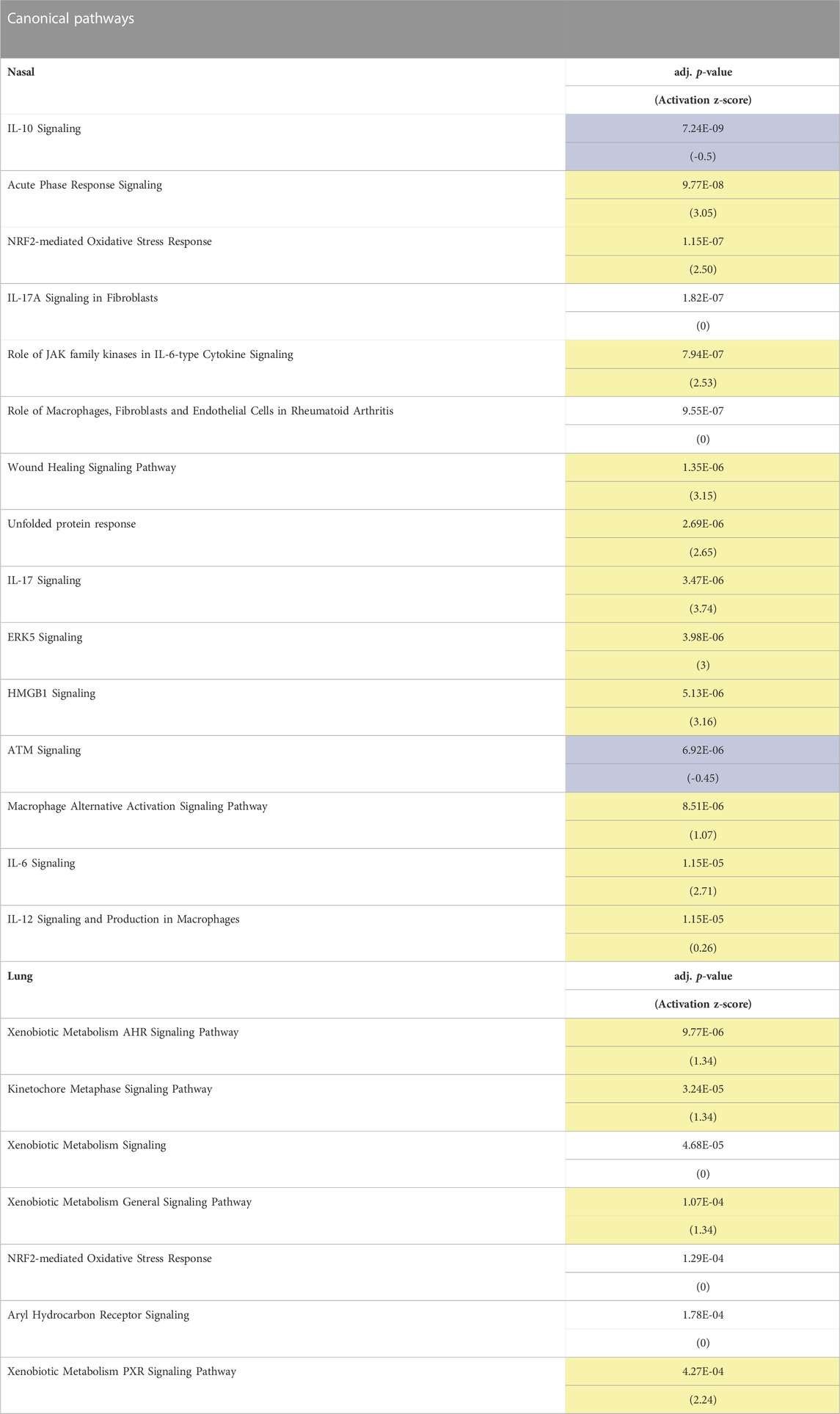
TABLE 1. Canonical pathways identified by Ingenuity Pathway Analysis (IPA) in nasal and lung tissue suggests activation of oxidative stress and inflammatory pathways in nasal tissue and xenobiotic metabolism pathways in lung tissue. Table shows top directionally predicated canonical pathways in nasal and lung tissue (adj. p < 0.1). Canonical pathways shown are based upon p-value of overlap of differentially expressed genes (DEGs) with IPA networks, and include activation z-score, which predicts the activation state of a pathway (positive values in yellow indicates pathway activation, negative values in blue indicates pathway inhibition, and no highlight is non-directional). Pathway analysis in lung tissue yielded fewer associated pathways due to fewer transcriptional changes.
Figure 5A shows 18 significant DEGs within the APR network in nasal tissue. Multiple genes associated with APR activation were enriched in nasal but not lung tissue, including Il6, Cxcl2, Hamp, Tnfrsf1a, and Serpine1, corroborating NALF and BALF pro-inflammatory cytokine involvement (Figure 5A). Genes Map2k3 and Map3k14, components of mitogen-activated protein kinase (MAPK) cascades in innate immune activation, were significantly enriched in nasal epithelium (Figure 5A). Acrolein’s effect on APR genes in the lung was smaller than that in the nasal tissue. Further, 18 DEGs comprised Nrf2-mediated oxidative signaling in the nose (Figure 5B). Various AP-1 subunit genes (Fos, Fosl1, Jun, Junb, and Jund) were enriched in the nose, along with Gclc and Gclm (Figure 5B). Among DEGs identified within these nasal pathways, only Hmox1 was significantly altered in the lung (Figures 5A, B).
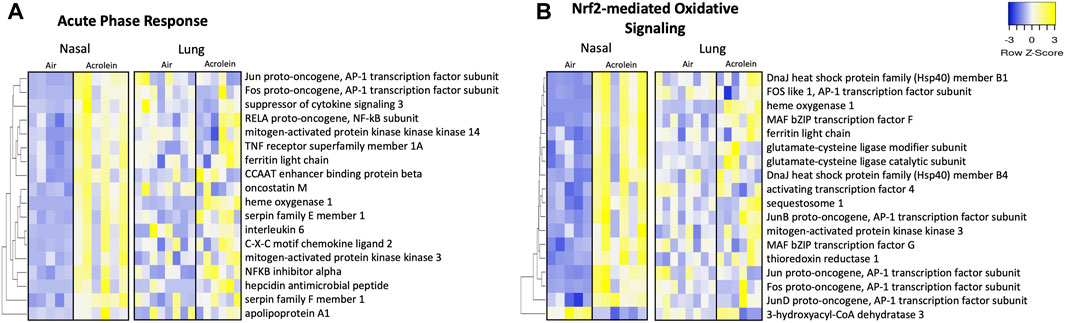
FIGURE 5. Acute phase response and Nrf2-mediated oxidative inflammatory signaling pathways are enriched in nasal but not lung tissue following acute acrolein inhalation. Inflammatory pathways of acute phase response (A) and Nrf2-mediated oxidative signaling (B) were among top enriched transcriptional pathways in nasal tissue. Nasal tissue mRNA heatmaps show differentially expressed genes (DEGs) within each pathway hierarchically clustered by average-linkage Euclidean distance. The same genes were selected from lung RNAseq results and ordered to match nasal clustering for tissue comparison of inflammatory and oxidative stress changes. DEGs were determined at an absolute fold change >20% and adj. p-value <.1 (nasal, n = 5–6; lung, n = 6–8). Yellow signifies increased gene expression and blue indicates decreased gene expression. Supplementary Table S3 shows Log2FC and adjusted p-value for each gene.
To explore the involvement of nuclear receptor signaling in upper and lower airway acrolein effects, we identified molecules pertaining to glucocorticoid receptor (GR) and G protein-coupled receptor (GPCR) signaling through IPA analysis (Figure 6), which is potentially affected based on our prior observation that circulating corticosterone and epinephrine are increased in rats after acrolein exposure (Snow et al., 2017; Alewel et al., 2023b). Overall, acrolein-exposed rats exhibited many gene expression changes modulated by GR and GPCR signaling in the nose that were not observed in the lung. Prominent genes enriched in the nasal GR signaling pathway included Hsp70 encoding genes (Hspa1a/Hspa1b, Hspa1l, and Hspa8) and Sgk1 (Figure 6A), which is a glucocorticoid-regulated kinase prompted by cellular stress (Jang et al., 2022). Multiple GPCR signaling network changes were observed in the nose following acrolein exposure, whereas these effects were not seen in the lung (Figure 6B). GPCR-mediated changes altering intracellular adenylate cyclase or cyclic-AMP (cAMP) levels were noted through increased nasal expression of genes such as Adra2a, Adora2a, Creb3l3, and Atf4. Among the 21 GR and 26 GPCR signaling mRNA expression changes noted in the nasal tissue, none of these genes were significantly changed in the lung (Figure 6).
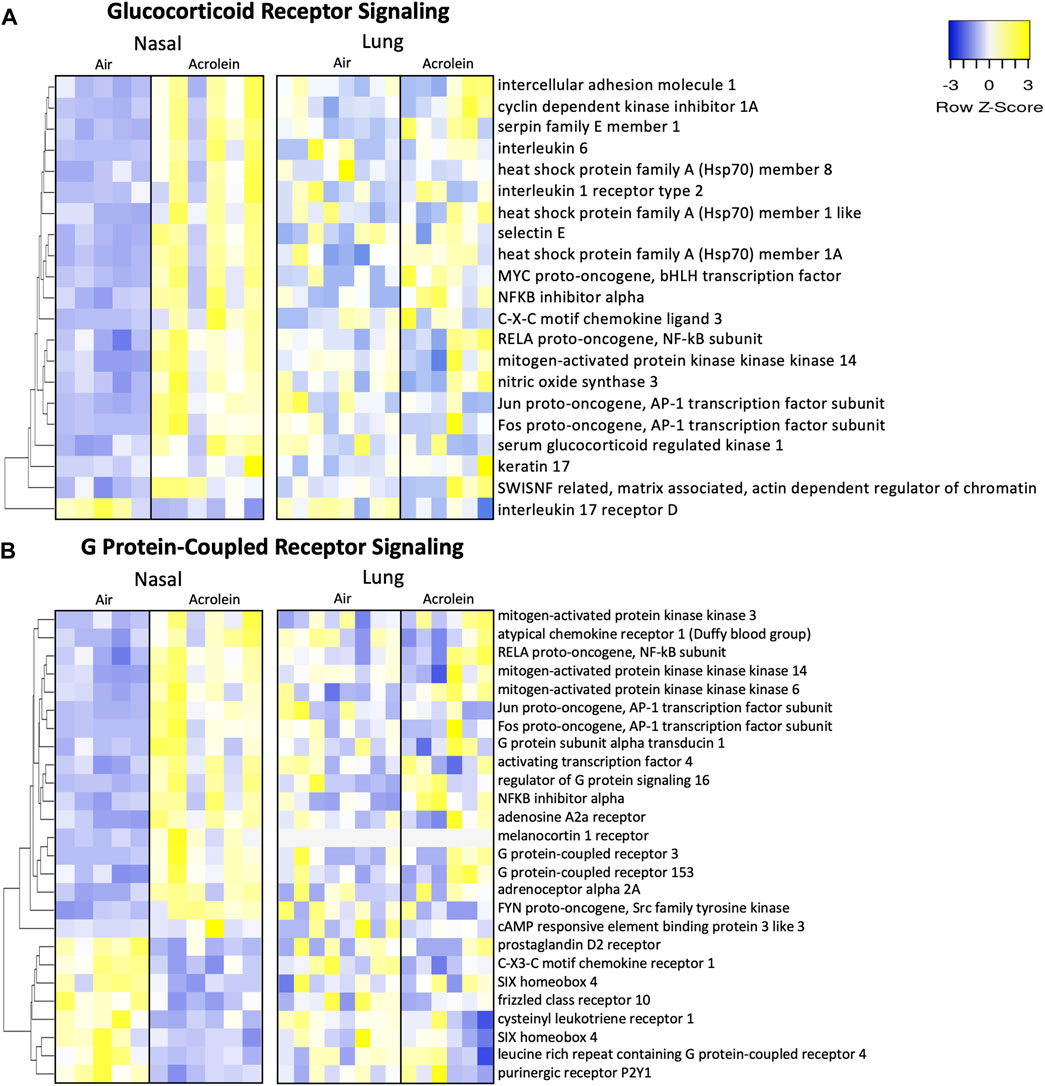
FIGURE 6. Acrolein-exposure alters glucocorticoid receptor (GR) and G protein-coupled receptor (GPCR) genes reflective of stress signaling in nasal epithelial but not lung tissue. Differentially expressed genes (DEGs) involved in GR signaling (A) and GPCR signaling (B) were identified in nasal tissue (n = 5-6) and hierarchically clustered by average-linkage Euclidean distance. These same genes were identified in lung (n = 6–8) and organized in the same manner for site-specific comparison-associated transcriptional alterations. Yellow indicates increased expression and blue indicates decreased expression (fold change >20%; adj. p-value <.1). Supplementary Table S4 shows Log2FC and adj. p-value for each gene.
Because one of the objectives of our transcriptomic analysis was to identify the involvement of adrenal-hormone signaling in acrolein airways effects, we evaluated specific glucocorticoid-responsive genes that are extensively validated as consistent markers of GR activity due to their gene loci often falling within glucocorticoid response elements (GRE) (Wang et al., 2004; Kan and Himes, 2021). Table 2 shows 9 such genes selected from nasal and lung RNAseq data. Ccl2, Cebpd, Per1, Sgk1, Thbd, Ccl20, and Gadd45b were significantly enriched in nasal tissue only, except for Cebpd which shared increased lung expression. Interestingly, Fkbp5, a prominent modulator of glucocorticoid signaling, was enriched only in the lung, also seen in prior ozone studies (Henriquez et al., 2021).
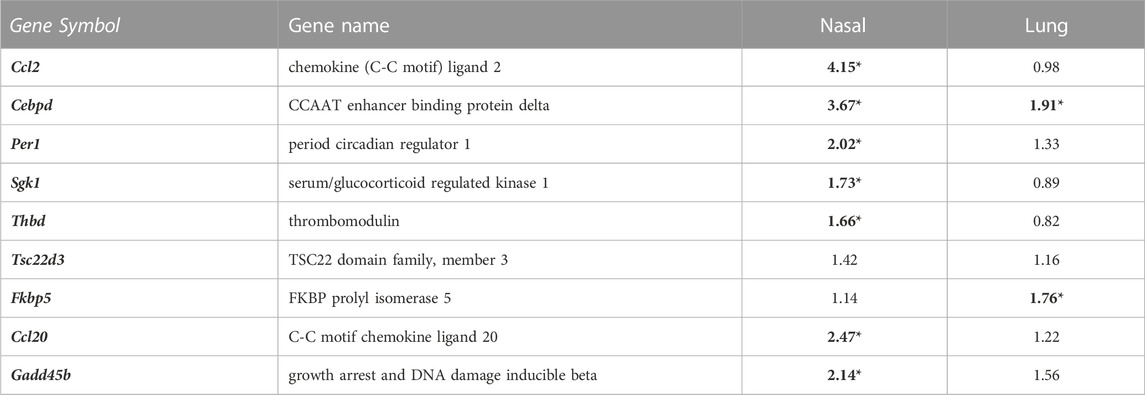
TABLE 2. Well characterized glucocorticoid-responsive genes are changed in nasal and lung tissue following acrolein exposure. Genes validated by prior ‘omics studies as consistently regulated by glucocorticoid-mediated cellular signaling are shown by fold change (FC) in nasal (n = 5-6) and lung tissue (n = 6–8), where a FC in bold with an * represents a significant change in expression following acrolein exposure (adj p-value <0.1). Supplementary Table S5 displays adj. p-value for each gene.
We used the IPA upstream predictor tool to evaluate cytokines, transcriptional activators, or chemicals which shared similar gene changes with acrolein-induced airway effects (Table 3). Here, our gene expression dataset was compared to IPA databases to provide the most likely regulators responsible for network changes in either nasal or lung datasets. Nasal acrolein-induced changes predicted activated regulation by lipopolysaccharide (LPS), multiple cytokines, CREB1, and inhibition by various chemical and biologic agents (Table 3). Further, ozone and cigarette smoke were predicted chemical toxicants with gene signatures similar to acrolein-induced nasal effects, which is consistent with acrolein as a major tobacco smoke constituent (Hikisz and Jacenik, 2023), (Supplementary Table S6). Forskolin and methylprednisolone were also suggested upstream predictors of nasal transcriptomic changes (Supplementary Table S6), implicating the involvement of epinephrine-mediated GPCR signaling via activation of cAMP and corticosteroid effects on GR in acrolein-induced upper airway effects. The fewer transcriptional changes in the lung, however, were predicted to be related to different substances. The lung prediction algorithm identified similarities to activation of aflatoxin B1, NFE2L2 (Nrf2), and Vegf, as well as inhibition by multiple transcriptional regulators and chemical agents (Table 3).
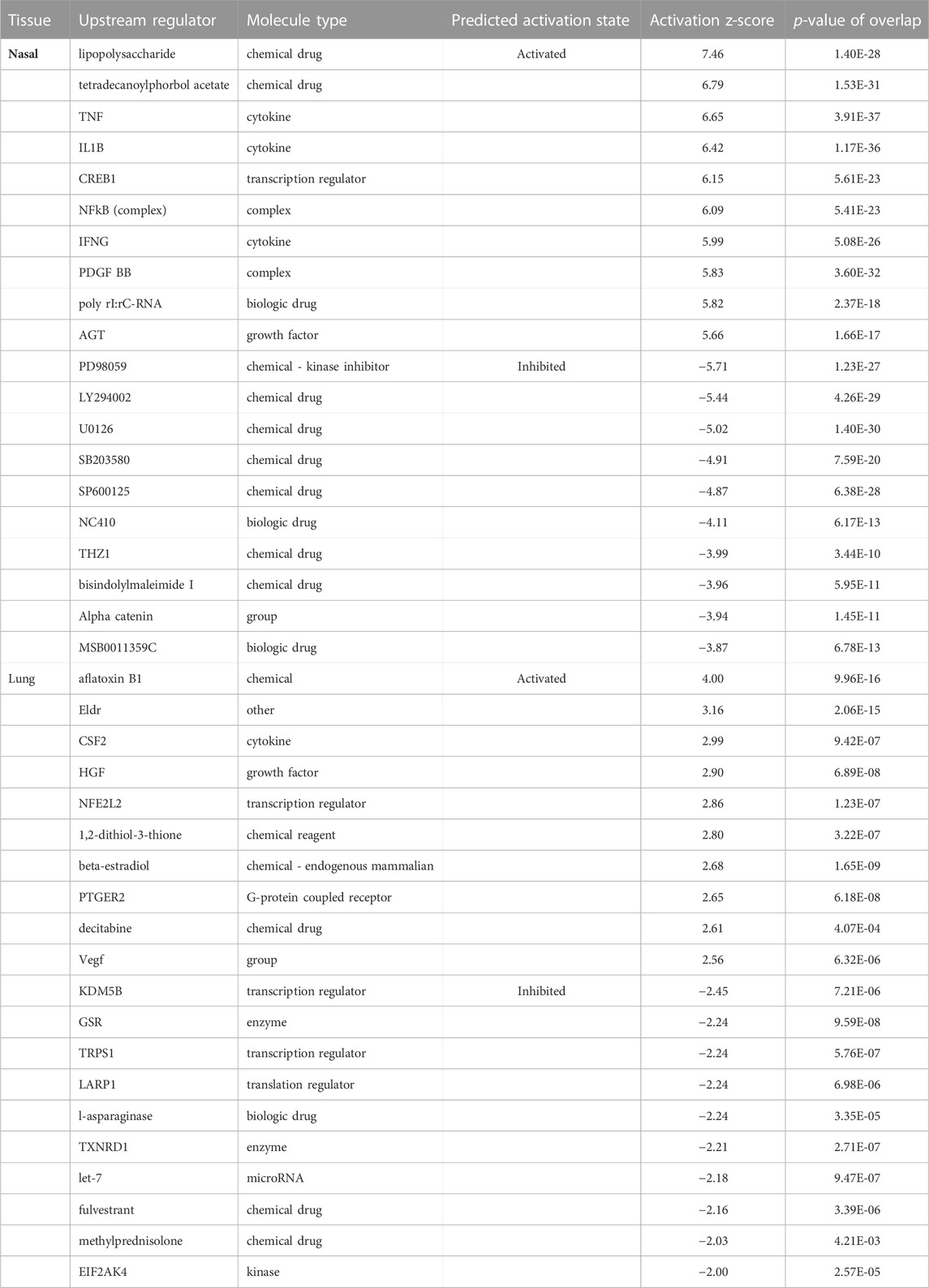
TABLE 3. Unbiased Ingenuity upstream predictor analysis of top predicted activators and inhibitors in nasal and lung tissue. Top 10 activated or inhibited upstream regulators in nasal and lung tissue associated with acrolein-induced transcriptional alterations are shown. IPA-identified upstream regulators based upon network associations with acrolein-induced transcriptional alterations in nasal or lung tissue. Activation z-score denotes regulator activation (>0) or inhibition (<0), where the magnitude of direction is depicted by higher absolute values. Supplementary Table S6 shows molecules falling within each regulator dataset, as well as additional regulators.
Volatile water-soluble vapors, such as acrolein, deposit in nasal airways of both rats and humans, but a greater proportion may deposit into nasal airways in rats than the tracheobronchial space in humans because of differences in airway morphology, cellular composition, breathing mode, and ventilation rate (Corley et al., 2012). Although computational approaches have been developed to predict airway injury in humans based on rodent inhalation dosimetry data (Cichocki et al., 2014), there remains considerable uncertainty in predicting human toxicity because sex- and site-specific variation in respiratory responses are not well defined. In this study, we assessed nasal (isolated nasal respiratory epithelial tissue) versus pulmonary responses to acrolein inhalation in rats to aid in understanding the molecular signatures of cellular effects in these compartments and how systemically released stress hormones might be modulating local nasal versus pulmonary injury and inflammation. Consistent with our previous findings (Alewel et al., 2023b), acute acrolein inhalation caused marked nasal injury and cytokine changes, with males being more sensitive than females. Transcriptomic analysis of nasal respiratory epithelium versus lung tissue of male rats revealed major differences in terms of the extent and type of changes following acrolein inhalation. There were 452 genes differentially expressed in acrolein-exposed nasal tissue versus only 95 genes in the lung. The changes in the nose were reflective of the activation of innate immune responses, characterized by NFκB, TNF, oxidative stress, Nrf2 signaling, and glucocorticoid and GPCR signaling. The lung showed fewer mRNA expression changes that were indicative of changes in xenobiotic metabolism and proliferation. These divergent responses may reflect the acrolein concentration encountered at the site of airway deposition, with likely localized influence of circulating stress hormones, and/or variance in site-specific cellular biology (including cell type) in the nose and lung.
Acute airway effects of aldehydes involve the induction of pro-inflammatory cytokine networks (van der Toorn et al., 2013; Sun et al., 2014; Xiong et al., 2018). Given that IL-6 was the only acute phase reactant increased in nasal tissue of both males and females, and in the BALF of males, this suggests a distinct mechanism that is not influenced by sex in the acrolein response that may serve as a generalized, sensitive indicator of inflammation. Higher TNF-α, IL-1β, and IFN-γ in acrolein-exposed male but not female NALF suggest sex-specific influence on these innate immune cytokines, perhaps through complex hormonal interactions. Glucocorticoids and steroidal hormones, including androgens/estrogens, are known to interactively regulate immune responses (Bereshchenko et al., 2018), which may underlie greater sensitivity of male rats to acrolein-induced inflammation under these conditions. Considering this exposure scenario has been shown to increase glucocorticoids only in males (Alewel et al., 2023b), further studies are needed to elucidate possible interactions between adrenal and gonadal hormones in sex-specific outcomes. Since acrolein induced nasal eosinophilic and neutrophilic extravasation in male rats (Snow et al., 2017; Alewel et al., 2023b), the cytokine results suggest a site-specific inflammatory response, whereby TNF-α, IFN-γ, and IL-1β play a critical signaling role in the nasal airways (Takeuchi and Akira, 2010; Aghasafari et al., 2019). Lastly, the lack of BALF cytokine changes despite increased inflammatory cells may indicate complex homeostatic responses to acrolein, whereby redistribution of circulating immune cells through adrenal-derived stress hormones (Dhabhar et al., 2012; Henriquez et al., 2017; Snow et al., 2017; Henriquez et al., 2018) might occur through the entire respiratory system under stress.
Previously reported inflammatory and oxidative stress-related gene expression and signaling in the nasal and tracheobronchial mucosa (Cichocki et al., 2014; Moghe et al., 2015) align well with transcriptional changes we observed in the nasal tissue after an acute acrolein exposure. In contrast to the few changes that were observed in the lung transcriptome in the present study, marked transcriptomic changes have been reported in the lungs of susceptible mouse strains after an acute acrolein inhalation, albeit at 3-4 times higher concentrations than what we used in the present study (Fabisiak et al., 2011). Further, C57BL/6J mice exposed to lethal concentrations of acrolein (50 ppm) showed pulmonary transcriptomic changes of inflammatory and oxidative cascades similar to current nasal results (Bein et al., 2021), suggesting the muted lung responses in the present study is related to limited lung deposition. Despite significantly lower oxidative and inflammatory changes in the lung, the pathway analysis of transcriptional data revealed activation of cell growth and proliferation-mediated processes by a long non-coding downstream RNA (Eldr) involved in cancer cell growth and promotion (Sur and Ray, 2022). It is likely that acrolein metabolites, which might have been encountered by the lung, may activate genes involved in systemically encountered small non-coding RNA-induced effects (Tang et al., 2011).
Top DEGs in the nose were most reflective of previously established molecular effects of acrolein toxicity, including the activation of Fos-Jun transcriptional machinery, as well as enhanced Atf3 that can cause ER stress and activation of cellular survival pathways (Chinenov and Kerppola, 2001; Haberzettl et al., 2009; Moghe et al., 2015). In the lung, aryl hydrocarbon receptor (AHR) and pregnane X receptor (PXR) xenobiotic signaling pathways were enriched, which are nuclear receptor pathways that coordinate xenobiotic metabolizing enzymes and cellular detoxification processes in response to environmental chemical stimuli (Oladimeji and Chen, 2018; Lamorte et al., 2021). Additionally, because of shared marked increased expression of Hmox1 and Slc7a11, a pair of genes that work in tandem to modulate iron homeostasis and cysteine/glutamate transport for cellular GSH biosynthesis (Poss and Tonegawa, 1997; Thompson and Burcham, 2008; Tang et al., 2021), it appears that acrolein-induced respiratory effects likely involve disrupted iron homeostasis processes in the nose and distal lung space.
Similar to ozone, acute acrolein inhalation increases circulating epinephrine and corticosterone (Snow et al., 2017; Alewel et al., 2023b). Ligand-activated GR and GPCR are two of the most transcriptionally active nuclear signaling receptors across the genome and are highly responsive to environmental stressors (Alhosaini et al., 2021; Frigo et al., 2021). Although adrenergic receptors (AR) and GR likely play a key role in mediating pulmonary health effects of various pollutants (Hodge et al., 2021), their involvement in nasal effects of acrolein is not established. The factors involved in acrolein stress, such as AP-1 and NFκB, are often co-regulated with GRE (Reddy et al., 2009). In this study, we noted GR and GPCR signaling pathways were enriched in the nasal tissue that included gene targets such as Hsp70, a co-chaperone essential for nuclear trafficking of GR (Pratt et al., 2006). Even though BALF analysis revealed no lung injury, glucocorticoid-responsive Fkbp5, encoding scaffolding proteins for GR assembly to modulate cellular sensitivity to glucocorticoids (Hartmann et al., 2021; Nold et al., 2021), was enriched only in the lung. In prior studies, we have shown the involvement of Fkbp5 in coordinating pulmonary and hypothalamic changes following exposure to ozone and particulate matter (Henriquez et al., 2019; Alewel et al., 2023a). Nasal mRNA expression was enriched for Adra2a, as well as the adenylate cyclase gene Adora2a, which both interact with the PI3K/AKT signaling pathway responsive to catecholamines (Shi et al., 2019; Ye et al., 2023). Genes modulated through catecholamine-induced cAMP intracellular messaging, such as Creb3l3, Atf4, and Ptgdr, were also enriched in the nose, perhaps linked with the activated cAMP-dependent transcription factors such as CREB1 predicted to be involved in upstream predictor analysis. Overall, these changes suggest that while GR and AR signaling mechanisms were activated in the nasal tissue, the lung also displayed some degree of glucocorticoid involvement in response to acrolein, but in a manner that was different than the nose.
Multiple nasal upstream regulators were predicted, as expected, to be similar to the effects of cigarette smoke, glucocorticoids, and inflammatory signaling regulators of oxidative stress. Chemicals or drugs that target molecular modes of acrolein toxicity, such as AP-1 and PI3K/AKT activity (Wan et al., 2022; Wu et al., 2022), were predicted as inhibited in IPA analysis, suggesting anti-inflammatory responses in the nose were inhibited. Interestingly, IPA predicted nasal mRNA effects significantly overlapped with prior ozone datasets, indicating airway effects of these volatile gases share similar yet site-specific oxidative and inflammatory responses. No top regulators were found to overlap between the nose and lung, although with low transcriptional alterations in the lung, there is a lower confidence in network prediction of upstream regulators. Nevertheless, multiple transcriptional regulators identified in acrolein-induced lung mRNA changes control cell cycle checkpoint and proliferation processes (Dey et al., 2008; Sun et al., 2012; Wu et al., 2014), suggesting upstream proto-oncogene effects in the lung that could have implications for dysregulated cellular growth over time.
It is important to highlight the limitations of this study, particularly as they pertain to the implications of these findings. The hypothesis generating observations in this study will need to be confirmed with targeted interventional approaches in both sexes. This nasal and lung transcriptional assessment did not involve long-term effects or temporality examination, as the changes observed immediately post exposure are likely reversible. A repeated or chronic acrolein exposure could trigger divergent mechanisms involved in inflammatory and stress signaling processes (Moghe et al., 2015), meaning respiratory toxicity outcomes of rodent or in vitro experiments should be analyzed in the context of the model being employed. Future investigations would benefit from assessing the potential habituation or desensitization of these nasal responses to intermittent or repeated acrolein exposure.
Here, we demonstrate that a single acrolein inhalation exposure produced nasal injury and inflammation while causing only a small inflammatory response in the lung, with the effects being more pronounced in male than female rats. Transcriptional responses in nasal versus lung tissue of acrolein-exposed male rats revealed quantitative and qualitative differences between tissues owing to expected depositional differences. Distinct transcriptional changes in the nasal tissue were characterized by the activation of major oxidative/antioxidative pathways, proinflammatory signaling, acute phase response, ER stress, glucocorticoid and GPCR signaling, and inhibition of anti-inflammatory mechanisms, whereas cellular proliferative responses and changes in xenobiotic metabolic processes were observed in the lung. Although pulmonary airways are likely not exposed to the same concentration of acrolein as nasal tissue, based on the distinct type of changes observed, it can be surmised that the biological processes that are affected between nasal airway versus pulmonary tissues, while influenced by similar circulating factors such as stress hormones and metabolites, are mechanistically different. Considering acute acrolein exposures are frequently encountered through wildfires, tobacco smoking, and anthropogenic pollution, these findings may help elucidate the pathophysiology of upper and lower airway disease affecting large segments of the population.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE247698.
The animal study was approved by United States Environmental Protection Agency Health Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
DA: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing–original draft, Writing–review and editing. TJ: Data curation, Formal Analysis, Software, Visualization, Writing–review and editing. KR: Investigation, Methodology, Writing–review and editing. MS: Investigation, Project administration, Resources, Writing–review and editing. AA-F: Formal Analysis, Resources, Writing–review and editing. SG: Investigation, Methodology, Validation, Writing–review and editing. PE: Investigation, Resources, Writing–review and editing. UK: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing–original draft, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the U.S. EPA Intramural Research funds. DA, KR, and TJ were supported, in part, by an appointment to the Research Participation Program at the Center for Public Health and Environmental Assessment at the U.S. EPA, administered by the Oak Ridge Institute for Science and Education through an interagency agreement (DE-SC0014664) between the U.S. Department of Energy and U.S. EPA.
Authors thank Dr. Aimen Farraj of the U.S. EPA and Dr. Gregory Smith of UNC Chapel Hill for their critical review of this manuscript. Dr. Mark Higuchi is acknowledged for coordinating inhalation exposures.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftox.2023.1280230/full#supplementary-material
Achanta, S., and Jordt, S. E. (2017). TRPA1: acrolein meets its target. Toxicol. Appl. Pharmacol. 324, 45–50. doi:10.1016/j.taap.2017.03.007
Agency for Toxic Substances and Disease Registry (ATSDR) (2007). Toxicological profile for acrolein. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
Aghasafari, P., George, U., and Pidaparti, R. (2019). A review of inflammatory mechanism in airway diseases. Inflamm. Res. official J. Eur. Histamine Res. Soc. 68 (1), 59–74. [et al. doi:10.1007/s00011-018-1191-2
Alarie, Y. (1973). Sensory irritation by airborne chemicals. CRC Crit. Rev. Toxicol. 2 (3), 299–363. doi:10.3109/10408447309082020
Alewel, D. I., Henriquez, A. R., Schladweiler, M. C., Grindstaff, R., Fisher, A. A., Snow, S. J., et al. (2023a). Intratracheal instillation of respirable particulate matter elicits neuroendocrine activation. Inhal. Toxicol. 35 (3-4), 59–75. doi:10.1080/08958378.2022.2100019
Alewel, D. I., Jackson, T. W., Vance, S. A., Schladweiler, M. C., Evansky, P. A., Henriquez, A. R., et al. (2023b). Sex-specific respiratory and systemic endocrine effects of acute acrolein and trichloroethylene inhalation. Toxicol. Lett. 382 (23), 22–32. doi:10.1016/j.toxlet.2023.05.005
Alhosaini, K., Azhar, A., Alonazi, A., and Al-Zoghaibi, F. (2021). GPCRs: the most promiscuous druggable receptor of the mankind. Saudi Pharm. J. SPJ official Publ. Saudi Pharm. Soc. 29 (6), 539–551. doi:10.1016/j.jsps.2021.04.015
Alwis, K. U., deCastro, B. R., Morrow, J. C., and Blount, B. C. (2015). Acrolein exposure in U.S. Tobacco smokers and non-tobacco users: NHANES 2005-2006. Environ. health Perspect. 123 (12), 1302–1308. doi:10.1289/ehp.1409251
Bautista, D. M., Jordt, S. E., Nikai, T., Tsuruda, P. R., Read, A. J., Poblete, J., et al. (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124 (6), 1269–1282. doi:10.1016/j.cell.2006.02.023
Bein, K., Birru, R. L., Wells, H., Larkin, T. P., Ge, T., and Leikauf, G. D. (2021). Sex-dependent acrolein sensitivity in mice is associated with differential lung cell, protein, and transcript changes. Physiol. Rep. 9 (19), e14997. doi:10.14814/phy2.14997
Bereshchenko, O., Bruscoli, S., and Riccardi, C. (2018). Glucocorticoids, sex hormones, and immunity. Front. Immunol. 9, 1332. doi:10.3389/fimmu.2018.01332
Bertin, J., Guo, Y., Wang, L., Srinivasula, S. M., Jacobson, M. D., Poyet, J. L., et al. (2000). CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J. Biol. Chem. 275 (52), 41082–41086. doi:10.1074/jbc.C000726200
Bromberg, P. A. (2016). Mechanisms of the acute effects of inhaled ozone in humans. Biochimica biophysica acta 1860 (12), 2771–2781. doi:10.1016/j.bbagen.2016.07.015
Chamanza, R., and Wright, J. A. (2015). A review of the comparative anatomy, histology, physiology and pathology of the nasal cavity of rats, mice, dogs and non-human primates. Relevance to inhalation Toxicology and human health risk assessment. J. Comp. pathology 153 (4), 287–314. doi:10.1016/j.jcpa.2015.08.009
Cheah, N. P., Pennings, J. L., Vermeulen, J. P., van Schooten, F. J., and Opperhuizen, A. (2013). In vitro effects of aldehydes present in tobacco smoke on gene expression in human lung alveolar epithelial cells. Toxicol. vitro Int. J. Publ. Assoc. BIBRA 27 (3), 1072–1081. doi:10.1016/j.tiv.2013.02.003
Chen, X., Yu, C., Kang, R., and Tang, D. (2020). Iron metabolism in ferroptosis. Front. cell Dev. Biol. 8, 590226. doi:10.3389/fcell.2020.590226
Chinenov, Y., and Kerppola, T. K. (2001). Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20 (19), 2438–2452. doi:10.1038/sj.onc.1204385
Cichocki, J. A., Smith, G. J., and Morris, J. B. (2014). Tissue sensitivity of the rat upper and lower extrapulmonary airways to the inhaled electrophilic air pollutants diacetyl and acrolein. Toxicol. Sci. official J. Soc. Toxicol. 142 (1), 126–136. doi:10.1093/toxsci/kfu165
Cloonan, S. M., and Choi, A. M. (2016). Mitochondria in lung disease. J. Clin. investigation 126 (3), 809–820. doi:10.1172/JCI81113
Corley, R. A., Kabilan, S., Kuprat, A. P., Carson, J. P., Minard, K. R., Jacob, R. E., et al. (2012). Comparative computational modeling of airflows and vapor dosimetry in the respiratory tracts of rat, monkey, and human. Toxicol. Sci. official J. Soc. Toxicol. 128 (2), 500–516. doi:10.1093/toxsci/kfs168
De Woskin, R., Greenberg, M., Pepelko, W., and Strickland, J. (2003). Toxicological review of acrolein (cas no. 107-02-08) in support of summary information on the integrated risk information system (Iris). Washington, DC: US Environmental Protection Agency.
Dey, B. K., Stalker, L., Schnerch, A., Bhatia, M., Taylor-Papidimitriou, J., and Wynder, C. (2008). The histone demethylase KDM5b/JARID1b plays a role in cell fate decisions by blocking terminal differentiation. Mol. Cell. Biol. 28 (17), 5312–5327. doi:10.1128/MCB.00128-08
Dhabhar, F. S., Malarkey, W. B., Neri, E., and McEwen, B. S. (2012). Stress-induced redistribution of immune cells--from barracks to boulevards to battlefields: a tale of three hormones--Curt Richter Award winner. Psychoneuroendocrinology 37 (9), 1345–1368. doi:10.1016/j.psyneuen.2012.05.008
Einhorn, I. N. (1975). Physiological and toxicological aspects of smoke produced during the combustion of polymeric materials. Environ. health Perspect. 11, 163–189. doi:10.1289/ehp.7511163
Fabisiak, J. P., Medvedovic, M., Alexander, D. C., McDunn, J. E., Concel, V. J., Bein, K., et al. (2011). Integrative metabolome and transcriptome profiling reveals discordant energetic stress between mouse strains with differential sensitivity to acrolein-induced acute lung injury. Mol. Nutr. food Res. 55 (9), 1423–1434. doi:10.1002/mnfr.201100291
Fowles, J., and Dybing, E. (2003). Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob. control 12 (4), 424–430. doi:10.1136/tc.12.4.424
Frigo, D. E., Bondesson, M., and Williams, C. (2021). Nuclear receptors: from molecular mechanisms to therapeutics. Essays Biochem. 65 (6), 847–856. doi:10.1042/EBC20210020
Gambeta, E., Chichorro, J. G., and Zamponi, G. W. (2020). Trigeminal neuralgia: an overview from pathophysiology to pharmacological treatments. Mol. pain 16, 1744806920901890. doi:10.1177/1744806920901890
Haberzettl, P., Vladykovskaya, E., Srivastava, S., and Bhatnagar, A. (2009). Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicol. Appl. Pharmacol. 234 (1), 14–24. doi:10.1016/j.taap.2008.09.019
Harkema, J. R. (1990). Comparative pathology of the nasal mucosa in laboratory animals exposed to inhaled irritants. Environ. health Perspect. 85, 231–238. doi:10.1289/ehp.85-1568334
Harkema, J. R., Hotchkiss, J. A., Barr, E. B., Bennett, C. B., Gallup, M., Lee, J. K., et al. (1999). Long-lasting effects of chronic ozone exposure on rat nasal epithelium. Am. J. Respir. cell Mol. Biol. 20 (3), 517–529. doi:10.1165/ajrcmb.20.3.3227
Hartmann, J., Bajaj, T., Klengel, C., Chatzinakos, C., Ebert, T., Dedic, N., et al. (2021). Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Rep. 35 (9), 109185. doi:10.1016/j.celrep.2021.109185
Hazari, M. S., Griggs, J., Winsett, D. W., Haykal-Coates, N., Ledbetter, A., Costa, D. L., et al. (2014). A single exposure to acrolein desensitizes baroreflex responsiveness and increases cardiac arrhythmias in normotensive and hypertensive rats. Cardiovasc. Toxicol. 14 (1), 52–63. doi:10.1007/s12012-013-9228-9
Henriquez, A., House, J., Miller, D. B., Snow, S. J., Fisher, A., Ren, H., et al. (2017). Adrenal-derived stress hormones modulate ozone-induced lung injury and inflammation. Toxicol. Appl. Pharmacol. 329, 249–258. doi:10.1016/j.taap.2017.06.009
Henriquez, A. R., House, J. S., Snow, S. J., Miller, C. N., Schladweiler, M. C., Fisher, A., et al. (2019). Ozone-induced dysregulation of neuroendocrine axes requires adrenal-derived stress hormones. Toxicol. Sci. official J. Soc. Toxicol. 172 (1), 38–50. doi:10.1093/toxsci/kfz182
Henriquez, A. R., Snow, S. J., Schladweiler, M. C., Miller, C. N., Dye, J. A., Ledbetter, A. D., et al. (2018). Adrenergic and glucocorticoid receptor antagonists reduce ozone-induced lung injury and inflammation. Toxicol. Appl. Pharmacol. 339, 161–171. doi:10.1016/j.taap.2017.12.006
Henriquez, A. R., Williams, W., Snow, S. J., Schladweiler, M. C., Fisher, C., Hargrove, M. M., et al. (2021). The dynamicity of acute ozone-induced systemic leukocyte trafficking and adrenal-derived stress hormones. Toxicology 458, 152823. doi:10.1016/j.tox.2021.152823
Hikisz, P., and Jacenik, D. (2023). The tobacco smoke component, acrolein, as a major culprit in lung diseases and respiratory cancers: molecular mechanisms of acrolein cytotoxic activity. Cells 12 (6), 879. doi:10.3390/cells12060879
Hodge, M. X., Henriquez, A. R., and Kodavanti, U. P. (2021). Adrenergic and glucocorticoid receptors in the pulmonary health effects of air pollution. Toxics 9 (6), 132. doi:10.3390/toxics9060132
Hristova, M., Spiess, P. C., Kasahara, D. I., Randall, M. J., Deng, B., and van der Vliet, A. (2012). The tobacco smoke component, acrolein, suppresses innate macrophage responses by direct alkylation of c-Jun N-terminal kinase. Am. J. Respir. cell Mol. Biol. 46 (1), 23–33. doi:10.1165/rcmb.2011-0134OC
Jang, H., Park, Y., and Jang, J. (2022). Serum and glucocorticoid-regulated kinase 1: structure, biological functions, and its inhibitors. Front. Pharmacol. 13, 1036844. doi:10.3389/fphar.2022.1036844
Kan, M., and Himes, B. E. (2021). Insights into glucocorticoid responses derived from omics studies. Pharmacol. Ther. 218, 107674. doi:10.1016/j.pharmthera.2020.107674
Krämer, A., Green, J., Pollard, J., and Tugendreich, S. (2014). Causal analysis approaches in ingenuity pathway analysis. Bioinforma. Oxf. Engl. 30 (4), 523–530. doi:10.1093/bioinformatics/btt703
Lamorte, S., Shinde, R., and McGaha, T. L. (2021). Nuclear receptors, the aryl hydrocarbon receptor, and macrophage function. Mol. aspects Med. 78, 100942. doi:10.1016/j.mam.2021.100942
Love, M. I., Huber, W., and Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 (12), 550. doi:10.1186/s13059-014-0550-8
Mazzone, S. B., and Undem, B. J. (2016). Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 96 (3), 975–1024. doi:10.1152/physrev.00039.2015
Miller, D. B., Karoly, E. D., Jones, J. C., Ward, W. O., Vallanat, B. D., Andrews, D. L., et al. (2015). Inhaled ozone (O3)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicol. Appl. Pharmacol. 286 (2), 65–79. doi:10.1016/j.taap.2015.03.025
Miller, D. B., Snow, S. J., Schladweiler, M. C., Richards, J. E., Ghio, A. J., Ledbetter, A. D., et al. (2016). Acute ozone-induced pulmonary and systemic metabolic effects are diminished in adrenalectomized rats. Toxicol. Sci. official J. Soc. Toxicol. 150 (2), 312–322. doi:10.1093/toxsci/kfv331
Moghe, A., Ghare, S., Lamoreau, B., Mohammad, M., Barve, S., McClain, C., et al. (2015). Molecular mechanisms of acrolein toxicity: relevance to human disease. Toxicol. Sci. official J. Soc. Toxicol. 143 (2), 242–255. doi:10.1093/toxsci/kfu233
Morris, J. B., Stanek, J., and Gianutsos, G. (1999). Sensory nerve-mediated immediate nasal responses to inspired acrolein. J. Appl. physiology 87 (5), 1877–1886. doi:10.1152/jappl.1999.87.5.1877
Nold, V., Richter, N., Hengerer, B., Kolassa, I. T., and Allers, K. A. (2021). FKBP5 polymorphisms induce differential glucocorticoid responsiveness in primary CNS cells - first insights from novel humanized mice. Eur. J. Neurosci. 53 (2), 402–415. doi:10.1111/ejn.14999
Oladimeji, P. O., and Chen, T. (2018). PXR: more than just a master xenobiotic receptor. Mol. Pharmacol. 93 (2), 119–127. doi:10.1124/mol.117.110155
Poss, K. D., and Tonegawa, S. (1997). Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. U. S. A. 94 (20), 10919–10924. doi:10.1073/pnas.94.20.10919
Pratt, W. B., Morishima, Y., Murphy, M., and Harrell, M. (2006). Chaperoning of glucocorticoid receptors. Handb. Exp. Pharmacol. (172), 111–138. doi:10.1007/3-540-29717-0_5
Reddy, T. E., Pauli, F., Sprouse, R. O., Neff, N. F., Newberry, K. M., Garabedian, M. J., et al. (2009). Genomic determination of the glucocorticoid response reveals unexpected mechanisms of gene regulation. Genome Res. 19 (12), 2163–2171. doi:10.1101/gr.097022.109
Ren, T., Jones, R. S., and Morris, M. E. (2022). Untargeted metabolomics identifies the potential role of monocarboxylate transporter 6 (MCT6/SLC16A5) in lipid and amino acid metabolism pathways. Pharmacol. Res. Perspect. 10 (3), e00944. doi:10.1002/prp2.944
Saunders, C. J., Reynolds, S. D., and Finger, T. E. (2013). Chemosensory brush cells of the trachea. A stable population in a dynamic epithelium. Am. J. Respir. cell Mol. Biol. 49 (2), 190–196. doi:10.1165/rcmb.2012-0485OC
Shi, L., Wu, Z., Miao, J., Du, S., Ai, S., Xu, E., et al. (2019). Adenosine interaction with adenosine receptor A2a promotes gastric cancer metastasis by enhancing PI3K-AKT-mTOR signaling. Mol. Biol. cell 30 (19), 2527–2534. doi:10.1091/mbc.E19-03-0136
Snow, S. J., McGee, M. A., Henriquez, A., Richards, J. E., Schladweiler, M. C., Ledbetter, A. D., et al. (2017). Respiratory effects and systemic stress response following acute acrolein inhalation in rats. Toxicol. Sci. official J. Soc. Toxicol. 158 (2), 454–464. doi:10.1093/toxsci/kfx108
Stevens, J. F., and Maier, C. S. (2008). Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. food Res. 52 (1), 7–25. doi:10.1002/mnfr.200700412
Sun, R., Zhang, Q., Guo, L., Chen, M. Y., Sun, Y., Cao, B., et al. (2012). HGF stimulates proliferation through the HGF/c-Met pathway in nasopharyngeal carcinoma cells. Oncol. Lett. 3 (5), 1124–1128. doi:10.3892/ol.2012.613
Sun, Y., Ito, S., Nishio, N., Tanaka, Y., Chen, N., and Isobe, K. (2014). Acrolein induced both pulmonary inflammation and the death of lung epithelial cells. Toxicol. Lett. 229 (2), 384–392. doi:10.1016/j.toxlet.2014.06.021
Sur, S., and Ray, R. B. (2022). Emerging role of lncRNA ELDR in development and cancer. FEBS J. 289 (11), 3011–3023. doi:10.1111/febs.15876
Takeuchi, O., and Akira, S. (2010). Pattern recognition receptors and inflammation. Cell 140 (6), 805–820. doi:10.1016/j.cell.2010.01.022
Tang, D., Chen, X., Kang, R., and Kroemer, G. (2021). Ferroptosis: molecular mechanisms and health implications. Cell Res. 31 (2), 107–125. doi:10.1038/s41422-020-00441-1
Tang, M. S., Wang, H. T., Hu, Y., Chen, W. S., Akao, M., Feng, Z., et al. (2011). Acrolein induced DNA damage, mutagenicity and effect on DNA repair. Mol. Nutr. food Res. 55 (9), 1291–1300. doi:10.1002/mnfr.201100148
Thompson, C. A., and Burcham, P. C. (2008). Genome-wide transcriptional responses to acrolein. Chem. Res. Toxicol. 21 (12), 2245–2256. doi:10.1021/tx8001934
United States Environmental Protection Agency (2006). 1999 national air toxics assessment. Washington DC: Office of Air Quality and Planning Standards.
van der Toorn, M., Slebos, D. J., de Bruin, H. G., Gras, R., Rezayat, D., Jorge, L., et al. (2013). Critical role of aldehydes in cigarette smoke-induced acute airway inflammation. Respir. Res. 14 (1), 45. doi:10.1186/1465-9921-14-45
Wan, P., Zhang, S., Ruan, Z., Liu, X., Yang, G., Jia, Y., et al. (2022). AP-1 signaling pathway promotes pro-IL-1β transcription to facilitate NLRP3 inflammasome activation upon influenza A virus infection. Virulence 13 (1), 502–513. doi:10.1080/21505594.2022.2040188
Wang, J. C., Derynck, M. K., Nonaka, D. F., Khodabakhsh, D. B., Haqq, C., and Yamamoto, K. R. (2004). Chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc. Natl. Acad. Sci. U. S. A. 101 (44), 15603–15608. doi:10.1073/pnas.0407008101
Wu, L., Wang, Y., Liu, Y., Yu, S., Xie, H., Shi, X., et al. (2014). A central role for TRPS1 in the control of cell cycle and cancer development. Oncotarget 5 (17), 7677–7690. doi:10.18632/oncotarget.2291
Wu, X., Pu, L., Chen, W., Zhao, Q., Wu, G., Li, D., et al. (2022). LY294002 attenuates inflammatory response in endotoxin-induced uveitis by downregulating JAK3 and inactivating the PI3K/Akt signaling. Immunopharmacol. Immunotoxicol. 44 (4), 510–518. doi:10.1080/08923973.2022.2055565
Xiong, R., Wu, Q., Muskhelishvili, L., Davis, K., Shemansky, J. M., Bryant, M., et al. (2018). Evaluating mode of action of acrolein toxicity in an in vitro human airway tissue model. Toxicol. Sci. official J. Soc. Toxicol. 166 (2), 451–464. doi:10.1093/toxsci/kfy226
Ye, H., Zhao, J., Xu, X., Zhang, D., Shen, H., and Wang, S. (2023). Role of adenosine A2a receptor in cancers and autoimmune diseases. Immun. Inflamm. Dis. 11 (4), e826. doi:10.1002/iid3.826
Yeager, R. P., Kushman, M., Chemerynski, S., Weil, R., Fu, X., White, M., et al. (2016). Proposed mode of action for acrolein respiratory toxicity associated with inhaled tobacco smoke. Toxicol. Sci. official J. Soc. Toxicol. 151 (2), 347–364. doi:10.1093/toxsci/kfw051
Keywords: acrolein, air pollution, nose, lung, glucocorticoids, cytokines, transcriptomics
Citation: Alewel DI, Jackson TW, Rentschler KM, Schladweiler MC, Astriab-Fisher A, Gavett SH, Evansky PA and Kodavanti UP (2023) Differential transcriptomic alterations in nasal versus lung tissue of acrolein-exposed rats. Front. Toxicol. 5:1280230. doi: 10.3389/ftox.2023.1280230
Received: 19 August 2023; Accepted: 06 November 2023;
Published: 27 November 2023.
Edited by:
Nikolai V. Ivanov, Philip Morris International, SwitzerlandReviewed by:
Ishita Choudhary, North Carolina State University, United StatesCopyright © 2023 Alewel, Jackson, Rentschler, Schladweiler, Astriab-Fisher, Gavett, Evansky and Kodavanti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Urmila P. Kodavanti, a29kYXZhbnRpLnVybWlsYUBlcGEuZ292
†ORCID: Devin I. Alewel, orcid.org/0000-0003-3415-9477; Thomas W. Jackson, orcid.org/0000-0002-7996-0412; Urmila P. Kodavanti, orcid.org/0000-0001-6333-1024
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.