
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Toxicol., 23 August 2023
Sec. Clinical Toxicology
Volume 5 - 2023 | https://doi.org/10.3389/ftox.2023.1173683
This article is part of the Research TopicThe impact of clinical and environmental toxicological exposures and eye healthView all 14 articles
Dry eye disease (DED) is a multifactorial condition that often presents with chronic symptoms of pain (that can be characterized as “dryness,” “burning,” and “irritation,” to name a few) and/or fluctuating or poor-quality vision. Given its multifactorial nature, several pathophysiologic mechanisms have been identified that can underlie symptoms, including tear film, ocular surface, and/or corneal somatosensory nerve abnormalities. Research has focused on understanding how environmental exposures can increase the risk for DED flares and negatively impact the tear film, the ocular surface, and/or nerve health. Given that DED is a common condition that negatively impacts physical and mental functioning, managing DED requires multiple strategies. These can include both medical approaches and modulating adverse environmental conditions, the latter of which may be a cost-effective way to avoid DED flares. Thus, an understanding of how environmental exposures relate to disease is important. This Review summarizes research on the relationships between environmental exposures and DED, in the hope that this information will engage healthcare professionals and patients to consider environmental manipulations in their management of DED.
Dry eye disease (DED) is a common ocular condition and a major cause of chronic ocular surface pain and/or fluctuating and poor-quality vision. It is a multifactorial condition, characterized by tear film instability, high or unstable osmolarity, ocular surface inflammation, and/or somatosensory abnormalities. DED does not have a “gold standard” definition, but instead is often referred to as an umbrella term under which various disease phenotypes fit (Galor et al., 2020; Villani et al., 2020). Given this complexity, it is not surprising that heterogeneity exists with respect to the pathophysiological pathways underlying the disease (Craig et al., 2017; Ganesalingam et al., 2019). Of these, this Review will focus on how environmental exposures may impact DED onset, severity, and persistence.
This Review is needed as less is known about the relationships between DED and adverse environmental exposures compared to other disease contributors. For example, T-cell-mediated inflammation has been studied in individuals with DED and comorbid Sjögren’s syndrome (SS), neurovascular instability has been examined in individuals with DED and rosacea, and neuropathic mechanisms have been probed in individuals with DED and comorbid migraine or fibromyalgia. Other studies have focused on behavioral factors (e.g., contact lens use and smoke exposure) and medications (e.g., antihistamines, antidepressants, and antihypertensives) as they relate to DED.
In comparison to these established relationships, less is known about the etiology of DED in response to adverse environmental conditions. Given that DED impacts physical and mental functioning, understanding the factors that contribute to the disorder is essential and can help providers improve care algorithms and deliver precision medicine. Furthermore, certain environmental manipulations may be more cost-effective than medical therapy in controlling severe and/or refractory symptoms. This Review will summarize the current knowledge on the toxicological mechanisms of environmental exposures as they relate to DED manifestations.
When examining studies on DED, it is important to understand the constellation of symptoms and signs that fall under the disorder. The diagnosis of DED is made by clinical examination, based on the presence of symptoms (e.g., that can be assessed with various validated questionnaires), slit lamp findings, and in-clinic point-of-care tests. Given that different risk factors may relate to different aspects of DED, it is important to examine disease definitions when reviewing epidemiological studies on DED.
For symptoms, ocular surface pain is a common complaint patients present with, with common descriptors that include “dryness,” “burning,” “aching,” and “tenderness,” to name a few. Pain symptoms can arise from nociceptive sources (activation of nociceptors due to abnormalities in peripheral tissues), neuropathic sources (abnormalities in somatosensory pathways to and from the cornea), or a combination of both (Stucky et al., 2001; Basbaum et al., 2009). Ocular surface pain, whether secondary to DED or other causes, is a major source of morbidity, and DED-associated chronic ocular surface pain is a leading cause of ophthalmic healthcare costs (Yu et al., 2011) and has deleterious effects on the quality of life and productivity (Goyal and Hamrah, 2016; Patel et al., 2019). Considering all symptoms of DED (pain and visual symptoms), cost-of-illness analyses have estimated the burden of DED at nearly $3.84 billion, including indirect costs (loss of work) (Yu et al., 2011).
Ocular surface pain can be quantified using standardized questionnaires, each aimed at eliciting different characteristics of pain. For example, the 5-Item Dry Eye Questionnaire (DEQ-5) assesses the frequency and intensity of dryness and discomfort, along with the frequency of tearing (Chalmers et al., 2010). The Ocular Surface Disease Index (OSDI) assesses the frequency of sensitivity to light, grittiness, and painful or sore eyes, along with visual symptoms, triggers, and quality of life implications (Schiffman et al., 2000). Pain-specific questionnaires have also been developed, most of which use a Likert-type Numerical Rating Scale (NRS), including the Ocular Pain Assessment Survey (OPAS; intensity, descriptors, and quality of life) (Qazi et al., 2016) and the Neuropathic Pain Symptom Inventory modified for the Eye (NPSI-E), the latter of which focuses on neuropathic descriptors of eye pain (Farhangi et al., 2019).
“Signs” of the disease are examined with in-clinic tests that assess ocular structure and function, with certain thresholds used as cut-offs for the clinical diagnosis of DED. These include tests that look for alterations in tear stability (e.g., tear breakup time (TBUT)) and production (e.g., Schirmer’s test, with or without anesthesia) and structural integrity (e.g., corneal and conjunctival staining using vital dyes such as fluorescein, lissamine green, and Rose Bengal), and assess corneal function and structure (Mehra et al., 2020; Patel and Sarantopoulos, 2023). Corneal function can be evaluated via corneal sensitivity (qualitatively assessed in the clinic with a cotton tip or floss or quantified in the research arena with an esthesiometer). Structural attributes are examined microscopically via in vivo confocal microscopy (IVCM); commonly reported findings include the presence of immune cells within the cornea (e.g., dendritic cells, most commonly noted in individuals with aqueous tear deficiency (ATD) in the setting of auto-immune disease) and corneal nerve abnormalities (e.g., decreased nerve density and increased nerve tortuosity, also common in individuals with systemic auto-immune diseases such as SS) (Hwang et al., 2021).
Risk relationships with environmental factors have been studied for several ocular and systemic conditions (Paschides et al., 1998; Syndulko et al., 1996; D’Amato et al., 2015; Michelozzi et al., 2009). Generally, studies classify exposures as “indoor” or “outdoor” (also known as ambient) when reporting associations. Studies on outdoor exposure are more common, even though we spend most of our time indoors, at least partially due to the availability of national ambient meteorological databases. Some commonly studied factors include air pollutants (e.g., ozone, O3; nitrogen dioxide, and NO2), aeroallergens (e.g., pollen, dander, mold, and dust), meteorological conditions [e.g., temperature and relative humidity (RH)], interaction effects (e.g., the effect of temperature and RH simultaneously, also known as heat stress), and behavioral factors (e.g., exposure to smoke, chemicals, medications, and contact lens use). It is important to note that studying the environment is challenging, regardless of the type of exposure—the study of ambient variables requires the integration of patient health data and environmental data with different spatiotemporal scales, which can result in exposure uncertainty (Kumar, 2016). On the other hand, accurate measurements of indoor variables can require special devices like climate control chambers (Calonge et al., 2018) to control indoor exposures or a special set-up to measure exposures.
Perhaps the least studied of all ambient variables, toxic exposure to temperature is thought to mainly affect ocular health by its influence on the tear film (Nagymihályi et al., 2004). Controlled chamber studies have described the direct impact of temperature on the tear film. Specifically, two controlled environment chambers in Europe (Abusharha and Pearce, 2013; Abusharha et al., 2016) exposed individuals to increasing ambient temperatures at constant RH and found that tear film parameters varied by temperature level. One study found that lipid layer thickness increased with increasing temperature (20–40 nm at 5°C and 10°C vs. 40–90 nm at 15°C, 20°C, and 25°C; p < 0.05) (Abusharha and Pearce, 2013). Similar findings were noted in the second study (median lipid layer thickness 20–40 nm at 5°C and 10°C vs. 40–90 nm at 15, 20, and 25°C; p < 0.005), but interestingly, this second study also found that the evaporation rate increased with temperature (0.06 μL/min at 5°C vs. 0.17 μL/min at 25°C; p < 0.005) (Abusharha et al., 2016). These findings are difficult to interpret, as other studies found that a thicker lipid layer led to a lower evaporation rate, thus having a protective effect on the ocular surface (Craig and Tomlinson, 1997; Giraldez et al., 2009). As such, further research is needed to understand how temperature impacts the risk of ocular surface disorders like DED, beyond its effects on lipid thickness.
On an epidemiological level, associations between temperature and DED have varied. A Taiwanese study of 25,818 subjects with known DED (not further defined) found that temperature was positively associated with a DED diagnosis (β = 1.01, 95% CI = 1.00 to 1.02; p < 0.001). In this model, RH had an inverse relationship with DED (β= 0.93, 95% CI = 0.91 to 0.95; p < 0.001), and NO2 had a positive relationship (β = 1.08, 95% CI = 1.04 to 1.11; p < 0.001) (Zhong et al., 2018). In comparison, a Taiwanese study of 351 patients with known DED (OSDI ≥ 13, TBUT ≤ 5, staining) reported that temperature was inversely related to symptoms (via OSDI; β = −0.84, 95% CI = −1.34 to −0.33; p < 0.005) and tear production (Schirmer’s: β= −0.73, 95% CI = −1.19 to −0.26; p < 0.005) (Ho et al., 2022). Further highlighting the variable findings on temperature, an American study that examined 3.41 million visits at Veteran Affairs (VA) eye clinics between July 2006 and July 2011 found that DED (via ICD9 code; diagnosed in 17.4% of the study population) was most frequently diagnosed in the winter and spring months, compared to the fall and summer (18.7% ± 0.98% and 18.5% ± 4.16%, respectively), with the highest frequency occurring in April (20.9% ± 0.14%) (Kumar et al., 2015). These data suggest that factors beyond temperature alone may impact DED presentation.
In summary, while there is evidence suggesting that the tear film and lipid layer are affected by temperature extremes, the findings are inconsistent as to which extreme of the temperature scale is most harmful. These findings may suggest that the relationship between temperature and DED is non-linear and is instead possibly a “U”-shaped curve, with a “Goldilocks” zone (temperatures below or above this zone having a detrimental impact on tear film health). In fact, the American Society of Heating, Refrigerating, and Air-Conditioning Engineers has recognized the concept and recommended that the indoor temperature be set between 20°C and 25°C (Abdul-Wahab et al., 2015). Further studies are necessary to understand and translate this recommendation to individuals with DED.
Exposure to temperature change is another important factor that may relate to the risk of DED. Studies examining temperature change often utilize the diurnal temperature range (DTR) as a measure of change, which measures the difference between the maximum and minimum daily temperature. A higher DTR has been reported as a risk factor for disease flares across several conditions, from asthma (Xu et al., 2013; Kim et al., 2014; Qiu et al., 2015; Li et al., 2016) to heart failure (Lim et al., 2012). It is hypothesized that exposure to abrupt temperature change may impact the function of immune cells involved in inflammatory and allergic presentations, specifically through altered release of cytokines and cytotoxic proteins (Lobefalo et al., 1999; Graudenz et al., 2006). The aforementioned American study, which studied visits to 3.41 million VA eye clinics across the United States between July 2006 and July 2011, reported on this association with respect to DED—the study found that change in temperature had more influence on DED presentation than absolute temperature throughout each season. The greatest decrease in symptom intensity (via OSDI, DEQ5, and NPSI-E) occurred in winter and summer, when the weather change from the previous season was less abrupt, compared to spring and autumn. The study hypothesized that abrupt meteorological changes may have a detrimental effect on the lacrimal unit (Kumar et al., 2015); however, further studies are necessary to test this hypothesis.
Low RH (e.g., desiccating stress) is a well-described risk factor for DED (Smith, 2007). Adequate production and stability of tears is essential to a healthy tear film, and any destabilization in these variables can lead to ocular surface diseases like DED. While the pathway is not entirely understood, studies have implicated a negative association between RH and tear osmolarity (e.g., induction of stress via a hyperosmolar mechanism) (González-García et al., 2007) and alterations in protein oxidation within the tear film and Meibomian lipids (Abusharha and Pearce, 2013) as potential causes of this relationship. In addition to this, RH has also been shown to directly affect tear film evaporation; specifically, aridity affects vapor concentration, which, together with tear film thickness, determines the evaporative flux at the ocular surface (Peng et al., 2014). In this manner, RH may also exert its effects on tear film health by influencing the tear evaporation rate, a finding described in several studies.
Describing these effects, one of the previously described European climate chamber studies also reported on the relationship between RH and tear dynamics. This study examined two conditions: RH set at 40% (normal) and at 5% (desiccating stress). Tear film abnormalities noted in the desiccating (low RH) environment included an increase in tear evaporation, a decrease in tear production, a decrease in lipid layer thickness, and an increase in ocular pain (specific data not provided; p < 0.05 for each) (Abusharha and Pearce, 2013). Supporting these human findings, a mouse study reported that exposure to low RH (RH = 18.5% ± 5.1%) for 28 days after an initial equal exposure to normal RH (RH = 50%–80%) led to decreased tear production via cotton thread wetting (baseline: ∼2.2 ± 0.2 mm; day 3: 1.4 ± 0.3 mm; p < 0.005; day 28: 1.3 ± 0.4 mm; p < 0.05) and increased fluorescein staining (baseline: ∼1.5 ± 1.5; day 3: 5.8 ± 2.2; p < 0.0001; day 28: 4.6 ± 2.3; p < 0.05) (Barabino et al., 2005). However, not all studies reported an inverse relationship between DED and RH—one English study of 10 individuals with mild–moderate DED (symptoms, TBUT<10 s, Schirmer<10 mm) and 10 controls exposed groups to varying RH (5%, 40%, and 70%, for 25 min on 3 separate days). As the RH increased from 5% to 70%, tear evaporation rates linearly decreased in both groups (∼100 g/m2/hr at 5%, ∼70 g/m2/hr at 40%, and ∼0 g/m2/hr at 70% for DED vs. ∼90 g/m2/hr at 5%, ∼40 g/m2/hr at 40%, and ∼0 g/m2/hr for controls; p < 0.05 between points in each group, respectively), supporting the results of the previous studies. However, tear stability (TBUT) followed a U-shaped curve in both groups with varying RH (4.90 ± 1.66 s at 5%, 6.31 ± 2.21 s at 40%, and 5.90 ± 1.91 s at 70% in the DED group vs. mean 17.80 ± 3.91 s at 5%, 20.70 ± 5.88 s at 40%, and 20.00 ± 5.35 s at 70% in controls), suggesting an optimal value at 40% RH (Madden et al., 2013).
Many epidemiological studies have noted relationships between DED and RH. A Taiwanese study of 25,818 subjects diagnosed with DED found that lower RH (β = 0.93, 95% CI = 0.91 to 0.95; p < 0.001) was associated with DED diagnosis, along with temperature and NO2 (Zhong et al., 2018). A Korean study of 16,824 participants from January 2010 to December 2012 found an inverse relationship between RH and DED symptoms (OR = 0.87; 95% CI = 0.77 to 0.98; p = 0.03) and diagnosis (OR = 0.86; 95% CI = 0.76 to 0.97; p = 0.01) (Hwang et al., 2016). Supporting this association, a Chinese case-crossover study of 5,062 individuals diagnosed with DED found that lower RH was associated with an increased risk for an outpatient DED diagnosis visit (specific data not provided; p < 0.05) (Mo et al., 2019). However, just as observed with the chamber studies, not all epidemiological studies have reported an inverse relationship—one American study of 97 individuals who underwent indoor RH monitoring instead found that RH was positively associated with symptoms (OSDI: r = 0.30, 95% CI = 0.07 to 0.49; p = 0.01) and Meibomian gland (MG) dropout (r = 0.27, 95% CI = 0.05 to 0.47; p = 0.02), and negatively associated with tear production (Schirmer: r = −0.25, 95% CI = −0.45 to 0.02; p = 0.03) (Huang et al., 2020). Of note, the group hypothesized that the noted association between RH and DED was not driven by RH alone, but by the interaction between RH and particle size via the hygroscopic effect (the ability of particulate matter (PM) to absorb water and increase in size under high RH). These findings suggest that, like with temperature, a U-shaped curve may describe the relationship with RH. In fact, the Environmental Protection Agency recommends an ideal RH level of 30%–50% (Wendt et al., 2004), providing credence to a potential “Goldilocks” zone.
Air pollutants can be divided into airborne PM and gas molecules, both of which are generated by indoor and outdoor sources, such as fossil combustion (e.g., automobile emissions and aerosolization of cooking and cleaning products) (Mandell et al., 2020). Air pollutants of special interest to ocular health, as outlined by the World Health Organization (WHO), are O3, NO2, sulfur dioxide (SO2), carbon monoxide (CO), and PM (Versura et al., 1999; Jung et al., 2018).
Air pollutants are hypothesized to impact ocular and periocular components variably, depending on their composition. While all types act as sources of inflammation, ultrafine PM particles can cross the corneal epithelium to induce stress in deeper layers of the eye, while larger particles can settle upon and physically damage (e.g., abrasion) the ocular surface and periocular lid margin (Mandell et al., 2020). Gaseous pollutants, including reactive gases [e.g., NO2, SO2, O3, and volatile organic compounds (VOCs)], react with the tear film and induce a local stress reaction (Mandell et al., 2020). Several mechanisms have been postulated, including the formation of direct irritant reagents at the ocular surface (e.g., solubilization of sulfur-containing compounds to create sulfurous or sulfuric acids) and activation of conjunctival antigen-presenting cells, leading to a pro-inflammatory response (Jung et al., 2018).
One climate control study focused on air pollutants and symptoms and signs of DED in humans and found a decrease in tear stability (via TBUT) after exposure. Specifically, a Danish study exposed 10 individuals to clean (41 μg/m3 dust) and polluted air (394 μg/m3 dust) in a randomized order for 3 h and reported a decrease in TBUT compared to baseline (specific data not provided; p < 0.05) (Pan et al., 2000). Epidemiological studies have consistently reported positive relationships between air pollution and DED. The Chinese case-crossover study of 5,062 individuals with DED identified that same-day exposure to PM2.5 (OR = 1.02, 95% CI = 1.01 to 1.03; p < 0.01) and PM10 (OR = 1.01, 95% CI = 1.003 to 1.02; p < 0.01) was a risk factor for a DED diagnosis visit, along with decreasing RH (Mo et al., 2019). Similarly, the Taiwanese study of 25,818 subjects with DED found that increasing NO2 (β = 1.08, 95% CI = 1.04 to 1.11; p < 0.001) was associated with a DED diagnosis, along with ambient temperature and RH (Zhong et al., 2018). In a similar fashion, a Korean study of 16,824 participants found positive relationships between O3 levels with DED symptoms (OR = 1.16; 95% CI = 1.02 to 1.30; p = 0.04) and DED diagnosis (OR = 1.21; 95% CI = 1.05 to 1.40; p = 0.008) (Hwang et al., 2016). Finally, a prospective Korean study of 43 patients with DED undergoing treatment (symptoms, TBUT, staining) noted that O3 (β = 0.33, 95% CI = 0.16 to 0.49; p < 0.001) and PM2.5 (β = 0.38, 95% CI = 0.06 to 0.70; p < 0.02) levels were positively associated with symptoms (via OSDI), while PM10 (β = −0.03, 95% CI = −0.045 to −0.01; p = 0.001) was negatively associated with tear stability (TBUT) (Kim et al., 2020).
In summary, studies have overwhelmingly reported a positive association between exposure to different outdoor air pollutants and various aspects of DED.
While allergy and DED are separate entities, DED is often comorbid with “ocular allergy” (Leonardi et al., 2021), and DED flares can occur as a result of exposure to allergens, both seasonally and perennially (Friedlaender, 2011; Ortega et al., 2022). One systematic review reported that ∼50% of individuals with allergic conjunctivitis (AC) have comorbid DED, and ∼20% of individuals with DED have comorbid AC (Akasaki et al., 2022). Other studies have found molecular links between DED and AC—an American study on 75 patients with symptoms or signs of DED reported that 17% of subjects (13/75) had high tear IgE (>1 ng/mL) and that this group was more likely to be exposed to allergens in their home (e.g., pets: OR = 11.5; p = 0.002; smoke: OR = 38.6; p = 0.008), supporting the idea of an allergic component to DED in some individuals (Dermer et al., 2019). Shared signs have also been noted between DED and allergy, for example, corneal epithelial disruptions assessed with Rose Bengal and fluorescein (Dogru et al., 2008). Overall, these findings suggest that allergens may impact various aspects of ocular surface health, including tear stability, mediators of inflammation, and mucin abnormalities, leading to sign overlap with DED.
No chamber studies have examined the association between allergens and DED, but epidemiological studies have reported positive links between ocular allergy and DED. In a Swedish study of 89 children aged 7–18 with pollen allergy (positive skin prick test or presence of IgE), ocular pain scores (pain Likert 0–3) increased linearly with pollen grain exposure over 42 days, until exposure to 150 grains/cm3, where the trend flattened (specific data not provided; p < 0.05) (Kiotseridis et al., 2013). In addition to pollen, studies have also examined mold spores, which have been classified as aeroallergens and as bioaerosols across different studies. In a study of 3,485 adults in China, individuals who lived in homes with more signs of mold (severity score quantified by the presence of mold/damp stains, moldy odor, dampness on bed/clothing, window pane condensation in winter, and water damage) had an increased risk of ocular pain compared to those who lived in homes with fewer signs (OR = 3.20, 95% CI = 1.67 to 6.15; p < 0.01) (Lu et al., 2016).
Overall, studies suggest a positive relationship between allergens and DED, most notably pain and tear stability. Of interest, environmental studies focusing on allergies have coincided with findings reported for DED—allergic diagnoses and symptoms have been positively linked to temperature, negatively to RH, and positively to air pollution (Reinikainen et al., 1992; Mendell et al., 2002; Wolkoff et al., 2003; Rozanova et al., 2009; Idarraga et al., 2020). Further studies are needed to examine the overlapping pathophysiology between allergy and ocular surface disease and their relationships to the environment.
Although not as well-studied, atmospheric pressure may also impact ocular health. Atmospheric pressure is especially important in high-altitude areas (e.g., mountainous regions and aircrafts mid-flight), where its value decreases (the amount of gas molecules in the air decreases, making the air less dense than that closer to the ground), as it is hypothesized that lower atmospheric pressure leads to increased tear film evaporation (Tesón et al., 2013). Supporting this idea, the previously discussed American VA-based study found that atmospheric pressure was a risk factor for a DED diagnosis—the risk of a DED diagnosis was 13% higher in patients residing in regions where atmospheric pressure was 1 standard deviation higher than the population mean (incidence rate ratio (IRR) = 1.13, 95% CI = 1.129 to 1.133; p < 0.001) (Galor et al., 2014). Further studies are needed to examine this association and to develop appropriate mitigation strategies.
Bioaerosols are small biological particles (0.001–100 μm in diameter) that are present in both ambient outdoor and indoor air and are characterized as another form of air pollutant in some studies. These molecules originate from endotoxins, glucans, mycotoxins, allergens, bacteria, and fungal spores and are made airborne by the handling of industrial/agricultural products (soil, plants, animals, etc.). Similar to other airborne particles (PM and allergens), concentrations of bioaerosols vary by meteorological conditions, seasonality, and by human and animal activity (Rock et al., 2021).
It is hypothesized that lipolytic enzymes and polar lipids secreted by eyelid-colonized bacteria may influence meibum composition and health, and thus relate this exposure to the risk of surface disorders like DED (Dougherty and McCulley, 1986). Unfortunately, there is a large paucity of studies that focused on this relationship, with only a few epidemiological studies having been conducted. Only one study has specifically examined bioaerosols in the context of DED—another Australian study obtained swabs from the inferior conjunctival fornix and lid margin of 66 individuals with DED (symptoms, TBUT <10s, staining >3 on Oxford) and 18 controls and found more colony-forming units (CFUs) in the DED vs. the control group (106 ± 82 CFUs vs. 12 ± 18 CFUs; p < 0.0001). Moreover, within the DED group, individuals with (n = 15) versus without (n = 51) MG dysfunction (eyelid thickening, irregularity, telangiectasia, gland loss, capping, or abnormal meibum) had higher CFUs on average (95 ± 66 CFUs vs. 12 ± 18 CFUs; p < 0.05) (Albietz and Lenton, 2006).
Given the lack of data, further studies examining both the relationship between DED and airborne bioaerosols as well as the molecular mechanism of injury are necessary. This is especially important given that there are no existing recommended indoor, outdoor, or occupational bioaerosol concentration standards in the United States.
While we have summarized studies examining the relationships between environmental factors and DED, there are considerations to keep in mind when analyzing these results. First, environmental factors affect one another, making it difficult to analyze the effect of an individual exposure with respect to ocular diseases like DED (D’Amato et al., 2015; Vocks et al., 2001; Pfab et al., 2010; Hong et al., 2016; Levetin and Van de Water, 2008; Ju et al., 1998; Mimura et al., 2014; Park et al., 2020). For example, higher temperatures can promote aeroallergen dispersion—some genes that encode pollen production work in a heat-dependent manner; thus, increasing temperature can promote the earlier initiation of flowering and enhanced allergenicity of aeroallergens (Ju et al., 1998; Levetin and Van de Water, 2008). Similarly, low RH can promote the suspension of airborne pollutants (PM2.5 and PM10) and airborne pollen levels (Wyon et al., 2002; Qiu et al., 2019). As previously discussed, RH can also impact PM size, known as the hygroscopic effect (PM can absorb airborne moisture in settings of high RH and inflate in size) (Huang et al., 2020). These confounding factors must be taken into consideration when examining the reported relationships between RH and ocular disease.
Second, population demographics vary across climate regions and may play a role in environmental susceptibility; this may impact comparisons across geographically diverse studies. For example, heat sensitivity is heightened in elderly women, patients with decreased mobility or dementia, those on medications that affect thermoregulation (diuretics or anticholinergics), and those with disorders that compromise thermoregulation (obesity, hypertension, pulmonary disease, and diabetes) (Kovats and Hajat, 2008; Kenny et al., 2010). In addition, individual differences in the ability to adapt to one’s environment may drive geographic differences (Hori et al., 1977). A Japanese study found that men in hot subtropical zones who later moved to colder temperate zones showed signs of superior heat acclimation than those who spent their lives in the temperate region, including less skinfold thickness (e.g., upper arm: 5.3 ± 2.3 mm vs. 7.7 ± 3.2 mm; p < 0.001) and more effective sweating with less salt wasting (0.022 ± 0.004 mEq/L vs. 0.029 ± 0.008 mEq/L; p < 0.05) (Hori et al., 1978). Several biologic modifications underlie climate adaptation, including a heat-dependent shearing mechanism for controlling blood flow (Carter et al., 2014), improved fluid balance and sweating mechanics (Périard et al., 2015), and changes in thermal behavior (e.g., brown adipose plasticity and metabolic enzyme activity) (Lee et al., 2014; Ning et al., 2016), and it is not known how these factors impact tear metrics, corneal epithelial cells, and corneal nerves. These factors may confound study results and account for variability across studies, along with other factors such as DED definitions and variance in methods for capturing environmental exposures.
The indoor environment is also an important potential contributor to DED (Mandell et al., 2020). Ocular irritation is a frequently reported complaint of office workers, with studies suggesting that beyond indoor meteorological exposures, activities like work-related tasks (concentration causing decreased blink rate) and behavioral factors (contact lenses, eye make-up, medications, and smoking) may also impact ocular health (Wolkoff et al., 2003; Rozanova et al., 2009).
DED has been associated with indoor temperature, RH, and air pollution (organic and inorganic). In one American study, 396 office workers working on two floors of the same building had ocular pain assessed weekly via a questionnaire (scale 0–25)—a 1°C decrease in temperature was associated with an increased severity of dryness, itching, and irritation [OR = −1.11 (per unit decrease in temperature), 95% CI = −1.76 to −0.47; p < 0.005] (Mendell et al., 2002). Next, similar to outdoor studies, low RH indoors has also been implicated in DED. In a Finnish study, 290 office workers located in two wings of the same building were crossed over between high humidity conditions (30%–40% RH) and “natural” conditions (20%–30%) for 3 weeks each (6 weeks total). Daily ocular pain symptoms (Likert 0–3) were worse on average while working in the low-RH conditions (0.39 vs. 0.35; p < 0.05) (Reinikainen et al., 1992). Similar findings were noted in a geographically diverse population—a study of 44 individuals in New Zealand had subjects work with and without a desktop humidifier (which increased RH by 5.4% ± 5.0%). This study found that 36% of participants noted improved ocular comfort scores while working with a humidifier, as compared to 5% in the non-humidifier group; p < 0.001) (Wang et al., 2017).
Studies focusing on at-home air PM have aligned with findings focusing on outdoor air pollution. Specifically, an American study of 97 individuals found that a 1 unit increase in PM2.5 was associated with increased OSDI (β = 0.59, 95% CI = 0.58 to 2.59; p = 0.002) and reduced tear production (Schirmer’s: β = −0.67, 95% CI = 0.75 to −0.03; p = 0.04) (Huang et al., 2020). In addition to these factors, building-related factors may also relate to DED—an American study evaluated the short-term effects of 88 subjects working in an older building (with a higher concentration of airborne PM (24,436 particles ≥0.5 μm/ft3) as compared to 102 subjects working in a newer building (12,313 particles ≥0.5 μm/ft3).
Like with outdoor air, few studies have examined indoor air bioaerosols and how they relate to ocular health. One American VA-based study examined the relationship with ocular health in 157 individuals seen at a VA eye clinic between October 2017 and October 2019. This study examined microbial presence in indoor air via bioaerosol concentrations (CFUs). Positive associations were noted between indoor air microbial load and the amount of corneal epithelial disruption (OR = 28.07, 95% CI = 1.8 to 443.8; p < 0.05) as well as with meibomian dropout (OR = 39.6, 95% CI = 1.8 to 875.2; p < 0.05). As expected, inter-meteorological relationships were noted; a 1% increase in RH was associated with a 3% increase in CFUs (OR = 0.03, 95% CI = 0.01 to 0.04; p < 0.001) (Rock et al., 2021).
Short-term exposures have also been studied as they relate to DED. An American study questioned 88 individuals as they left an older building (with a higher concentration of airborne PM (24,436 particles ≥0.5 μm/ft3) as compared to 102 subjects who left a newer building (12,313 particles ≥0.5 μm/ft3). When adjusting for other variables (e.g., building and time interaction), there was a 1% increase in the odds of reporting worsening DED symptoms per hour spent in the older versus newer building (OR = 1.01; 95% CI = 1.00 to 1.02; p < 0.05). In multivariate analyses, subjects working in the older building for longer periods (upwards of 3 h) were more likely to report pain (OR 3.89, 95% CI = 1.21 to 12.5; p < 0.05) than those working in the newer building (Idarraga et al., 2020).
Overall, the literature supports findings that are similar to what has been noted with respect to outdoor exposures, as various indoor exposures have been found to relate to various aspects of DED.
Smoking is a behavioral factor that has been connected to DED, among other ocular conditions, including macular degeneration, glaucoma, and cataracts (Makrynioti et al., 2020). Smoke exposure can lead to tear film instability, secondary to a direct irritant action, through free-radical formation or the promotion of lipid peroxidation at the tear film (Sahai and Malik, 2005; Sayin et al., 2014). Studying this question with a focus on e-cigarettes, an American study examined 49 e-cigarette flavoring liquids and analyzed ROS production (via electron paramagnetic resonance (EPR)) as well as synthetic lipid peroxidation in vitro (analyzed for secondary lipid oxidation products using a thiobarbituric acid reactive substances (TBARS) assay kit). The study found that 43% of the e-cigarette flavorants analyzed led to a significant increase in free-radical production as compared to a flavor-free liquid (PG:GLY) (specific data not provided; p < 0.05 each). In addition, the effects of these liquids on lipid peroxidation were also measured in vitro, and significant increases in lipid peroxidation were noted for several flavorants, especially those that contained linalool (4 mg/mL), piperonal (1.6 mg/mL), and citral (4 mg/mL) (257%, 197%, and 205% increase in peroxidation rate vs. PG:GLY, respectively; specific data not provided; p < 0.05) (Bitzer et al., 2018). For reference, similar lipid peroxidase abnormalities have been noted in non-smokers with DED (95), suggesting that similar downstream mechanisms of DED can be caused by a variety of insults.
Epidemiological studies have been mixed with respect to the impact of smoking on ocular health. Some studies have found positive relationships between smoking and DED—in a Turkish study of 49 smokers and 53 non-smokers, tear stability (TBUT: 8.24 ± 2.39 s vs.11.15 ± 1.94 s; p < 0.0001) and tear production (Schirmer’s 13.30 ± 4.63 vs. 15.45 ± 4.11; p = 0.02) were both decreased in the smoking group. However, the values were still within normal ranges in both groups (Sayin et al., 2014). Supporting these findings, the Beaver Dam study of 3,583 individuals found that DED symptoms were present in 534 patients (14.4%) and that both past (OR = 1.22, 95% CI = 0.97 to 1.52; p < 0.05) and current smoking status (OR = 1.82, 95% CI = 1.36 to 2.46; p < 0.05) acted as risk factors for symptom presence (Moss et al., 2000). Other studies, however, have not found smoking to be a risk factor for DED—a meta-analysis of 10 studies (two cohort and eight cross-sectional studies) reported no relationship between DED diagnosis and a smoking history, when considering the impact of age and gender (OR = 1.16, 95% CI = 0.83 to 1.64; p = 0.38). However, the same study presented a subsequent sensitivity analysis in which only general (non-hospital) populations were included, and in this sub-analysis, the association became significant (OR = 1.50, 95% CI = 1.08 to 2.09; p = 0.02) (Xu et al., 2016).
In summary, the effects of smoking on DED are not entirely understood. While studies have demonstrated the direct negative effects of smoking on ocular health in vitro, results have been mixed when examined on the epidemiological level. Of growing interest are the health effects of other forms of smoking, such as vaping, for which preliminary studies have also demonstrated toxic effects on ocular health (Isa et al., 2019; Martheswaran et al., 2021).
The impact of office work has been studied with respect to ocular disease (Huang et al., 2020), with the focus centered on the use of video display units (VDUs; e.g., computer screens) and reading tasks suggesting altered blink rates due to these tasks negatively affecting ocular health (Wolkoff, 2020). A Saudi study demonstrated a time-dependent positive association between ocular discomfort scores and visual tasks—in this study, 40 healthy men who read from a book and an electronic tablet for 15 min each found that the blink rate decreased significantly under both reading conditions (19.74 ± 9.12 blinks/min at baseline to 11.35 ± 0.20 and 14.93 ± 10.90 blinks/min for book and a tablet; p < 0.05 each). Concurrently, ocular discomfort scores [via a visual analog scale (VAS)] increased significantly from baseline values at all time intervals (5, 10, and 15 min) during both forms of reading. While still being explored, studies suggest that prolonged VDU use has a negative impact on ocular health.
As previously stated, several exposures have been linked to ocular surface disorders like DED. However, the mechanisms that link a specific environmental insult to a specific facet of DED have not been fully elucidated. Some potential mechanisms include hyperactivation of pro-inflammatory cytokines and reactive oxygen species (ROS) (Zheng et al., 2014; Dogru et al., 2018; Ma et al., 2021), pathological apoptosis of epithelia (corneal and conjunctival) (Yeh et al., 2003; Stern et al., 2004), impaired activation of protective autophagy mechanisms (Wang et al., 2019; Liu et al., 2020), and tear film unit glandular dysfunction [e.g., lacrimal and meibomian dysfunction as a result of immune cell infiltration (Hikichi et al., 1993) and hyperkeratinization] (Jester et al., 1981; Yu et al., 2021).
Only a few studies have examined the molecular mechanisms that underlie the impact of adverse ambient conditions (RH, temperature) on the eye, with most focusing on animal or in vitro human cell models. One Canadian study examined tear cytokine levels after an incident of desiccating stress in volunteers with known DED (n = 8, diagnostic criteria not provided) and healthy controls (n = 8)—individuals sat in an environmental chamber with a controlled temperature (23°C ± 3°C), relative humidity (10% ± 3%), and air velocity (3–5 ft/s) for 180 min. Basal tears were collected before and after exposure to the low-RH environment, and tears were analyzed for cytokines (via V-plex assay). Individuals with DED had higher baseline IL-2 levels than controls (1.11 ± 0.83 pg/mL vs. 0.45 ± 0.37 pg/mL; p < 0.05). Post-exposure, IL-2 levels significantly increased in the DED group compared to baseline (1.57 ± 0.91 pg/mL vs. 1.11 ± 0.83 pg/mL; p < 0.05). On the other hand, no significant changes were noted in the control group after exposure (0.45 ± 0.37 pg/mL vs. 0.47 ± 0.15 pg/mL, p > 0.05) (Subbaraman et al., 2014). In summary, preliminary findings suggest that inflammatory mediators may link desiccating stress to tear abnormalities, with individuals differentially impacted based on baseline disease status.
A larger body of literature has focused on the molecular consequences of air pollution (PM and reactive gases). One study exposed mice to PM10 (50 µL PM10 eye drop four times daily for 14 days to the right eye). Expression of pro-inflammatory molecules in the cornea (TNF-α; NF-κB) increased when compared to non-exposed eyes (specific data not provided; p < 0.05 for each). Furthermore, an increased level of apoptosis was noted in the corneal superficial and basal epithelia in the PM10-treated group (specific data not provided; p < 0.05 for each) (Li et al., 2017). Other in vitro human (Tau et al., 2013) and animal model studies (Li et al., 2019) have similarly reported increased tear cytokines after exposure to air pollutants. Another noted mechanism is corneal epithelial oxidative stress— an in vitro Chinese study that studied the effects of air pollution (up to 320 μg/100 μL of PM) on human corneal epithelial cells found a dose–response relationship between PM concentration and oxidative stress (via 8-hydroxy-2' -deoxyguanosine (8OHdG): 214 ± 6.50 pg/mL with 5 μg/100 μL of PM vs. 400 ± 38.8 pg/mL with 80 μg/100 μL of PM; p < 0.005) (Xiang et al., 2016). Finally, altered cell autophagy has also been noted due to PM—an in vitro study found that human corneal epithelial cells exposed to PM2.5 (50 μg/mL) had changed to autophagy; increased autophagosome formation was noted via immunofluorescence of epithelial cell LC3B (an autophagy-associated marker; ∼80% of total cells expressing autophagy post exposure vs. ∼45% in non-exposed; p < 0.01). Interestingly, this effect did not occur linearly. Western blot analysis showed that the expression of LC3B decreased during the first 4 h of exposure and then slowly returned to the baseline before increasing with longer exposure periods (Fu et al., 2017). This suggests a time-dependent role in autophagy that requires further study.
Other studies have focused on molecular mechanisms related to reactive gas exposure, like O3. One animal study exposed mice to O3 (0.5 or 2.0 ppm of O3 for 3 h in a whole-body exposure chamber) and noted conjunctival goblet cell damage on IVCM and a dose-dependent increase in tear cytokines (via BD cytometric bead array) post exposure as compared to baseline. Specifically, IL-1β, IL-6, IL-17, interferon (IFN)-γ, and NF-κB translocation and transcriptional activity levels all significantly increased at 1 week and 4 weeks after exposure in both experimental groups (specific data not provided; p < 0.05 for each) (Lee et al., 2013). In summary, several molecular pathways of injury have been attributed to toxic exposure to air pollution and reactive gases, including proinflammatory cytokine release, corneal oxidative stress, and alteration in normal apoptosis and autophagy mechanisms.
Molecular mechanisms have also been studied for smoking. One rat model examined corneal health after cigarette smoke exposure via a smoking chamber (six daily episodes, each 3 h long to 300 mL of x 5 days). Immunohistochemical analysis reported increased oxidative stress in the corneal epithelium and lacrimal glands after exposure (via 8OhdG: specific data not provided; p < 0.05 for each) (Higuchi et al., 2011). This has also been investigated in humans—a Japanese study exposed 12 healthy individuals to smoking in a controlled chamber for 5 min. Increased tear inflammatory cytokines, most notably IL-6, were reported at both 5 min and 24 h post exposure compared to pre-exposure (specific data not provided; p < 0.05 for each). In addition, tear abnormalities were noted in the form of increased tear evaporation (post: 3.34 ± 2.04 (10–7) g/cm2/s vs. pre: 1.84 ± 1.19 (10–7) g/cm2/s; p < 0.05) (Rummenie et al., 2008). The authors hypothesized that the change in evaporation was related to lipid layer peroxidation and damage, as this has been observed after cigarette smoking in other human studies (Choi et al., 2016; Bitzer et al., 2018).
Shared molecular pathways have also been found that link ocular allergy and DED (Proud et al., 1990; Albrecht and Dittrich, 2018). Mucin layer dysfunction has been implicated in both ocular allergy and DED independently (Davidson and Kuonen, 2004; Rabensteiner et al., 2019). The mucus layer, adjacent to the corneal epithelium, functions as part of the tear film to lubricate and protect the cornea, anchor the aqueous layer to the corneal epithelium, and modulate shearing forces, and dysfunction in this layer has been demonstrated in patients with known DED. In particular, studies have reported reduced or altered expression of mucins in the bulbar and tarsal conjunctiva of individuals with DED (Pflugfelder et al., 1997; Danjo et al., 1998). Demonstrating this finding in ocular allergy, a Japanese study that examined 18 individuals with atopic keratoconjunctivitis and 14 controls found alterations in corneal epithelium mucin transcription in atopic eyes. Specifically, increased MUC16 expression (501 copies/ng vs. 143 copies/ng in controls; p = 0.001) and decreased MUC5AC expression (311 copies/ng vs. 1,006 copies/ng in controls; p = 0.001) were reported (Dogru et al., 2008). Overall, this suggests pathologic changes to mucus layer protein expression in a similar manner to those observed in individuals with DED.
In summary, the research has implicated several molecular mechanisms, including inflammation, oxidative stress, and altered apoptosis and autophagy, as underlying causes that may explain the association between toxic environmental and behavioral exposures and risk for ocular diseases like DED. Given the lack of studies examining these relationships, especially with respect to variables such as temperature and allergen exposure, further research is needed to fully understand these relationships.
Several mitigation strategies exist that can target outdoor and indoor environmental conditions, with varying levels of difficulty and cost. For patients with severe or refractory disease, environmental modulation should be considered. Understandably, mitigation strategies for the outdoor environment are difficult. With regards to direct contact exposures (e.g., pollutants and aeroallergens), providers have recommended frequent hand washing, wearing wrap-around glasses or goggles, the use of pollen screens, and tracking local forecasted levels to mitigate outdoor exposures (Bergmann et al., 2021).
Mitigation strategies for the indoor environment are more plausible, given that the space is smaller and thus more controllable. Options include maintaining temperature and humidity in the “Goldilocks” zone (with the use of air conditioning and humidifiers). According to EPA guidelines, the optimal indoor RH should be set between 30% and 50%. According to the American Society of Heating, Refrigerating, and Air-Conditioning Engineers, the indoor temperature should be set between 20°C and 25°C (Abdul-Wahab et al., 2015). Managing indoor sources of pollution is another important strategy. Steps to reduce indoor PM levels include replacing filters on central heating and cooling systems, installing air purifiers, and avoiding unvented stoves and fireplaces (Mandell et al., 2020). In addition to this, removing sources of mold growth (paper, sheetrock (drywall), and carpet) is also a possible strategy (Vance et al., 2016). While not studied directly in DED, similar environmental controls have been found to be beneficial in 937 children with atopic asthma. In a US-based trial, caretakers in the intervention group were asked to perform mitigation behaviors that were tailored to each child’s skin-test-sensitization results for 1 year. These included high-efficiency air purifiers, allergen-impermeable covers on mattresses and pillows, and specific allergy interventions such as pest control for children with cockroach allergies. In the control group, no interventions were undertaken. Families were contacted every 2 months and asked about the number of days with symptoms such as wheezing, chest tightness, cough, disturbed sleep, or decreased playtime due to asthma in the last 2 weeks before the phone interview. The group that underwent interventions had fewer active symptom days than controls (3.39 ± 0.12 days vs. 4.20 ± 0.12 in a 14-day period; p < 0.001) (Morgan et al., 2004). Similar approaches can be considered for DED.
Our Review highlights that environmental and behavioral exposures can impact the risk of DED diagnosis, symptoms, and signs, both in individuals with pre-existing DED and in healthy individuals. The studies summarized in this article suggest positive relationships between DED and weather extremes and air pollution, including PM, gases, allergens, and bioaerosols. In addition, links to behavioral factors like smoking have been reported, albeit with inconsistency in findings across studies. Data suggest that these environmental components may contribute to aspects of DED through a variety of molecular mechanisms (Table 1). The pathophysiologic mechanisms that underlie the noted associations require further study to elucidate causal pathways, but several theories have been included in the Review (Figure 1). Given these findings, we suggest mitigation factors should be considered in appropriate patients (Alves et al., 2014); indoor factors such as air filters to minimize pollutant and allergen levels or tighter control of indoor RH and temperature may be the most cost-effective solutions for those most at risk. In the meantime, these associations can be incorporated into clinical practice by discussing exposure avoidance and/or mitigation for susceptible patients (Rozanova et al., 2009).
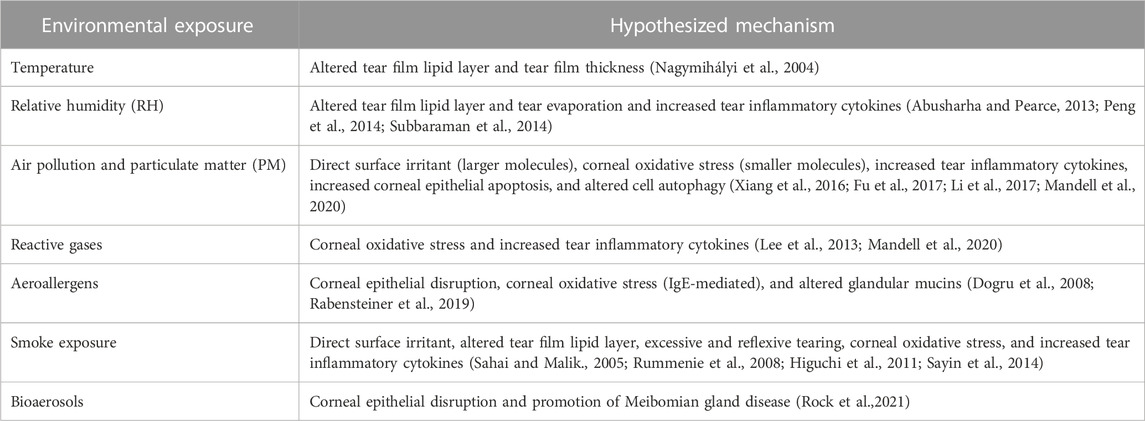
TABLE 1. Summary of hypothesized mechanisms underlying toxic environmental exposures that may predispose to DED.
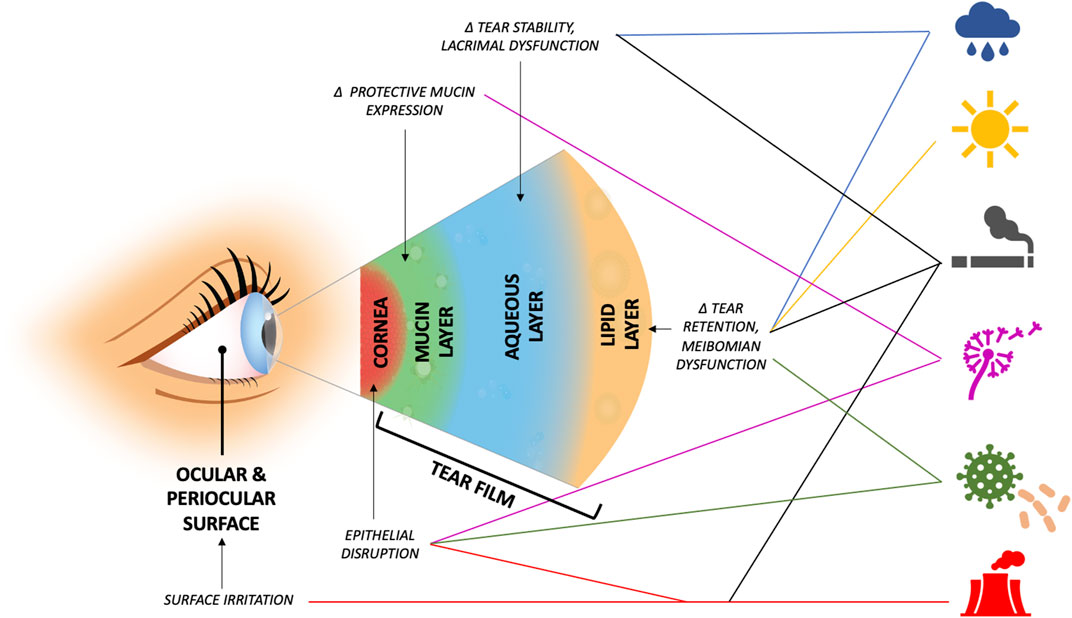
FIGURE 1. Suggested areas of dysfunction and possible underlying mechanisms that predispose to ocular surface disease by environmental exposure (exposures, top to bottom, by icon: relative humidity, ambient temperature, smoking, aeroallergens, bioaerosols, air pollutants, and reactive gases). Δ = alteration in.
Conceptualization, AG; supervision, NK and AG; writing—original draft, SP; writing—review and editing, RM, NK, and AG. All authors contributed to the article and approved the submitted version.
This study was supported by the National Eye Institute R01EY026174 (AG and NK), Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (AG), Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (AG), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (AG) and Vision Research Program (VRP) W81XWH-20-1-0820 (AG), National Eye Institute R01EY026174 (AG) and R61EY032468 (AG), NIH Center Core Grant P30EY014801 (institutional), and Research to Prevent Blindness Unrestricted Grant GR004596 (institutional).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdul-Wahab, S. A., En, S. C. F., Elkamel, A., Ahmadi, L., and Yetilmezsoy, K. (2015). A review of standards and guidelines set by international bodies for the parameters of indoor air quality. Atmos. Pollut. Res. 6 (5), 751–767. doi:10.5094/apr.2015.084
Abusharha, A. A., Pearce, E. I., and Fagehi, R. (2016). Effect of ambient temperature on the human tear film. Eye Contact Lens Sci. Clin. Pract. 42 (5), 308–312. doi:10.1097/ICL.0000000000000210
Abusharha, A. A., and Pearce, E. I. (2013). The effect of low humidity on the human tear film. Cornea 32 (4), 429–434. doi:10.1097/ICO.0b013e31826671ab
Akasaki, Y., Inomata, T., Sung, J., Nakamura, M., Kitazawa, K., Shih, K. C., et al. (2022). Prevalence of comorbidity between dry eye and allergic conjunctivitis: A systematic review and meta-analysis. J. Clin. Med. 11 (13), 3643. doi:10.3390/jcm11133643
Albietz, J. M., and Lenton, L. M. (2006). Effect of antibacterial honey on the ocular flora in tear deficiency and meibomian gland disease. Cornea 25 (9), 1012–1019. doi:10.1097/01.ico.0000225716.85382.7b
Albrecht, M., and Dittrich, A. M. (2018). Cytokines in sensitization to aeroallergens. Allergol. Sel. 2 (1), 94–100. doi:10.5414/ALX1480E
Alves, M., Novaes, P., Morraye, MdA., Reinach, P. S., and Rocha, E. M. (2014). Is dry eye an environmental disease? Arq. Bras. Oftalmol. 77, 193–200. doi:10.5935/0004-2749.20140050
Barabino, S., Shen, L., Chen, L., Rashid, S., Rolando, M., and Dana, M. R. (2005). The controlled-environment chamber: A new mouse model of dry eye. Investigative Ophthalmol. Vis. Sci. 46 (8), 2766–2771. doi:10.1167/iovs.04-1326
Basbaum, A. I., Bautista, D. M., Scherrer, G., and Julius, D. J. C. (2009). Cellular and molecular mechanisms of pain. Cell. 139 (2), 267–284. doi:10.1016/j.cell.2009.09.028
Bergmann, K-C., Berger, M., Klimek, L., Pfaar, O., Werchan, B., Werchan, M., et al. (2021). Nonpharmacological measures to prevent allergic symptoms in pollen allergy: A critical review. Allergol. Sel. 5, 349–360. doi:10.5414/ALX02294E
Bitzer, Z. T., Goel, R., Reilly, S. M., Elias, R. J., Silakov, A., Foulds, J., et al. (2018). Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic. Biol. Med. 120, 72–79. doi:10.1016/j.freeradbiomed.2018.03.020
Calonge, M., Labetoulle, M., Messmer, E. M., Shah, S., Akova, Y. A., Boboridis, K. G., et al. (2018). Controlled adverse environment chambers in dry eye research. Curr. Eye Res. 43 (4), 445–450. doi:10.1080/02713683.2017.1420197
Carter, H. H., Spence, A. L., Atkinson, C. L., Pugh, C. J. A., Naylor, L. H., and Green, D. J. (2014). Repeated core temperature elevation induces conduit artery adaptation in humans. Eur. J. Appl. Physiology 114 (4), 859–865. doi:10.1007/s00421-013-2817-2
Chalmers, R. L., Begley, C. G., and Caffery, B. (2010). Validation of the 5-item dry eye questionnaire (DEQ-5): discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Contact Lens Anterior Eye 33 (2), 55–60. doi:10.1016/j.clae.2009.12.010
Choi, W., Lian, C., Ying, L., Kim, G. E., You, I. C., Park, S. H., et al. (2016). Expression of lipid peroxidation markers in the tear film and ocular surface of patients with non-sjogren syndrome: potential biomarkers for dry eye disease. Curr. Eye Res. 41 (9), 1143–1149. doi:10.3109/02713683.2015.1098707
Craig, J. P., Nichols, K. K., Akpek, E. K., Caffery, B., Dua, H. S., Joo, C. K., et al. (2017). TFOS DEWS II definition and classification report. Ocul. Surf. 15 (3), 276–283. doi:10.1016/j.jtos.2017.05.008
Craig, J. P., and Tomlinson, A. (1997). Importance of the lipid layer in human tear film stability and evaporation. Optometry Vis. Sci. 74 (1), 8–13. doi:10.1097/00006324-199701000-00014
D’Amato, G., Holgate, S. T., Pawankar, R., Ledford, D. K., Cecchi, L., Al-Ahmad, M., et al. (2015). Meteorological conditions, climate change, new emerging factors, and asthma and related allergic disorders. A statement of the World Allergy Organization. World Allergy Organ. J. 8 (1), 25–52. doi:10.1186/s40413-015-0073-0
Danjo, Y., Watanabe, H., Tisdale, A. S., George, M., Tsumura, T., Abelson, M. B., et al. (1998). Alteration of mucin in human conjunctival epithelia in dry eye. Invest. Ophthalmol. Vis. Sci. 39 (13), 2602–2609.
Davidson, H. J., and Kuonen, V. J. (2004). The tear film and ocular mucins. Vet. Ophthalmol. 7 (2), 71–77. doi:10.1111/j.1463-5224.2004.00325.x
Dermer, H., Theotoka, D., Lee, C. J., Chhadva, P., Hackam, A. S., Galor, A., et al. (2019). Total tear IgE levels correlate with allergenic and irritating environmental exposures in individuals with dry eye. J. Clin. Med. [Internet] 8 (10), 1627. doi:10.3390/jcm8101627
Dogru, M., Kojima, T., Simsek, C., and Tsubota, K. (2018). Potential role of oxidative stress in ocular surface inflammation and dry eye disease. Investigative Ophthalmol. Vis. Sci. 59 (14), DES163–DES8. doi:10.1167/iovs.17-23402
Dogru, M., Matsumoto, Y., Okada, N., Igarashi, A., Fukagawa, K., Shimazaki, J., et al. (2008). Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy 63 (10), 1324–1334. doi:10.1111/j.1398-9995.2008.01781.x
Dougherty, J. M., and McCulley, J. P. (1986). Bacterial lipases and chronic blepharitis. Investigative Ophthalmol. Vis. Sci. 27 (4), 486–491.
Farhangi, M., Feuer, W., Galor, A., Bouhassira, D., Levitt, R. C., Sarantopoulos, C. D., et al. (2019). Modification of the neuropathic pain symptom inventory for use in eye pain (NPSI-eye). Pain 160 (7), 1541–1550. doi:10.1097/j.pain.0000000000001552
Friedlaender, M. H. (2011). Ocular allergy. Curr. Opin. allergy Clin. Immunol. 11 (5), 477–482. doi:10.1097/ACI.0b013e32834a9652
Fu, Q., Lyu, D., Zhang, L., Qin, Z., Tang, Q., Yin, H., et al. (2017). Airborne particulate matter (PM2.5) triggers autophagy in human corneal epithelial cell line. Environ. Pollut. 227, 314–322. doi:10.1016/j.envpol.2017.04.078
Galor, A., Felix, E. R., Feuer, W., Levitt, R. C., and Sarantopoulos, C. D. (2020). Corneal nerve pathway function in individuals with dry eye symptoms. Ophthalmology 128, 619–621. doi:10.1016/j.ophtha.2020.07.061
Galor, A., Kumar, N., Feuer, W., and Lee, D. J. (2014). Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmology 121 (4), 972–973. doi:10.1016/j.ophtha.2013.11.036
Ganesalingam, K., Ismail, S., Sherwin, T., and Craig, J. P. (2019). Molecular evidence for the role of inflammation in dry eye disease. Clin. Exp. Optometry 102 (5), 446–454. doi:10.1111/cxo.12849
Giraldez, M. J., Naroo, S. A., and Resua, C. G. (2009). A preliminary investigation into the relationship between ocular surface temperature and lipid layer thickness. Contact Lens Anterior Eye 32 (4), 177–180. doi:10.1016/j.clae.2009.06.004
González-García, M. J., González-Sáiz, A., Bdl, F., Morilla-Grasa, A., Mayo-Iscar, A., San-José, J., et al. (2007). Exposure to a controlled adverse environment impairs the ocular surface of subjects with minimally symptomatic dry eye. Investigative Ophthalmol. Vis. Sci. 48 (9), 4026–4032. doi:10.1167/iovs.06-0817
Goyal, S., and Hamrah, P. (2016). Understanding neuropathic corneal pain—Gaps and current therapeutic approaches. Semin. Ophthalmol. 31 (1–2), 59–70. doi:10.3109/08820538.2015.1114853
Graudenz, G. S., Landgraf, R. G., Jancar, S., Tribess, A., Fonseca, S. G., Faé, K. C., et al. (2006). The role of allergic rhinitis in nasal responses to sudden temperature changes. J. Allergy Clin. Immunol. 118 (5), 1126–1132. doi:10.1016/j.jaci.2006.07.005
Higuchi, A., Ito, K., Dogru, M., Kitamura, M., Mitani, F., Kawakita, T., et al. (2011). Corneal damage and lacrimal gland dysfunction in a smoking rat model. Free Radic. Biol. Med. 51 (12), 2210–2216. doi:10.1016/j.freeradbiomed.2011.09.025
Hikichi, T., Yoshida, A., and Tsubota, K. (1993). Lymphocytic infiltration of the conjunctiva and the salivary gland in Sjögren's syndrome. Archives Ophthalmol. 111 (1), 21–22. doi:10.1001/archopht.1993.01090010023009
Ho, W. T., Chiu, C. Y., and Chang, S. W. (2022). Low ambient temperature correlates with the severity of dry eye symptoms. Taiwan J. Ophthalmol. 12 (2), 191–197. doi:10.4103/tjo.tjo_25_21
Hong, J., Zhong, T., Li, H., Xu, J., Ye, X., Mu, Z., et al. (2016). Ambient air pollution, weather changes, and outpatient visits for allergic conjunctivitis: A retrospective registry study. Sci. Rep. 6, 23858. doi:10.1038/srep23858
Hori, S., Ohnaka, M., Shiraki, K., Tsujita, J., Yoshimura, H., Saito, N., et al. (1977). Comparison of physical characteristics, body temperature and basal metabolism between Thai and Japanese in a neutral temperature zone. Jpn. J. Physiology 27 (5), 525–538. doi:10.2170/jjphysiol.27.525
Hori, S., Tsujita, J., Tanaka, N., and Mayuzumi, M. (1978). Studies on heat tolerance of subtropical natives after migration to a temperate zone. Int. J. Biometeorology 22 (2), 82–93. doi:10.1007/BF01552887
Huang, A., Janecki, J., Galor, A., Rock, S., Menendez, D., Hackam, A. S., et al. (2020). Association of the indoor environment with dry eye metrics. JAMA Ophthalmol. 138 (8), 867–874. doi:10.1001/jamaophthalmol.2020.2237
Hwang, J., Dermer, H., and Galor, A. (2021). Can in vivo confocal microscopy differentiate between sub-types of dry eye disease? A review. Clin. Exp. Ophthalmol. 49 (4), 373–387. doi:10.1111/ceo.13924
Hwang, S. H., Choi, Y. H., Paik, H. J., Wee, W. R., Kim, M. K., and Kim, D. H. (2016). Potential importance of ozone in the association between outdoor air pollution and dry eye disease in South Korea. JAMA Ophthalmol. 134 (5), 503–510. doi:10.1001/jamaophthalmol.2016.0139
Idarraga, M. A., Guerrero, J. S., Mosle, S. G., Miralles, F., Galor, A., and Kumar, N. (2020). Relationships between short-term exposure to an indoor environment and dry eye (DE) symptoms. J. Clin. Med. 9 (5), 1316. doi:10.3390/jcm9051316
Isa, N. A. M., Koh, P. Y., and Doraj, P. (2019). The tear function in electronic cigarette smokers. Optometry Vis. Sci. 96 (9), 678–685. doi:10.1097/OPX.0000000000001422
Ju, A., Helander, M. L., and Savolainen, J. (1998). Genetic and environmental factors affecting the allergenicity of birch (Betula pubescens ssp. czerepanovii [Orl] Hämet-ahti) pollen. Clin. Exp. Allergy 28 (11), 1384–1388. doi:10.1046/j.1365-2222.1998.00404.x
Jester, J. V., Nicolaides, N., and Smith, R. E. (1981). Meibomian gland studies: histologic and ultrastructural investigations. Investigative Ophthalmol. Vis. Sci. 20 (4), 537–547.
Jung, S. J., Mehta, J. S., and Tong, L. (2018). Effects of environment pollution on the ocular surface. Ocular Surf. 16 (2), 198–205. doi:10.1016/j.jtos.2018.03.001
Kenny, G. P., Yardley, J., Brown, C., Sigal, R. J., and Jay, O. (2010). Heat stress in older individuals and patients with common chronic diseases. CMAJ 182 (10), 1053–1060. doi:10.1503/cmaj.081050
Kim, J., Lim, Y., and Kim, H. (2014). Outdoor temperature changes and emergency department visits for asthma in seoul, korea: A time-series study. Environ. Res. 135, 15–20. doi:10.1016/j.envres.2014.07.032
Kim, Y., Choi, Y. H., Kim, M. K., Paik, H. J., and Kim, D. H. (2020). Different adverse effects of air pollutants on dry eye disease: ozone, PM2.5, and PM10. Environ. Pollut. 265, 115039. doi:10.1016/j.envpol.2020.115039
Kiotseridis, H., Cilio, C. M., Bjermer, L., Tunsäter, A., Jacobsson, H., and Dahl, Å. (2013). Grass pollen allergy in children and adolescents-symptoms, health related quality of life and the value of pollen prognosis. Clin. Transl. allergy 3 (1), 19–12. doi:10.1186/2045-7022-3-19
Kovats, R. S., and Hajat, S. (2008). Heat stress and public health: A critical review. Annu. Rev. Public Health 29 (1), 41–55. doi:10.1146/annurev.publhealth.29.020907.090843
Kumar, N., Feuer, W., Lanza, N. L., and Galor, A. (2015). Seasonal variation in dry eye. Ophthalmology 122 (8), 1727–1729. doi:10.1016/j.ophtha.2015.02.013
Kumar, N. (2016). The exposure uncertainty analysis: the association between birth weight and trimester specific exposure to particulate matter (PM2.5 vs. PM10). Int. J. Environ. Res. Public Health 13, 906. doi:10.3390/ijerph13090906
Lee, H., Kim, E. K., Kang, S. W., Kim, J. H., Hwang, H. J., and Kim, T. I. (2013). Effects of ozone exposure on the ocular surface. Free Radic. Biol. Med. 63, 78–89. doi:10.1016/j.freeradbiomed.2013.05.006
Lee, P., Smith, S., Linderman, J., Courville, A. B., Brychta, R. J., Dieckmann, W., et al. (2014). Temperature-acclimated Brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63 (11), 3686–3698. doi:10.2337/db14-0513
Leonardi, A., Modugno, R. L., and Salami, E. (2021). Allergy and dry eye disease. Ocular Immunol. Inflamm. 29 (6), 1168–1176. doi:10.1080/09273948.2020.1841804
Levetin, E., and Van de Water, P. (2008). Changing pollen types/concentrations/distribution in the United States: fact or fiction? Curr. Allergy Asthma Rep. 8 (5), 418–424. doi:10.1007/s11882-008-0081-z
Li, J., Tan, G., Ding, X., Wang, Y., Wu, A., Yang, Q., et al. (2017). A mouse dry eye model induced by topical administration of the air pollutant particulate matter 10. Biomed. Pharmacother. 96, 524–534. doi:10.1016/j.biopha.2017.10.032
Li, K., Ni, H., Yang, Z., Wang, Y., Ding, S., Wen, L., et al. (2016). Effects of temperature variation between neighbouring days on daily hospital visits for childhood asthma: A time-series analysis. Public Health 136, 133–140. doi:10.1016/j.puhe.2016.04.002
Li, X., Kang, B., Eom, Y., Lee, H. K., Kim, H. M., and Song, J. S. (2019). The protective effect of a topical mucin secretagogue on ocular surface damage induced by airborne carbon black exposure. Investigative Ophthalmol. Vis. Sci. 60 (1), 255–264. doi:10.1167/iovs.18-25964
Lim, Y-H., Hong, Y-C., and Kim, H. (2012). Effects of diurnal temperature range on cardiovascular and respiratory hospital admissions in Korea. Sci. total Environ. 417-418, 55–60. doi:10.1016/j.scitotenv.2011.12.048
Liu, Z., Chen, D., Chen, X., Bian, F., Gao, N., Li, J., et al. (2020). Autophagy activation protects ocular surface from inflammation in a dry eye model in vitro. Int. J. Mol. Sci. 21 (23), 8966. doi:10.3390/ijms21238966
Lobefalo, L., D'Antonio, E., Colangelo, L., Loggia, G. D., Di Gioacchino, M., Angelucci, D., et al. (1999). Dry eye in allergic conjunctivitis: role of inflammatory infiltrate. Int. J. Immunopathol. Pharmacol. 12 (3), 205873929901200304.
Lu, C., Deng, Q., Li, Y., Sundell, J., and Norbäck, D. (2016). Outdoor air pollution, meteorological conditions and indoor factors in dwellings in relation to sick building syndrome (SBS) among adults in China. Sci. Total Environ. 560, 186–196. doi:10.1016/j.scitotenv.2016.04.033
Ma, B., Zhou, Y., Liu, R., Zhang, K., Yang, T., Hu, C., et al. (2021). Pigment epithelium-derived factor (PEDF) plays anti-inflammatory roles in the pathogenesis of dry eye disease. Ocular Surf. 20, 70–85. doi:10.1016/j.jtos.2020.12.007
Madden, L. C., Tomlinson, A., and Simmons, P. A. (2013). Effect of humidity variations in a controlled environment chamber on tear evaporation after dry eye therapy. Eye Contact Lens 39 (2), 169–174. doi:10.1097/ICL.0b013e318283dfc6
Makrynioti, D., Zagoriti, Z., Koutsojannis, C., Morgan, P. B., and Lagoumintzis, G. (2020). Ocular conditions and dry eye due to traditional and new forms of smoking: A review. Contact Lens Anterior Eye 43 (3), 277–284. doi:10.1016/j.clae.2020.02.009
Mandell, J. T., Idarraga, M., Kumar, N., and Galor, A. (2020). Impact of air pollution and weather on dry eye. J. Clin. Med. 9 (11), 3740. doi:10.3390/jcm9113740
Martheswaran, T., Shmunes, M. H., Ronquillo, Y. C., and Moshirfar, M. (2021). The impact of vaping on ocular health: A literature review. Int. Ophthalmol. 41 (8), 2925–2932. doi:10.1007/s10792-021-01842-w
Mehra, D., Cohen, N. K., and Galor, A. J. O. (2020). Ocular surface pain: A narrative review. Ophthalmol. Ther. 9, 1–21. doi:10.1007/s40123-020-00263-9
Mendell, M. J., Fisk, W. J., Petersen, M. R., Hines, C. J., Dong, M., Faulkner, D., et al. (2002). Indoor particles and symptoms among office workers: results from a double-blind cross-over study. Epidemiology 13, 296–304. doi:10.1097/00001648-200205000-00010
Michelozzi, P., Accetta, G., De Sario, M., D'Ippoliti, D., Marino, C., Baccini, M., et al. (2009). High temperature and hospitalizations for cardiovascular and respiratory causes in 12 European cities. Am. J. Respir. Crit. Care Med. 179 (5), 383–389. doi:10.1164/rccm.200802-217OC
Mimura, T., Ichinose, T., Yamagami, S., Fujishima, H., Kamei, Y., Goto, M., et al. (2014). Airborne particulate matter (PM2.5) and the prevalence of allergic conjunctivitis in Japan. Sci. Total Environ. 487, 493–499. doi:10.1016/j.scitotenv.2014.04.057
Mo, Z., Fu, Q., Lyu, D., Zhang, L., Qin, Z., Tang, Q., et al. (2019). Impacts of air pollution on dry eye disease among residents in hangzhou, China: A case-crossover study. Environ. Pollut. 246, 183–189. doi:10.1016/j.envpol.2018.11.109
Morgan, W. J., Crain, E. F., Gruchalla, R. S., O'Connor, G. T., Kattan, M., Evans, R., et al. (2004). Results of a home-based environmental intervention among urban children with asthma. N. Engl. J. Med. 351 (11), 1068–1080. doi:10.1056/NEJMoa032097
Moss, S. E., Klein, R., and Klein, B. E. K. (2000). Prevalence of and risk factors for dry eye syndrome. Archives Ophthalmol. 118 (9), 1264–1268. doi:10.1001/archopht.118.9.1264
Nagymihályi, A., Dikstein, S., and Tiffany, J. M. (2004). The influence of eyelid temperature on the delivery of meibomian oil. Exp. Eye Res. 78 (3), 367–370. doi:10.1016/s0014-4835(03)00197-0
Ning, H., Wang, Z., and Ji, Y. (2016). Thermal history and adaptation: does a long-term indoor thermal exposure impact human thermal adaptability? Appl. Energy 183, 22–30. doi:10.1016/j.apenergy.2016.08.157
Ortega, N. A., Díaz, S. G., Hamsho, J. M., Noriega, K. M., Weinmann, A. M., Avilán, R. G., et al. (2022). Dry eye syndrome in patients with allergic eye disease. J. Allergy Clin. Immunol. 149 (2), AB217. doi:10.1016/j.jaci.2021.12.709
Pan, Z., Mølhave, L., and Kjaergaard, S. K. (2000). Effects on eyes and nose in humans after experimental exposure to airborne office dust. Indoor air 10 (4), 237–245. doi:10.1034/j.1600-0668.2000.010004237.x
Park, J-E., Son, W-S., Ryu, Y., Choi, S. B., Kwon, O., and Ahn, I. (2020). Effects of temperature, humidity, and diurnal temperature range on influenza incidence in a temperate region. Influenza Other Respir. Viruses 14 (1), 11–18. doi:10.1111/irv.12682
Paschides, C. A., Stefaniotou, M., Papageorgiou, J., Skourtis, P., and Psilas, K. (1998). Ocular surface and environmental changes. Acta Ophthalmol. Scand. 76 (1), 74–77. doi:10.1034/j.1600-0420.1998.760113.x
Patel, S., Felix, E. R., Levitt, R. C., Sarantopoulos, C. D., and Ajjocm, G. (2019). Dysfunctional coping mechanisms contribute to dry eye symptoms. J. Clin. Med. 8 (6), 901. doi:10.3390/jcm8060901
Patel, S., and Sarantopoulos, K. D. (2023). Pathways and mechanisms of ocular pain and photophobia in dry eye disease. Dry. Eye Dis. 2023, 229–240. doi:10.1016/b978-0-323-82753-9.00005-9
Peng, C-C., Cerretani, C., Braun, R. J., and Radke, C. J. (2014). Evaporation-driven instability of the precorneal tear film. Adv. Colloid Interface Sci. 206, 250–264. doi:10.1016/j.cis.2013.06.001
Périard, J. D., Racinais, S., and Sawka, M. N. (2015). Adaptations and mechanisms of human heat acclimation: applications for competitive athletes and sports. Scand. J. Med. Sci. Sports 25 (S1), 20–38. doi:10.1111/sms.12408
Pfab, F., Valet, M., Sprenger, T., Huss-Marp, J., Athanasiadis, G. I., Baurecht, H. J., et al. (2010). Temperature modulated histamine-itch in lesional and nonlesional skin in atopic eczema – A combined psychophysical and neuroimaging study. Allergy 65 (1), 84–94. doi:10.1111/j.1398-9995.2009.02163.x
Pflugfelder, S. C., Tseng, S. C. G., Yoshino, K., Monroy, D., Felix, C., and Reis, B. L. (1997). Correlation of goblet cell density and mucosal epithelial membrane mucin expression with rose bengal staining in patients with ocular irritation. Ophthalmology 104 (2), 223–235. doi:10.1016/s0161-6420(97)30330-3
Proud, D., Sweet, J., Stein, P., Settipane, R. A., Kagey-Sobotka, A., Friedlaender, M. H., et al. (1990). Inflammatory mediator release on conjunctival provocation of allergic subjects with allergen. J. Allergy Clin. Immunol. 85 (5), 896–905. doi:10.1016/0091-6749(90)90075-f
Qazi, Y., Hurwitz, S., Khan, S., Jurkunas, U. V., Dana, R., and Hamrah, P. (2016). Validity and reliability of a novel ocular pain assessment Survey (OPAS) in quantifying and monitoring corneal and ocular surface pain. Ophthalmology 123 (7), 1458–1468. doi:10.1016/j.ophtha.2016.03.006
Qiu, H., Yu, I. T. S., Tse, L. A., Chan, E. Y. Y., Wong, T. W., and Tian, L. (2015). Greater temperature variation within a day associated with increased emergency hospital admissions for asthma. Sci. Total Environ. 505, 508–513. doi:10.1016/j.scitotenv.2014.10.003
Qiu, L., Liu, F., Zhang, X., and Gao, T. (2019). Difference of airborne particulate matter concentration in urban space with different green coverage rates in baoji, China. Int. J. Environ. Res. public health 16 (8), 1465. doi:10.3390/ijerph16081465
Rabensteiner, D. F., Rabensteiner, J., Horwath-Winter, J., Lang-Loidolt, D., Wedrich, A., Heidinger, A., et al. (2019). Extracts of different pollen species and their effect on human tear fluid and an epithelial cell line. Cutan. Ocular Toxicol. 38 (1), 93–103. doi:10.1080/15569527.2018.1530259
Reinikainen, L. M., Jaakkola, J. J., and Seppänen, O. (1992). The effect of air humidification on symptoms and perception of indoor air quality in office workers: A six-period cross-over trial. Archives Environ. Health Int. J. 47 (1), 8–15. doi:10.1080/00039896.1992.9935938
Rock, S., Galor, A., and Kumar, N. (2021). Indoor airborne microbial concentration and dry eye. Am. J. Ophthalmol. 223, 193–204. doi:10.1016/j.ajo.2020.10.003
Rozanova, E., Heilig, P., and Godnić-Cvar, J. (2009). The eye--a neglected organ in environmental and occupational medicine: an overview of known environmental and occupational non-traumatic effects on the eyes. Arh. Hig. Rada Toksikol. 60 (2), 205–215. doi:10.2478/10004-1254-60-2009-1869
Rummenie, V. T., Matsumoto, Y., Dogru, M., Wang, Y., Hu, Y., Ward, S. K., et al. (2008). Tear cytokine and ocular surface alterations following brief passive cigarette smoke exposure. Cytokine 43 (2), 200–208. doi:10.1016/j.cyto.2008.05.011
Sahai, A., and Malik, P. (2005). Dry eye: prevalence and attributable risk factors in a hospital-based population. Indian J. Ophthalmol. 53 (2), 87–91. doi:10.4103/0301-4738.16170
Sayin, N., Kara, N., Pekel, G., and Altinkaynak, H. (2014). Effects of chronic smoking on central corneal thickness, endothelial cell, and dry eye parameters. Cutan. Ocular Toxicol. 33 (3), 201–205. doi:10.3109/15569527.2013.832688
Schiffman, R. M., Christianson, M. D., Jacobsen, G., Hirsch, J. D., and Reis, B. L. (2000). Reliability and validity of the ocular surface disease Index. Arch. Ophthalmol. 118 (5), 615–621. doi:10.1001/archopht.118.5.615
Smith, J. A. (2007). The epidemiology of dry eye disease. Acta Ophthalmol. Scand. 85, 0. doi:10.1111/j.1600-0420.2007.01063_2858.x
Stern, M. E., Gao, J., Siemasko, K. F., Beuerman, R. W., and Pflugfelder, S. C. (2004). The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp. Eye Res. 78 (3), 409–416. doi:10.1016/j.exer.2003.09.003
Stucky, C. L., Gold, M. S., and Zhang, X. (2001). Mechanisms of pain. Mech. pain 98 (21), 11845–11846. doi:10.1073/pnas.211373398
Subbaraman, L. N., McCanna, D. J., Lorentz, H. I., Soong, F., Salapatek, A. M., and Jones, L. W. (2014). Tear cytokines in non-dry eye and dry eye participants after exposure to a low humidity environmental exposure chamber. Investigative Ophthalmol. Vis. Sci. 55 (13), 3682.
Syndulko, K., Jafari, M., Woldanski, A., Baumhefner, R. W., and Tourtellotte, W. W. (1996). Effects of temperature in multiple sclerosis: A review of the literature. J. Neurologic Rehabilitation 10 (1), 23–34. doi:10.1177/154596839601000104
Tau, J., Novaes, P., Matsuda, M., Tasat, D. R., Saldiva, P. H., and Berra, A. (2013). Diesel exhaust particles selectively induce both proinflammatory cytokines and mucin production in cornea and conjunctiva human cell lines. Investigative Ophthalmol. Vis. Sci. 54 (7), 4759–4765. doi:10.1167/iovs.12-10541
Tesón, M., González-García, M. J., López-Miguel, A., Enríquez-de-Salamanca, A., Martín-Montañez, V., Benito, M. J., et al. (2013). Influence of a controlled environment simulating an in-flight airplane cabin on dry eye disease. Investigative Ophthalmol. Vis. Sci. 54 (3), 2093–2099. doi:10.1167/iovs.12-11361
Vance, P. H., Schaeffer, F., Terry, P., Trevino, E., and Weissfeld, A. S. (2016). Mold causes and effects “in a Material World”. Clin. Microbiol. Newsl. 38 (14), 111–116. doi:10.1016/j.clinmicnews.2016.06.004
Versura, P., Profazio, V., Cellini, M., Torreggiani, A., and Caramazza, R. (1999). Eye discomfort and air pollution. Ophthalmologica 213 (2), 103–109. doi:10.1159/000027401
Villani, E., Bonsignore, F., Cantalamessa, E., Serafino, M., and Nucci, P. (2020). Imaging biomarkers for dry eye disease. Eye Contact Lens 46 (2), S141–s5. doi:10.1097/ICL.0000000000000650
Vocks, E., Busch, R., Fröhlich, C., Borelli, S., Mayer, H., and Ring, J. (2001). Influence of weather and climate on subjective symptom intensity in atopic eczema. Int. J. Biometeorology 45 (1), 27–33. doi:10.1007/s004840000077
Wang, B., Peng, L., Ouyang, H., Wang, L., He, D., Zhong, J., et al. (2019). Induction of DDIT4 impairs autophagy through oxidative stress in dry eye. Investigative Ophthalmol. Vis. Sci. 60 (8), 2836–2847. doi:10.1167/iovs.19-27072
Wang, M. T., Chan, E., Ea, L., Kam, C., Lu, Y., Misra, S. L., et al. (2017). Randomized trial of desktop humidifier for dry eye relief in computer users. Optometry Vis. Sci. 94 (11), 1052–1057. doi:10.1097/OPX.0000000000001136
Wendt, R., Aglan, H., Livengood, S., Khan, M., Ibrahim, E., Heidenreich, M., et al. (2004). “Indoor air quality of an energy-efficient, healthy house with mechanically induced fresh air,” in Technical and Symposium Papers—2004 Annual Meeting of the American Society of Heating, Refrigerating and Air-Conditioning Engineers, June 26–30, 2004 110 (PART II), 77–84.
Wolkoff, P. (2020). Dry eye symptoms in offices and deteriorated work performance – a perspective. Build. Environ. 172, 106704. doi:10.1016/j.buildenv.2020.106704
Wolkoff, P., Skov, P., Franck, C., and Petersen, L. N. (2003). Eye irritation and environmental factors in the office environment--hypotheses, causes and a physiological model. Scand. J. Work Environ. Health 29 (6), 411–430. doi:10.5271/sjweh.748
Wyon, D., Fang, L., Meyer, H., Sundell, J., Weirsoee, C., SederbergOlsen, N., et al. (2002). “Limiting criteria for human exposure to low humidity indoors,” in 9th international conference on indoor air quality and climate (Rotterdam (Netherlands): in-house publishing).
Xiang, P., He, R-W., Han, Y-H., Sun, H-J., Cui, X-Y., and Ma, L. Q. (2016). Mechanisms of housedust-induced toxicity in primary human corneal epithelial cells: oxidative stress, proinflammatory response and mitochondrial dysfunction. Environ. Int. 89-90, 30–37. doi:10.1016/j.envint.2016.01.008
Xu, L., Zhang, W., Zhu, X. Y., Suo, T., Fan, X. Q., and Fu, Y. (2016). Smoking and the risk of dry eye: A meta-analysis. Int. J. Ophthalmol. 9 (10), 1480–1486. doi:10.18240/ijo.2016.10.19
Xu, Z., Huang, C., Su, H., Turner, L. R., Qiao, Z., and Tong, S. (2013). Diurnal temperature range and childhood asthma: A time-series study. Environ. Health 12 (1), 12. doi:10.1186/1476-069X-12-12
Yeh, S., Song, X. J., Farley, W., Li, D-Q., Stern, M. E., and Pflugfelder, S. C. (2003). Apoptosis of ocular surface cells in experimentally induced dry eye. Investigative Ophthalmol. Vis. Sci. 44 (1), 124–129. doi:10.1167/iovs.02-0581
Yu, J., Asche, C. V., and Fairchild, C. J. J. C. (2011). The economic burden of dry eye disease in the United States: A decision tree analysis. Cornea 30 (4), 379–387. doi:10.1097/ICO.0b013e3181f7f363
Yu, L., Yu, C., Dong, H., Mu, Y., Zhang, R., Zhang, Q., et al. (2021). Recent developments about the pathogenesis of dry eye disease: based on immune inflammatory mechanisms. Front. Pharmacol. 12, 732887. doi:10.3389/fphar.2021.732887
Zheng, Q., Ren, Y., Reinach, P. S., She, Y., Xiao, B., Hua, S., et al. (2014). Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye. Exp. Eye Res. 125, 1–8. doi:10.1016/j.exer.2014.05.001
Keywords: dry eye disease, ocular surface pain, environment, toxicology, temperature, humidity, air pollutant, allergy
Citation: Patel S, Mittal R, Kumar N and Galor A (2023) The environment and dry eye—manifestations, mechanisms, and more. Front. Toxicol. 5:1173683. doi: 10.3389/ftox.2023.1173683
Received: 17 May 2023; Accepted: 07 August 2023;
Published: 23 August 2023.
Edited by:
Marcelo Dutra Arbo, Federal University of Rio Grande do Sul, BrazilReviewed by:
Danni Lyu, Zhejiang University, ChinaCopyright © 2023 Patel, Mittal, Kumar and Galor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anat Galor, YWdhbG9yQG1lZC5taWFtaS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.