- 1Tecnologico de Monterrey, Escuela de Medicina y Ciencias de La Salud, Monterrey, Mexico
- 2Department of Ophthalmology, Foster Center for Ocular Immunology at Duke Eye Center, Duke University School of Medicine, Durham, NC, United States
- 3Asociación Para Evitar La Ceguera en México, I.A.P, Mexico City, Mexico
- 4Unidad Oftalmología, Departamento de Especialidades, Facultad de Medicina, Universidad de La Frontera, Temuco, Chile
Ocular surface disease (OSD), a disorder affecting the lacrimal and meibomian glands and the corneal and conjunctival epithelium, is a well-known complication of topical glaucoma therapy. OSD can present as a new or pre-existing condition that virtually any anti-glaucoma formulation can exacerbate. As such, both glaucoma and OSD frequently coexist. Typical OSD symptoms include ocular discomfort, redness, burning, and dryness, whereas signs include periorbital and eyelid skin pigmentation, conjunctival scarring, and superficial punctate keratitis. Pressure-lowering eyedrops can cause toxic, allergic, and inflammatory reactions on the ocular surface. The latter can result from either preservatives or direct toxicity from the active molecule. Although usually mild, OSD can cause significant symptoms that lead to poor quality of life, decreased compliance to therapy, glaucoma progression, and worse visual outcomes. Given the chronic nature of glaucoma, lack of curative therapy, and subsequent lifelong treatment, addressing OSD is necessary. This manuscript aims to provide an up-to-date overview of OSD’s signs, symptoms, and pathogenic mechanisms from glaucoma therapy toxicity.
1 Introduction
Glaucoma is a group of diseases leading to progressive optic neuropathy, characterized by visual field and optic nerve head changes (Aguayo Bonniard et al., 2016). It is the primary cause of irreversible blindness worldwide, with a prevalence ranging from 2.0% in Europeans to 7.3% among individuals of African descent (Sun et al., 2022). Unfortunately, the lack of a cure renders glaucoma therapy lifelong (Aguayo Bonniard et al., 2016). Moreover, disease progression requiring more than one anti-glaucomatous agent occurs in approximately 40% of glaucoma patients. The latter results in chronic exposure and toxicity to the active molecules and associated preservatives (Anwar et al., 2013).
Ocular surface disease (OSD) represents a spectrum of diseases, including conjunctivitis, lid disease, allergic manifestations, superficial punctate keratitis, and dry eye disease (DED) (Fechtner et al., 2010; Saade et al., 2015; Gomes et al., 2017). Both glaucoma and OSD are prevalent in the elderly and frequently coexist in the same patient (Fechtner et al., 2010). Up to 66% of patients with severe OSD have glaucoma (Fechtner et al., 2010), whereas the prevalence of OSD in patients using topical anti-glaucoma agents is as high as 59% (Zhang et al., 2019). Patients with prior DED, those exposed to preserved pressure-lowering medications (PLMs), and those requiring ≥1 agent (Saade et al., 2015), are at an increased risk of experiencing worse OSD symptoms. Furthermore, dry eye symptoms can result in increased patient depression, anxiety, and poor quality of life (QoL), which, in turn, is associated with poor compliance to glaucoma therapy and an increased risk of glaucoma progression (Stringham et al., 2018; Tirpack et al., 2019; Rodriguez-Garcia et al., 2022). Thus, addressing OSD in patients with glaucoma is necessary.
This review discusses the pathogenic mechanisms and diagnosis of ocular surface toxicity induced by anti-glaucoma agents, with emphasis on the newer drugs: the rho-kinase (ROCK) inhibitors and nitric oxide (NO)-donating prostaglandin analogs (PGAs).
2 Mechanism of action of pressure-lowering medications
The mechanism of action of PLMs can be divided into those decreasing the aqueous humor production and those increasing the trabecular meshwork or uveoscleral outflow. Regarding the latter, PGAs are the first-line PLM for managing ocular hypertension (OHT) and glaucoma. They exert their effects by binding to E prostanoid and F prostanoid receptors, leading to ciliary muscle relaxation and increased aqueous humor outflow through the uveoscleral pathway (Yamagishi-Kimura et al., 2022). PGAs also induce the expression of matrix metalloproteinases (MMPs) that disrupt the extracellular matrix (ECM), leading to increased trabecular meshwork outflow. Hence, PGAs lower intraocular pressure (IOP) by increasing flow through both pathways (Heo et al., 2020).
Table 1 describes the mechanism of action of each PLM class. However, in the following subsections, we will discuss the mechanism of action of the newer classes of PLMs: the ROCK inhibitors and the NO-donating PGAs.
2.1 Rho-kinase (ROCK) inhibitors
Rho-associated coiled-coil-containing protein kinase (ROCK) is the most studied downstream effector of RhoA, a guanosine triphosphate (GTP)-ase member of the Rho subfamily of the Ras protein family. Rho is activated in the GTP-binding state. The process is aided by bioactive receptors like endothelin-1 and transforming growth factor (TGF)-β, which activates GTPase activating proteins (GAP) and guanine nucleotide exchange factors (GEFs), leading to Rho activation through GTP-binding (Berrino and Supuran, 2019).
ROCKs exhibit two isoforms (ROCK-I and ROCK-II) expressed in many body organs, with varying extents of the subtype involved in each organ and cell tissue. Within the eye, ROCKs are expressed in the trabecular meshwork. In a calcium-independent manner, ROCKs contract the trabecular meshwork by phosphorylating LIM kinases and myosin light chain (MLC) phosphatase. This creates resistance to aqueous humor outflow in the trabecular meshwork (Berrino and Supuran, 2019; Bhargava et al., 2022). ROCK inhibitors decrease actin fiber density in the trabecular meshwork and increase endothelial cell permeability in Schlemm’s canal, ultimately counteracting this outflow resistance (Moura-Coelho et al., 2019).
Netarsudil, a specific ROCK inhibitor, has an amplified impact as it is also a norepinephrine transporter (NET) inhibitor that further decreases aqueous humor production and venous pressure in episcleral vessels (Bhargava et al., 2022).
2.2 Nitric oxide (NO)-donating prostaglandin analogs (PGAs)
Under physiologic conditions, NO is expressed by trabecular meshwork cells, Schlemm’s canal, and the ciliary body. NO reduces IOP by increasing aqueous humor outflow from the trabecular meshwork by activating the soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate (cGMP) pathway and by decreasing aqueous humor production through ion channel modulation. cGMP regulates the action of various downstream effectors, including protein kinase G (PKG), that relaxes vascular smooth muscle (Mao et al., 2020). Via the NO/sGC/cGMP pathway, PKG activates MLC phosphatase, which, in turn, dephosphorylates MLC, leading to the relaxation of trabecular meshwork and Schlemm’s canal cells. The hindmost decreases aqueous humor outflow resistance (Gao et al., 2017). In addition, inducible NO synthase can be activated in trabecular meshwork cells when anterior chamber perfusion pressure becomes elevated (Wu and Ma, 2012).
Latanoprostene bunod is a NO-donating prostaglandin F2α analog recently approved by the FDA (2017) for managing glaucoma (Krauss et al., 2011). When topically administered, the compound is split into the conventional drug and NO, potentiating the IOP-lowering effect (Krauss et al., 2011; Mao et al., 2020). Thus, NO-donating PGAs offer two mechanisms of action. The NO component promotes outflow through the conventional pathway, and the prostaglandin component facilitates flow through the uveoscleral pathway space (Cavet et al., 2015).
3 General mechanisms of ocular surface toxicity caused by pressure-lowering medications
The lacrimal functional unit (LFU) is comprised of the eyelids, meibomian glands (MGs), the main and accessory lacrimal glands, the lacrimal drainage system, the ocular surface (cornea and conjunctiva), and the intertwined innervation (Baudouin et al., 2013). A healthy ocular surface relies on the LFU, which is responsible for the adequate production, distribution, and clearance of the tear film. The latter, in turn, preserves homeostasis of the ocular surface epithelium and protects it from physical damage and exposure (Kawakita, 2018). Dysfunction of one or more components of the LFU 1) hinders the composition of tears, and thus its ability to protect the surface epithelium; 2) disrupts the innate and adaptive immune and inflammatory pathways that protect the ocular surface from external stimuli (i.e., exposure, infection); and 3) stimulate the production of proinflammatory cytokines [i.e., interferon (IFN)-γ, interleukin (IL)-1, −6, −8, tumor necrosis factor (TNF)-α, intercellular adhesion molecule (ICAM)-1, among others] by the ocular surface immune and epithelial cells (Roy et al., 2022).
In this regard, PLMs for the management of OHT and glaucoma have been shown to cause damage to the LFU through a myriad of mechanisms, including a decrease in conjunctival goblet cell (GC) density, squamous metaplasia (Baudouin et al., 2008), conjunctival human leukocyte antigen (HLA)-DR overexpression (Baudouin et al., 2008), and disruption of the degrading-remodeling effect between MMPs and tissue inhibitors of metalloproteinases (TIMPs) in the ECM compounds, including collagen fibers (Karli et al., 2018). Moreover, the need for fixed combinations, commonly required by patients exhibiting disease progression and lifelong treatment, as well as the vehicles and preservatives contained in drug formulations [i.e., benzalkonium chloride (BAK)], will result in a significant number of patients experiencing ocular surface damage (Tiedemann et al., 2019; Rodriguez-Garcia et al., 2022). Additionally, an impression cytology study reported significant overexpression of C-C chemokine receptors (CCR)-type 4 and 5 in the conjunctival epithelium of glaucoma subjects chronically treated with PLMs compared with controls (Baudouin et al., 2008). CCR4 is expressed by the Th2 pathway, which is involved in IgE-mediated allergic diseases, whereas CCR5 is expressed by the Th1 pathway, which has a role in type IV hypersensitivity reactions and the immune response to infections (Baudouin et al., 2005) (Figure 1). These findings suggest that, aside from the inflammatory and toxic mechanisms, allergy may also play a role in the ocular surface damage experienced by glaucoma patients treated with PLMs. Table 2 and Table 3 present the ocular surface disease manifestations of PLMs preserved with BAK and other additives, respectively (Figure 2).
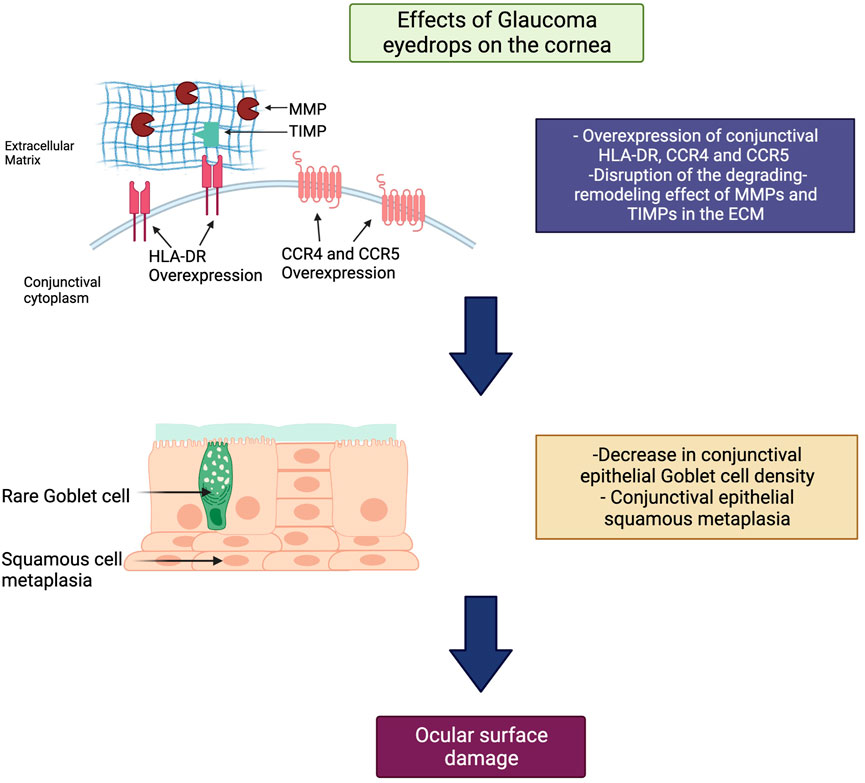
FIGURE 1. General mechanism of ocular surface toxicity due to pressure-lowering medications (PLMs). Within the ocular surface, PLMs cause overexpression of C-C chemokine receptors (CCR)-type 4 and 5, leading to an IgE-mediated allergic reaction on the ocular surface. Also, they cause to disrupt the degrading-remodeling balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in the extracellular matrix (ECM). The latter results in decreased goblet cell density, squamous metaplasia, and ocular surface damage. Footnote: HLA-DR, human leukocyte antigen DR isotype; MMPs, matrix metalloproteinases; TIMPs, tissue inhibitors of metalloproteinases; ECM, extracellular matrix; CCR, C-C chemokine receptor.
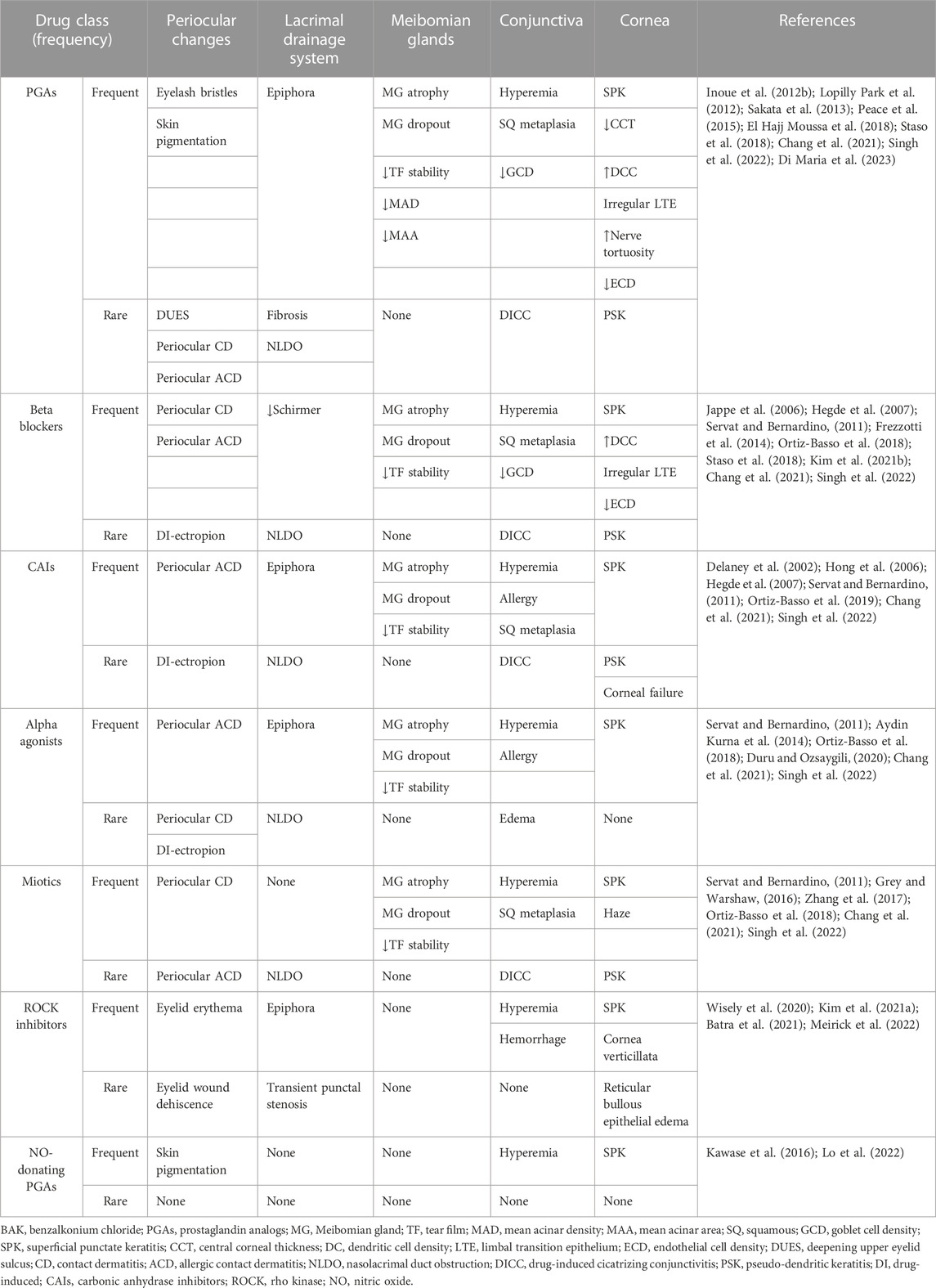
TABLE 2. Human studies reporting ocular surface disease manifestations of BAK-containing pressure-lowering medications.
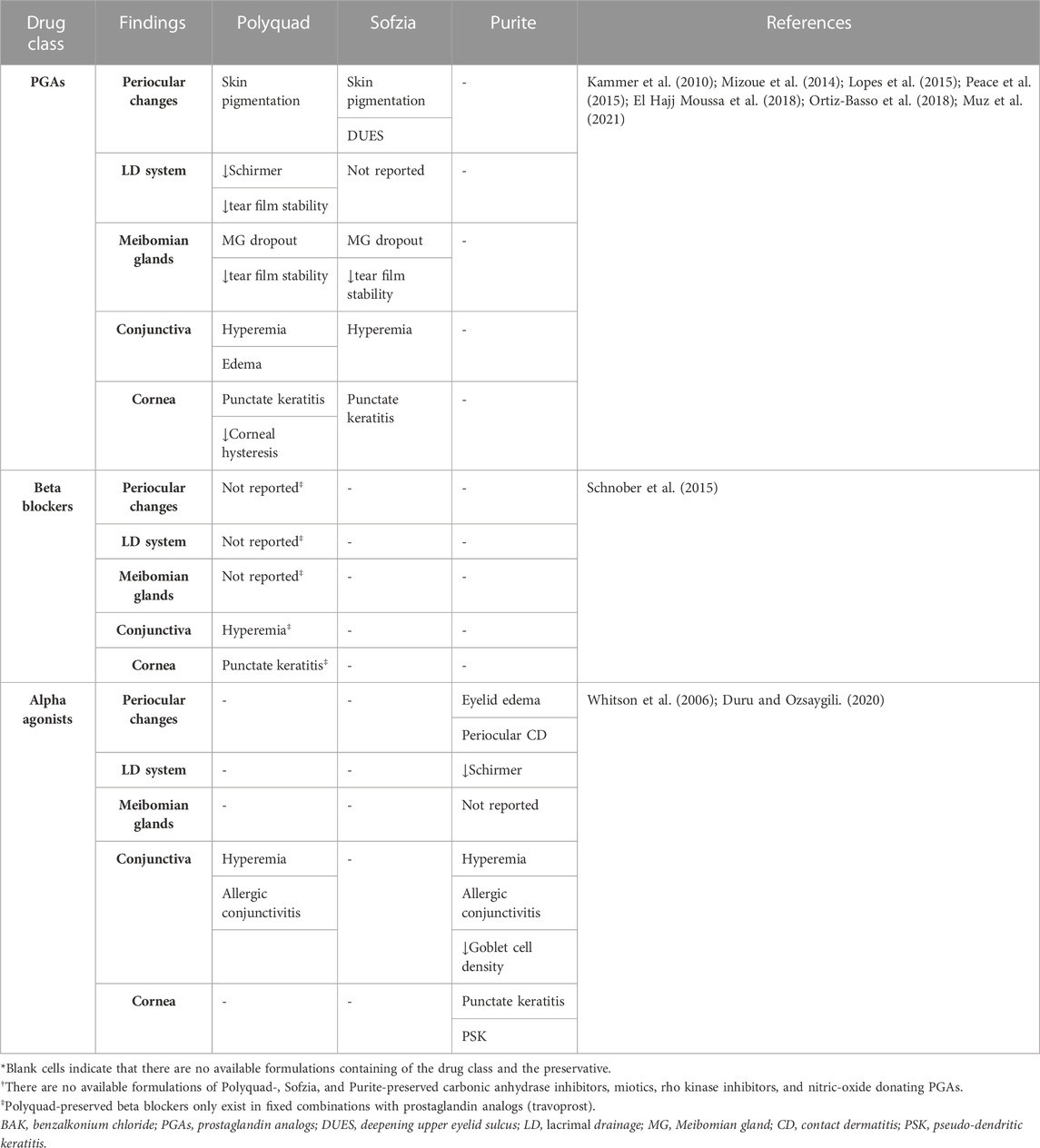
TABLE 3. Human studies reporting ocular surface disease manifestations of non-BAK preserved pressure lowering medications.*†
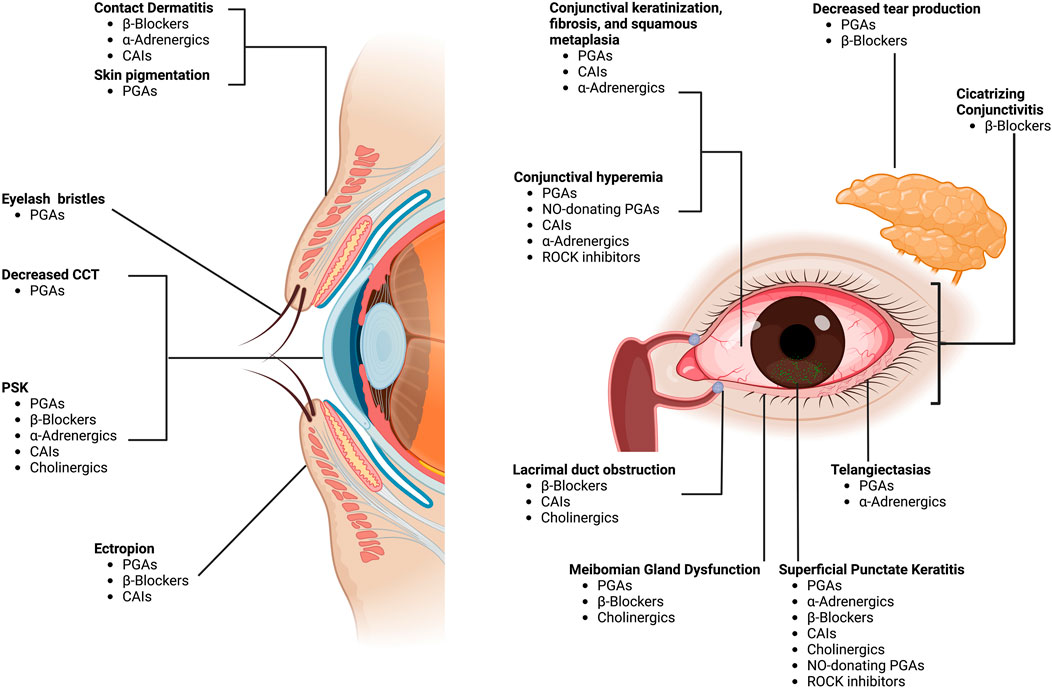
FIGURE 2. Ocular surface adverse effects of pressure-lowering medications. Footnote: CAIs, carbonic anhydrase inhibitors; PGAs, prostaglandin analogs; CCT, central corneal thickness; PSK, pseudodendritic sterile keratitis; NO, nitric oxide; ROCK, rho-kinase. “Created with BioRender.com”.
4 Specific ocular surface disease changes caused by pressure-lowering medications
4.1 β-Adrenergic antagonists (β-blockers)
4.1.1 Contact dermatitis
Periocular contact dermatitis is a frequent adverse effect of topical anti-glaucomatous agents, mainly β-blockers (Horcajada-Reales et al., 2015) (Figure 3A). It may present as erythema with or without eczema and crusting of the eyelids. Koch et al. reported sensitization to a single β-blocker despite previous exposure to other β-blockers in three patients (Koch, 1995). This was also reported by Perez-Rodriguez et al. in a patient sensitized to 0.005% latanoprost but not to 0.03% bimatoprost (Perez-Rodriguez et al., 2008) and by Geyer et al. in 15 patients with proven allergy to 0.5% apraclonidine but without cross-reactivity to 0.25% clonidine hydrochloride (Geyer et al., 2000). Contrariwise, other authors report positive patch testing for multiple β-blockers, suggesting cross-sensitization (Horcajada-Reales et al., 2015). While some authors suggest cross-reactivity between multiple β-blockers might result from a common lateral aliphatic chain acting as an antigenic determinant others hypothesize that positive reactions could be related to multiple sensitizations instead of cross-reactivity. Allergic contact dermatitis in patients naïve to other PLMs from the same or other group has been reported with dorzolamide, brimonidine tartrate, and cholinergic agonists (i.e., pilocarpine) (Grey and Warshaw, 2016).
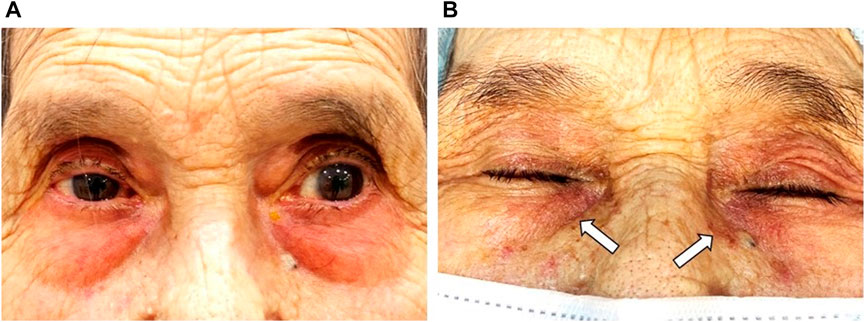
FIGURE 3. A 91-year-old female patient with a history of chronic angle-closure glaucoma treated with benzalkonium chloride (BAK)-containing 0.03% bimatoprost qHs and a fixed combination of 0.5% timolol maleate/2% dorzolamide BID eyedrops for at least 10 years. (A) Significant erythema of the periorbital skin and lid margin are consistent with allergic contact dermatitis. (B) After 4 weeks of treatment with ocular lubricants and preservative-free (PF) IOP-lowering eyedrops, there was a significant reduction of the periorbital skin and lid margin erythema. However, residual skin pigmentation (white arrows) is observed. Footnote: Written informed consent was obtained from both patients to publish the clinical images.
Interestingly, other studies report the development of a clinically apparent allergic reaction but with negative patch testing. Giordano-Labadie et al. reported the case of a patient who developed negative-patch chronic eczema for timolol, carteolol, and befunolol. The authors suggested cross-reactivity occurred after drugs were metabolized to a common aldehyde rather than a reaction to an individual hapten (Giordano-Labadie et al., 1997). Written informed consent was obtained from all patients to publish the clinical images used throughout the manuscript.
4.1.2 Meibomian gland dysfunction (MGD)
Sullivan and coworkers hypothesized that the drug action of anti-glaucomatous agents might contribute to DED development by a direct effect on MGs (Zhang et al., 2017; Han et al., 2018; Han et al., 2020). They performed a series of experiments in which they cultured immortalized human MG epithelial cells (iHMGEC) with different concentrations of several α-adrenergic agonists (brimonidine, clonidine, phenylephrine) (Han et al., 2018), dorzolamide (Han et al., 2020), pilocarpine, and timolol (Zhang et al., 2017). Using clinical doses of 0.5% timolol and 4% pilocarpine (See Section 4.5.2), Zhang et al. demonstrated they both caused significant cell atrophy and death of iHMGEC (Zhang et al., 2017). Regarding timolol, MG dropout might be associated with the blockade of β3-adrenoreceptors, which has been found in MGs of murine models (Knop et al., 2011). These receptors mediate fat oxidation and increase lipolysis; thus, beta-adrenergic blockade might cause detrimental effects on iHMGEC (Zhang et al., 2017). Arici et al. reported significantly lower TFBUT scores, a surrogate marker of increased evaporation due to MGD, in patients treated with BAK-containing 0.5% betaxolol or 0.5% timolol eyedrops compared with controls (Arici et al., 2000). Table 4 presents the recent relevant ex-vivo and in-vitro human studies reporting OSD manifestations of preserved PLMs.
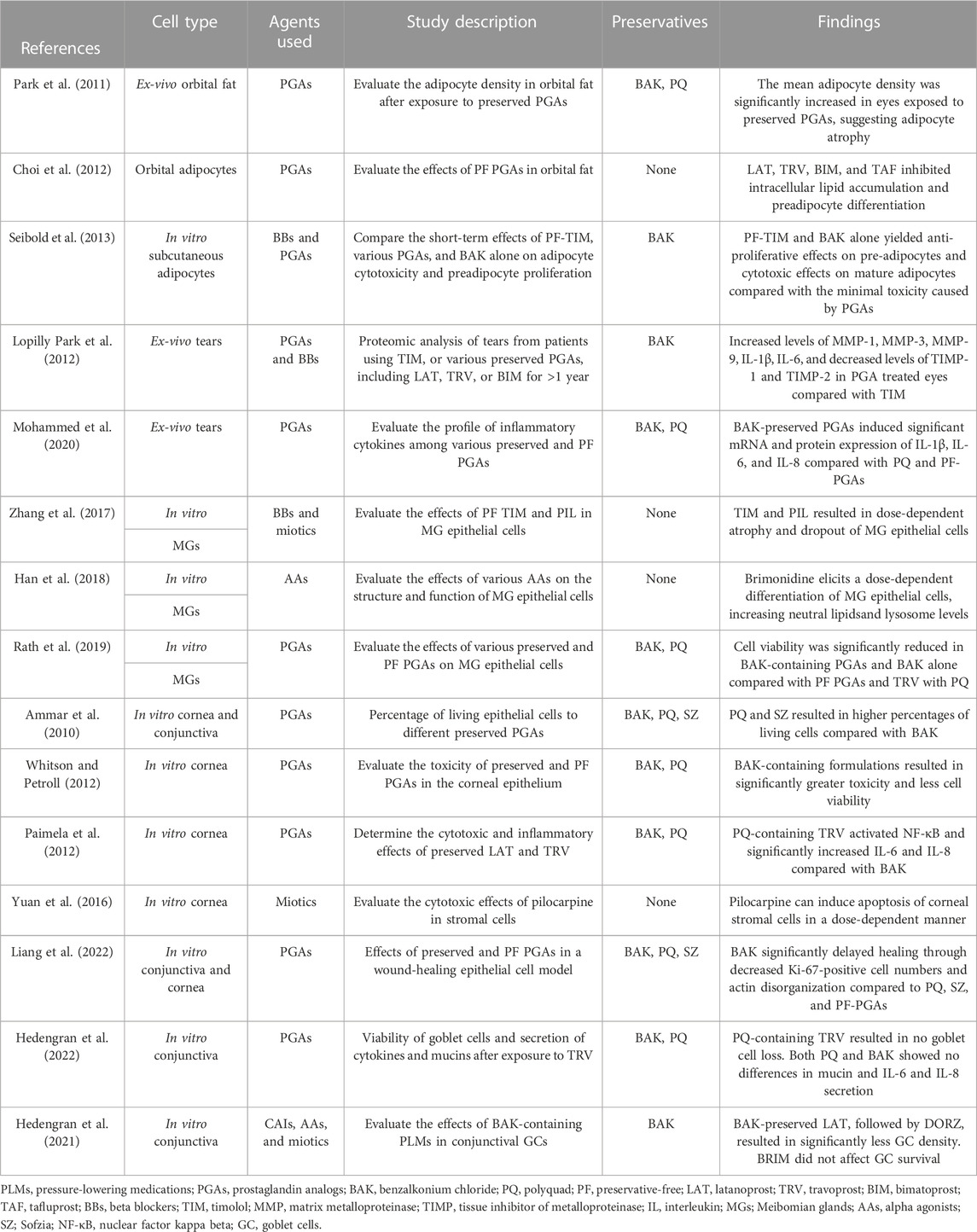
TABLE 4. Recent relevant ex-vivo and in-vitro human studies reporting ocular surface disease manifestations of PLMs.
4.1.3 conjunctival goblet cell (GC) dropout
β-blockers cause abnormal keratinization, squamous metaplasia, inflammation leading to GC loss, and subconjunctival fibrosis (Singh et al., 2022). An impression cytology study reported that 50% and 55% of samples treated with BAK-containing 0.5% betaxolol or 0.5% timolol, respectively, were classified as grade 2, defined as the presence of large and multinucleated epithelial cells and a marked reduction of GCs, or grade 3, defined as even larger epithelial cells and complete absence of GCs (Arici et al., 2000). Terai et al. performed a histological analysis of human conjunctiva, evaluating the effect of BAK-containing 0.5% timolol and 0.005% latanoprost on MMPs and TIMPs expression and ECM organization (Terai et al., 2009). Compared with latanoprost, timolol-treated eyes exhibited overexpression of CD68 antibodies, an indicator of inflammatory infiltration. The latter suggests that chronic exposure to timolol eyedrops might result in conjunctival scarring and the potential for filtering surgery (trabeculectomy) failure (Terai et al., 2009).
A study performed by Aydin Kurna and coworkers evaluated the effect of different anti-glaucoma formulations, including preserved and preservative-free (PF)-timolol, and preserved formulations of latanoprost, bimatoprost, travoprost, and brimonidine (Aydin Kurna et al., 2014). At the 12-month follow-up, a significant increase in superior-central and inferior-nasal squamous metaplasia was observed in the brimonidine and both preserved and PF-timolol maleate groups. In PGA-treated eyes, an increase in the inferior-nasal squamous metaplasia was only reported in the BAK-containing travoprost group. Regarding GC loss, significant superior-central and inferior-nasal loss were observed in the PF-timolol and BAK-travoprost groups. In contrast, a consequential inferior-nasal loss was documented in the preserved-latanoprost and -brimonidine groups (Aydin Kurna et al., 2014).
4.1.4 drug-induced cicatrizing conjunctivitis (DICC)
DICC, also known as “pseudo-pemphigoid,” is the development of conjunctival scarring after exposure to an inciting agent (Singh et al., 2022). It may be non-progressive or progressive, depending on whether the scarring process stabilizes (or not) after the withdrawal of the inciting agent (Singh et al., 2022). Although DICC can be associated with any anti-glaucoma medication, β-blockers are, by far, the most frequently reported. In 41 patients with DICC, β-blocker exposure was reported in 36 cases (88%). Timolol maleate was the culprit in 73% of cases (Thorne et al., 2004). The pathogenic mechanism of DICC consists of an inflammatory and immunological process leading to limbal stem cell deficiency, subconjunctival fibrosis, and fornix foreshortening, mainly of the inferior bulbar and palpebral conjunctiva (Vazirani et al., 2020). However, ocular signs may involve the entire surface, including punctum scarring, periocular hypopigmentation, obstructive MGD, eyelash overgrowing (distichiasis), malposition (trichiasis), and lid margin keratinization (Singh et al., 2022).
Histopathological findings of DICC are very similar to those encountered in other cicatrizing conditions such as ocular mucous membrane pemphigoid (OMMP), notably showing increased proliferation of the basal cells of the conjunctival epithelium, marked infiltration of inflammatory cells such as macrophages, neutrophils, and T-lymphocytes in the acute phase, and fibroblast stimulation resulting in fibrosis in the chronic phase (Elder and Lightman, 1994; Singh et al., 2022). However, a notable distinction between DICC and OMMP can be made with direct immunofluorescence (DIF). While DIF following a conjunctival biopsy of a patient with OCP shows linear deposition of IgA, IgG, IgM, and complement C3 on the conjunctival epithelial basement membrane zone, DIF in pseudo-pemphigoid cases such as DICC is usually negative for these observations and require clinical diagnoses (Singh et al., 2022).
Gibran reported the case of an 85-year-old woman with an 8-year BAK-containing latanoprost and apraclonidine use to manage pseudo-exfoliative glaucoma in her left eye (Gibran, 2004). Symptoms included painful red eye and blurred vision, whereas signs included keratoconjunctivitis sicca, fornix foreshortening, and corneal scarring with active neovascularization in the left eye. The right eye was normal (Gibran, 2004). A similar case of a patient exposed to multiple BAK-containing drugs, including latanoprost, dorzolamide, brinzolamide, pilocarpine, and brimonidine, was also reported by Kahana et al. (Kahana et al., 2007). In both cases, BAK was deemed responsible for the development of DICC (Gibran, 2004; Kahana et al., 2007).
4.1.5 Lacrimal drainage obstruction (LDO)
Topical anti-glaucoma agents can cause isolated canalicular and lacrimal occlusion, as well as a more extensive cicatrizing process known as drug-induced cicatrizing conjunctivitis (DICC, See Section 4.1.4) (Kashkouli et al., 2008). Narioka et al. found a decrease in the lumen width of the nasolacrimal drainage (NLD) system after exposure to 0.5% timolol, located mainly in the middle and lower regions (Narioka and Ohashi, 2007). These findings imply that timolol caused vasodilation of the blood vessels in the NLD system’s cavernous body, suggesting that the autonomic nervous system may partially control tear drainage through the NLD system (Narioka and Ohashi, 2007). In a large prospective and controlled case series of 627 eyes from 384 patients, Kashkouli et al. reported significant lacrimal drainage obstruction (LDO) in patients using combined formulations of timolol/dorzolamide and timolol/dorzolamide/pilocarpine. Timolol alone did not cause substantial obstruction, which suggests that fixed combinations of PLMs had an increased risk of LDO (Kashkouli et al., 2008).
4.2 Prostaglandin analogs (PGAs)
4.2.1 Skin pigmentation
Eyelid pigmentation (0%–26%) and eyelash bristles (0%–77%) represent a frequent periorbital manifestation associated with PGAs, with an increased frequency if used for >3 months (S et al., 2018) (Figure 3B). Inoue et al. reported there were no significant differences in the frequency (4%–6%) of eyelid pigmentation after >3 months of latanoprost, tafluprost, bimatoprost, travoprost, or isopropyl unoprostone use (Inoue et al., 2012a). Eyelash bristles, however, occurred significantly less with unoprostone (8%) compared with the other four drugs (26%–54%).
4.2.2 Meibomian gland dysfunction (MGD)
Mocan et al. reported a significantly higher prevalence of MGD in glaucoma patients managed with PGA monotherapy than those treated with other PLMs (92% vs 58%). The obstructive form of MGD was documented in 96% of patients from the PGA group (Mocan et al., 2016). Moreover, other ocular surface parameters, including the Ocular Surface Disease Index (OSDI), tear-film breakup time (TFBUT), lissamine green staining, and Schirmer scores were significantly worse in patients treated with PGA monotherapy compared to healthy controls (Mocan et al., 2016). The pathogenic mechanisms of MGD in patients treated with PGA remains poorly understood. Some authors suggest that subclinical inflammation of the conjunctiva results in MG dropout and dysfunction (Agnifili et al., 2018). Agnifili et al. reported a significant reduction in the mean acinar area (MAA) and density (MAD), which are respective surrogates of reduced meibum production and glandular dropout, and higher interstice inhomogeneity, which reflects MG and tarsal inflammation, in patients treated with PGAs (Agnifili et al., 2018). These findings were significantly higher in preservative PGA-treated patients than those managed with PF-PGAs. In another study, Arita et al. reported significantly higher lid margin abnormalities (i.e., vascular tortuosity, irregular lid margin, replacement of the mucocutaneous junction, and plugged MG orifices) (Figures 4A,B), which are associated with MGD and conjunctival inflammation, and higher Meibo-scores, implying increased MG dropout, in patients treated with PGA compared with β-blocker treated eyes and healthy controls (Arita et al., 2012) (Figure 5). The authors suggest that the lid margin abnormalities in glaucoma-treated eyes support the hypothesis that subclinical inflammation predates MG alterations (Arita et al., 2012). Recurrent inflammation resulting from prolonged exposure to PGA might lead to meibum stagnation with subsequent keratinization of MG orifices (i.e., obstructive MGD) (S et al., 2018).
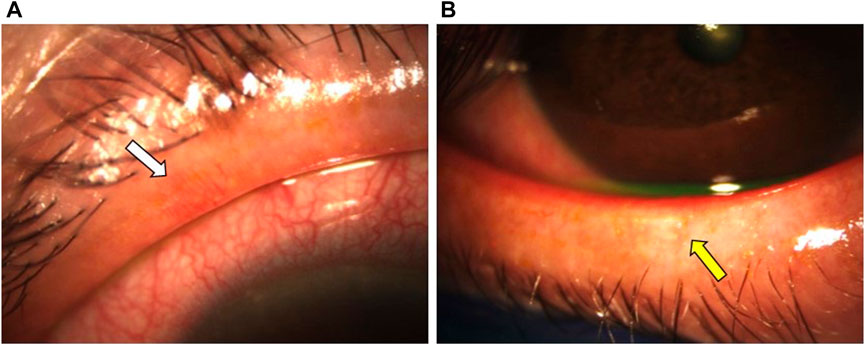
FIGURE 4. A 61-year-old female patient with a 7-year history of primary open-angle glaucoma (POAG) was treated with BAK-containing 0.005% latanoprost qHs eyedrops. (A,B) Significant lid margin erythema, telangiectasias (white arrow), meibomian gland clogging (yellow arrow). Footnote: Written informed consent was obtained from the patient to publish the clinical images.
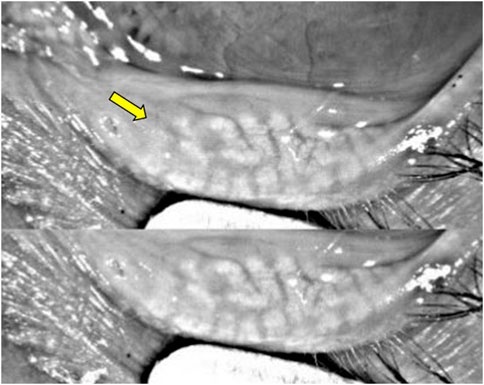
FIGURE 5. Keratograph analysis from a patient with a 12-year history of POAG treated with BAK-containing 0.005% latanoprost qHs eyedrops showing significant meibomian gland dropout (yellow arrow).
4.2.3 Conjunctival hyperemia
A recent meta-analysis performed by Tang et al. reported that the frequency of conjunctival hyperemia was significantly higher in bimatoprost (40%) compared with travoprost (39%) and latanoprost (28%) (Tang et al., 2019). Although unclear, PGAs are suspected of inducing the production of NO synthase, which may lead to conjunctival hyperemia due to their vasodilatory properties (Astin et al., 1994) (Figure 6A).
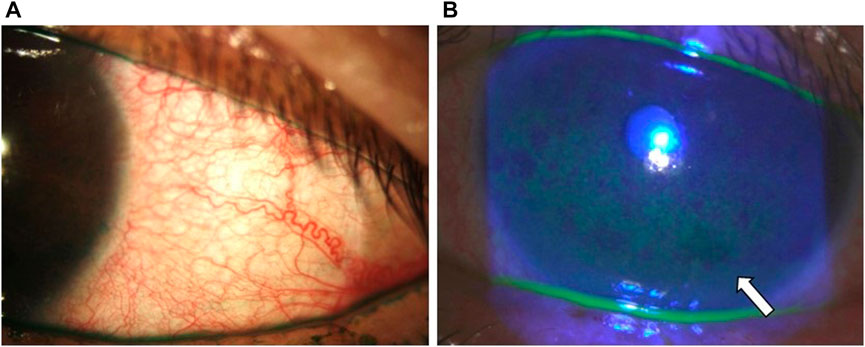
FIGURE 6. Clinical photographs of the patient from Figure 4 showing (A) conjunctival hyperemia with lissamine green staining and (B) central and inferior corneal staining (white arrow).
4.2.4 Conjunctival goblet cell (GC) dropout
Human studies report GC loss after long-term treatment with BAK-containing PGA eyedrops and after short-term exposure to BAK alone (Pisella et al., 2004). On the other hand, Mastropasqua et al. described an increase in GC density after 6 months of therapy with PF-tafluprost. This finding can be explained by the ability of PG to stimulate the secretion and proliferation of mucin in numerous mucosal surfaces, including the conjunctiva (Mastropasqua et al., 2013). Interestingly, the authors report a transient increase in GC density after 1 month of preserved latanoprost. The GC density, however, returned to baseline after 6 months, suggesting that over time, the positive effect of the PG derivative is nullified by the preservative (Mastropasqua et al., 2013).
4.2.5 Tear film
Using the Schirmer test I, Agnifili et al. compared tear production between healthy controls and groups of patients using several anti-glaucoma regimes, including combinations of preserved PGAs with timolol maleate and PF-bimatoprost and timolol (Agnifili et al., 2018). The tear production of healthy controls was significantly higher than in all therapy groups, including the fixed combination of PF-bimatoprost and timolol (18.4 ± 5.5 mm vs. 9.8 ± 3.5 mm). Interestingly, the unfixed combination of latanoprost and timolol yielded significantly worse Schirmer scores than the fixed combinations of timolol and different PGAs (latanoprost, travoprost, and bimatoprost) (Agnifili et al., 2018).
4.2.6 Corneal thickness
A significant reduction in the central corneal thickness (CCT) was documented in human eyes after 8 weeks of treatment with either 0.03% bimatoprost, 0.004% travoprost, or 0.005% latanoprost compared with controls. No difference between PGAs was found (Hatanaka et al., 2009). Human studies have shown central corneal thinning after long-term exposure to PGAs, possibly due to increased activity of MMPs relative to TIMPs in the corneal epithelium and stroma (Lopilly Park et al., 2012). Upregulation of MMPs, mainly MMP-2 and -9, has been reported in stromal tissue after corneal ablative procedures and corneal erosions (Jadczyk-Sorek et al., 2022). In another study, Lopilly Park et al. did not find a significant reduction in the CCT of human eyes after 1 year of treatment with PGAs (Lopilly Park et al., 2012). In the same study, however, the authors reported that rabbit corneas exhibited corneal thinning caused by a decrease in collagen type 1 after PGA treatment. Also, a significant increase in MMP-1 and MMP-9 and a reduction in TIMP-1 were found in rabbit corneas (Lopilly Park et al., 2012). Differences in the collagen distribution between the human and rabbit corneas might also explain humans’ lack of significant corneal thinning. Table 5 presents the recent relevant in-vivo, ex-vivo, and in-vitro animal studies reporting OSD manifestations of preserved PLMs.
TABLE 5. Recent relevant in-vivo, ex-vivo, and in-vitro animal studies reporting ocular surface disease manifestations of PLMs.
4.2.7 Pseudodendritic keratitis (PSK)
PSK is uncommon in patients using PLMs. It is characterized by pseudodendritic central and lower corneal lesions surrounded by SPK (Chang et al., 2021). A recent retrospective case series reported 31 events (19 patients) of PSK, 52% of them associated with PGAs and 97% to BAK-containing PLMs (Chang et al., 2021). Management includes using therapeutic soft contact lens, lubricant eyedrops, andto discontinuation, decrease, or change of the PLM used (Chang et al., 2021). Due to their similarities, PSK could get confused with herpetic simplex keratitis. However, the latter is characterized by dendrites with central fluorescein staining and terminal bulbs that can be found anywhere in the cornea (Chang et al., 2021).
4.3 Carbonic anhydrase inhibitors (CAIs)
4.3.1 Contact dermatitis
Delaney et al. reported periocular contact dermatitis in 14 patients after 20.4 weeks of initiating BAK-containing dorzolamide. Of those, 13 patients were using preserved β-blockers for 34.2 months (Delaney et al., 2002). Dermatitis wholly resolved in 8 cases (57%) after discontinuing dorzolamide, and in the remaining 6 (43%), resolution occurred after the topical β-blocker was also stopped. Negative patch testing to β-blockers, BAK, and dorzolamide was reported in all cases. The authors hypothesized that a false-negative reaction, or simply irritation rather than sensitization, might have occurred (Delaney et al., 2002).
4.3.2 Drug-induced ectropion
Hegde et al. reported 13 patients who developed drug-induced ectropion after exposure to dorzolamide (7 cases, 53%), brimonidine (3 cases, 23%), and other agents, including β-blockers, latanoprost, and preserved lubricant eyedrops (Hegde et al., 2007). This effect was reversible after drug discontinuation and a short course of topical steroids in 9 cases. However, the remaining patients who did not receive steroids were successfully managed with surgical correction (Hegde et al., 2007).
4.3.3 Meibomian gland dysfunction (MGD)
Han et al. reported that, compared with the therapeutic concentration (50 µg/mL) of dorzolamide, a 10-fold higher concentration of 500 µg/mL significantly reduces iHMGEC proliferation while increasing iHMGEC differentiation (Han et al., 2020). The authors suggest that dorzolamide might enhance the expression of hypoxia-inducible factor (HIF)-1α, which facilitates iHMGEC differentiation by triggering a cellular response to hypoxic stress. However, the therapeutic concentration of dorzolamide did not elicit such an effect (Han et al., 2020).
4.3.4 Conjunctival hyperemia
he incidence of hyperemia in dorzolamide users ranges from 7% to 21% (Adamsons et al., 1998; Ott et al., 2005); however, brinzolamide, another CAI, has a lower prevalence (<3%) of hyperemia due to its physiological pH, which enhances tolerability and compliance (Lusthaus and Goldberg, 2017).
4.4 α-Adrenergic agonists
4.4.1 Meibomian gland dysfunction (MGD)
Brimonidine triggers the upregulation of sterol regulatory element-binding protein (SREBP)-1, a lipogenesis regulator that synthesizes lipid, cholesterol, and fatty acid production enzymes. Moreover, brimonidine enhances the conversion of SREBP-1 to its mature form, thus promoting lipid accumulation and differentiation iHMGECs (Han et al., 2018). The authors conclude that dry eye symptoms associated with brimonidine, including blurry vision, ocular discomfort, and DED, might be related to corneal toxicity, GC loss, and conjunctival inflammation rather than MG dropout (Han et al., 2020).
4.4.2 Conjunctival allergy
Allergic reactions are another frequent side effect of alpha agonists. Although unclear, the pathogenic mechanism could be related to a volume decrease and subsequent widening of the intracellular spaces between conjunctival cells, leading to an entry portal for external allergens (Yeh et al., 2021). Butler et al. reported an incidence of allergic reaction in 48% of apraclonidine users (Araie et al., 2015). This side effect was more common in women and led to drug discontinuation after an average latency of 4.7 months (Butler et al., 1995). In the case of brimonidine, the incidence of allergy ranges from 4.7% to 25%, occurring at a mean time interval of 6–9 months (Yeh et al., 2021). The prevalence of hyperemia is 13% and 8% for apraclonidine and brimonidine users, respectively (Robin et al., 1995; Mundorf et al., 2003).
4.5 Cholinergic agents
4.5.1 Contact dermatitis
Allergic contact dermatitis has been previously reported in topical pilocarpine users. O’Donnell and Foulds presented a patient with negative patch testing to topical PLM ingredients at days 4 and 7 (O'Donnell and Foulds, 1993). However, using the prick-testing method with unpreserved pilocarpine elicited a 10-mm wheal after 30 min, a finding suggestive of contact urticaria. In a similar fashion, Cusano et al. described of a patient with 1-year history of eyelid dermatitis and negative patch testing, but who developed an inflammatory reaction after repeated open application Test with pilocarpine eye drops (Cusano et al., 1993).
4.5.2 Meibomian gland dysfunction (MGD)
Zhang et al. reported significant cell atrophy and death of cultured immortalized human MG epithelial cells (iHMGEC) with 0.04% pilocarpine, a tenfold lower than the clinical dose (Zhang et al., 2017). The standard 0.4% pilocarpine-induced impaired cellular adherence, perinuclear vesicle accumulation, which heralds cell death, and decreased survival of iHMGEC. Although elusive, pilocarpine-induced MG dropout might be related to its effects on muscarinic acetylcholine receptor 3, present in iHMGEC (Wu et al., 2020).
4.6 Rho-kinase (ROCK) inhibitors
4.6.1 Postoperative eyelid wound dehiscence
It is defined as a break or split in the eyelid after a previously closed surgical incision site (Sandy-Hodgetts et al., 2015). Kim et al. reported the case of an 81-year-old male with glaucoma managed with PLMs, including 0.02% netarsudil. The patient underwent upper eyelid Mohs surgery and lid repair due to basal cell carcinoma. Interestingly, the patient suffered three episodes of wound dehiscence, requiring repair in two (Kim et al., 2021). After the last episode, the patient discontinued the netarsudil eyedrops, and 2 weeks later, he developed granulation tissue, and the wound healed appropriately. In diabetic foot ulcer rat models, overexpression of ROCK1 has been shown to increase wound healing (Wang et al., 2022). Inhibition of MLC-phosphatases by the ROCK pathway leads to long-lasting contraction of myofibroblasts, which is required for wound healing (Saha et al., 2022). Thus, ROCK inhibition with netarsudil could lead to poor wound healing.
4.6.2 Conjunctival hyperemia
The Rho Kinase Elevated IOP Treatment (ROCKET) Trials reported an incidence of conjunctival hyperemia of 50%–53% and 59% in eyes receiving 0.02% netarsudil once and twice daily, respectively. These results were significantly higher than the 8%–11% incidence of conjunctival hyperemia in eyes receiving 0.5% timolol twice daily (Serle et al., 2018). In a rabbit model, Watabe and coworkers demonstrated that ROCK inhibitors caused vasodilation of the ciliary arteries due to a calcium-independent mechanism. This contrasts with the PLMs tafluprost, a PGA, and levobunolol, a β-blocker, which cause relaxation of the ciliary arteries by decreasing calcium concentration in the intracellular space (Watabe et al., 2011). As stated above, ROCKs contract the trabecular meshwork by phosphorylation of MLCs. Thus, conjunctival hyperemia associated with ROCK inhibitors could be related to vasodilation of ciliary arteries due to smooth muscle relaxation secondary to MLC phosphorylation (Watabe et al., 2011).
4.6.3 Subconjunctival hemorrhage
Singh et al. reported a higher incidence of subconjunctival hemorrhage in patients managed with once-a-day 0.02% netarsudil eyedrops compared with twice-a-day 0.5% timolol (17% vs 2%). Among patients managed with netarsudil, the hemorrhage was mild in 92% of cases, self-limiting in 96%, and requiring drug discontinuation in 1% (Singh et al., 2020).
4.6.4 Drug-induced cicatrizing conjunctivitis (DICC)
Meirick et al. reported 16 patients who developed reversible punctum stenosis after an average time of 14 months of 0.02% netarsudil use. Of those, 13 (81%) patients had symptomatic epiphora, leading to drug discontinuation in 7 cases. Histopathological analysis from the conjunctiva and punctum of one patient showed nonspecific lymphocytic inflammation without eosinophils (Meirick et al., 2022). This contrasts with the findings encountered in eyes with the non-reversible scarring inflammation in DICC, which is typically associated with β-blockers (Singh et al., 2022). Punctal scarring has not been reported with ripasudil, another ROCK inhibitor. Compared with ripasudil, netarsudil has a NET inhibitor function. However, the effect of NET inhibition and punctum scarring remains unknown (Meirick et al., 2022).
4.6.5 Corneal edema
Wisely et al. reported five patients who developed reticular bullous corneal epithelial edema after a mean time of 5.4 (range: 2–8) weeks of netarsudil use (Wisely et al., 2020). Four patients had a prior history of corneal edema due to different causes. The remaining patient had a previous history of anterior uveitis, which predisposes to corneal edema. In all cases, the epithelial edema resolved after a mean time of 7.4 (range: 2–12) weeks of discontinuing netarsudil (Wisely et al., 2020).
ROCKs and tight junctions, including zonula occludins (ZO)-1, oversee osmotic regulation in epithelial surfaces (Chmiel and Gardel, 2022). ROCK inhibitors could lead to epithelial edema by increasing the permeability of tight junctions, thus allowing fluid to percolate from the corneal stroma into the epithelium (Chmiel and Gardel, 2022; Lyons et al., 2022). Corneas with a prior history of developing corneal edema might be more susceptible to ROCK inhibition (Wisely et al., 2020). However, the latter remains unknown.
4.7 Nitric oxide (NO)-donating prostaglandin analogs (PGAs)
4.7.1 Conjunctival hyperemia
The prevalence of hyperemia associated with latanoprostene bunod ranges from 2% to 18% (Lo et al., 2022). Besides the NO synthase induced vasodilation (See Section 4.1.3), another potential mechanism of conjunctival hyperemia could be related to the pro-inflammatory properties of the prostaglandin F2α molecule itself (Kawase et al., 2016).
4.7.2 Superficial punctate keratitis (SPK)
In an open-label clinical study of healthy subjects, Araie et al. reported a prevalence of 54.2% of SPK among healthy users of once-a-day 0.024% latanoprostene bunod. However, in all cases, the SPK was mild and clinically insignificant (Araie et al., 2015). In a recent meta-analysis of randomized controlled trials (RCTs), the prevalence of SPK ranged from 1.1% to 4.3% (Lo et al., 2022).
5 Preservatives
Preservative agents, including BAK, polyquaternium-1 (Polyquad), Sofzia®, and Purite®, influence corneal penetration of the active substance through their surface wetting properties and provide bacteriostatic activity (Servat and Bernardino, 2011). Within the eye, the lipophilic nature of most preservatives renders immediate binding to ocular tissues after application. However, many animal and human studies have shown that preservatives are culprits of inducing or worsening ophthalmic formulations’ toxic, allergic, and inflammatory reactions, including PLMs (Kim et al., 2015; Muz et al., 2021).
BAK is the most frequently used preservative in ophthalmic preparations (Muz et al., 2021). It is commonly used as a cationic surfactant, a phase transfer agent in the chemical industry, and a biocidal agent due to its activity against fungi and Gram-positive and Gram-negative bacteria (Muz et al., 2021). However, BAK-preserved formulations have been shown to trigger dose-dependent inflammation, tear instability, increased osmolarity, corneal and conjunctival epithelial cytotoxicity, squamous metaplasia, and GC loss (Kim et al., 2015). To reduce the toxicity of BAK-preserved formulations, less toxic formulations were designed, including Sofzia®, Polyquad, and Purite®. However, PF antiglaucoma formulations are available and should be considered first-line, mainly in patients with preexisting OSD.
Damage to the limbal stem cells (LSCs) and corneal epitheliopathy has been associated with multiple PLMs. However, studies suggest that preservatives, rather than the active ingredient, have been identified as culprits. LSCs, which have a high proliferation, differentiation, and migration capacity, reside in the corneoscleral limbus (Guclu et al., 2021). LSCD results from an impaired function and reduced number of LSCs which, in turn, can lead to corneal conjunctivalization, persistent epithelial defects (PEDs), scarring, and vision loss. The concept of “iatrogenic LSCD” in eyes managed with PLMs was first coined by Schwartz and Holland (Schwartz and Holland, 2001). Güçlü et al. evaluated the limbal epithelium thickness (LET) in patients treated with either one, two, three, or four-drug regimens of BAK-containing anti-glaucoma medications (Guclu et al., 2021). The authors found no difference in the LET between treated groups; however, it was significantly lower compared to non-treated healthy eyes (64.1 ± 9.1 µm vs. 76.0 ± 11.5 µm). Moreover, there was a positive correlation between increased LET and increased Schirmer (r = 0.4), TFBUT (r = 0.37), and central corneal epithelial thickness (CCET; r = 0.37) (Guclu et al., 2021). A decreased significant LET is also reported in DED patients (Francoz et al., 2011). PLMs might decrease LET due to increased epithelial turnover or chronic inflammation (Francoz et al., 2011). On the other hand, the association between decreased LET and decreased CCET may result from decreased proliferation, differentiation, and migration of reduced LSCs (Guclu et al., 2021).
A morphologic IVCM study by Mastropasqua et al. analyzed the density of dendritic cells (DCs) and the regularity of the limbal transition epithelium (LTE) in eyes treated with single, double, and triple or more anti-glaucoma eyedrops regimes (Mastropasqua et al., 2015). Eyes managed with preserved β-blockers and preserved PGAs exhibited higher DCs density and worsened LTE irregularity compared with PF-drugs. A higher DC density results from BAK-induced local immune system activation, whereas the LTE irregularity, observed as punctate reflecting elements with IVCM analysis, represents an additional sign of inflammation (Mastropasqua et al., 2015). Moreover, eyes treated with preserved drugs significantly increased HLA-DR and IL-6 compared with PF drugs. This supports the theory that inflammation might be the initial cascade step leading to limbal abnormalities (Mastropasqua et al., 2015).
Superficial punctate keratitis (SPK) encircles a group of corneal epithelial lesions with varying morphology and can be observed as corneal staining at the slit-lamp. The prevalence of SPK in anti-glaucoma eyedrop users is reported to be as high as 70% (Lajmi et al., 2021) (Figure 6B). Using in vivo confocal microscopy (IVCM), Mastropasqua et al. reported that the central corneal DC density significantly increased in patients with preserved compared to those receiving PF PGAs and β-blockers (Mastropasqua et al., 2016). Additionally, the corneal DC density significantly correlated with corneal staining and OSDI scores (p < 0.001). These results resemble those found in the limbal epithelium, suggesting an increased inflammatory response in the corneal epithelium with a subsequent increase in signs and symptoms (Mastropasqua et al., 2016). Ye et al. reported a significant association between increased fluorescein staining and epithelial thickness in the central and paracentral cornea, indicating that abnormal staining might predict corneal epithelial thinning (Ye et al., 2022). SPK is also reported in 5%–10% of patients using netarsudil and in 1%–4% of latanoprostene bunod users (Batra et al., 2021; Lo et al., 2022).
6 Ocular surface disease and quality of life in glaucoma patients
Quality of life (QoL) refers to patients’ perception of their daily wellbeing (Kumar et al., 2020). Unfortunately, QoL can be severely affected in patients with glaucoma and OSD, which often coexist (Camp et al., 2015; Tirpack et al., 2019). Studies report a significant association between the increased number of PLMs and decreased emotional wellbeing scores, with African American patients experiencing worse QoL scores (Camp et al., 2015). Abegão Pinto et al. prospectively evaluated the change in visual-related QoL, assessed by the Glaucoma Symptom Scale (GSS), in patients with glaucoma after switching from preserved IOP-lowering therapy to PF-timolol/dorzolamide fixed combination (TDFC). After 8 weeks of treatment with PF-TDFC, there was a significant improvement in the GSS-related symptom, function, and total scores (Abegao Pinto et al., 2014). Accordingly, Kumar et al. found significantly worse QoL scores between patients using BAK-containing travoprost and the PF-travoprost and control groups (Kumar et al., 2020). Interestingly, there was no difference between the reported QoL in the PF-travoprost and control groups, suggesting the harmful role of preservatives in OSD and QoL in glaucoma patients (Kumar et al., 2020).
7 Future directions
OSD symptoms are detrimental to glaucoma patients’ perceived QoL, compliance to therapy, reliability of diagnostic tests, poor surgical outcomes, and disease progression and visual outcomes (Zhang et al., 2019). Thus, addressing OSD in glaucoma is crucial. A survey-based study reported that 100% of Canadian glaucoma specialists agreed that a good approach to OSD in patients might improve QoL, whereas 97% agreed that it could result in enhanced glaucoma outcomes (Muzychuk et al., 2020). Furthermore, only 22% agreed that OSD is currently being managed appropriately, and 92% agreed that a stepwise approach should be undertaken to address OSD in glaucoma. Accordingly, the authors proposed an algorithm consisting of 1) optimizing topical glaucoma medications by using combined and PF formulations; 2) promoting ocular surface health (i.e., PF-lubricants and gels, omega-3 fatty acid supplementation); 3) enhancing OSD therapy with steroids immunomodulatory drugs (i.e., cyclosporine A, autologous serum); and 4) considering surgical interventions (Muzychuk et al., 2020). In this regard, minimally invasive glaucoma surgery and slow delivery systems such as the bimatoprost implant have emerged as a possible solution for reducing IOP with fewer ocular surface adverse effects compared with traditional surgical and non-surgical IOP-lowering methods (Schoenberg et al., 2015; Schweitzer et al., 2020; Shirley, 2020). A recent prospective cohort study reported a significant reduction in the number of PLMs, a substantial improvement in OSDI and TFBUT scores, and conjunctival hyperemia in patients who underwent combined cataract surgery with trabecular micro-bypass stent(s) implantation (Schweitzer et al., 2020). More randomized prospective trials with extensive follow-up are required to assess the direct impact of MIGS on the ocular surface.
8 Conclusion
OSD is a frequently overlooked condition resulting from glaucoma therapy, with age, use of BAK-containing and multiple anti-glaucoma medications, concomitant systemic comorbidities, and previous DED as the most frequent associated risk factors. Eye care specialists must remain aware of the adverse effects of PLMs and, thus, actively inquire about ocular surface symptoms. Upon diagnosis of OSD, a severity-based, stepladder approach consisting of optimizing glaucoma treatment by switching to fixed and PF combinations, using PF ocular lubricants, prescribing short courses of topical steroids with or without immunomodulatory therapy, and considering early surgical intervention are required to enhance medication adherence and improve glaucoma outcomes.
Author contributions
Design of the work: RR-L, SK, and LW-G. Conceptualization: NA, HM, and VP. Literature investigation and selection: RR-L, NA, HM, SK, MQ-G, LW-G, and CC. Main writing: RR-L. Manuscript reviewing: RR-L, NA, HM, SK, MQ-G, LW-G, and CC. Figures and table editing: RR-L, NA, MQ-G, LW-G, and CC. Critical reviewing: LW-G, CC, and VP. Project coordination: VP. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abegao Pinto, L., Vandewalle, E., Gerlier, L., Stalmans, I., and Cosopt, U. D. S. S. G. (2014). Improvement in glaucoma patient quality of life by therapy switch to preservative-free timolol/dorzolamide fixed combination. Ophthalmologica 231 (3), 166–171. doi:10.1159/000356468
Adamsons, I. A., Polis, A., Ostrov, C. S., and Boyle, J. E. (1998). Two-year safety study of dorzolamide as monotherapy and with timolol and pilocarpine. J. Glaucoma 7 (6), 395–401. doi:10.1097/00061198-199812000-00007
Agarwal, P., and Agarwal, R. (2018). Trabecular meshwork ECM remodeling in glaucoma: Could RAS be a target? Expert Opin. Ther. Targets 22 (7), 629–638. doi:10.1080/14728222.2018.1486822
Agnifili, L., Mastropasqua, R., Fasanella, V., Brescia, L., Scatena, B., Oddone, F., et al. (2018). Meibomian gland features and conjunctival goblet cell density in glaucomatous patients controlled with prostaglandin/timolol fixed combinations: A case control, cross-sectional study. J. Glaucoma 27 (4), 364–370. doi:10.1097/IJG.0000000000000899
Aguayo Bonniard, A., Yeung, J. Y., Chan, C. C., and Birt, C. M. (2016). Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin. Drug Metab. Toxicol. 12 (11), 1279–1289. doi:10.1080/17425255.2016.1209481
Alm, A., and Nilsson, S. F. (2009). Uveoscleral outflow--a review. Exp. Eye Res. 88 (4), 760–768. doi:10.1016/j.exer.2008.12.012
Ammar, D. A., Noecker, R. J., and Kahook, M. Y. (2010). Effects of benzalkonium chloride-preserved, polyquad-preserved, and sofZia-preserved topical glaucoma medications on human ocular epithelial cells. Adv. Ther. 27 (11), 837–845. doi:10.1007/s12325-010-0070-1
Anwar, Z., Wellik, S. R., and Galor, A. (2013). Glaucoma therapy and ocular surface disease: Current literature and recommendations. Curr. Opin. Ophthalmol. 24 (2), 136–143. doi:10.1097/ICU.0b013e32835c8aba
Araie, M., Sforzolini, B. S., Vittitow, J., and Weinreb, R. N. (2015). Evaluation of the effect of latanoprostene bunod ophthalmic solution, 0.024% in lowering intraocular pressure over 24 h in healthy Japanese subjects. Adv. Ther. 32 (11), 1128–1139. doi:10.1007/s12325-015-0260-y
Arici, M. K., Arici, D. S., Topalkara, A., and Guler, C. (2000). Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin. Exp. Ophthalmol. 28 (2), 113–117. doi:10.1046/j.1442-9071.2000.00237.x
Arita, R., Itoh, K., Maeda, S., Maeda, K., Furuta, A., Tomidokoro, A., et al. (2012). Effects of long-term topical anti-glaucoma medications on meibomian glands. Graefes Arch. Clin. Exp. Ophthalmol. 250 (8), 1181–1185. doi:10.1007/s00417-012-1943-6
Astin, M., Stjernschantz, J., and Selen, G. (1994). Role of nitric oxide in PGF2 alpha-induced ocular hyperemia. Exp. Eye Res. 59 (4), 401–407. doi:10.1006/exer.1994.1124
Ayaki, M., Iwasawa, A., and Niwano, Y. (2012). Cell viability score as an integrated indicator for cytotoxicity of benzalkonium chloride-containing antiglaucoma eyedrops. Biocontrol Sci. 17 (3), 121–128. doi:10.4265/bio.17.121
Aydin Kurna, S., Acikgoz, S., Altun, A., Ozbay, N., Sengor, T., and Olcaysu, O. O. (2014). The effects of topical antiglaucoma drugs as monotherapy on the ocular surface: A prospective study. J. Ophthalmol. 2014, 460483. doi:10.1155/2014/460483
Batra, M., Gupta, S., Nair, A. B., Dhanawat, M., Sandal, S., and Morsy, M. A. (2021). Netarsudil: A new ophthalmic drug in the treatment of chronic primary open angle glaucoma and ocular hypertension. Eur. J. Ophthalmol. 31 (5), 2237–2244. doi:10.1177/11206721211008783
Baudouin, C., Aragona, P., Messmer, E. M., Tomlinson, A., Calonge, M., Boboridis, K. G., et al. (2013). Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul. Surf. 11 (4), 246–258. doi:10.1016/j.jtos.2013.07.003
Baudouin, C., Liang, H., Bremond-Gignac, D., Hamard, P., Hreiche, R., Creuzot-Garcher, C., et al. (2005). CCR 4 and CCR 5 expression in conjunctival specimens as differential markers of T(H)1/T(H)2 in ocular surface disorders. J. Allergy Clin. Immunol. 116 (3), 614–619. doi:10.1016/j.jaci.2005.05.033
Baudouin, C., Liang, H., Hamard, P., Riancho, L., Creuzot-Garcher, C., Warnet, J. M., et al. (2008). The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T-helper 1 and T-helper 2 pathways. Ophthalmology 115 (1), 109–115. doi:10.1016/j.ophtha.2007.01.036
Bausher, L. P., and Horio, B. (1995). Regulation of cyclic AMP production in adult human ciliary processes. Exp. Eye Res. 60 (1), 43–48. doi:10.1016/s0014-4835(05)80082-x
Berrino, E., and Supuran, C. T. (2019). Rho-kinase inhibitors in the management of glaucoma. Expert Opin. Ther. Pat. 29 (10), 817–827. doi:10.1080/13543776.2019.1670812
Bhargava, M., Sen, S., Bhambhani, V., Paul, R. S., and Dutta, C. (2022). Reticular epithelial corneal edema as a novel side-effect of Rho Kinase Inhibitors: An Indian scenario. Indian J. Ophthalmol. 70 (4), 1163–1170. doi:10.4103/ijo.IJO_2865_21
Butler, P., Mannschreck, M., Lin, S., Hwang, I., and Alvarado, J. (1995). Clinical experience with the long-term use of 1% apraclonidine. Incidence of allergic reactions. Arch. Ophthalmol. 113 (3), 293–296. doi:10.1001/archopht.1995.01100030047020
Camp, A., Wellik, S. R., Tzu, J. H., Feuer, W., Arheart, K. L., Sastry, A., et al. (2015). Dry eye specific quality of life in veterans using glaucoma drops. Cont. Lens Anterior Eye 38 (3), 220–225. doi:10.1016/j.clae.2015.02.001
Cavet, M. E., Vollmer, T. R., Harrington, K. L., VanDerMeid, K., and Richardson, M. E. (2015). Regulation of endothelin-1-induced trabecular meshwork cell contractility by latanoprostene bunod. Invest. Ophthalmol. Vis. Sci. 56 (6), 4108–4116. doi:10.1167/iovs.14-16015
Chang, H. L., Kuo, B. I., Wu, J. H., Huang, W. L., Su, C. C., and Chen, W. L. (2021). Anti-glaucoma agents-induced pseudodendritic keratitis presumed to be herpetic simplex keratitis: A clinical case series. Sci. Rep. 11 (1), 21443. doi:10.1038/s41598-021-01073-0
Chmiel, T. A., and Gardel, M. L. (2022). Confluence and tight junction dependence of volume regulation in epithelial tissue. Mol. Biol. Cell. 33 (11), ar98. doi:10.1091/mbc.E22-03-0073
Choi, H. Y., Lee, J. E., Lee, J. W., Park, H. J., Lee, J. E., and Jung, J. H. (2012). In vitro study of antiadipogenic profile of latanoprost, travoprost, bimatoprost, and tafluprost in human orbital preadiopocytes. J. Ocul. Pharmacol. Ther. 28 (2), 146–152. doi:10.1089/jop.2011.0160
Cusano, F., Luciano, S., Capozzi, M., and Verrilli, D. A. (1993). Contact dermatitis from pilocarpine. Contact Dermat. 29 (2), 99. doi:10.1111/j.1600-0536.1993.tb03495.x
Delaney, Y. M., Salmon, J. F., Mossa, F., Gee, B., Beehne, K., and Powell, S. (2002). Periorbital dermatitis as a side effect of topical dorzolamide. Br. J. Ophthalmol. 86 (4), 378–380. doi:10.1136/bjo.86.4.378
Di Maria, A., Tredici, C., Cozzupoli, G. M., Vinciguerra, P., and Confalonieri, F. (2023). Effects of prostaglandin analogues on epiphora persistence after EN-DCR: A hypothesis-generating study. Eur. J. Ophthalmol. 33 (1), 182–187. doi:10.1177/11206721221106138
Duru, Z., and Ozsaygili, C. (2020). Preservative-free versus preserved brimonidine %0.15 preparations in the treatment of glaucoma and ocular hypertension: Short term evaluation of efficacy, safety, and potential advantages. Cutan. Ocul. Toxicol. 39 (1), 21–24. doi:10.1080/15569527.2019.1680685
El Hajj Moussa, W. G., Farhat, R. G., Nehme, J. C., Sahyoun, M. A., Schakal, A. R., Jalkh, A. E., et al. (2018). Comparison of efficacy and ocular surface disease Index score between bimatoprost, latanoprost, travoprost, and tafluprost in glaucoma patients. J. Ophthalmol. 2018, 1319628. doi:10.1155/2018/1319628
Elder, M. J., and Lightman, S. (1994). The immunological features and pathophysiology of ocular cicatricial pemphigoid. Eye (Lond) 8, 196–199. doi:10.1038/eye.1994.45
Fechtner, R. D., Godfrey, D. G., Budenz, D., Stewart, J. A., Stewart, W. C., and Jasek, M. C. (2010). Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea 29 (6), 618–621. doi:10.1097/ICO.0b013e3181c325b2
Francoz, M., Karamoko, I., Baudouin, C., and Labbe, A. (2011). Ocular surface epithelial thickness evaluation with spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 52 (12), 9116–9123. doi:10.1167/iovs.11-7988
Frezzotti, P., Fogagnolo, P., Haka, G., Motolese, I., Iester, M., Bagaglia, S. A., et al. (2014). In vivo confocal microscopy of conjunctiva in preservative-free timolol 0.1% gel formulation therapy for glaucoma. Acta Ophthalmol. 92 (2), e133–e140. doi:10.1111/aos.12261
Gao, N., Tsai, M. H., Chang, A. N., He, W., Chen, C. P., Zhu, M., et al. (2017). Physiological vs. pharmacological signalling to myosin phosphorylation in airway smooth muscle. J. Physiol. 595 (19), 6231–6247. doi:10.1113/JP274715
Geyer, O., Schmidt, K. G., Pianka, P., Neudorfer, M., and Lazar, M. (2000). Clonidine provides an allergy-free alternative in glaucoma patients with proven allergy to apraclonidine. Graefes Arch. Clin. Exp. Ophthalmol. 238 (2), 149–152. doi:10.1007/pl00007883
Gibran, S. K. (2004). Unilateral drug-induced ocular pseudopemphigoid. Eye (Lond) 18 (12), 1270. doi:10.1038/sj.eye.6701385
Giordano-Labadie, F., Lepoittevin, J. P., Calix, I., and Bazex, J. (1997). Contact allergy to beta blockaders in eye drops: Cross allergy? Ann. Dermatol Venereol. 124 (4), 322–324.
Gomes, J. A. P., Azar, D. T., Baudouin, C., Efron, N., Hirayama, M., Horwath-Winter, J., et al. (2017). TFOS DEWS II iatrogenic report. Ocul. Surf. 15 (3), 511–538. doi:10.1016/j.jtos.2017.05.004
Grey, K. R., and Warshaw, E. M. (2016). Allergic contact dermatitis to ophthalmic medications: Relevant allergens and alternative testing methods. Dermatitis 27 (6), 333–347. doi:10.1097/DER.0000000000000224
Guclu, H., Cinar, A. K., Cinar, A. C., Akaray, I., Sambel Aykutlu, M., Sakallioglu, A. K., et al. (2021). Corneal epithelium and limbal region alterations due to glaucoma medications evaluated by anterior segment optic coherence tomography: A case-control study. Cutan. Ocul. Toxicol. 40 (2), 85–94. doi:10.1080/15569527.2021.1902341
Han, X., Liu, Y., Kam, W. R., and Sullivan, D. A. (2018). Effect of brimonidine, an α2 adrenergic agonist, on human meibomian gland epithelial cells. Exp. Eye Res. 170, 20–28. doi:10.1016/j.exer.2018.02.009
Han, X., Yang, S., Kam, W. R., Sullivan, D. A., and Liu, Y. (2020). The carbonic anhydrase inhibitor dorzolamide stimulates the differentiation of human meibomian gland epithelial cells. Curr. Eye Res. 45 (12), 1604–1610. doi:10.1080/02713683.2020.1772832
Hatanaka, M., Vessani, R. M., Elias, I. R., Morita, C., and Susanna, R. (2009). The effect of prostaglandin analogs and prostamide on central corneal thickness. J. Ocul. Pharmacol. Ther. 25 (1), 51–53. doi:10.1089/jop.2007.0125
Hedengran, A., Begun, X., Mullertz, O., Mouhammad, Z., Vohra, R., Bair, J., et al. (2021). Benzalkonium chloride-preserved anti-glaucomatous eye drops and their effect on human conjunctival goblet cells in vitro. Biomed. Hub. 6 (2), 69–75. doi:10.1159/000517845
Hedengran, A., Freiberg, J. C., Hansen, P. M., Jacobsen, J., Larsen, S. W., Harloff-Helleberg, S., et al. (2022). Generic benzalkonium chloride-preserved travoprost eye drops are not identical to the branded polyquarternium-1-preserved travoprost eye drop: Effect on cultured human conjunctival goblet cells and their physicochemical properties. Acta Ophthalmol. 100 (7), 819–827. doi:10.1111/aos.15163
Hegde, V., Robinson, R., Dean, F., Mulvihill, H. A., and Ahluwalia, H. (2007). Drug-induced ectropion: What is best practice? Ophthalmology 114 (2), 362–366. doi:10.1016/j.ophtha.2006.09.032
Heo, J. Y., Ooi, Y. H., and Rhee, D. J. (2020). Effect of prostaglandin analogs: Latanoprost, bimatoprost, and unoprostone on matrix metalloproteinases and their inhibitors in human trabecular meshwork endothelial cells. Exp. Eye Res. 194, 108019. doi:10.1016/j.exer.2020.108019
Hong, S., Lee, C. S., Seo, K. Y., Seong, G. J., and Hong, Y. J. (2006). Effects of topical antiglaucoma application on conjunctival impression cytology specimens. Am. J. Ophthalmol. 142 (1), 185–186. doi:10.1016/j.ajo.2006.02.056
Horcajada-Reales, C., Rodriguez-Soria, V. J., and Suarez-Fernandez, R. (2015). Allergic contact dermatitis caused by timolol with cross-sensitivity to levobunolol. Contact Dermat. 73 (6), 368–369. doi:10.1111/cod.12448
Inoue, K., Shiokawa, M., Higa, R., Sugahara, M., Soga, T., Wakakura, M., et al. (2012a). Adverse periocular reactions to five types of prostaglandin analogs. Eye (Lond) 26 (11), 1465–1472. doi:10.1038/eye.2012.195
Inoue, K., Shiokawa, M., Sugahara, M., Higa, R., Wakakura, M., and Tomita, G. (2012b). Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin. Ophthalmol. 6, 111–116. doi:10.2147/OPTH.S27489
Jadczyk-Sorek, K., Garczorz, W., Bubala-Stachowicz, B., Francuz, T., and Mrukwa-Kominek, E. (2022). Increased matrix metalloproteinase-2 and matrix metalloproteinase-3 concentrations in corneal epithelium of patients with recurrent corneal erosions. J. Ophthalmol. 2022, 5024037. doi:10.1155/2022/5024037
Jappe, U., Uter, W., Menezes de Padua, C. A., Herbst, R. A., and Schnuch, A. (2006). Allergic contact dermatitis due to beta-blockers in eye drops: A retrospective analysis of multicentre surveillance data 1993-2004. Acta Derm. Venereol. 86 (6), 509–514. doi:10.2340/00015555-0162
Jiang, X. Y., Yang, P. S., Xiao, O., Yu, K., Wang, S. Y., Yang, S. J., et al. (2022). Effects of PPAR-γ and RXR-α on mouse meibomian gland epithelial cells during inflammation induced by latanoprost. Exp. Eye Res. 224, 109251. doi:10.1016/j.exer.2022.109251
Kahana, A., Marcet, M. M., Albert, D. M., and Thliveris, A. T. (2007). Drug-induced cicatrising granulomatous conjunctivitis. Br. J. Ophthalmol. 91 (5), 691–692. doi:10.1136/bjo.2006.099085
Kam, W. R., and Sullivan, D. A. (2011). Neurotransmitter influence on human meibomian gland epithelial cells. Invest. Ophthalmol. Vis. Sci. 52 (12), 8543–8548. doi:10.1167/iovs.11-8113
Kammer, J. A., Katzman, B., Ackerman, S. L., and Hollander, D. A. (2010). Efficacy and tolerability of bimatoprost versus travoprost in patients previously on latanoprost: A 3-month, randomised, masked-evaluator, multicentre study. Br. J. Ophthalmol. 94 (1), 74–79. doi:10.1136/bjo.2009.158071
Karli, S., Ayala-Haedo, J. A., Feuer, W. J., Fernandez, M., Dubovy, S., and Wester, S. T. (2018). Effect of prostaglandin analogs on matrix metalloproteinases and tissue inhibitor of metalloproteinases in eyelid muscle specimens. Clin. Ophthalmol. 12, 2039–2046. doi:10.2147/OPTH.S178106
Kashkouli, M. B., Rezaee, R., Nilforoushan, N., Salimi, S., Foroutan, A., and Naseripour, M. (2008). Topical antiglaucoma medications and lacrimal drainage system obstruction. Ophthalmic Plast. Reconstr. Surg. 24 (3), 172–175. doi:10.1097/IOP.0b013e3181706829
Kawakita, T. (2018). Regeneration of lacrimal gland function to maintain the health of the ocular surface. Invest. Ophthalmol. Vis. Sci. 59 (14), DES169–DES173. doi:10.1167/iovs.17-23576
Kawase, K., Vittitow, J. L., Weinreb, R. N., Araie, M., and Group, J. S. (2016). Long-term safety and efficacy of latanoprostene bunod 0.024% in Japanese subjects with open-angle glaucoma or ocular hypertension: The JUPITER study. Adv. Ther. 33 (9), 1612–1627. doi:10.1007/s12325-016-0385-7
Kiland, J. A., Gabelt, B. T., and Kaufman, P. L. (2004). Studies on the mechanism of action of timolol and on the effects of suppression and redirection of aqueous flow on outflow facility. Exp. Eye Res. 78 (3), 639–651. doi:10.1016/j.exer.2003.11.001
Kim, H. M., Tran, A. Q., Yang, C., and Dagi Glass, L. R. (2021a). Netarsudil-related eyelid wound dehiscence. J. Glaucoma 30 (2), 206–208. doi:10.1097/IJG.0000000000001698
Kim, J. H., Kim, E. J., Kim, Y. H., Kim, Y. I., Lee, S. H., Jung, J. C., et al. (2015). In vivo effects of preservative-free and preserved prostaglandin analogs: Mouse ocular surface study. Korean J. Ophthalmol. 29 (4), 270–279. doi:10.3341/kjo.2015.29.4.270
Kim, M., Jang, H., and Rho, S. (2021b). Risk factors for periorbital dermatitis in patients using dorzolamide/timolol eye drops. Sci. Rep. 11 (1), 17896. doi:10.1038/s41598-021-97565-0
Knop, E., Knop, N., Millar, T., Obata, H., and Sullivan, D. A. (2011). The international workshop on meibomian gland dysfunction: Report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest. Ophthalmol. Vis. Sci. 52 (4), 1938–1978. doi:10.1167/iovs.10-6997c
Koch, P. (1995). Allergic contact dermatitis due to timolol and levobunolol in eyedrops, with no cross-sensitivity to other ophthalmic beta-blockers. Contact Dermat. 33 (2), 140–141. doi:10.1111/j.1600-0536.1995.tb00530.x
Krauss, A. H., Impagnatiello, F., Toris, C. B., Gale, D. C., Prasanna, G., Borghi, V., et al. (2011). Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating prostaglandin F2α agonist, in preclinical models. Exp. Eye Res. 93 (3), 250–255. doi:10.1016/j.exer.2011.03.001
Kumar, S., Singh, T., Ichhpujani, P., Vohra, S., and Thakur, S. (2020). Correlation of ocular surface disease and quality of life in Indian glaucoma patients: BAC-preserved versus BAC-free travoprost. Turk J. Ophthalmol. 50 (2), 75–81. doi:10.4274/tjo.galenos.2019.29000
Lajmi, H., Hmaied, W., Achour, B. B., and Zahaf, A. (2021). Risk factors for ocular surface disease in Tunisian users of preserved antiglaucomatous eye drops. J. Curr. Ophthalmol. 33 (2), 128–135. doi:10.4103/JOCO.JOCO_226_20
Leary, K. A., Lin, K. T., Steibel, J. P., Harman, C. D., and Komaromy, A. M. (2021). Safety and efficacy of topically administered netarsudil (Rhopressa) in normal and glaucomatous dogs with ADAMTS10-open-angle glaucoma (ADAMTS10-OAG). Vet. Ophthalmol. 24 (1), 75–86. doi:10.1111/vop.12734
Lee, H. J., Jun, R. M., Cho, M. S., and Choi, K. R. (2015). Comparison of the ocular surface changes following the use of two different prostaglandin F2α analogues containing benzalkonium chloride or polyquad in rabbit eyes. Cutan. Ocul. Toxicol. 34 (3), 195–202. doi:10.3109/15569527.2014.944650
Liang, H., Baudouin, C., Daull, P., Garrigue, J. S., and Brignole-Baudouin, F. (2022). In vitro corneal and conjunctival wound-healing assays as a tool for antiglaucoma prostaglandin formulation characterization. Front. Biosci. (Landmark Ed. 27 (5), 147. doi:10.31083/j.fbl2705147
Liang, H., Brignole-Baudouin, F., Pauly, A., Riancho, L., and Baudouin, C. (2011). Polyquad-preserved travoprost/timolol, benzalkonium chloride (BAK)-preserved travoprost/timolol, and latanoprost/timolol in fixed combinations: A rabbit ocular surface study. Adv. Ther. 28 (4), 311–325. doi:10.1007/s12325-011-0003-7
Lin, C. W., Sherman, B., Moore, L. A., Laethem, C. L., Lu, D. W., Pattabiraman, P. P., et al. (2018). Discovery and preclinical development of netarsudil, a novel ocular hypotensive agent for the treatment of glaucoma. J. Ocul. Pharmacol. Ther. 34 (1-2), 40–51. doi:10.1089/jop.2017.0023
Lo, T. C., Chen, Y. Y., Hung, M. C., and Chou, P. (2022). Latanoprostene bunod 0.024% in the treatment of open-angle glaucoma and ocular hypertension: A meta-analysis. J. Clin. Med. 11 (15), 4325. doi:10.3390/jcm11154325
Lopes, J. F., Hubatsch, D. A., and Amaris, P. (2015). Effect of benzalkonium chloride-free travoprost on intraocular pressure and ocular surface symptoms in patients with glaucoma previously on latanoprost: An open-label study. BMC Ophthalmol. 15, 166. doi:10.1186/s12886-015-0151-7
Lopilly Park, H. Y., Kim, J. H., Lee, K. M., and Park, C. K. (2012). Effect of prostaglandin analogues on tear proteomics and expression of cytokines and matrix metalloproteinases in the conjunctiva and cornea. Exp. Eye Res. 94 (1), 13–21. doi:10.1016/j.exer.2011.10.017
Lusthaus, J. A., and Goldberg, I. (2017). Brimonidine and brinzolamide for treating glaucoma and ocular hypertension; a safety evaluation. Expert Opin. Drug Saf. 16 (9), 1071–1078. doi:10.1080/14740338.2017.1346083
Lyons, L. J., Wu, K. Y., Baratz, K. H., and Sit, A. J. (2022). Honeycomb epithelial edema associated with rho kinase inhibition: A case series and review of the literature. Cornea 41 (2), 243–248. doi:10.1097/ICO.0000000000002694
Mao, Y. J., Wu, J. B., Yang, Z. Q., Zhang, Y. H., and Huang, Z. J. (2020). Nitric oxide donating anti-glaucoma drugs: Advances and prospects. Chin. J. Nat. Med. 18 (4), 275–283. doi:10.1016/S1875-5364(20)30035-2
Mastropasqua, L., Agnifili, L., Fasanella, V., Curcio, C., Ciabattoni, C., Mastropasqua, R., et al. (2013). Conjunctival goblet cells density and preservative-free tafluprost therapy for glaucoma: An in vivo confocal microscopy and impression cytology study. Acta Ophthalmol. 91 (5), e397–e405. doi:10.1111/aos.12131
Mastropasqua, R., Agnifili, L., Fasanella, V., Curcio, C., Brescia, L., Lanzini, M., et al. (2015). Corneoscleral limbus in glaucoma patients: In vivo confocal microscopy and immunocytological study. Invest. Ophthalmol. Vis. Sci. 56 (3), 2050–2058. doi:10.1167/iovs.14-15890
Mastropasqua, R., Agnifili, L., Fasanella, V., Lappa, A., Brescia, L., Lanzini, M., et al. (2016). In vivo distribution of corneal epithelial dendritic cells in patients with glaucoma. Invest. Ophthalmol. Vis. Sci. 57 (14), 5996–6002. doi:10.1167/iovs.16-20333
Meirick, T. M., Mudumbai, R. C., Zhang, M. M., and Chen, P. P. (2022). Punctal stenosis associated with topical netarsudil use. Ophthalmology 129 (7), 765–770. doi:10.1016/j.ophtha.2022.02.025
Mincione, F., Scozzafava, A., and Supuran, C. T. (2007). The development of topically acting carbonic anhydrase inhibitors as anti-glaucoma agents. Curr. Top. Med. Chem. 7 (9), 849–854. doi:10.2174/156802607780636735
Mizoue, S., Nakano, T., Fuse, N., Iwase, A., Matsumoto, S., and Yoshikawa, K. (2014). Travoprost with sofZia® preservative system lowered intraocular pressure of Japanese normal tension glaucoma with minimal side effects. Clin. Ophthalmol. 8, 347–354. doi:10.2147/OPTH.S57640
Mocan, M. C., Uzunosmanoglu, E., Kocabeyoglu, S., Karakaya, J., and Irkec, M. (2016). The association of chronic topical prostaglandin analog use with meibomian gland dysfunction. J. Glaucoma 25 (9), 770–774. doi:10.1097/IJG.0000000000000495
Mohammed, I., Kulkarni, B., Faraj, L. A., Abbas, A., Dua, H. S., and King, A. J. (2020). Profiling ocular surface responses to preserved and non-preserved topical glaucoma medications: A 2-year randomized evaluation study. Clin. Exp. Ophthalmol. 48 (7), 973–982. doi:10.1111/ceo.13814
Moura-Coelho, N., Tavares Ferreira, J., Bruxelas, C. P., Dutra-Medeiros, M., Cunha, J. P., and Pinto Proenca, R. (2019). Rho kinase inhibitors-a review on the physiology and clinical use in Ophthalmology. Graefes Arch. Clin. Exp. Ophthalmol. 257 (6), 1101–1117. doi:10.1007/s00417-019-04283-5
Mundorf, T., Williams, R., Whitcup, S., Felix, C., and Batoosingh, A. (2003). A 3-month comparison of efficacy and safety of brimonidine-purite 0.15% and brimonidine 0.2% in patients with glaucoma or ocular hypertension. J. Ocul. Pharmacol. Ther. 19 (1), 37–44. doi:10.1089/108076803762718097
Muz, O. E., Dagdelen, K., Pirdal, T., and Guler, M. (2021). Comparison of BAK-preserved latanoprost and polyquad-preserved travoprost on ocular surface parameters in patients with glaucoma and ocular hypertension. Int. Ophthalmol. 41 (11), 3825–3835. doi:10.1007/s10792-021-01947-2
Muzychuk, A., Racine, L., Robert, M. C., Birt, C., Penner, V., Harasymowycz, P., et al. (2020). Management of ocular surface disease in glaucoma: A survey of Canadian glaucoma specialists. J. Glaucoma 29 (12), 1162–1172. doi:10.1097/IJG.0000000000001659
Narioka, J., and Ohashi, Y. (2007). Effects of beta-adrenergic antagonist on width of nasolacrimal drainage system lumen. J. Ocul. Pharmacol. Ther. 23 (5), 467–475. doi:10.1089/jop.2007.0025
Nilsson, S. F., Drecoll, E., Lutjen-Drecoll, E., Toris, C. B., Krauss, A. H., Kharlamb, A., et al. (2006). The prostanoid EP2 receptor agonist butaprost increases uveoscleral outflow in the cynomolgus monkey. Invest. Ophthalmol. Vis. Sci. 47 (9), 4042–4049. doi:10.1167/iovs.05-1627
Nyborg, N. C., and Nielsen, P. J. (1995). Beta-adrenergic receptors regulating vascular smooth muscle tone are only localized to the intraocular segment of the long posterior ciliary artery in bovine eye. Surv. Ophthalmol. 39 (1), S66–S75. doi:10.1016/s0039-6257(05)80075-x
O'Donnell, B. F., and Foulds, I. S. (1993). Contact allergic dermatitis and contact urticaria due to topical ophthalmic preparations. Br. J. Ophthalmol. 77 (11), 740–741. doi:10.1136/bjo.77.11.740
Ortiz-Basso, T., Galmarini, A., Vigo, R. L., Gonzalez-Barlatay, J. M., and Premoli, E. J. (2018). The relationship between topical anti-glaucoma medications and the development of lacrimal drainage system obstruction. Arq. Bras. Oftalmol. 81 (6), 490–493. doi:10.5935/0004-2749.20180095
Ott, E. Z., Mills, M. D., Arango, S., Getson, A. J., Assaid, C. A., and Adamsons, I. A. (2005). A randomized trial assessing dorzolamide in patients with glaucoma who are younger than 6 years. Arch. Ophthalmol. 123 (9), 1177–1186. doi:10.1001/archopht.123.9.1177
Paimela, T., Ryhanen, T., Kauppinen, A., Marttila, L., Salminen, A., and Kaarniranta, K. (2012). The preservative polyquaternium-1 increases cytoxicity and NF-kappaB linked inflammation in human corneal epithelial cells. Mol. Vis. 18, 1189–1196.
Park, J., Cho, H. K., and Moon, J. I. (2011). Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn. J. Ophthalmol. 55 (1), 22–27. doi:10.1007/s10384-010-0904-z
Peace, J. H., Ahlberg, P., Wagner, M., Lim, J. M., Wirta, D., and Branch, J. D. (2015). Polyquaternium-1-Preserved travoprost 0.003% or benzalkonium chloride-preserved travoprost 0.004% for glaucoma and ocular hypertension. Am. J. Ophthalmol. 160 (2), 266–274.e1. doi:10.1016/j.ajo.2015.04.041
Perez-Rodriguez, E., Gonzalez-Perez, R., Poza, P., Feliciano, L., Lopez-Correcher, B., and Matheu, V. (2008). Contact dermatitis caused by latanoprost-containing eye drops with good tolerance to bimatoprost eye drops. Contact Dermat. 58 (6), 370–371. doi:10.1111/j.1600-0536.2007.01297.x
Pisella, P. J., Debbasch, C., Hamard, P., Creuzot-Garcher, C., Rat, P., Brignole, F., et al. (2004). Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: An ex vivo and in vitro study. Invest. Ophthalmol. Vis. Sci. 45 (5), 1360–1368. doi:10.1167/iovs.03-1067
Pisella, P. J., Fillacier, K., Elena, P. P., Debbasch, C., and Baudouin, C. (2000). Comparison of the effects of preserved and unpreserved formulations of timolol on the ocular surface of albino rabbits. Ophthalmic Res. 32 (1), 3–8. doi:10.1159/000055579
Rath, A., Eichhorn, M., Trager, K., Paulsen, F., and Hampel, U. (2019). In vitro effects of benzalkonium chloride and prostaglandins on human meibomian gland epithelial cells. Ann. Anat. 222, 129–138. doi:10.1016/j.aanat.2018.12.003
Robin, A. L., Ritch, R., Shin, D., Smythe, B., Mundorf, T., and Lehmann, R. P. (1995). Topical apraclonidine hydrochloride in eyes with poorly controlled glaucoma. The apraclonidine maximum tolerated medical therapy study group. Trans. Am. Ophthalmol. Soc. 93, 421–438.
Rodriguez-Garcia, A., Babayan-Sosa, A., Ramirez-Miranda, A., Santa Cruz-Valdes, C., Hernandez-Quintela, E., Hernandez-Camarena, J. C., et al. (2022). A practical approach to severity classification and treatment of dry eye disease: A proposal from the Mexican dry eye disease expert panel. Clin. Ophthalmol. 16, 1331–1355. doi:10.2147/OPTH.S351898
Roy, N. S., Yu, Y., Ying, G. S., Maguire, M. G., Asbell, P. A., and Group, D. S. (2022). Effect of omega-3 on HLA-DR expression by conjunctival cells and tear cytokine concentrations in the dry eye assessment and management study. Eye Contact Lens 48 (9), 384–390. doi:10.1097/ICL.0000000000000916
Saade, C. E., Lari, H. B., Berezina, T. L., Fechtner, R. D., and Khouri, A. S. (2015). Topical glaucoma therapy and ocular surface disease: A prospective, controlled cohort study. Can. J. Ophthalmol. 50 (2), 132–136. doi:10.1016/j.jcjo.2014.11.006
Saha, B. C., Kumari, R., Kushumesh, R., Ambasta, A., and Sinha, B. P. (2022). Status of Rho kinase inhibitors in glaucoma therapeutics-an overview. Int. Ophthalmol. 42 (1), 281–294. doi:10.1007/s10792-021-02002-w
Sakata, R., Shirato, S., Miyata, K., and Aihara, M. (2013). Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn. J. Ophthalmol. 57 (2), 179–184. doi:10.1007/s10384-012-0219-3
Sandy-Hodgetts, K., Carville, K., and Leslie, G. D. (2015). Determining risk factors for surgical wound dehiscence: A literature review. Int. Wound J. 12 (3), 265–275. doi:10.1111/iwj.12088
Schnober, D., Hubatsch, D. A., and Scherzer, M. L. (2015). Efficacy and safety of fixed-combination travoprost 0.004%/timolol 0.5% in patients transitioning from bimatoprost 0.03%/timolol 0.5% combination therapy. Clin. Ophthalmol. 9, 825–832. doi:10.2147/OPTH.S80880
Schoenberg, E. D., Blake, D. A., Swann, F. B., Parlin, A. W., Zurakowski, D., Margo, C. E., et al. (2015). Effect of two novel sustained-release drug delivery systems on bleb fibrosis: An in vivo glaucoma drainage device study in a rabbit model. Transl. Vis. Sci. Technol. 4 (3), 4. doi:10.1167/tvst.4.3.4
Schwartz, G. S., and Holland, E. J. (2001). Iatrogenic limbal stem cell deficiency: When glaucoma management contributes to corneal disease. J. Glaucoma 10 (6), 443–445. doi:10.1097/00061198-200112000-00001
Schweitzer, J. A., Hauser, W. H., Ibach, M., Baartman, B., Gollamudi, S. R., Crothers, A. W., et al. (2020). Prospective interventional cohort study of ocular surface disease changes in eyes after trabecular micro-bypass stent(s) implantation (iStent or iStent inject) with phacoemulsification. Ophthalmol. Ther. 9 (4), 941–953. doi:10.1007/s40123-020-00290-6
Seibold, L. K., Ammar, D. A., and Kahook, M. Y. (2013). Acute effects of glaucoma medications and benzalkonium chloride on pre-adipocyte proliferation and adipocyte cytotoxicity in vitro. Curr. Eye Res. 38 (1), 70–74. doi:10.3109/02713683.2012.733055
Serle, J. B., Katz, L. J., McLaurin, E., Heah, T., Ramirez-Davis, N., Usner, D. W., et al. (2018). Two phase 3 clinical trials comparing the safety and efficacy of netarsudil to timolol in patients with elevated intraocular pressure: Rho kinase elevated IOP treatment trial 1 and 2 (ROCKET-1 and ROCKET-2). Am. J. Ophthalmol. 186, 116–127. doi:10.1016/j.ajo.2017.11.019
Servat, J. J., and Bernardino, C. R. (2011). Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs Aging 28 (4), 267–282. doi:10.2165/11588830-000000000-00000
Shahidullah, M., To, C. H., Pelis, R. M., and Delamere, N. A. (2009). Studies on bicarbonate transporters and carbonic anhydrase in porcine nonpigmented ciliary epithelium. Invest. Ophthalmol. Vis. Sci. 50 (4), 1791–1800. doi:10.1167/iovs.08-2487
Shin, H. Y., Lee, H. S., Lee, Y. C., and Kim, S. Y. (2015). Effect of brimonidine on the B cells, T cells, and cytokines of the ocular surface and aqueous humor in rat eyes. J. Ocul. Pharmacol. Ther. 31 (10), 623–626. doi:10.1089/jop.2015.0067
Shirley, M. (2020). Bimatoprost implant: First approval. Drugs Aging 37 (6), 457–462. doi:10.1007/s40266-020-00769-8
Singh, I. P., Fechtner, R. D., Myers, J. S., Kim, T., Usner, D. W., McKee, H., et al. (2020). Pooled efficacy and safety profile of netarsudil ophthalmic solution 0.02% in patients with open-angle glaucoma or ocular hypertension. J. Glaucoma 29 (10), 878–884. doi:10.1097/IJG.0000000000001634
Singh, S., Donthineni, P. R., Shanbhag, S. S., Senthil, S., Ong, H. S., Dart, J. K., et al. (2022). Drug induced cicatrizing conjunctivitis: A case series with review of etiopathogenesis, diagnosis and management. Ocul. Surf. 24, 83–92. doi:10.1016/j.jtos.2022.02.004
Staso, D. I. S., Agnifili, L., Cecannecchia, S., and Ciancaglini, M. (2018). In vivo analysis of prostaglandins-induced ocular surface and periocular adnexa modifications in patients with glaucoma. Vivo 32 (2), 211–220. doi:10.21873/invivo.11227
Stringham, J., Ashkenazy, N., Galor, A., and Wellik, S. R. (2018). Barriers to glaucoma medication compliance among veterans: Dry eye symptoms and anxiety disorders. Eye Contact Lens 44 (1), 50–54. doi:10.1097/ICL.0000000000000301
Sugrue, M. F., Mallorga, P., Schwam, H., Baldwin, J. J., and Ponticello, G. S. (1990). A comparison of L-671,152 and MK-927, two topically effective ocular hypotensive carbonic anhydrase inhibitors, in experimental animals. Curr. Eye Res. 9 (6), 607–615. doi:10.3109/02713689008999600
Sun, Y., Chen, A., Zou, M., Zhang, Y., Jin, L., Li, Y., et al. (2022). Time trends, associations and prevalence of blindness and vision loss due to glaucoma: An analysis of observational data from the global burden of disease study 2017. BMJ Open 12 (1), e053805. doi:10.1136/bmjopen-2021-053805
Taketani, Y., Yamagishi, R., Fujishiro, T., Igarashi, M., Sakata, R., and Aihara, M. (2014). Activation of the prostanoid FP receptor inhibits adipogenesis leading to deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy. Invest. Ophthalmol. Vis. Sci. 55 (3), 1269–1276. doi:10.1167/iovs.13-12589
Tang, W., Zhang, F., Liu, K., and Duan, X. (2019). Efficacy and safety of prostaglandin analogues in primary open-angle glaucoma or ocular hypertension patients: A meta-analysis. Med. Baltim. 98 (30), e16597. doi:10.1097/MD.0000000000016597
Terai, N., Schlotzer-Schrehardt, U., Lampel, J., Bohm, A. G., Rummelt, C., Schmidt, E., et al. (2009). Effect of latanoprost and timolol on the histopathology of the human conjunctiva. Br. J. Ophthalmol. 93 (2), 219–224. doi:10.1136/bjo.2008.140186
Thorne, J. E., Anhalt, G. J., and Jabs, D. A. (2004). Mucous membrane pemphigoid and pseudopemphigoid. Ophthalmology 111 (1), 45–52. doi:10.1016/j.ophtha.2003.03.001
Tiedemann, D., Mouhammad, Z. A., Utheim, T. P., Dartt, D. A., Heegaard, S., Petrovski, G., et al. (2019). Conjunctival goblet cells, the overlooked cells in glaucoma treatment. J. Glaucoma 28 (4), 325–333. doi:10.1097/IJG.0000000000001168
Tirpack, A. R., Vanner, E., Parrish, J. M., Galor, A., Hua, H. U., and Wellik, S. R. (2019). Dry eye symptoms and ocular pain in veterans with glaucoma. J. Clin. Med. 8 (7), 1076. doi:10.3390/jcm8071076
Vazirani, J., Donthineni, P. R., Goel, S., Sane, S. S., Mahuvakar, S., Narang, P., et al. (2020). Chronic cicatrizing conjunctivitis: A review of the differential diagnosis and an algorithmic approach to management. Indian J. Ophthalmol. 68 (11), 2349–2355. doi:10.4103/ijo.IJO_604_20
Wang, H., Wang, X., Liu, X., Zhou, J., Yang, Q., Chai, B., et al. (2022). miR-199a-5p plays a pivotal role on wound healing via suppressing VEGFA and ROCK1 in diabetic ulcer foot. Oxid. Med. Cell. Longev. 2022, 4791059. doi:10.1155/2022/4791059
Watabe, H., Abe, S., and Yoshitomi, T. (2011). Effects of Rho-associated protein kinase inhibitors Y-27632 and Y-39983 on isolated rabbit ciliary arteries. Jpn. J. Ophthalmol. 55 (4), 411–417. doi:10.1007/s10384-011-0048-9
Whitson, J. T., Ochsner, K. I., Moster, M. R., Sullivan, E. K., Andrew, R. M., Silver, L. H., et al. (2006). The safety and intraocular pressure-lowering efficacy of brimonidine tartrate 0.15% preserved with polyquaternium-1. Ophthalmology 113 (8), 1333–1339. doi:10.1016/j.ophtha.2006.03.025
Whitson, J. T., and Petroll, W. M. (2012). Corneal epithelial cell viability following exposure to ophthalmic solutions containing preservatives and/or antihypertensive agents. Adv. Ther. 29 (10), 874–888. doi:10.1007/s12325-012-0057-1
Wisely, C. E., Liu, K. C., Gupta, D., Carlson, A. N., Asrani, S. G., and Kim, T. (2020). Reticular bullous epithelial edema in corneas treated with netarsudil: A case series. Am. J. Ophthalmol. 217, 20–26. doi:10.1016/j.ajo.2020.04.002
Wu, J., Li, X., Zhou, P., and Li, X. (2020). M(3) but not M(4) muscarinic receptors in the rostromedial tegmental nucleus are involved in the acquisition of morphine-induced conditioned place preference. Eur. J. Pharmacol. 882, 173274. doi:10.1016/j.ejphar.2020.173274
Wu, R. Y., and Ma, N. (2012). Expression of nitric oxide synthase and guanylate cyclase in the human ciliary body and trabecular meshwork. Chin. Med. J. Engl. 125 (1), 129–133. doi:10.3760/cma.j.issn.0366-6999.2012.01.024
Yamagishi-Kimura, R., Honjo, M., and Aihara, M. (2022). The roles played by FP/EP3 receptors during pressure-lowering in mouse eyes mediated by a dual FP/EP3 receptor agonist. Invest. Ophthalmol. Vis. Sci. 63 (2), 24. doi:10.1167/iovs.63.2.24
Ye, Y., Xu, Y., Yang, Y., Fan, Y., Liu, P., Yu, K., et al. (2022). Wide corneal epithelial thickness mapping in eyes with topical antiglaucoma therapy using optical coherence tomography. Transl. Vis. Sci. Technol. 11 (1), 4. doi:10.1167/tvst.11.1.4
Yeh, P. H., Cheng, Y. C., Shie, S. S., Lee, Y. S., Shen, S. C., Chen, H. S., et al. (2021). Brimonidine related acute follicular conjunctivitis: Onset time and clinical presentations, a long-term follow-up. Med. Baltim. 100 (29), e26724. doi:10.1097/MD.0000000000026724
Young, T. L., Higginbotham, E. J., Zou, X. L., and Farber, M. D. (1990). Effects of topical glaucoma drugs on fistulized rabbit conjunctiva. Ophthalmology 97 (11), 1423–1427. doi:10.1016/s0161-6420(90)32392-8
Yuan, X. L., Wen, Q., Zhang, M. Y., and Fan, T. J. (2016). Cytotoxicity of pilocarpine to human corneal stromal cells and its underlying cytotoxic mechanisms. Int. J. Ophthalmol. 9 (4), 505–511. doi:10.18240/ijo.2016.04.05
Zhang, X., Vadoothker, S., Munir, W. M., and Saeedi, O. (2019). Ocular surface disease and glaucoma medications: A clinical approach. Eye Contact Lens 45 (1), 11–18. doi:10.1097/ICL.0000000000000544
Keywords: alpha-adrenergic agonists, beta blockers, carbonic anhydrase inhibitors, dry eye disease, nitric oxide-donating prostaglandin analogs, ocular surface disease, prostaglandin analogs, rho-kinase inhibitors
Citation: Ruiz-Lozano RE, Azar NS, Mousa HM, Quiroga-Garza ME, Komai S, Wheelock-Gutierrez L, Cartes C and Perez VL (2023) Ocular surface disease: a known yet overlooked side effect of topical glaucoma therapy. Front. Toxicol. 5:1067942. doi: 10.3389/ftox.2023.1067942
Received: 12 October 2022; Accepted: 14 July 2023;
Published: 21 July 2023.
Edited by:
Anat Galor, University of Miami, United StatesReviewed by:
Mee Kum Kim, Seoul National University, Republic of KoreaPramila Singh, Charles River Laboratories, France
Naoyuki Tanimoto, University of Kiel, Germany
Copyright © 2023 Ruiz-Lozano, Azar, Mousa, Quiroga-Garza, Komai, Wheelock-Gutierrez, Cartes and Perez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victor L. Perez, dmljdG9yLnBlcmV6LnF1aW5vbmVzQGR1a2UuZWR1
 Raul E. Ruiz-Lozano
Raul E. Ruiz-Lozano Nadim S. Azar2
Nadim S. Azar2 Hazem M. Mousa
Hazem M. Mousa Manuel E. Quiroga-Garza
Manuel E. Quiroga-Garza Lorena Wheelock-Gutierrez
Lorena Wheelock-Gutierrez Cristian Cartes
Cristian Cartes Victor L. Perez
Victor L. Perez