
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Toxicol. , 16 March 2022
Sec. Neurotoxicology
Volume 4 - 2022 | https://doi.org/10.3389/ftox.2022.826488
This article is part of the Research Topic Methods and Protocols in Neurotoxicology View all 6 articles
Neurological hazard assessment of industrial and pesticidal chemicals demands a substantial amount of time and resources. Caenorhabditis elegans is an established model organism in developmental biology and neuroscience. It presents an ideal test system with relatively fewer neurons (302 in hermaphrodites) versus higher-order species, a transparent body, short lifespan, making it easier to perform neurotoxic assessment in a time and cost-effective manner. Yet, no regulatory testing guidelines have been developed for C. elegans in the field of developmental and adult neurotoxicity. Here, we describe a set of morphological and behavioral assessment protocols to examine neurotoxicity in C. elegans with relevance to cholinergic and dopaminergic systems. We discuss the homology of human genes and associated proteins in these two signaling pathways and evaluate the morphological and behavioral endpoints of C. elegans in the context of published adverse outcome pathways of neurodegenerative diseases. We conclude that C. elegans neurotoxicity testing will not only be instrumental to eliminating mammalian testing in neurological hazard assessment but also lead to new knowledge and mechanistic validation in the adverse outcome pathway framework.
There is a major impetus to develop and implement alternatives to mammalian testing for ethical, temporal, and financial reasons. Here, model organism assays such as the nematode Caenorhabditis elegans can provide valuable tools for neurotoxicity assessment. These assays provide new knowledge on key events (KEs), like changes in acetylcholine (Ach) levels. This is essential for the development of adverse outcome pathways (AOPs) that provide a framework to inform the mechanisms of action and downstream KEs that ultimately result in an adverse effect. AOPs can then support alternative models for chemical testing by building on modular KEs, each of which needs to be measurable for an adequate assessment of the KE’s necessity in the AOP itself (OECD, 2018). Testing the molecular initiating event and the downstream events stemming from that initial perturbation is key to the development of alternative testing. Essentially, AOPs can be utilized to develop and test hypotheses and guide research by highlighting specific assays that can be used to assess each step leading to adverse outcomes.
Toxicologists often use organismal-level adverse outcomes as measures to determine the toxicity of a given chemical, such as tremors, gross activity, and response to stimuli including light and touch. AOPs with similar late outcomes but differing initiating events tied to assays at several points may elucidate which molecular pathway is responsible for the outcomes seen at the organismal level. This can accelerate toxicity testing by eliminating molecular pathways using fewer assays; for instance, differentiating, between the neurological effects of mAchRs and nAchRs for their roles in particular neurodegenerative conditions (AOP 281; https://aopwiki.org/aops). Further, having multiple assays attached to each AOP’s molecular pathway can indicate to researchers a way to discover which step of the pathway is being affected and provide insight in ways to more thoroughly test by providing a curated list of assays.
Conventional guideline testing for neurotoxicity (e.g., U.S. EPA OPPTS 870.6200 Neurotoxicity Screening Battery and 870.6300 Developmental Neurotoxicity Study) plays an important role in the hazard assessment of chemical use, such as pesticide applications. However, these methods also require a large number of animals and are labor-, time-, and cost-intensive. They also do not cover the critical effects that are translational to many human neurological conditions. One omission in neurodegenerative diseases. Recently AOP developments have expanded towards utilizing conserved biological systems between species to allow for relevant measurements in model organisms, including invertebrates. Using this framework can give us a method by which C. elegans testing can effectively fill some knowledge gaps and translate assay results to evaluate certain effects in mammalian systems.
This article presents a set of behavioral and morphological assessment protocols related to acetylcholine and dopamine neurons in C. elegans. These assays are connected to the AOP framework (Figure 1), which demonstrates the biological objects that these assays allow for testing. We have demonstrated protocols for five assays, with three for behavioral and two for morphological assessments. Behavioral assays include 1-Nonanol assay, Aldicarb assay and Levamisole assay; morphological assessment entails assessment of neurodegeneration for dopaminergic and cholinergic neurons.
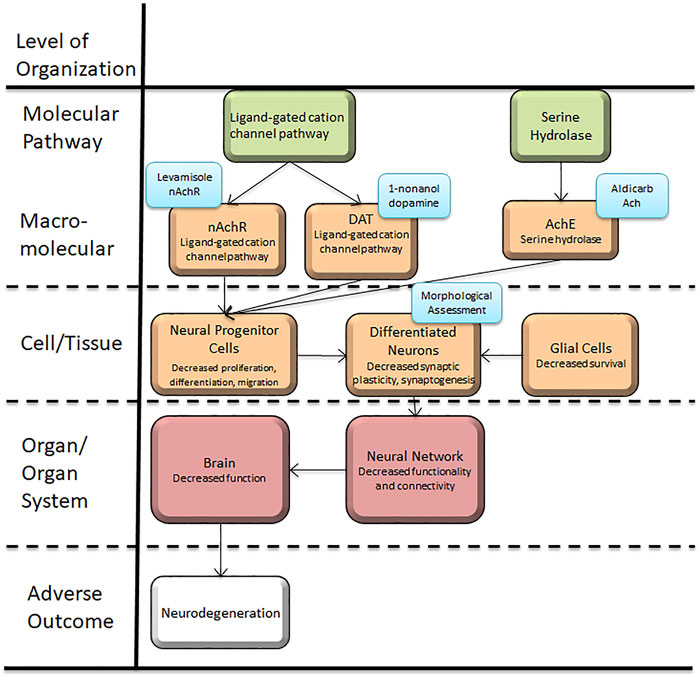
FIGURE 1. Caenorhabditis elegans neurotoxicity testing in the Adverse Outcome Pathway Framework. This article presents a set of behavioral and morphological assessment protocols related to acetylcholine and dopamine neurons and function in C. elegans, which is applicable to conserved systems in target organisms using the AOP framework. These assays can provide new information to further the mechanistic studies and hazard assessments related to Alzheimer’s and Parkinson’s disease.
C. elegans exhibit olfaction based repulsive behavior towards various chemicals such as 1-nonanol, 1-octanol, 1-nonanone, etc. (Bargmann et al., 1993). Dopamine signaling has been known to play a significant role in the aversive behavior and these odorant based repulsion assays have been employed to indirectly measure dopamine levels (Kimura et al., 2010; Baidya et al., 2014; Sammi et al., 2018). Notably mutations in cat-2 (tyrosine hydroxylase, responsible for synthesis of dopamine) has been shown to decrease this repulsive behavior (Baidya et al., 2014; Sammi et al., 2018). On the other hand treatment with exogenous dopamine (Baidya et al., 2014), over expression of cat-2 or inhibition of dat-1 leads to a quicker response or curtailed repulsion time (Sammi et al., 2018). Notably, dat-1 is responsible for uptake of dopamine in the presynapse; inhibition or mutation in dat-1 leads to a longer presence of dopamine in synapse (Sawin et al., 2000). Thus, 1-nonanol assay is an established assay for indirect measurement of dopamine levels (Kimura et al., 2010; Fatima et al., 2014; Smita et al., 2017; Sammi et al., 2019).
Synaptic transmission of acetylcholine (Ach) requires exocytosis of Ach to the synaptic cleft. Within the synapse, Acetylcholinesterase (AchE) acts to breaks down Ach (McHardy et al., 2017). Aldicarb is an AchE inhibitor that blocks the breakdown of Ach (Johnson and Russell, 1983; Lue et al., 1984). The resultant accumulation of Ach, which corresponds to Key Event 10 “Acetylcholine accumulation in synapses” (AOP Wiki, 2021a), leads to flexion of muscles, evident in the form of paralysis in worms. Percentage of paralysis at a given point of time corresponds to the Ach levels (Mahoney et al., 2006). Hence Aldicarb assay can be utilized to indirectly measure the relative Ach levels. Conventionally, at a given time point the higher percentage of worms paralyzed relates to a relative increase in Ach neurotransmission.
Ach receptors are broadly classified into two subtypes, nicotinic Ach receptors (nAchR) (Albuquerque et al., 1995; Dani and Bertrand, 2007) and muscarinic Ach receptors (Eglen, 2005). Levamisole acts on nAchR culminating in spastic paralysis in nematodes (McHugh et al., 2020). While the former assay (Aldicarb assay) is a measure of augmented or curtailed neurotransmission, levamisole assay measures the activity of nAchR (Qian et al., 2008; Sammi et al., 2017; Trivedi et al., 2017), which corresponds to Key Event 559 “Activation, Nicotinic acetylcholine receptor” (AOP Wiki, 2021b). A combination of two behavioral assays (Aldicarb and Levamisole assay) suffices to provide the mechanistic insight in terms of total cholinergic transmission along with the relative effect on nAchR activity. The mechanism of aldicarb and levamisole assays has been demonstrated in Figure 2.
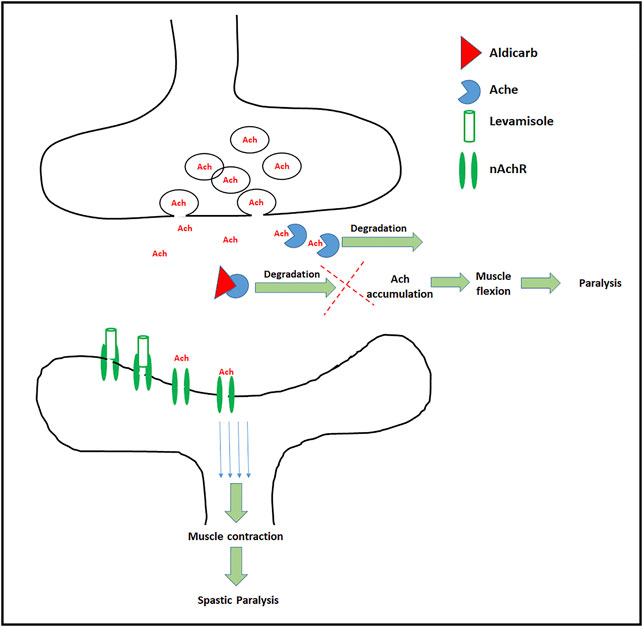
FIGURE 2. Schematic depiction of targets and effects of Aldicarb and Levamisole: In the normal conditions Ach is synthesized in pre synapse, and transported to the synapse. At the synaptic cleft, Ach binds to the Ach receptors (only nAchR shown), which results in the transfer of action potential across neurons. Aldicarb (red triangle) blocks the AchE which leads to build up of Ach causing muscle flexion and paralysis. On the other hand levamisole, a nAchR agonist (transparent cylinder) binds to the nicotinic Ach receptor, ensures continuous action potential culminating in muscle contraction and spastic paralysis.
Morphological assessment of cholinergic and dopaminergic neurons relies on the expression of green fluorescence protein (GFP) under the control of promoters for the genes specific to each neuron type. For the assessment of cholinergic and dopaminergic neurodegeneration GFP is expressed under the control of promoter for unc-17 and dat-1 respectively. Genes unc-17 and dat-1 encodes vesicular Ach transporter (Alfonso et al., 1993; Zhu et al., 2001) and dopamine transporter (Sawin et al., 2000; McDonald et al., 2007) respectively. Transparent nature of C. elegans body makes the visualization of these neurons easier under the microscope. Later sections in this article have elaborated the scoring strategies for neurodegeneration.
In order to study and exemplify these behavioral and morphological protocols, worms were treated with different doses of chlorpyrifos (CPF), an organophosphate pesticide (Ozkan et al., 2014). Acetylcholinesterase (AchE) inhibition has long been researched as a primary mechanism of action of CPF toxicity, though dopaminergic neurotoxicity has recently received a significant attention (Ozkan et al., 2014; Zhang et al., 2015; Ali et al., 2019). Further discussion will explore how these behavioral and morphological assays can provide new knowledge to advance mechanistic studies and hazard assessments related to diseases associated with morphological and functional affliction of these neurons. Broadly, cholinergic aberrations have been associated with diseases such as Alzheimer’s disease (Francis et al., 1999; Schliebs, 2005), Parkinson’s disease (Muller and Bohnen, 2013; Hall et al., 2014), Huntington’s disease (Smith et al., 2006), and Schizophrenia (Gibbons and Dean, 2016). On a similar note, curtailed dopamine function and morphology is attributed to Parkinson’s disease (Cacabelos, 2017; Masato et al., 2019; Marogianni et al., 2020). This article is focused to identify the protocols which can be easily applied to ascertain the effect on morphology and function of both types of neurons. Utilization of C. elegans as an alternate approach offers a significant advancement over the conventional approaches involving invertebrates, along with an edge over costly and time consuming approaches.
(1) 1-Nonanol (Acros Organic, Cat No: AC157471000; Pubchem Compound CID: 8914)
(2) Aldicarb (Sigma, Cat No: 33386-100MG; Pubchem Compound CID: 9570071)
(3) Tetramisole hydrochloride (Levamisole) (Sigma, Cat No: T1512-2G; Pubchem Compound CID: 68628)
(4) Chlorpyriphos Neat (Ultra Scientific, Cat No: PST-480; Pubchem Compound CID: 2730)
(5) Agar (Fisher, Cat No: BP1423-500; Pubchem Compound CID: 71571511)
(6) Peptone (Fisher, Cat No: BP1420-500; Pubchem Compound CID: 9257)
(7) Sodium chloride (Fisher, Cat No: 527L3; Pubchem Compound CID: 5234)
(8) Cholesterol (Alfa Aesar, Cat No: A11470-30; Pubchem Compound CID: 5997)
(9) Calcium chloride dihydrate (Fisher, Cat No: C79-500; Pubchem Compound CID: 5284359)
(10) Magnesium Sulfate (Fisher, Cat No: M65-500; Pubchem Compound CID: 24083)
(11) Potassium phosphate monobasic (Fisher, Cat No: BP362-500; Pubchem Compound CID: 516951)
(12) Potassium chloride (Macron Chemicals, Cat No: 685804; Pubchem Compound CID: 4873)
(13) Uracil (Sigma, Cat No: U0750-5G; Pubchem Compound CID: 1174)
(14) 5-Fluorodeoxyuridine (FUDR) (MP Biomedicals, Cat No: 105551; Pubchem Compound CID: 5790)
(15) Sodium azide (Alfa Aesar, Cat No: 14314; Pubchem Compound CID: 33557)
(16) N2 (Caenorhabditis genetics center, University of Minnesota)
(17) BZ555 (Caenorhabditis genetics center, University of Minnesota)
(18) LX929 (Caenorhabditis genetics center, University of Minnesota)
(19) Escherichia Coli OP50 (Caenorhabditis genetics center, University of Minnesota)
(20) Fluorescence Microscope (Olympus Bx-53)
(21) Centrifuge (Eppendorf refrigerated centrifuge S424R)
(22) Stereo-zoom microscope (Olympus SZ61)
Caenorhabditis elegans strains, Bristol N2, BZ555 (egls1[dat-1p::GFP]), LX929 (vsIs48 [unc-17::GFP]), and Escherichia coli OP50, were obtained from Caenorhabditis Genetics Centre, (University of Minnesota, Minnesota). C. elegans strains were grown on nematode growth medium (NGM) with E. coli OP50 as food at 22°C (Stiernagle, 2006). Briefly NGM was prepared by adding sodium chloride (50 mM), peptone (2.5 g/L), and agar (17 g/L) in 975 ml double-distilled water and autoclaved (Allen-Bradley ADV Plus) for 40 min at 15 lb/in2. One milliliter of 5 mg/ml cholesterol solution (prepared in ethanol), 1 mM calcium chloride (autoclaved), and 1 mM magnesium sulfate (autoclaved), and 25 mM potassium dihydrogen phosphate (autoclaved) was added after the medium was cooled at 60°C. The media was poured in Petri dishes.
To obtain an age-synchronized population, embryos were isolated using sodium hypochlorite treatment. Isolated embryos were stored overnight in M9 buffer (3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 ml 1 M MgSO4, H2O to 1 L. Sterilize by autoclaving (Stiernagle, 2006)) at 15°C to obtain an age-synchronized L1 staged worms, as described previously (Sammi et al., 2019). Worms were pelleted through centrifugation at 2000 rpm for 3 min and counted under the stereozoom microscope.
Worms (L1) were centrifuged at 2,000 rpm for 3 min to concentrate the worm suspension. Worms were counted in a 1 µl drop at least three times and averaged. A fixed number of worms (∼200) was added to each well (Culture volume: 500 µl). Alternatively, treatment can also be administered on solid media (through mixing toxicant with molten NGM before pouring) or through mixing with bacterial food (E. coli OP50) seeded onto solid media.
For behavioral assays (Aldicarb, Levamisole, and 1-nonanol assay), worms were exposed to different concentrations of CPF in liquid culture using complete K medium. K medium-complete was prepared by adding 1 ml cholesterol (5 mg/ml), 1 ml 1 M Calcium chloride, 1 ml 1 M Magnesium sulfate to K medium (2.36 g KCl, 3 g NaCl, in 1 L dH2O) (Boyd et al., 2012) For 48 h.
For neurodegeneration assays, worms were exposed to toxicants at L1 and L4 stages as described above. In addition, liquid culture was supplemented with 50 μg/ml 5-fluoro-2′-deoxyuridine (5-FUdR) to prevent the hatching of eggs.
Note: Although we have used liquid culture to treat worms, experiments can also be conducted on solid media as described above (Concentration of 5-fluoro-2′-deoxyuridine for NGM: 100 μg/ml).
Supplementation with FUDR makes it easier to conduct studies, however it might be an additional confounding factor due to alteration of mitochondrial biology (Rooney et al., 2014). Quantification of mitochondrial stress can be evaluated using methods described in Luz et al., 2016 (Luz et al., 2016). On the other hand, not adding FUDR might dilute the dose of exposure when the eggs hatch. In this case, it is advisable to transfer the worms to fresh plates, when doing experiments without FUDR.
(1) Day 2 (48 h post-treatment): Wash worms with M9 Buffer three to four times in 1.5 ml centrifuge tubes followed with centrifugation at 2,000 rpm for 3 minutes. The supernatant is to be discarded after every wash and fresh M9 buffers should be added to worm pellet. Place a drop of worm suspension (made by suspending worm pellet in 100 µl of M9 buffer) on NGM plates (typically 60 mm or 90 mm plate).
(2) Let the drop of worm suspension dry; separate the worms using a poking lash, if necessary.
(3) Add 20 µl of 1-nonanol on the cap of 1.5–2 ml centrifuge tube.
(4) Dip the poking lash gently into 1-nonanol, removing extra by touching the brim of the centrifuge tube.
(5) Keep the poking lash close to the head region on the agar surface, avoid contact with the worm, and start the stopwatch. Stop the watch as the worms exhibit repulsion, as shown in the Supplementary Videos S1. (Repulsion time typically ranges between 1.200 to 2.000 s in wild type, untreated worms)
(6) Take readings for up to 20 worms per replicate. Calculate the average repulsion time per replicate.
Tips:
(1) Avoid the presence of food on the NGM surface. Worms can be shifted to the different areas using a poking lash.
(2) Do not use same poking lash for 1-nonanol and transferring worms (1-nonanol is sticky in nature; using the same lash will pre-expose the worms to 1-nonanol).
(3) Avoid touching the worms with the poking lash. Any worms touched accidentally should be disregarded from the study (touch will evoke a mechano-sensory response which will serve as a confounding factor as an additional response).
(4) Avoid too much 1-nonanol on the poking lash since it might get transferred onto the NGM surface. In addition, this might alter the worm behavior since pre-exposed worms are likely to exhibit enhanced response (Kimura et al., 2010).
(5) Repulsion behavior can be characterized as an avoidance behavior in response to 1-nonanol. Worms might exhibit a complete 180° reversal or 90° bend followed by movement away from the lash. While it is strongly recommended to keep the criteria same, both of these behavior qualify as repulsion.
(6) It is recommended to keep the magnification consistent throughout the experiment so as to keep the distance between the worms and lash uniform.
Note: Several positive and negative controls can be used for this assay. For positive control, Bupropion HCL and UA57 (cat-2/TH overexpression) can be used. Similarly, MT15620 (cat-2/TH mutant) can be used as a negative control (Sammi et al., 2018)
(1) Prepare NGM–Aldicarb plates by diluting 100 mM Aldicarb (prepared in ethanol) stock to 1:200, making the final concentration to 0.5 mM in NGM.
(2) Pour the molten NGM-Aldicarb in plates in 12 wells cell culture plates(3 ml per well) alternatively, 6 well (3 ml per well), 35 mm (3 ml), or 60 mm plates (10 ml) can be used.
(3) Avoid bubbles and be consistent with the volume of media being poured in all the plates. Bubbles can be removed using 200 µl tips.
(1) Day 2 (48 h post-treatment): Wash worms with M9 Buffer three to four times in 1.5 ml centrifuge tubes followed with centrifugation at 2,000 rpm for 3 minutes. The supernatant is to be discarded after every wash and fresh M9 buffers should be added to worm pellet.
(2) Transfer some worms (∼30) from the worm suspension (made by resuspending the worm pellet in 100 µl of M9 buffer) onto the NGM-Aldicarb plates. Too many worms on the plates can be diluted by adding some M9 buffer and then removing it.
(3) Let the buffer dry. The worms tend to clump together; it is better to separate them with a poking lash as the buffer dries.
(1) Score for the percentage of worms paralyzed by counting the number of paralyzed worms at regular intervals (say 30 min) using a stereo zoom microscope. It is recommended to consider the time point when approximately 50% of worms have been paralyzed in control.
(2) The worms can be prodded using a poking lash (usually prepared by sticking an eyelash to a 10 µl tip) to confirm for paralysis, as shown in the Supplementary Videos S2.
(3) Percentage of worms paralyzed can be calculated with respect to the total number of worms and compared with control. Typically, it is ideal to consider the time point when 50% of the worms are paralyzed in control. This will allow the identification of both positive and negative effects on neurotransmission.
Tips:
(1) Avoid bubbles when pouring the media since worms tend to burrow inside the agar.
(2) The final concentration of the Aldicarb can be standardized between 0.5 and 1 mM depending upon the speed of paralysis and sensitivity or resolution desired. Briefly an early paralysis (high aldicarb concentration) might overlook the minor differences, while delayed paralysis (Low aldicarb concentration) will allow identification of minor differences across the individual groups.
(3) It is better to use freshly made plates. Typically plates can be used for a month when stored at 4°C. Using two different batches of Aldicarb-NGM plates (prepared on different days) for a replicate might vary the time points specific to paralysis and hence is not recommended.
(4) Plates can be stored at 4°C.
(5) Keep the criteria uniform for prodding the worms (for instance, if a worm does not move after prodding three times, it should be considered as paralyzed).
(6) On the day of the experiment, keep the plates covered after the worm suspension has dried. Excessive drying leads to formation of gaps between the media and walls of the plate. This is quite common for plates with a small diameter.
(7) Paralysis is visible in body muscles first, and worms might still show head movements. The criteria to consider a worm paralyzed (paralysis in body muscles Vs complete paralysis) should be same across all groups.
(8) Any worms lost should be disregarded from the study. Instead, it is better to recount the total number of worms at the end of the experiment (Some worms disappear during the experiment due to the tendency to burrow into agar).
(9) Avoid excessive drying or damaging the agar surface.
Note: Several positive and negative controls can be used for this assay. AchE inhibitors such as donepezil, galantamine can be used as a positive control, while a fair number of genetic mutations conferring resistance to aldicarb can be used as negative control (Table 1).
(1) Prepare fresh 1M stocks of levamisole in M9 buffer. Dilute the Levamisole stock to 2x working concentration (final concentration may range from 25 to 200 µM) using M9 buffer (day 2). We have used a 100 and 400 µM Levamisole concentration (2X). This concentration can be varied to suit the time gap required between readings. For example, a lower concentration can be used if a longer time gap between subsequent readings is desired. Furthermore, a lower concentration increases the resolution of the assay, warranting identification of minor differences between the groups. A very high concentration [as used to depict the false positive (4 mM)] will jeopardize the purpose of assay.
(2) Day 2 (48 h post-treatment): Wash worms with M9 Buffer three to four times in 1.5 ml centrifuge tubes followed with centrifugation at 2,000 rpm for 3 minutes. The supernatant is to be discarded after every wash and fresh M9 buffers should be added to worm pellet.
(3) Add 20 µl of worm suspension (made by resuspending worm pellet in 100 µl of M9 buffer) containing 20 to 30 worms in each well of 96 well plates.
(4) Gently vortex the plate to spread the worm suspension evenly in each well. Alternatively, this can also be achieved by gentle tapping the plate along the X-Y axis.
(5) Add an equal volume of 2x working stocks of levamisole and mix immediately by vortexing or tapping.
(1) Score for the percentage of worms paralyzed by counting the number of paralyzed worms at regular intervals (say 5–10 min) using a stereo zoom microscope. A lower concentration of levamisole can be used to get longer time windows for counting the paralyzed worms.
(2) Percentage of worms paralyzed can be calculated with respect to the total number of worms and compared with the control. Typically, it is ideal to consider the time point when 50% of the worms are paralyzed in control. This allows identification of both positive and negative modulation of nAchR activity.
Tips:
(1) The final concentration of levamisole can be standardized between 25 and 200 µM or even higher depending upon the speed of paralysis.
(2) Avoid excess volumes in the wells since it will make scoring difficult due to the planar difference.
Note: Several positive and negative controls can be used for this assay. AchE inhibitors such as donepezil should also serve as a positive control, while a fair number of genetic mutations conferring resistance to levamisole can be used as negative control (Table 2)
(1) Day 3 (72 h post-treatment): Wash worms with M9 Buffer three to four times in 1.5 ml centrifuge tubes followed with centrifugation at 2,000 rpm for 3 minutes. The supernatant is to be discarded after every wash and fresh M9 buffers should be added to worm pellet.
(2) Anesthetize worms by adding 10 µl of 100 mM Sodium azide to 100 µl of worm suspension.
(3) Mount the worms onto the slides and seal using transparent nail paint.
(4) Visualize worms under the FITC filter (Excitation/Emission: 485/520 nm).
(1) Given that cholinergic neurons are approximate 120 in number (Rand, 2007) and have a dense neural network, it is best to adopt a straightforward approach by scoring the worms on the basis of neuronal loss in the head region with scoring worms as “with” or “without” neuronal damage (Sammi et al., 2019). Researchers have also adopted similar approaches for scoring dopaminergic neurodegeneration (Chikka et al., 2016). A relatively easy practice focuses on head neurons and nerve rings as described previously (Sammi et al., 2019).
(2) Any neurons with broken dendrites, damaged/missing cell bodies are scored as damaged. Worms with apparent neuronal damage are scored as affected, and the percentage of worms lacking damage is calculated. A minimum of 20 worms per replicate should be scored.
(1) Day 3 (72 h post-treatment): Wash worms with M9 Buffer three to four times in 1.5 ml centrifuge tubes followed with centrifugation at 2,000 rpm for 3 minutes. The supernatant is to be discarded after every wash and fresh M9 buffers should be added to worm pellet.
(2) Anesthetize worms by adding 10 µl of 100 mM Sodium azide to 100 µl of worm suspension.
(3) Mount the worms onto the slides and seal using transparent nail paint.
(4) Visualize worms under the FITC filter (Excitation/Emission: 485/520 nm).
(1) Given that dopaminergic neurons are less (eight) in number, it is relatively easy to count all the neurons and assess the effect on individual neuronal subpopulations. Hence all the neurons can be assessed for neurodegeneration and scored. It is by far an adequately detailed approach (Sammi et al., 2018) compared to the other strategies, summarized in Table 3. Any neurons with broken dendrites, damaged/missing cell bodies are scored as damaged. Alternatively, in an approach similar to that of cholinergic neurons, worms with apparent neuronal damage can be scored as affected, and the percentage of worms lacking damage is calculated. A minimum of 20 worms per replicate should be scored. Extent of neurodegeneration can be represented as percentage of intact neurons. While there is no specific reason to represent neuronal damage as percentage of intact neurons; we found this as one of the most common way of presenting neuronal damage (Yao et al., 2010; Settivari et al., 2013; Ray et al., 2014). Alternatively, data can also be shown as percentage of neuronal loss.
Repeat each experiment a minimum of three times. Then, calculate analysis of variance (ANOVA) using Dunnett’s post hoc test (to compare with control) or Sidak’s post hoc test (for two-way ANOVA). Each experiment was conducted with a minimum of three independent replicates, comprising of a minimum of 20 worms. For behavioral assays, any worms showing movement defects prior to the assay were disregarded from the study.
Assessment of effect on dopamine associated behavior was conducted through 1-nonanol assay. Repulsive movement in response to 1-nonanol is associated with dopamine levels, where a higher repulsion time relates to decrease dopamine levels and vice versa. Repulsion time was normalized with respect to control. In comparison to that of CPF 0 µM (1.000 ± 0.000), a significant increase in repulsion time was observed in worms treated with CPF 1 µM (1.613 ± 0.089, p = 0.0156), CPF 2.5 µM (1.980 ± 0.071, p = 0.0004), CPF 5 µM (2.224 ± 0.113, p < 0.0001), CPF 10 µM (2.992 ± 0.090, p < 0.0001) and CPF 25 µM (3.268 ± 0.235, p < 0.0001) (Figure 3A). As noted earlier prolonged repulsion time relates to lower dopamine levels (Baidya et al., 2014; Sammi et al., 2018), thus identifying the negative effects of CPF on dopamine levels.
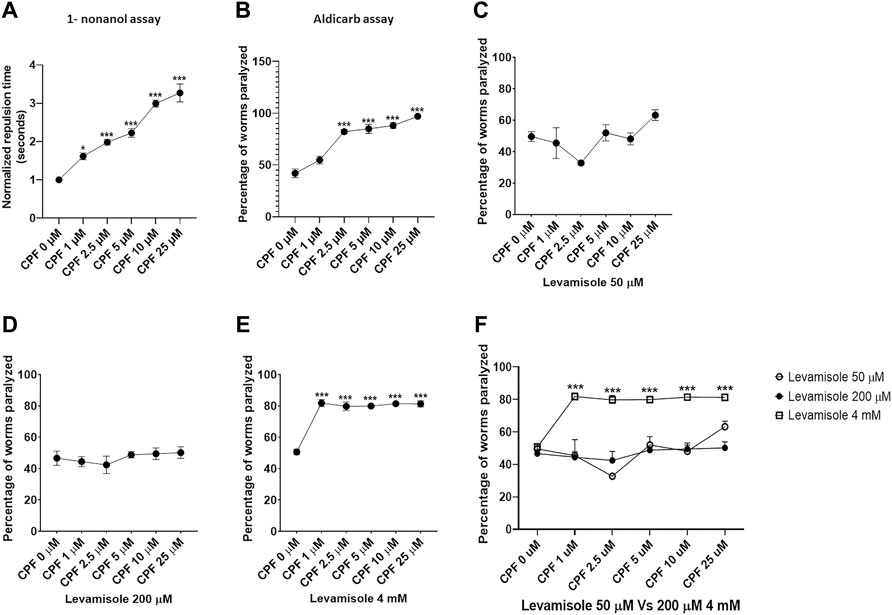
FIGURE 3. Behavior assays for evaluation of dopamine levels, Acetylcholine levels and nicotinic acetylcholine receptor activity. (A) 1-nonanol assay: CPF treatment exhibited increased repulsion time in a dose-dependent manner which corresponds to the lowered levels of dopamine. (B) Aldicarb assay: CPF treatment led to an increase in the percentage of worms paralyzed, indicating augmented levels of acetylcholine in a concentration-dependent manner. (C) Levamisole assay: CPF treatment exhibited no effect on nAchR activity at Levamisole concentration 50 µM. (D) Levamisole assay: CPF treatment exhibited no effect on nAchR activity at Levamisole concentration 200 µM. (E) Levamisole assay: CPF treatment exhibited significant increase in nAchR activity at above optimum Levamisole concentration 4 mM resulting in false positive results due to saturation effect. (F) Comparison of the effect of Levamisole across three different concentrations All experiments were conducted in three independent replicates. For 1-nonanol assay a minimum of 20 worms were analyzed per replicate, whereas 20 to 30 worms were analyzed for Aldicarb assay and Levamisole assay. Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. *p < 0.05, **p < 0.005, and ***p < 0.001 (n = 3).
Assessment of acetylcholine-associated behavior was conducted through a pair of two assays, Aldicarb assay, and Levamisole assay. The former assay determines the effect on cholinergic transmission and is based on aldicarb-induced inhibition of acetylcholinesterase. AchE inhibition results in a buildup of acetylcholine, causing flexion of muscles, evident as paralysis. The latter assay uses levamisole, which is a nAchR agonist. Overstimulation of nAchR causes spastic paralysis in worms. Therefore, higher levels of Ach (Mahoney et al., 2006) or augmented nAchR (Qian et al., 2008) activity are expected to increase the number of worms paralyzed.
Aldicarb assay is an indirect method to measure relative cholinergic transmission. We studied the effect of CPF on cholinergic transmission through aldicarb assay. A higher percentage of paralyzed worms at a given point of time indicates increased Ach levels and vice versa. A significant increase in percentage of paralyzed worms was observed in worms treated with CPF 2.5 µM (82.143 ± 2.062, p < 0.0001), CPF 5 µM (84.899 ± 4.392, p < 0.0001), CPF 10 µM (88.072 ± 2.896, p < 0.0001) and CPF 25 µM (96.940 ± 0.394, p < 0.0001) in comparison to that of CPF 0 µM (41.910 ± 4.088) (Figure 3B). The results indicated increased cholinergic transmission.
After ascertaining increased effect of CPF on aldicarb assay, we studied the effect of CPF on nAchR using levamisole assay. In order to ascertain the effect of levamisole concentration on outcome of the assay we ran the assay on three different concentrations of levamisole (two optimum and one 20 time times above the upper limit). At 50 μM and 200 µM levamisole concentration CPF did not show any effect on nAchR activity (Figures 3C,D). However at 4 mM Levamisole concentration, we did observe an increase in percentage of worms paralyzed. In comparison to control (50.667 ± 1.824), a significant increase in percentage of worms paralyzed at doses CPF 1 µM (81.782 ± 2.213, p < 0.0001), CPF 2.5 µM (79.733 ± 2.719, p < 0.0001), CPF 5 µM (79.911 ± 0.679, p < 0.0001), CPF 10 µM (81.421 ± 0.598, p < 0.0001) and CPF 25 µM (81.288 ± 1.998, p < 0.0001) was observed (Figure 3E). These findings show that CPF does not alter nAchR activity. Comparison of assay for optimum levamisole concentration Vs false positive (4 mM) has been shown in Figure 3F.
Evaluation of the effect on cholinergic neurons was done using reporter strain for cholinergic neurons (LX929). C. elegans has approximately 120 cholinergic neurons (Rand, 2007), and hence it is quite tedious to score individual neurons and relate them to neuronal damage. A straightforward approach instead is to identify the worms with neuronal damage and score them as affected (Sammi et al., 2018). Given that CPF, an organophosphate, causes developmental delay, the effect on cholinergic neurons was assessed with treatment at two different stages, L1 and L4.
Any worm exhibiting damaged neurons (broken dendrites or loss of neurons) was marked as affected, and the percentage of worms involved was calculated.
In case of worms treated at L1 stage (Figures 4A–C), a significant decrease in percentage of worms lacking damaged neurons at doses, CPF 50 µM (61.667 ± 4.410, p = 0.0001), CPF 100 µM (45.000 ± 5.000, p < 0.0001), CPF 250 µM (38.333 ± 3.333, p < 0.0001) and CPF 500 µM (36.667 ± 6.009, p < 0.0001) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 4G).
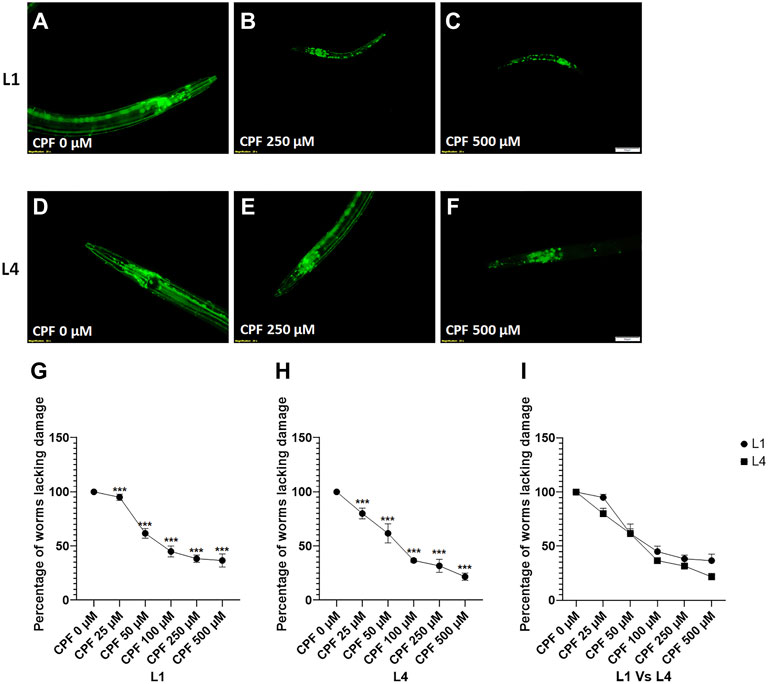
FIGURE 4. Cholinergic neurodegeneration and its assessment: (A–C) LX929 worms at L1 stage exposed to different concentrations of CPF (0–500 µM). (D–F) LX929 worms at L4 stage were exposed to different concentrations of CPF (0–500 µM). (G–I) Worms exhibiting neuronal damage, indicated by loss of neuron or dendrite breaks were marked as affected and graphs were plotted for the percentage of worms lacking neuronal damage Vs concentration for worms treated at L1 (G) and L4 (H) stages. (I) a comparison between the effects on neuronal damage for worms treated at L1 and L4 stages. A minimum of 20 worms were analyzed per replicate. All experiments were conducted in three independent replicates Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. *p < 0.05, **p < 0.005, and ***p < 0.001 (n = 3). Scale bar represents 50 µm. Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. *p < 0.05, **p < 0.005, and ***p < 0.001 (n = 3).
In case of worms treated at L4 stage (Figures 4D–F), a significant decrease in percentage of worms lacking damaged neurons at doses, CPF 50 µM (61.667 ± 8.819, p = 0.0007), CPF 100 µM (36.667 ± 1.667, p < 0.0001), CPF 250 µM (31.667 ± 6.009, p < 0.0001) and CPF 500 µM (21.667 ± 3.333, p < 0.0001) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 4H). The damage to cholinergic neurons for the worms treated at L1 and L4 stage was statistically insignificant (Figure 4I).
C. elegans hermaphrodites have eight dopaminergic neurons, comprising of 3 types of neuronal subpopulations: 4 cephalic sensilla (CEP), two anterior deirid (ADE), and two posterior deirid (PDE) (Sulston et al., 1975; Sammi et al., 2018). This makes it easier to score in comparison to other neuronal populations, which are large in number. Hence, keeping this in mind, we demonstrate both the methods, one that scores on the basis percentage of worms lacking damage (similar to cholinergic neurons) and the second based on the percentage of intact neurons with respect to individual subpopulations (Sammi et al., 2018; Sammi et al., 2019). Given that PDE neurons are late-born neurons (Wicks and Rankin, 1996), this assessment method also indicates development delay of molting arrest, if any.
In case of worms treated at L1 stage (Figures 5A–C), a significant decrease in percentage of worms lacking damaged neurons at doses, CPF 50 µM (13.333 ± 6.009, p < 0.0001), CPF 100 µM (0.000 ± 0.000, p < 0.0001), CPF 250 µM (0.000 ± 0.000, p < 0.0001) and CPF 500 µM (0.000 ± 0.000, p < 0.0001) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 5G).
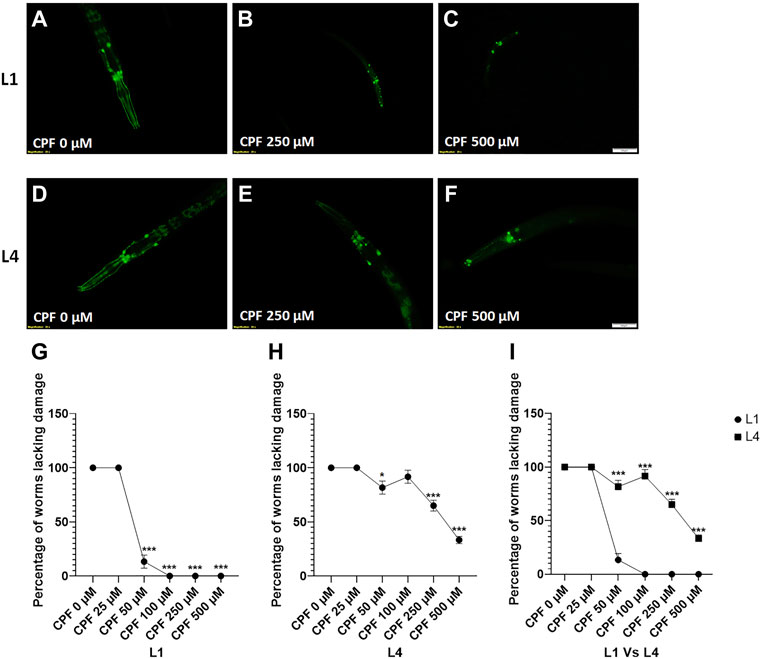
FIGURE 5. Dopaminergic neurodegeneration and its assessment: (A–C) BZ555 worms at L1 stage exposed to different concentrations of CPF (0–500 µM). (D–F) BZ555 worms at L4 stage exposed to different concentrations of CPF (0–500 µM). (G–I) Worms exhibiting neuronal damage, indicated by loss of neuron or dendrite breaks were marked as affected and graphs were plotted for the percentage of worms lacking neuronal damage Vs concentration for worms treated at L1 (G) and L4 (H) stages. (I) a comparison between the effects on neuronal damage for worms treated at L1 and L4 stages. A minimum of 20 worms were analyzed per replicate. All experiments were conducted in three independent replicates Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. *p < 0.05, **p < 0.005, and ***p < 0.001 (n = 3). Scale bar represents 50 µm. Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test (A to H) and two-way ANOVA followed by Sidak’s test for I. *p < 0.05, **p < 0.005, and ***p < 0.001 (n = 3).
In case of worms treated at L4 stage (Figures 5D–F), a significant decrease in percentage of worms lacking damaged neurons at doses, CPF 50 µM (13.33 ± 6.009, p = 0.0384), CPF 250 µM (65.000 ± 5.000, p = 0.0004) and CPF 500 µM (33.333 ± 3.333, p < 0.0001) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 4G). These results showed a significant difference in extent of neurodegeneration which was dependent on the molting stage (Figure 5I)
Next, we scored the neurodegeneration on the basis of neuronal subpopulations. In case of worm treated at L1 stage, a significant decrease in percentage of intact neurons was observed for total neurons at doses CPF 50 µM (70.000 ± 6.683, p = 0.0432), CPF 100 µM (54.375 ± 6.505, p = 0.0031), CPF 250 µM (39.792 ± 2.114, p = 0.0003) and CPF 500 µM (48.750 ± 3.750, p = 0.0030) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 6A). For CEP neurons, a significant decrease in percentage of intact neurons was observed at doses CPF 250 µM (69.167 ± 3.975, p = 0.0028) and CPF 500 µM (69.167 ± 6.548, p = 0.0028) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 6B). For ADE neurons, a significant decrease in percentage of intact neurons was observed at doses CPF 100 µM (61.667 ± 12.611, p = 0.0069), CPF 250 µM (33.333 ± 10.240, p < 0.0001), and CPF 500 µM (46.667 ± 0.833, p = 0.0005) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 6C). For PDE neurons, a significant decrease in percentage of intact neurons was observed at doses CPF 50 µM (26.667 ± 7.949, p < 0.0001), CPF 100 µM (0.000 ± 0.000, p < 0.0001), CPF 250 µM (0.833 ± 0.833, p < 0.0001), and CPF 500 µM (0.000 ± 0.000, p < 0.0001) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 6D)
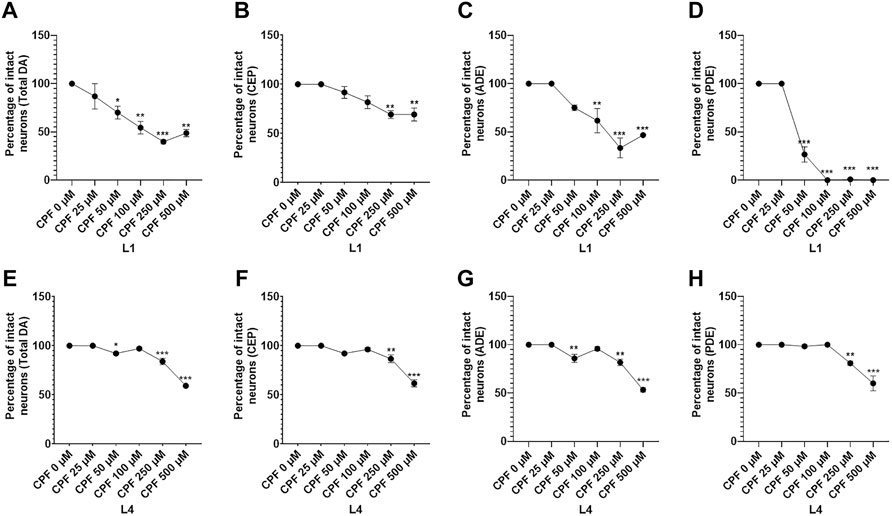
FIGURE 6. Detailed evaluation of dopaminergic cell death with respect to neuron subtypes: (A–D) Graphical representation of the dopaminergic cell death with respect to neuronal subtype for total (A), CEP (B), ADE (C), and PDE (D) for worms treated at the L1 stage. (E,F) Graphical representation of the dopaminergic cell death with respect to neuronal subtype for total (E), CEP (F), ADE (G), and PDE (H) for worms treated at L4 stage. All experiments were conducted in three independent replicates Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. *p < 0.05, **p < 0.005, and ***p < 0.001 (n = 3). Data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. *p < 0.05, **p < 0.005, and ***p < 0.001 (n = 3).
In case of worm treated at L4 stage, a significant decrease in percentage of intact neurons was observed for total neurons at doses CPF 50 µM (92.083 ± 1.267, p = 0.0145), CPF 250 µM (83.958 ± 3.234, p < 0.0001) and CPF 500 µM (59.167 ± 0.417, p < 0.0001) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 6E). For CEP neurons, a significant decrease in percentage of intact neurons was observed at doses CPF 250 µM (86.667 ± 3.975, p = 0.0076) and CPF 500 µM (61.667 ± 3.703, p < 0.0001) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 6F). For ADE neurons, a significant decrease in percentage of intact neurons was observed at doses CPF 50 µM (85.833 ± 4.167, p = 0.0076), CPF 250 µM (81.667 ± 3.333, p = 0.0011), and CPF 500 µM (53.333 ± 2.205, p < 0.0001) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 6G). For PDE neurons, a significant decrease in percentage of intact neurons was observed at doses CPF 250 µM (80.833 ± 2.205, p = 0.0062), and CPF 500 µM (60.000 ± 7.638, p < 0.0001) in comparison to that of CPF 0 µM (100 ± 0.000) (Figure 6H). The above results indicated neurotoxic effects of CPF on dopaminergic neurons while also highlighting that the effects were more severe when worms were exposed at early stages and also that an alternative approach to assessment of neurodegeneration should be adopted if the toxicant in consideration poses developmental defects.
In this article, we list a combination of methodologies as an alternative to mammalian testing with respect to neurological hazard assessment for industrial and pesticides. C. elegans is a transparent, simple model organism that shares considerable homology in terms of neurotransmitters and mechanisms. In addition, ease of culture, short life span, and availability of reporter strains and mutants offer additional advantages in conducting time and cost-efficient research for safety assessment and delving into the underlying mechanisms. These critical mechanistic endpoints are crucial to define or discern AOPs. For the purpose of this study, CPF was utilized, given widespread agricultural use, being one of the most used pesticides (Ozkan et al., 2014) and linked to multiple neurological diseases (Pallotta et al., 2017; Voorhees et al., 2019; Miller et al., 2021). Environmental pollutants, including pesticides and heavy metals, have gained considerable attention as risk factors for neurodegenerative disorders (Chin-Chan et al., 2015). With an enlarged focus on the safety assessment of toxicants, a need has been constantly felt to identify new approaches or methodologies for assessing pesticides as risk factors. While higher model systems such as rodents share immense similarities with humans, their large-scale use involves several time and cost constraint factors. While in vitro models often an alternative, they are unable to replicate the features of an in vivo model. C. elegans serves as an intermediate model with shared features of both systems. In this direction we have demonstrated three behavioral assays for the assessment of dopaminergic and cholinergic function and two assays for the ascertaining neurodegeneration in cholinergic and dopaminergic neurons. While the main focus of this article is on neurotoxicological assessment, C. elegans is also an established model to study toxicity (Hunt, 2017; Xiong et al., 2017; Gao et al., 2018) which can also be compared with neurotoxicological findings. The above assays rely on conserved nature of biochemical pathways, genes and neurotransmitters. A limitation to C. elegans is that C. elegans neural network is not the exact representation of the mammalian nervous system, which is relatively more complex in nature (Alexander et al., 2014).
Assessment of DA and Ach-based behavior has been demonstrated through behavioral assays. The first assay, the 1-nonanol assay, is indirectly used to ascertain dopamine levels. 1-nonanol elicits a chemo-repulsive behavior in worms, where nematodes with lower levels of dopamine exhibit delay in exhibiting repulsive behavior and vice versa (Smita et al., 2017; Sammi et al., 2018). A similar behavior has also been shown by other chemicals such as 1-octanol and 2-nonanone (Bargmann et al., 1993). In response to CPF, worms showed a dose-dependent delay in repulsion time, indicating mitigated dopamine levels. The 1-nonanol assay can serve as an indirect method for quantification of dopamine levels in worms. We have previously validated this assay by employing various positive (DAT inhibitor and nematodes overexpressing cat-2/tyrosine hydroxylase) and negative controls (nematodes with a mutation in cat-2/tyrosine hydroxylase gene) (Sammi et al., 2018). These controls can be utilized to validate or compare the results. Similar findings have also been reported with other chemicals such as 1-octanol where cat-2 mutants have shown to exhibit a longer repulsion time (Baidya et al., 2014). These positive and negative controls can be utilized to validate the assay and also to draw a comparison with the neurotoxicants in question. Post hoc tests such as Tukey’s should be employed to determine intergroup variance.
We demonstrated assays for Ach-based behavior through a combination of two assays, Aldicarb and Levamisole assay. Aldicarb is an AchE inhibitor, while levamisole is a nAchR agonist; these assays can determine the effect on neurotransmission and nAchR activity, respectively (Mahoney et al., 2006). Aldicarb inhibits AchE leading to accumulation of Ach, causing flexion of muscles, which can be scored as paralyzed worms (Mahoney et al., 2006), while levamisole exhibits paralysis through nAchR (Qian et al., 2008). A higher percentage of paralyzed worms in Aldicarb and Levamisole assay indicate elevated Ach and nAchR activity levels, respectively (Sammi et al., 2017). CPF being an AchE inhibitor, exhibited a dose-dependent increase in the percentage of worms paralyzed emanating from accumulation of Ach. A condition where a pesticide or toxicant exhibits deleterious effect on Ach neurons, Ach levels, and nAchR, a decreased paralysis is expected. To test the second aspect of cholinergic transmission, being nAchR activity, Levamisole assay was performed at three different concentrations. The first two concentrations, 50 μM and 200 µM are the optimum concentrations for Levamisole assay and did not exhibit any effect, implying that CPF did not alter nAchR response. While there was no effect on nAchR activity, a close look at the 50 μM Vs 200 µM showed that at lower concentration, (although insignificant) there was some difference between groups, which got smoothened as at higher Levamisole concentration. Paradoxically, at a very high concentration (20 times of upper range) the results turned to be false positive, because of saturation due to excess levamisole. These results although statistically significant were biologically insignificant, since higher concentration of levamisole led to saturation and any effect observed was only due to accumulation of Ach.
These behavioral assays can also employ various mutants as negative controls listed in Tables 1, 2 for aldicarb and levamisole assay, respectively. Furthermore, these listed controls can also be employed to determine the mechanism of neurotoxicants. As an alternative approach RNAi specific to key genes can also be performed to discern the mechanism. In addition, an intergroup comparison can also be made to determine the relative alteration with respect to the negative or positive controls. The concentration of both levamisole and Aldicarb can be altered to increase or decrease the sensitivity/resolution of assay. Although a lower concentration of aldicarb/levamisole will increase the time required for paralysis, but it will also enhance the sensitivity to minor differences across the groups.
Next, we demonstrate the assessment of neurodegeneration for both Ach and DA neurons. Various groups have adopted diverse approaches for scoring neurons as damaged as listed in Table 3. Nematode cholinergic neurons are 120 in number, and hence it is tedious to assess every single neuron. Strains expressing GFP under the control of unc-17 promoter have been used previously to identify the degeneration in cholinergic neurons (Benedetto et al., 2010; Sammi et al., 2018; Sammi et al., 2019). Adopting a straightforward approach seems more feasible where a worm with one or more damaged neurons is scored as affected, and the percentage of worms lacking neuronal damage is calculated. While various researchers have used different approaches to assess neurodegeneration (explained later in discussion), we suggest adopting a simple approach where a neuron is scored as damaged upon loss/breakage in dendrites or cell body. Similar techniques have also been used in other studies previously for DA neurons (Chikka et al., 2016). Nematodes have four molting stages (Lazetic and Fay, 2017), which could be susceptible to arrest when exposed to toxicants or pesticides. Hence, we recommend that studies be conducted with exposure at both L1 and L4 stages for detailed assessment of neurodegeneration. This is particularly important for two reasons, one to determine if the toxicant exerts developmental delay/defect and the second to determine if early or late-stage neurons vary in terms of relative vulnerability. We do not observe any significant difference in the case of Ach neurons The anomaly can be justified by two reasons, one being that the number of cholinergic neurons is significantly larger than the dopaminergic neurons and second that the correlation between neuron subtype and the developmental stage was not accounted for. Although this poses a limitation, but it seems a more practical and straightforward approach where large number of neurons is concerned.
In case of dopaminergic neurodegeneration studies, we observed a significant difference in percentage of intact neurons between worms treated at L1 stage Vs L4 stage. This was partly due to the fact that PDE neurons are late-born neurons (Wicks and Rankin, 1996) and fail to appear in case of molting arrest and that ADE neurons exhibited increased vulnerability when worms were exposed at a L1 stage. Dopaminergic neurons being small in number also offer an added advantage to study the sub-populations easily. Not only do our results indicate a fair difference in the vulnerability of neuronal subpopulations, but they also highlight that exposure to the early neurons is more likely to exhibit damage than matured neurons. This is certainly important from the translational viewpoint since it can be associated with an increased risk of fetus or newborns to neurotoxicants. We also compared the methodologies, summarized in Table 3, followed by other research groups for the assessment of DA degeneration, and found that our approach is more detailed and robust. While some researchers have used relatively more straightforward approaches (Settivari et al., 2013; Ray et al., 2014; Chikka et al., 2016), some have delved into the distinct morphological patterns, such as branching, wavy patterns, and breakage in neurons (Wu et al., 2015; Bijwadia et al., 2021). A similar, more detailed approach has been used by Shefali et al., 2021, where they have used confocal microscopy and designed a seven-point scale for assessment of dendrite morphology in great detail (Bijwadia et al., 2021).
Overall the findings from these five assays were in agreement with the previous findings. CPF is a known AchE inhibitor (Yen et al., 2011; Reiss et al., 2012); no effect of CPF has been reported on nAchR activity in C. elegans. Additionally, CPF has also been shown to exhibit cholinergic neurodegeneration in SN56 basal forebrain cholinergic neurons (del Pino et al., 2015) and alter cholinergic neurochemistry in developing rats. Similarly, CPF has been shown to exhibit detrimental effects on dopaminergic cells in vitro (Lou and Li, 2016) and in young adult rats (Zhang et al., 2015).
Our results were in consensus with the findings from available literature, validating the assays to be used as new approach methodologies for replacing mammalian testing in assessment of neurological hazard. In summary, we believe that C. elegans as a model system is a candidate for new approach methodologies with significant potential to substitute mammalian testing in neurological hazard assessment. Given the characteristic features of both in vitro and higher model organisms, C. elegans presents as a cost and time-effective in vivo model. Using the AOP framework, significant perturbations from normal biological functions can potentially be applied to conserved systems in target organisms outside of C. elegans. The KEs relating to Ach activity (AOP-Wiki) and nicotinic Ach receptor activation (AOP-Wiki) demonstrate existing potential applications of the levamisole and aldicarb assays for chemical testing. With further developments, the AOP Wiki may expand to applications dealing with the endpoints observed using the 1-nonanol assay and morphological assessments of applicable neurons in C. elegans. In addition to testing of neuro-toxicants, the same assays can also be utilized for testing lead molecules for their positive effect on neurotransmission as well as neuroprotection.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health (R01ES025750 to JC; K99ES032488 to SRS) and the Ralph W. and Grace M. Showalter Research Trust (to JC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Caenorhabditis elegans and bacterial strains employed in this study were provided by the C. elegans Genetics Center, University of Minnesota, MN, United States, funded by the NIH National Center for Research Resources (NCRR).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftox.2022.826488/full#supplementary-material
Supplementary Video S1 | Wild type C. elegans age synchronized from L1 and cultured in complete M9 with OP50 for 48 h in liquid media exposed to 1% DMSO in M9 for 10 min on NGM agar plate before recording begins. Lash dipped in 1-nonanol between each worm. Magnification is 20X.
Supplementary Video S2 | Adult wild type C. elegans placed on 0.5 mM aldicarb NGM plate shown at 3 states: 1) not paralyzed 2) complete paralysis, defined by no response to 3 head touches with poking lash, 3) partial paralysis, defined by difficulty in movement, passes head touch test. Nematodes were grown on NGM agar with OP50. Magnification is 20X.
Ach, acetylcholine; AchE, Acetylcholinesterase; DA, Dopamine; nAchR, nicotinic acetylcholine receptor; AOP, Adverse outcome pathway; NAM, New approach methodology.
Albuquerque, E. X., Pereira, E. F. R., Castro, N. G., Alkondon, M., Reinhardt, S., Schröder, H., et al. (1995). Nicotinic Receptor Function in the Mammalian Central Nervous Systema. Ann. N. Y Acad. Sci. 757, 48–72. doi:10.1111/j.1749-6632.1995.tb17464.x
Alexander, A. G., Marfil, V., and Li, C. (2014). Use of Caenorhabditis elegans as a Model to Study Alzheimerâ€s Disease and Other Neurodegenerative Diseases. Front. Genet. 5, 279. doi:10.3389/fgene.2014.00279
Alfonso, A., Grundahl, K., Duerr, J. S., Han, H.-P., and Rand, J. B. (1993). The Caenorhabditis elegans Unc-17 Gene: a Putative Vesicular Acetylcholine Transporter. Science 261, 617–619. doi:10.1126/science.8342028
Alfonso, A., Grundahl, K., Mcmanus, J., and Rand, J. (1994). Cloning and Characterization of the Choline Acetyltransferase Structural Gene (Cha-1) from C. elegans. J. Neurosci. 14, 2290–2300. doi:10.1523/jneurosci.14-04-02290.1994
Ali, S. J., Ellur, G., Patel, K., and Sharan, K. (2019). Chlorpyrifos Exposure Induces Parkinsonian Symptoms and Associated Bone Loss in Adult Swiss Albino Mice. Neurotox Res. 36, 700–711. doi:10.1007/s12640-019-00092-0
AOP Wiki (2021a) Aop-Wiki Key Event 10 “Acetylcholine Accumulation in Synapses” [Online]. Available at: https://aopwiki.org/events/10 (Accessed 11 07, 2021).
AOP Wiki (2021b) Aop-Wiki Key Event 559 “Activation, Nicotinic Acetylcholine Receptor” [Online]. Available at: https://aopwiki.org/events/559 (Accessed 11 07, 2021).
Baidya, M., Genovez, M., Torres, M., and Chao, M. Y. (2014). Dopamine Modulation of Avoidance Behavior in Caenorhabditis elegans Requires the NMDA Receptor NMR-1. PLoS One 9, e102958. doi:10.1371/journal.pone.0102958
Bargmann, C. I., Hartwieg, E., and Horvitz, H. R. (1993). Odorant-selective Genes and Neurons Mediate Olfaction in C. elegans. Cell 74, 515–527. doi:10.1016/0092-8674(93)80053-h
Bastiani, C. A., Gharib, S., Simon, M. I., and Sternberg, P. W. (2003). Caenorhabditis elegans Gαq Regulates Egg-Laying Behavior via a PLCβ-independent and Serotonin-dependent Signaling Pathway and Likely Functions Both in the Nervous System and in Muscle. Genetics 165, 1805–1822. doi:10.1093/genetics/165.4.1805
Benedetto, A., Au, C., Avila, D. S., Milatovic, D., and Aschner, M. (2010). Extracellular Dopamine Potentiates Mn-Induced Oxidative Stress, Lifespan Reduction, and Dopaminergic Neurodegeneration in a BLI-3-dependent Manner in Caenorhabditis elegans. PLoS Genet. 6, e1001084. doi:10.1371/journal.pgen.1001084
Bijwadia, S. R., Morton, K., and Meyer, J. N. (2021). Quantifying Levels of Dopaminergic Neuron Morphological Alteration and Degeneration in Caenorhabditis elegans. JoVE 177, e62894. doi:10.3791/62894
Boyd, W. A., Smith, M. V., and Freedman, J. H. (2012). Caenorhabditis elegans as a Model in Developmental Toxicology. Methods Mol. Biol. 889, 15–24. doi:10.1007/978-1-61779-867-2_3
Cacabelos, R. (2017). Parkinson's Disease: From Pathogenesis to Pharmacogenomics. Int. J. Mol. Sci. 18, 551. doi:10.3390/ijms18030551
Chikka, M. R., Anbalagan, C., Dvorak, K., Dombeck, K., and Prahlad, V. (2016). The Mitochondria-Regulated Immune Pathway Activated in the C. elegans Intestine Is Neuroprotective. Cell Rep. 16, 2399–2414. doi:10.1016/j.celrep.2016.07.077
Chin-Chan, M., Navarro-Yepes, J., and Quintanilla-Vega, B. (2015). Environmental Pollutants as Risk Factors for Neurodegenerative Disorders: Alzheimer and Parkinson Diseases. Front. Cel. Neurosci. 9, 124. doi:10.3389/fncel.2015.00124
Culetto, E., Baylis, H. A., Richmond, J. E., Jones, A. K., Fleming, J. T., Squire, M. D., et al. (2004). The Caenorhabditis elegans Unc-63 Gene Encodes a Levamisole-Sensitive Nicotinic Acetylcholine Receptor α Subunit. J. Biol. Chem. 279, 42476–42483. doi:10.1074/jbc.m404370200
Dani, J. A., and Bertrand, D. (2007). Nicotinic Acetylcholine Receptors and Nicotinic Cholinergic Mechanisms of the central Nervous System. Annu. Rev. Pharmacol. Toxicol. 47, 699–729. doi:10.1146/annurev.pharmtox.47.120505.105214
Doi, M., and Iwasaki, K. (2002). Regulation of Retrograde Signaling at Neuromuscular Junctions by the Novel C2 Domain Protein AEX-1. Neuron 33, 249–259. doi:10.1016/s0896-6273(01)00587-6
Eglen, R. M. (2005). Muscarinic Receptor Subtype Pharmacology and Physiology. Prog. Med. Chem. 43, 105–136. doi:10.1016/s0079-6468(05)43004-0
Fatima, S., Haque, R., Jadiya, P., Farooq, S., Kumar, L., and Nazir, A. (2014). Ida-1, the Caenorhabditis elegans Orthologue of Mammalian Diabetes Autoantigen IA-2, Potentially Acts as a Common Modulator between Parkinson's Disease and Diabetes: Role of Daf-2/Daf-16 Insulin like Signalling Pathway. PLoS One 9, e113986. doi:10.1371/journal.pone.0113986
Fitzgerald, J., Kennedy, D., Viseshakul, N., Cohen, B. N., Mattick, J., Bateman, J. F., et al. (2000). UNCL, the Mammalian Homologue of UNC-50, Is an Inner Nuclear Membrane RNA-Binding protein11Published on the World Wide Web on 10 August 2000. Brain Res. 877, 110–123. doi:10.1016/s0006-8993(00)02692-5
Fleming, J. T., Squire, M. D., Barnes, T. M., Tornoe, C., Matsuda, K., Ahnn, J., et al. (1997). Caenorhabditis elegansLevamisole Resistance Geneslev-1,unc-29, andunc-38Encode Functional Nicotinic Acetylcholine Receptor Subunits. J. Neurosci. 17, 5843–5857. doi:10.1523/jneurosci.17-15-05843.1997
Francis, P. T., Palmer, A. M., Snape, M., and Wilcock, G. K. (1999). The Cholinergic Hypothesis of Alzheimer's Disease: a Review of Progress. J. Neurol. Neurosurg. Psychiatry 66, 137–147. doi:10.1136/jnnp.66.2.137
Gao, S., Chen, W., Zeng, Y., Jing, H., Zhang, N., Flavel, M., et al. (2018). Classification and Prediction of Toxicity of Chemicals Using an Automated Phenotypic Profiling of Caenorhabditis elegans. BMC Pharmacol. Toxicol. 19, 18. doi:10.1186/s40360-018-0208-3
Gibbons, A., and Dean, B. (2016). The Cholinergic System: An Emerging Drug Target for Schizophrenia. CPD 22, 2124–2133. doi:10.2174/1381612822666160127114010
Halevi, S., Mckay, J., Palfreyman, M., Yassin, L., Eshel, M., Jorgensen, E., et al. (2002). The C.Elegansric-3 Gene Is Required for Maturation of Nicotinic Acetylcholine Receptors. EMBO J. 21, 1012–1020. doi:10.1093/emboj/21.5.1012
Hall, D. H., and Hedgecock, E. M. (1991). Kinesin-related Gene Unc-104 Is Required for Axonal Transport of Synaptic Vesicles in C. elegans. Cell 65, 837–847. doi:10.1016/0092-8674(91)90391-b
Hall, H., Reyes, S., Landeck, N., Bye, C., Leanza, G., Double, K., et al. (2014). Hippocampal Lewy Pathology and Cholinergic Dysfunction Are Associated with Dementia in Parkinson's Disease. Brain 137, 2493–2508. doi:10.1093/brain/awu193
Harada, S., Hori, I., Yamamoto, H., and Hosono, R. (1994). Mutations in the Unc-41 Gene Cause Elevation of Acetylcholine Levels. J. Neurochem. 63, 439–446. doi:10.1046/j.1471-4159.1994.63020439.x
Harris, T. W., Hartwieg, E., Horvitz, H. R., and Jorgensen, E. M. (2000). Mutations in Synaptojanin Disrupt Synaptic Vesicle Recycling. J. Cell Biol 150, 589–600. doi:10.1083/jcb.150.3.589
Hunt, P. R. (2017). The C. elegans Model in Toxicity Testing. J. Appl. Toxicol. 37, 50–59. doi:10.1002/jat.3357
Johnson, C. D., and Russell, R. L. (1983). Multiple Molecular Forms of Acetylcholinesterase in the NematodeCaenorhabditis Elegans. J. Neurochem. 41, 30–46. doi:10.1111/j.1471-4159.1983.tb11811.x
Kimura, K. D., Fujita, K., and Katsura, I. (2010). Enhancement of Odor Avoidance Regulated by Dopamine Signaling in Caenorhabditis elegans. J. Neurosci. 30, 16365–16375. doi:10.1523/jneurosci.6023-09.2010
Lazetic, V., and Fay, D. S. (2017). Molting in C. elegans. Worm 6, e1330246. doi:10.1080/21624054.2017.1330246
Lewis, J., Elmer, J., Skimming, J., Mclafferty, S., Fleming, J., and Mcgee, T. (1987). Cholinergic Receptor Mutants of the Nematode Caenorhabditis elegans. J. Neurosci. 7, 3059–3071. doi:10.1523/jneurosci.07-10-03059.1987
Lou, Y., and Li, Y. (2016). Effects of Chlorpyrifos on Dopaminergic Neuronal Viability with Activation of Microglia. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 33, 506–511.
Lue, L. P., Lewis, C. C., and Melchor, V. E. (1984). The Effect of Aldicarb on Nematode Population and its Persistence in Carrots, Soil and Hydroponic Solution. J. Environ. Sci. Health B 19, 343–354. doi:10.1080/03601238409372435
Luz, A. L., Lagido, C., Hirschey, M. D., and Meyer, J. N. (2016). In Vivo Determination of Mitochondrial Function Using Luciferase-Expressing Caenorhabditis elegans: Contribution of Oxidative Phosphorylation, Glycolysis, and Fatty Acid Oxidation to Toxicant-Induced Dysfunction. Curr. Protoc. Toxicol. 69, 25.8.1–25.8.22. doi:10.1002/cptx.10
Mahoney, T. R., Luo, S., and Nonet, M. L. (2006). Analysis of Synaptic Transmission in Caenorhabditis elegans Using an Aldicarb-Sensitivity Assay. Nat. Protoc. 1, 1772–1777. doi:10.1038/nprot.2006.281
Marogianni, C., Sokratous, M., Dardiotis, E., Hadjigeorgiou, G. M., Bogdanos, D., and Xiromerisiou, G. (2020). Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson's Disease. Int. J. Mol. Sci. 21, 8421. doi:10.3390/ijms21228421
Maruyama, H., Rakow, T. L., and Maruyama, I. N. (2001). Synaptic Exocytosis and Nervous System Development Impaired in Caenorhabditis elegans Unc-13 Mutants. Neuroscience 104, 287–297. doi:10.1016/s0306-4522(01)00097-5
Masato, A., Plotegher, N., Boassa, D., and Bubacco, L. (2019). Impaired Dopamine Metabolism in Parkinson's Disease Pathogenesis. Mol. Neurodegeneration 14, 35. doi:10.1186/s13024-019-0332-6
Mcdonald, P. W., Hardie, S. L., Jessen, T. N., Carvelli, L., Matthies, D. S., and Blakely, R. D. (2007). Vigorous Motor Activity in Caenorhabditis elegans Requires Efficient Clearance of Dopamine Mediated by Synaptic Localization of the Dopamine Transporter DAT-1. J. Neurosci. 27, 14216–14227. doi:10.1523/jneurosci.2992-07.2007
Mchardy, S. F., Wang, H.-Y. L., Mccowen, S. V., and Valdez, M. C. (2017). Recent Advances in Acetylcholinesterase Inhibitors and Reactivators: an Update on the Patent Literature (2012-2015). Expert Opin. Ther. Patents 27, 455–476. doi:10.1080/13543776.2017.1272571
Mchugh, M., Williams, P., Verma, S., Powell-Coffman, J. A., Robertson, A. P., and Martin, R. J. (2020). Cholinergic Receptors on Intestine Cells of Ascaris suum and Activation of nAChRs by Levamisole. Int. J. Parasitol. Drugs Drug Resist. 13, 38–50. doi:10.1016/j.ijpddr.2020.04.002
Miller, D. R., Mcclain, E. S., Dodds, J. N., Balinski, A., May, J. C., Mclean, J. A., et al. (2021). Chlorpyrifos Disrupts Acetylcholine Metabolism across Model Blood-Brain Barrier. Front. Bioeng. Biotechnol. 9, 622175. doi:10.3389/fbioe.2021.622175
Müller, M. L. T. M., and Bohnen, N. I. (2013). Cholinergic Dysfunction in Parkinson's Disease. Curr. Neurol. Neurosci. Rep. 13, 377. doi:10.1007/s11910-013-0377-9
Nonet, M. L., Grundahl, K., Meyer, B. J., and Rand, J. B. (1993). Synaptic Function Is Impaired but Not Eliminated in C. elegans Mutants Lacking Synaptotagmin. Cell 73, 1291–1305. doi:10.1016/0092-8674(93)90357-v
OECD (2018). User’s Handbook Supplement To The Guidance Document For Developing And Assessing AOPs. 233 ed. Co. Cork, Ireland: OECD Environment, Health and Safety Publications.
Ozkan, U., Osun, A., Basarslan, K., Senol, S., Kaplan, I., and Alp, H. (2014). Effects of Intralipid and Caffeic Acid Phenethyl Ester on Neurotoxicity, Oxidative Stress, and Acetylcholinesterase Activity in Acute Chlorpyriphos Intoxication. Int. J. Clin. Exp. Med. 7, 837–846.
Pallotta, M. M., Ronca, R., Carotenuto, R., Porreca, I., Turano, M., Ambrosino, C., et al. (2017). Specific Effects of Chronic Dietary Exposure to Chlorpyrifos on Brain Gene Expression-A Mouse Study. Int. J. Mol. Sci. 18, 2467. doi:10.3390/ijms18112467
Patikoglou, G. A., and Koelle, M. R. (2002). An N-Terminal Region of Caenorhabditis elegans RGS Proteins EGL-10 and EAT-16 Directs Inhibition of Gαo VersusGαq Signaling. J. Biol. Chem. 277, 47004–47013. doi:10.1074/jbc.m208186200
Pino, J. d., Moyano, P., Anadon, M. J., García, J. M., Díaz, M. J., García, J., et al. (2015). Acute and Long-Term Exposure to Chlorpyrifos Induces Cell Death of Basal Forebrain Cholinergic Neurons through AChE Variants Alteration. Toxicology 336, 1–9. doi:10.1016/j.tox.2015.07.004
Qian, H., Robertson, A. P., Powell‐Coffman, J. A., and Martin, R. J. (2008). Levamisole Resistance Resolved at the Single‐channel Level inCaenorhabditis Elegans. FASEB j. 22, 3247–3254. doi:10.1096/fj.08-110502
Ray, A., Martinez, B. A., Berkowitz, L. A., Caldwell, G. A., and Caldwell, K. A. (2014). Mitochondrial Dysfunction, Oxidative Stress, and Neurodegeneration Elicited by a Bacterial Metabolite in a C. elegans Parkinson's Model. Cell Death Dis 5, e984. doi:10.1038/cddis.2013.513
Reiss, R., Neal, B., Lamb, J. C., and Juberg, D. R. (2012). Acetylcholinesterase Inhibition Dose-Response Modeling for Chlorpyrifos and Chlorpyrifos-Oxon. Regul. Toxicol. Pharmacol. 63, 124–131. doi:10.1016/j.yrtph.2012.03.008
Reynolds, N. K., Schade, M. A., and Miller, K. G. (2005). Convergent, RIC-8-dependent Gα Signaling Pathways in the Caenorhabditis elegans Synaptic Signaling Network. Genetics 169, 651–670. doi:10.1534/genetics.104.031286
Richmond, J. E., and Jorgensen, E. M. (1999). One GABA and Two Acetylcholine Receptors Function at the C. elegans Neuromuscular junction. Nat. Neurosci. 2, 791–797. doi:10.1038/12160
Rooney, J. P., Luz, A. L., González-Hunt, C. P., Bodhicharla, R., Ryde, I. T., Anbalagan, C., et al. (2014). Effects of 5′-Fluoro-2-Deoxyuridine on Mitochondrial Biology in Caenorhabditis elegans. Exp. Gerontol. 56, 69–76. doi:10.1016/j.exger.2014.03.021
Saifee, O., Wei, L., and Nonet, M. L. (1998). The Caenorhabditis elegans Unc-64 Locus Encodes a Syntaxin that Interacts Genetically with Synaptobrevin. MBoC 9, 1235–1252. doi:10.1091/mbc.9.6.1235
Sammi, S. R., Agim, Z. S., and Cannon, J. R. (2018). From the Cover: Harmane-Induced Selective Dopaminergic Neurotoxicity in Caenorhabditis elegans. Toxicol. Sci. 161, 335–348. doi:10.1093/toxsci/kfx223
Sammi, S. R., Foguth, R. M., Nieves, C. S., De Perre, C., Wipf, P., Mcmurray, C. T., et al. (2019). Perfluorooctane Sulfonate (PFOS) Produces Dopaminergic Neuropathology in Caenorhabditis elegans. Toxicol. Sci. 172, 417–434. doi:10.1093/toxsci/kfz191
Sammi, S. R., Trivedi, S., Rath, S. K., Nagar, A., Tandon, S., Kalra, A., et al. (2017). 1-Methyl-4-propan-2-ylbenzene from Thymus Vulgaris Attenuates Cholinergic Dysfunction. Mol. Neurobiol. 54, 5468–5481. doi:10.1007/s12035-016-0083-0
Sawin, E. R., Ranganathan, R., and Horvitz, H. R. (2000). C. elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway. Neuron 26, 619–631. doi:10.1016/s0896-6273(00)81199-x
Schliebs, R. (2005). Basal Forebrain Cholinergic Dysfunction in Alzheimer's Disease - Interrelationship with β-amyloid, Inflammation and Neurotrophin Signaling. Neurochem. Res. 30, 895–908. doi:10.1007/s11064-005-6962-9
Settivari, R., Vanduyn, N., Levora, J., and Nass, R. (2013). The Nrf2/SKN-1-dependent Glutathione S-Transferase π Homologue GST-1 Inhibits Dopamine Neuron Degeneration in a Caenorhabditis elegans Model of Manganism. Neurotoxicology 38, 51–60. doi:10.1016/j.neuro.2013.05.014
Smita, S. S., Raj Sammi, S., Laxman, T. S., Bhatta, R. S., and Pandey, R. (2017). Shatavarin IV Elicits Lifespan Extension and Alleviates Parkinsonism in Caenorhabditis elegans. Free Radic. Res. 51, 954–969. doi:10.1080/10715762.2017.1395419
Smith, R., Chung, H., Rundquist, S., Maat-Schieman, M. L. C., Colgan, L., Englund, E., et al. (2006). Cholinergic Neuronal Defect without Cell Loss in Huntington's Disease. Hum. Mol. Genet. 15, 3119–3131. doi:10.1093/hmg/ddl252
Stiernagle, T. (2006). Maintenance Of C. elegans. Wormbook: C. elegans Res. Community, 1–11. doi:10.1895/wormbook.1.101.1
Sulston, J., Dew, M., and Brenner, S. (1975). Dopaminergic Neurons in the nematode Caenorhabditis elegans. J. Comp. Neurol. 163, 215–226. doi:10.1002/cne.901630207
Trivedi, S., Maurya, P., Sammi, S. R., Gupta, M. M., and Pandey, R. (2017). 5-Desmethylnobiletin Augments Synaptic ACh Levels and Nicotinic ACh Receptor Activity: A Potential Candidate for Alleviation of Cholinergic Dysfunction. Neurosci. Lett. 657, 84–90. doi:10.1016/j.neulet.2017.08.010
Voorhees, J. R., Remy, M. T., Erickson, C. M., Dutca, L. M., Brat, D. J., and Pieper, A. A. (2019). Occupational-like Organophosphate Exposure Disrupts Microglia and Accelerates Deficits in a Rat Model of Alzheimer's Disease. NPJ Aging Mech. Dis. 5, 3. doi:10.1038/s41514-018-0033-3
Weimer, R. M., Richmond, J. E., Davis, W. S., Hadwiger, G., Nonet, M. L., and Jorgensen, E. M. (2003). Defects in Synaptic Vesicle Docking in Unc-18 Mutants. Nat. Neurosci. 6, 1023–1030. doi:10.1038/nn1118
Wicks, S. R., and Rankin, C. H. (1996). The Integration of Antagonistic Reflexes Revealed by Laser Ablation of Identified Neurons Determines Habituation Kinetics of the Caenorhabditis elegans Tap Withdrawal Response. J. Comp. Physiol. A. 179, 675–685. doi:10.1007/bf00216131
Wu, Q., Cao, X., Yan, D., Wang, D., and Aballay, A. (2015). Genetic Screen Reveals Link between the Maternal Effect Sterile Gene Mes-1 and Pseudomonas Aeruginosa-Induced Neurodegeneration in Caenorhabditis elegans. J. Biol. Chem. 290, 29231–29239. doi:10.1074/jbc.m115.674259
Xiong, H., Pears, C., and Woollard, A. (2017). An Enhanced C. elegans Based Platform for Toxicity Assessment. Sci. Rep. 7, 9839. doi:10.1038/s41598-017-10454-3
Yao, C., El Khoury, R., Wang, W., Byrd, T. A., Pehek, E. A., Thacker, C., et al. (2010). LRRK2-mediated Neurodegeneration and Dysfunction of Dopaminergic Neurons in a Caenorhabditis elegans Model of Parkinson's Disease. Neurobiol. Dis. 40, 73–81. doi:10.1016/j.nbd.2010.04.002
Yen, J., Donerly, S., Levin, E. D., and Linney, E. A. (2011). Differential Acetylcholinesterase Inhibition of Chlorpyrifos, Diazinon and Parathion in Larval Zebrafish. Neurotoxicology and Teratology 33, 735–741. doi:10.1016/j.ntt.2011.10.004
Zhang, J., Dai, H., Deng, Y., Tian, J., Zhang, C., Hu, Z., et al. (2015). Neonatal Chlorpyrifos Exposure Induces Loss of Dopaminergic Neurons in Young Adult Rats. Toxicology 336, 17–25. doi:10.1016/j.tox.2015.07.014
Keywords: pesticides, adverse outcome pathway, chlorpyrifos, dopamine, acetylcholine, new approach methodologies (NAM), nicotinic acetylcholine receptor
Citation: Sammi SR, Jameson LE, Conrow KD, Leung MCK and Cannon JR (2022) Caenorhabditis elegans Neurotoxicity Testing: Novel Applications in the Adverse Outcome Pathway Framework. Front. Toxicology 4:826488. doi: 10.3389/ftox.2022.826488
Received: 30 November 2021; Accepted: 07 February 2022;
Published: 16 March 2022.
Edited by:
Ellen Fritsche, Leibniz-Institut für Umweltmedizinische Forschung (IUF), GermanyReviewed by:
Gaylia Jean Harry, National Institute of Environmental Health Sciences (NIH), United StatesCopyright © 2022 Sammi, Jameson, Conrow, Leung and Cannon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maxwell C. K. Leung, bWNrbGV1bmdAYXN1LmVkdQ==; Jason R. Cannon, Y2Fubm9uanJAcHVyZHVlLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.