
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Toxicol. , 01 October 2021
Sec. Nanotoxicology
Volume 3 - 2021 | https://doi.org/10.3389/ftox.2021.737158
This article is part of the Research Topic Novel Methods for Labeling Nanomaterials for Biological and Environmental Tracing View all 4 articles
 Amalie Thit1*
Amalie Thit1* Monica Hamann Sandgaard1
Monica Hamann Sandgaard1 Joachim Sturve2
Joachim Sturve2 Catherine Mouneyrac3
Catherine Mouneyrac3 Anders Baun4
Anders Baun4 Henriette Selck1
Henriette Selck1For engineered metal nanoparticles (NPs), such as copper oxide (CuO) NPs, the sediment is recognized as a major compartment for NP accumulation. Sediment-dwelling organisms, such as the worm Tubifex tubifex, will be at particular risk of metal and metal NP exposure. However, a range of complex transformation processes in the sediment affects NP bioavailability and toxicity as the contamination ages. The objective of this study was to examine bioaccumulation and adverse effects of CuO NPs in T. tubifex compared to dissolved Cu (administered as CuCl2) and the influence of aging of spiked sediment. This was done in a 28-day exposure experiment with T. tubifex incubated in clean sediment or freshly spiked sediment with different concentrations of dissolved Cu (up to 230 μg g−1 dw) or CuO NPs (up to 40 μg g−1 dw). The experiment was repeated with the same sediments after it had been aged for 2 years. To obtain a distinct isotopic signature compared to background Cu, both Cu forms were based on the stable isotope 65Cu (>99%). The 28-day exposure to sediment-associated dissolved 65Cu and 65CuO NPs resulted in a clear concentration-dependent increase in the T. tubifex65Cu body burden. However, despite the elevated 65Cu body burdens in exposed worms, limited adverse effects were observed in either of the two experiments (e.g., above 80% survival in all treatments, low or no effects on the growth rate, feeding rate, and reproduction). Organisms exposed to aged sediments had lower body burdens of 65Cu than those exposed to freshly spiked sediments and we suggest that aging decreases the bioavailability of both 65Cu forms. In this study, the use of a stable isotope made it possible to use environmentally realistic Cu concentrations and, at the same time, differentiate between newly accumulated 65Cu and background Cu in experimental samples despite the high background Cu concentrations in sediment and T. tubifex tissue. Realistic exposure concentrations and aging of NPs should preferably be included in future studies to increase environmental realism to accurately predict the environmental risk of metal NPs.
Engineered metal nanoparticles (NPs), such as copper oxide (CuO) NPs, have unique properties as a result of their small size (1–100 nm) and high surface-to-volume ratio compared to their larger counterparts (Nel et al., 2006). CuO NPs and other Cu-containing NPs have vast applications, including antifouling paint, bioactive coatings, cosmetics, electronics, health products, inks, lubricants, plastics, solar cells, and batteries (Nanotechproject, 2021; Cioffi et al., 2005; Park et al., 2007; Wang et al., 2008; Perelshtein et al., 2009; Ren et al., 2009; Anyaogu et al., 2008). Therefore, there has been a dramatic increase in the use of Cu-containing particles over the past decades. As the production of CuO NPs increases the release of these particles via wastewater and agricultural runoff, release from weathered surfaces treated with NP-coatings and antifouling paints (Lammel et al., 2020) will likely cause increased exposure in the aquatic environment. In 2010, the annual production of Cu NPs was estimated to 200 tons year−1 and the release of these particles was estimated to 11 tons year−1 (Keller et al., 2013).
Aquatic sediments have been recognized as a major compartment for metal NP accumulation (Praetorius et al., 2012; Thit et al., 2015a; Cross et al., 2015). For example, the use of CuO NP-containing wood coatings in Europe alone could increase sediment concentration of CuO NPs by about 400 ng kg−1 annually (Caballero-Guzman and Nowack, 2018). According to Garner et al. (2017), CuO NP accumulation in the sediment compartment may lead to concentrations in the ng to μg kg−1 range (Garner et al., 2017). Due to this inevitable increase in sediment CuO NP concentration, sediment-dwelling, and especially deposit-feeding organisms such as the worm Tubifex tubifex, will be at particular risk of metal and metal NP exposure (Ozoh, 1992; Selck et al., 1998; Luoma and Rainbow, 2008; Croteau et al., 2011a; Burton, 2013; Thit et al., 2015a). In the few studies that have been conducted with sediment exposures, toxicity and bioaccumulation of CuO particles of varying sizes and shapes have been reported for sediment-dwelling snails, mussels, and worms (Gomes et al., 2012; Pang et al., 2012; Buffet et al., 2013; Pang et al., 2013; Ramskov et al., 2014; Thit et al., 2015a; Thit et al., 2015b; Thit et al., 2016; Thit and Selck, 2021).
In the aquatic environment, NPs will undergo a series of transformations. In the water column, CuO NPs will likely only marginally dissolve and rather agglomerate/aggregate, favoring their deposition onto sediment (Baun et al., 2008; Klaine et al., 2008; Quik et al., 2012; Thit et al., 2015a; Keller et al., 2017). In aquatic sediments, a range of transformation processes will occur, but the complex sediment matrix makes it very difficult to predict NP transformations in this compartment (Baun et al., 2017). Furthermore, increased contact time between metal NP and sediment (aging) may affect the bioavailability and toxicity of NPs in sediment. Compared to the literature on transformations occurring in the pelagic zone, little is known about these processes in sediment and the influence of aging on bioaccumulation and toxicity. Soil research has reported that prolonged contact time between NPs and soil generally decreased the toxicity of NPs (Jośko and Oleszczuk, 2013).
In the present study, the influence of aging on the toxicity and bioavailability of CuO NPs was studied using the freshwater oligochaete T. tubifex due to its important role in the ecosystem as a bioturbator greatly influencing the milieu (Matisoff et al., 1999; Anschutz et al., 2012; Thit et al., 2020), and as prey for a range of species, including fish, leading to potential trophic transfer of CuO NPs (Lammel et al., 2020). The objective of the study was to examine the potential bioaccumulation and adverse effects of CuO NPs compared to dissolved Cu and the influence of aging of spiked sediment on these processes. The study specifically addressed whether 1) bioaccumulation differed between Cu treatments (CuO NPs vs. dissolved Cu), 2) CuO NPs and dissolved Cu caused adverse effects in T. tubifex (in terms of altered reproduction, survival, growth, burrowing behavior, and feeding), and 3) aging of spiked sediment affected the bioaccumulation and adverse effects of CuO NPs and dissolved Cu. Since Cu is an essential metal and ubiquitously present in sediment and worm tissue, both Cu forms used in the present study were enriched in 65Cu (>99%) to obtain a distinct isotopic signature compared to background Cu in samples (Croteau et al., 2014; Zhang et al., 2019).
The 65CuCl2 stock solution was prepared by dissolving commercially purchased isotopically enriched 65CuCl2 (99.7% enrichment, Lot: 55-9, Trace Sciences International, United States) in Milli-Q water. Isotopically modified 65CuO NPs were synthesized as described previously (Lammel et al., 2021) using enriched 65Cu as a precursor (>99% purity, Trace Sciences International, United States) and CuO NPs from the same batch were used in the present study. Briefly, 65Cu was dissolved in a mixture of HCl and H2O2 followed by solvent removal on rotatory evaporator under vacuum at 90°C to obtain a powder of 65CuCl2. 65Cu NPs were synthesized by thermolysis of 65Cu-oleate obtained by reflux of a mixture of 65CuCl2 and sodium-oleate in water, ethanol, and hexane. Oxidation of the 65Cu NPs resulted in the formation of 65CuO NPs that was subsequently isolated via centrifugation and resuspended in 1 mmol/L sodium citrate solution. Characterization is presented in (Lammel et al., 2021). Briefly, primary particle size (20 nm), morphology (spherical), and surface chemistry (mixture phase of CuO, Cu2O, and Cu) were determined using TEM imaging (JEOL2100F at 200 kV) and X-ray diffraction (Bruker D8 advance diffractometer with a copper target).
T. tubifex were originally purchased from Bonnies Dyrecenter (DK), where they have been stored at 4°C. The worms were acclimatized in the laboratory in glass aquaria with T. tubifex medium (media preparation described below) by gradually increasing temperature and storing at experimental conditions for 24 h (20°C in 16 h light and 8 h dark) before exposure. Aeration was provided using a pump, a silicon tube, and an aeration stone.
Tubifex medium (artificial freshwater) was prepared following OECD guideline 315. Briefly, 2 L stock solution was made by dissolving calcium chloride (11.76 g/L CaCl2-2H2O, 10035-04-8, Merck), magnesium sulfate (4.93 g/L MgSO4-7H2O, 10034-99-8, Merck), sodium bicarbonate (2.59 g/L, NaHCO3, 144-55-8, Merck), and potassium chloride (0.23 g/L KCl, 7447-40-7, Merck) in deionized water. Subsequently, the total volume was made up to 25 L with deionized water, aerated until oxygen saturation was achieved, and stored at 20°C.
Sediment was collected from a shallow area by Munkholmbroen in Isefjord, Roskilde, Denmark (55°40′25″N 11°48′44″E), in early spring 2019 by scraping off the top few centimeters of the sediment surface as described in (Ramskov et al., 2015). Subsequently, sediment was sieved at the site (<500 µm) using natural brackish water from the site to remove coarse debris and macrofauna. After settling (>72 h), the overlying water was carefully removed using a siphon and the sediment was frozen at −20°C to kill micro- and meiofauna. Then, the sediment was thawed and sieved to <63 µm with T. tubifex medium, and the overlying water was removed carefully after the sediment had settled after about a week. Sediment was rinsed by mixing with clean, aerated T. tubifex medium to obtain relevant salinity and homogenized thoroughly by hand mixing. Sediment was left to settle and the overlying water carefully removed, avoiding removal of organic material. Before spiking, a homogenous sediment slur was obtained using an immersion blender. The sediment was stored at 4°C in the dark until experimental use. Sediment aliquots were collected for the dry weight (dw): wet weight (ww) ratio determinations and organic matter content as described below.
The sediment was spiked (spring, 2019) by adding a known amount of the 65CuCl2 or 65CuO NP stock to a known amount of wet sediment. Four exposure concentrations per treatment were chosen to cover the range of environmentally relevant Cu concentrations and covering both low non-toxic and toxic concentrations (up to 230 µg 65Cu g−1 dw sediment). A lower concentration range was chosen for CuO NPs (up to 40 µg 65Cu g−1 dw sediment) than dissolved Cu as we expect the environmental concentrations to be lower for this Cu form. Uncontaminated control sediment was treated similarly, and the total volume of Milli-Q water added was equal among all treatments. Sediment (control and all Cu concentrations) was mixed thoroughly with a spoon and subsequently covered with parafilm and aluminum foil and placed on a shaking table for 72 h at room temperature (about 20°C) to obtain homogenous Cu distribution in the sediment. Sediment aliquots were collected to determine 65Cu concentration. Sediment was used immediately hereafter for experiments with freshly spiked sediment. The remaining spiked sediment was aged at 4°C in the dark for 2 years (23 months, until spring 2021).
The dw:ww ratio was determined for both freshly spiked (0.202 ± < 0.01; n = 6) and aged sediment (0.204 ± <0.01; n = 6) after drying sediment aliquots for 24 h at 105°C. The organic matter (OM) content (in freshly spiked sediment 18.1 ± 0.1%; n = 6 and aged sediment 18.6 ± 0.4%; n = 6) was determined after loss on ignition (>2 h at 550°C) on dry sediment aliquots.
The setup was similar for both experiments (freshly spiked sediment and aged sediment). For all ten treatments, the respective sediment was transferred into each individual exposure container (25 ml scintillation vials: 2.5 cm in diameter and 5 cm in height). The amount of sediment per container corresponded to 1.5 g dw sediment (7.45 ± 0.03 g ww; n = 100, in experiment 1 with freshly spiked sediment; 7.37 ± 0.06 g ww; n = 100, in experiment 2 with aged sediment). The 9 ml aerated Tubifex media were gently added and exposure containers were covered with a lid and kept under experimental conditions overnight to allow sediment to settle. Healthy, sexually mature worms (with clitellum) of approximately similar size and age were carefully separated from the culture and pooled in groups of four. The worms were kept in six-well multi-well plates with aerated Tubifex media to empty their guts overnight. This depuration time was chosen in order to 1) be equal to depuration time after exposures (see below) to allow determination of worm growth rate during exposures and 2) to allow sufficient time to obtain fully empty guts (Thit and Selck, 2021). On the day of experimental initiation, 2/3 of the overlying water was decanted from the vials and replaced with newly aerated water and resuspended sediment was allowed to settle for 2 h. Immediately before exposure initiation, worms were weighed in groups of four (i.e., mean wet weight of each group: 15.71 ± 5.93 mg, n = 200 groups; corresponding to approximately 4 mg ww per worm in average).
In both experiments, exposures were initiated by adding four T. tubifex to each replicate exposure container with ten replicates per treatment and ten treatments in total (five concentrations for 65CuCl2 and five for 65CuO NPs), resulting in a total of 400 worms per experiment. Four worms per replicate were chosen to allow sufficient biomass for 65Cu body burden measurements. The time for each individual worm to burrow into the sediment was recorded for all treatments every minute for the first 10 min and then every 5–10 min for up to 2 h. Burrowing of worms was assessed as the number of worms (out of four) in each of the following categories: 1) worms in the overlying water, 2) partially buried (i.e., head only burrowed in the sediment), and 3) fully burrowed in sediment. Survival was noted, and fecal layer thickness was recorded at five selected days during exposures until day 27 of exposure. The fecal layer thickness was assessed following Thit et al. (2020). Briefly, the thickness of the fecal layer was measured at a predetermined place on the exposure container using an electronic caliper (Biltema, Dk), with 0.1 mm precision.
The test beakers were provided with air using silicone tubes with a needle connected to an aquarium pump. The beakers were covered with a plastic lid with holes for the air supply. Test beakers were placed in a climate cabinet at 20°C with a 16:8 h light-dark cycle. The test beakers were monitored every second day during the exposure period and evaporated water was replaced with DI water when needed. The pH of the overlying water was ∼7.5.
At experimental termination (i.e., day 28), approximately 7.5 ml of the overlying water, and subsequently fecal layer, was carefully removed from exposure containers using a pasture pipette, avoiding disturbing the sediment. Sediment was mixed carefully to get a homogenous slur and subsamples were retrieved for Cu concentration determination at Tend (making sure no worms or cocoons were in the sample) and frozen at -20°C. The remaining sediment (+worms and cocoons) was sieved through a 125 µm sieve and the sediment was washed with Tubifex media to expose surviving worms and cocoons and fecal matter. The adult worms were carefully retrieved from the sieve, washed, and transferred to multi-wells, where they were allowed to depurate overnight. The following day, the worms were weighed on an analytical scale to determine growth (weight change) and then frozen at −20°C. In the first experiment (i.e., with freshly spiked sediment), the cocoons were removed from the sieve and transferred to a multi-well with aerated Tubifex media and counted to examine fecundity. The cocoons were kept under experimental conditions for up to 3 months to examine the effects of treatment on hatching time and hatching efficiency. Every second day the number of hatched cocoons and the number of hatched worms were counted. Empty cocoons and newly hatched worms were removed from the wells. On the same census days, 2/3 of the water was removed and replaced with aerated Tubifex media. This process of collecting and counting cocoons, eggs, and newly born worms was very time-consuming and practically extremely challenging and was therefore only included in the first experiment (i.e., with freshly spiked sediment).
Cu concentrations in all samples (water, sediment, and worm tissue) were measured by inductively coupled plasma-mass spectrometry (ICP-MS, 7,900, Agilent) as previously described (Thit and Selck, 2021). Tissue and sediment samples were dried at 40°C for at least 48 h. All samples were subsequently digested according to ISO15587-2. Briefly, sediment and tissue samples were weighed into Teflon™ inserts (Milestone, Germany) and dissolved with 65% ultrapure nitric acid (HNO3) and Milli-Q water (1:1). Three Teflon™ inserts were placed in each Weflon™ vial (Milestone, Germany) containing 10 ml Milli-Q water and 2 ml 30% H2O2. Samples were heated in a microwave oven (START D Microwave Digestion System, Milestone, Germany) and subsequently transferred into volumetric flasks (resulting in 8% HNO3). Finally, Cu concentration in each sample was determined directly after digestion or after a short storage period (<24 h). A series of standard Cu solutions (8% HNO3) was used to calibrate Cu concentrations (six standards were selected from 0, 0.1, 2, 5, 10, 50, 100, 1,000 to 5,000 μg Cu L−1 to cover the range of expected Cu concentrations in the sample batch). During each analytical run, at least one of these standards was re-analyzed (approximately for every ten samples analyzed) to check for analytical drift and were all in agreement with expected Cu concentrations. To account for instrument drift and change in sensitivity, an internal standard (germanium 74Ge) was added to all samples. Samples were analyzed in duplicate (each analysis averaged 32 measurements) for the naturally occurring stable isotopes 63Cu and 65Cu by ICP-MS (Agilent). Samples were set to zero if their Cu concentrations inferred from 63Cu were higher than those of 65Cu.
Cu recovery was examined using a control solution with known Cu concentration (miljøkontrol 100 µg Cu L−1), certified mussel tissue (European reference materials, ERM®—CE278k; 5.98 μg Cu g−1 dw tissue), and certified freshwater sediment (RIZA, Trace metals WD CRM-CNS301-050; 44.2 μg Cu g−1 dw sediment), which were digested and analyzed together with different sample batches (at least one reference sample per batch). Results of control solution, certified tissue, and sediment were in good agreement with the certificate of analysis (control: 97.03 μg L−1 ± 3.2, n = 3; mussel: 5.47 ± 0.7 μg Cu g−1 dw tissue, n = 3; sediment: 40.1 ± 2.6 μg Cu g−1 dw sediment, n = 13, respectively). All equipment used for sample digestions was thoroughly acid-washed before use.
Concentrations of newly accumulated or added 65Cu (referred to as 65Cu in the following) were calculated based on ICP-MS measurements of the two naturally abundant stable isotopes, 65Cu and 63Cu (Croteau et al., 2004; Thit and Selck, 2021). Briefly, the relative abundance of the two isotopes in natural Cu samples in the absence of a spike (p65) was set to 0.309. Concentrations of newly accumulated 65Cu in the experimental organisms ([65Cu]org) were calculated as the product of p65 and the total Cu concentrations inferred by the ICP-MS software from 65Cu intensity ([T65Cu]):
The original load of tracer ([65Cu]org0) that occurred in each sample in the absence of a 65Cu spike was calculated as the product of p65 and the total Cu concentrations inferred from the intensity of the most abundant Cu isotope, ([T63Cu]):
The net tracer uptake (Δ[65Cu]org) was derived from the total Cu concentration inferred from 65Cu signal ([65Cu]org) minus the pre-existing concentration of tracer ([65Cu]org0):
All statistical analyses were conducted using SYSTAT, version 13, and graphical data presentations were made using SigmaPlot for windows, version 14. (Systat Software, Inc., San Jose, CA, United States). All data are presented as mean ± one standard deviation (SD). Whether data fitted the assumptions for parametric analysis, i.e., normally distributed data with homogeneous variances, was tested using Kolmogorov–Smirnov and Levene’s test, respectively. One-way Analysis of Variance (one-way ANOVA) was conducted when data met the requirements for parametric tests. Tukey’s Honestly Significant Difference Test was used when significant differences were detected to determine significant pairwise differences between samples. When data (or transformed data) did not meet the requirements for parametric analysis, a nonparametric Kruskal–Wallis analysis on ranks was performed, followed by Conover–Inman Test for all pairwise comparisons. When comparing the two samples, a Student’s two-sample t-test was conducted. Differences were considered significant when p ≤ 0.05.
The average background Cu concentration in the control sediment (sieved to <63 μm) was 72.5 ± 7.4 μg Cu g−1 dry weight (dw) sediment (n = 11). Note that the high background Cu concentration is a result of the small sediment grain size compared to, for example, the same sediment sieved to greater grain size (<125 μm; 23 µg Cu g−1 dw sediment) (Thit and Selck, 2021). As expected, the newly added 65Cu concentration was negligible (0.1 ± 0.1 μg 65Cu g−1 dry weight sediment; n = 11). Sediment 65Cu concentrations were measured immediately after spiking (T0, freshly spiked), after 28 days of exposure in freshly spiked sediment (Tend, freshly spiked), at exposure initiation of experiments in aged sediment (T0, aged), and after 28 days in experiments with aged sediment (Tend, aged). Measured sediment 65Cu concentrations increased with increasing nominal concentrations for experiments with both freshly spiked and aged sediment at both the beginning and end of exposure (Table 1). In the following, exposure 65Cu concentrations are reported as the mean measured concentrations at the initiation of exposures with fresh sediment (i.e., for 65CuCl2: 0, 18, 53, 112, 227 µg 65Cu g−1 dw sediment and for 65CuO NP: 0, 3, 8.9, 18, 36.5 µg 65Cu g−1 dw sediment). In general, there were limited statistically significant changes in exposure concentrations from the beginning to the end of exposures in either of the two experiments (see Supplementary Material, SI, for statistical comparison). Furthermore, 65Cu levels at the beginning of exposures with newly spiked and aged sediment in control sediment were not statistically significantly different from each other (p = 0.694 and 0.648 for 65CuCl2 and 65CuO NP, respectively). Sediment 65Cu concentrations in the majority of the treatments were slightly higher after aging than right after spiking (significant for most concentrations; see SI for further info).
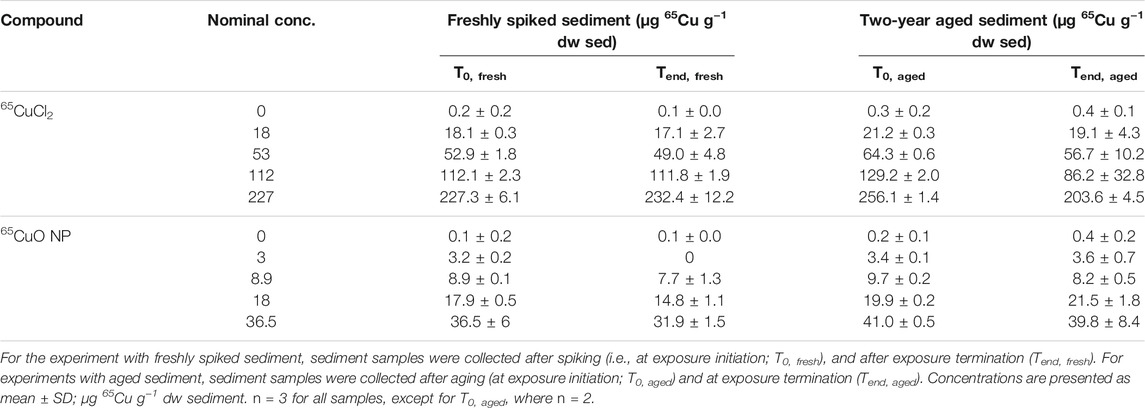
TABLE 1. Sediment 65Cu concentrations from experiments with freshly spiked sediment and sediment aged for 2 years.
Background Cu concentrations (total Cu) in overlying water of control sediment were 18.8 ± 5.3 μg L−1 (n = 8) for freshly spiked sediment and 12.0 ± 5.1 μg L−1 (n = 6) for aged sediment. 65Cu concentrations in overlying water (unfiltered samples containing dissolved Cu and Cu sorbed to colloids) collected at the termination of experiments with freshly spiked sediment and aged sediment from both 65CuCl2 and 65CuO NP exposures increased significantly with increasing sediment concentrations (all four p-values <0.001; Kruskal–Wallis; Table 2).
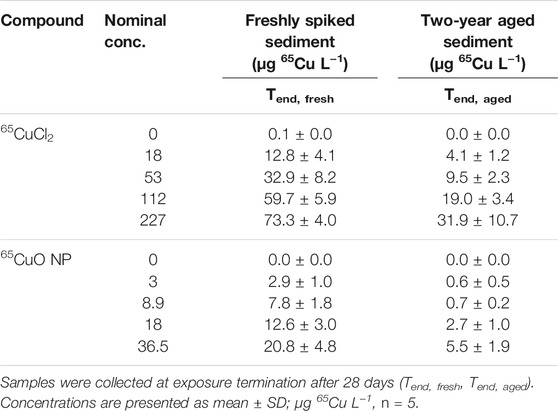
TABLE 2. Water 65Cu concentrations from experiments with freshly spiked sediment and sediment aged for 2 years.
In controls (with unspiked control sediment), 65Cu concentrations in overlying water were close to zero and did not significantly differ between experiments with freshly spiked or aged sediment (p >0.05; two-sample t-test). However, 65Cu concentrations in overlying water at the end of exposures in freshly spiked sediment were significantly higher than those from aged sediment for both 65CuCl2 and 65CuO NP (all p-values <0.01; two-sample t-test).
The background concentration of Cu (total Cu) in control worms was 30.3 ± 10.3 µg Cu g−1 dw tissue. As expected, the concentration of newly added 65Cu in T. tubifex from control treatments (i.e., sediment with no added 65Cu) was negligible at 0.2 ± 0.2 μg g−1 dw tissue (n = 20).
In both treatments, the added 65Cu was bioavailable and resulted in an increased 65Cu weight-specific body burden (WS-BB) in T. tubifex after 28 days of exposure. The WS-BB of worms exposed in sediment with 65CuCl2 in freshly spiked or aged sediment was significantly affected by 65Cu concentration (both p <0.001; Kruskal–Wallis). In freshly spiked sediment, the WS-BB of worms in all treatments was significantly different from control and from each other (all p-values <0.001; Conover–Inman). In aged sediment, the WS-BB differed significantly from the control for worms exposed at 227 µg 65Cu g−1 dw sediment and marginally at 112 µg 65Cu g−1 dw sediment (p = 0.018 and 0.052, respectively).
For 65CuO NPs, the WS-BB of worms in both freshly spiked and aged sediments differed significantly among concentrations (both p-values <0.001; Kruskal–Wallis). In freshly spiked sediment, the WS-BB of worms in all treatments was significantly higher than that in control and differed among concentrations (p-values <0.01 for all comparisons to control and p-values <0.05 for all comparisons between other treatments; Conover–Inman). In aged sediment, only the WS-BB of worms in the highest concentration, 36.5μg g−1 dw sediment, was significantly higher than that in control (p = 0.015).
As seen in Figure 1, bioaccumulation of 65Cu after exposure to 65CuCl2 and 65CuO NPs seemed to increase similarly with increasing sediment exposure concentration in both experiments with freshly spiked or aged sediment. The 65Cu WS-BB for organisms exposed to newly spiked sediment was higher than that for organisms exposed to aged sediment compared to worms exposed to similar 65Cu form and concentration.
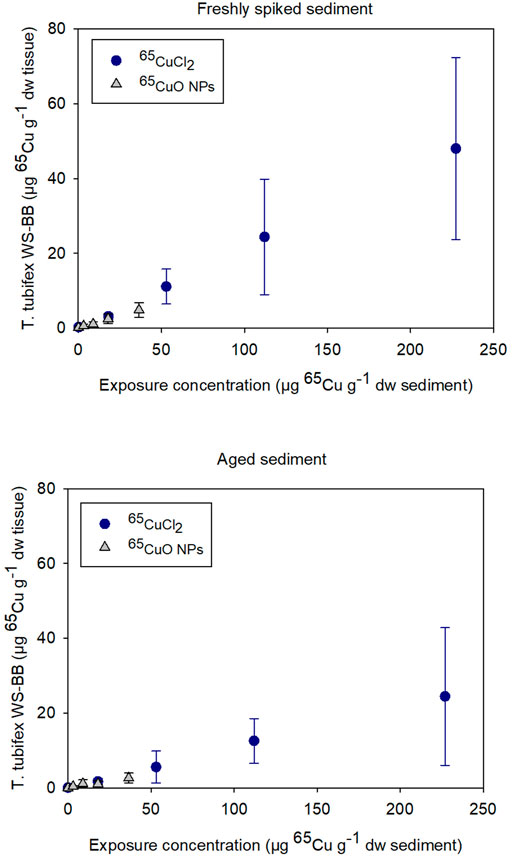
FIGURE 1. Weight-specific body burden (WS-BB) of newly accumulated 65Cu in T. tubifex after 28-day exposure to five concentrations of 65CuCl2 or 65CuO NPs in freshly spiked or 2-year aged sediment. WS-BB is presented as mean µg 65Cu g−1 dw tissue ±SD, n = 8–10.
For 65CuCl2, there was a significant difference between WS-BB in worms exposed to freshly spiked and aged sediment in sediments with 18, 53, and 227 µg 65Cu g−1 dw sediment (p <0.001, 0.013, and 0.029, respectively; two-sample t-test; Supplementary Figure S1). For control worms and those exposed to 112 µg 65Cu g−1 dw sediment, there was no significant difference (p > 0.05). For 65CuO NPs, the WS-BB of worms in freshly spiked sediment was significantly higher than that in aged sediment, when exposed to the two highest concentrations, 18 and 36.5 µg 65Cu g−1 dw sediment (p = 0.010 and 0.012, respectively; Two-sample t-test). Thus, the 28-day exposures to 65CuCl2 and 65CuO NPs in freshly spiked or aged sediment revealed that 65Cu accumulation in T. tubifex was influenced by 65Cu exposure concentration and aging but not on 65Cu form (Figure 1).
About 90% of T. tubifex survived during experiments with freshly spiked sediment and aged sediment, 88–98% in freshly spiked sediment, and 81–100% in aged sediment (Figure 2). No influence of 65Cu treatment, exposure concentration, or sediment aging on T. tubifex survival was detected during exposures.
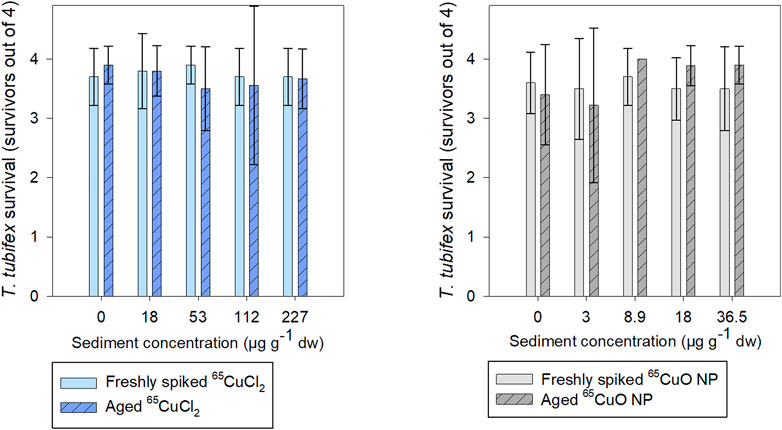
FIGURE 2. Number of surviving T. tubifex out of four per replicate after exposure in freshly spiked sediment (full bars) or aged sediment (scratched bars). Worms were exposed in sediment with 65CuCl2 (blue) or 65CuO NPs (gray) at five concentrations of 65CuCl2 or 65CuO NPs for 28 days. Bars represent the mean number of surviving worms ±SD, n = 10.
As seen in Figure 3, the burrowing activity of T. tubifex in control sediments was not affected by the aging of sediment. There was no significant difference in the mean burrowing time in freshly spiked sediment (11 ± 18 min) and aged sediment (10 ± 12 min) (p = 0.814; two-sample t-test). Furthermore, in spiked sediments, there was no significant effect of 65Cu treatment, concentration, or sediment aging on burrowing time (freshly spiked sediment with 65CuCl2: p = 0.140; aged sediment with 65CuCl2: p = 0.592; freshly spiked sediment for both treatments: p = 0.192; freshly spiked sediment with 65CuO NP: p = 0.374; aged sediment with 65CuO NP: p = 0.568; aged sediment for both treatments: p = 0.283; Kruskal–Wallis). Thus, the 28-day exposures to 65CuCl2 and 65CuO NPs in freshly spiked or aged sediment did not result in any detectable influence of 65Cu form, 65Cu exposure concentration, or aging on T. tubifex survival and burrowing activity (Figure 2).
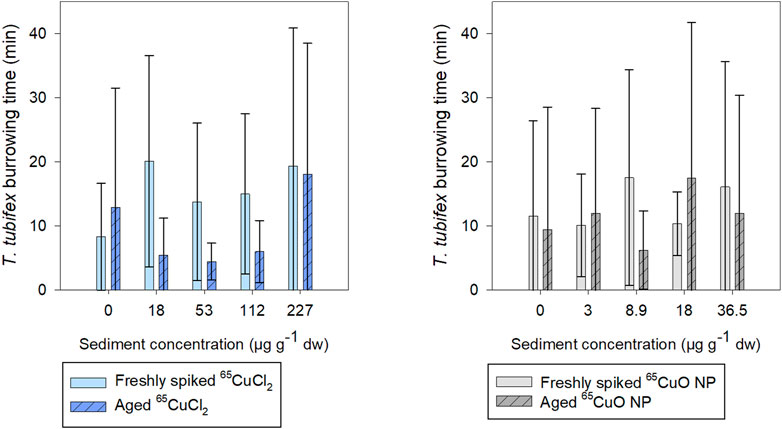
FIGURE 3. Time until T. tubifex was completely buried in sediment after initiation of exposure in freshly spiked sediment (full bars) or aged sediment (scratched bars). Worms were exposed in sediment with 65CuCl2 [blue, (A)] or 65CuO NPs [gray, (B)] at five concentrations. Bars represent the mean time for all four worms to be completely burrowed ±SD, n = 10.
Weight-specific growth rate (WS-GR) of control worms was close to zero (as expected for sexually mature worms) during exposures in freshly spiked and aged sediment (Figure 4). Furthermore, there were no significant differences in WS-GR between the two control groups (65CuCl2 or 65CuO NPs) in either of the two experiments (p = 0.846 in freshly spiked sediment and 0.786 in aged sediment; two-sample t-test). In spiked sediments, there were no significant effects of 65Cu treatments or 65Cu concentration on WS-GR in neither freshly spiked nor aged sediment (in freshly spiked sediment: p = 0.251 for 65CuCl2 and p = 0.718 for 65CuO NPs; in aged sediment: p = 0.472 for 65CuCl2 and p = 0.108 for 65CuO NPs; ANOVA).
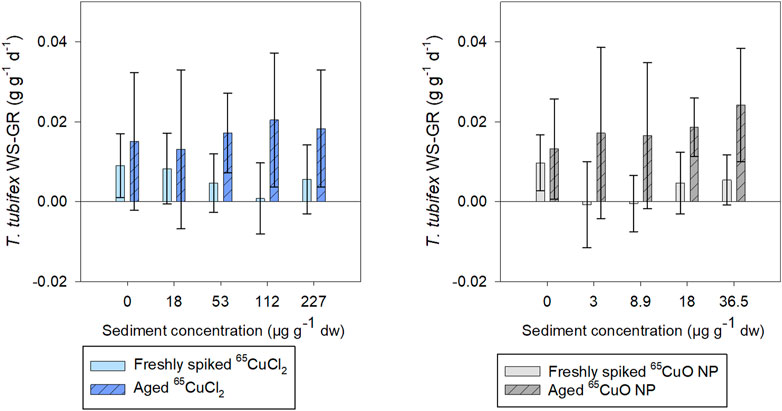
FIGURE 4. T. tubifex weight-specific growth rate (WS-GR) during exposure in freshly spiked sediment (full bars) or aged sediment (scratched bars). Worms were exposed in sediment with 65CuCl2 [blue, (A)] or 65CuO NPs [gray, (B)] at five different concentrations for 28 days. Bars represent the mean WS-GR ± SD, n = 10.
In experiments with freshly spiked sediment, worms were slightly bigger and had slightly lower growth rates than worms in aged sediment (0.009 and 0.014 g g−1 ww tissue d−1, respectively; n = 20 per sample), though not significantly different (p = 0.204; two-sample t-test).
WS-GR of worms from 65CuCl2 or 65CuO NP control treatments did not differ significantly between experiments with freshly spiked sediment and aged sediment (p = 0.328 and 0.448, respectively; two-sample t-test). However, the WS-GR of worms in freshly spiked sediment differed significantly from those exposed to aged sediment with 65CuCl2 for all treatments (18, 112, and 227; p-values = 0.030, 0.007, and 0.041, respectively) except at 53 µg 65Cu g−1 dw sediment (p = 0.233; two-sample t-test). For worms exposed to 65CuO NPs, WS-GR also differed between the two experiments (8.9, 18, and 36.5 μg g−1 dw sediment; p-values = 0.018, 0.001, and 0.002, respectively; two-sample t-test) except at the lowest exposure concentration of 3 µg 65Cu g−1 dw sediment (p = 0.057). Thus, T. tubifex weight-specific growth rate (WS-GR) was not affected by 65Cu form or exposure concentration in sediment but was affected by 2 years of aging of sediment with 65CuCl2 and 65CuO NPs.
It was found that fecal layer increased throughout exposures for all treatments in both freshly spiked and aged sediment indicating that worms were actively feeding throughout both experiments (Supplementary Figure S2 in SI). In freshly spiked sediment, there was a tendency for worms in the control treatments to produce slightly more fecal matter throughout exposures compared to worms exposed to 65CuCl2 (Supplementary Figure S2). However, after 27 days of exposure, there was no effect of 65Cu concentration on worm fecal matter production in freshly spiked or aged sediment with 65CuCl2 (p = 0.192 and 0.067, respectively; ANOVA) or 65CuO NP (p = 0.080 and 0.180, respectively; ANOVA) (Figure 5).
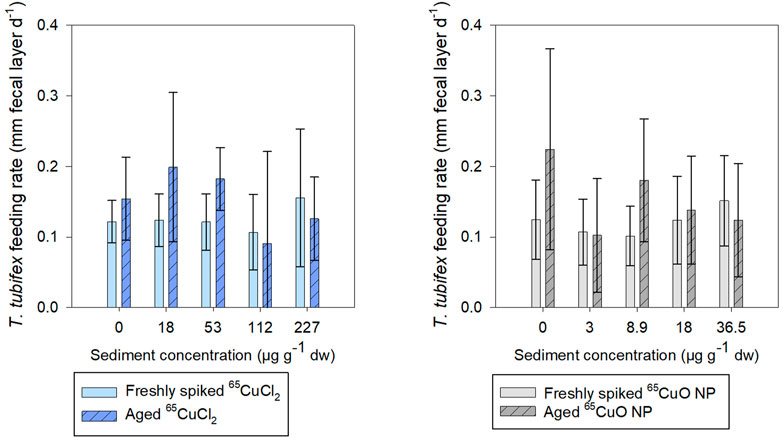
FIGURE 5. T. tubifex feeding rate measured as increase in fecal layer thickness (mm d−1) at day 27 of exposure in freshly spiked (full bars) or aged (schratched bars) sediment with 65CuCl2 [blue, (A)] or 65CuO NPs [gray, (B)] at five different concentrations. Fecal layer thickness was measured with calipers at a pre-destined place of the exposure vial. mean ± SD, n = 10.
There was no influence of aging on the fecal matter production of control worms, and limited effects were observed on feeding of worms in sediments spiked with either 65Cu form. For worms exposed to 65CuCl2, there was no significant difference between fecal matter production in freshly spiked sediment and aged sediment (control, 18, 36.5, 112, and 227 µg 65Cu g−1 dw sediment; p = 0.142, 0.058, 0.722, and 0.422, respectively; two-sample t-test) except at 36.5 µg 65Cu g−1 dw sediment (p = 0.005).
For worms exposed to 65CuO NPs, there was no significant difference between fecal matter production in freshly spiked sediment and aged sediment (control, 3, 18, and 36.5 µg 65Cu g−1 dw sediment; p = 0.063, 0.881, 0.647, and 0.412, respectively; two-sample t-test) except at 8.9 µg 65Cu g−1 dw sediment (p = 0.022). Thus, limited influence of 65Cu treatment, exposure concentration, or aging on feeding rate was observed during exposures.
The mean number of cocoons produced during 28 days of exposure in freshly spiked sediment was about 15 for T. tubifex in all treatments. Hatching efficiency after 2.5 months after exposure termination was generally about 75% and above 60% in all treatments. There was a tendency for increased hatchability with increasing exposure concentration in both 65Cu treatments. However, there was no significant effect of 65Cu sediment concentration on the number of cocoons or hatching efficiency for either 65CuCl2 (p = 0.532 and 0.223, respectively; Kruskal–Wallis) or 65CuO NPs (p = 0.929 and 0.301, respectively; Kruskal–Wallis) (Figure 6). Thus, limited influence 65Cu treatment or exposure concentration on T. tubifex reproduction was observed. Reproduction was only assessed for experiments in freshly spiked sediment and therefore no data are presented for reproduction in aged sediment.
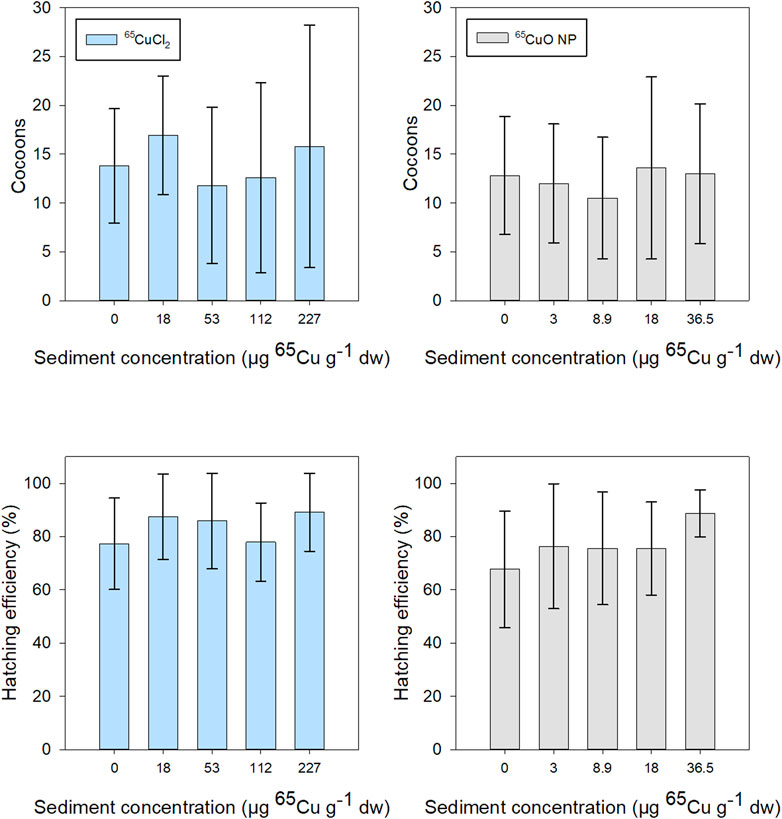
FIGURE 6. T. tubifex reproduction during exposures in freshly spiked sediment with 65CuCl2 (blue) or 65CuO NPs (gray) at five different concentrations. Reproductive output is presented as the mean ± SD number of cocoons at the end of exposure and hatchability (number of cocoons hatched, 2.5 months after exposure termination). n = 10.
In the present study, background Cu concentrations in overlying water of sediments were up to about 70 µg 65Cu L−1, which is in the range of naturally occurring Cu concentrations in freshwater (0.2–100 μg L−1) (Cole et al., 1984; Misra et al., 2012a). The sediment exposure concentrations (up to 230 µg 65Cu g−1 dw sediment for dissolved Cu, administered as 65CuCl2) were selected to reflect the range of Cu sediment concentrations occurring in uncontaminated (often 5–50 µg Cu g−1 dw sediment) to highly contaminated sites (mg Cu g−1 dw range). In the present study, background Cu concentrations in sediment sieved to 63 µm ( ̴ 70 µg Cu g−1 sediment) also reflected naturally occurring background Cu concentrations. Though concentrations of CuO NPs in sediments are expected to increase considerably in the future (Caballero-Guzman and Nowack, 2018), the estimated sediment concentrations of CuO NPs are currently in the ng Cu g−1 range (Garner et al., 2017). Thus, lower exposure concentrations of up to 40 µg 65Cu g−1 dw sediment for 65CuO NPs were selected to increase environmental realism. Though these concentrations are still higher than the generally expected concentrations of CuO NPs in the environment, point sources may lead to hot spots with higher CuO NP concentrations. Exposures to CuO NPs will be chronic and aging should be considered when examining the risk of these NPs in the environment (Lowry et al., 2012).
Transformations of CuO NPs (as well as Cu administered as CuCl2) will inevitably occur in the environment, affecting bioavailability and toxicity. Compared to the literature on transformations occurring in the pelagic zone, little is known about transformations in sediment. The complex and heterogenic sediment matrix combined with the dynamic and stochastic nature of the environmental system makes it very difficult to predict these transformation processes in the sediment compartment (Lowry et al., 2012; Cross et al., 2015). However, aggregation/agglomeration, sorption on solids, adsorption of macromolecules, such as proteins, on the particle surface (referred to as corona formation), sulfidation, dissolution, and redox reactions in the environment are expected to be pronounced for metal oxide NPs (Lowry et al., 2012; Ma et al., 2014; Cross et al., 2015; Baun et al., 2017). During the 2 years of aging in the present study, transformations, such as reduction, have likely been pronounced due to the low oxygen levels present in the sediment. Though the solubility of CuO NPs in the aquatic environment (at neutral pH) has generally been reported to be low (below 3% of the original mass) (Misra et al., 2012a; Ma et al., 2014; Thit et al., 2017a; Thit et al., 2017b), dissolution rate depends on the surrounding environment. For instance, decreased pH increases the dissolution of CuO NPs (Thit et al., 2017a) and available ligands present may either enhance or decrease dissolution. For instance, adsorption of ligands, such as organic acids, can affect dissolution directly or indirectly by influencing aggregation/agglomeration, which decreases surface area and thus dissolution (Mudunkotuwa et al., 2012). In addition, Cu has a high affinity for sulfur and will likely bind to inorganic sulfur in sediment. Ma et al. (2014) have reported increased dissolution after sulfidation of CuO NPs. However, the sulfidation process may also lead to the formation of a relatively insoluble metal-sulfide shell that alters surface charge and increases aggregation/agglomeration (Lowry et al., 2012; Ma et al., 2014), which may likely lead to decreased Cu bioavailability (Wang et al., 2013). Thus, copper sulfide is expected to be the predominant form of Cu in sediment under the sulfate-reducing conditions that have likely existed during the 2 years of aging (without stirring or aeration) in the present study. These conditions are also likely to occur in the aquatic environment and will be predominant in, for instance, sewer pipes and wastewater treatment plants (Ma et al., 2014). In addition, the relative distribution of CuO NPs between sediment grains and pore waters will likely change during aging (Cross et al., 2015). It was beyond the scope of the present study to characterize 65CuO NPs in sediments, but measurements of 65Cu distribution between sediment and overlying water revealed that 65Cu concentration in overlying water decreased and sediment concentrations increased slightly during 2 years of aging. This may indicate that the distribution of Cu changes to increase adsorption to sediment grains and lessen concentrations in pore water and overlying water.
The high background Cu concentrations in sediments and T. tubifex tissue (∼ 30 µg Cu g−1 dw tissue) highlight the importance of a tracer when using a relatively low environmentally realistic exposure concentration of omnipresent metals such as Cu. Enriched stable isotope tracers make it possible to distinguish background Cu from newly accumulated 65Cu. Thus, it allows the detection of 65Cu body burdens in minute tissue samples, such as from T. tubifex (a few mg), despite the high Cu background levels in their tissue. One of the many advantages of this particular labeling method is that the label is incorporated into the NP during synthesis to have a unique isotopic composition throughout (Zhang et al., 2019). This makes the label more robust and reliable than labels attached to the surface, such as fluorescent markers, which can possibly be released (Schür et al., 2019). An array of papers can be consulted for further discussion on the technique (Croteau et al., 2011b; Dybowska et al., 2011; Misra et al., 2012b; Zhang et al., 2019).
The findings on similar 65Cu WS-BB following exposure to the two different 65Cu forms are well in line with the published literature on sediment exposures of sediment-dwelling organisms to CuO NPs and dissolved Cu (Thit et al., 2015a; Thit et al., 2016; Thit and Selck, 2021). In the present study, the increases in 65Cu WS-BB are likely related to both water and sediment exposures. Concentrations in sediment were considerably higher than those in overlying water and may likely have contributed considerably to 65Cu uptake during exposures, as previously forecasted using the biodynamic model based on experiments using the same species and 65CuO NPs (Thit and Selck, 2021) and another oligochaete (Ramskov et al., 2015).
The lower T. tubifex WS-BB of 65Cu after 2 years of sediment aging found in the present study indicates that bioavailability decreases with prolonged aging. Although it has been suggested that transformations, such as sulfidation, may likely decrease bioavailability in sediment (Wang et al., 2013), there is no consensus on whether aged NPs are more or less bioavailable and toxic than pristine particles in sediment (Ma et al., 2014). However, soil research is well in line with the findings in the present study and have reported that bioaccumulation (Tatsi et al., 2018) and toxicity (Jośko et al., 2016) decrease with prolonged aging/contact time between NPs and soil (Jośko and Oleszczuk, 2013; Jośko et al., 2016; Tatsi et al., 2018).
The limited adverse effects observed for either of the two 65Cu forms found in the present study may likely be a result of using relatively low, environmentally realistic exposure concentrations (Thit and Selck, 2021), the high tolerance of T. tubifex that makes this organism especially suitable for bioaccumulation studies, and high variation in some of the tested endpoints. The experiment was designed to elucidate bioaccumulation of the two 65Cu forms during the 28-day exposure and the influence of aging. The design proved very useful for this purpose.
T. tubifex survival was generally high in all treatments (>80%) during exposures to both treatments and in both freshly spiked and aged sediments. There was a slight tendency of decreased survival with increasing 65CuCl2 concentrations in both experiments, but the changes were not sufficient to eliminate that this is coincidental. Thus, no considerable differences were observed between the two 65Cu treatments or between the two experiments. The absence of adverse effects is similar to the findings of a previously published study (Thit and Selck, 2021), where no effects on T. tubifex survival or burrowing were observed. T. tubifex burrowing data showed high variation and were, in general, a difficult endpoint to assess in setups with four individuals per exposure container. This endpoint has previously been reported as an especially environmentally relevant endpoint (Thit et al., 2015a; Thit et al., 2020). Furthermore, CuO NPs have previously been reported to affect the burrowing behavior of sediment-dwelling species (Buffet et al., 2011) and we suggest investigating this endpoint further in the future, e.g., by collecting and weighing the fecal matter produced at the end of the exposure.
Both Cu and CuO NPs have previously been reported to affect the feeding behavior of T. tubifex (Thit et al., 2020; Thit and Selck, 2021) and other sediment-dwelling organisms (Ramskov et al., 2015) and is a highly environmentally relevant endpoint. However, in the present study, we observed limited effects of 65Cu treatment on T. tubifex feeding (slightly higher for controls than worms exposed to 65CuCl2 during exposures in freshly spiked sediment). Aging also only slightly affected feeding (at one concentration for each of the two 65Cu forms). Though WS-GR of control worms did not differ between experiments with freshly spiked sediment and aged sediment, interestingly, aging increased WS-GR of exposed worms in both 65Cu treatments, likely as a result of their slightly smaller size. Further investigation into the influence of aging on this endpoint is warranted.
The number of cocoons produced during 28 days was about 15 per sample (4 per worm) in all treatments and is in line with values of 5–18 cocoons per worm over a period of 72 days, reported in the literature (Kaster, 1980). CuO NPs have previously been reported to increase the hatching of zebrafish eggs (Thit et al., 2017b). Similarly, a tendency of increased hatching efficiency with increasing exposure concentration was observed in both 65Cu treatments. While impaired reproduction has significant implications for populations in the environment, it was very challenging practically and very time-consuming to examine reproduction in the present setup. For future studies, we suggest examining reproduction in smaller-scale experiments keeping a minimum of 10 replicates (and suggest using more due to the high variability in this endpoint) and using a lower number of treatments and fewer endpoints.
The use of stable isotopically labeled 65Cu made it possible to detect 65Cu accumulation in minute tissue samples following exposure at environmentally realistic concentrations. Both 65CuO NPs and dissolved 65Cu sediment exposures resulted in increased 65Cu WS-BB in the freshwater oligochaete T. tubifex. The WS-BBs of 65Cu were lower in organisms exposed to sediments that were aged for 2 years than immediately after the initial spiking with 65CuCl2 and 65CuO NP. This indicates that transformations in sediment decrease the bioavailability of 65Cu in sediment. Our findings did not reveal whether prolonged aging affects the toxicity of 65CuO NPs in sediments. However, the decrease in organism uptake of 65Cu in the experiments with aged sediments suggests that a decrease in toxicity is likely to occur. These findings may aid in the understanding of bioaccumulation behavior and toxicity of metal NPs under environmentally realistic conditions. Realistic exposure concentrations and aging of NPs should preferably be included in future studies to increase environmental realism to predict the environmental risk of metal NPs accurately.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AT and MS carried out the experiment. AT analyzed the data and wrote the manuscript with support from HS and AB. CM, JS, and MS commented on the manuscript. AT, MS, and HS conceived the original idea with support from AB, CM, and JS. AT, HS, AB, JS, and CM obtained funding for the project. HS supervised the project.
This research was financed by the Villum Foundation (NanoTransfer Grant, reference number: 00010592).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to the Villum Foundation for financing the research (Nanotransfer Grant 00010592) and to Xianjin Cui and Eugenia Valsami-Jones for providing 65CuO NPs. We would like to thank Mette Flodgaard for conducting ICP-MS measurements and assistance during the work and Rikke Guttesen and Anne Busk Faarborg for assistance in the laboratory.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ftox.2021.737158/full#supplementary-material
Anschutz, P., Ciutat, A., Lecroart, P., Gérino, M., and Boudou, A. (2012). Effects of Tubificid Worm Bioturbation on Freshwater Sediment Biogeochemistry. Aquat. Geochem. 18 (6), 475–497. doi:10.1007/s10498-012-9171-6
Anyaogu, K. C., Fedorov, A. V., and Neckers, D. C. (2008). Synthesis, Characterization, and Antifouling Potential of Functionalized Copper Nanoparticles. Langmuir. 24 (8), 4340–4346. doi:10.1021/la800102f
Baun, A., Hartmann, N. B., Grieger, K., and Kusk, K. O. (2008). Ecotoxicity of Engineered Nanoparticles to Aquatic Invertebrates: a Brief Review and Recommendations for Future Toxicity Testing. Ecotoxicology. 17 (5), 387–395. doi:10.1007/s10646-008-0208-y
Baun, A., Sayre, P., Steinhäuser, K. G., and Rose, J. (2017). Regulatory Relevant and Reliable Methods and Data for Determining the Environmental Fate of Manufactured Nanomaterials. NanoImpact. 8, 1–10. doi:10.1016/j.impact.2017.06.004
Buffet, P.-E., Richard, M., Caupos, F., Vergnoux, A., Perrein-Ettajani, H., Luna-Acosta, A., et al. (2013). A Mesocosm Study of Fate and Effects of CuO Nanoparticles on Endobenthic Species (Scrobicularia Plana, Hediste Diversicolor). Environ. Sci. Technol. 47 (3), 130110104824003. doi:10.1021/es303513r
Buffet, P.-E., Tankoua, O. F., Pan, J.-F., Berhanu, D., Herrenknecht, C., Poirier, L., et al. (2011). Behavioural and Biochemical Responses of Two Marine Invertebrates Scrobicularia Plana and Hediste Diversicolor to Copper Oxide Nanoparticles. Chemosphere. 84 (1), 166–174. doi:10.1016/j.chemosphere.2011.02.003
Burton, G. A. (2013). Assessing Sediment Toxicity: Past, Present, and Future. Environ. Toxicol. Chem. 32 (7), 1438–1440. doi:10.1002/etc.2250
Caballero-Guzman, A., and Nowack, B. (2018). Prospective Nanomaterial Mass Flows to the Environment by Life Cycle Stage From Five Applications Containing CuO, DPP, FeO , CNT and SiO2. J. Clean. Prod. 203, 990–1002. doi:10.1016/j.jclepro.2018.08.265
Cioffi, N., Ditaranto, N., Torsi, L., Picca, R. A., Sabbatini, L., Valentini, A., et al. (2005). Analytical Characterization of Bioactive Fluoropolymer Ultra-Thin Coatings Modified by Copper Nanoparticles. Anal. Bioanal. Chem. 381 (3), 607–616. doi:10.1007/s00216-004-2761-4
Cole, R. H., Frederick, R. E., Healy, R. P., and Rolan, R. G. (1984). Preliminary Findings of the Priority Pollutant Monitoring Project of the Nationwide-Urban-Runoff-Program. J. Water Pollut. Control. Fed. 56 (7), 898–908.
Cross, R. K., Tyler, C., and Galloway, T. S. (2015). Transformations that Affect Fate, Form and Bioavailability of Inorganic Nanoparticles in Aquatic Sediments. Environ. Chem. 12 (6), 627–642. doi:10.1071/en14273
Croteau, M.-N., Dybowska, A. D., Luoma, S. N., and Valsami-Jones, E. (2011a). A Novel Approach Reveals that Zinc Oxide Nanoparticles Are Bioavailable and Toxic after Dietary Exposures. Nanotoxicology. 5 (1), 79–90. doi:10.3109/17435390.2010.501914
Croteau, M.-N., Misra, S. K., Luoma, S. N., and Valsami-Jones, E. (2011b). Silver Bioaccumulation Dynamics in a Freshwater Invertebrate after Aqueous and Dietary Exposures to Nanosized and Ionic Ag. Environ. Sci. Technol. 45 (15), 6600–6607. doi:10.1021/es200880c
Croteau, M.-N., Luoma, S. N., Topping, B. R., and Lopez, C. B. (2004). Stable Metal Isotopes Reveal Copper Accumulation and Loss Dynamics in the Freshwater BivalveCorbicula. Environ. Sci. Technol. 38 (19), 5002–5009. doi:10.1021/es049432q
Croteau, M.-N., Misra, S. K., Luoma, S. N., and Valsami-Jones, E. (2014). Bioaccumulation and Toxicity of CuO Nanoparticles by a Freshwater Invertebrate after Waterborne and Dietborne Exposures. Environ. Sci. Technol. 48 (18), 10929–10937. doi:10.1021/es5018703
Dybowska, A. D., Croteau, M.-N., Misra, S. K., Berhanu, D., Luoma, S. N., Christian, P., et al. (2011). Synthesis of Isotopically Modified ZnO Nanoparticles and Their Potential as Nanotoxicity Tracers. Environ. Pollut. 159 (1), 266–273. doi:10.1016/j.envpol.2010.08.032
Garner, K. L., Suh, S., and Keller, A. A. (2017). Assessing the Risk of Engineered Nanomaterials in the Environment: Development and Application of the nanoFate Model. Environ. Sci. Technol. 51 (10), 5541–5551. doi:10.1021/acs.est.6b05279
Gomes, T., Pereira, C. G., Cardoso, C., Pinheiro, J. P., Cancio, I., and Bebianno, M. J. (2012). Accumulation and Toxicity of Copper Oxide Nanoparticles in the Digestive Gland of Mytilus galloprovincialis. Aquat. Toxicol. 118-119, 72–79. doi:10.1016/j.aquatox.2012.03.017
Jośko, I., and Oleszczuk, P. (2013). Influence of Soil Type and Environmental Conditions on ZnO, TiO2 and Ni Nanoparticles Phytotoxicity. Chemosphere. 92 (1), 91–99. doi:10.1016/j.chemosphere.2013.02.048
Jośko, I., Oleszczuk, P., and Skwarek, E. (2016). The Bioavailability and Toxicity of ZnO and Ni Nanoparticles and Their Bulk Counterparts in Different Sediments. J. Soils Sediments. 16 (6), 1798–1808. doi:10.1007/s11368-016-1365-x
Kaster, J. L. (1980). The Reproductive Biology of Tubifex Tubifex Muller (Annelida:Tubificidae). Am. Midland Naturalist. 104 (2), 364–366. doi:10.2307/2424877
Keller, A. A., Adeleye, A. S., Conway, J. R., Garner, K. L., Zhao, L., Cherr, G. N., et al. (2017). Comparative Environmental Fate and Toxicity of Copper Nanomaterials. Nanoimpact. 7, 28–40. doi:10.1016/j.impact.2017.05.003
Keller, A. A., McFerran, S., Lazareva, A., and Suh, S. (2013). Global Life Cycle Releases of Engineered Nanomaterials. J. Nanopart Res. 15 (6), 1692. doi:10.1007/s11051-013-1692-4
Klaine, S. J., Alvarez, P. J. J., Batley, G. E., Fernandes, T. F., Handy, R. D., Lyon, D. Y., et al. (2008). Nanomaterials in the Environment: Behavior, Fate, Bioavailability, and Effects. Environ. Toxicol. Chem. 27 (9), 1825–1851. doi:10.1897/08-090.1
Lammel, T., Thit, A., Cui, X., Mouneyrac, C., Baun, A., Valsami-Jones, E., et al. (2021). Dietary Uptake and Effects of Copper in Sticklebacks at Environmentally Relevant Exposures Utilizing Stable Isotope-Labeled 65CuCl2 and 65CuO NPs. Sci. Total Environ. 757, 143779. doi:10.1016/j.scitotenv.2020.143779
Lammel, T., Thit, A., Cui, X., Mouneyrac, C., Baun, A., Valsami-Jones, E., et al. (2020). Trophic Transfer of CuO NPs From Sediment to Worms (Tubifex Tubifex) to Fish (Gasterosteus aculeatus): a Comparative Study of Dissolved Cu and NPs Enriched With a Stable Isotope Tracer (65Cu). Environ. Sci. Nano. 7 (8), 2360–2372. doi:10.1039/d0en00227e
Lowry, G. V., Gregory, K. B., Apte, S. C., and Lead, J. R. (2012). Transformations of Nanomaterials in the Environment. Environ. Sci. Technol. 46 (13), 6893–6899. doi:10.1021/es300839e
Luoma, S., and Rainbow, P. (2008). Metal Contamination in Aquatic Environments: Science and Lateral Management. New York: Cambridge University Press.
Ma, R., Stegemeier, J., Levard, C., Dale, J. G., Noack, C. W., Yang, T., et al. (2014). Sulfidation of Copper Oxide Nanoparticles and Properties of Resulting Copper Sulfide. Environ. Sci. Nano. 1 (4), 347–357. doi:10.1039/c4en00018h
Matisoff, G., Wang, X., and McCall, P. L. (1999). Biological Redistribution of Lake Sediments by Tubificid Oligochaetes: Branchiura Sowerbyi and Limnodrilus Hoffmeisteri/Tubifex Tubifex. J. Great Lakes Res. 25 (1), 205–219. doi:10.1016/s0380-1330(99)70729-x
Misra, S. K., Dybowska, A., Berhanu, D., Croteau, M. N., Luoma, S. N., Boccaccini, A. R., et al. (2012a). Isotopically Modified Nanoparticles for Enhanced Detection in Bioaccumulation Studies. Environ. Sci. Technol. 46 (2), 1216–1222. doi:10.1021/es2039757
Misra, S. K., Dybowska, A., Berhanu, D., Croteau, M. N., Luoma, S. N., Boccaccini, A. R., et al. (2012b). Isotopically Modified Nanoparticles for Enhanced Detection in Bioaccumulation Studies. Environ. Sci. Technol. 46 (2), 1216–1222. doi:10.1021/es2039757
Mudunkotuwa, I. A., Pettibone, J. M., and Grassian, V. H. (2012). Environmental Implications of Nanoparticle Aging in the Processing and Fate of Copper-Based Nanomaterials. Environ. Sci. Technol. 46 (13), 7001–7010. doi:10.1021/es203851d
Nanotechproject (2021). Available at: https://nanotechproject.tech/cpi/ (Accessed July, , 2021).
Nel, A., Xia, T., Madler, L., and Li, N. (2006). Toxic Potential of Materials at the Nanolevel. Science. 311 (5761), 622–627. doi:10.1126/science.1114397
Ozoh, P. T. E. (1992). The Importance of Adult Hediste (Nereis) Diversicolor in Managing Heavy Metal Pollution in Shores and Estuaries. Environ. Monit. Assess. 21 (3), 165–171. doi:10.1007/bf00399685
Pang, C., Selck, H., Banta, G. T., Misra, S. K., Berhanu, D., Dybowska, A., et al. (2013). Bioaccumulation, Toxicokinetics, and Effects of Copper From Sediment Spiked With Aqueous Cu, Nano-CuO, or Micro-CuO in the Deposit-Feeding Snail,Potamopyrgus Antipodarum. Environ. Toxicol. Chem. 32, 1561–1573. doi:10.1002/etc.2216
Pang, C., Selck, H., Misra, S. K., Berhanu, D., Dybowska, A., Valsami-Jones, E., et al. (2012). Effects of Sediment-Associated Copper to the Deposit-Feeding Snail, Potamopyrgus Antipodarum: a Comparison of Cu Added in Aqueous Form or as Nano- and Micro-CuO Particles. Aquat. Toxicol. 106-107, 114–122. doi:10.1016/j.aquatox.2011.10.005
Park, B. K., Kim, D., Jeong, S., Moon, J., and Kim, J. S. (2007). Direct Writing of Copper Conductive Patterns by Ink-Jet Printing. Thin Solid Films. 515 (19), 7706–7711. doi:10.1016/j.tsf.2006.11.142
Perelshtein, I., Applerot, G., Perkas, N., Wehrschuetz-Sigl, E., Hasmann, A., Guebitz, G., et al. (2009). CuO-Cotton Nanocomposite: Formation, Morphology, and Antibacterial Activity. Surf. Coat. Technology. 204 (1-2), 54–57. doi:10.1016/j.surfcoat.2009.06.028
Praetorius, A., Scheringer, M., and Hungerbühler, K. (2012). Development of Environmental Fate Models for Engineered Nanoparticles-A Case Study of TiO2 Nanoparticles in the Rhine River. Environ. Sci. Technol. 46 (12), 6705–6713. doi:10.1021/es204530n
Quik, J. T. K., Stuart, M. C., Wouterse, M., Peijnenburg, W., Hendriks, A. J., and van de Meent, D. (2012). Natural Colloids Are the Dominant Factor in the Sedimentation of Nanoparticles. Environ. Toxicol. Chem. 31 (5), 1019–1022. doi:10.1002/etc.1783
Ramskov, T., Selck, H., Banta, G., Misra, S. K., Berhanu, D., Valsami-Jones, E., et al. (2014). Bioaccumulation and Effects of Different-Shaped Copper Oxide Nanoparticles in the Deposit-Feeding Snail Potamopyrgus Antipodarum. Environ. Toxicol. Chem. 33 (9), 1976–1987. doi:10.1002/etc.2639
Ramskov, T., Thit, A., Croteau, M.-N., and Selck, H. (2015). Biodynamics of Copper Oxide Nanoparticles and Copper Ions in an Oligochaete - Part I: Relative Importance of Water and Sediment as Exposure Routes. Aquat. Toxicol. 164, 81–91. doi:10.1016/j.aquatox.2015.04.022
Ren, G., Hu, D., Cheng, E. W. C., Vargas-Reus, M. A., Reip, P., and Allaker, R. P. (2009). Characterisation of Copper Oxide Nanoparticles for Antimicrobial Applications. Int. J. Antimicrob. Agents. 33 (6), 587–590. doi:10.1016/j.ijantimicag.2008.12.004
Schür, C., Rist, S., Baun, A., Mayer, P., Hartmann, N. B., and Wagner, M. (2019). When Fluorescence Is Not a Particle: The Tissue Translocation of Microplastics in Daphnia Magna Seems an Artifact. Environ. Toxicol. Chem. 38 (7), 1495–1503. doi:10.1002/etc.4436
Selck, H., Forbes, V., and Forbes, T. (1998). Toxicity and Toxicokinetics of Cadmium in Capitella Sp. I:relative Importance of Water and Sediment as Routes of Cadmium Uptake. Mar. Ecol. Prog. Ser. 164, 167–178. doi:10.3354/meps164167
Tatsi, K., Shaw, B. J., Hutchinson, T. H., and Handy, R. D. (2018). Copper Accumulation and Toxicity in Earthworms Exposed to CuO Nanomaterials: Effects of Particle Coating and Soil Ageing. Ecotoxicology Environ. Saf. 166, 462–473. doi:10.1016/j.ecoenv.2018.09.054
Thit, A., Banta, G. T., Palmqvist, A., and Selck, H. (2020). Effects of Sediment-Associated Cu on Tubifex Tubifex - Insights Gained by Standard Ecotoxicological and Novel, but Simple, Bioturbation Endpoints. Environ. Pollut. 266, 115251. doi:10.1016/j.envpol.2020.115251
Thit, A., Banta, G. T., and Selck, H. (2015a). Bioaccumulation, Subcellular Distribution and Toxicity of Sediment-Associated Copper in the Ragworm Nereis Diversicolor: The Relative Importance of Aqueous Copper, Copper Oxide Nanoparticles and Microparticles. Environ. Pollut. 202, 50–57. doi:10.1016/j.envpol.2015.02.025
Thit, A., Dybowska, A., Købler, C., Kennaway, G., and Selck, H. (2015b). Influence of Copper Oxide Nanoparticle Shape on Bioaccumulation, Cellular Internalization and Effects in the Estuarine Sediment-Dwelling Polychaete, Nereis Diversicolor. Mar. Environ. Res. 111, 89–98. doi:10.1016/j.marenvres.2015.06.009
Thit, A., Huggins, K., Selck, H., and Baun, A. (2017a). Acute Toxicity of Copper Oxide Nanoparticles to Daphnia Magna Under Different Test Conditions. Toxicol. Environ. Chem. 99 (4), 665–679. doi:10.1080/02772248.2016.1249368
Thit, A., Skjolding, L. M., Selck, H., and Sturve, J. (2017b). Effects of Copper Oxide Nanoparticles and Copper Ions to Zebrafish ( Danio rerio ) Cells, Embryos and Fry. Toxicol. Vitro. 45, 89–100. doi:10.1016/j.tiv.2017.08.010
Thit, A., Ramskov, T., Croteau, M.-N., and Selck, H. (2016). Biodynamics of Copper Oxide Nanoparticles and Copper Ions in an Oligochaete - Part II: Subcellular Distribution Following Sediment Exposure. Aquat. Toxicol. 180, 25–35. doi:10.1016/j.aquatox.2016.08.011
Thit, A., and Selck, H. (2021). Biodynamics and Adverse Effects of CuO Nanoparticles and CuCl2 in the Oligochaete T. Tubifex: Cu Form Influence Biodynamics in Water, but Not Sediment. Nanotoxicology. 15, 673. doi:10.1080/17435390.2021.1913657
Wang, F., Bi, Q.-L., Wang, X.-B., and Liu, W.-M. (2008). Sliding Friction and Wear Performance of Ti6Al4V in the Presence of Surface-Capped Copper Nanoclusters Lubricant. Tribology Int. 41 (3), 158–165. doi:10.1016/j.triboint.2007.07.010
Wang, Z., von dem Bussche, A., Kabadi, P. K., Kane, A. B., and Hurt, R. H. (2013). Biological and Environmental Transformations of Copper-Based Nanomaterials. ACS nano. 7 (10), 8715–8727. doi:10.1021/nn403080y
Keywords: metal, nanomaterial, bioavailability, effect, aging, freshwater, sediment, transformation
Citation: Thit A, Sandgaard MH, Sturve J, Mouneyrac C, Baun A and Selck H (2021) Influence of Aging on Bioaccumulation and Toxicity of Copper Oxide Nanoparticles and Dissolved Copper in the Sediment-Dwelling Oligochaete Tubifex tubifex: A Long-Term Study Using a Stable Copper Isotope. Front. Toxicology 3:737158. doi: 10.3389/ftox.2021.737158
Received: 06 July 2021; Accepted: 16 August 2021;
Published: 01 October 2021.
Edited by:
Peng Zhang, University of Birmingham, United KingdomReviewed by:
Zhiyong Zhang, Institute of High Energy Physics (CAS), ChinaCopyright © 2021 Thit, Sandgaard, Sturve, Mouneyrac, Baun and Selck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amalie Thit, YXRoaXRqQHJ1Yy5kaw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.