
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Syst. Neurosci., 18 July 2022
Volume 16 - 2022 | https://doi.org/10.3389/fnsys.2022.923576
 Antonella LoMauro1†
Antonella LoMauro1† Maria Takeko Molisso1,2†
Maria Takeko Molisso1,2† Francesca Mameli2
Francesca Mameli2 Fabiana Ruggiero2
Fabiana Ruggiero2 Roberta Ferrucci3,4
Roberta Ferrucci3,4 Chiara Dellarosa5
Chiara Dellarosa5 Giada Aglieco2
Giada Aglieco2 Andrea Aliverti1
Andrea Aliverti1 Sergio Barbieri2
Sergio Barbieri2 Maurizio Vergari2*
Maurizio Vergari2*Psychological distress among healthcare professionals, although already a common condition, was exacerbated by the COVID-19 pandemic. This effect has been generally self-reported or assessed through questionnaires. We aimed to identify potential abnormalities in the electrical activity of the brain of healthcare workers, operating in different roles during the pandemic. Cortical activity, cognitive performances, sleep, and burnout were evaluated two times in 20 COVID-19 frontline operators (FLCO, median age 29.5 years) and 20 operators who worked in COVID-19-free units (CFO, median 32 years): immediately after the outbreak of the pandemic (first session) and almost 6 months later (second session). FLCO showed higher theta relative power over the entire scalp (FLCO = 19.4%; CFO = 13.9%; p = 0.04) and lower peak alpha frequency of electrodes F7 (FLCO = 10.4 Hz; CFO = 10.87 Hz; p = 0.017) and F8 (FLCO = 10.47 Hz; CFO = 10.87 Hz; p = 0.017) in the first session. FLCO parietal interhemispheric coherence of theta (FLCO I = 0.607; FLCO II = 0.478; p = 0.025) and alpha (FLCO I = 0.578; FLCO II = 0.478; p = 0.007) rhythms decreased over time. FLCO also showed lower scores in the global cognitive assessment test (FLCO = 22.72 points; CFO = 25.56; p = 0.006) during the first session. The quantitative evaluation of the cortical activity might therefore reveal early signs of changes secondary to stress exposure in healthcare professionals, suggesting the implementation of measures to prevent serious social and professional consequences.
Stress among healthcare professionals working in hospitals and medical environments is a common feature. Healthcare professionals usually work long shifts, often overnight, subsequently suffering from sleep deprivation (Ganesan et al., 2019; Di Muzio et al., 2020). In addition, they deal with responsibility and emergencies, performing difficult procedures and treating critically ill patients. If healthcare professionals are not provided with suitable emotional support, the resulting heavy emotional load may therefore lead to severe consequences. Immediate interventions are essential to enhance psychological resilience and improve the healthcare systems' capacity (Pappa et al., 2020). Previous studies established that healthcare workers, especially those assigned to Intensive Care Units, are commonly affected by anxiety, post-traumatic stress syndrome (PTSD), and stress-related disorders, such as fatigue, burnout, and lack of motivation and accomplishment (Mealer et al., 2007; Golubic et al., 2009). This may affect the cognitive performance of clinicians: considering that memory and concentration are essential for healthcare professions, as they impact the decision-making process, the quality of care provided to patients may worsen and, in extreme cases, even personal lives can be jeopardized (Leblanc, 2009).
Although mental stress has been a common issue worldwide for decades, the COVID-19 pandemic has led to a widespread mental crisis whose social and health implications could last for many years ahead (Imperatori et al., 2014; Shevlin et al., 2020). Due to their frontline work with COVID-19 patients, healthcare workers are today even more exposed to the risk of developing physical and mental health issues (Babore et al., 2020; Melnyk et al., 2020; Alonso et al., 2021; Gilleen et al., 2021; Ranieri et al., 2021; Schneider et al., 2021; Testoni et al., 2021; Tauro et al., 2022). Since 2020, many healthcare workers have been facing longer work shifts and a heavier emotional load, dealing with critically ill patients and deaths in a climate of deep uncertainty, living with the fear of being infected and of infecting people close to them, also because of a general lack of adequate personal protective equipment (Ehrlich et al., 2020). Results on the impact of the first wave of the COVID-19 pandemic on the mental health of very large cohorts of healthcare workers have already been published. Alonso et al. administered a cross-sectional, web-based survey to more than 9,000 Spanish healthcare workers, finding that 45.7% of the subjects suffered from mental health conditions such as anxiety, panic attacks, and substance abuse, while 14.5% of them suffered from a disabling mental disorder (Alonso et al., 2021). Gilleen et al. also based their research on an online survey, reporting that nearly a third of the 2,000 British respondents suffered from moderate to severe levels of anxiety and depression (Gilleen et al., 2021). It is therefore clear that the psychological wellbeing of healthcare workers was negatively affected by the pandemic, and this might prove to be a serious problem in the future: therefore, it is crucial for this category of workers to address their mental health issues. However, the existing literature on healthcare work during the COVID-19 pandemic is mostly based on self-reporting through questionnaires, surveys, and interviews, while no study was conducted to assess the presence of potential abnormalities in the brain waves or in the electrical activity of the brain in healthcare workers: it is still undetermined if and how those issues are reflected in the cortical activity in this specific category of operators. The idea behind the present study came after an association between high levels of cumulative life stress and aberrant resting-state EEG was recently found in different categories of patients (Marshall and Cooper, 2017; Ehrhardt et al., 2021; Berretz et al., 2022). Moreover, studies recording EEG signals for mental stress evaluation found stress-related altered rhythms by extracting quantitative parameters from the signals (Seo and Lee, 2010), while spectral analysis of EEG showed decreased alpha power, mainly in the posterior region, and increased theta power in the central region and beta power in the frontal region (Begić et al., 2001). Additionally, it was found that, with increased levels of stress, the frontal connectivity in beta frequencies decreased, while theta and alpha connectivities increased in the parietal region (Imperatori et al., 2014; Lee et al., 2014). Functional connectivity is, therefore, an interesting and useful indicator for the quantification of the interactions between different neuronal networks, as it evaluates the temporal correlation between two or more spatially distant regions (Fingelkurts et al., 2005). Finally, during the COVID-19 pandemic, sleep disturbances were also reported by frontline health care workers, mainly insomnia and sleep disruptions (Stewart et al., 2021).
For these reasons, we conducted a pilot study to ascertain if—and how—recording brain activity might help in objectively identifying the consequences of severe stress exposure. We expect the COVID-19 pandemic to be such a heavy factor of stress in healthcare professionals to negatively affect not only the perceived sleep quality and burnout on working environment but also the cognitive performances, especially memory skills, and to impact on brain activity. In particular, we expect to detect increased theta power and decreased alpha power and peak alpha frequency, together with an increased alpha coherence, as suggested by previous findings (Begić et al., 2001; Imperatori et al., 2014; Lee et al., 2014). In addition to the subjective self-reported symptoms and perception, the EEG analysis would objectivize the evaluation of the operators' condition. Providing an objective and, hopefully, a more sensitive outcome would have important social and professional implications, as exposure to stress may result in physical and mental disorders.
The aim of the present study was to evaluate the impact of severe stress exposure on healthcare professionals who have worked in direct contact with patients with COVID-19 after the pandemic “first wave” (Spring 2020). More specifically, we were interested in investigating potential changes in cortical activity, cognitive performances, the insurgence of burnout, and sleep quality. A quantitative evaluation of these items was therefore implemented and administrated two times through dedicated tests in order to detect possible consequences of severe stress exposure not only immediately after the outbreak of the sanitary emergency but also after a few months. This would allow the authors to draw a comparison between acute and recovery phases. Finally, as a stressful situation sets off a chain of physical reactions, such as a temporary acceleration of breathing and heart rate (Muraoka et al., 1998; Tan et al., 2011; Kim et al., 2018; Bustamante-Sánchez et al., 2020), we also aimed to assess if the two aforementioned parameters might provide additional and useful information.
This single-center, observational longitudinal study was conducted at the Neurophysiopathology Unit of Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico of Milan, Italy, in collaboration with Politecnico di Milano for data analysis. The protocol of the study was drawn up in conformity with Good Clinical Practice norms of the European Union and with the current revision of the Declaration of Helsinki and was approved by the Research Ethics Board of the IRCCS Ca' Granda Ospedale Maggiore Policlinico (439_2020).
The study cohort comprised healthcare professionals working at Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico. The subjects were divided into two groups (Table 1), one of the frontline COVID-19 operators (FLCO) and one of the operators who worked in COVID-19-free units (CFO) during the outbreak of the pandemic (March-May 2020) and also after that time. Subjects were first enrolled among the staff of the Neuroscienze e salute mentale (Neuroscience and mental health) department, who were invited by e-mail to participate in the study and provided with information about the study goal and procedures. All participants volunteered and gave written consent. The inclusion criteria were the following: age <60 years, no neurological disorders, no post-traumatic syndrome disorder (assessed by interview before starting each of the two study sessions), no chronic therapy, and no leave of absence since the beginning of the pandemic. Healthcare workers were considered FLCOs when their entire shift required them to be in direct contact with patients with COVID-19 in order to have identical frequency and duration of exposure and proximity to patients.
All subjects were evaluated in the afternoon, at the end of their morning shift, and cognitive performances were tested resorting to the Italian version of the Montreal Cognitive Assessment (MoCA) and the Stroop Color and Word Test (SCWT), followed by the Pittsburgh Sleep Quality Index (PSQI) and the Maslach Burnout Inventory-General Survey (MBI-GS). Additionally, electroencephalogram (EEG) and electrocardiogram (EKG) signals were simultaneously recorded. Recordings and tests were carried out separately and administrated by the same operator. Both groups of subjects were evaluated two times: in a first session (18 May to 22 July 2020), immediately after the first epidemic wave, to evaluate stress in the acute phase, and in a second session (1 October to 12 November 2020), almost 6 months after, to evaluate possible chronic issues or recovery stress. All subjects did not take any naps during the morning work shift and did not take any psychostimulant drugs before the session. The caffeine intake was limited to one h before the acquisition of data.
Brain activity was measured by EEG recording administered by qualified neurophysiologists in a quiet room, with all windows closed in order to minimize the noises coming from outside the building. It was recorded in the resting-state condition for 8 min, during which subjects were seated on a comfortable chair with their eyes closed. Subjects were instructed to stay awake and, whenever they were drifting into sleep, the neurophysiologist resorted to low audio input to help the subject keep vigilant (Jobert et al., 2013). The subjects were instrumented with 19 bridge silver and silver chloride electrodes fitted on a plastic prewired head cap and placed on the scalp according to the 10–20 system (namely: Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, Cz, P3, P4, Pz, T3, T4, T5, T6, O1, and O2), with an electroconductive paste applied between the electrodes and the skin to guarantee optimal connection (Micromed System Plus Electroencephalograph, Galileo). The signals were recorded with a 512 Hz sampling frequency and filtered by an offline first order zero-phase Butterworth band-pass filter, with cutoff frequencies set, respectively, at 1.6 and 70 Hz to remove frequencies outside the range of interest. All segments of the signal affected by ocular, muscular, or other types of artifacts were removed manually and by means of independent component analysis and then a 50 Hz notch filter was applied to remove power line noise. After this pre-processing, the main EEG rhythms and dominant frequencies were extracted by spectral and connectivity analyses. Delta (1.6–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), gamma1 (30–50 Hz), and gamma2 (50–70 Hz) frequency bands were considered. Because we considered 0.5 Hz increments bins for all the bands, we had therefore acquired the 1.5 and 2 Hz data points, but the lower cutoff for the delta frequency band was 1.6 Hz (Jobert et al., 2013; Malver et al., 2014). For this reason, we performed a linear interpolation between the two discrete data points (1.5 and 2 Hz) with a window length of 2 s and 0.1 Hz increment to estimate the new data point of 1.6 Hz.
Welch's averaged modified periodogram method was implemented to determine the power spectral density (PSD) of the brain activity recorded by each electrode with a 2-s window length. The PSD curves' relative powers (i.e., the ratios between the area under the curve in each frequency band and the total area of the curve) were calculated to estimate how each rhythm contributes to the entire. Then, dominant frequencies (i.e., frequencies corresponding to the peaks of the power spectrum) were calculated first for the entire frequency range (Main Dominant Frequency) and then for the theta and alpha frequency bands (Peak Theta Frequency and Peak Alpha Frequency), being the main frequency bands related to stress and cognitive impairment (Klimesch, 1999; Begić et al., 2001; Hayes et al., 2012). Both parameters were computed for every single electrode as well as for each cerebral region of interest: i.e., central (C3, Cz, C4, F3, Fz, and F4 electrodes), posterior (T5, T6, P3, P4, Pz, O1 and O2 electrodes), occipital (O1 and O2 electrodes), and global (i.e., the entire scalp). Because the activity recorded by Fp1 and Fp2 electrodes was severely affected by residual ocular artifacts, which are mostly muscular artifacts, they were excluded from the analysis.
Besides spectral analysis, the connectivity analysis was also applied to detect the presence of temporal correlations among brain activities produced by spatially distant cortical areas. The coherence among activities recorded by electrodes located on the same cortical region but on opposite hemispheres was therefore calculated (Jorge et al., 2017). Interhemispheric coherence was firstly evaluated for theta, alpha, and beta rhythms, and then the entire frequency range was considered (Matlab R2020a and the plugin EEGLAB 2019.1 toolbox).
One electrode was placed on the left arm and a second one on the right arm in order to record a one-lead EKG for cardiac activity monitoring during EEG recording. The duration of the EKG recording was also 8 min. The number of heartbeats was obtained through the implementation of the Pan-Tompkins algorithm, a method that detects the QRS complexes in an EKG signal (Pan and Tompkins, 1985). Then, the standard deviation of R-R intervals (SDRR) was derived as a time-domain measurement of heart rate variability (HRV) (Tan et al., 2011; Järvelin-Pasanen et al., 2018; Kim et al., 2018). Finally, because the respiratory signals can be indirectly derived by the EKG signal, the respiratory sinus arrhythmia (RSA) method was implemented to calculate the breathing rate (Cysarz et al., 2008).
All participants underwent a comprehensive neuropsychological assessment, including the Italian version of the Montreal Cognitive Assessment (MoCA), the Stroop Color and Word Test (SCWT), the Pittsburgh Sleep Quality Index (PSQI), and the Maslach Burnout Inventory-General Survey (MBI-GS).
The test screens the global cognitive functioning to detect cognitive impairment and it is composed of six items, each focused on a specific cognitive domain: (1) memory [0–5 points]; (2) visuospatial ability [0–4 points]; (3) executive function [0–4 points]; (4) attention and working memory [0–6 points]; (5) language [0–6 points]; and (6) orientation in time and space [0–6 points]. A single score is assigned to each item according to the performance, and then, a total score is calculated by adding up all single scores and normalized according to the educational level of the subject, up to a maximum value of 30 points. The higher the total score, the better the cognitive performances are. Cognitive functions are considered normal with a total score of ≥15.51 points (Nasreddine et al., 2005; Smith et al., 2007; Santangelo et al., 2015).
This is a neuropsychological test used to evaluate working memory and selective attention, processing speed, and cognitive flexibility, as cognitive processes are associated with the frontal lobe of the brain. The test assesses the ability to inhibit cognitive interference, which occurs when the processing of a stimulus feature affects the simultaneous processing of another attribute of the same stimulus (Stroop, 1935; Caffarra et al., 2002). The SCWT we administered is divided into three parts: reading, denomination, and interference. The SCWT was administered by means of physical cards and subjects were asked to read three different tables as fast as they could. Two of them represented the “congruous condition” in which participants are required to read names of colors (henceforth referred to as “color-words”) printed in black ink (W) and then name different color patches (C). Conversely, in the third table, named “color-word (CW) condition,” color words were printed in an inconsistently colored ink (for instance, the word “red” is printed in green ink). Thus, in this incongruent condition, participants were required to name the color of the ink instead of reading the word. The test was evaluated by counting the interference effect error (the number of mistakes/omissions made by the subject) and by the interference effect time (the time required to accomplish the test). Both the interference effect error and the interference effect time were obtained by subtracting the mean values of reading and denomination parts from the values of the third interference part with the formula: WC – [(W + C)/2], indicating the degree to which the person has control over interference. A participant has good cognitive performances with 0 or few mistakes and a very short time of execution.
This is a self-rated questionnaire that evaluated the quality of sleep and its associated disturbances in the month preceding the test (Buysse et al., 1989). It is composed of 19 questions combined to assess the following seven items of sleep-related specific aspects: 1. subjective sleep quality; 2. sleep latency; 3. sleep duration; 4. sleep efficiency; 5. sleep disturbances; 6. use of sleep medications; and 7. daytime dysfunctions. Each item is scored between 0 (absence of disturbances) and 3 (serious problems associated with the specific sleep aspect). All scores are then summed together to obtain a global PSQI score, ranging between 0 and 21 points. The higher the total score, the worse the sleep quality is.
This is a self-rated questionnaire used to assess the perceived burnout in the working environment (Maslach et al., 1996; Mealer et al., 2009), including healthcare professionals (Hallberg and Sverke, 2006) and social workers (Kim and Ji, 2009). The questionnaire asks participants 22 questions. The subject has to score each question with a number of points between 0 (never perceived) and 6 (felt everyday), depending on how often they feel the situation or emotion described. The questions are then combined into 3 main items that assess burnout: (1) emotional exhaustion [0–54 points]; (2) depersonalization [0–30 points]; and (3) personal accomplishment [0–48 points]. Each item is usually described in a qualitative way by using the terms low, moderate, or high. Higher scores of emotional exhaustion and depersonalization, together with low scores of personal accomplishment, are indicators of burnout.
After computing all the parameters from each test, a statistical analysis was performed to determine the presence of statistically significant differences between data according to two factors: groups (FLCO vs. CFO) and session times (acute phase vs. chronic phase). The Kolmogorov-Smirnov test was applied to verify the distribution of the datasets and data were not normally distributed. For this reason, non-parametric tests were used for the statistical analysis of data. The Kruskal–Wallis and the Friedman tests were performed: the former was used to compare FLCO and CFO groups for each session; the latter was used to compare the two sessions within each group. A significance level equal to 5% was considered significant for both tests. The statistical analysis was performed in MatLab 2020a (MathWorks, Natick, MA). We considered theta relative power in the global region as our primary outcome. We could not find relevant published data to base a sample size calculation on, as theta relative power in the global region was never performed before on this kind of operator. For this reason, we ran a pilot study on 8 operators (4 FLCO: 3 women, median age: 34.5 years; 3 nurses; 1 intermediate care technician and 4 CFO: 3 women, median age: 40.2 years; 3 nurses; 1 intermediate care technician) to compute the mean (FLCO: 11.4; CFO: 14.8) and standard deviation (FLCO: 3.4; CFO: 2.1) of theta relative power in the global region. The difference between these two independent means provided an effect size of 1.203 that, with a type-1 error probability α = 0.05, a power (1-β, with β being type-2 error probability) of 0.95, and an allocation ratio of 1, resulted in a sample size of 38 subjects (19 FLCO and 19 CFO). The power analysis was performed in G*Power 3.1.9.4 software.
A total of 40 healthcare professionals volunteered to participate in the study: 20 frontline COVID-19 operators (FLCO) and 20 operators who worked in a COVID-19-free unit (CFO). The main characteristics of both groups are summarized in Table 1.
Because of the huge amount of data collected and in order to make the results more readable, we decided to report only statistically significant results with clinical implications, in CFO and FLCO groups at the first session and/or in CFO and FLCO groups in the second session and/or within the CFO group between the two sessions and/or within the FLCO group between the two sessions. All the other results of this exploratory analysis can be found in Supplementary Material.
Among the power spectral density curves relative powers, significance was found only for the theta frequency band. In the first session, the theta relative power of FLCO was significantly higher than CFO when considering the central, posterior, and global regions. The theta relative power of FLCO also remained higher in the second session but only in the central region (Figure 1). The Peak Alpha Frequency was systematically lower in FLCO in the first session for electrodes F7 and F8 (Figure 2).
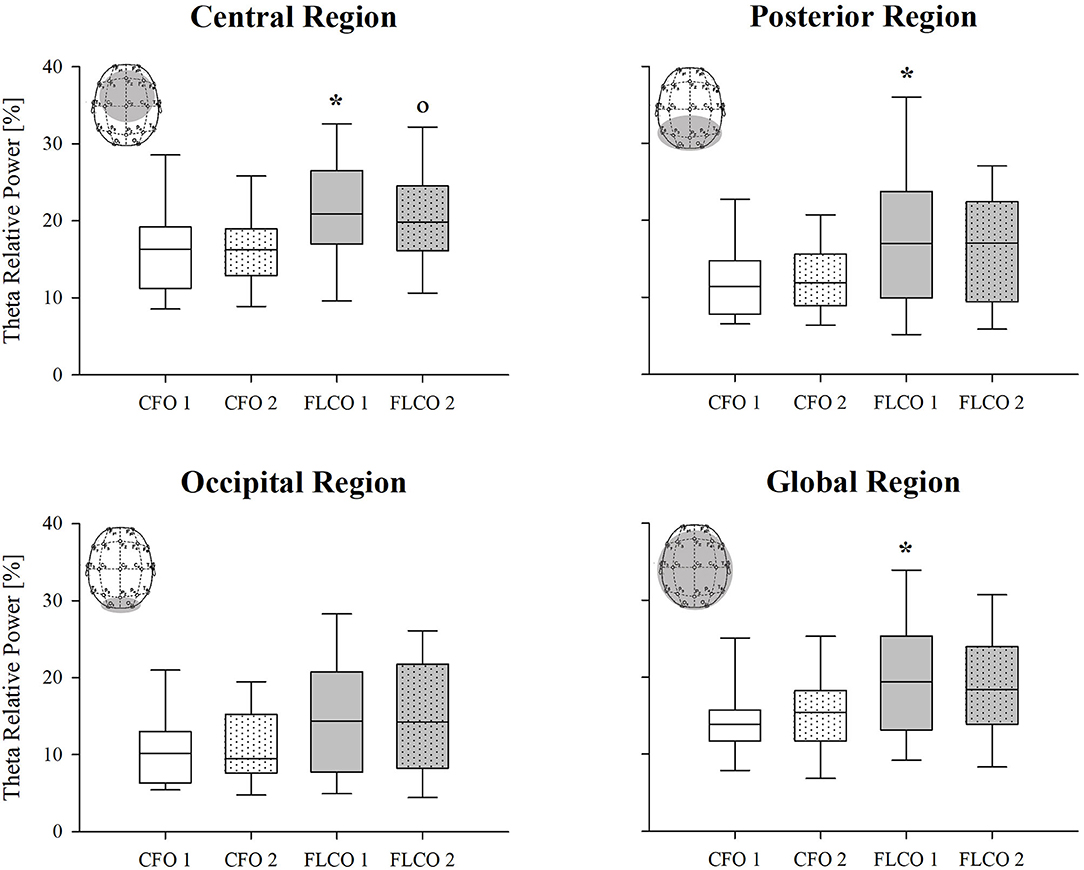
Figure 1. A box-and-whisker plot representing the median (line within the box), the interquartile range (length of the box), the 90th and 10th percentiles (whiskers above and below the box) of the electroencephalographic derived theta relative power in central (top left panel), posterior (top right panel), occipital (bottom left panel), and global regions (bottom right panel) in operators who worked in COVID-19-free wards and departments (CFO, white) and in frontline COVID-19 operators during the pandemic (FLCO, gray) during the first (1) and the second sessions (2). *p < 0.05 CFO1 vs. FLCO1; °p < 0.05 CFO2 vs. FLCO2.
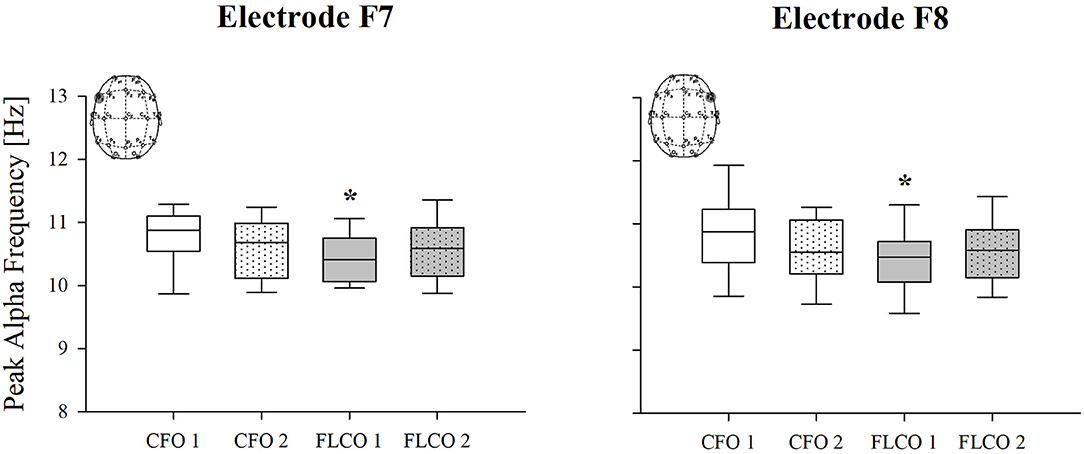
Figure 2. A box-and-whisker plot representing the median (line within the box), the interquartile range (length of the box), the 90th and 10th percentiles (whiskers above and below the box) of the electroencephalographic derived peak alpha frequency (PAF) in F7 (left panel) and F8 electrodes (right panel) in operators who worked in COVID-19-free wards and departments (CFO, white) and in frontline COVID-19 operators during the pandemic (FLCO, gray) during the first (1) and the second sessions (2). *p < 0.05 CFO1 vs. FLCO1.
A significantly higher interhemispheric coherence of both the alpha and theta rhythms was found in FLCO in the first session between two couple of electrodes, P3-P4 and T3-T4, although they were both significantly reduced in the second session between electrodes P3-P4. When considering electrodes C3-C4, only the interhemispheric theta coherence was higher in FLCO in the first session (Figure 3).
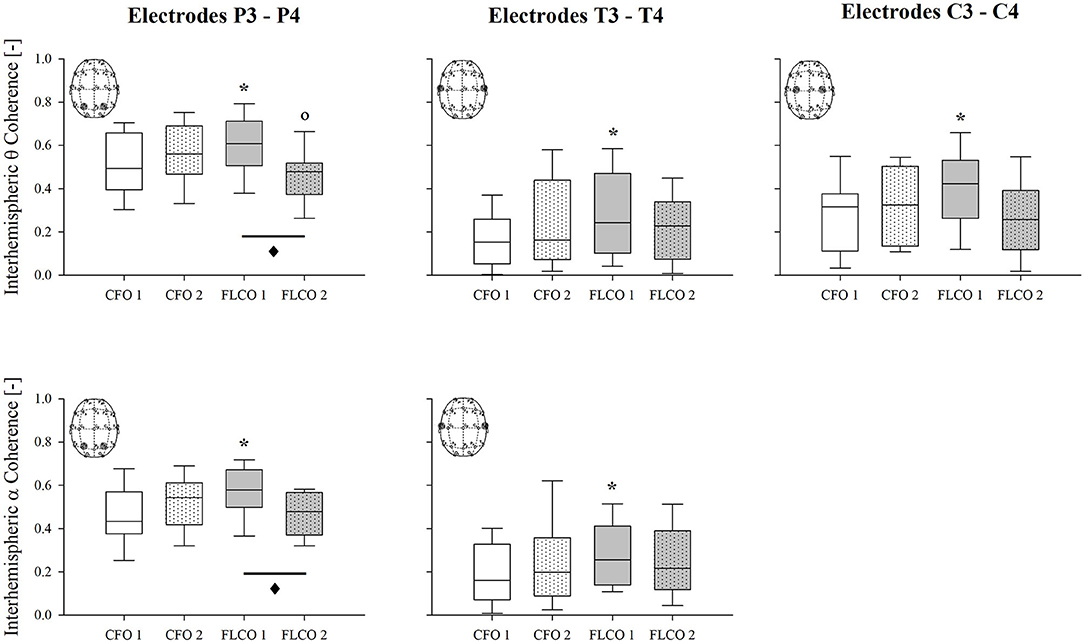
Figure 3. A box-and-whisker plot representing the median (line within the box), the interquartile range (length of the box), the 90th and 10th percentiles (whiskers above and below the box) of the electroencephalographic derived interhemispheric theta coherence in P3-P4 (top left panel), T3-T4 (top central panel), C3-C4 (top right panel) electrodes and interhemispheric alpha coherence in P3-P4 (bottom left panel) and T3-T4 electrodes (bottom central panel) in operators who worked in COVID-19-free wards and departments (CFO, white) and in frontline COVID-19 operators during the pandemic (FLCO, gray) during the first (1) and the second sessions (2). *p < 0.05 CFO1 vs. FLCO1; °p < 0.05 CFO2 vs. FLCO2; ♦p < 0.05 FLCO1 vs. FLCO2.
During the first session, differences were also found in the EKG with FLCO being characterized by higher median heart rate (FLCO: 78.24 bpm; CFO: 71.1; p = 0.024) and consequently reduced R-R interval (FLCO: 0.767 s; CFO: 0.853 s; p = 0.0207). However, median HRV did not differ between the two groups during the first (FLCO: 83.1; CFO: 78.1; p = 0.705) and second (FLCO: 56.7; CFO: 60.1; p = 0.955) sessions, although it changed between the two sessions in both FLCO (p = 0.011) and CFO (p = 0.025) groups.
The MoCA test scores were lower in the FLCO group at the first session in language and memory items as well as in the total score. The language item score and the total score still remained lower in the second session (Figure 4), as well as executive functions and attention/working memory items.
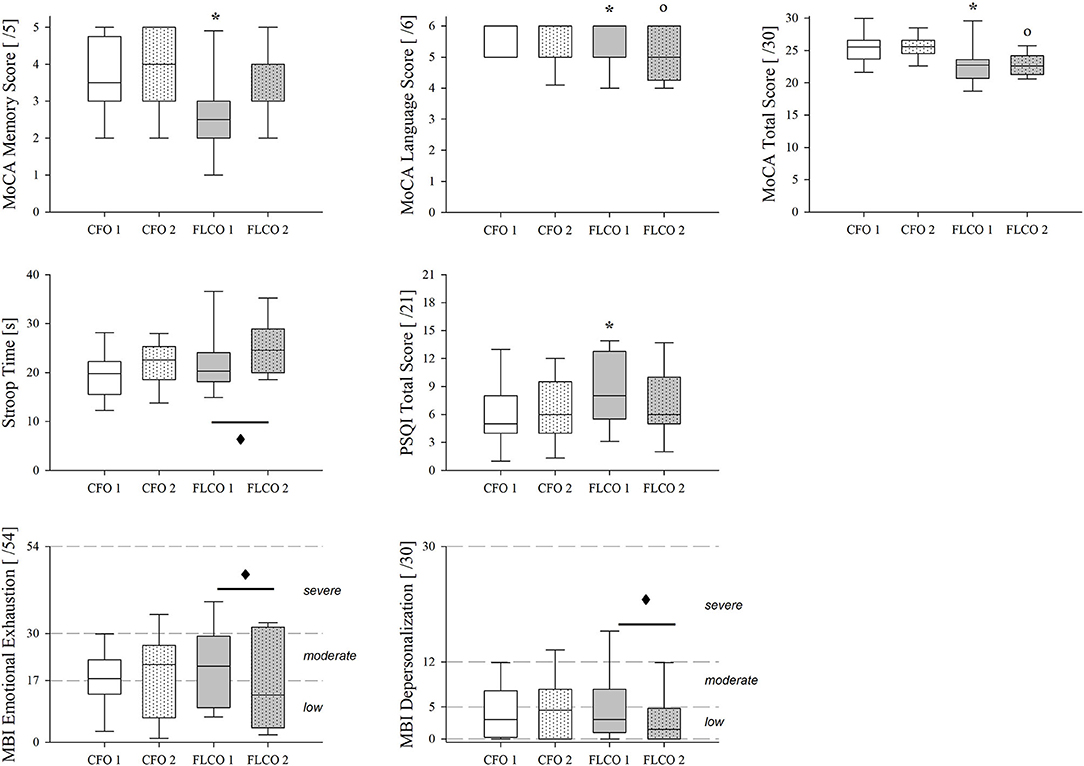
Figure 4. A box-and-whisker plot representing the median (line within the box), the interquartile range (length of the box), the 90th and 10th percentiles (whiskers above and below the box) of cognitive tests, and surveys results: MoCA memory score (top left panel), MoCA language score (top central panel), MoCA total score (top right panel), Stroop Color and Word Test time duration (middle left panel), Pittsburgh Sleep Quality Index Total Score (middle central panel), MBI-GS Emotional Exhaustion (bottom left panel), and depersonalization (bottom central panel) in operators who worked in COVID-19-free wards and departments (CFO, white) and in frontline COVID-19 operators during the pandemic (FLCO, gray) during the first (1) and the second sessions (2). *p < 0.05 CFO1 vs. FLCO1; °p < 0.05 CFO2 vs. FLCO2; ♦: p < 0.05 FLCO1 vs. FLCO2.
The time duration of the Stroop Color and Word Test changed only in the FLCO group, increasing during the second session (Figure 4).
A higher Pittsburgh Sleep Quality Index total score characterized the FLCO group in the first session of measurements (Figure 4).
According to the Maslach Burnout Inventory-General Survey, the emotional exhaustion and the depersonalization in FLCO group were reduced between the first and second sessions (Figure 4).
To our best knowledge, none of the published studies on the effects of severe stress exposure in healthcare professionals working in hospitals during the COVID-19 pandemic was based on the evaluation and analysis of the electrical activity of the subjects' brains. Although our pilot study was conducted on a relatively small population, we found a significantly altered pattern in frontline COVID-19 operators compared to those who worked in COVID-19-free units. The potential abnormalities were the following: higher theta relative power, lower peak alpha frequency, higher interhemispheric coherence of both alpha and theta rhythms, and higher heart rate. Frontline COVID-19 operators were also characterized by lower MoCA test scores and higher Pittsburgh Sleep Quality Index scores. This reduction in cognitive performance and sleep quality may be attributed to stress exposure, but direct testing is still needed.
We might infer such differences to the additional stress of working directly with patients affected by a—till then—unknown virus.
We observed that theta power was higher in the FLCO group on the entire scalp. However, the main significant differences were detected in electrodes located in the central region, in particular on the central axis and on the right hemisphere (data not shown), which is involved in the regulation of emotions (Hugdahl, 2005). Theta band is associated with emotions and, therefore, variations of this specific band indicate alterations in the emotion field. Thus, theta power is an objective parameter to assess those alterations, aside from the subject reports and perceptions (Zhang et al., 2012, 2013; Kraus et al., 2020). In addition, theta power is easily calculated and highly reproducible in different studies. For all the above reasons, it was beneficial to include theta power in our analysis.
As expected, alpha was the dominant rhythm of the EEG signals, because cortical activities were recorded in a resting state condition with closed eyes. However, in our pilot study, it did not significantly differ between the two groups. This is in contrast with previous studies showing that a decreased alpha rhythm is associated with stress and stress-related disorders in combat veterans with post-traumatic stress disorder (Begić et al., 2001).
Instead, the peak alpha frequency significantly differed, being lower in the FLCO group's activity recorded by electrodes F7 and F8. Previous studies showed lower PAF to be associated with worse cognitive performances, especially memory skills (Klimesch, 1994, 1999). Therefore, PAF also seems to be a promising indicator of reduced cognitive function in healthcare operators, but this must be confirmed by future dedicated studies.
Both the significances in the relative powers and in the peak frequency were detected in the first measurement, while the differences between the two groups appeared reduced in the second one.
We can speculate about the mechanism behind these results. The increase in the spectral power of the theta rhythm and the reduction of the peak alpha frequency parameters are simultaneously modified secondary to exposure to high levels of stress (Kraus et al., 2020). The mental fatigue induced by such high attentional and cognitive demands might have played a central role. Indeed, mental fatigue is the main cause of reduced alertness and may trigger fatigue, irritability, and loss of motivation (Tanaka et al., 2012).
Increased theta frequencies, particularly in the frontal-central regions, may be caused by sleepiness, which might result from excessive workloads. Furthermore, a large literature links increased theta rhythm to conditions characterized by strong emotional (Zhang et al., 2012, 2013) and visual impact (Cavallaro et al., 2010), as well as to pathological conditions such as major depression, panic attacks, and generalized anxiety (Begić et al., 2001; Aftanas et al., 2003).
Interesting results came also from the connectivity analysis. A similar trend was found for almost all couples of electrodes: with the exception of frontal F3-F4 electrodes, the FLCO group showed higher coherence values in the first measurement and lower values in the second measurement.
The most important differences were detected in electrodes T3-T4 and P3-P4 for the theta and alpha bands. Although the interpretation of connectivity analysis is still debated, we can speculate it to be a potential index of stress since it was already associated with stress in healthy subjects, although assessed by different parameters (Alonso et al., 2015; Khosrowabadi, 2018) and used to determine the presence and the severity of post-traumatic stress disorder (Modarres et al., 2019).
The MoCA test total score, a rapid screening instrument for mild cognitive dysfunction, highlighted an overall worsening performance for the FLCO group in both sessions. The main functions involved were attention, working memory, visuospatial abilities, and language items. The subjects scored between 23 and 26, indicating a mild but noticeable cognitive decline in terms of mild forgetfulness, mild disorientation, and mild impairment in problem-solving. Interestingly, memory skill decreased in the first measurement but it improved in the second one, with a trend similar to the peak alpha frequency results. Similarly, the FLCO group was characterized by lower overall sleep quality in the first measurement as indicated by the Pittsburgh Sleep Quality Index, with improvements in the second session. The Maslach Burnout Inventory-General Survey scores detected a low to moderate level of burnout, with improvements in emotional exhaustion and depersonalization over time in the FLCO group. Finally, also the modifications that emerged from the Stroop Test confirmed slightly altered cognitive function in the FLCO group compared to the CFO group. Indeed, literature shows that mental fatigue resulting from a high level of stress exposure might determine a deficit of attention revealed by Stroop Test and highlighted by EEG modifications in terms of connectivity (Hassanin et al., 2021) and increased theta activity (Sánchez-Moguel et al., 2022).
Even if these results showed differences between the two groups, they did not reveal the presence of alarming sleep disorders, cognitive deficits, or levels of burnout.
Finally, the heart rate was slightly higher in the first session of the FLCO group, although still within physiological values. As we know, a stressful situation sets off a chain of events: the body releases adrenaline, a hormone causing a momentaneous acceleration of breathing and heart rate (Muraoka et al., 1998; Tan et al., 2011; Kim et al., 2018; Bustamante-Sánchez et al., 2020). However, heart rate variability analysis did not show any difference between the two groups but only within the two sessions in both groups. In addition, breathing rates did not present any differences between groups or measurements.
Taken together, from EEG signal analysis, we could claim that theta power, PAF, and coherence were the main cortical features affected by the stressful experience of healthcare professionals belonging to the FLCO group. In particular, significant differences were detected, respectively, in the central, frontal, and parieto-temporal areas, namely the regions where the structures involved in human stress response are located. This furtherly prove the solidity of our results.
Comparing EEG signals and cognitive tests to survey scores of, respectively, objective and subjective measurements, we noticed an interesting anomaly: despite the altered electroencephalographic and cognitive parameters, we found that the subjects did not perceive burnout. This might suggest that (1) subjective tools for sleep and burnout investigations might be weak in the assessment of the burden caused by a stressful situation, such as working on the frontline at the beginning of a severe pandemic or (2) EEG measurements might be more sensible and able to identify the reaction to severe stress exposure, anticipating the subjective sensation felt by the subjects. We are inclined toward the second option, with the FLCO participants not experiencing burnout while higher stress levels might be reasonably speculated (although not measured). In any case, introducing an objective evaluation to integrate the results of subjective surveys in future research is definitely worthwhile to identify early prodrome of possible issues.
It is important to underline that our subjects were deliberately selected among workers assigned continuously to full-time shifts and with no previous diagnosis of post-traumatic stress disorder. This is another strong point of our study, as it further confirms the sensitivity of the method in identifying early-altered signs secondary to severe stress exposure.
A potential limitation of this study might be the low number of subjects enrolled, particularly if compared to studies resorting to web-based surveys that were able to address large cohorts (Alonso et al., 2021; Gilleen et al., 2021). However, this was a pilot study aimed at verifying the potentiality of EEG as a means to identify the effects of severe stress exposure even in operators not yet affected by a severe stress syndrome, when no data could be found in the literature. Of course, the results should be further strengthened by enrolling larger cohorts of operators, but our preliminary data are encouraging since they already highlight important differences. We do not believe the mixed occupational roles (and hence small sample size for each role) of the CFO group to be confounding, as it reflected the real distribution of the working people at that specific time. Indeed, Lombardy was the epicenter of the first pandemic wave, characterized by a full lockdown of all activities not primarily related to the sanitary emergency (Villani et al., 2020). Some healthcare professionals were banned by the COVID-19 wards and therefore they could not be frontline COVID-19 operators by definition, while others (like the three intermediate care technicians of our FLCO group) were reassigned to a COVID-19 ward and needed to adapt to a new situation of full contact with patients. In that specific moment of uncertainty and dramatic situation in hospitals and clinics, the related changes were more likely a consequence of the proximity to severely ill patients rather than to a specific professional role.
Another limitation that goes beyond the design of the present study is the lack of a benchmark in terms of quantitative EEG analysis in the general population. Indeed, various factors of data analysis methods were proposed to contribute to several contradictory results. Some of these factors include a lack of standardized protocol, the brain region of interest, the stressor type, the recording duration, and proper EEG processing (Katmah et al., 2021).
We opted for quantitative electroencephalography, which distinguishes itself from clinical EEG by the application of complex mathematical approaches and computing scientific methods. Its quantitative nature enables a quantitative description of the waveforms of EEG signals feature extraction: analysis of specific frequency band and signal complexity, analysis of connectivity, and network analysis. The role of quantitative EEG is not necessarily an immediate diagnosis but provides additional insights that can be paired with other diagnostic evaluations in order to get the objective information necessary for a precise diagnosis, correct disease severity assessment, and specific treatment response evaluation (Livint Popa et al., 2020; Höller, 2021). This is the main strength and innovation provided by this pilot study.
Having operators who worked in COVID-19-free wards and departments as subjects is another strong point of the study, as it provides a useful control group to refer to. Although sex was equally distributed between groups but not within groups, we do not believe that this parameter might have played a role. As far as we know, EEG tracing does not differ between men and women.
The lack of direct measures (either subjective or objective) of stress or anxiety or depression is a major limitation. This was a pilot study mainly aimed at understanding the potential role of EEG in this kind of situation and cohort. The pilot study plays a role, among others, in evaluating the limits of the protocol in order to improve it upon the study design prior to a full-scale research project (In, 2017). We are aware that a direct measure of stress or anxiety or depression would be essential. In spite of this important issue, we believe that the results of the present pilot study highlight the potentiality of cortical activity as an objective means of assessment, with the potential use in occupational medicine. However, studies based on large cohorts have already shown how the pandemic has severely increased the level of stress among healthcare operators. Because there is evidence of an association between EEG modification and acute period stress (Katmah et al., 2021), we might infer the differences found between the two groups to the different levels of stress exposure at work. Finally, when we designed the study, we assumed that the second measurement would have happened in an improved epidemiological situation, both in Italy and worldwide, with less pressure on hospitals. Unfortunately, our second measurement coincided with the beginning of a second emergency crisis: therefore, it was not a recovery phase, as originally intended, but instead a second acute phase. Despite the circumstances, in the second measurement, the FLCO group showed a lower alteration, suggesting that some sort of chronic adaptive mechanism was in place in the FLCO group to cope with the prolonged stressful situation. For this reason, it would be useful to add a third data acquisition session at the very end of the sanitary emergency in order to detect potential symptoms of stress-related psychological disorders. Indeed, this type of illness usually does not arise immediately after the occurrence of an experienced traumatic event but after a given amount of time (Salazar de Pablo et al., 2020).
Additionally, this technique of evaluation secondary to stress exposure could be implemented beyond the medical field. For example, employees belonging to other working categories may have a lower threshold of stress tolerance compared to healthcare workers, and EEG could be therefore used as a new clinical evaluation tool for preventive interventions in occupational medicine to identify early signs of burnout. Indeed, occupational medicine is not based only on consolidated data but evolves according to emerging scientific evidence (Cristaudo, 2020). Stress and its negative impact have become a growing problem not only in daily lives but also in occupational medicine, as the different systems of the human body, such as the nervous, immune, cardiovascular, and gastrointestinal systems, are affected. Thus, objective evaluation and analysis of prolonged stress exposure are very important to detect stress-induced alteration to prevent significant health problems. The use of EEG evaluation could also be extended to patients suffering from COVID-19 since they are at risk of developing mental disorders like anxiety, depression, or post-traumatic distress syndrome (Rogers et al., 2020). Once again, this objective evaluation might have some important social and economic implications, considering that the virus has already infected over 230 million people worldwide (COVID-19 Map - Johns Hopkins Coronavirus Resource Center, 2022).
In conclusion, working in direct contact with patients with COVID-19 could be effectively considered an important cause of mental stress. One of the most likely negative outcomes of increased levels of stress, fatigue, and excessive workload in healthcare professionals is burnout. This might have important social and professional implications, as exposure to stress, especially for prolonged periods, may result in physical and mental disorders. Indeed, stress is a social determinant of health, and in order to avoid the risk of medical negligence, an unbiased, objective, and automated method for the diagnosis of stress should be adopted alongside the current self-reporting assessment methods.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Research Ethics Board of the IRCCS Ca' Granda Ospedale Maggiore Policlinico (439_2020). The patients/participants provided their written informed consent to participate in this study.
Conceptualization and methodology: MV and AL. Software and formal analysis: MM. Investigation: FM, FR, CD, and GA. Resources: RF, AA, and SB. Data curation: MM and AL. Writing original draft preparation and writing, reviewing, and editing: AL, MM, and MV. Supervision: RF, AA, and SB. Project administration: MV. All authors have read and agreed to the published version of the manuscript.
Publication costs were funded by Grant Ricerca Corrente, Italian Ministry of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank all the operators who kindly volunteered to be part of our study, and also Dr. Myriam Neglia, Dr. Mimma Sternativo, and Dr. Elisabetta Corona for their help during the recruitment of the subjects, and Dr. Elena Balzano for her help in editing the content. This paper is dedicated to the loving memory of Ornella Crisigiovanni.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnsys.2022.923576/full#supplementary-material
Aftanas, L. I., Pavlov, S. V., Reva, N. V., and Varlamov, A. A. (2003). Trait anxiety impact on the EEG theta band power changes during appraisal of threatening and pleasant visual stimuli. Int. J. Psychophysiol. 50, 205–212. doi: 10.1016/S0167-8760(03)00156-9
Alonso, J., Romero, S., Ballester, M., Antonijoan, R., and Mañanas, M. (2015). Stress assessment based on EEG univariate features and functional connectivity measures. Physiol. Meas. 36, 1351–165. doi: 10.1088/0967-3334/36/7/1351
Alonso, J., Vilagut, G., Mortier, P., Ferrer, M., Alayo, I., and Aragón-Peña, A. (2021). Mental health impact of the first wave of COVID-19 pandemic on Spanish healthcare workers: a large cross-sectional survey. Rev. Psiquiatr. Salud Ment. 14, 90–105. doi: 10.1016/j.rpsm.2020.12.001
Babore, A., Lombardi, L., Viceconti, M. L., Pignataro, S., Marino, V., and Crudele, M. (2020). Psychological effects of the COVID-2019 pandemic: perceived stress and coping strategies among healthcare professionals. Psychiatry Res. 293, 113366. doi: 10.1016/j.psychres.2020.113366
Begić, D., Hotujac, L., and Jokić-Begić, N. (2001). Electroencephalographic comparison of veterans with combat-related post-traumatic stress disorder and healthy subjects. Int. J. Psychophysiol. 40, 167–172. doi: 10.1016/S0167-8760(00)00153-7
Berretz, G., Packheiser, J., Wolf, O. T., and Ocklenburg, S. (2022). Acute stress increases left hemispheric activity measured via changes in frontal alpha asymmetries. iScience 25, 103841. doi: 10.1016/j.isci.2022.103841
Bustamante-Sánchez, A., Tornero-Aguilera, J., Fernández-Elías, V., Hormeño-Holgado, A., Dalamitros, A., and Clemente-Suárez, V. (2020). Effect of stress on autonomic and cardiovascular systems in military population: a systematic review. Cardiol. Res. Pract. 2020, 7986249. doi: 10.1155/2020/7986249
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., and Kupfer, D. J. (1989). The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. doi: 10.1016/0165-1781(89)90047-4
Caffarra, P., Vezzadini, G., Dieci, F., Zonato, F., and Venneri, A. (2002). A short version of the Stroop test: normative data in an Italian population sample. Nuova Riv. Neurol. 12, 111–115.
Cavallaro, F. I., Cacace, I., Del Testa, M., Andre, P., Carli, G., De Pascalis, V., et al. (2010). Hypnotizability-related EEG alpha and theta activities during visual and somesthetic imageries. Neurosci. Lett. 470, 13–18. doi: 10.1016/j.neulet.2009.12.044
COVID-19 Map - Johns Hopkins Coronavirus Resource Center. Johns Hopkins University & Medicine (2022). Available online at: https://coronavirus.jhu.edu/map.html (accessed September 27, 2021).
Cristaudo, A.. (2020). Scientific evidence and guidelines in occupational medicine. Med. Lav. 111, 87–98.
Cysarz, D., Zerm, R., Bettermann, H., Frühwirth, M., Moser, M., and Kröz, M. (2008). Comparison of respiratory rates derived from heart rate variability, ECG amplitude, and nasal/oral airflow. Ann. Biomed. Eng. 36, 2085–2094. doi: 10.1007/s10439-008-9580-2
Di Muzio, M., Diella, G., Di Simone, E., Novelli, L., Alfonsi, V., Scarpelli, S., et al. (2020). Nurses and night shifts: poor sleep quality exacerbates psychomotor performance. Front. Neurosci. 14, 579938. doi: 10.3389/fnins.2020.579938
Ehrhardt, N. M., Fietz, J., Kopf-Beck, J., Kappelmann, N., and Brem, A. K. (2021). Separating EEG correlates of stress: cognitive effort, time pressure, and social-evaluative threat. Eur. J. Neurosci. 55, 2464–2473. doi: 10.1111/ejn.15211
Ehrlich, H., McKenney, M., and Elkbuli, A. (2020). Protecting our healthcare workers during the COVID-19 pandemic. Am. J. Emerg. Med. 38, 1527–1528. doi: 10.1016/j.ajem.2020.04.024
Fingelkurts, A. A., Fingelkurts, A. A., and Kähkönen, S. (2005). Functional connectivity in the brain–is it an elusive concept? Neurosci. Biobehav. Rev. 28, 827–836. doi: 10.1016/j.neubiorev.2004.10.009
Ganesan, S., Magee, M., Stone, J. E., Mulhall, M. D., Collins, A., and Howard, M. E. (2019). The impact of shift work on sleep, alertness and performance in healthcare workers. Sci. Rep. 9, 4635. doi: 10.1038/s41598-019-40914-x
Gilleen, J., Santaolalla, A., Valdearenas, L., Salice, C., and Fusté, M. (2021). Impact of the COVID-19 pandemic on the mental health and well-being of UK healthcare workers. BJPsych Open 7, e88. doi: 10.1192/bjo.2021.42
Golubic, R., Milosevic, M., Knezevic, B., and Mustajbegovic, J. (2009). Work-related stress, education and work ability among hospital nurses. J. Adv. Nurs. 65, 2056–2066. doi: 10.1111/j.1365-2648.2009.05057.x
Hallberg, U., and Sverke, M. (2006). Construct validity of the maslach burnout inventory: two swedish health care samples. Eur. Psychol. 11, 320–338. doi: 10.1027/1015-5759.20.4.320
Hassanin, O., Al-Shargie, F., Tariq, U., and Al-Nashash, H. (2021). asymmetry of regional phase synchrony cortical networks under cognitive alertness and vigilance decrement states. IEEE Trans. Neural Syst. Rehabil. Eng. 29, 2378–2387. doi: 10.1109/TNSRE.2021.3125420
Hayes, J. P., VanElzakker, M. B., and Shin, L. M. (2012). Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Front. Integr. Neurosci. 6, 89. doi: 10.3389/fnint.2012.00089
Höller, Y.. (2021). Quantitative EEG in cognitive neuroscience. Brain Sci. 11, 517. doi: 10.3390/brainsci11040517
Hugdahl, K.. (2005). Symmetry and asymmetry in the human brain. Eur. Rev. 13, 119–133. doi: 10.1017/S1062798705000700
Imperatori, C., Farina, B., Quintiliani, M. I., Onofri, A., Castelli Gattinara, P., and Lepore, M. (2014). Aberrant EEG functional connectivity and EEG power spectra in resting state post-traumatic stress disorder: a sLORETA study. Biol. Psychol. 102, 10–17. doi: 10.1016/j.biopsycho.2014.07.011
In, J.. (2017). Introduction of a pilot study. Korean J. Anesthesiol. 70, 601. doi: 10.4097/kjae.2017.70.6.601
Järvelin-Pasanen, S., Sinikallio, S., and Tarvainen, M. P. (2018). Heart rate variability and occupational stress-systematic review. Ind. Health 56, 500–511. doi: 10.2486/indhealth.2017-0190
Jobert, M., Wilson, F., Roth, T., Ruigt, G., Anderer, P., and Drinkenburg, W. (2013). Guidelines for the recording and evaluation of pharmaco-sleep studies in man: the International Pharmaco-EEG Society (IPEG). Neuropsychobiology 67, 127–167. doi: 10.1159/000343449
Jorge, M. S., Spindola, L., Katata, J. H. B., and Anghinah, R. (2017). Alpha band EEG coherence in healthy nonagenarians. Arq. Neuropsiquiatr. 75, 609–613. doi: 10.1590/0004-282x20170102
Katmah, R., Al-Shargie, F., Tariq, U., Babiloni, F., Al-Mughairbi, F., and Al-Nashash, H. (2021). A review on mental stress assessment methods using EEG Signals. Sensors 21, 5043. doi: 10.3390/s21155043
Khosrowabadi, R.. (2018). Stress and perception of emotional stimuli: long-term stress rewiring the brain. Basic Clin. Neurosci. 9, 107–120. doi: 10.29252/nirp.bcn.9.2.107
Kim, H., Cheon, E., Bai, D., Lee, Y., and Koo, B. (2018). Stress and heart rate variability: a meta-analysis and review of the literature. Psychiatry Investig. 15, 235–245. doi: 10.30773/pi.2017.08.17
Kim, H., and Ji, J. (2009). Factor structure and longitudinal invariance of the maslach burnout inventory. Res. Soc. Work Pract. 19, 325–339. doi: 10.1177/1049731508318550
Klimesch, W.. (1994). The Structure of Long-term Memory: A Connectivity Model for Semantic Processing. Mahwah, NJ: Taylor and Francis Inc.
Klimesch, W.. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res. Rev. 29, 169–195. doi: 10.1016/S0165-0173(98)00056-3
Kraus, J., Roman, R., Lacinová, L., Lamoš, M., Brázdil, M., and Fredrikson, M. (2020). Imagery-induced negative affect, social touch and frontal EEG power band activity. Scand. J. Psychol. 61, 731–739. doi: 10.1111/sjop.12661
Leblanc, V. R.. (2009). The effects of acute stress on performance: Implications for health professions education. Acad. Med. 84(10 Suppl.), S25–S33. doi: 10.1097/ACM.0b013e3181b37b8f
Lee, S. H., Yoon, S., Kim, J. I., Jin, S. H., and Chung, C. K. (2014). Functional connectivity of resting state EEG and symptom severity in patients with post-traumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 51, 51–57. doi: 10.1016/j.pnpbp.2014.01.008
Livint Popa, L., Dragos, H., Pantelemon, C., Verisezan Rosu, O., and Strilciuc, S. (2020). The role of quantitative EEG in the diagnosis of neuropsychiatric disorders. J. Med. Life 13, 8–15. doi: 10.25122/jml-2019-0085
Malver, L. P., Brokjær, A., Staahl, C., Graversen, C., Andresen, T., and Drewes, A. M. (2014). Electroencephalography and analgesics. Br. J. Clin. Pharmacol. 77, 72–95. doi: 10.1111/bcp.12137
Marshall, A. C., and Cooper, N. R. (2017). The association between high levels of cumulative life stress and aberrant resting state EEG dynamics in old age. Biol. Psychol. 127, 64–73. doi: 10.1016/j.biopsycho.2017.05.005
Maslach, C., Jackson, S., and Leiter, M. (1996). Maslach Burnout Inventory Manual, 3rd Edn. Mountain View, CA: CPP, Inc.
Mealer, M., Burnham, E. L., Goode, C. J., Rothbaum, B., and Moss, M. (2009). The prevalence and impact of post traumatic stress disorder and burnout syndrome in nurses. Depress. Anxiety 26, 1118–1126. doi: 10.1002/da.20631
Mealer, M. L., Shelton, A., Berg, B., Rothbaum, B., and Moss, M. (2007). Increased prevalence of post-traumatic stress disorder symptoms in critical care nurses. Am. J. Respir. Crit. Care Med. 175, 693–697. doi: 10.1164/rccm.200606-735OC
Melnyk, B. M., Kelly, S. A., Stephens, J., and Dhakal, K. (2020). McGovern C, Tucker S et al. Interventions to improve mental health, well-being, physical health, and lifestyle behaviors in physicians and nurses: a systematic review. Am. J. Health Promot. 34, 929–941. doi: 10.1177/0890117120920451
Modarres, M., Opel, R., Weymann, K., and Lim, M. (2019). Strong correlation of novel sleep electroencephalography coherence markers with diagnosis and severity of posttraumatic stress disorder. Sci. Rep. 9, 4247. doi: 10.1038/s41598-018-38102-4
Muraoka, M., Carlson, J., and Chemtob, C. (1998). Twenty-four-hour ambulatory blood pressure and heart rate monitoring in combat-related posttraumatic stress disorder. J. Trauma. Stress 11, 473–484. doi: 10.1023/A:1024400628342
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., and Collin, I. (2005). The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Pan, J., and Tompkins, W. J. A. R. (1985). Detection algorithm. IEEE Trans. Biomed. Eng. 32, 230–236. doi: 10.1109/TBME.1985.325532
Pappa, S., Ntella, V., Giannakas, T., Giannakoulis, V. G., Papoutsi, E., and Katsaounou, P. (2020). Prevalence of depression, anxiety, and insomnia among healthcare workers during the COVID-19 pandemic: a systematic review and meta-analysis. Brain Behav. Immun. 88, 901–907. doi: 10.1016/j.bbi.2020.05.026
Ranieri, J., Guerra, F., Perilli, E., Passafiume, D., Maccarone, D., and Ferri, C. (2021). Prolonged COVID 19 outbreak and psychological response of nurses in Italian healthcare system: cross-sectional study. Front. Psychol. 12, 608413. doi: 10.3389/fpsyg.2021.608413
Rogers, J., Chesney, E., Oliver, D., Pollak, T., McGuire, P., Fusar-Poli, P., et al. (2020). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 7, 611–627. doi: 10.1016/S2215-0366(20)30203-0
Salazar de Pablo, G., Vaquerizo-Serrano, J., Catalan, A., Arango, C., Moreno, C., Ferre, F., et al. (2020). Impact of coronavirus syndromes on physical and mental health of health care workers: systematic review and meta-analysis. J. Affect. Disord. 275, 48–57. doi: 10.1016/j.jad.2020.06.022
Sánchez-Moguel, S. M., Baravalle, R., González-Salinas, S., Rosso, O. A., Fernández, T., and Montani, F. A. (2022). signal energy in the elderly: a wavelet analysis of event-related potentials during a stroop task. J. Neurosci. Methods 376, 109608. doi: 10.1016/j.jneumeth.2022.109608
Santangelo, G., Siciliano, M., Pedone, R., Vitale, C., Falco, F., and Bisogno, R. (2015). Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 36, 585–591. doi: 10.1007/s10072-014-1995-y
Schneider, J., Talamonti, D., Gibson, B., and Forshaw, M. (2021). Factors mediating the psychological well-being of healthcare workers responding to global pandemics: a systematic review. J. Health Psychol. 135910532110127. doi: 10.1177/13591053211012759
Seo, S., and Lee, J. (2010). “Stress and EEG,” in Convergence and Hybrid Information Technologies, ed M. Crisan (Rijeka, Croatia: InTech). doi: 10.5772/9651
Shevlin, M., McBride, O., Murphy, J., Miller, J. G., Hartman, T. K., Levita, L., et al. (2020). Anxiety, depression, traumatic stress and COVID-19-related anxiety in the UK general population during the COVID-19 pandemic. BJPsych Open 6, e125. doi: 10.1192/bjo.2020.109
Smith, T., Gildeh, N., and Holmes, C. (2007). The Montreal cognitive assessment: validity and utility in a memory clinic setting. Can. J. Psychiatry 52, 329–332. doi: 10.1177/070674370705200508
Stewart, N. H., Koza, A., Dhaon, S., Shoushtari, C., Martinez, M., and Arora, V. M. (2021). Sleep Disturbances in frontline health care workers during the COVID-19 pandemic: social media survey study. J. Med. Internet Res. 23, e27331. doi: 10.2196/27331
Stroop, J. R.. (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol. 18, 643–662. doi: 10.1037/h0054651
Tan, G., Dao, T., Farmer, L., Sutherland, R., and Gevirtz, R. (2011). Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): a pilot study. Appl. Psychophysiol. Biofeedback 36, 27–35. doi: 10.1007/s10484-010-9141-y
Tanaka, M., Shigihara, Y., Ishii, A., Funakura, M., Kanai, E., and Watanabe, Y. (2012). Effect of mental fatigue on the central nervous system: an electroencephalography study. Behav. Brain Funct. 8, 48. doi: 10.1186/1744-9081-8-48
Tauro, E., Gorini, A., Caglio, C., Gabanelli, P., and Caiani, E. G. (2022). COVID-19 and mental disorders in healthcare Personnel: a novel framework to develop Personas from an online survey. J. Biomed. Inform. 126, 103993. doi: 10.1016/j.jbi.2022.103993
Testoni, I., Franco, C., Gallo Stampino, E., Iacona, E., Crupi, R., and Pagano, C. (2021). Facing COVID-19 between sensory and psychoemotional stress, and instrumental deprivation: a qualitative study of unmanageable critical incidents with doctors and nurses in two hospitals in Northern Italy. Front. Psychol. 12, 622894. doi: 10.3389/fpsyg.2021.622894
Villani, L., McKee, M., Cascini, F., Ricciardi, W., and Boccia, S. (2020). Comparison of deaths rates for COVID-19 across Europe during the first wave of the COVID-19 pandemic. Front. Public Heal. 8, 620416. doi: 10.3389/fpubh.2020.620416
Zhang, D., Wang, L., Luo, Y., and Luo, Y. (2012). Individual differences in detecting rapidly presented fearful faces. PLoS ONE 7, e49517. doi: 10.1371/journal.pone.0049517
Keywords: COVID-19, stress exposure, EEG, healthcare workers, mental fatigue
Citation: LoMauro A, Molisso MT, Mameli F, Ruggiero F, Ferrucci R, Dellarosa C, Aglieco G, Aliverti A, Barbieri S and Vergari M (2022) EEG Evaluation of Stress Exposure on Healthcare Workers During COVID-19 Emergency: Not Just an Impression. Front. Syst. Neurosci. 16:923576. doi: 10.3389/fnsys.2022.923576
Received: 19 April 2022; Accepted: 01 June 2022;
Published: 18 July 2022.
Edited by:
Mariella Pazzaglia, Sapienza University of Rome, ItalyReviewed by:
Valentina Alfonsi, Sapienza University of Rome, ItalyCopyright © 2022 LoMauro, Molisso, Mameli, Ruggiero, Ferrucci, Dellarosa, Aglieco, Aliverti, Barbieri and Vergari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maurizio Vergari, bWF1cml6aW8udmVyZ2FyaUBwb2xpY2xpbmljby5taS5pdA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.