
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Syst. Neurosci., 06 January 2023
Volume 16 - 2022 | https://doi.org/10.3389/fnsys.2022.1088587
This article is part of the Research TopicDistributed networks: new outlooks on cerebellar function - Volume IIView all 19 articles
The ability of the brain to change structure and function in response to experience accounts for its ability to successfully adapt to the environment in both learning processes and unique phases, such as during development and repair. On this basis, the occurrence of the brain, cognitive, and neural reserves has been advanced to explain the discrepancies between the extent of neurological damage and the severity of clinical manifestations described in patients with different life span experiences. Research on this topic highlighted the neuroprotective role of complex stimulations, allowing the brain to better cope with the damage. This framework was initially developed by observing patients with Alzheimer's disease, and it has since been progressively expanded to multifarious pathological states. The cerebellum is known to be particularly responsive to experience through extensive plastic rearrangements. The neuroprotective value exerted by reserve mechanisms appears to be suitable for basic neuronal plasticity in the cerebellum. Thus, it is of primary interest to deepen our understanding of how life experiences modify individuals' cerebellar morphology and functionality. The present study is aimed at analyzing the evidence provided on this topic by animal and human studies. For animals, we considered the studies in which subjects were submitted to enhanced stimulations before the damage occurred. For humans, we considered studies in which previous lifelong high-level experiences were associated with superior cerebellar abilities to cope with injury. Detailed indications of the processes underlying cerebellar reserves may be important in proposing effective interventions for patients suffering from pathologies that directly or indirectly damage cerebellar functionality.
In neurological disorders, the underlying neuropathology and the clinical/cognitive/behavioral symptoms may not be directly related. Indeed, subjects may suffer from severe cerebral damage without corresponding severe functional impairment and vice versa. To explain this gap, Stern proposed the reserve hypothesis ~30 years ago (Stern et al., 1992). According to this hypothesis, a number of experiential factors can exert a protective action on the brain by making it more resilient to neurological damage. Such a protective action is possible since the brain is characterized by high-level plasticity that is present throughout the entire life span (Stern, 2009, 2012; Barulli and Stern, 2013).
Several activities are considered reserve builders. In particular, human studies have shown that various cognitive (reading, painting, writing, etc.), social (volunteering, participating in group leisure activities, marital status, etc.), and physical (running, walking, swimming, biking, etc.) activities; healthy lifestyle habits (diet, smoking, drinking, etc.); and education and occupational attainment, alone or in combination, build a reserve warehouse that can be spent to withstand physiological and/or pathological cognitive decline processes (Serra et al., 2018; Serra and Gelfo, 2019). These factors construct a sort of reserve in terms of structure (brain reserve: brain volume, parenchymal fraction, gray/white matter volume, lesion load, cortical thickness, gyrification, etc.) and function (cognitive reserve: memory, language, executive functions, learning, general cognition, etc.) of the brain, as well as the interaction between them (neural reserve: brain network efficiency, capacity, or flexibility; metabolism; etc.). The integrity of the structure leads to increased efficiency of the neural networks that in turn produce more efficient cognitive functions (Serra et al., 2017a, 2018).
In animal models, several studies have been performed on the neuroprotective effects of exposure to enhanced stimulations before the occurrence of brain damage by using the classical paradigm of environmental enrichment (Diamond et al., 1966; Rosenzweig et al., 1978; Rosenzweig and Bennett, 1996). This experimental paradigm is based on rearing the animals (typically rodents) in conditions in which the laboratory standards are enhanced by manipulating factors that mimic the ones considered reserve builders in humans, namely, the cognitive factor (objects multifarious in nature, shape, size, and color, frequently rearranged and replaced to expose the animals to the novelty); the social factor (increased number of individuals in the same cage in comparison to the laboratory standards); and the physical factor (cages bigger than the standard; shelves, ladders, and running wheels; supplementary diets; etc.) (Mandolesi et al., 2017; Sampedro-Piquero and Begega, 2017; Balietti and Conti, 2022). Animal models allow the investigation of the reserve in all its components, such as the brain, cognitive, and neural components. Furthermore, the use of animals makes available the direct investigation of a large number of structural, biological, and behavioral indices, also ensuring the possibility of high-level control of experimental variables (Petrosini et al., 2009; Gelfo et al., 2018).
The reserve mechanisms have been studied directly in the brain, and several studies have documented the association between different levels of reserve and brain structural/functional changes in several neurological conditions. In contrast, the relationship between reserve measures and the cerebellum has been described in a handful of studies and specifically investigated to a lesser extent (Mitoma et al., 2020; Bordignon et al., 2021). More focused evidence on the relationship between reserve measures and cerebellar structures is available in animal models (Gelfo and Petrosini, 2022a,b). This situation is on the whole very surprising since the plastic properties of the cerebellum are currently well-known (Luciani, 1893; Carulli et al., 2004; Cesa and Strata, 2005; Bosman and Konnerth, 2009; Cheron et al., 2016; Mitoma et al., 2021). Cerebellar involvement in cognitive and emotional functions in addition to motor functions has been increasingly ascertained (Schmahmann, 1997; Laricchiuta et al., 2015; Jang and Kim, 2019; De Zeeuw et al., 2021; Picerni et al., 2021, 2022; Petrosini et al., 2022; van Dun et al., 2022). Schmahmann (2019) reports that the cerebellum maintains behavior (in terms of cognition and emotions) around a homeostatic baseline by unconsciously using implicit learning and by adapting actions to context. The cerebellar posterior lobe plays a role in maintaining cognitive and emotional functions. Indeed, posterior lobe lesions produce cerebellar cognitive affective syndrome characterized by executive dysfunctions, visuospatial disorders, language difficulty, and affective dysregulation (Schmahmann, 2019). Distributed cerebro-cerebellar networks are involved in motor, cognitive, and emotional functions. In particular, the dorsal and ventral attentional networks, the frontoparietal network, the default mode network, and the salience network have been mapped into focal areas of the posterior lobe of the cerebellum. This cortico-cerebellar organization accounts for the functional connectivity between the cerebellum and associative brain areas that are involved in higher-level behaviors (Schmahmann, 2019).
A recent study investigated the association between cognitive reserve and several brain structures, including the cerebellum, in a cohort of young healthy subjects (Conti et al., 2021). A total of 77 healthy individuals with a median age of 27.5 years were recruited and underwent the Cognitive Reserve Index, a comprehensive questionnaire investigating several cognitive reserve factors, including education, occupation, and leisure activities. The authors found associations between the Cognitive Reserve Index and the gray matter volume of the left cerebellum. Moreover, they also found low functional connectivity of the right cerebellum with the executive control network (Conti et al., 2021).
In addition, a large number of animal studies have indicated that exposure to environmental enrichment is able to induce plastic rearrangements in the cerebellum of healthy rodents. Such plastic changes are associated with the potentiation of cognitive performance (Gelfo and Petrosini, 2022b). Most studies have investigated the effects of exposure to enhanced stimulation at an early age by reporting changes in cerebellar synaptogenesis (De Bartolo et al., 2015; Kim et al., 2019), neurotrophin and neurotransmitter expression (Naka et al., 2002; Angelucci et al., 2009; Vazquez-Sanroman et al., 2013), chromatin levels (Uphouse, 1978), and electrophysiological activity (Eshra et al., 2019). However, the provided frame is not fully clarified because of some conflicting results. A lack of effects following early exposure to environmental enrichment is reported in relation to cerebellar synaptogenesis (Nithianantharajah et al., 2004; Pascual and Bustamante, 2013), neurotrophin and neurotransmitter expression (Naka et al., 2002; Vazquez-Sanroman et al., 2013), and chromatin levels (Uphouse and Tedeschi, 1979). Scarce evidence is available in relation to the neuroprotective effects of environmental enrichment when the animals are exposed later in life. Nevertheless, some indications suggest a beneficial effect on spatial learning associated with changes in cerebellar volume and polypeptide levels (Horvath et al., 2015; Scholz et al., 2015).
In this view, it is possible to advance the specific and complex construct of the cerebellar reserve, which includes, on the one hand, the capacity of the cerebellar structure to efficiently compensate for the damage and, on the other hand, the neuroprotective effects of enhanced experiences on cerebellar structure (Mitoma et al., 2020, 2022; Bordignon et al., 2021; Gelfo and Petrosini, 2022a,b). As a consequence, the present review aims to analyze the available literature on the neuroprotective effects of enriched experiences in humans and animals on the occurrence of neural damage that involves cerebellar functionality. To address this issue, we considered only the studies that specifically investigated the association between the current concept of brain/cognitive/neural reserve and the cerebellum.
Since the seminal work of Schmahmann, “The Cerebellum and Cognition” (1997), it has become clear that the cerebellum exerts a concomitant role in motor and cognitive functions (Koziol et al., 2014). While in animal models, the cerebellar plastic response to complex stimulations is widely documented (Cutuli et al., 2011; Gelfo and Petrosini, 2022a,b), in humans, it is less clear whether the cerebellum is sensitive to environmental stimuli to the point of changing its structures and/or function. Neuroimaging studies suggest that compensatory cerebellar reorganization might be present in neurodegenerative diseases, such as Alzheimer's disease (AD). Although several studies have documented cerebellar involvement in cognitive and emotional processes (Toniolo et al., 2018, 2020; Olivito et al., 2020; Petrosini et al., 2022), few studies have directly investigated the role of the cerebellum in the framework of brain/cognitive/neural reserve in clinical populations. Serra et al. (2017a) found a significantly decreased brain functional connectivity in a large network involving fronto-temporo-cerebellar nodes by comparing a group of patients with amnestic-mild cognitive impairment (a-MCI) and high educational level (a proxy measure of cognitive reserve) against a-MCI patients with low CR. In particular, significant differences in the correlations between pairs of nodes in a-MCI patients with high cognitive reserve have been found mainly between bilateral BA10 and bilateral cerebellar lobule 9, bilateral BA10 and cerebellar vermis, bilateral BA43 and cerebellar vermis, and, finally, right BA37 and right cerebellar lobule 10. In the same study, abnormalities in the local circuit properties in several nodes of the cerebellum were found in a-MCI and in AD patients with high cognitive reserve. Specifically, the betweenness centrality and the nodal degree (both measures of the efficiency of the cerebral–cerebellar networks; the betweenness centrality is calculated as the ratio of all the shortest paths passing through a given node; the nodal degree is the number of connections for each node) have been found to be reduced in bilateral lobule 10 and the left Crus II in a-MCI patients with high cognitive reserve compared with a-MCI patients with low cognitive reserve. In addition, healthy elderly controls with high cognitive reserve compared with those with low cognitive reserve showed reduced nodal degree in Crus I and Crus II and in lobules 8 and 9. Finally, AD patients with high cognitive reserve showed reduced nodal efficiency in the vermis (Serra et al., 2017a). The authors concluded that the reduced connectivity in this large network involving the cerebellum accounted for the cognitive disorders observed in the patients according to the cognitive reserve framework. Indeed, the cognitive reserve model postulates that individuals with high cognitive reserve levels need to accumulate more neuropathology than those with lower cognitive reserve levels to show the same rate of cognitive dysfunction (Stern and Barulli, 2019).
In a recent study on the relationship between structural changes in the brain and the cerebellar structure (Serra et al., 2017b), the cerebellum was found to be related to cognitive reserve (computed through a latent variable derived from memory and general cognition scores). In this study, direct correlations between cognitive reserve measures and gray matter volumes of the cerebellum, mainly in Crus I and lobules 4 and 7, were found in both a-MCI patients and healthy controls (Serra et al., 2017b).
A recent FDG-PET study (Canosa et al., 2021) on patients with amyotrophic lateral sclerosis (ALS) found an inverse correlation between the metabolism of the medial frontal lobe and the cerebellum only in the patient group and not in the control group. The authors hypothesized that this finding might be due to the recruitment of cerebellar reserve networks (Canosa et al., 2021).
Even in multiple sclerosis (MS), it has been described that there is a relationship between the cerebellum and cognitive reserve (Rocca et al., 2019). Namely, in patients with MS, an association was found between gray matter atrophy of the left cerebellum and memory and cognitive reserve measures. The authors concluded that in MS, cognitive reserve plays a protective role in preserving cognition, moderating the effect of structural damage on memory performance without affecting the progression of the disease (Rocca et al., 2019).
Details on the studies cited in this section are provided in Table 1.
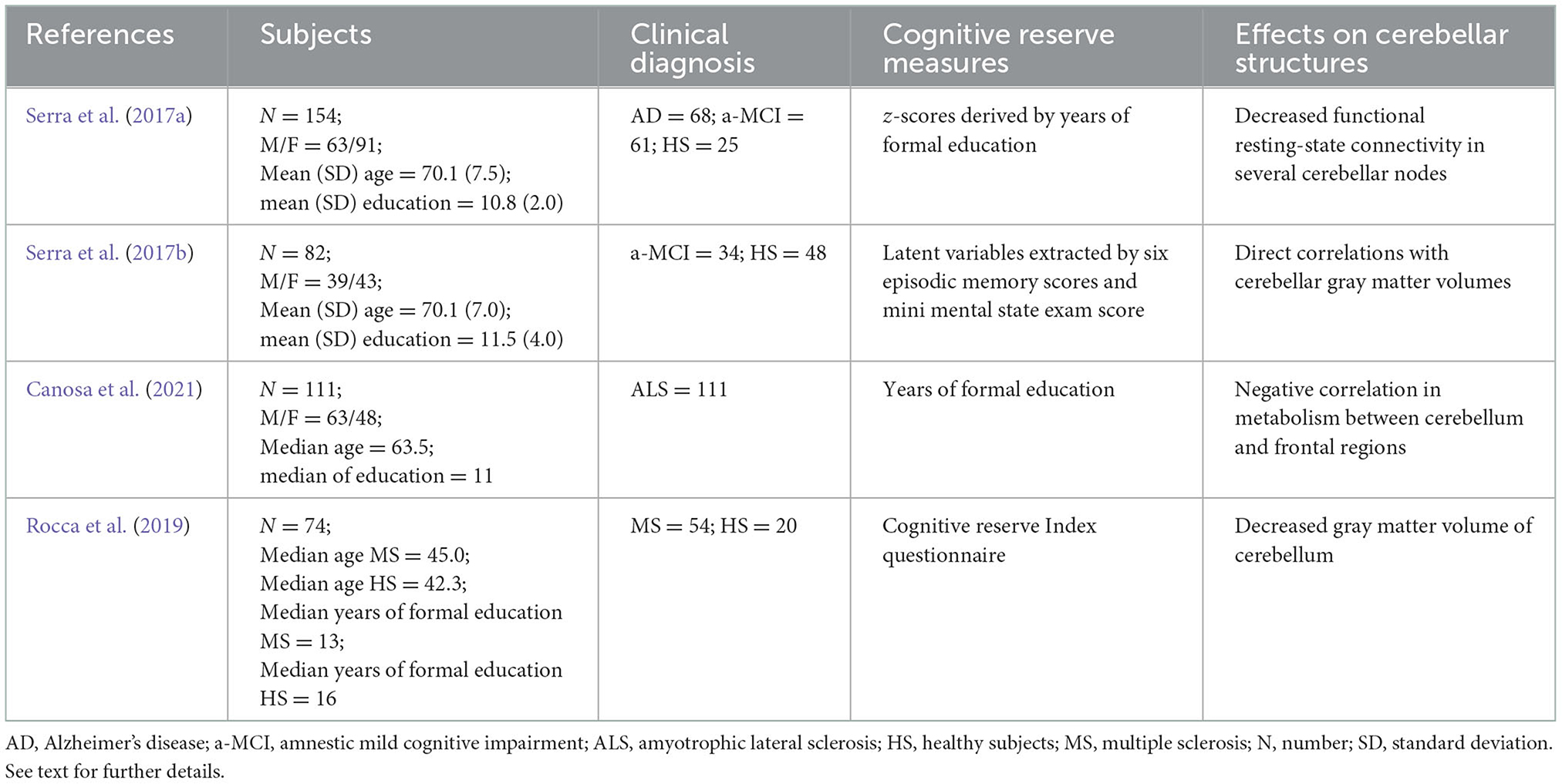
Table 1. Studies on the neuroprotective effects of the exposure to enhanced stimulations in human neuropathological conditions involving cerebellar functionality.
By analyzing animal studies focused on the neuroprotective effects of exposure to enhanced environmental stimulations before the occurrence of brain damage that affects cerebellar functionality and cognition, it is possible to detect that this issue is still scarcely addressed. Some evidence is provided about the neuroprotective effects of environmental enrichment in models of the Rett syndrome and cerebellar trauma. However, the reported findings are not completely consistent.
Rett syndrome is characterized by the progressive loss of motor and cognitive abilities, previously acquired in the early postnatal phases. It is caused by mutations in the X-linked methyl CpG-binding protein 2 (Mecp2) gene and is predominant in females. Such a disease is mimicked in Mecp2 mutant mouse models showing most impairments that characterize Rett syndrome patients, namely, deficits in motor, cognitive, social, and emotional competencies (Harris, 2021). By using one of these models—male Mecp2tm1Jae mice—Lonetti et al. (2010) reported that early exposure to environmental enrichment from 10 days of age for 50 days prevented the impairment in motor learning shown by standard-reared male homozygous Mecp2 mutant mice. This functional neuroprotective effect was accompanied by the increased density of cerebellar inhibitory synapses shown by the enriched mutant mice compared with the standard-reared mice. Moreover, in female heterozygous Mecp2 mutants, the same protocol of environmental enrichment prevented deficits in spatial learning and anxiety-related behaviors. Conversely, Nag et al. (2009) reported that the exposure to environmental enrichment of male Mecp2 mutant mice from 21 to 44 days of age did not improve the performance in contextual or cued fear conditioning, although it ameliorated the locomotor deficits. In association, it did not affect cerebellar volume.
A series of studies have been dedicated to the analysis of the neuroprotective effects of long-term exposure to environmental enrichment in a rat model of hemicerebellectomy, realized by surgically ablating half of the vermis and one entire cerebellar hemisphere of rats (Burello et al., 2012). Typically, the lesion induces postural and locomotor asymmetries that are almost completely compensated in ~3 weeks, accompanied by persistent impairment in complex motor behavior, spatial learning, and memory performance (Foti et al., 2011). Spontaneous cerebellar compensation is associated with plastic rearrangements in Purkinje cell spine size and density (Gelfo et al., 2016) and in the expression of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) in the spared hemicerebellum (Gelfo et al., 2011). By using this model, Foti et al. (2011) showed that the exposure to environmental enrichment from weaning onward induced an anticipation of locomotor and postural compensation of ~1 week in animals submitted to hemicerebellectomy on the 75th postnatal day. In addition, impairments in complex motor abilities and spatial learning and memory were almost completely prevented (Foti et al., 2011; Gelfo et al., 2016). Regarding morphological findings, Gelfo et al. (2016) reported that enriched rats maintained the amelioration of Purkinje cell dendritic spine size and density induced by environmental enrichment, as also shown by the enriched unlesioned animals, without displaying the additional increase induced by the lesion. Regarding the expression of neurotrophins, Gelfo et al. (2011) similarly showed that the expression of BDNF was not additionally increased in the spared hemicerebellum of the enriched lesioned animals with respect to the standard-reared lesioned animals. Such a finding indicates that structural and molecular upheavals are not necessary for enriched brains. The exposure to environmental enrichment ideally shapes neural connectivity, making further rearrangements unnecessary in cases of lesions. However, hemicerebellar NGF levels were additionally increased by the exposure to environmental enrichment and by the lesion.
Finally, it is appropriate to add the findings of some studies investigating the specific neuroprotective effect of a single component of environmental enrichment, such as exercise, in a rat model of chronic cerebral hypoperfusion. Notably, chronic cerebral hypoperfusion, thought to be the main cause of vascular dementia, is caused by brain thromboembolic events and induces progressive cognitive decline (Lee et al., 2021). By using a model of bilateral common carotid artery occlusion executed in adult rats (12 weeks of age), Lee et al. (2018) reported that the exposure of the animals to treadmill exercise from 4 weeks of age for 8 weeks reduced the deficits in spatial navigation due to subsequent chronic cerebral hypoperfusion. Such a cognitive effect was accompanied by reduced levels of reactive astrocytes and microglial activation in the cerebellum, reduced loss of Purkinje cells, and apoptotic cell death in the cerebellum. In a subsequent study (Lee et al., 2021) in the same model, it was demonstrated that preventive exposure to treadmill exercise from 4 weeks of age for 8 weeks induced a reduction in spatial working memory impairment associated with enhanced mitochondrial calcium retention capacity.
Details on the studies cited in this section are provided in Table 2.

Table 2. Studies on the neuroprotective effects of the exposure to enhanced stimulations in neuropathological animal models involving cerebellar functionality.
Until now, the elective locus of studies on reserve mechanisms has been the whole brain. Most studies have been driven by the evidence that different levels of reserve are associated with brain structural/functional changes in several neurological conditions. However, the specific relationship between reserve measures and individual brain areas has been scarcely described. In particular, only sporadic studies have addressed the link between reserve mechanisms and the cerebellum. This is very surprising, given the well-known plastic properties of the cerebellar networks and their involvement in high-level cognitive and emotional functioning. In recent years, some authors have addressed the specific construct of the cerebellar reserve. This construct, in accordance with the recent literature, encompasses both the capacity of the cerebellar structure to successfully compensate for the damage and the neuroprotective effects of lifelong enhanced experiences on the cerebellar structure. The present review falls within this framework and summarizes the available evidence on the neuroprotective effects of enriched experiences in humans and animals in pathological conditions involving cerebellar functionality. To this end, we included studies that documented the association between the current concept of the brain/cognitive/neural reserve and the cerebellum.
Such a literature review allowed us to reveal in both humans and animals the existence of reserve mechanisms specifically involving the structure, biology, and functionality of the cerebellum. However, these studies are still scarce and do not exhaustively deepen our understanding. Moreover, the evidence provided is not completely clear since the findings reported are sometimes conflicting. According to the key role of the cerebellum in the regulation of high-level cognitive and affective processes, the reported suggestions emphasized the importance of planning specific investigations disentangling the peculiar involvement of the cerebellum in reserve mechanisms. Such an issue has to be addressed in terms of both the dynamics and extension of the reserve development process. It is also important to promote the study of cerebellar reserve mechanisms in relation to the actions of different and specific reserve builders to design more personalized and tuned prevention and rehabilitation interventions in both healthy and pathological populations.
All authors contributed to the design and conceptualization, literature search, and writing of the review and approved the final submitted version.
The research was partially funded by the Italian Ministry of Health, Ricerca Corrente.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Angelucci, F., De Bartolo, P., Gelfo, F., Foti, F., Cutuli, D., Bossù, P., et al. (2009). Increased concentrations of nerve growth factor and brain-derived neurotrophic factor in the rat cerebellum after exposure to environmental enrichment. Cerebellum 8, 499–506. doi: 10.1007/s12311-009-0129-1
Balietti, M., and Conti, F. (2022). Environmental enrichment and the aging brain: is it time for standardization? Neurosci. Biobehav. Rev. 139, 104728. doi: 10.1016/j.neubiorev.2022.104728
Barulli, D., and Stern, Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn. Sci. 17, 502–509. doi: 10.1016/j.tics.2013.08.012
Bordignon, A., Devita, M., Sergi, G., and Coin, A. (2021). “Cerebellar cognitive reserve”: a possible further area of investigation. Aging Clin. Exp. Res. 33, 2883–2886. doi: 10.1007/s40520-021-01795-1
Bosman, L. W. J., and Konnerth, A. (2009). Activity-dependent plasticity of developing climbing fiber–purkinje cell synapses. Neuroscience 162, 612–623. doi: 10.1016/j.neuroscience.2009.01.032
Burello, L., De Bartolo, P., Gelfo, F., Foti, F., Angelucci, F., and Petrosini, L. (2012). Functional recovery after cerebellar damage is related to GAP-43-mediated reactive responses of pre-cerebellar and deep cerebellar nuclei. Exp. Neurol. 233, 273–282. doi: 10.1016/j.expneurol.2011.10.016
Canosa, A., Palumbo, F., Iazzolino, B., Peotta, L., Di Pede, F., Manera, U., et al. (2021). The interplay among education, brain metabolism, and cognitive impairment suggests a role of cognitive reserve in amyotrophic lateral sclerosis. Neurobiol. Aging 98, 205–213. doi: 10.1016/j.neurobiolaging.2020.11.010
Carulli, D., Buffo, A., and Strata, P. (2004). Reparative mechanisms in the cerebellar cortex. Prog. Neurobiol. 72, 373–398. doi: 10.1016/j.pneurobio.2004.03.007
Cesa, R., and Strata, P. (2005). Axonal and synaptic remodeling in the mature cerebellar cortex. Progr. Brain Res. 148, 45–56. doi: 10.1016/S0079-6123(04)48005-4
Cheron, G., Márquez-Ruiz, J., and Dan, B. (2016). Oscillations, timing, plasticity, and learning in the cerebellum. Cerebellum 15, 122–138. doi: 10.1007/s12311-015-0665-9
Conti, L., Riccitelli, G. C., Preziosa, P., Vizzino, C., Marchesi, O., Rocca, M. A., et al. (2021). Effect of cognitive reserve on structural and functional MRI measures in healthy subjects: a multiparametric assessment. J. Neurol. 268, 1780–1791. doi: 10.1007/s00415-020-10331-6
Cutuli, D., Rossi, S., Burello, L., Laricchiuta, D., De Chiara, V., Foti, F., et al. (2011). Before or after does it matter? Different protocols of environmental enrichment differently influence motor, synaptic and structural deficits of cerebellar origin. Neurobiol. Dis. 42, 9–20. doi: 10.1016/j.nbd.2010.12.007
De Bartolo, P., Florenzano, F., Burello, L., Gelfo, F., and Petrosini, L. (2015). Activity-dependent structural plasticity of purkinje cell spines in cerebellar vermis and hemisphere. Brain Struct. Funct. 220, 2895–2904. doi: 10.1007/s00429-014-0833-6
De Zeeuw, C. I., Lisberger, S. G., and Raymond, J. L. (2021). Diversity and dynamism in the cerebellum. Nat. Neurosci. 24, 160–167. doi: 10.1038/s41593-020-00754-9
Diamond, M. C., Law, F., Rhodes, H., Lindner, B., Rosenzweig, M. R., Krech, D., et al. (1966). Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comp. Neurol. 128, 117–125. doi: 10.1002/cne.901280110
Eshra, A., Hirrlinger, P., and Hallermann, S. (2019). enriched environment shortens the duration of action potentials in cerebellar granule cells. Front. Cell. Neurosci. 13, 289. doi: 10.3389/fncel.2019.00289
Foti, F., Laricchiuta, D., Cutuli, D., De Bartolo, P., Gelfo, F., Angelucci, F., et al. (2011). Exposure to an enriched environment accelerates recovery from cerebellar lesion. Cerebellum 10, 104–119. doi: 10.1007/s12311-010-0236-z
Gelfo, F., Cutuli, D., Foti, F., Laricchiuta, D., De Bartolo, P., Caltagirone, C., et al. (2011). Enriched environment improves motor function and increases neurotrophins in hemicerebellar lesioned rats. Neurorehabil. Neural Repair 25, 243–252. doi: 10.1177/1545968310380926
Gelfo, F., Florenzano, F., Foti, F., Burello, L., Petrosini, L., and De Bartolo, P. (2016). Lesion-induced and activity-dependent structural plasticity of purkinje cell dendritic spines in cerebellar vermis and hemisphere. Brain Struct. Funct. 221, 3405–3426. doi: 10.1007/s00429-015-1109-5
Gelfo, F., Mandolesi, L., Serra, L., Sorrentino, G., and Caltagirone, C. (2018). The neuroprotective effects of experience on cognitive functions: evidence from animal studies on the neurobiological bases of brain reserve. Neuroscience 370, 218–235. doi: 10.1016/j.neuroscience.2017.07.065
Gelfo, F., and Petrosini, L. (2022a). Is it possible to develop a cerebellar reserve? Neural Regen. Res. 17, 994–996. doi: 10.4103/1673-5374.324836
Gelfo, F., and Petrosini, L. (2022b). Environmental enrichment enhances cerebellar compensation and develops cerebellar reserve. Int. J. Environ. Res. Public Health 19, 5697. doi: 10.3390/ijerph19095697
Harris, J. C. (2021). Animal models of neurodevelopmental disorders with behavioral phenotypes. Curr. Opin. Psychiatry 34, 87–93. doi: 10.1097/YCO.0000000000000675
Horvath, G., Kiss, P., Nemeth, J., Lelesz, B., Tamas, A., and Reglodi, D. (2015). Environmental enrichment increases PACAP levels in the CNS of adult rats. Neuro. Endocrinol. Lett. 36, 143–147.
Jang, D. C., and Kim, S. J. (2019). Plasticity leading to cerebellum-dependent learning: two different regions, two different types. Pflugers Arch. 471, 927–934. doi: 10.1007/s00424-019-02282-3
Kim, H.-W., Oh, S., Lee, S. H., Lee, S., Na, J.-E., Lee, K. J., et al. (2019). Different types of multiple-synapse boutons in the cerebellar cortex between physically enriched and ataxic mutant mice. Microsc. Res. Tech. 82, 25–32. doi: 10.1002/jemt.23054
Koziol, L. F., Budding, D., Andreasen, N., D'Arrigo, S., Bulgheroni, S., Imamizu, H., et al. (2014). Consensus paper: the cerebellum's role in movement and cognition. Cerebellum 13, 151–177. doi: 10.1007/s12311-013-0511-x
Laricchiuta, D., Petrosini, L., Picerni, E., Cutuli, D., Iorio, M., Chiapponi, C., et al. (2015). The embodied emotion in cerebellum: a neuroimaging study of alexithymia. Brain Struct. Funct. 220, 2275–2287. doi: 10.1007/s00429-014-0790-0
Lee, J. M., Kim, C. J., Park, J. M., Song, M. K., and Kim, Y. J. (2018). Effect of treadmill exercise on spatial navigation impairment associated with cerebellar Purkinje cell loss following chronic cerebral hypoperfusion. Mol. Med. Rep. 17, 8121–8128. doi: 10.3892/mmr.2018.8893
Lee, J. M., Park, J., Lee, J. H., Kwak, H. B., No, M. H., Heo, J. W., et al. (2021). Low-intensity treadmill exercise protects cognitive impairment by enhancing cerebellar mitochondrial calcium retention capacity in a rat model of chronic cerebral hypoperfusion. J. Exerc. Rehabil. 17, 324–330. doi: 10.12965/jer.2142544.272
Lonetti, G., Angelucci, A., Morando, L., Boggio, E. M., Giustetto, M., and Pizzorusso, T. (2010). Early environmental enrichment moderates the behavioral and synaptic phenotype of MeCP2 null mice. Biol. Psychiatry 67, 657–665. doi: 10.1016/j.biopsych.2009.12.022
Luciani, L. (1893). Il Cervelletto. Nuovi Studi Di Fisiologia Normale E Patologica. Philos. Rev. 2, 475–477. doi: 10.2307/2175726
Mandolesi, L., Gelfo, F., Serra, L., Montuori, S., Polverino, A., Curcio, G., et al. (2017). Environmental factors promoting neural plasticity: insights from animal and human studies. Neural Plast. e7219461. doi: 10.1155/2017/7219461
Mitoma, H., Buffo, A., Gelfo, F., Guell, X., Fuc,à, E., Kakei, S., et al. (2020). Consensus Paper. Cerebellar reserve: from cerebellar physiology to cerebellar disorders. Cerebellum 19, 131–153. doi: 10.1007/s12311-019-01091-9
Mitoma, H., Kakei, S., and Manto, M. (2022). Development of cerebellar reserve. Cells 11, 3013. doi: 10.3390/cells11193013
Mitoma, H., Kakei, S., Yamaguchi, K., and Manto, M. (2021). Physiology of cerebellar reserve: redundancy and plasticity of a modular machine. Int. J. Mol. Sci. 22, 4777. doi: 10.3390/ijms22094777
Nag, N., Moriuchi, J. M., Peitzman, C. G. K., Ward, B. C., Kolodny, N. H., and Berger-Sweeney, J. E. (2009). Environmental enrichment alters locomotor behaviour and ventricular volume in Mecp21lox mice. Behav. Brain Res. 196, 44–48. doi: 10.1016/j.bbr.2008.07.008
Naka, F., Shiga, T., Yaguchi, M., and Okado, N. (2002). An enriched environment increases noradrenaline concentration in the mouse brain. Brain Res. 924, 124–126. doi: 10.1016/S0006-8993(01)03257-7
Nithianantharajah, J., Levis, H., and Murphy, M. (2004). Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiol. Learn. Mem. 81, 200–210. doi: 10.1016/j.nlm.2004.02.002
Olivito, G., Serra, L., Marra, C., Di Domenico, C., Caltagirone, C., Toniolo, S., et al. (2020). Cerebellar dentate nucleus functional connectivity with cerebral cortex in Alzheimer's disease and memory: a seed-based approach. Neurobiol. Aging. 89, 32–40. doi: 10.1016/j.neurobiolaging.2019.10.026
Pascual, R., and Bustamante, C. (2013). Early postweaning social isolation but not environmental enrichment modifies vermal purkinje cell dendritic outgrowth in rats. Acta Neurobiol. Exp. 73, 387–393.
Petrosini, L., De Bartolo, P., Foti, F., Gelfo, F., Cutuli, D., Leggio, M.G., and Mandolesi, L. (2009). On whether the environmental enrichment may provide cognitive and brain reserves. Brain Res. Rev. 61, 221–239. doi: 10.1016/j.brainresrev.2009.07.002
Petrosini, L., Picerni, E., Termine, A., Fabrizio, C., Laricchiuta, D., and Cutuli, D. (2022). The cerebellum as an embodying machine. Neuroscientist 10738584221120187. doi: 10.1177/10738584221120187
Picerni, E., Laricchiuta, D., Piras, F., Petrosini, L., Spalletta, G., and Cutuli, D. (2022). Cerebellar engagement in the attachment behavioral system. Sci. Rep. 12, 13571. doi: 10.1038/s41598-022-17722-x
Picerni, E., Laricchiuta, D., Piras, F., Vecchio, D., Petrosini, L., Cutuli, D., et al. (2021). Macro- and micro-structural cerebellar and cortical characteristics of cognitive empathy towards fictional characters in healthy individuals. Sci. Rep. 11, 8804. doi: 10.1038/s41598-021-87861-0
Rocca, M. A., Riccitelli, G. C., Meani, A., Pagani, E., Del Sette, P., Martinelli, V., et al. (2019). Cognitive reserve, cognition, and regional brain damage in MS: a 2 -year longitudinal study. Mult. Scler. 25, 372–381. doi: 10.1177/1352458517750767
Rosenzweig, M. R., and Bennett, E. L. (1996). Psychobiology of plasticity: effects of training and experience on brain and behavior. Behav. Brain Res. 78, 57–65. doi: 10.1016/0166-4328(95)00216-2
Rosenzweig, M. R., Bennett, E. L., Hebert, M., and Morimoto, H. (1978). Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 153, 563–576. doi: 10.1016/0006-8993(78)90340-2
Sampedro-Piquero, P., and Begega, A. (2017). Environmental enrichment as a positive behavioral intervention across the lifespan. Curr. Neuropharmacol. 15, 459–470. doi: 10.2174/1570159X14666160325115909
Schmahmann, J. D. (2019). The cerebellum and cognition. Neurosci. Lett. 688, 62–75. doi: 10.1016/j.neulet.2018.07.005
Scholz, J., Allemang-Grand, R., Dazai, J., and Lerch, J. P. (2015). Environmental enrichment is associated with rapid volumetric brain changes in adult mice. Neuroimage 109, 190–198. doi: 10.1016/j.neuroimage.2015.01.027
Serra, L., Bruschini, M., Di Domenico, C., Gabrielli, G. B., Marra, C., Caltagirone, C., et al. (2017b). Memory is not enough: the neurobiological substrates of dynamic cognitive reserve. J. Alzheimers Dis. 58, 171–184. doi: 10.3233/JAD-170086
Serra, L., and Gelfo, F. (2019). What Good Is the Reserve? A translational perspective for the managing of cognitive decline. Neural Regen. Res. 14, 1219–1220. doi: 10.4103/1673-5374.251328
Serra, L., Gelfo, F., Petrosini, L., Di Domenico, C., Bozzali, M., and Caltagirone, C. (2018). Rethinking the reserve with a translational approach: novel ideas on the construct and the interventions. J. Alzheimers Dis. 65, 1065–1078. doi: 10.3233/JAD-180609
Serra, L., Mancini, M., Cercignani, M., Di Domenico, C., Spanò, B., Giulietti, G., et al. (2017a). Network-based substrate of cognitive reserve in Alzheimer's Disease. J. Alzheimers Dis. 55, 421–430. doi: 10.3233/JAD-160735
Stern, Y. (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004
Stern, Y. (2012). Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 11, 1006–1012. doi: 10.1016/S1474-4422(12)70191-6
Stern, Y., Alexander, G. E., Prohovnik, I., and Mayeux, R. (1992). Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann. Neurol. 32, 371–375. doi: 10.1002/ana.410320311
Stern, Y., and Barulli, D. (2019). Cognitive reserve. Handb. Clin. Neurol. 167, 181–190. doi: 10.1016/B978-0-12-804766-8.00011-X
Toniolo, S., Serra, L., Olivito, G., Caltagirone, C., Mercuri, N. B., Marra, C., et al. (2020). Cerebellar white matter disruption in alzheimer's disease patients: a diffusion tensor imaging study. J. Alzheimers Dis. 74, 615–624. doi: 10.3233/JAD-191125
Toniolo, S., Serra, L., Olivito, G., Marra, C., Bozzali, M., and Cercignani, M. (2018). Patterns of cerebellar gray matter atrophy across Alzheimer's Disease progression. Front. Cell. Neurosci. 12, 430. doi: 10.3389/fncel.2018.00430
Uphouse, L. (1978). In vitro RNA synthesis by chromatin from three brain regions of differentially reared rats. Behav. Biol. 22, 39–49. doi: 10.1016/S0091-6773(78)91989-2
Uphouse, L., and Tedeschi, B. (1979). Environmental enrichment and brain chromatin. Behav. Neural Biol. 25, 268–270. doi: 10.1016/S0163-1047(79)90636-8
van Dun, K., Manto, M., and Meesen, R. (2022). Cerebellum and neurorehabilitation in emotion with a focus on neuromodulation. Adv. Exp. Med. Biol. 1378, 285–299. doi: 10.1007/978-3-030-99550-8_18
Keywords: cerebellar reserve, brain/cognitive/neural reserve, cerebellum, cognition, neuroprotection, environmental enrichment, humans, animal models
Citation: Gelfo F, Serra L and Petrosini L (2023) New prospects on cerebellar reserve: Remarks on neuroprotective effects of experience in animals and humans. Front. Syst. Neurosci. 16:1088587. doi: 10.3389/fnsys.2022.1088587
Received: 03 November 2022; Accepted: 15 December 2022;
Published: 06 January 2023.
Edited by:
Jimena Laura Frontera, Institut Pasteur, FranceReviewed by:
Shinji Kakei, Jissen Women's University, JapanCopyright © 2023 Gelfo, Serra and Petrosini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Gelfo,  Zi5nZWxmb0Boc2FudGFsdWNpYS5pdA==
Zi5nZWxmb0Boc2FudGFsdWNpYS5pdA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.